Abstract
Objective:
Breast cancer is the most common cancer in Indonesia, with Indonesia’s breast cancer mortality rate being the highest among Southeast Asian countries. This study aims to evaluate the cost-effectiveness and budget impacts of adding trastuzumab to chemotherapy versus chemotherapy alone for HER2-positive breast cancer patients in Indonesia.
Methods:
We performed a Markov model-based economic evaluation to assess cost-effectiveness, cost–utility, and budget impact. Utility data, direct medical costs, and indirect costs were obtained primarily from interviewing patients. Clinical effectiveness data, on the other hand, were obtained from systematic reviews and real-world data and represented through progression free survival, overall survival, and quality-adjusted life years (QALYs).
Result:
From a healthcare provider’s perspective, the total costs for the combined group were USD 14,516, while chemotherapy alone cost USD 7,489. While the cost-effectiveness analysis showed that the combination group had a higher total cost by USD 7,027, PFS was longer in the chemotherapy alone group, with a difference of 2.2 months. The ICER was USD 17,307 for every QALY gained. The total cost of adding trastuzumab over a 5-year period was USD 589 million.
Conclusion:
In conclusion, this economic evaluation suggests that the addition of trastuzumab to standard chemotherapy is not cost-effective in terms of PFS and OS compared with chemotherapy alone.
Key Words: HER2-positive, breast cancer, trastuzumab, economic evaluation, cost-effectiveness
Introduction
Breast cancer currently ranks first as the most common cancer worldwide, even surpassing lung cancer (World Health Organization, 2020). Breast cancer was found to have the highest incidence of all cancers among the Indonesian population and was the leading cause of death, with an estimated mortality rate of 9.6%. Overexpression of human epidermal growth factor receptor type 2 (HER2) has been observed in 20–30% of breast cancers (Owens et al., 2004). The recombinant monoclonal antibody trastuzumab, which binds to HER2 receptors, has been shown to be effective in improving clinical response (Buzdar et al., 2005), disease free survival (Cameron et al., 2017), and the overall survival rate of HER2-positive breast cancer patients (Okamoto et al., 2020; Gajria and Chandarlapaty, 2011; Paracha et al., 2020; Balduzzi et al., 2014).
While the use of trastuzumab for breast cancer patients seems promising, the costs associated with this drug are very high and can potentially impose significant burdens on healthcare systems. In the United Kingdom (UK), the cost of trastuzumab at its full price can reach up to GBP 90,000 per patient. The UK National Institute for Health and Care Excellence (NICE) rejected the routine funding scheme for trastuzumab in 2015 because of its high cost. Nevertheless, further negotiation with the pharmaceutical company reached a win–win solution to include trastuzumab in the new fast track funding scheme for new cancer drugs in the UK (NHS England, 2017). Evidence from high-income countries (HIC) reported that trastuzumab may be cost-effective, with incremental cost-effectiveness ratios (ICERs) ranging from USD 6,018 to 78,929 per quality-adjusted life year (QALY) gained (Garrison et al., 2007; Kurian et al, 2007; Skedgel et al, 2009; Seferina et al., 2017; Leung et al., 2016). However, evidence from upper- and lower-middle income countries is still insufficient. Some studies revealed that ICERs of trastuzumab ranged from USD 3,526 to 174,901 per QALY gained in upper-middle income countries, which mostly exceeded their thresholds (Chen et al., 2009; Genuino et al., 2021).
Despite the well-known clinical effectiveness of trastuzumab in HER2-positive breast cancer patients, access to this drug presents another challenging issue. Brazilian regulatory authorities, for instance, approved the use of trastuzumab for metastatic diseases in 2000. However, the drug was initially only accessible by privately insured patients and did not become available to the public until 2017 (Henrique et al., 2019). In China, patients’ access to this drug was highly influenced by the region in which individuals resided. Less than 10% of patients in resource-limited regions used trastuzumab, in contrast with nearly 90% of patients in resource-abundant regions (Li et al., 2017). Access to this drug continues to vary among countries.
Trastuzumab has been listed as an essential drug by the World Health Organization. However, the high cost of this drug and the issue of its accessibility have constrained its implementation across many health systems. Assessing the cost-effectiveness of trastuzumab according to country context is necessary for policy makers to inform about health financing decisions. Investments in advanced cancer drugs in middle-income countries are still contentious, considering challenges, such as disparities between different regions and income levels in accessing drugs and issues in achieving universal health coverage. The Government of Indonesia need to define its priorities for the allocation of funding resources to advanced cancer drugs such as trastuzumab. Therefore, this study was performed in order to evaluate the cost-effectiveness and budget impacts of adding trastuzumab to chemotherapy treatment versus chemotherapy alone for HER2-positive breast cancer patients in Indonesia.
Materials and Methods
The economic evaluation comparing trastuzumab and chemotherapy to chemotherapy alone was designed to assess cost-effectiveness, cost-utility, and budget impact. The primary outcome of the cost-effectiveness analysis is the incremental cost-effectiveness ratio (ICER), which is calculated by dividing the incremental costs by the incremental health outcomes, expressed in clinical outcomes and quality-adjusted life years (QALYs). The clinical outcomes were represented by progression free survival (PFS) and overall survival (OS).
Data sources
The data sources used for this modeling were reports from epidemiological studies, registration data, claim data, market forecast research data, published literature on clinical effectiveness, and disease progression data.
Clinical effectiveness
Systematic search were performed using various electronic databases, such as PubMed, the Cochrane Library, EMBASE, and Google Scholar. The keywords applied to this study were “trastuzumab”, “Herceptin”, “HER-2 antibodies”, and “cancer” (and its synonyms, such as “neoplasm”, “malignancy”, “malignant tumor”, and “tumor” by adding OR), combined with AND for “metastatic breast cancer”. The search criteria were restricted to research that was in English, accessible in full text, published post-2000, and related to clinical trials. The search was undertaken in 2018. Articles in the form of editorials, observational studies, posters, and reviews were not included in the systematic review. In terms of real-world data, a retrospective cohort study was conducted using medical records of HER2-positive metastatic breast cancer patients from November 2018 to February 2019. The subjects were recruited from four type A hospital located in different regions, namely Java, Bali, and Kalimantan . Hospitals in Indonesia are classified into four types based on the type of service provided and the capacity of the bed, namely type A, B, C, and D. Type A hospital is the highest ranking, in which the most advanced services with high range of subspecialists medical service capabilities and more accommodations for patients. A total of 120 patients were included in this study, consisting of 58 patients who received chemotherapy alone and 62 patients who received a combination of trastuzumab and chemotherapy. The clinical effectiveness was measured by comparing OS and PFS between those received the combination therapy versus chemotherapy alone. Based on the guidelines of the American Cancer Society (ACS) and the National Cancer Comprehensive Network (NCCN) 2006, the following chemotherapy can be used as a single agent for breast cancer, i.e., doxorubicin, epirubicin, paclitaxel, dosetaxel, capesitabin, gemcitabine, and for the combination of CAF (cyclophosphamide + doxorubicin + fluorouracil), FEC (fluurouracil + epirubicin + cyclophosphamide), AC (doxorubicin + cyclophosphamide), ET (epirubicin + paclitaxel), CMF, doxetaxel/capesitabin, GT (gemcitabine + paclitaxel), other active agents can also be used, namely cisplatin, carboplatin, etoposide, vinblastine, and fluorouracil infusion.
Costs
Direct medical costs were collected from primary data, i.e., patients’ billing details in outpatient and inpatient units. Direct medical costs included drug costs, costs associated with medical devices, hospitalization costs, medical personnel costs, and laboratory and administrative costs. Direct non-medical costs were derived from interview results with patients and/or caregivers. Direct non-medical costs consisted of transportation costs, meal costs, accommodation costs, and caregivers’ costs. The indirect costs calculated for this study included costs associated with loss of productivity among patients and their caregivers. Both direct non-medical costs and indirect costs were obtained by interviewing patients and their caregivers. All costs were calculated in Indonesian rupiahs in 2018 (1 USD = 14,481 IDR).
Utility
Utility data were obtained through direct interviews with patients using the EuroQoL (EQ)-5D-5L instrument. We used the EQ-5D-5L value set for Indonesia. The inclusion criterion was patients with metastatic breast cancer, both in the progression-free and progression state.
Cost-effectiveness analysis
We used real-world data in the cost-effectiveness analysis. Given that our research was from the perspective of healthcare providers (i.e., hospitals), we only included direct medical costs. The time period of the CEA spanned from the start of therapy until the last follow-up.
Cost-utility analysis
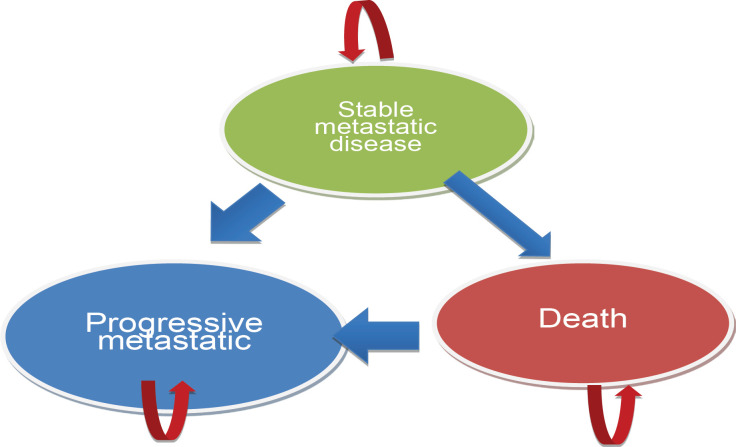
A Markov model was constructed to assess the cost-utility of adjuvant trastuzumab for HER2-positive breast cancer patients. The model incorporated three health states, namely stable metastatic disease, progressive metastatic disease, and death. The length for each cycle was 6 months, with a lifespan commencing at the age of 40 years. The discount rate applied in this study was 3%. We modelled the lifetime costs and consequences of treating a cohort of patients with metastatic breast cancer starting at the age of 40 years using societal perspective. Patients entered the model through stable state. Per simulation cycle, patients could remain in stable state or moved to progressive state or die, based on transition probabilities. The Markov model is shown in Figure 1 .
Figure 1.
Three Health States for the Markov Model
Budget impact analysis
Budget impact analysis (BIA) was performed to assess the financial consequences and to evaluate the affordability of adopting trastuzumab in the benefits package. The costs included in the BIA were direct medical costs. We estimated the budget impact for a period of 5 years from a payer’s perspective. Parameter inputs such as prevalence, incidence, and population numbers were obtained from the Central Bureau of Statistics, Basic Health Survey, data from hospitals, and expert judgment.
Sensitivity analysis
In order to assess the robustness of the model, one-way sensitivity and probabilistic sensitivity analyses were performed. The variables tested in the one-way sensitivity analysis included cost, clinical effectiveness, and discount rate. In the base case scenario, the value used in the model was the average of each parameter. One-way sensitivity analyses were based on the variation from the lowest to highest values. The probabilistic sensitivity analysis was performed with 1,000 iterations and a threshold of three times the gross domestic product (GDP) per capita.
Results
Clinical effectiveness
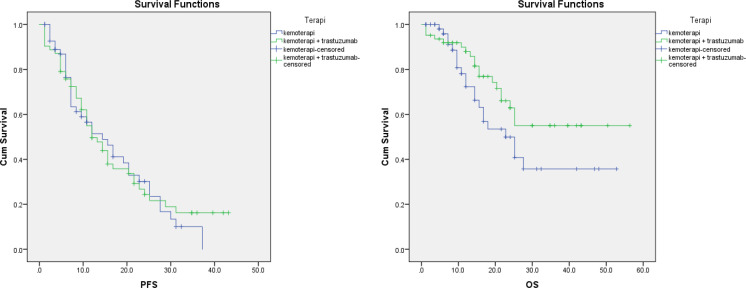
The median progression free survival (PFS) of the trastuzumab and chemotherapy combination group was 12 months; this was lower than the chemotherapy alone group, which was 14.4 months (p > 0.05). In contrast, the median of overall survival (OS), the combined group was better than the chemotherapy alone group (p > 0.05). Nevertheless, both PFS and OS derived from real-world data showed no significant difference between these two groups. Meanwhile, results from a systematic review revealed that the addition of trastuzumab to chemotherapy provided a significant improvement in OS (RR: 0.79; 95% CI: 0.65-0.96) as well as PFS (RR: 0.51; 95% CI: 0.42-0.62) compared with chemotherapy alone in patients with metastatic breast cancer.
Cost effectiveness
From a healthcare provider’s perspective, the total cost for the combined group was USD 14,516; for the chemotherapy alone group, the total cost was USD 7,489. The cost-effectiveness analysis showed a higher total cost in the combination group of USD 7,027; however, PFS was longer in the Chemotherapy alone group with a difference of 2.2 months.
Cost utility analysis
The input parameters consisted of transitional probabilities based on the natural course of metastatic breast cancer, clinical effectiveness, cost utility, and discount rate. Data for transitional probabilities were obtained from literature reviews, cost and utility data were derived from primary data, and a 3% discount rate was applied. In terms of QALY, the incremental QALY was 0.789 years. From a healthcare provider’s perspective, the incremental cost-effectiveness ratio (ICER) was USD 17,307 per QALY gained. The ICER value was three times above the GDP value per capita of Indonesia in 2017 (i.e., USD 11,532).
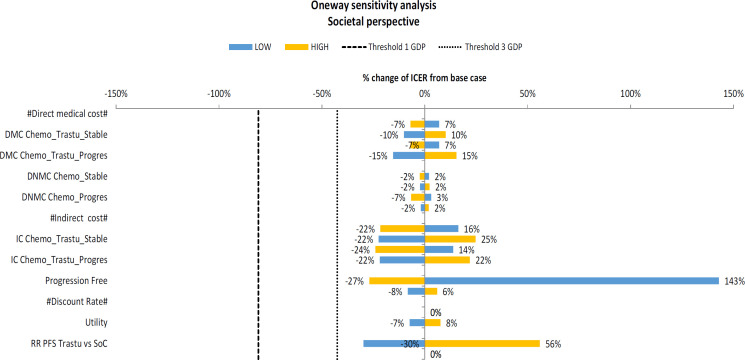
The one-way sensitivity analysis of parameters in the model showed that cost-effectiveness of trastuzumab was sensitive to utility in the progression free state. The range of values of these variables caused a large shift in the ICER value (143%). Nevertheless, the variation in each variable did not affect the conclusion that the ICER value of adding trastuzumab to chemotherapy is above the cost-effectiveness threshold used in Indonesia.
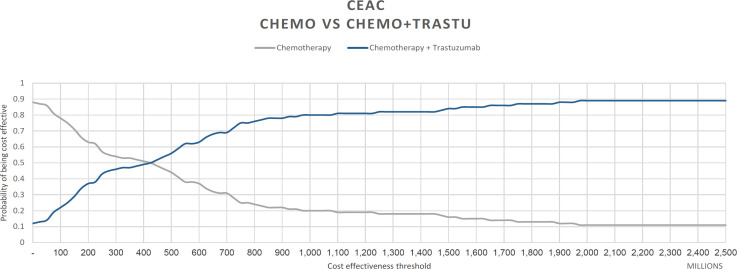
The result of probabilistic sensitivity analysis was described by the cost-effectiveness acceptability curve (CEAC) in Figure 4. The CEAC revealed that the probability of adding trastuzumab to chemotherapy for HER2-positive breast cancer patients and having it be cost-effective was only 17% at the threshold of three times the Indonesian GDP, and it would be more than 90% cost-effective if the threshold was increased by 172 thousand dollars . The price of trastuzumab in the market can reach up to USD 1,726 per ampoule. We run a simulation to calculate the ICER if the price of trastuzumab was reduced to merely one-fourth of the original price. Using the provider’s perspective, the ICER would be USD 9,485 per QALY gained, below the threshold of three times the Indonesian GDP.
Figure 4.
Cost-Effectiveness Acceptability Curve of Trastuzumab and Chemotherapy vs. Chemotherapy Alone
Budget impact analysis
The input parameters used to calculate the budget impact were population numbers, population growth rate, incidence of breast cancer, proportion of metastatic breast cancer, and proportion of HER2-positive breast cancer. The total estimated cost for the addition of trastuzumab to chemotherapy for all HER2-positive metastatic breast cancer patients in Indonesia for 5 years would be USD 589 million, while the total cost for chemotherapy alone, if given to all patients for 5 years, would be USD 340 million. Therefore, if all the HER2-positive metastatic breast cancer patients switched their treatment from chemotherapy alone to trastuzumab plus chemotherapy for 5 years, then the additional required cost would be USD 249 million.
Figure 2.
Kaplan–Meier Curve for the Comparison of PFS and OS between the Trastuzumab Plus Chemotherapy Group and the Chemotherapy Alone Group
Figure 3.
Tornado Diagram of the One-Way Sensitivity Analysis Comparing Trastuzumab and Chemotherapy vs. Chemotherapy Alone
Discussion
Our study indicates that adding trastuzumab to standard chemotherapy for patients with metastatic breast cancer is not cost-effective in Indonesia in terms of PFS, OS, and QALY. Furthermore, the estimated cost is substantially significant. The result appears to be inconsistent with previous publications (Norum, 2006; Ferrusi et al., 2011; Chan et al., 2009) in high-income western countries, in which adjuvant therapy trastuzumab was reported to be a cost-effective treatment for HER2-positive metastatic breast cancer patients. Moreover, a systematic review of trastuzumab, and its economic evaluation as an adjuvant therapy conducted in several Asian countries, also found trastuzumab to be a cost-effective treatment option. Nevertheless, these Asian countries are considered high-income countries. Studies performed in India and African countries yielded similar results to our findings (Gershon et al., 2019; Gupta et al., 2020).
In our study, the incremental QALY gained was 0.798, which was not different from previous publications. Nevertheless, we did not see a gain of incremental life years when we used the real-world data. Our systematic review found that adding trastuzumab to chemotherapy would improve OS and PFS significantly compared with chemotherapy alone, RR: 0.79, 95% CI: 0.65-0.96 and RR: 0.51; 95% CI: 0.42-0.62, respectively. However, the outcomes from real-world data showed no significant differences in terms of PFS and OS. This is understandable considering that clinical trials are always carried out in an ideal setting. In clinical trials , all variables that can affect outcomes are firmly controlled, ranging from accurate and careful diagnosis, treatment procedures, strict treatment schedules, uniform, and strict standards for administering therapy, and accurate reporting of the outcomes. Meanwhile, in a real setting, this is not entirely possible. A patient’s condition may be completely different from the patients enrolled in a clinical trial. There is a possibility that the patient would receive delayed therapy. In the context of assessing health technology, policy makers need to decide whether the health technology is more or less useful after being applied to a real setting.
Aside from the benefits obtained from an intervention, the health financing system must consider affordability aspects. Trastuzumab, in this case, was proven not to be cost-effective, and it is not possible to finance it in a long-term. Health technology may be useful in a clinical trial setting, but the benefits in real settings are questionable, and therefore, may not be worth the cost .
Our study also has limitations. Evidence on the clinical effectiveness were obtained from the study conducted in non-Asian population, that had different setting with the situation in Indonesia. Therefore, we also did a cohort retrospective study using data from Indonesian population to reflect the real world data. Nevertheless, the observation period in the cohort study was too short and lacking to generate the OS and PFS outcome. More research is needed to capture a longer observation period.
In conclusion, this economic evaluation suggests that the addition of trastuzumab to standard chemotherapy is not cost-effective in terms of progression free survival and overall survival compared to chemotherapy alone. As such, it requires a much higher budget than the cost of chemotherapy alone in Indonesia. The government may need to opt for price negotiation of trastuzumab to facilitate the entry of biosimilar products at a cheaper price. Our findings can help guide decision makers in considering whether trastuzumab offers good value for money and would be affordable to breast cancer patients in Indonesia.
Author Contribution Statement
Conceptualization, E.K., D.E., B.H., and M.N.; methodology, E.K., D.E., B.H., and M.N.; data curation, E.K., D.E., L.C.K., W.R.P., and R.T.P.; investigation, R.P.F., A.Y., D.A.A.N., K.W.T., R.K., I.W.S., and M.D.P.; formal analysis, E.K., D.E., and L.C.K.; writing—original draft preparation, L.C.K.; writing—review and editing, E.K., D.E., and L.C.K; supervision, E.K., E.H., B.H., and M.N.; project administration, R.P.F., A.Y., D.A.A.N., K.W.T., R.K., I.W.S., and M.D.P.; funding acquisition, E.K., E.H., B.H., and M.N. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
We thank the patients who participated in this study and the staff at Dharmais Hospital, Bethesda Hospital, Dr Sardjito Hospital, Ulin General Hospital, and Sanglah General Hospital for providing the data. We appreciate the support and guidance from the Center of Financing and Health Insurance, Ministry of Health, Government of Indonesia, and Indonesia Health Technology Assessment Committee throughout the study. We are also grateful to the National Health Insurance Agency for sharing the data with us.
Funding Statement
This work was supported by the National Health Insurance Agency (BPJS Kesehatan) as part of the Health Technology Assessment study under the Center of Financing and Health Insurance (PPJK), Ministry of Health, Government of Indonesia.
Approval
Informed consent was obtained from all subjects involved in this study.
Ethical Declaration
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Faculty of Medicine Gadjah Mada University-Dr. Sardjito General Hospital (protocol no: KE/FK/1020/EC/2018; 24 September 2018).
Data Availability
The data presented in this study are available from the corresponding author upon request.
Study Registration
The study was not registered in any registering dataset.
Conflict of Interest
The authors declare no conflicts of interest.
References
- Blue A, Pink B, Green C, et al. TITLE. Asian Pac J Cancer Prev. 2000;2000:322–4. [Google Scholar]
- Balduzzi S, Mantarro S, Guarneri V, et al. Trastuzumab-Containing Regimens For Metastatic Breast Cancer. Cochrane Database Syst Rev. 2014;6:CD006242. doi: 10.1002/14651858.CD006242.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzdar AU, Ibrahim NK, Francis D, et al. significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–85. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. 11 Years’ Follow-Up Of Trastuzumab After Adjuvant Chemotherapy In Her2-Positive Early Breast Cancer: Final Analysis Of The Herceptin Adjuvant (HERA) Trial. Lancet. 2017;389:1195–1205. doi: 10.1016/S0140-6736(16)32616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AL, Leung HW, Lu CL, et al. Cost-Effectiveness of Trastuzumab As Adjuvant Therapy For Early Breast Cancer: A Systematic Review. Ann Pharm. 2009;43:296–303. doi: 10.1345/aph.1L504. [DOI] [PubMed] [Google Scholar]
- Chen W, Jiang Z, Shao Z, et al. An Economic Evaluation Of Adjuvant Trastuzumab Therapy In Her2-Positive Early Breast Cancer. Value Health. 2009;12(SUPPL.3) doi: 10.1111/j.1524-4733.2009.00634.x. [DOI] [PubMed] [Google Scholar]
- Ferrusi IL, Leighl NB, Kulin NA, et al. Do Economic Evaluations Of Targeted Therapy Provide Support For Decision Makers? J Oncol Pract. 2011;7:36–45. doi: 10.1200/JOP.2011.000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajria D, Chandarlapaty S. Her2-Amplified Breast Cancer: Mechanisms Of Trastuzumab Resistance And Novel Targeted Therapies. Exp Rev Anticancer Ther. 2011;11:263–75. doi: 10.1586/era.10.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison LP, Lubeck D, Lalla D, et al. Cost-Effectiveness Analysis Of Trastuzumab In The Adjuvant Setting For Treatment Of Her2-Positive Breast Cancer. Cancer. 2007;110:489–98. doi: 10.1002/cncr.22806. [DOI] [PubMed] [Google Scholar]
- Genuino AJ, Gloria MAJ, Chaikledkaew U, et al. Economic Evaluation Of Adjuvant Trastuzumab Therapy For Her2-Positive Early-Stage Breast Cancer: Systematic Review And Quality Assessment. Expert Rev Pharmacoecon Outcomes Res. 2021;21:1001–10. doi: 10.1080/14737167.2020.1819795. [DOI] [PubMed] [Google Scholar]
- Gershon N, Berchenko Y, Hall PS, et al. Cost Effectiveness And Affordability Of Trastuzumab In Sub-Saharan Africa For Early Stage Her2-Positive Breast Cancer. Cost Eff Resour Alloc. 2019:17. doi: 10.1186/s12962-019-0174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Rohan , Verma K, et al. Cost Effectiveness Of Trastuzumab For Management Of Breast Cancer In India. JCO Global Oncol. 2020;6:205–16. doi: 10.1200/JGO.19.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrique BC, Reinert T, Werutsky G. Access To High-Cost Drugs For Advanced Breast Cancer In Latin America, Particularly TrastuzumaB. Ecancermedicalscience. 2019:13. doi: 10.3332/ecancer.2019.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian AW, Thompson RN, Gaw AF, et al. A Cost-Effectiveness Analysis Of Adjuvant Trastuzumab Regimens In Early Her2/Neu-Positive Breast Cancer. J Clin Oncol. 2007;25:634–41. doi: 10.1200/JCO.2006.06.3081. [DOI] [PubMed] [Google Scholar]
- Leung W, Kvizhinadze G, Nair N, et al. Adjuvant Trastuzumab In Her2-Positive Early Breast Cancer By Age And Hormone Receptor Status: A Cost-Utility Analysis. PLoS Med. 2016;13:e1002067. doi: 10.1371/journal.pmed.1002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang S, Wang Y, et al. Disparities Of Trastuzumab Use In Resource-Limited Or Resource-Abundant Regions And Its Survival Benefit On Her2 Positive Breast Cancer: A Real-World Study From China. Oncologist. 2017;22:1333–8. doi: 10.1634/theoncologist.2017-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norum J. The Cost-Effectiveness Issue Of Adjuvant Trastuzumab In Early Breast Cancer. Exp Opinion Pharm. 2006;7:1617–25. doi: 10.1517/14656566.7.12.1617. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Tajiri W, Ueo H, et al. Efficacy Of Adjuvant Combination Therapy With Trastuzumab And Chemotherapy In Her2-Positive Early Breast Cancer: A Single Institutional Cohort STUDY FROM CLINICAL PRACTICE. Anticancer Res. 2020;40:3315–23. doi: 10.21873/anticanres.14314. [DOI] [PubMed] [Google Scholar]
- Owens MA, Horten BC, da Silva MM. Her2 Amplification Ratios By Fluorescence In Situ Hybridization And Correlation With Immunohistochemistry In A Cohort Of 6556 Breast Cancer Tissues. Clin Breast Cancer. 2004;5:63–9. doi: 10.3816/cbc.2004.n.011. [DOI] [PubMed] [Google Scholar]
- Paracha N, Reyes A, Diéras V, et al. Evaluating The Clinical Effectiveness And Safety Of Various Her2-Targeted Regimens After Prior Taxane/Trastuzumab In Patients With Previously Treated, Unresectable, Or Metastatic Her2-Positive Breast Cancer: A Systematic Review And Network Meta-Analysis. Breast Cancer Res Treat. 2020;180:597–609. doi: 10.1007/s10549-020-05577-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seferina SC, Ramaekers BLT, de Boer M, et al. Cost And Cost-Effectiveness Of Adjuvant Trastuzumab In The Real World Setting: A Study Of The Southeast Netherlands Breast Cancer Consortium, 2017;8:79223-233. doi: 10.18632/oncotarget.16985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skedgel C, Rayson D, Younis T. The Cost-Utility Of Sequential Adjuvant Trastuzumab In Women With Her2/Neu-Positive Breast Cancer: An Analysis Based On Updated Results From The Hera Trial. Value Health. 2009;12:641–8. doi: 10.1111/j.1524-4733.2009.00511.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization. The Global Cancer Burden. In: Wild CP, Weiderpass E, editors. World Cancer Report: Cancer Research for Cancer Prevention. IARC; 2020. pp. 15–34. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available from the corresponding author upon request.