Abstract
Introduction:
Cholangiocarcinoma (CCA) is the second most common type of primary liver cancer. Several factors, such as epigenetic changes in promoter genes, gene expression, and microRNAs (miR), can contribute to genomic instability in cancer. This study aimed at evaluating the expression of VEGF, miRs 145-3p, and 101-3p in patients with CCA and their potential as biomarkers for diagnosis and prognosis of CCA.
Material and methods :
Sixty two patients were studied. Out of these 62 patients, 41 cases had confirm CCA and 21 cases had hepatopathies complications. The RNA was extracted from a paraffined tissue block, and then the synthesis of cDNA was performed. The analysis of the expression of VEGF, miR-145-3p, and miR-101-3p was carried out by polymerase chain reaction in real time.
Results:
The findings revealed that miRs 145-3p and 101-3p were under expressed in the case group compared to the control group (0.46; 0.17; P = 0.0001, respectively). VEGF was overexpressed in the case group compared to the control group (11.8; P = 0.0001). An increase in miR-145-3p expression level was observed in patients with perihilar CCA compared to those with distal CCA (0.51 ± 0.41; 0.17 ± 0.13; P = 0.0698). Survival rate analysis showed that 41.9% of patients with intrahepatic CCA and 31.5% of patients with extrahepatic CCA were free from death within 11 months, leading to a significant difference (P> 0.05).
Conclusion:
The underexpression of miRNAs, tumor suppressors, the overexpression of VEGF, smoking, and aging were associated with CCA based on our findings. It seems that the reduced expression of the studies miRNAs and increased expression of VEGF can contribute to a decrease in survival rate of patients with tumor in their intrahepatic bile ducts.
Key Words: Gene expression, MicroRNAs, bile duct diseases, molecular biology
Introduction
Cholangiocarcinoma (CCA) is the second most common type of primary liver cancer, responsible for 10 to 15% of hepatobiliary tumors. According to its anatomical location, CCA can be classified as intrahepatic CCA (iCCA), perihilar CCA (pCCA), and distal CCA (dCCA) (Banales et al., 2020; Massironi et al., 2020).
Although associated with inflammatory diseases and malformations in the bile ducts, its etiology is unknown in most cases (Jing et al., 2020). Studies show that treatments, such as chemotherapy and radiotherapy, have a limited efficacy and no significant effect on patient survival (Gringeri et al., 2020). Photodynamic therapy (PDT), as a disease site-specific treatment modality, has shown promising results, becoming the standard treatment for unresectable CCA. However, in general, CCA cells do not respond to PDT and this therapy is currently palliative and not curative (Moole et al., 2017).
Total tumor resection is another option for possible treatment of CCA in only 20-30% of patients since most cases are in an advanced stage at the time of diagnosis (Moole et al., 2017). Nevertheless, lifestyle habits, comorbidities, and aging reduce the efficacy of this treatment. The average survival of patients with unresectable tumors is 6 to 12 months, and , palliative treatments are indicated for the most cases (Brito et al., 2015).
Gradual accumulation of mutations in cancer-related genes and chromosomal changes are involved in human carcinogenesis (Yvamoto et al., 2015). Several types of cells, including fibroblasts, hypertrophic smooth muscle cells, chondrocytes, and osteoblasts (Yvamoto et al., 2015), produce vascular endothelial growth factor (VEGF), whose gene is located on the human chromosome 6p21.3. It is an angiogenesis promoter factor, which constitutes an important step in the development of cancer and contributes to the growth of the primary tumor, invasion, and metastasis in several types of cancer (Peng et al., 2019).
Another important molecular mechanism is microRNAs (miRNAs), which are small fragments of endogenous non-coding protein RNA and composed of 17-24 nucleotides that regulate the expression of target genes, thus blocking their translation. They have regulatory roles in gene expressionand bind to complementary siteson 3’- untranslated regions (3’ UTRs), repressing the target messenger RNA or promoting its degradation through cleavage (Ingenito et al., 2019).
Evidence indicates that some miRNAs function as oncogenes or tumor suppressors along with influencing various biological processes, such as cell proliferation, migration, invasion, differentiation, and apoptosis, especially in cancer cells (Yin et al., 2020). Promising results have been observed in the treatment of several neoplasms after using circulating miRNAs as predictors (Yin et al., 2020). The identification of molecular alterations in miRNAs and candidate genes for CCA biomarkers may contribute to clarify the molecular mechanisms involved in the pathogenesis of CCA, thus assisting with the early diagnosis of the disease and choosing new therapeutic interventions.
In this context, miR-145 stands out acting directly on VEGF function. In addition, it is overexpressed in biliary tumors, suggesting its performance as a tumor suppressor. on the other hand, mir-101-3p promotes apoptosis and decreases proliferation and metastasis (Xu et al., 2017). Thus, the aim of this study was to evaluate the expression of VEGF and miRNAs involved in oncogenesis in patients with CCA, in addition to comorbidities, lifestyle, clinical profile, and survival.
Materials and Methods
Patients
This study was carried out at São José do Rio Preto Medical School (FAMERP) in partnership with the State University of Campinas Medical Science School (FMC / UNICAMP). To do this study, 41 patients with CCA, whose samples of tumor tissue were collected from 2006 to 2020 and stored in paraffin blocks, and 21 individuals without clinical signs of hepatopathies were selected regardless of their sex, ethnic group, and age.
Patients with CCA (case group) were selected after histopathological confirmation of their paraffined blocks by the HB / FAMERP and FCM / UNICAMP Pathology Services . The patients’ clinical, demographic, and lifestyle information was extracted using computerized medical records and a questionnaire. Waiver of the Informed Consent Form (ICF) was requested as this material had already been collected and; therefore, it presented no additional risks to the patients.
The control group included patients who referred to the General Surgery Service of HB / FAMERP. Their biological sample was obtained during video laparoscopic cholecystectomy. The patients were informed about the objective and procedure of the study. This study protocol was approved by the Research Ethics Committee REC / FAMERP (CAAE Nº 42513115.7.0000.5415) before initiation.
Analysis of the expressions of miR-145-3p, miR-101-3p, and VEGF
For the case group, total RNA was extracted from their paraffin-embedded tissue samples by “ReliaPrep ™ FFPE Total RNA Miniprep System” (Promega Biotechnology - Brazil) according to the manufacturer’s protocol. For the control group, the collected material was stored immediately in RNAlater for RNA extraction by the Trizol method according to the manufacturer’s protocol. The concentration and purity were analyzed using NanoDrop-ND-1000 (Thermo Scientific - USA) according to the manufacturer’s manual and by considering the sample absorbance (proportion of 260/280 and 1.7 - 2.0, respectively).
The reverse transcription of miRNAs and the obtaining of cDNA for VEGF analysis were performed using the RT primers kit (Taq Man MicroRNA Reverse Transcription, Applied Biosystems, Foster City, CA, USA) and High Capacity cDNA® (Applied Biosystems), respectively. The real-time polymerase chain reaction (qPCR) was conducted using the StepOne Plus ™ Real Time-PCR® system according to the manufacturer (Applied Biosystems). The samples were processed at 50 ° C for 2 minutes, 95 ° C for 10 minutes, followed by 40 cycles of 95 ° C for 15 seconds and 60 ° C for 1 minute. All samples were tested in triplicate and expressed as relative difference in the number of times in relation to the calibrator (controls). Negative controls were included for all reactions.
Statistical analysis
Statistical analysis was performed using the following softwares: IBM® SPSS®Statistics software, version 20.0 (IBM Corporation, Armonk, New York, USA), StatsDirect, and GraphPad. The descriptive analysis of the variables and the results were presented as median, minimum, and maximum. Fisher’s exact test was used to analyze frequency of the variables in relation to the clinical-demographic profile, lifestyle, and comorbidities. Kruskal Wallis test was applied for the analysis of non-parametric quantitative variables when comparing three or more groups , and Mann Whitney for two groups and parametric methods (analysis of continuous quantitative variables with normal distribution). The relative expression of miRs145-3p and 101-3p was calculated by the 2-∆∆Ct method, and the reaction normalized by the housekeeping genes U6 and RNU48, and for VEGF the ACTB and RPLPO genes . To evaluate the discriminative power of molecular markers and determine cut-off points, sensitivity, and specificity values, a ROC curve (receiver operating characteristic) was used and areas under the curve ≥0.7 were considered as having clinical relevance. Logistic regression analysis was applied to verify the chance of occurrence of the event (CCA) in the presence of different variables by using the Kramer-Tukey multiple comparison test. Patient survival was assessed in months using Kaplan-Meier actuarial curve. The time of survival was defined as period from diagnosis to the date of death of each patient. To compare groups, an alpha error of 5% was considered.
Results
In terms of lifestyle habits, the findings revealed that smoking prevailed in the case group (36.6%; P = 0.034), while the frequency of alcohol use was similar in both groups (study group = 24.4%; control group = 23.8%; P> 0.05). Two groups did not differ significantly in terms of systemic arterial hypertension (SAH: 24.4%; 23.8%, respectively) and diabetes mellitus (DM: 9.75%; 19.0%, respectively; P> 0.05) frequencies. On the other hand, body mass index (BMI) ≥25 kg / m2 stood out in the controls (66.6%, P = 0.012). Regarding demographic characteristics, the findings indicated that the mean age of patients in the case group was significantly (median = 56 years) higher than those in the control group (median = 39 years; P = 0.0003). The frequency of females in both groups differed significantly (case group = 51.2%; control group = 80.9%; P = 0.0456).
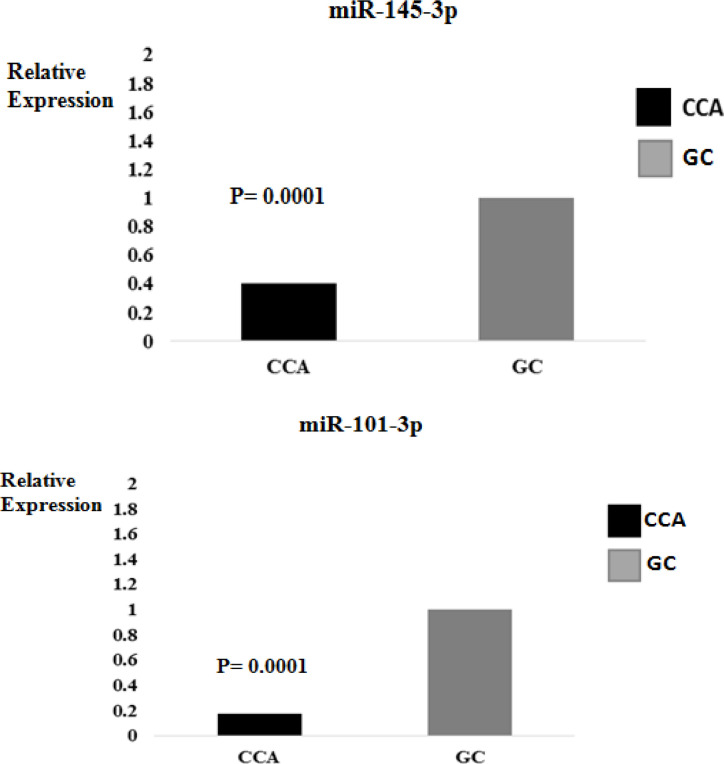
The frequency of iCCA (46.3%) was the highest, followed by pCCA (36.6%) and dCCA (17.1%) (Table 1). Concerning gene expressions, it was found that mir-145-3p and mir-101-3p were under-expressed in the case group compared to the control group (0.46; 0.17; P = 0.0001, respectively) (Figure 1). On the other hand, there was an overexpression of VEGF in the case group compared to the controls (11.8; P = 0.0001). No association was detected between VEGF expression and miRNAs (P> 0.05, data not shown). The findings demonstrated that the expression of miR-145-3p was highlighted in patients with pCCA (0.51 ± 0.41), followed by iCCA (0.41 ± 0.36) and dCCA (0.17 ± 0.13), while led to no significant difference between groups (P> 0.05). For miR-101-3p, patients with dCCA (0.94 ± 1.41) stood out, followed by iCCA (1.18 ± 1.27), and pCCA (0.83 ± 0.83), while led to no significant difference between groups (P> 0.05).
Table 1.
Information on Demographic Characteristics, Lifestyle, Comorbidities, and Clinical Classification in Patients with Cholangiocarcinoma (CCA) and Individuals without Signs of the Disease (Control Group)
| Variables | CCA (N =41) | Control group (N = 21) | p-value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Lifestyle habits | |||||
| Smoking | 15 | 36.60 | 2 | 9.5 | 0.0343 |
| Alcohol | 10 | 24.40 | 5 | 23.8 | 1 |
| Comorbidities | |||||
| SAH | 10 | ff | 5 | 23.8 | 1 |
| Diabetes mellitus | 4 | 9.75 | 4 | 19 | 0.4291 |
| BMI | |||||
| ≥25 kg/m2 | 9 | 21.90 | 14 | 66.6 | 0.0124 |
| Age (years) | |||||
| Median | 56 | 39 | 0.0003 | ||
| Minimum | 30 | 24 | |||
| Maximum | 79 | 67 | |||
| Gender | |||||
| Male | 20 | 48.80 | 4 | 19.1 | 0.0456 |
| Female | 21 | 51.20 | 17 | 80.9 | |
p, significance level <0.05/Mann Whitney and Fisher Test; N, Number of individuals de indivíduos; DM, diabetes mellitus; SAH, systemic arterial hypertension; BMI, body mass index
Figure 1.
Relative Expression of miR-145-3p and miR-101-3p (mean value of 2-ddCt) in Patients with Cholangiocarcinoma (CCA) and Individuals without the Disease (CG)
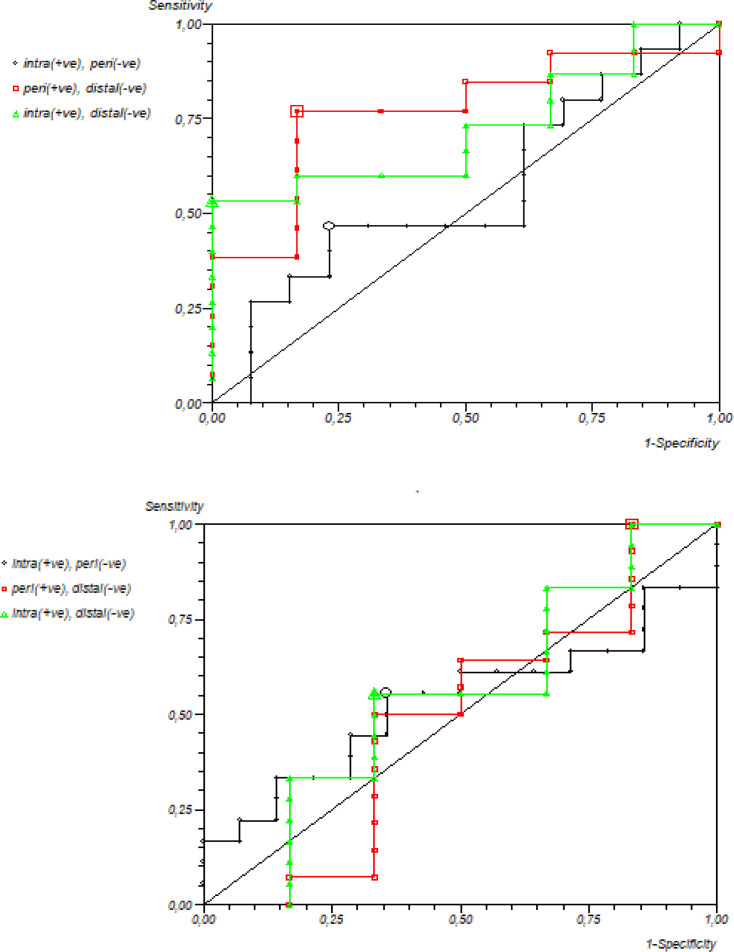
The evaluation of the discriminatory power of miR-145-3p and miR-101-3p in relation to tumor subtypes of iCCA and dCCA (Figure 2) revealed no association (area under the curve - AUC = 0.63 and 0.55, sensitivity of 53% and 55%, and specificity of 100% and 66%, respectively). No association in regard to pCCA and iCCA was found, either(AUC = 0.46; 0.55, sensitivity of 46%; 55%, and specificity of 76 %; 64%, respectively). However, miR-145-3p showed relevant discriminatory power between pCCA versus dCCA (AUC = 0.76; 0.51, 76%; 100% sensitivity and 83%; 16% specificity).
Figure 2.
Receiver Operator Characteristic (ROC) Curve of the Levels of Relative Expression of miR-145-3p and miR-101-3p Considering Patients with Intrahepatic, Perihilar and Distal Cholangiocarcinoma (CCA)
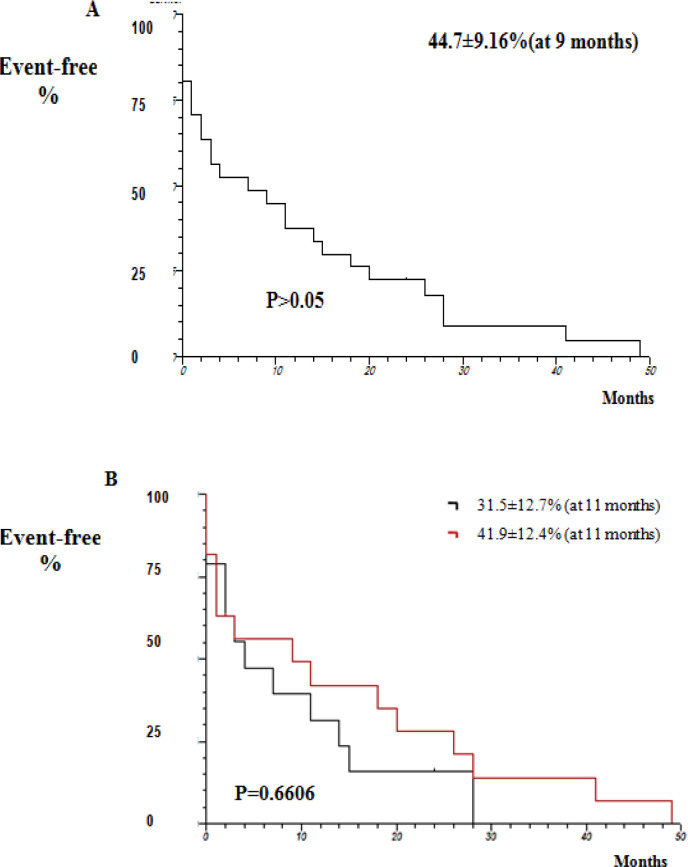
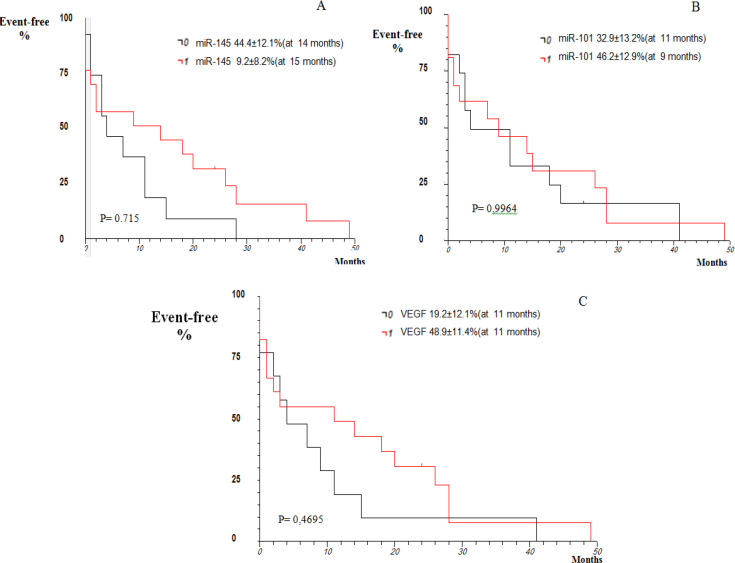
Considering survival analysis, the findings demonstrated that 44.7 ± 9.16% of patients were free from death within nine months (P> 0.05; Figure 3A). The comparison between intrahepatic and extrahepatic CCA showed an increase in event / death free duration in patients with intrahepatic CCA (41.9 ± 12.5%) compared to those with extrahepatic CCA (31.5 ± 12.7%; P = 0.6606; Figure 3B). According to our findings, there was a similarity in the expression of the microRNAS and VEGF in patients with CCA (P> 0.05; Figure 4).
Figure 3.
Actuarial Kaplan-Meier Curve Regarding Event-Free Survival Analysis of Patients with CholangiocarciNoma. Actuarial Kaplan-Meier Curve; Log Rank Test, with significance level of <0.05
Figure 4.
Kaplan-Meier Actuarial Curve regarding Survival Analysis Related to the Expression of miR-145-3p, miR-101-3p, and VEGF in Patients with Cholangiocarcinoma
The logistic regression analysis to identify independent variables, and the respective chance of the individual having CCA, presented the equation logit Y = -2.564949 = -2.564949 +43.035593 miR-101 +62.821957 mir-145 +25, 505954 VEGF -25.671698 Cholangitis -35.690944 Cholelithiasis -18.494324 Smoking -18.970156 Alcoholism -21.191733 Hypertension -1.724476 Diabetes, revealing no significant difference between the factors involved (P> 0.05).
Discussion
In this study, lifestyle habits, comorbidities, logistic regression, and survival were compared in patients with CCA and controls. In addition, the association of these variables with expression of miR-145, miR-101, and VEGF was assessed. In relation to lifestyle habits, smoking was more frequent in the case group (36.6%) based on our findings, suggesting its association with CCA. Similarly, Huang et al., (2017) found that the carcinogenic compounds of tobacco, such as 2-acetylaminofluorene and 4-aminobiphenyl, might be an initiator of tumorigenesis in hepatocytes, causing hepatocarcinoma and CCA (Petrick et al., 2018). The N-nitrous compounds and nicotine are some of the main carcinogens present in cigarettes, which are able to modify several pathways and impair endothelial function, cell proliferation, and migration, thus leading to the formation of tumors (Petrick et al., 2018). In contrast, an Asian study found no association between smoking and CCA and introduced other risk factors for the disease (Huang et al., 2017 ).
Alcohol consumption frequency was similar in the groups based on our findings, which was similar to findings of a study done by Ye et al., (2013). However, another study showed higher risk of developing this disease in patients who consumed at least 80g of alcohol a day (Shaib et al., 2007).
Among comorbidities, SAH frequency was shown to be similar in both groups. It should be noted that there were patients with controlled hypertension who were under treatment with beta-blockers in both groups. However, a prospective study evaluated hypertension and the risk of developing neoplasms over 12 years concluded that the incidence of cancer in patients with SAH was higher (Koene et al., 2016). Admittedly, hypertension is related to angiogenic factors, especially high levels of VEGF, and it may be an important factor inducing the formation of new blood vessels. Similarly, a study suggested that angiotensin II, a key hormone in vasoconstriction, stimulated the production of VEGF, thus potentiating the development or progression of cancer (Koene et al., 2016).
In this study, patients and controls did not differ significantly in terms of DM . In contrast, in Thai casuistry, patients with CCA and diabetes had a higher mortality rate compared to those without DM (Saengboonmee et al., 2015). This difference in findings can be due to this fact that DM has a higher incidence in males (International Diabetes Federation, 2013) though there was a prevalence of females in both groups studied here. In our study, BMI ≥25 kg /m2 prevailed in the control group. However, epidemiological studies demonstrated an association between obesity and several types of cancer (Mansour, 2013). According to a previous study, patients with a BMI ≥30 kg /m2 had a 1.5 greater risk for CCA compared to those with a BMI >25 kg / m2 (Grainge et al., 2009). The adipose tissue secretes several molecules in the circulation. Some of these molecules, known as adipokines, have an important role in cell modulation, leading to the development of cancer (Mansour, 2013).
In this study, it was observed that the mean age of patients with CCA was higher, which was similar to findings of a study done in the North American population (Sahai et al., 2018). Notably, several molecular, cellular, and physiological changes are associated with age, which may contribute to an increased risk of cancer. Thus, at the cellular level, underlying aging processes, such as increased oxidative stress, macromolecular damage, genomic instability, senescence, unregulated cell growth, and differentiation can trigger the development of cancer (Hwang et al., 2020).
It was also detected that the prevalence of females in patients with CCA was higher, which was in line with Petrick et al.’ study findings (Petrick et al., 2020). Admittedly, cholangiocytes express α and β estrogen receptors, which are potentially mediated by interleukin-6 (IL-6) or VEGF, this hormone plays an important role in cholangiocarcinogenesis (Petrick et al., 2020).
Elevated levels of estradiol in women is associated with iCCA (Mancinelli et al., 2010). Regarding tumor subtypes, iCCA and pCCA stood out in our patients, which was in line with Banales et al.’ study findings(Banales et al., 2016). The increasing incidence of iCCA in recent decades outstands, especially in western countries, due to risk factors such as viral and parasitic infections, with tropism by the hepatocyte (Bartsch et al., 2019).
In this study, miR-145-3p was shown to be reduced in patients with CCA, similar to a study done in European population (Goeppert et al., 2019). Admittedly, miR-145 acts as a silencer that targets various tumor-specific genes, and for that, it is known as an important tumor suppressor (Xu et al., 2019). Molecular mechanisms related to tumor suppression by this miRNA showed that p53, the main suppressive agent, binds directly to the promoter region of pre-miR-145 (Goto et al., 2017). The same occurred for miR-101-3p according to Sun et al., (2019), who observed reduced expression of miR-101 in hepatocellular carcinoma (HCC) and cell lines promoting apoptosis and suppressing tumorigenicity in HCC cells. Thus, it seems that a decrease in miR-145-3p expression is associated with increased tumor invasion and lymph node metastasis, suggesting that the negative regulation of this miRNA may play a role in tumor progression (Xu et al., 2019).
In this study, we found an overexpression of VEGF, which was similar to findings of a study done on Chinese population (Pan et al., 2020). Tumor remodeling and angiogenesis are processes that require activation of multiple vascular components, such as division of endothelial cells, degradation of the vascular basement membrane, degradation of the peripheral extracellular matrix, and migration of endothelial cells. In the aforementioned study, VEGF high expression was associated with the development and progression of the disease (Pan et al., 2020). Life habits and comorbidities were not associated with the expression of neither microRNAs nor VEGF in this study. The same was observed for tumor subtypes, which should be evaluated in future prospective studies using larger sample size.
Regarding the survival rate, this study showed that 44% of patients were free from the death within nine months of follow-up, which was in line with a study done by Sweigert et al., (2020). CCA is a silent disease in the early stages and often is diagnosed in advanced stages, , resulting in a worse prognosis and low survival rate (Banales et al., 2020). On the other hand, in this study, it was found that patients with extrahepatic CCA had increased survival rate compared to those with intrahepatic type, the second most common primary malignant tumor of the liver accounting for 10-15% of hepatobiliary neoplasms (Ahn e Kang, 2020).
Due to anatomopathological heterogeneity and lack of specific symptoms, the clinical symptoms, the type of surgical resection, and the prognosis of the intrahepatic type were different between patients with extrahepatic CCA and extrahepatic ones. Therefore, the prognosis and targeted treatment is limited, causing low survival rate (Ahn e Kang, 2020). In addition, an association was detected between decreased survival rate and expression of microRNAs and VEGF although it was not significant. These findings were similar to studies carried out in other populations (Zeng et al., 2020). Reduced expression of tumor suppressors, namely mir-101 and mir-145, was associated with reduced survival in several types of cancer. Both of them play an important role in inhibiting cell invasion and migration and promoting apoptosis (Dong et al., 2018; Xu et al., 2019).
Regarding VEGF expression, it was found that its overexpression was associated with decreased survival rate though it did not lead to a significant difference. These findings were also in line with those of an Asian study done by Wang et al (Wang et al., 2020). Several factors regulate the occurrence and development of CCA, such as proliferation, apoptosis, and tumor angiogenesis, which are closely related to biological behavior of (Xu et al., 2019). Cancer-associated fibroblasts promote angiogenesis through the production of several molecules, including VEGF, fibroblast growth factor (FGF), and interleukin-6 (IL-6). They also promote the production of VEGF-A, IL-10, and transforming growth factor beta (TGFβ), which causes the polarization of the macrophage towards the pro-angiogenic phenotype M2 (Simone et al., 2017).
Based on the results of logistic regression analysis to identify independent variables and the respective chance of belonging to the group with CCA, there was no association between variables analyzed, such as life habits, comorbidities, and genetic profile, and risk of developing CCA. These findings were in contrast with those reported by Petrick et al., (2017) in an American population .
Cancer is a multifactorial disease, involving environmental and genetic factors, making populations with different cultures and habits even more heterogeneous and subsequently contributing to divergences and difficulty in comparative analysis between studies. This challenge can be overcome through prospective studies f evaluating specific subgroups.
In this study, the under-expression of miR-145-3p and miR-101-3p, tumor suppressors, and the overexpression of VEGF, an important angiogenic factor, were associated with the risk of developing CCA. In addition, according to our findings, smoking and aging increased the risk of CCA. It seems that reduced expression of miRNAs and increased expression of VEGF can decrease the survival rate of patients with tumors in their intrahepatic bile ducts.
Author Contribution Statement
All authors worked on data analysis and manuscript preparation. The final manuscript was already approved by all authors.
Acknowledgements
We thank the following research development agencies for the financial support: São Paulo Research Foundation (FAPESP; Grant: 2018/00356-3), National Council for Scientific and Technological Development - CNPq (400988 / 2016-0 - Research Grants).
Funding Statement
We thank National Council for Scientific and Technological Development (CNPq; Grants 400988/2016-0 and 301704/2017-1) and São Paulo Research Foundation (FAPESP; Grant: 2018/00356-3) for their financial support of this research. We are also grateful to the São José do Rio Preto Medical School Multi-User Laboratory (FAMERP/LMU) for their support regarding the use of their microtome and StepOne Plus (Applied Biosystems).
Ethics approval
This study was approved by the Research Ethics Committee of REC / FAMERP (CAAE Nº 42513115.7.0000.5415).
Conflict of interest
The authors declared that there is no conflict of interest regarding the publication of this article.
References
- Ahn KS, Kang KJ. Molecular heterogeneity in intrahepatic cholangiocarcinoma. World J Hepatol. 2020;12:1148–57. doi: 10.4254/wjh.v12.i12.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banales JM, Cardinale V, Carpino G, et al. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA) Nat Rev Gastroenterol Hepatol. 2016;13:261–80. doi: 10.1038/nrgastro.2016.51. [DOI] [PubMed] [Google Scholar]
- Banales JM, Marin JJG, Angela Lamarca, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557–88. doi: 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch F, Baumgart J, Tripke V, et al. Resection of intrahepatic cholangiocarcinoma in elderly patients - is it reasonable? BMC Surg. 2019;19:157. doi: 10.1186/s12893-019-0620-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito AF, Abrantes AM, Encarnação JC, Tralhão JG, Botelho MF. Cholangiocarcinoma: From molecular biology to treatment. Med Oncol. 2015;32:245. doi: 10.1007/s12032-015-0692-x. [DOI] [PubMed] [Google Scholar]
- Dong X, Liu Y. Expression and significance of miR-24 and miR-101 in patients with advanced gastric cancer. Oncol Lett. 2018;16:5769–74. doi: 10.3892/ol.2018.9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeppert B, Truckenmueller F, Ori A, et al. Profiling of gallbladder carcinoma reveals distinct miRNA profiles and activation of STAT1 by the tumor suppressive miRNA-145-5p. Sci Rep. 2019;9:4796. doi: 10.1038/s41598-019-40857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Kurozumi A, Arai T, et al. Impact of novel miR-145-3p regulatory networks on survival in patients with castration-resistant prostate câncer. Br J Cancer. 2017;117:409–20. doi: 10.1038/bjc.2017.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainge MJ, West J, Solaymani-Dodaran M, Aithal GP, Card TR. The antecedents of biliary cancer: a primary care case-control study in the United Kingdom. Br J Cancer. 2009;100:178–80. doi: 10.1038/sj.bjc.6604765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gringeri E, Gambato M, Sapisochin G, et al. Cholangiocarcinoma as an Indication for Liver Transplantation in the Era of Transplant Oncology. J Clin Med. 2020;9:1353. doi: 10.3390/jcm9051353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, You L, Xie W, Ning L, Lang J. Smoking and risk of cholangiocarcinoma: a systematic review and meta-analysis. Oncotarget. 2017;8:100570–81. doi: 10.18632/oncotarget.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JH, McGovern J, Minett GM, et al. Mobilizing serum factors and immune cells through exercise to counteract age-related changes in cancer risk. Exerc Immunol Rev. 2020;26:80–99. [PubMed] [Google Scholar]
- Ingenito F, Roscigno G, Affinito A, et al. The Role of Exo-miRNAs in Cancer: A Focus on Therapeutic and Diagnostic Applications. Int J Mol Sci. 2019;20:4687. doi: 10.3390/ijms20194687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Diabetes Federation. IDF Diabetes Atlas. 6th ed Brussels, Belgium: International Diabetes Federation: 2013. [Google Scholar]
- Jing X, Chen Y, Chen Y, et al. Down-regulation of USP8 Inhibits Cholangiocarcinoma Cell Proliferation and Invasion. Cancer Manag Res. 2020;12:2185–94. doi: 10.2147/CMAR.S234586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koene RJ, Prizment AE, Blaes A, Konety SH. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation. Author Manuscript Circulation. 2016;133:1104–14. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancinelli R, Onori P, Demorrow S, et al. Role of sex hormones in the modulation of cholangiocyte function. World J Gastrointest Pathophysiol. 2010;1:50–62. doi: 10.4291/wjgp.v1.i2.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massironi S, Pilla L, Elvevi A, et al. New and Emerging Systemic Therapeutic Options for Advanced Cholangiocarcinoma. Cells. 2020;9:688. doi: 10.3390/cells9030688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moole H, Tathireddy H, Dharmapuri S, et al. Success of photodynamic therapy in palliating patients with nonresectablecholangiocarcinoma: A systematic review and meta-analysis. World J Gastroenterol. 2017;23:1278–88. doi: 10.3748/wjg.v23.i7.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan S, Hu Y, Hu M, et al. S100A8 facilitates cholangiocarcinoma metastasis via upregulation of VEGF through TLR4/NFκB pathway activation. Int J Oncol. 2020;56:101–12. doi: 10.3892/ijo.2019.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsi MA. Obesity and cholangiocarcinoma. World J Gastroenterol. 2013;19:457–62. doi: 10.3748/wjg.v19.i4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng T, Deng X, Tian F, et al. The interaction of LOXL2 with GATA6 induces VEGFA expression and angiogenesis in cholangiocarcinoma. Int J Oncol. 2019;55:657–70. doi: 10.3892/ijo.2019.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrick JL, Campbell PT, Koshiol J, et al. Tobacco, alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: The Liver Cancer Pooling Project. Br J Cancer. 2018;118:1005–12. doi: 10.1038/s41416-018-0007-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrick JL, Úna CM, Zhang X, et al. Exogenous hormone use, reproductive factors and risk of intrahepatic cholangiocarcinoma among women: results from cohort studies in the Liver Cancer Pooling Project and the UK Biobank. Br J Cancer. 2020;123:316–24. doi: 10.1038/s41416-020-0835-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrick JL, Yang B, Altekruse SF, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States. A population-based study in SEER-Medicare. 2017;12:e0186643. doi: 10.1371/journal.pone.0186643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saengboonmee C, Seubwai W, Wongkham C, Wongkham S. Diabetes mellitus: Possible risk and promoting factors of cholangiocarcinoma: association of diabetes mellitus and cholangiocarcinoma. Cancer Epidemiol. 2015;39:274–8. doi: 10.1016/j.canep.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Sahai V, Catalano PJ, Zalupski MM, et al. Nab-Paclitaxel and Gemcitabine as First-line Treatment of Advanced or Metastatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2018;4:1707–12. doi: 10.1001/jamaoncol.2018.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaib YH, El-Serag HB, Nooka AK, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a hospital-based case-control study. Am J Gastroenterol. 2007;102:1016–21. doi: 10.1111/j.1572-0241.2007.01104.x. [DOI] [PubMed] [Google Scholar]
- Simone V, Brunetti O, Lupo L, et al. Targeting Angiogenesis in Biliary Tract Cancers: An Open Option. Int J Mol Sci. 2017;18:418. doi: 10.3390/ijms18020418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Zhang Q, Wu Z, Xue N. miR-101-3p sensitizes hepatocellular carcinoma cells to oxaliplatin by inhibiting Beclin-1-mediated autophagy. Int J Clin Exp Pathol. 2019;12:2056–65. [PMC free article] [PubMed] [Google Scholar]
- Sweigert PJ, Eguia E, Janjua H, et al. Does resection improve overall survival for intrahepatic cholangiocarcinoma with nodal metastases? Surg Open Sci. 2020;2:107–12. doi: 10.1016/j.sopen.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhu H, Zhao Y, et al. Comprehensive molecular profiling of intrahepatic cholangiocarcinoma in the Chinese population and therapeutic experience. J Transl Med. 2020;18:273. doi: 10.1186/s12967-020-02437-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Li J, Jiang F, Cai K, Ren G. The Effect and Mechanism of Vascular Endothelial Growth Factor (VEGF) on Tumor Angiogenesis in Gallbladder Carcinoma Iran. J Public Health. 2019;48:713–21. [PMC free article] [PubMed] [Google Scholar]
- Xu F, Liao J, Xiang G, et al. MiR-101 and doxorubicin codelivered by liposomes suppressing malignant properties of hepatocellular carcinoma. Cancer Med. 2017;6:651–61. doi: 10.1002/cam4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Zhang Y, Tang J, et al. The Prognostic Value and Regulatory Mechanisms of microRNA-145 in Various Tumors: A Systematic Review and Meta-analysis of 50 Studies. Cancer Epidemiol Biomarkers Prev. 2019;28:867–81. doi: 10.1158/1055-9965.EPI-18-0570. [DOI] [PubMed] [Google Scholar]
- Ye XH, Huai JP, Ding J, et al. Smoking, alcohol consumption, and the risk of extrahepatic cholangiocarcinoma: a meta-analysis. World J Gastroenterol. 2013;19:8780–8. doi: 10.3748/wjg.v19.i46.8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Chai Z, Sun X, et al. Overexpression of microRNA-96 is associated with poor prognosis and promotes proliferation, migration and invasion in cholangiocarcinoma cells via MTSS1. Exp Ther Med. 2020;19:2757–65. doi: 10.3892/etm.2020.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvamoto EY, Ferreira R, Nogueira V, et al. Influence of vascular endothelial growth factor and alpha-fetoprotein on hepatocellular carcinoma. Genet Mol Res. 2015;14:17453–62. doi: 10.4238/2015.December.21.16. [DOI] [PubMed] [Google Scholar]