Abstract
Background:
The human papillomavirus (HPV) is an important public health problem that can cause cervical cancer. HPVs were classified into high-risk (HR-HPV) and low-risk (LR-HPV) types. In this study, we aimed to determine the prevalence and genotype distribution of HR-HPV infection in Samsun province in Turkey.
Methods:
Cervical smear samples taken from 5406 women over a 23-month period were evaluated for the presence of HPV infection. The detection of HPV genotypes was performed using RT-PCR technology. HPV detection and genotyping were performed using RT-PCR method. HR- HPV types are divided into 3 groups as type 16, type 18 and other types (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, with or without type 16 and 18). The results were evaluated statistically.
Results:
The mean age of HR-HPV positive patients was 39.56 years (20-68 years). The prevalance of HR-HPV types did not differ between different age groups (p˃0.05). Overall, 9.17% of women (496/5406 samples) were found to be positive at least one type of HR-HPV. HPV type 16 was detected in 28.62%, type 18 in 9.67%, and other types in 78.83%. The most common HR-HPV type was other types (p˂0.001). Type 16 was most common than type 18 (p˂0.001). The patients were evaluated by dividing them into 6 age groups. Type 16 positivity was higher in 30-39 ages while type18 and other types positivity were higher in the 40-49 age group. When the 23-month period of HPV test was evaluated according to months and seasons, the highest prevalance was seen in June 2021 and Summer 2021.
Conclusion:
To our knowledge, this is the first large-scale study of HR-HPV prevalence and genotype distribution among women in Samsun Province of Turkey. The other types containing one or more types made up the majority of the studied population.
Key Words: Cervical cancer, HPV, genotyping, RT-PCR
Introduction
Cervical cancer (CC), the fourth most frequently diagnosed cancer, is the fourth major death cause of women, showing an estimated 342,000 deaths and 604,000 new cases worldwide in 2020 (Sung et al.,2021). CC is increasing in number every year, and the patient population is getting younger. Thus, CC endangers women’s life and health as a significant disease (Olusola et al., 2019). An essential step in human development and reproduction is to focus on women’s health. We still focus on prevention at present due to CC. Hence, the factors affecting the development and occurrence of cervical cancer should be considered for our research and discussion.
Human papillomavirus (HPV), the most prevalent sexually transmitted infection globally, negatively impacts personal social life (Chesson et al., 2014). HPV belongs to the Papillomaviridae family as a small, double-stranded DNA virus which is classified into two groups: low-risk HPVs (LR-HPVs), which are responsible for cutaneous and anogenital warts, and HPVs (HR-HPVs), which cause anogenital cancers, and oropharyngeal cancers, including anal, vulvar, vaginal, CC, and penile cancers (Buchanan et al., 2016). More than 150 HPV genotypes have been characterized up to the present time, and the genital tract is infected by about 40 of them (Bernard et al., 2010). LR- HPV types include types 6, 11, 42, 43, 42, and 44. HR-HPV types include types 16, 18, 31, 33, 34, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68. Some HPV types less frequently found in cancers are included in the high-risk group but are frequently found in squamous intraepithelial lesions (Burd, 2003). The cervical epithelium infection with HR-HPV causes more than 95% of CCs (Muñoz et al., 2003). These types are largely different in their geographic distributions. For example, different regions of the world face the five most common HR-HPV types as follows: North America, 16, 53, 18, 51, 31; Southern Europe, 16, 66, 45, 31, 42; South America, 16, 58, 18, 45, 31; Western Europe, 16, 18, 31, 35, 33; and Eastern Europe, 16, 31, 18, 66, 39 (AlObaid et al., 2014, Zhang et al., 2014, Moosa et al., 2014). In a large-scale study with women in Turkey, Dursun et al. reported that the most common HPV types in patients with abnormal cytology were as follows: 16, 6, 31 , 11, 18, and 33, respectively (Dursun et al., 2013).
CC is considered nearly completely preventable because of the highly effective primary and secondary prevention measures. Therefore, we aimed to determine the prevalence of HR-HPV infection and genotype distribution in cervical swabs in Samsun province in Turkey.
Materials and Methods
Study population
This retrospective study was conducted on 5406 women [median age 43.87 years (min-max:20-84)] from 232 219 patients examined between February 2020 and December 2021 in the Gynecology and Obstetrics Clinic of Samsun Training and Research Hospital, Samsun, Turkey. The cervical exfoliated cells of the included women were collected by the clinician using a specialized cervical brush. All patients were of the same ethnic origin and the same geographic area. Four hundred and ninety-six women with positive HR-HPV were divided into 6 groups according to their age and evaluated. HR-HPV types were evaluated into 3 groups: type 16, type 18, and others (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 with or without 16, 18). After the approval from the same hospital’s Institutional Ethics Board, we used the hospital’s electronic database, which contains multiple data fields on laboratory variables for patients. The principles outlined in the Declaration of Helsinki were followed. The Local Ethics Committee of the Human Research of the same hospital approved the study (GOKA/2021/20/10).
HR-HPV Detection
The detection of HPV genotypes was tested by the Cobas x480 Instrument (Mannheim/GERMANY) based real-time polymerase chain reaction (RT-PCR) technology. The presence of HPV 18, HPV 16, and a pool of 12 other HR-HPV genotypes can be qualitatively assayed with the Cobas HPV test. This method uses the same primers and probes and provides genotyping for types 16 and 18. DNA extraction and purification were done according to the manufacturer’s instructions. Each sample had an internal control, β-globin, to monitor cell adequacy, and each run included a set of HPV positive and negative controls. The PreservCyt Solution samples were vortexed and placed on the sample carrier, and the reagents (lysis buffer, wash buffer, and elution buffer) were loaded in respective reagent reservoir carriers. After sample and reagent loading, DNA preparation was completed automatically, and final DNA products were collected into a microwell plate. Subsequently, the microwell plate containing DNA was manually sealed and loaded on the Analyzer, and the amplification was completed.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics. Visual and analytical methods were used to determine whether the variables were normally distributed or not. Non-normally distributed variables were described using the median and the IQR. Pearson’s χ2test and the Mann-Whitney U test were used to analyze the data, as appropriate. P- value of ˂0.05 was considered statistically significant.
Results
Cervical smears were obtained over 23 months, and an HPV test was performed in 5406 of 232 219 patients examined in the Clinic during the study. The analysis results showed that at least one type of HR-HPV positivity was detected in 9.17% of the women. The mean age of HR-HPV-positive patients was 39.56. The patients were evaluated by dividing them into 6 age groups. The prevalence of HR-HPV infection was the highest at 40-49 years. Total and HR-HPV prevalence classified according to different age groups is presented in Table 1.
Table 1.
Prevalence of Patients with Total and HR- HPV Infection in Different Age Groups
| Ages | Total patients n:5406 (%) | HR-HPV patients n: 496 (%) |
|---|---|---|
| Mean age | 43.87 (20-84) | 39.56 (20-68) |
| 20-29 | 439 (8.12) | 98 (19.76) |
| 30-39 | 1300(24.05) | 137 (27.63) |
| 40-49 | 2259 (41.79) | 179 (36.08) |
| 50-59 | 1035 (19.15) | 65 (13.11) |
| 60-69 | 312 (5.77) | 17 (3.42) |
| 70≥ | 61 (1.13) | 0 (0) |
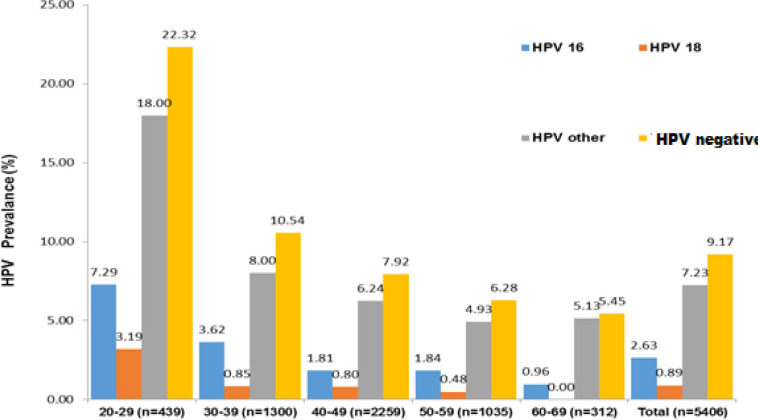
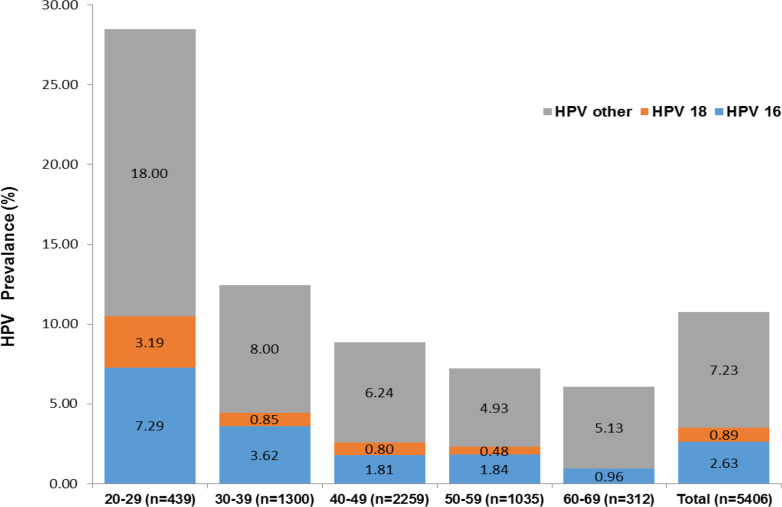
HR- HPV types are divided into 3 groups of type 16, type 18, and other types. The other types contained one or more types. There was no significant difference between the groups in terms of mean age. Also, the prevalence of HR-HPV types did not differ between different age groups.HPV type 16 was detected in 142 samples, type 18 in 48 samples, and other types in 391 samples. The most common HR-HPV type included other types. Type 16 was higher in 30-39 ages while type 18 and other types were higher in the 40-49 age group. Type 16 was higher than type 18. The distribution of HR-HPV types according to the age group is shown in Table 2.
Table 2.
Distrubition of HR-HPV Type 16, Type 18 and Other Types According to Age Groups
| Ages | Type 16 patients n: 142 (%) | Type 18 patients n: 48 (%) | Patients with other types n: 391 (%) | P |
|---|---|---|---|---|
| Mean age | 38.30 ± 10.22 | 37.66 ± 9.77 | 39.54 ± 10.47 | >0.05 |
| 20-29 | 32 (22.54) | 14 (29.16) | 79 (20.20) | >0.05 |
| 30-39 | 47 (33.09) | 11 (22.92) | 104 (26.59) | >0.05 |
| 40-49 | 41 (28.87) | 18 (37.6) | 141 (36.07) | >0.05 |
| 50-59 | 19 (13.39) | 5 (10.41) | 51 (13.05) | >0.05 |
| 60-69 | 3 (2.11) | 0 (0) | 16 (4.09) | >0.05 |
| 70≥ | 0 (0) | 0 (0) | 0 (0) | >0.05 |
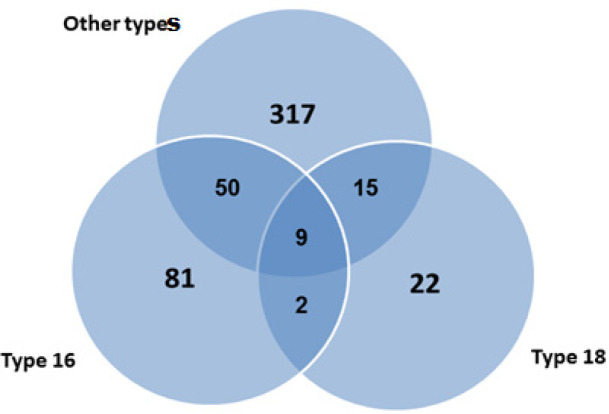
In the distribution of HR-HPV types, other types and type 16 positivity was found in 50 patients, other types and type 18 were positive in 15 patients, type 16 and type 18 positivity was found together in 2 patients. There were 103 patients with a single type. Type 16, type 18, and other types were found to be positive together in 9 of 496 patients, at least one of which was positive for HPV.
The distribution of 5406 patients tested according to age groups was evaluated. The prevalence and age distribution of patients for HPV tests are presented in Figure 2. Then, we evaluated the distribution of HR-HPV types according to prevalence and age groups in all women for whom HPV testing was requested. The other types with the highest prevalence were seen in the 20-29 age group. The prevalence and distribution of HR-HPV types according to age groups are summarized in Figure 3.
Figure 2.
The Prevalance and Distribution of All Patients According toAge Groups
Figure 3.
The Prevalance and Distribution of HR-HPV by Age Groups
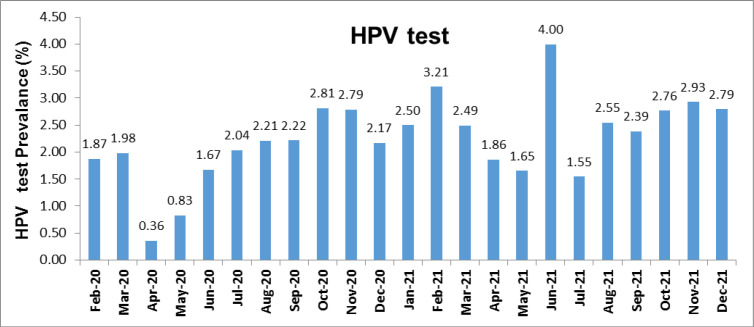
We examined the prevalence of HPV testing performed during the 23-month period during which the study was conducted. During this period, the highest HPV test prevalence was found in June 2021. The prevalence of HPV tests by month is presented in Figure 4.
Figure 4.
HPV Test Prevalence According to Months
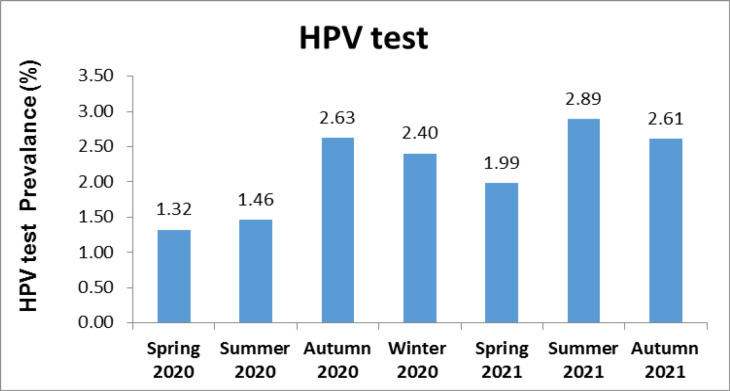
Then, we evaluated the HPV prevalence of 7 seasons in this period. The highest prevalence of HPV testing was seen in the summer 2021.
Figure 1.
The Distribution of HR-HPV Types
Figure 5.
HPV Test Prevalence According to Seasons
Discussion
Despite the same diseases associated with HPV infection based on the viral types (HR-HPV or LR-HPV) all over the world, HPV genotypic prevalence does not vary geographically. Policy-making for strengthening vaccination programs in these specific regions will be facilitated by knowing the genotypic prevalence in a specified continental region, less strongly, developing broad-spectrum vaccines’ new generation. The health and socioeconomic burdens related to HPV infection will be reduced by reinforcing the vaccination programs or selecting the vaccine influenced by the most prevalent genotypes. Several studies have investigated the HPV genotyping profiles in various populations all over the world, and HPV16 and 18 appear to be highly prevalent in CC.
CC, a global health problem, is the 12th most common malignancy in women in Turkey (https://hpvcentre.net/statistics/reports/TUR_FS.pdf). CC screening tests in healthy-looking women with no symptoms are performed to detect pre-cancerous and cancerous lesions. Unfortunately, developing countries diagnose cancer with a delay until advanced stages appear and the symptoms occur. In addition, therapies are usually not sufficient in the advanced stages, increasing the mortality rates (Sankaranarayanan, 2014). The oldest and the most common tests are cytological screening tests (PAP-smears). Despite the effective reduction of the incidence and mortality using this technique in most developed countries, developing countries will not reach the same progress. Turkey has recently started the CC screening program. Thus, the real prevalence of HPV infections is not represented by even multi-centered studies. In addition, different research results depend on not only the socio-cultural and regional differences but also the selection of the study group and the cause of hospital admission. A twelve-centered study in Turkey reported HPV positivity as 57%, 27%, and 25% in patients with abnormal cytology, normal cytology, and all patients, respectively ( Dursun et al., 2013).
Therefore, to create a representative regional map, we aimed to determine if HR-HPV is prevalent among women in Samsun (Turkey) and identify the most prevalent genotypes in the city. Samsun province, located in the northern part of Turkey, is relatively developed economically and culturally. Some studies have been performed to evaluate the prevalence and genotype of HPV in Turkey, most of which are based on small samples. The prevalence of HR-HPV was reported to be 33.5%, 36.3%, 8.5%, 10.07%, 23.9%, and 2.79% in their studies, respectively (Aydogan et al., 2018, Alacam et al., 2021, Akcali et al., 2013, Bayram et al., 2015, Eroglu and Asgin 2020, Kulhan et al., 2017). In a study conducted on 2234 Turkish women, HPV positivity was found 38.05%, and the most common type was HPV 16 (Hancer et al., 2018). Overall, HPV-DNA positivity in Italy was identified in 35.9% of the women (Piana et al., 2011). A larger sample size (5406) of the women was screened in this study. We found at least one type of HR-HPV in 9.17% of the women. According to 2021 data, in Turkey, it was reported that the most common HPV types seen in women with high-grade cervical lesions were type 16, 31, 18, 51, 33, 45, 52, 35, 58, and 39 (https://hpvcentre.net/statistics/reports/TUR_FS.pdf). We evaluated HR-HPV cases in three groups of type 16, type 18, and other types based on the data. Wang et al. reported that prevalence rates of type 16 and 18 were 3.95%, and 3.27% in Chinese women, respectively (Wang et al. 2021). In a study conducted with Mexican women, the most common types were type 16 (43%) and 18 (42%) (López Rivera et al., 2012). Italian women from North Sardinia, the prevalence types 16 and 18 were 54.3% and 5.2%, respectively ( Piana et al., 2011). In a study by Demir et al. in Turkish women with a normal smear, type 16 and type 18 were found 3.6% and 0.4%, respectively (Demir et al., 2012). In a large-scale study on 11 624 women, the prevalence rates of type 16 and 18 were 11.25% and 2.27% (Kulhan et al. 2017). In the present study, other types, with or without type 16 and type 18, constituted the majority of cases (78.83%). Single type HR-HPV (type 16 and type 18) was found in 103 women (20.76%). We determined types 16 and 18 as 28.62% and 9.67%. The HR-HPV prevalence and type distribution were found in different studies with different results. We think that this difference is due to geographical region, the methods used to diagnose HPV, the education level of the patients admitted to the hospital, and their awareness of HPV infection.
Age is an essential factor that is related to HPV infection. Importantly, young women have frequent sex. The literature shows that young women are more susceptible to having multiple partners. In addition, since they are not sensitized, their immune systems are susceptible to HPV infection (Qian et al., 2017). From another viewpoint, immune functions are lower in women during menopausal, decreasing viral clearance rates and increasing HPV infection rates. Thus, the age distribution of HPV infection has two peaks: women >54 years and 15–24-year-old ( Kim et al. 2013, Wang et al. 2012). However, the Caribbean, Latin America, and most countries in Africa show a second prevalence peak from 45 years onwards (Bruni et al., 2010). In a study, Demir et al. observed that HPV prevalence was the highest in the age group of 25-29 years (Demir et al., 2012). In the current study, type 16 was more common in the 30-39-year-old group, but type 18 and other types were more common in the 40-49-year-old group. According to age group, there was no significant difference in the distribution type 16, type 18, and other types (Table 2).
The Ministry of Health announced the first case of COVID-19 in Turkey in March 2020. Schools were closed on March 13, and some other precautions were executed, requiring special permission for government personnel to leave the country and not allowing spectators at sporting events. All scientific, sports, artistic, and cultural activities have been put off on March 19 (Bozkurt et al., 2020). Considering this information, we evaluated the distribution of HPV test requests by months and seasons during the study period. Examination of the monthly and seasonal distribution of HPV test prevalence showed that the prevalence was low during the closure period during the pandemic period and started to increase after June 2021. The peak prevalence of HPV tests (monthly and seasonal) was determined as of June 2021 and Summer 2021, in line with the end of the closure. It can be interpreted that these HPV-positive patients hesitated to come to the hospital in the first 18 months of the pandemic period, and then, this hesitation was overcome. Although the prevalence of HPV testing in the first six months of the pandemic was low, it fluctuated in monthly and seasonal graphs.
The study’s limitations are that our study was conducted only on the patients who attended gynecology polyclinics of the health institution in the Black Sea region of Turkey. Although our results do not reflect Turkey in general, it is an advantageous study for directing the vaccination studies in our province. Our subsequent work will evaluate the types by classifying the samples as pathological.
The present study evaluated HR-HPV infection in gynecological outpatients in Samsun from 2020 to 2021. To our knowledge, this is the first large-scale study on HR-HPV prevalence and genotype distribution among women in Samsun Province of Turkey. The other types containing one or more types constitute most of the studied population. Our results will shed light on vaccination studies in our province.
Author Contribution Statement
Concept: Mehmet Hakan TASKIN, Ergin KARIPTAS; Design: Mehmet Hakan TASKIN, Muhammet Ali ORUC; Data Collection and/or Processing: Mehmet Hakan TASKIN, Ergin KARIPTAS; Analysis and/or Interpretation: Mehmet Hakan TASKIN; Literature Search: Ayse Feyda NURSAL, Muhammet Ali ORUC; Writing Manuscript: Ayse Feyda NURSAL; Critical Review: Mehmet Hakan TASKIN, Ergin KARIPTAS, Ayse Feyda NURSAL, Muhammet Ali ORUC.
Acknowledgments
Ethics Committee Approval
Ethical committee approval was received from the Ethics Committee of Samsun Training and Research Hospital Clinical Research Ethics Committee (date and number of approval: (GOKA/2021/20/10).
Financial Disclosure
The authors declared that this study has received no financial support.
Conflict of Interest
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
References
- Akcali S, Goker A, Ecemis T, Kandiloglu AR, Sanlidag T. Human papilloma virus frequency and genotype distribution in a Turkish population. Asian Pac J Cancer Prev. 2013;14:503–36. doi: 10.7314/apjcp.2013.14.1.503. [DOI] [PubMed] [Google Scholar]
- Alacam S, Bakir A. Human Papillomavirus Prevalence and Genotype Distribution in Cervical Swab Samples in Istanbul, Turkey. J Infect Dev Ctries. 2021;15:1190–96. doi: 10.3855/jidc.14663. [DOI] [PubMed] [Google Scholar]
- AlObaid A, Al-Badawi IA, Al-Kadri H, et al. Human papillomavirus prevalence and type distribution among women attending routine gynecological examinations in Saudi Arabia. BMC Infect Dis. 2014;14:643. doi: 10.1186/s12879-014-0643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydogan S, Yazgan A, Tas EE, et al. The presence and distribution of high risk HPV types in simultaneous cervical cytology samples. Turk Hij Den Biyol Derg. 2018;75:13–20. [Google Scholar]
- Bayram A, Derici YK, Yilmaz NO, et al. Prevalence of high–risk human papillomavirus in women from Turkey. Clin Obstet Gynecol Reprod Med. 2015;1:84–86. [Google Scholar]
- Bernard HU, Burk RD, Chen Z, et al. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology. 2010;401:70–9. doi: 10.1016/j.virol.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt B, Egrilmez S, Sengor T, Yildirim O, Irkec M. The COVID-19 Pandemic: Clinical Information for Ophthalmologists. Turk J Ophthalmol. 2020;50:59–63. doi: 10.4274/tjo.galenos.2020.29805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni L, Diaz M, Castellsague X, et al. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202:1789–99. doi: 10.1086/657321. [DOI] [PubMed] [Google Scholar]
- Buchanan TR, Graybill WS, Pierce JY. Morbidity and mortality of vulvar and vaginal cancers: Impact of 2-, 4-, and 9-valent HPV vaccines. Hum Vaccines Immunother. 2016;12:1352–56. doi: 10.1080/21645515.2016.1147634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd EM. Human Papillomavirus and Cervical Cancer. Clin Microbiol Rev. 2003;16:1–17. doi: 10.1128/CMR.16.1.1-17.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir ET, Ceyhan M, Simsek M, et al. The prevalence of different HPV types in Turkish women with a normal Pap smear. J Med Virol. 2012;84:1242–7. doi: 10.1002/jmv.23333. [DOI] [PubMed] [Google Scholar]
- Dursun P, Ayhan A, Mutlu L, et al. HPV types in HPV types in Turkey: multicenter hospital based evaluation of 6388 patients in Turkish gynecologic oncology group centers. Turk Patoloji Derg. 2013;29:210–16. doi: 10.5146/tjpath.2013.01188. [DOI] [PubMed] [Google Scholar]
- Eroglu S, Asgin N. Frequency and genotype distribution of high-risk human papilloma virus types in Karabuk province, Turkey: A hospital based cross-sectional study. Ann Med Res. 2020;27:765–9. [Google Scholar]
- Feng S. Analysis of risk factors and preventive health care of cervical cancer. Elect J Pract Gynecol Endocrinol. 2018;5:11–4. [Google Scholar]
- Hancer VS, Buyukdogan M, Bylykbashi I, Oksuz B, Acar M. Prevalence of Human Papilloma Virus Types in Turkish and Albanian Women. J Cytol. 2018;35:252–54. doi: 10.4103/JOC.JOC_162_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Kim JJ, Kim S. Type-specific prevalence of high-risk human papillomavirus by cervical cytology and age: Data from the health check-ups of 7,014 Korean women. Obstet Gynecol Sci. 2013;56:110–20. doi: 10.5468/OGS.2013.56.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulhan M, Kulhan NG, Seven Y, et al. Estimation of the prevalence and distribution of HPV genotypes and identification of related risk factors among Turkish women. Contemp Oncol (Pozn) 2017;21:218–23. doi: 10.5114/wo.2017.69591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López Rivera MG, Flores MO, Villalba Magdaleno JD, Sánchez Monroy V. Prevalence of human papillomavirus in women from Mexico City. Infect Dis Obstet Gynecol. 2012;2012:384758. doi: 10.1155/2012/384758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosa K, Alsayyad AS, Quint W, Gopala K, DeAntonio R. An epidemiological study assessing the prevalence of human papillomavirus types in women in the Kingdom of Bahrain. BMC Cancer. 2014;14:905. doi: 10.1186/1471-2407-14-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz N, Bosch FX, de Sanjosé S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- Olusola P Banerjee HN, Philley JV, Dasgupta S. Human papilloma virus-associated cervical cancer and health disparities. Cells. 2019;8:622. doi: 10.3390/cells8060622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L, Zhang Y, Cui D, et al. Analysis of epidemiological trends in human papillomavirus infection among gynaecological outpatients in Hangzhou, China, 2011–2015. BMC Infect Dis. 2017;17:393. doi: 10.1186/s12879-017-2498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piana A, Sotgiu G, Castiglia P, et al. Prevalence and type distribution of human papillomavirus infection in women from North Sardinia, Italy. BMC Public Health. 2011;11:785. doi: 10.1186/1471-2458-11-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- Wang L, Yu C, Ni X, et al. Prevalence characteristics of human papillomavirus (HPV) infection among women receiving physical examinations in the Shangcheng District, Hangzhou city, China. Sci Rep. 2021;11:Article number: 16538. doi: 10.1038/s41598-021-96131-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Wei H, Wang N, et al. The prevalence and role of human papillomavirus genotypes in primary cervical screening in the northeast of China. BMC Cancer. 2012;12:160. doi: 10.1186/1471-2407-12-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Fei MD, Jiang Y, et al. The diversity of human papillomavirus infection among human immunodeficiency virus-infected women in Yunnan, China. Virol J. 2014;11:202. doi: 10.1186/s12985-014-0202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]