Abstract
Objective:
This study aimed to determine the presence of Epstein-Barr Virus (EBV) and Human papillomavirus (HPV) in breast cancer with patients from Northeast of Brazil, considering the molecular subtypes and also taking in account the relation with TP53 immunoexpression.
Methods:
Seventy-five samples of invasive breast carcinoma with no special type were selected from pathology archives at Federal University of Ceará. EBV was detected by In situ hybridization (ISH) and immunohistochemistry (IHC) and HPV was detected by PCR. ISH was performed using EBER1 probe (Shibata et al., 1991; Bacchi et al., 1996) while IHC was performed on histological formalin-fixed paraffin-embedded tissue samples (Hsu et al., 1981). PCR methodology (Haws et al., 2004) was used to amplify the genetic material of human papillomavirus. The amplification products were electrophoretic analyzed on 1% agarose gel. The data analyses were carried out using the statistical software EPINFO® version 6.04d and SPSS version 17.0 (SPSS Inc., Chicago, IL). Statistically significant differences were evaluated by the chi-square test and Fisher’s exact test and correlations between groups were analyzed by Spearman’s and Pearson’s rank correlation coefficient.
Results:
69.4% of the cases were EBNA1 positives by IHC. EBNA1 positive tumors had lower Ki-67 index (0-40%), while EBNA1 negative cases had relevant higher Ki-67 index (41-100%) (p = 0.06). EBV was present in all tumor grades, with a high frequency in grade I and III tumors comparing to EBNA1 negative cases. No HPV positive cases were observed.
Conclusion:
Regarding the results from this study, we support the hypothesis that EBV can be involved on breast tumorigenesis.
Key Words: Breast, biomarkers, epidemiology, molecular oncology
Introduction
Breast cancer (BC) is the major cause of cancer mortality among women in less developed regions. Although some risk factors are well described, it has been estimated that It is stimated that, in only 20% of breast cancers worldwide, known risk factors have been identified (Torre et al., 2017). Recently, it was suggested an association between some viral infections and BC development as Human Papillomavirus (HPV) and Epstein-Barr virus (EBV) (Pai et al., 2016; Khodabandehlou et al., 2019; Jin et al., 2020; Mastoraki et al., 2020).
EBV is a gamma herpes virus that infects roughly 95% of world’s population, most happening in early childhood. The virus mainly infects B cells from the lymphocytes and oropharyngeal epithelial cells (Yin et al., 2019). After EBV infection, the virus usually becomes latent and can adopt different latency patterns expressing different viral elements depending on the affected tissue (Kimura, 2018). EBV was the first human virus directly involved in carcinogenesis being a well-established etiological factor of Burkitt’s lymphoma, nasopharyngeal cancer, Hodgkin’s disease and cancers associated with immunodeficiency (Marques-Piubelli et al., 2020). In BC, the presence of EBV is a controversial issue, the majority of publications have not managed to provide convincing evidence that the virus is truly present in human mammary epithelium or is directly associated with breast cancer (Joshi and Buehring, 2012; Glaser et al., 2017).
EBV detection uses the viral elements expressed in the latency pattern as target. In situ hybridization, targeting EBV-encoded small noncoding RNAs (EBER), is usually the methodology of choice since these RNAs are expressed in almost all latently programs of infected cell. However, in tumor cells, the EBV latent program differs according to the tissue. In breast cancer, the latency pattern of EBV is not fully known, but the EBNA1 presence and absence of Latent Membrane Protein 1 (LMP1) is always reported, while the Latent Membrane Protein 1 2A (LMP2A) and Epstein-Barr Virus Encoded Small RNA (EBERs) could be present or absent (Lima and Rabenhorst, 2006; Lorenzetti et al., 2010).
HPV is a papillomavirus that infect approximately 12% of the world population varying according to demographic characteristics and access to healthcare (Colpani et al., 2020). HPV shows a tropism for squamous epithelium and the expression of its E6 gene has been related to degradation of tumor suppressor protein TP53 (Brianti et al., 2017). Approximately 15 HPVs are categorized as high-risk, The most common are HPV 16, 18, 31, 33, 52 and 58 , which have a well-established role in cervical carcinoma (Balci et al., 2019). HPV detection could be rich achieved by in situ hybridization, however, it is not a highly sensitive method.
The association of HPV with cancers development such as cervical, anogenital, upper digestive tract region, head and neck and skin are already well established (Brianti et al., 2017; Hu and Ma, 2018; Tumban, 2019; Tommasino, 2019). Some studies have reported a relationship with breast carcinogenesis, but their detection in mammary tumor tissue varies greatly from 0 to 86% (Di Lonardo et al., 1992; Gopalkrishna et al., 1996; Hennig et al., 1999; León et al., 2009; Hachana et al., 2010).
Taking in account the necessity of more studies focusing viruses and breast cancer, this study aimed to determine the presence of EBV and HPV in breast cancer with patients from Northeast of Brazil, considering the molecular subtypes and also taking in account the relation with TP53 immunoexpression.
Materials and Methods
Clinical specimens
Seventy-five samples of invasive breast carcinoma with no special type were selected from pathology archives at Federal University of Ceará in this study. Histological and clinical data were collected from medical records. The present study was approved by the Hospital Ethics Committee of the Assis Chateaubriand Maternity School, Ceará, Brazil.
In situ hybridization (ISH) for EBER1
ISH was performed using EBER1 probe described by Shibata (1991) following previous report (Bacchi et al., 1996). The cases were considered positive when there was nuclear staining in tumor cells, regardless of the percentage of labeled cells. Cases of gastric adenocarcinomas previously tested and found positive for EBV were used as positive reaction controls.
Immunohistochemistry (IHC) for EBNA1 and breast cellular markers
Immunohistochemistry was performed on histological formalin-fixed paraffin-embedded tissue samples according to a previously described protocol (Hsu et al., 1981). For EBNA1 (Abcam®) and for cellular proteins ER, PR and TP53 (Spring Bioscience®) the antibodies were diluted 1:50, HER2 and Ki67 (Spring Bioscience®) - dilution 1:80, CK5/6 (Biocare medical®) dilution 1:70 and CK8/18 (Biocare medical®) dilution 1:150. In each round of IHC, a known positive clinical sample was included for each marker as positive controls. For the EBNA1, a negative control was performed excluding the antibody of the inespecific .
DNA isolation
Eight 10µm sections were cut from FFPE tissue and collected in sterile tubes. After cutting each sample, the blade was cleaned with ethanol to avoid contamination. Total DNA was extracted from each sample using QIAamp DNA FFPE Tissue Kit (QIAGEN, Hilden, Germany) according to manufacturer’s protocol. For each sample, Quality of isolated DNA was checked by PCR β-globin gene primers as internal control.
HPV detection (NESTED-PCR)
The polymerase chain reaction (PCR) methodology was used to amplify the genetic material of human papillomavirus present in the studied samples. The degenerated oligonucleotides PGMY09 (5-CGT CCM ARR GGA WAC TGATC- 3) and PGMY11 (5-GCM CAG GGW CAT AAY AAT GG-3) were used to detect the DNA viral. The amplification products were used in a nested PCR with the oligonucleotides GP5+ (5-TTTGTTACTGTGGTAGATACTAC-3) and GP6+ (5-CTTATACTAAATGTCAAATAAAAA-3), which generate an internal sequence of 150 bp (Haws et al., 2004). The amplification products were electrophoretic analyzed on 1% agarose gel.
Statistical analyses
The data analyses were carried out using the statistical software EPINFO® version 6.04d and SPSS version 17.0 (SPSS Inc., Chicago, IL). Statistically significant differences were evaluated by the chi-square test and Fisher’s exact test and correlations between groups were analyzed by Spearman’s and Pearson’s rank correlation coefficient. The results were considered statistically significant when p-values were ≤ 0.05.
Results
Patients and tumor characteristics
The median age of the 75 patients included in this study was 55 years old (ranging from 28 to 100 years old). Of these, 21 women were younger than 45 years, 32.3% (22/68) were smokers, 13.2% (9/68) drank alcohol, 20% (14/70) were nulliparous and 8.57% (6/70) were uniparous. Familiar or previous history of cancer (any type) was observed in 16 cases (23%), of these, five cases were familial breast cancer. Absence of the above risk factors was observed in 50.7% (34/67) of women. Just few patients were diagnosed in early stage (IA).
Luminal A (34.7%) and luminal B (30.5%) were the most common subtypes, followed by HER2 (14.7%) and triple negative tumors: normal like (14.7%) and basal like (5.3%). TP53 was present in 31% of tumors. According to cut off age, tumors grade III were more frequent in women over 45 years old (54.3 versus 27.8%). HER2 subtype were slightly more frequent in women >45 years old (15.4% versus 10%, respectively), while basal like subtype was more frequent in women ≤45 years old (10% versus 3.8%).
EBV in breast cancer: EBER1 and EBNA1 detection
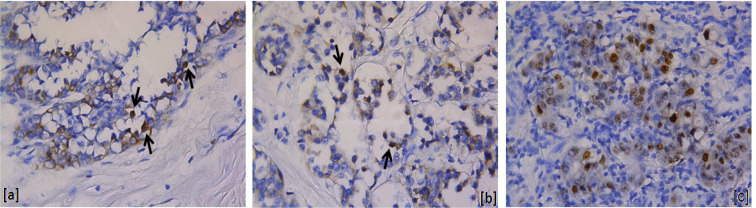
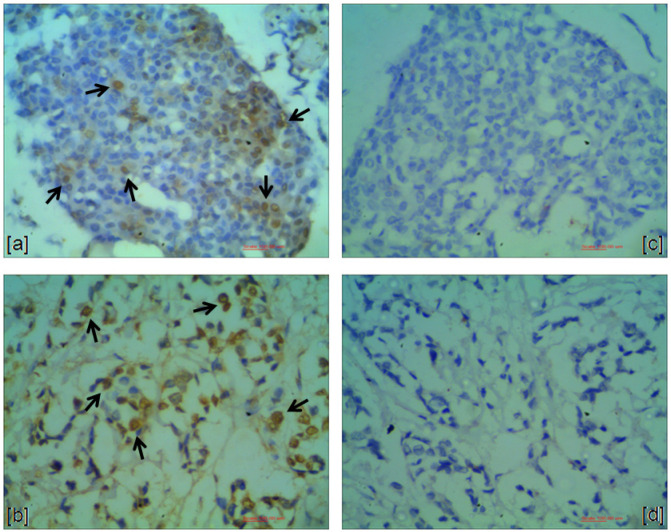
EBV-EBER1 positivity was observed in five cases (6.8%), all of them displaying weak nuclear staining in a few tumor cells (Figure 1). Unlike, EBV-EBNA1 immunostaining was present in 69.4% (50/72) of the studied cases. The stained was strong in all cases (Figure 2). Three cases were excluded from EBNA1 analysis because they did not have sufficient tumors cells. All EBV positive cases by ISH (EBER1) were also EBNA1 positive.
Figure 1.
EBER1-Positive Cases. a and b, Breast carcinomas: nuclear staining in a few tumor cells (arrows); c, Positive control (gastric adenocarcinoma). (400X)
Figure 2.
EBNA1-Positive Cases. a and b, Breast carcinomas: nuclear staining in a large number of tumor cells (arrows); c and d, Negative controls. (400X)
EBNA1 positive cases were slightly more common among smokers (76.2% versus 65.9%), those women who had at least one child (71.4% versus 67.9%), did not have drink alcohol (69.6% versus 66.7%) and had breastfeed (73.8% versus 70%). Conversely, EBNA1 positive cases were less frequent in patients with previous or familiar history of any kind of cancer (33.3% versus 70.3% and 64.3% versus 71.2% respectively).
Table 1 shows pathological profile and protein expression in EBER1 positive cases and table 2 shows the clinical and histopathological data according to the EBNA1 status. No statistical differences in molecular markers studied, histopathological parameters and cutoff age were observed between EBNA1 positive and negative cases. However, it is important to highlight that EBNA1 positive cases were present in all tumor grades with a high frequency in grade I and III tumors comparing to EBNA1 negative cases.
Table 1.
Pathological Profile and Protein Expression (ER, PR, HER2, TP53, Ki67, CK5/6 and CK8/18) in EBV Positive (EBER1) Cases (n= 5)
| In situ hibridization | Immunohistochemistry | Histopathology | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ER | PR | HER2 | TP53 | Ki67 | CK5/6 | CK8/18 | ||||||||||
| Age | R | S | R | LI | R | LI | R | E | R | LI | R | LI | R | R | G | S. Tumor |
| 55 | + | F | + | 60 | + | 20 | - | - | - | 0 | + | 99 | - | + | III | T2 |
| 45 | + | F | + | 5 | - | 0 | - | - | + | 80 | + | 8 | - | + | I | T2 |
| 64 | + | F | + | 40 | - | 0 | - | + | - | 0 | + | 35 | - | + | I | T1 |
| 54 | + | F | + | 80 | + | 40 | - | + | - | 0 | + | 20 | - | + | III | T1 |
| 47 | + | F | + | 80 | + | 80 | + | +++ | + | 95 | + | 25 | - | + | III | T3 |
ER, estrogen receptor; PR, progesterone receptor; HER2, epidermal growth factor receptor 2; CK, cytokeratin; R, result; S, staining; F, focal;
LI, labelling index (%); E, score; G, grade; S, tumor: tumor size.
Table 2.
Clinical, Histopathological and Protein Expression in EBV Positive (EBNA1) Cases (n=50)
| EBV positive % (n) |
EBV negative % (n) |
P | |
|---|---|---|---|
| Age | |||
| ≤45 years old (20) | 28 (14) | 27.3 (6) | 0.94 |
| >45 years old (52) | 72 (36) | 72.7 (16) | |
| Grade | |||
| I (15) | 27.3 (12) | 15 (3) | 0.22 |
| II (17) | 22.7 (10) | 35 (7) | 0.30 |
| III (32) | 50 (22) | 50 (10) | 1.00 |
| Lymph node status | |||
| Positive (33) | 75 (24) | 75 (9) | 0.64 |
| Negative (11) | 25 (8) | 25 (3) | |
| Molecular markers | |||
| ER+ (52) | 72 (36) | 72.7 (16) | 0.94 |
| PR+ (41) | 58 (29) | 54.5 (12) | 0.78 |
| Her2+ (10) | 14 (07) | 13.6 (03) | 0.64 |
| Ki67+ (50) | 68 (34) | 72.7 (16) | 0.68 |
| P53+ (23) | 34 (17) | 27.3 (06) | 0.57 |
| Molecular subtypes | |||
| Luminal A (23) | 36 (18) | 31.8 (7) | |
| Luminal B (24) | 30 (15) | 31.8 (7) | 0.082 |
| Her2 (10) | 14 (7) | 13.6 (3) | 0.96 |
| Basal like (04) | 06 (3) | 4.5 (1) | 0.80 |
| Normal like (11) | 14 (7) | 18.2 (4) | 0.64 |
| Triple negative (15) | 20 (10) | 22.7 (05) | 0.79 |
ER, estrogen receptor; PR, progesterone receptor.
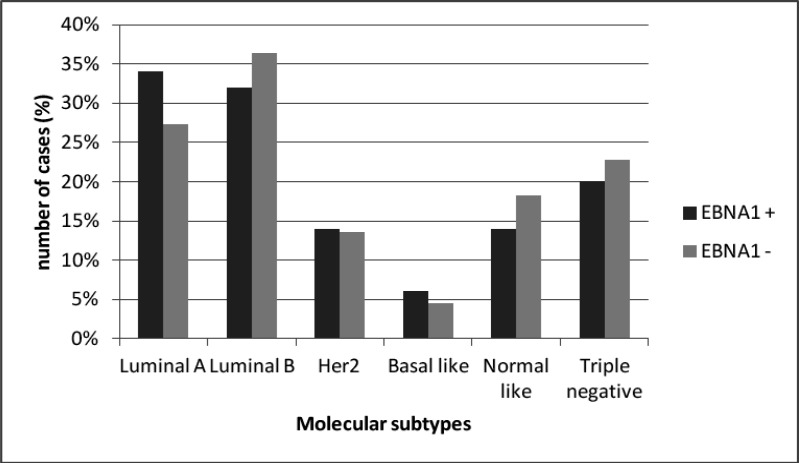
Taking in account the molecular subtypes (Figure 3), it was interesting to observed that luminal A subtype was slight more frequent in EBNA1 positive tumors while luminal B was slight more frequent in EBNA1 negative tumors. Additionally, normal simile was more frequent in EBNA1 negative tumors than in positive. It is interesting fica repetido Also, in patients ≤45 years old, all triple negative tumors were EBV positive, while in patients >45 years old, this subtype Inverta EBV negative was more frequent. was more frequently EBV negative (31.3% versus 13.9%).
Figure 3.
Molecular Subtypes Distribution between EBV-Positive and EBV-Negative Tumors
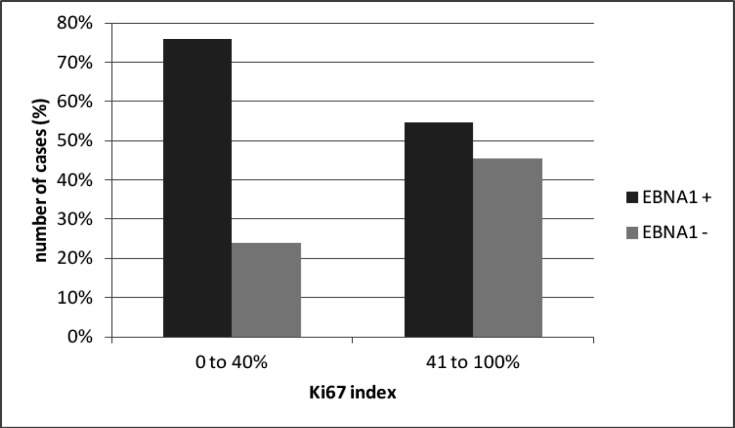
The difference among luminal molecular subtypes suggested a relation with Ki-67 (the molecular marker that differentiates these two subtypes). Considering Ki-67 index, the majority EBNA1 positive tumors had low Ki-67 index (0 to 40%), while EBNA1 negative cases had higher Ki-67 index (41 to 100%) (p = 0.06) (Figure 4).
Figure 4.
Ki67 Index in EBV-Positive and EBV Negative Tumors (p = 0.06)
HPV in breast cancer
HPV was tested in 54 samples analysis (21 cases were excluded due not having enough material available for DNA extraction). In this study, all tested samples were negative for the HPV DNA presence.
Discussion
In the present study, the most interesting results were the presence of EBV and absence of HPV in breast cancer cells. In fact, in the literature, EBV is largely associated with mammary carcinogenesis (Jin et al., 2020).
It was interesting to observe that EBV positivity was 6.67% when the target was EBER1 (by ISH) and 69.4% when the target was EBNA1 (by IHC).These results were according to the literature where EBV frequencies were higher when the target was EBNA1 comparing to EBER1. The frequency of EBV (EBNA1) in breast cancer is variable in the studies around the world (Chu et al., 2001; Grinstein et al., 2002; Murray et al., 2003; Preciado, 2003; Preciado et al., 2005; Fawzy et al., 2008; Joshi et al., 2009; Lorenzetti et al., 2010; Glenn et al., 2012). Only three studies did not find the EBV presence using EBNA1 as target (Deshpande et al., 2002; Herrman and Niedobitek, 2003; Aguayo et al., 2011). The EBV-EBNA1 frequency found in the current study (69%) was closer to the frequency found in Indian patients (54.9%), also using IHC (Joshi et al., 2009).
On the other hand, using EBER as target, the majority of the studies were EBV negative (Luqmani and Shousha, 1995; Chu et al., 1998; Glaser et al., 1998; Bonnet et al., 1999; Brink et al., 2000; Dadmanesh et al., 2001; Kijima et al., 2001; Deshpande et al., 2002; Herrman and Niedobitek, 2003; Murray et al., 2003; Perrigoue et al., 2005; Lorenzetti et al., 2010) or in very low frequencies, ranging from 1% to 20% (McCall et al., 2001; Fina et al., 2001; Trabelsi et al., 2008; Yahia et al., 2014). In only one study (from patients from Iraq and Egipt) EBV-EBER1 positivity were quite high (28% and 45%, respectively) (Zekri et al., 2012). This observation points out that EBER1, is not a constant element in breast cancer as in other EBV related cancer and could explain the few EBER1 staining cells in our study. One aspect to be raised when EBER is not abundant is the quality of the paraffin-embedded procedures. Fina (2001) found 31.8% EBV breast cancer positivity by PCR followed by HIS in paraffin-embedded and in frozen tissue for virus location (EBER1/2) and found that the signal intensity EBER1/2 was lower in paraffin-embedded sections than in frozen sections, justifying the few infected cells observed in this study using EBER as target. From that, our and the above results confirm the observation of Preciado (2005) study, in which in breast pattern latency, the EBNA1 protein is always present and EBER1 is not a constant element.
Considering the literature above cited, breast cancer -EBV associated, is worldwide spread with no specific region or development condition. There are many published studies about EBV frequency in North America, Europe and Middle East using various techniques for EBV detection. Conversely, there are few studies from south hemisphere. In South America, EBV positivity varies from 31 to 37.6% using EBNA1 as target as target. Corroborating twith this study, EBV was not detected when (Lorenzetti et al., 2010) and Chile (Aguayo et al., 2011). A previous Brazilian study (Ribeiro-Silva et al., 2004) with patients from Ribeirão Preto (São Paulo, Brazil) found EBV in 37.6% of the breast cancer cases, using IHC for EBNA1, which are a lower frequency than the current study showing differences in EBV frequency according Brazil regions.
In our study, the higher EBV frequency found in grade I (80% among the grade I was EBV+) in accordance with other studies (Chu et al., 2001; Preciado et al., 2005; Zekri et al., 2012; Mazouni et al., 2015; Reza et al., 2015) raises investigations on the role of this virus in the grade I breast cancer carcinogenesis. EBV also seems not to be associated to any molecular subtype, however, the highest EBV frequencies was in luminal A, followed by luminal B as it was found by Mazouni (2015). Considering the proliferative status though Ki67 analyzes, it was observed, besides didn’t rich the significance (p=0.06), that low Ki67 index was present in EBV positive tumors, while high Ki67 index was present in EBV negative tumors, suggesting that tumors EBV positive are less proliferative. There are no previous studies evaluating Ki67 in EBV positive breast cancers but in B-cell Non-Hodgkin lymphomas, it was not significantly related with any tested proliferation marker (Chabay et al., 2011). Beside EBV from the results above described could be linked to a potential better prognosis, it is important to highlight the relevant presence of EBV among the basal like subtype (75%) that has a poor prognosis. In addition the frequency of TP53 was slightly higher in EBV positive tumors, although contrasting to Glenn (2012) study, on which was found higher TP53 frequency in EBV negative sample. From that, the EBV positive breast cancer need more studies including therapeutic response.
On the other hand, HPV was found negative in the present study and in some others (Bratthauer et al., 1992; Wrede et al., 1992; Gopalkrishna et al., 1996; Lindel et al., 2007; Cremoux et al., 2008; Hachana et al., 2010; Hedau et al., 2011; Chang et al., 2012; Ahangar-Oskouee et al., 2014). However most of the published studies have reported HPV presence in BC. The presence of HPV in breast cancer vary widely in published studies worldwide, being high-risk HPVs present in 15.9% to 63.6% of breast cancers in European women (Di Lonardo et al., 1992; Henning et al., 1999; Widschwendter et al., 2004; Kroupis et al., 2006; Frega et al., 2012); at about 49% in women from Oceania (Kan et al., 2005 ; Glenn et al., 2012); in 5.7% to 86% in women from United States (Liu et al., 2001; Villiers et al., 2005; Baltzel et al., 2012); in 11 to 74% in women from Asian (Tsai et al., 2005; Gumus et al., 2006; Choi et al., 2007; Akil et al., 2008; Khan et al., 2008; Liang and Tian, 2008; Sigarood et al., 2012; Ahangar-Oskouee et al., 2014; Choi et al., 2016) and in 4.47% to 29.4% in South American women, including Mexican (Mendizabal-Ruiz et al., 2009; León et al., 2009) and Brazilian women with 24.75% using sequencing methodology (Damin et al., 2004).
These differences are attributed to demographic features, type of sample and different sensitivity of the methods used. In this study, the method was high sensitive, but HPV could be more associated to a specific subtype and underrepresented in this study. There is also a possibility that the genetic material of HPV could underwent some degradation due of paraffin-embedded process.
In conclusion, this study supports the hypothesis that EBV, but not HPV, may be involved in tumorigenesis of some breast tumor types, since the presence of the virus in high frequency was observed in low grade and luminal A tumors. Besides no significant association was observed for histopathological parameters for EBV associated breast carcinoma, the data of this study instigate more investigation for role of EBV in breast carcinogenesis.
Author Contribution Statement
The authors confirm contribution to the paper as follows: study conception and design: Oliveira ES, Rabenhorst SHB; data collection: Oliveira ES, Ferreira MVP, Rahal P, Branco MBC; analysis and interpretation of results: Oliveira ES, Rabenhorst SHB; draft manuscript preparation: Oliveira ES, Branco MBC, Rabenhorst SHB. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgment
This study was approved by the Hospital Ethics Committee of the Assis Chateaubriand Maternity School, Ceará, Brazil. It has also been part of a PhD thesis supported by Federal University of Ceará (UFC). No conflict of interests or external funding were present in this paper.
References
- Aguayo F, Khan N, Koriyama C, et al. Human papillomavirus and Epstein-Barr virus infections in breast cancer from chile. Infect Agent Cancer. 2011;6:1–7. doi: 10.1186/1750-9378-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahangar-Oskouee M, Shahmahmoodi S, Jalilvand S, et al. No detection of ‘high-risk’ human papillomaviruses in a group of Iranian women with breast cancer. Asian Pac J Cancer Prev. 2014;15:4061–5. doi: 10.7314/apjcp.2014.15.9.4061. [DOI] [PubMed] [Google Scholar]
- Akil N, Yasmeen A, Kassab A, et al. High-risk human papillomavirus infections in breast cancer in Syrian women and their association with Id-1 expression: a tissue microarray study. Br J Cancer. 2008;99:404–7. doi: 10.1038/sj.bjc.6604503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchi CE, Bacchi MM, Rabenhorst SH, et al. AIDS-related lymphoma in Brazil - Histopathology, immunophenotype, and association with Epstein-Barr virus. Am J Clin Pathol. 1996;1996:230–7. doi: 10.1093/ajcp/105.2.230. [DOI] [PubMed] [Google Scholar]
- Balci FL, Uras C, Feldman SM. Is human papillomavirus associated with breast cancer or papilloma presenting with pathologic nipple discharge? Cancer Treat Res Commun. 2019;19:100122. doi: 10.1016/j.ctarc.2019.100122. [DOI] [PubMed] [Google Scholar]
- Baltzell K, Buehring GC, Krishnamurthy S, et al. Limited evidence of human papillomavirus in [corrected] breast tissue using molecular in situ methods. Cancer. 2012;118:1212–20. doi: 10.1002/cncr.26389. [DOI] [PubMed] [Google Scholar]
- Bonnet M, Guinebretiere JM, Kremmer E, et al. Detection of Epstein-Barr virus in invasive breast cancers. J Natl Cancer Inst. 1999;91:1376–81. doi: 10.1093/jnci/91.16.1376. [DOI] [PubMed] [Google Scholar]
- Bratthauer GL, Tavassoli FA, O’Leary TJ. Etiology of breast carcinoma: no apparent role for papillomavirus types 6/11/16/18. Pathol Res Pract. 1992;188:384–6. doi: 10.1016/S0344-0338(11)81229-X. [DOI] [PubMed] [Google Scholar]
- Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017;40:80–5. [PubMed] [Google Scholar]
- Brink AA, ten Berge RL, van den Brule AJ, et al. Epstein-Barr virus is present in neoplastic cytotoxic T cells in extranodal, and predominantly in B cells in nodal T non-Hodgkin lymphomas. J Pathol. 2000;191:400–6. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH658>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Chabay P, Lara J, Lorenzetti M, et al. Epstein Barr virus in relation to apoptosis markers and patients’ outcome in pediatric B-cell non-Hodgkin lymphoma. Cancer Lett. 2011;307:221–6. doi: 10.1016/j.canlet.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Chang P, Wang T, Yao Q, et al. Absence of human papillomavirus in patients with breast cancer in north-west China. Med Oncol. 2012;29:521–5. doi: 10.1007/s12032-011-9945-5. [DOI] [PubMed] [Google Scholar]
- Chen WG, Chen YY, Bacchi MM, et al. Genotyping of Epstein-Barr virus in Brazilian Burkitt’s lymphoma and reactive lymphoid tissue Type A with a high prevalence of deletions within the latent membrane protein gene. Am J Pathol. 1996;148:17–23. [PMC free article] [PubMed] [Google Scholar]
- Choi J, Kim C, Lee HS, et al. Detection of Human Papillomavirus in Korean Breast Cancer Patients by Real-Time Polymerase Chain Reaction and Meta-Analysis of Human Papillomavirus and Breast Cancer. J Pathol Transl Med. 2016;50:442–50. doi: 10.4132/jptm.2016.07.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y-L, Cho EY, Kim JH, et al. Detection of human papillomavirus DNA by DNA chip in breast carcinomas of Korean women. Tumour Biol. 2007;28:327–32. doi: 10.1159/000124238. [DOI] [PubMed] [Google Scholar]
- Chu JS, Chen CC, Chang KJ. In situ detection of Epstein-Barr virus in breast cancer. Cancer Lett. 1998;124:53–7. doi: 10.1016/s0304-3835(97)00449-7. [DOI] [PubMed] [Google Scholar]
- Chu PG, Chang KL, Chen YY, Chen WG, Weiss LM. No significant association of Epstein-Barr virus infection with invasive breast carcinoma. Am J Pathol. 2001;159:571–8. doi: 10.1016/S0002-9440(10)61728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford GM, Smith JS, Aguado T, Franceschi S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br J Cancer. 2003;89:101–5. doi: 10.1038/sj.bjc.6601024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpani V, Soares Falcetta F, Bacelo Bidinotto A, et al. Prevalence of human papillomavirus (HPV) in Brazil: A systematic review and meta-analysis. PLoS One. 2020;15:e0229154. doi: 10.1371/journal.pone.0229154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coser J, da Rocha Boeira T, Simon D, et al. Prevalence and genotypic diversity of cervical human papillomavirus infection among women from an urban center in Brazil. Genet Mol Res. 2013;12:4276–85. doi: 10.4238/2013.February.19.3. [DOI] [PubMed] [Google Scholar]
- Cremoux P de, Thioux M, Lebigot I, et al. No evidence of human papillomavirus DNA sequences in invasive breast carcinoma. Breast Cancer Res Treat. 2008;109:55–8. doi: 10.1007/s10549-007-9626-4. [DOI] [PubMed] [Google Scholar]
- Crombie JL, LaCasce AS. Epstein Barr Virus Associated B-Cell Lymphomas and Iatrogenic Lymphoproliferative Disorders. Front Oncol. 2019;9:109. doi: 10.3389/fonc.2019.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadmanesh F, Peterse JL, Sapino A, Fonelli A, Eusebi V. Lymphoepithelioma-like carcinoma of the breast: lack of evidence of Epstein-Barr virus infection. Histopathology. 2001;38:54–61. doi: 10.1046/j.1365-2559.2001.01055.x. [DOI] [PubMed] [Google Scholar]
- Damin APS, Karam R, Zettler CG, Caleffi M, Alexandre COP. Evidence for an association of human papillomavirus and breast carcinomas. Breast Cancer Res Treat. 2004;84:131–7. doi: 10.1023/B:BREA.0000018411.89667.0d. [DOI] [PubMed] [Google Scholar]
- Deshpande CG, Badve S, Kidwai N, Longnecker R. Lack of expression of the Epstein-Barr Virus (EBV) gene products, EBERs, EBNA1, LMP1, and LMP2A, in breast cancer cells. Lab Invest. 2002;82:1193–9. doi: 10.1097/01.lab.0000029150.90532.24. [DOI] [PubMed] [Google Scholar]
- Di Lonardo A, Venuti A, Marcante ML. Human papillomavirus in breast cancer. Breast Cancer Res Treat. 1992;21:95–100. doi: 10.1007/BF01836955. [DOI] [PubMed] [Google Scholar]
- Fawzy S, Sallam M, Awad NM. Detection of Epstein-Barr virus in breast carcinoma in Egyptian women. Clin Biochem. 2008;41:486–92. doi: 10.1016/j.clinbiochem.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Fina F, Romain S, Ouafik L, et al. Frequency and genome load of Epstein-Barr virus in 509 breast cancers from different geographical areas. Br J Cancer. 2001;84:783–90. doi: 10.1054/bjoc.2000.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frega A, Lorenzon L, Bononi M, et al. Evaluation of E6 and E7 mRNA expression in HPV DNA positive breast cancer. Eur J Gynaecol Oncol. 2012;33:164–7. [PubMed] [Google Scholar]
- Glaser SL, Ambinder RF, DiGiuseppe JA, Horn-Ross PL, Hsu JL. Absence of Epstein-Barr virus EBER-1 transcripts in an epidemiologically diverse group of breast cancers. Int J Cancer. 1998;75:555–8. doi: 10.1002/(sici)1097-0215(19980209)75:4<555::aid-ijc10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Glaser SL, Hsu JL, Gulley ML. Epstein-Barr virus and breast cancer: state of the evidence for viral carcinogenesis. Cancer Epidemiol Biomarkers Prev. 2004;13:688–97. [PubMed] [Google Scholar]
- Glaser SL, Canchola AJ, Keegan THM, et al. Variation in risk and outcomes of Epstein-Barr virus-associated breast cancer by epidemiologic characteristics and virus detection strategies: an exploratory study. Cancer Causes Control. 2017;28:273–87. doi: 10.1007/s10552-017-0865-3. [DOI] [PubMed] [Google Scholar]
- Glenn WK, Heng B, Delprado W, et al. Epstein-Barr virus, human papillomavirus and mouse mammary tumour virus as multiple viruses in breast cancer. PLoS One. 2012;7:e48788. doi: 10.1371/journal.pone.0048788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalkrishna V, Singh UR, Sodhani P, et al. Absence of human papillomavirus DNA in breast cancer as revealed by polymerase chain reaction. Breast Cancer Res Treat. 1996;39:197–202. doi: 10.1007/BF01806186. [DOI] [PubMed] [Google Scholar]
- Grinstein S, Preciado MV, Gattuso P, et al. Demonstration of Epstein-Barr virus in carcinomas of various sites. Cancer Res. 2002;62:4876–8. [PubMed] [Google Scholar]
- Grm HS, Bergant M, Banks L. Human papillomavirus infection, cancer & therapy. Indian J Med Res. 2009;130:277–85. [PubMed] [Google Scholar]
- Gumus M, Yumuk PF, Salepci T, et al. HPV DNA frequency and subset analysis in human breast cancer patients’ normal and tumoral tissue samples. J Exp Clin Cancer Res. 2006;25:515–21. [PubMed] [Google Scholar]
- Hachana M, Ziadi S, Amara K, et al. No evidence of human papillomavirus DNA in breast carcinoma in Tunisian patients. Breast J. 2010;19:541–4. doi: 10.1016/j.breast.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Hachana M, Amara K, Ziadi S, et al. Investigation of Epstein-Barr virus in breast carcinomas in Tunisia. Pathol Res Pract. 2011;207:695–700. doi: 10.1016/j.prp.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Harkins LE, Matlaf LA, Soroceanu L, et al. Detection of human cytomegalovirus in normal and neoplastic breast epithelium. Herpesviridae. 2010;1:8. doi: 10.1186/2042-4280-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haws ALF, He Q, Rady PL, et al. Nested PCR with the PGMY09/11 and GP5(+)/6(+) primer sets improves detection of HPV DNA in cervical samples. J Virol Methods. 2004;122:87–93. doi: 10.1016/j.jviromet.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Hedau S, Kumar U, Hussain S, et al. Breast cancer and human papillomavirus infection: no evidence of HPV etiology of breast cancer in Indian women. BMC Cancer. 2011;11:27. doi: 10.1186/1471-2407-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng B, Glenn WK, Ye Y, et al. Human papilloma virus is associated with breast cancer. Br J Cancer. 2009;101:1345–50. doi: 10.1038/sj.bjc.6605282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig EM, Suo Z, Thoresen S, et al. Human papillomavirus 16 in breast cancer of women treated for high grade cervical intraepithelial neoplasia (CIN III) Breast Cancer Res Treat. 1999;53:121–35. doi: 10.1023/a:1006162609420. [DOI] [PubMed] [Google Scholar]
- Herrmann K, Niedobitek G. Lack of evidence for an association of Epstein–Barr virus infection with breast carcinoma. Breast Cancer Res. 2003;5:13–7. doi: 10.1186/bcr561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–80. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hu Z, Ma D. The precision prevention and therapy of HPV-related cervical cancer: new concepts and clinical implications. Cancer Med. 2018;7:5217–36. doi: 10.1002/cam4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii Y, Tanaka K, Kanda T. Mutational analysis of human papillomavirus type 16 major capsid protein L1: the cysteines affecting the intermolecular bonding and structure of L1-capsids. Virology. 2003;308:128–36. doi: 10.1016/s0042-6822(02)00099-5. [DOI] [PubMed] [Google Scholar]
- Jin Q, Su J, Yan D, Wu S. Epstein-Barr Virus Infection and Increased Sporadic Breast Carcinoma Risk: A Meta-Analysis. MPP. 2020;29:195–200. doi: 10.1159/000502131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi D, Quadri M, Gangane N, Joshi R, Gangane N. Association of Epstein Barr Virus Infection (EBV) with Breast Cancer in Rural Indian Women. PLoS One. 2009;4:e8180. doi: 10.1371/journal.pone.0008180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi D, Buehring GC. Are viruses associated with human breast cancer? Scrutinizing the molecular evidence. Breast Cancer Res Treat. 2012;135:1–15. doi: 10.1007/s10549-011-1921-4. [DOI] [PubMed] [Google Scholar]
- Kadivar M, Monabati A, Joulaee A, Hosseini N. Epstein-Barr virus and breast cancer: lack of evidence for an association in Iranian women. Pathol Oncol Res. 2011;17:489–92. doi: 10.1007/s12253-010-9325-z. [DOI] [PubMed] [Google Scholar]
- Kan C-Y, Iacopetta BJ, Lawson JS, Whitaker NJ. Identification of human papillomavirus DNA gene sequences in human breast cancer. Br J Cancer. 2005;93:946–8. doi: 10.1038/sj.bjc.6602778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan NA, Castillo A, Koriyama C, et al. Human papillomavirus detected in female breast carcinomas in Japan. Br J Cancer. 2008;99:408–14. doi: 10.1038/sj.bjc.6604502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodabandehlou N, Mostafaei S, Etemadi A, et al. Human papilloma virus and breast cancer: the role of inflammation and viral expressed proteins. BMC Cancer. 2019;19:61. doi: 10.1186/s12885-019-5286-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijima Y, Hokita S, Takao S, et al. Epstein-Barr virus involvement is mainly restricted to lymphoepithelial type of gastric carcinoma among various epithelial neoplasms. J Med Virol. 2001;64:513–8. doi: 10.1002/jmv.1079. [DOI] [PubMed] [Google Scholar]
- Kimura H. EBV in T-/NK-Cell Tumorigenesis. Adv Exp Med Biol. 2018;1045:459–75. doi: 10.1007/978-981-10-7230-7_21. [DOI] [PubMed] [Google Scholar]
- Kroupis C, Markou A, Vourlidis N, Dionyssiou-Asteriou A, Lianidou ES. Presence of high-risk human papillomavirus sequences in breast cancer tissues and association with histopathological characteristics. Clin Biochem. 2006;39:727–31. doi: 10.1016/j.clinbiochem.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Kudela E, Nachajova M, Danko J. Is the HPV virus responsible for the development of breast cancer? Breast J. 2019;25:1053–4. doi: 10.1111/tbj.13399. [DOI] [PubMed] [Google Scholar]
- León DC de, Montiel DP, Nemcova J, et al. Human papillomavirus (HPV) in breast tumors: prevalence in a group of Mexican patients. BMC Cancer. 2009;9:26. doi: 10.1186/1471-2407-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W, Tian H. Hypothetic association between human papillomavirus infection and breast carcinoma. Med Hypotheses. 2008;70:305–7. doi: 10.1016/j.mehy.2007.05.032. [DOI] [PubMed] [Google Scholar]
- Lima MAP de, Rabenhorst SHB. Associação do vírus Epstein-Barr (EBV) com tumores sólidos. Rev Bras Cancerol. 2006;2006:87–96. [Google Scholar]
- Lindel K, Forster A, Altermatt HJ, Greiner R, Gruber G. Breast cancer and human papillomavirus (HPV) infection: No evidence of a viral etiology in a group of Swiss women. Breast J. 2007;16:172–7. doi: 10.1016/j.breast.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Liu Y, Klimberg VS, Andrews NR, et al. Human papillomavirus DNA is present in a subset of unselected breast cancers. J Hum Virol. 2001;4:329–34. [PubMed] [Google Scholar]
- Lorenzetti MA, De Matteo E, Gass H, et al. Characterization of Epstein Barr Virus Latency Pattern in Argentine Breast Carcinoma. PLoS One. 2010;5:e13603. doi: 10.1371/journal.pone.0013603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luqmani Y, Shousha S. Presence of epstein-barr-virus in breast-carcinoma. Int J Oncol. 1995;6:899–903. doi: 10.3892/ijo.6.4.899. [DOI] [PubMed] [Google Scholar]
- Marques-Piubelli ML, Salas YI, Pachas C, et al. Epstein-Barr virus-associated B-cell lymphoproliferative disorders and lymphomas: a review. Pathology. 2020;52:40–52. doi: 10.1016/j.pathol.2019.09.006. [DOI] [PubMed] [Google Scholar]
- Mastoraki A, Schizas D, Gkiala A, et al. Human Papilloma Virus infection and breast cancer development: Challenging theories and controversies with regard to their potential association. J BUON. 2020;25:1295–301. [PubMed] [Google Scholar]
- Mazouni C, Fina F, Romain S, et al. Outcome of Epstein-Barr virus-associated primary breast cancer. Mol Clin Oncol. 2015;3:295–8. doi: 10.3892/mco.2014.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall SA, Lichy JH, Bijwaard KE, et al. Epstein-Barr virus detection in ductal carcinoma of the breast. J Natl Cancer Inst. 2001;93:148–50. doi: 10.1093/jnci/93.2.148. [DOI] [PubMed] [Google Scholar]
- Mendizabal-Ruiz AP, Morales JA, Ramírez-Jirano LJ, et al. Low frequency of human papillomavirus DNA in breast cancer tissue. Breast Cancer Res Treat. 2009;114:189–94. doi: 10.1007/s10549-008-9989-1. [DOI] [PubMed] [Google Scholar]
- Mohamed MM, Al-Raawi D, Sabet SF, El-Shinawi M. Inflammatory breast cancer: New factors contribute to disease etiology: A review. J Adv Res. 2014;5:525–36. doi: 10.1016/j.jare.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PG, Lissauer D, Junying J, et al. Reactivity with A monoclonal antibody to Epstein-Barr virus (EBV) nuclear antigen 1 defines a subset of aggressive breast cancers in the absence of the EBV genome. Cancer Res. 2003;63:2338–43. [PubMed] [Google Scholar]
- Pai T, Gupta S, Gurav M, et al. Evidence for the association of Epstein-Barr Virus in breast cancer in Indian patients using in-situ hybridization technique. Breast J. 2018;24:16–22. doi: 10.1111/tbj.12828. [DOI] [PubMed] [Google Scholar]
- Perrigoue JG, den Boon JA, Friedl A, et al. Lack of association between EBV and breast carcinoma. Cancer Epidemiol Biomarkers Prev. 2005;14:809–14. doi: 10.1158/1055-9965.EPI-04-0763. [DOI] [PubMed] [Google Scholar]
- Preciado MV. Lack of evidence for an association of Epstein-Barr virus infection with breast carcinoma--another point of view. Breast Cancer Res. 2003;5:13–7. doi: 10.1186/bcr599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preciado MV, Chabay PA, De Matteo EN, et al. Epstein-Barr virus in breast carcinoma in Argentina. Arch Pathol Lab Med. 2005;129:377–81. doi: 10.5858/2005-129-377-EVIBCI. [DOI] [PubMed] [Google Scholar]
- Reza MA, Reza MH, Mahdiyeh L, Mehdi F, Hamid ZN. Evaluation Frequency of Merkel Cell Polyoma, Epstein-Barr and Mouse Mammary Tumor Viruses in Patients with Breast Cancer in Kerman, Southeast of Iran. Asian Pac J Cancer Prev. 2015;16:7351–7. doi: 10.7314/apjcp.2015.16.16.7351. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Silva A, Ramalho LNZ, Garcia SB, Zucoloto S. Does the correlation between EBNA-1 and p63 expression in breast carcinomas provide a clue to tumorigenesis in Epstein-Barr virus-related breast malignancies? Braz J Med Biol Res. 2004;37:89–95. doi: 10.1590/s0100-879x2004000100013. [DOI] [PubMed] [Google Scholar]
- Shibata D, Tokunaga M, Uemura Y, et al. Association of Epstein-Barr virus with undifferentiated gastric carcinomas with intense lymphoid infiltration. Lymphoepithelioma-like carcinoma. Am J Pathol. 1991;139:469–74. [PMC free article] [PubMed] [Google Scholar]
- Sigaroodi A, Nadji SA, Naghshvar F, et al. Human Papillomavirus Is Associated with Breast Cancer in the North Part of Iran. Sci World J. 2012;2012:e837191. doi: 10.1100/2012/837191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MP, Kurzrock R. Epstein-Barr virus and cancer. Clin Cancer Res. 2004;10:803–21. doi: 10.1158/1078-0432.ccr-0670-3. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson DA. Epstein-Barr virus: exploiting the immune system. Nat Rev Immunol. 2001;1:75–82. doi: 10.1038/35095584. [DOI] [PubMed] [Google Scholar]
- Tommasino M. HPV and skin carcinogenesis. Papillomavirus Res. 2019;7:129–31. doi: 10.1016/j.pvr.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre LA, Islami F, Siegel RL, Ward EM, Jemal A. Global Cancer in Women: Burden and Trends. Cancer Epidemiol Biomarkers Prev. 2017;26:444–57. doi: 10.1158/1055-9965.EPI-16-0858. [DOI] [PubMed] [Google Scholar]
- Trabelsi A, Rammeh S, Stita W, et al. [Detection of Epstein-Barr virus in breast cancers with lymphoid stroma] Ann Biol Clin (Paris) 2008;66:59–62. doi: 10.1684/abc.2008.0191. [DOI] [PubMed] [Google Scholar]
- Tsai J-H, Tsai C-H, Cheng M-H, et al. Association of viral factors with non-familial breast cancer in Taiwan by comparison with non-cancerous, fibroadenoma, and thyroid tumor tissues. J Med Virol. 2005;75:276–81. doi: 10.1002/jmv.20267. [DOI] [PubMed] [Google Scholar]
- Tumban E. A Current Update on Human Papillomavirus-Associated Head and Neck Cancers. Viruses. 2019;11:922. doi: 10.3390/v11100922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa LL, Sichero L, Rahal P, et al. Molecular variants of human papillomavirus types 16 and 18 preferentially associated with cervical neoplasia. J Gen Virol. 2000;81:2959–68. doi: 10.1099/0022-1317-81-12-2959. [DOI] [PubMed] [Google Scholar]
- Villiers E-M de, Sandstrom RE, zur Hausen H, Buck CE. Presence of papillomavirus sequences in condylomatous lesions of the mamillae and in invasive carcinoma of the breast. Breast Cancer Res. 2005;7:1–11. doi: 10.1186/bcr940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widschwendter A, Brunhuber T, Wiedemair A, Mueller-Holzner E, Marth C. Detection of human papillomavirus DNA in breast cancer of patients with cervical cancer history. J Clin Virol. 2004;31:292–7. doi: 10.1016/j.jcv.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Wrede D, Luqmani YA, Coombes RC, Vousden KH. Absence of HPV 16 and 18 DNA in breast cancer. Br J Cancer. 1992;65:891–4. doi: 10.1038/bjc.1992.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahia ZA, Adam AA, Elgizouli M, et al. Epstein Barr virus: a prime candidate of breast cancer aetiology in Sudanese patients. Infect Agent Cancer. 2014;9:9. doi: 10.1186/1750-9378-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Qu J, Peng Q, Gan R. Molecular mechanisms of EBV-driven cell cycle progression and oncogenesis. Med Microbiol Immunol. 2019;208:573–83. doi: 10.1007/s00430-018-0570-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757–68. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- Zekri A-RN, Bahnassy AA, Mohamed WS, et al. Epstein-Barr virus and breast cancer: epidemiological and molecular study on Egyptian and Iraqi women. J Egypt Natl Canc Inst. 2012;24:123–31. doi: 10.1016/j.jnci.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Zur Hausen H. Papillomavirus infections - a major cause of human cancers. Biochim Biophys Acta. 1996;1288:55–78. doi: 10.1016/0304-419x(96)00020-0. [DOI] [PubMed] [Google Scholar]