Abstract
Tumor necrosis factor (TNF) has generally been regarded as a protective cytokine in host defense against bacterial infections. In the present study, we evaluated the role of TNF in the acute phase of infection by Yersinia enterocolitica by using mice rendered genetically deficient in TNF receptor p55 (TNFRp55−/−). Unexpectedly, TNFRp55−/− mice showed more effective resistance to the bacteria, reflected in enhanced bacterial clearance and less tissue damage, than did control C57BL/6 mice. C57BL/6 mice showed evidence of extensive apoptosis in the spleen accompanied by a selective decrease in the CD4+-T-cell population of splenocytes, whereas TNFRp55−/− mice were spared these changes. The splenocytes from TNFRp55−/− mice also maintained a robust gamma interferon IFN-γ response to mitogenic stimulation, while the comparable response in C57BL/6 mice was impaired. In addition, splenocytes harvested from infected mice demonstrated lower production of interleukin-10 IL-10 in TNFRp55−/− mice than in C57BL/6 mice. These findings suggest that Yersinia can induce TNFRp55-mediated apoptosis of splenocytes in the acute phase of the infection and that alteration of T-cell-generated cytokines can dramatically alter the early events in host defense against this pathogen.
Yersinia enterocolitica is a gram-negative pathogen that causes enteritis and enterocolitis in humans and rodents (17). Systemic infections can cause abscesses and granulomatous lesions in the spleen and liver (12). Furthermore, immunopathological sequelae of Yersinia infection such as reactive arthritis are well recognized (17). An effective cell-mediated immune response required to overcome infection with Yersinia is controlled by different cell types and their respective cytokines (1, 5, 8, 9, 11). It has been demonstrated that in the first 3 days after Y. enterocolitica infection, innate host defense mechanisms, particularly involving Mac-1+ phagocytes and NK cells, play a role in controlling the growth of this pathogen in host tissues (2, 4). Thereafter, a specific T-cell response accounts for resolution of the interaction (2, 4), with CD4+ Th1 cells playing a critical protective role (5). A balance between Th1 and Th2 cytokines has been proposed to influence the outcome of Yersinia infection (8), especially in the early stages of host defense. Gamma interferon (IFN-γ), produced by NK cells and CD4+ T cells, is associated with resistance of mice to Yersinia (3), and interleukin-12 (IL-12) is essential in enhancing IFN-γ production (8). In contrast, anti-IL-4 antibodies render BALB/c mice resistant to Yersinia (8). IL-10 acts antagonistically to IL-12 during yersinosis in BALB/c mice (11).
Tumor necrosis factor (TNF), produced primarily by monocytes and T cells, has also been considered a critical cytokine in activating macrophages in the protective host response to Yersinia. Increased TNF levels are associated with host resistance to Yersinia (13). However, TNF is a pleiotropic cytokine that can exert both beneficial and detrimental effects in bacterial infections (6), and the precise biologic mechanisms underlying the action of TNF in Yersinia infection have not yet been defined. TNF exerts its biologic activity via two receptors, TNFRp55 and TNFRp75. TNFRp55 is known to mediate most of the effects of TNF, including cytotoxicity mediated by necrosis and apoptosis, activation of macrophages, and up-regulation of adhesion molecules (6). Studies of TNFRp55 gene-targeted mice have revealed that in the absence of TNFRp55, the mice are highly susceptible to infection with Listeria monocytogenes, Mycobacterium tuberculosis, and Salmonella enterica serovar Typhimurium (18, 20, 23). A recent study by Bohn et al. demonstrated that TNFRp55-mediated mechanisms are essential for clearance of infection with Y. enterocolitica WA-314, a less virulent strain of serotype O:8 (10). However, mice lacking TNFRp55 are able to eliminate Leishmania major at the site of infection, although they do not heal their lesion (37, 48). A recent study also showed that TNF receptor signaling is not required for early control of Toxoplasma gondii but is critical for the prevention of toxoplasmic encephalitis later in infection (51). These studies demonstrated that macrophage activation appears to be largely unimpaired in the early phase of the infections in TNFRp55−/− mice, suggesting that cells other than macrophages are the primary effector of TNF receptor-dependent resistance during a certain stages of microbial infections.
Using a murine model of hematogenous Y. enterocolitica infection, we have recently demonstrated that TNFRp55 plays a critical role in host resistance to chronic infection with Y. enterocolitica strain 8081 (54). TNFRp55−/− mice succumbed to infection with Y. enterocolitica after 2 weeks, with a higher mortality rate than C57BL/6 mice. Increased bacterial growth in the liver, spleen, and lungs was observed in TNFRp55−/− mice compared with control mice on day 14 after infection. This was associated with impaired macrophage bactericidal activity including nitric oxide production and oxidative burst activity. Of interest, we observed that TNFRp55−/− mice appeared capable of limiting acute infection with different numbers of Y. enterocolitica bacteria (54) and that all the mice survived the acute infection. To explore the mechanisms whereby TNFRp55−/− mice respond in the acute phase of infection with Y. enterocolitica, we have investigated the cellular responses to Yersinia at the early stage in TNFRp55−/− and C57BL/6 mice. In the present study, we observed more efficient bacterial clearance and reduced tissue damage at the early stage of the infection in TNFRp55−/− mice. The early defense against Y. enterocolitica in TNFRp55−/− mice is associated with less apoptosis in splenocytes and the presence of increased total numbers of CD4+ T cells compared with those in control C57BL/6 mice.
MATERIALS AND METHODS
Mice.
Breeding pairs of homozygous TNFRp55−/− mice on a C57BL/6 background (38) were kindly provided by T. Mak (Amgen Institute and the University of Toronto, Toronto, Canada) and bred under specific-pathogen-free conditions. Control C57BL/6 mice were purchased from Charles River (Montreal, Canada). All mice were maintained at the animal facility in the Toronto Hospital, Western Division. For infections we used 6- to 10-week-old mice of both sexes that were housed in microisolators.
Infection of animals.
Y. enterocolitica strain 8081, a virulent serotype O:8 strain harboring the pYV plasmid, was used in the experiments (26). The presence of the virulence plasmid was confirmed by immunofluorescence staining using plasmid-encoded fibrillar outer membrane protein Yad A-specific monoclonal antibody 8D1 (kindly provided by Dr. J. Heesemann, Max von Pettenkofer Institute, Munich, Germany) (41). The bacteria were cultivated at 24°C in Luria broth (LB) (Difco, Detroit, Mich.), harvested during the log phase, and frozen at −80°C in LB containing 25% glycerol. Freshly thawed bacteria were prepared in phosphate-buffered saline (PBS), and mice were inoculated intravenously in a tail vein with 0.2 ml of the bacterial solution. The number of bacteria administered was determined by plating serial dilutions of the inoculum on Yersinia selective agar (Difco) and Luria broth agar (Difco), and the CFU were counted after an incubation period of 40 h at 24°C. TNFRp55−/− and C57BL/6 mice were inoculated with 350 CFU of the bacteria and were sacrificed on day 5 after infection. Blood samples were obtained before death. The organs including spleen, liver, lungs, and kidneys were collected for analysis.
Histologic examination and apoptosis detection.
Histopathologic examination of the liver and spleen was performed after routine fixation, decalcification, and paraffin embedding. Tissue sections were stained with hematoxylin-eosin, and the results were evaluated by two observers, each of whom read the slides blindly. The specimens were evaluated with regard to abscesses, necrotic lesions, and infiltrating inflammatory cells. Apoptotic cells were detected by the TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling) technique (7) using a kit (Boehringer Mannheim, Quebec, Canada) and the assay was conducted as described in the manufacturer's protocol. The sections were stained with terminal deoxynucleotidyl transferase and counterstained with methyl green. DNase-treated sections were used as a positive control. Negative controls were made by omitting the transferase.
Determination of bacterial growth.
For quantitation of bacterial numbers, a limiting-dilution in vitro culture was performed as previously described (52). Briefly, organs including the spleen, liver, lungs, and kidneys were aseptically removed and homogenized. Serial dilutions were plated on Yersinia selective agar plates. CFU were counted after incubation of the plates for 40 h at 24°C.
Measurement of in vitro intracellular killing of bacteria by PEC.
Peritoneal exudate cells (PEC) were obtained by intraperitoneal injection with thioglycolate broth and were harvested 3 days later. They were washed and resuspended in balanced salt solution and were incubated for 15 min at 37°C, with rotary stirring, with live Y. enterocolitica in balanced salt solution containing 5% mouse serum. Then the samples were washed three times, 100 μg of gentamicin per ml was added, and the samples were kept on ice for 75 min to remove extracellular bacteria. Subsequently, the samples were incubated for 90 min and the cells were lysed with 1% Triton X-100 for 5 min to release intracellular bacteria. Serial dilutions were made with 0.1 ml plated on an LB agar plate. CFU were counted after incubation of the plate for 40 h at 24°C.
Measurement of nitrite in supernatants from PEC and in sera.
For measurement of nitric oxide production, PEC were prepared as above and cultured at 106 cells/ml in 96-well tissue culture plates containing MEM medium (10% fetal calf serum, 2 mM l-glutamine, 50 U of penicillin per ml, and 50 U of streptomycin per ml). The cells were stimulated with 100 U of murine recombinant IFN-γ (rIFN-γ) (Genzyme, Cambridge, Mass.) per ml and 1,000 pg of rTNF-α (Genzyme) per ml. The supernatants were collected at 72 h to assess nitric oxide production. Nitrite levels in the supernatants and in sera were measured by the Griess reaction (25), using NaNO2 (Sigma, St. Louis, Mo.) as the standard with a detection limit of 1.66 μm.
Detection of oxidative burst activity in phagocytes in heparinized whole blood.
The oxidative burst activity of monocytes and granulocytes was quantified with a test kit (Bursttest; Orpegen Pharma, Heidelberg, Germany). In brief, 100 μl of freshly heparinized whole blood was incubated with the optimized Y. enterocolitica for 10 min at 37°C in a water bath. The samples were then mixed with 20 μl of substrate solution (fluorogenic dihydrorhodamine 123) and incubated for another 10 min at 37°C. The samples were lysed and washed with washing solution. Subsequently, 200 μl of DNA staining solution was added, and the samples were incubated for 10 min at 0°C. The cells were analyzed by flow cytometry using CellQuest software in a FACScan apparatus (Becton Dickinson, Mountain View, Calif.). Dihydrorhodamine 123 is oxidized to rhodamine 123 in the presence of reactive oxidatant intermediates such as H2O2 or O2. Upon excitation at 488 nm, rhodamine 123 emits a fluorescent signal that can be detected at 525 nm. The relevant granulocyte or monocyte/macrophage clusters were gated in the software program in the scatter diagram, and its green fluorescence histogram (FL1) was analyzed. The mean fluorescence was correlated with oxidation quantity per individual phagocyte.
Analysis of cell surface markers of splenocytes by flow cytometry.
Splenocytes from uninfected and infected mice were prepared as previously described (53). Briefly, mouse spleens were passed through a nylon mesh and erythrocytes were depleted by hypotonic lysis. The resulting single cells were suspended in 1% bovine serum albumin–PBS and were subsequently analyzed for expression of cell markers by flow cytometry. The cells were incubated with 10 μg of murine immunoglobulin G per ml for 15 min on ice for Fc receptor blocking. Subsequently, the cells were stained with various antibodies for 30 min at 4°C. For single-color analysis, the cells were stained with fluorescein isothiocyanate-conjugated antibodies specific for mouse Mac-1, Fas, or intercellular cell adhesion molecule 1 (ICAM-1) or with phycoerythrin-conjugated anti-B220 (Sigma). For two-color analysis, cells were stained with a combination of phycoerythrin-conjugated anti-CD8 (Sigma) and fluorescein-conjugated anti-CD4 (Sigma) or phycoerythrin-conjugated anti-NK1.1 (PharMingen, San Diego, Calif.) and fluorescein isothiocyanate-conjugated anti-CD3 (PharMingen). Fluorescein isothiocyanate- or phycoerythrin-conjugated rat immunoglobulin G2a served as isotype-matched negative controls (Sigma). At the end of the incubation, the cells were washed and were resuspended in 1% bovine serum albumin–PBS for analysis in a FACScan cytometer with CellQuest TM software (Becton Dickinson). The stained cells were gated using forward and side scatter encompassing lymphocytes or leukocytes.
Cell culture and cytokine assessment.
Splenocytes were prepared as above and suspended in MEM complete medium, and 106 live cells/ml were incubated with or without 2 μg of concanavalin A (ConA) (Sigma) per ml. The cultures were maintained in 24-well plates at 37°C in 5% CO2 under 95% humidity. The supernatants were collected after 24 h for the detection of TNF and IL-4 or after 72 h for the detection of IFN-γ and IL-10. These time points represent optimal responses as assessed in a pilot study (data not shown). The cytokine levels in culture supernatants and in sera were determined by an enzyme-linked immunosorbent assay (ELISA) or bioassay. IFN-γ and IL-10 levels were quantified using an ELISA system (PharMingen) as described previously (54). TNF levels were measured by a bioassay using clone 13 of the WEHI 164 cell line as the target cells (19) (kindly provided by T. Mosmann, University of Alberta) as described previously (52). IL-4 levels were determined by measuring the proliferation of the CT-4S cell line (28) blocked with anti-IL-2 supernatants as described previously (14). Recombinant murine IL-4, IL-10, IFN-γ, and TNF-α were purchased from Genzyme Corp.
Statistical analysis.
Differences between mean values were analyzed by the Mann-Whitney U test and the Student t test. Data are presented as mean and standard deviation obtained from five mice per group.
RESULTS
Increased resistance to Y. enterocolitica infection at early stage in TNFRp55−/− mice is accompanied by decreased apoptosis in the spleen.
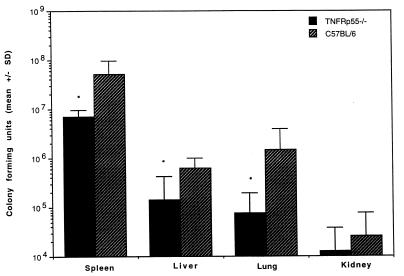
To evaluate the impact of TNFRp55 on the early response to Yersinia infection, TNFRp55−/− and control C57BL/6 mice were inoculated intravenously with 350 CFU of Y. enterocolitica and sacrificed on day 5 after infection. At this time point, no animals had died of the infection and no clinical evidence of arthritis was observed in either groups. Unexpectedly, TNFRp55−/− mice displayed more efficient bacterial clearance from the spleen, liver, lungs, and kidneys (Fig. 1). CFU counts were fourfold lower in the spleen and sevenfold lower in the liver in TNFRp55−/− mice compared with C57BL/6 mice. In parallel with the less efficient bacterial clearance in spleens, C57BL/6 mice developed obvious splenomegaly and abscess formation, while TNFRp55−/− mice had lower spleen weights and fewer abscesses.
FIG. 1.
Bacterial growth in different organs from TNFRp55−/− and C57BL/6 mice 5 days after infection with 350 CFU of Y. enterocolitica. Each bar represents the mean and standard deviation (SD) for five mice. ∗, P < 0.05.
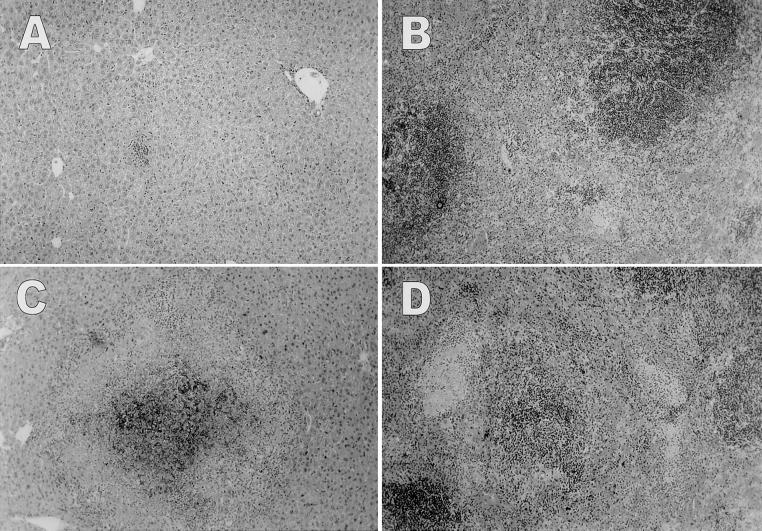
We examined the histologic pathology of liver and spleen tissues in mice infected with Y. enterocolitica, since these are the organs affected most extensively by Yersinia infection (4). The livers of TNFRp55−/− mice displayed mild sinusoidal and periportal inflammation, with small foci of inflammatory infiltrates consisting mainly of mononuclear cells (Fig. 2A). Similar low-grade inflammatory changes was seen in the spleens (Fig. 2B). Only rare microabscesses were observed in the sinuses of the spleens and only in a minority of the animals. In marked contrast to TNFRp55−/− mice, C57BL/6 mice displayed extensive pyogenic lesions including numerous macro- or microabscesses in both the liver (Fig. 2C) and the spleen (Fig. 2D). Large areas of confluent inflammatory infiltrates consisted of a mixed population of granulocytes and mononuclear cells in necrotic foci of liver and spleen parenchyma. In addition, marked sinusoidal congestion, increased extramedullary hematopoiesis, atrophic white pulp, and collections of phagocytes were detected in the spleens of C57BL/6 mice.
FIG. 2.
Representative histopathology of livers and spleens from TNFRp55−/− and C57BL/6 mice. TNFRp55−/− mice displayed mild sinusoidal and periportal inflammation in the liver (A) and small foci of inflammation in the sinuses of the spleen (B). In contrast, C57BL/6 mice showed extensive pyogenic lesions in the liver (C) and marked sinusoidal congestion, necrotic lesions, and atrophic white pulp in the spleen (D). Sections were excised from the mice on day 5 after inoculation with 350 CFU of Y. enterocolitica per mouse and stained with hematoxylin and eosin (five mice per group). Magnification, ×25.
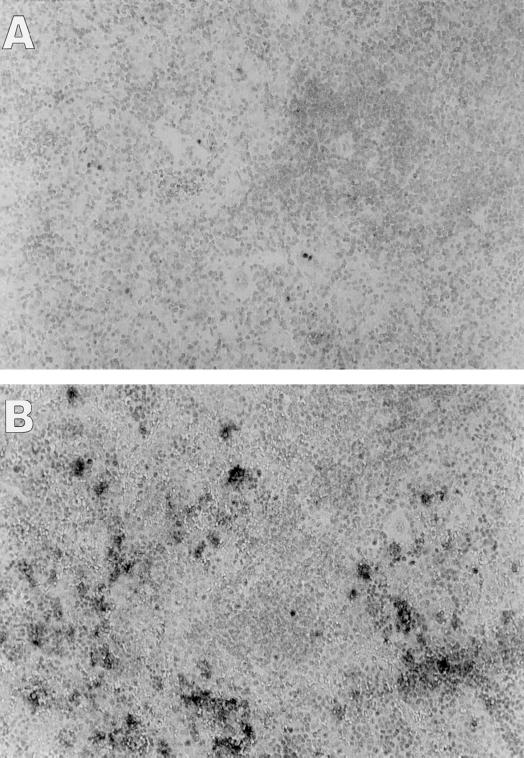
To analyze infection-induced cell death, the sections of livers and spleens in TNFRp55−/− and C57BL/6 mice were stained in the TUNEL assay. No significant difference with respect to apoptosis in hepatocytes could be detected outside of the abscesses between the groups (data not shown). However, TUNEL staining revealed a marked increase in cell death in the spleens of C57BL/6 mice (Fig. 3B) compared with TNFRp55−/− mice (Fig. 3A). TUNEL-positive cells in C57BL/6 mice clearly displayed the morphological hallmarks of programmed cell death characterized by dark, condensed chromatin juxtaposed against the nuclear membrane, nuclear fragmentation, and cell shrinkage. Furthermore, there was a notable absence of inflammatory reaction around dead cells and the TUNEL-positive cells were seen at sites remote from abscess foci. In contrast to these changes, the morphology of most of the spleen cells in TNFRp55−/− mice was intact and only rare scattered cells were stained in the TUNEL assay.
FIG. 3.
Apoptosis in spleens from TNFRp55−/− (A) and C57BL/6 (B) mice 5 days after infection with 350 CFU of Y. enterocolitica. Apoptotic cells were identified using the TUNEL assay and from morphologic features such as nuclear fragmentation and condensation of chromatin. Methyl green was used as the counterstain. Magnification, ×25.
Phagocyte microbicidal activity does not differ at early stage of the infection between TNFRp55−/− and C57BL/6 mice.
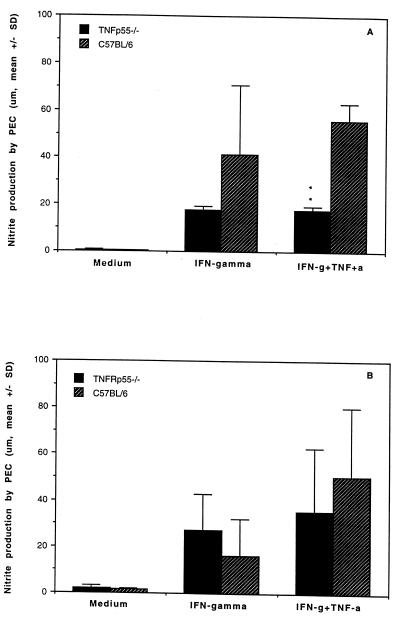
To determine whether the early defense against the bacteria in TNFRp55−/− was correlated with the microbicidal capacity of macrophages, macrophages obtained from infected mice on day 5 after infection were evaluated using PEC elicited by thioglycolate broth. The intracellular killing capacity was assessed by coculture of PEC and live bacteria for 90 min. TNFRp55−/− and C57BL/6 mice displayed an equal intracellular killing capacity (71.0% ± 7.8% and 73.3% ± 11.3%, respectively), indicating that TNFRp55−/− mice had no altered macrophage killing of Yersinia by PEC at this early stage of the infection. Nitric oxide production was evaluated in sera as well as in culture supernatants from PEC. As shown in Fig. 4, although decreased nitric oxide generation by PEC stimulated with rIFN-γ and rTNF-α was found in uninfected TNFRp55−/− mice, no statistical difference in NO production between the groups was observed on day 5 after infection. Also, there was no difference with respect to serum NO production between the groups at this time point (TNFRp55−/− mice, 20.0 ± 8.1 μM; C57BL/6 mice, 21.0 ± 5.0 μM).
FIG. 4.
Nitric oxide production by PEC stimulated with IFN-γ or IFN-γ plus TNF-α in TNFRp55−/− and C57BL/6 mice. (A) Nitric oxide production in uninfected mice. (B) Nitric oxide production in mice on day 5 after infection. The data were obtained from five mice per group. ∗∗, P < 0.01. SD, standard deviation.
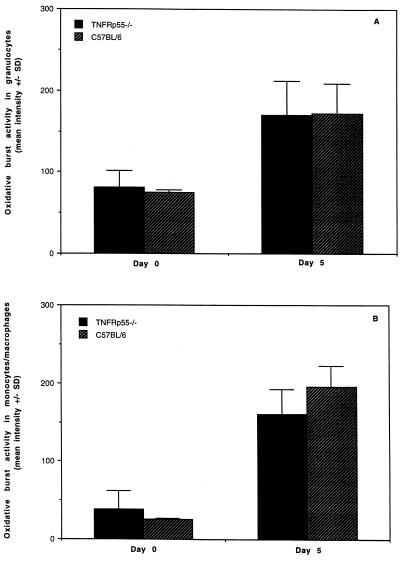
The oxidative-burst activity of phagocytes was detected in whole blood taken from uninfected mice or from infected mice 5 days after infection. Cells were stimulated with opsonized Yersinia, and the formation of reactive oxidants during the oxidative burst was determined by flow cytometry. Degranulation of granulocytes and monocytes/macrophages, indicated by a reduction of side-scatter signals, occurred similarly in uninfected and infected TNFRp55−/− and C57BL/6 mice after stimulation with the bacteria. The percentages of cells in the granulocyte gate significantly increased in infected mice versus uninfected mice (TNFRp55−/− mice, 8.78% ± 2.06% versus 40.34% ± 15.46%; C57BL/6 mice, 9.74% ± 5.26% versus 41.83% ± 11.73%). Significantly higher fluorescence intensities in monocytes/macrophages and granulocytes demonstrated that oxidative-burst formation was increased in both the infected TNFRp55−/− and C57BL/6 mice (Fig. 5). However, no significant differences between the groups could be detected. Collectively, these data indicate that TNF signaling via p55 did not alter macrophage microbicidal activity in terms of NO production or oxidative-burst activity at early stages of Y. enterocolitica infection.
FIG. 5.
Oxidative-burst activity of phagocytes in heparinized whole blood from noninfected or infected TNFRp55−/− and C57BL/6 mice. (A) Oxidative-burst activity in granulocytes. (B) Oxidative burst activity in monocytes/macrophages. The data were obtained from five mice per group. SD, standard deviation.
The early resistance in TNFRp55−/− mice is associated with increased IFN-γ and decreased IL-10 production by splenocytes.
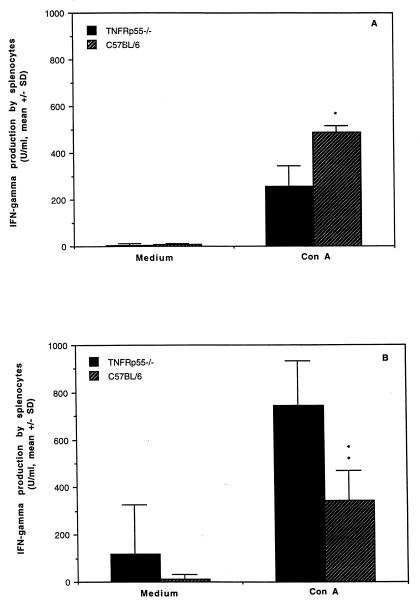
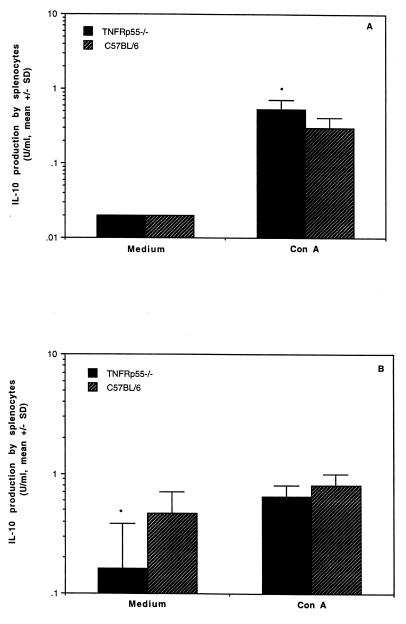
Cytokine levels were measured in sera and in culture supernatants of splenocytes from uninfected mice or day 5 after infection. Comparable levels of TNF were detected in the sera and the culture supernatants of splenocytes stimulated with ConA in both groups (data not shown). IFN-γ production by splenocytes was lower in response to ConA in uninfected TNFRp55−/− mice (258 ± 84 U/ml) than in the controls (490 ± 26 U/ml) (Fig. 6A). Strikingly, almost threefold-increased levels of IFN-γ in response to ConA were detected in TNFRp55−/− mice (743 ± 191 U/ml) (Fig. 6B) following Yersinia infection, while C57BL/6 mice (344 ± 123 U/ml) displayed decreased IFN-γ production upon stimulation with ConA compared with that before infection. Elevated IFN-γ levels in TNFRp55−/− mice were also noticed in the supernatants obtained from unstimulated cultures of splenocytes (Fig. 6B) and in the sera (40 ± 22 versus 21 ± 7) compared with C57BL/6 mice, although no statistical difference was observed. In contrast to IFN-γ production, IL-10 levels in culture supernatants of splenocytes stimulated with ConA were higher in uninfected TNFRp55−/− mice than in the controls (Fig. 7A). However, significantly decreased IL-10 levels in TNFRp55−/− mice were observed in unstimulated splenocytes compared with the controls on day 5 after infection (Fig. 7B). Low levels of IL-4 were detected, but no difference could be detected in infected mice between the groups (data not shown).
FIG. 6.
IFN-γ production in culture supernatants of splenocytes in TNFRp55−/− and C57BL/6 mice. (A) IFN-γ production in uninfected mice. (B) IFN-γ production in mice on day 5 after infection. The data represent the mean and standard deviation for five mice per group. ∗, P < 0.05; ∗∗, P < 0.01.
FIG. 7.
IL-10 production in culture supernatants of splenocytes in TNFRp55−/− and C57BL/6 mice. (A) IL-10 production in uninfected mice. (B) IL-10 production in mice on day 5 after infection. The data represent the mean and standard deviation for five mice per group. ∗, P < 0.05.
The frequencies of spleen CD4+ T cells in TNFRp55−/− mice are less decreased at early stages during the infection.
To further evaluate cell death in the splenocyte population, the viability of splenic mononuclear cells was assessed by trypan blue staining and the proportion of lymphocyte subsets was assessed by flow cytometry analysis. In C57BL/6 mice, 25% of the splenocytes died compared with only 10% of the splenocytes in TNFRp55−/− mice. As demonstrated in Table 1, phenotypic expression of CD4+, CD8+, NK, and B220+ cells was similar in lymphocytes from the uninfected TNFRp55−/− and C57BL/6 mice. Following infection with Y. enterocolitica, no statistical differences with respect to CD8+, NK, and B220+ cells were found between the groups. However, markedly decreased frequencies of CD4+ T cells were detected in C57BL/6 mice, while TNFRp55−/− mice displayed only a slightly decreased percentage of CD4+ T cells. The total numbers of splenic CD4+ T cells in infected TNFRp55−/− mice reflected a 4.5-fold increase (13.1 × 106 ± 2.1 × 106) compared with baseline values (2.9 × 106 ± 1.0 × 106). The total number of CD4+ T cells (10.2 × 106 ± 1.7 × 106) in C57BL/6 mice was smaller than in TNFRp55−/− mice on day 5 after infection and only 2.5-fold increased compared with that before infection (3.9 × 106 ± 1.7 × 106). The percentage of Mac-1+ cells was lower in both uninfected and infected TNFRp55−/− mice than in controls (Table 1). In addition, no statistical differences were observed with respect to the expression of Fas, NK1.1 T cells, and CD62L between the groups (data not shown).
TABLE 1.
Spleen lymphocyte populations in TNFRp55−/− and C57BL/6 mice
| Cell type | % of cells expressing the markera
|
|||
|---|---|---|---|---|
| Uninfected
|
Infectedb
|
|||
| TNFRp55−/− | C57BL/6 | TNFRp55−/− | C57BL/6 | |
| CD4+ | 17.64 ± 0.81 | 19.77 ± 3.02 | 15.34 ± 6.59 | 7.62 ± 2.31c |
| CD8+ | 6.81 ± 0.72 | 8.05 ± 1.23 | 9.74 ± 2.08 | 6.91 ± 2.29 |
| NK | 3.94 ± 0.45 | 3.69 ± 0.41 | 7.42 ± 0.49 | 6.08 ± 2.67 |
| B220+ | 71.62 ± 1.73 | 68.85 ± 3.04 | 64.07 ± 9.36 | 75.12 ± 6.14 |
| Mac-1+ | 9.6 ± 0.6 | 13.6 ± 0.8c | 7.0 ± 1.1 | 17.0 ± 2.2d |
The mean percentage of positive cells ± SD was obtained for five mice per group.
Five days after infection with 350 CFU of Y. enterocolitica.
P < 0.05 versus infected TNFRp55−/− mice.
P < 0.01 versus infected TNFRp55−/− mice.
DISCUSSION
The role of TNFRp55 in the early host response to Yersinia infection was investigated by the use of TNFRp55−/− mice. TNFRp55−/− mice proved to be relatively more resistant to Yersinia infection at the early stages, as manifested by more efficient bacterial clearance and reduced tissue damage compared with the controls. It appears that TNF signaling via TNFRp55 was deleterious rather than beneficial in the early host response to Yersinia infection. In studying the cellular sources accounting for the increased resistance, we found no difference with respect to macrophage microbicidal activity between the groups, indicating that the absence of TNFRp55 signaling did not influence the macrophage function in the early response to Yersinia and that macrophage microbicidal activity is not a determining factor in the early defense against Yersinia. Likewise, the comparable increase in numbers of circulating granulocytes in the groups indicated that this was not a differential variable. In addition, the frequencies of NK cells, a cell type that has been proposed to be critical in early Yersinia infection (8), did not show any difference between the groups. In contrast, the significant and dramatic decrease in the percentages of splenic CD4+ T cells in C57BL/6 mice was not observed in TNFRp55−/− mice. C57BL/6 mice demonstrated extensive cell death in the spleen, to a far greater degree than was observed in TNFRp55−/− mice. Concomitant with these cellular events, cytokine analyses demonstrated increased IFN-γ and decreased IL-10 production in TNFRp55−/− mice compared with C57BL/6 mice. Thus, in the acute phase of infection, TNFRp55-mediated signaling events precipitate a relative decline in splenic CD4+ T cells, with important consequences for the host response to the pathogen.
Previous studies have noted an association between the virulence of Yersinia pestis and suppression of host TNF production (35, 36). More recently, Schmidt et al. (46) have observed that the 39-kDa V antigen of Yersinia spp. suppresses TNF production through a pathway involving activated CD4+ and CD8+ T cells. These studies have largely utilized in vitro systems and have not addressed this issue following in vivo challenge of an animal with Y. enterocolitica. Whatever countermeasures Yersinia may possess (such as Vag) against host defensive cytokines, it is evident in our in vivo system that they are inadequate in suppressing a host TNF response in the acute phase of the infection, of which the sequela is splenocyte apoptosis. TNF can induce cytotoxicity either by necrosis or by apoptosis (22). It is known that TNFRp55-mediated apoptosis is mediated through a death domain, homologous to the Fas death domain (30, 47, 49). Yersinia-triggered apoptosis has been demonstrated in examining the interaction of Y. enterocolitica with macrophages or epithelial cells (32, 43). Interestingly, epithelial HeLa cells undergo apoptosis upon Yersinia infection only when TNF-α is present simultaneously, indicating that Yersinia-induced apoptosis is in part mediated through the TNF signaling pathway (43). Recently, Monack et al. have reported that Y. pseudotuberculosis infection in BALB/c mice induces apoptosis of Mac-1+ cells in the mesenteric lymph nodes and spleens (33), but the effect on the T-cell populations was not examined. In the present study, the cellular basis of the extensive apoptosis remains to be defined. It is of interest that a higher degree of apoptosis in spleens in C57BL/6 mice than in TNFRp55−/− mice was associated with a much greater decline in the total numbers and percentages of CD4+ T cells.
It has been recognized that death of activated T cells can be mediated by interaction between TNF and TNF receptors (15, 44, 50). Activation-induced CD4+-T-cell death by apoptosis is a prominent feature of a number of infectious diseases, such as human immunodeficiency virus infection (29), experimental Chagas' disease (31), and Schistosoma mansoni infection (21). In these studies, selective triggering of CD4+-T-cell death resulted in a global immunosuppression in infected hosts. In the present study, the proportion of splenic CD4+ T cells markedly decreased in C57BL/6 mice, which correlated with dramatically increased numbers of apoptotic cells, as demonstrated by the TUNEL assay. This suggests that Yersinia infection might trigger CD4+ T cells to undergo programmed death from the TNFRp55 signaling. In contrast to C57BL/6 mice, TNFRp55−/− mice displayed less apoptosis in their spleens, associated with more efficient bacterial clearance and reduced tissue damage, indicating that the induction of apoptosis may figure critically in early resistance to Yersinia. In addition to apoptosis, large necrotic lesions and scattered necrotic cells in the spleens of C57BL/6 mice might also be TNFRp55 dependent, since TNF has been suggested to be needed for lysis of microbe-infected cells (39). It should noted that not only the numbers but also the activities might also be altered in CD4+ T cells in the absence of TNFRp55, since TNF can suppress the T-cell response via modulation of T-cell receptor signaling (16). In accordance with this finding, several studies have shown that chronic TNF-α administration reduces both the number and function of splenic T cells and thus induces a preferential inhibition of cell-mediated immunity (24, 40).
CD4+ T cells are believed to mediate acquired immunity in bacterial infection, generally by means of cytokine production. The extent to which CD4+ T cells contribute to the early stage of Yersinia infection has not been defined. CD4+ T cells play a critical role in resistance to Yersinia by producing Th1 cytokines such as IFN-γ. It has been shown that NK cells are the major source of IFN-γ production on day 3 (2, 4). Thereafter, activated CD4+ T cells produce most of the IFN-γ following infection with Yersinia (2, 4). IFN-γ derived from NK cells can be compensated by CD4+-T-cell production, while IFN-γ derived from CD4+ T cells is essential and irreplaceable in terms of protection against Yersinia (8). In our experiments, the markedly increased total number of CD4+ T cells and the pronounced production of IFN-γ on day 5 after infection are probably related events and might be one of the mechanisms for the early defense against Yersinia in the absence of TNFRp55. Another postulated role for CD4+ T cells in the early stage of Yersinia infection is that they could provide signals for the early activation of B cells, macrophages, and dendritic cells through CD28-B7 and CD40-CD40L interactions. These activated cells might then contribute to the course of the infection by enhanced presentation of antigens, cytokine release, and antibody production. This notion is supported by a previous study demonstrating that administration of a sublethal dose of Y. enterocolitica causes the death of T-cell-deficient nude mice from severe infection within the first few days (4). Moreover, the studies with experimental Leishmania major infection have also suggested that αβ-positive T cells, especially CD4+ T cells, may be crucial in early protection against microbes, since depletion of NK or γδ-positive T cells did not reverse disease outcome in resistant mice (42, 45).
IFN-γ, in synergy with TNF-α, is known to be a key mediator in resistance to Yersinia infection. Early and enhanced IFN-γ mRNA is associated with a state of heightened resistance to Yersinia infection (9). Administration of recombinant IFN-γ rendered susceptible BALB/c mice resistant to infection (8). Moreover, anti-IFN-γ treatment abrogated the resistance to the infection (3). IFN-γ is believed to be one of the essential stimulators of macrophage microbicidal activity and therefore is crucial for resistance to Yersinia infection (3, 9). In the present study, decreased IFN-γ production by splenocytes in response to ConA was found in uninfected TNFRp55−/− mice, indicating that TNF is required for optimal IFN-γ production, as demonstrated previously (27). The elevated IFN-γ production following infection resulted in part from the increased absolute number of Th1-type CD4+ T cells in these mice. TNFRp55−/− mice might also develop rapid regulatory feedback mechanisms upon administration of the bacteria. For instance, decreased IL-10 levels might contribute to the increased level of IFN-γ, since IL-10 downregulates CD4+ Th1 cytokine production (34). High levels of IFN-γ may be sufficient to effectively activate macrophages in the early events following infection in the absence of TNFRp55. In this regard, we do not exclude the possibility that TNFRp75 compensates for the absence of TNFRp55 in activating macrophages at early stages of the infection.
A hypothesis proposed herein might explain the disparate roles of TNFRp55 signaling during Y. enterocolitica infection. At early stages of the infection, Yersinia-triggered, TNFRp55-mediated apoptosis of CD4+ T cells occurs in normal mice. TNFRp55−/− mice are spared this relative depletion of T cells and are able to mount a protective IFN-γ response acutely. Increased IFN-γ and decreased IL-10 might be the key effectors for this early control of Yersinia in TNFRp55−/− mice. As the infection proceeds, macrophages activated by TNF signaling via TNFRp55 may become critical for clearance of the bacteria. It may be that the enhanced temporary presence of bacteria and death of splenocytes in C57BL/6 mice at early stages is a necessary component of an effective adaptive immune response to Yersinia, resulting in later resistance to Yersinia. Such a hypothesis suggests a dynamic balance between beneficial and deleterious roles for TNFRp55 in bacterial infection and defines TNF as a key player in orchestrating host reactivity during the different stages of the bacterial infection.
ACKNOWLEDGMENTS
This work was supported by the Arthritis Society and the Medical Research Council of Canada. Y.-X. Zhao is a recipient of research fellowship award from the Medical Research Council of Canada.
We thank J. Wither, Department of Immunology, University of Toronto, for kindly providing IFN-γ and IL-10 ELISA reagents.
REFERENCES
- 1.Autenrieth I B, Beer M, Bohn E, Kaufmann S H E, Heeseman J. Immune responses to Yersinia enterocolitica in susceptible BALB/c and resistant C57BL/6 mice: an essential role for gamma interferon. Infect Immun. 1994;62:2590–2599. doi: 10.1128/iai.62.6.2590-2599.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Autenrieth I B, Hantschmann P, Heymer B, Heeseman J. Immunohistological characterization of the cellular immune response against Yersinia enterocolitica in mice: evidence for the involvement of T lymphocytes. Immunobiology. 1993;187:1–16. doi: 10.1016/S0171-2985(11)80241-X. [DOI] [PubMed] [Google Scholar]
- 3.Autenrieth I B, Heeseman J. In vivo neutralization of tumor necrosis factor alpha and interferon-gamma abrogates resistance to Yersinia enterocolitica in mice. Med Microbiol Immunol. 1992;181:333–338. doi: 10.1007/BF00191545. [DOI] [PubMed] [Google Scholar]
- 4.Autenrieth I B, Vogel V, Preger S, Heymer B, Heeseman J. Experimental Yersinia enterocolitica infection in euthymic and T-cell-deficient athymic nude C57BL/6 mice: comparison of time course, histomorphology, and immune response. Infect Immun. 1993;61:2585–2595. doi: 10.1128/iai.61.6.2585-2595.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Autenrieth I B, Tingle A, Reske-Kunz A, Heesemann J. T lymphocytes mediate protection against Yersinia enterocolitica in mice: characterization of murine T-cells clones specific for Y. enterocolitica. Infect Immun. 1992;60:1140–1149. doi: 10.1128/iai.60.3.1140-1149.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bemelmans M H A, van Tits L J H, Buurman W A. Tumor necrosis factor. Crit Rev Immunol. 1996;16:1–11. doi: 10.1615/critrevimmunol.v16.i1.10. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Sasson S, Sherman Y, Gavrieli Y. Identification of dying cells: in situ staining. Methods Cell Biol. 1995;46:29–39. [PubMed] [Google Scholar]
- 8.Bohn E, Autenrieth I B. IL-12 is essential for resistance against Yersinia enterocolitica by triggering IFN-γ production in NK cells and CD4+ T cells. J Immunol. 1996;156:1458–1468. [PubMed] [Google Scholar]
- 9.Bohn E, Heesemann J, Ehlers S, Autenrieth I B. Early gamma interferon mRNA expression is associated with resistance of mice against Yersinia enterocolitica. Infect Immun. 1994;62:3027–3032. doi: 10.1128/iai.62.7.3027-3032.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohn E, Schmitt E, Bielfeldt C, Noll A, Schulte R, Autenrieth I. Ambiguous role of interleukin-12 in Yersinia enterocolitica infection in susceptible and resistant mouse strains. Infect Immun. 1998;66:2213–2220. doi: 10.1128/iai.66.5.2213-2220.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bohn E, Sing A, Zumbihl R, Bieleldt C, Okamura H, Kurimoto M, Heesemann J, Autenrieth I B. IL-18 (IFN-γ-inducing factor) regulates early cytokine production in, and promotes resolution of, bacterial infection in mice. J Immunol. 1998;160:299–307. [PubMed] [Google Scholar]
- 12.Bouza E, Dominguez A, Meseguer M, Buzon L, Boixeda M, Revillo M J, de Rafael L, Martinez Beltran J. Yersinia enterocolitica septicemia. Am J Clin Pathol. 1980;74:404–409. doi: 10.1093/ajcp/74.4.404. [DOI] [PubMed] [Google Scholar]
- 13.Buedack S S, Knieschies A E, Rollinghoff M, Beuscher H U. Tumor necrosis factor-alpha expression induced by anti-YopB antibodies coincides with protection against Yersinia enterocolitica infection in mice. Med Microbiol Immunol. 1997;185:223–229. doi: 10.1007/s004300050034. [DOI] [PubMed] [Google Scholar]
- 14.Chu N R, DeBenedette M A, Niclas Stiernholm B J, Barber B H, Watts T H. Role of IL-12 and 4-1BB ligand in cytokine production by CD28+ and CD28− T cells. J Immunol. 1997;158:3081–3089. [PubMed] [Google Scholar]
- 15.Clement M-V, Stamenkovic I. Fas and tumor necrosis factor receptor-mediated cell death: similarities and distinctions. J Exp Med. 1994;180:557–567. doi: 10.1084/jem.180.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cope A, Liblau R, Yang X, Congia M, Laudanna C, Schreiber R, Probert L, Kollias G, McDevitt H. Chronic tumor necrosis factor alters T cell responses by attenuating T cell receptor signaling. J Exp Med. 1997;185:1573. doi: 10.1084/jem.185.9.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cover T L, Aber R C. Yersinia enterocolitica. N Engl J Med. 1989;321:16–24. doi: 10.1056/NEJM198907063210104. [DOI] [PubMed] [Google Scholar]
- 18.Endres R, Luz A, Schulze H, Neubauer H, Futterer A, Holland S M, Wagner H, Pfeffer K. Listeriosis in p47phox−/− and TRp55−/− mice: protection despite absence of ROI and susceptibility despite presence of RNI. Immunity. 1997;7:419–432. doi: 10.1016/s1074-7613(00)80363-5. [DOI] [PubMed] [Google Scholar]
- 19.Espevik T, Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986;95:99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- 20.Everest P, Roberts M, Gougan G. Susceptibility to Salmonella typhimurium infection and effectiveness of vaccination in mice deficient in the tumor necrosis factor alpha p55 receptor. Infect Immun. 1998;66:3355–3364. doi: 10.1128/iai.66.7.3355-3364.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fallon P, Smith P, Dunne D. Type 1 and type 2 cytokine-producing mouse CD4+ and CD8+ T cell in acute Schistomoma mansoni infection. Eur J Immunol. 1998;28:1408–1416. doi: 10.1002/(SICI)1521-4141(199804)28:04<1408::AID-IMMU1408>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 22.Fiers W, Beyaert R, Boone E, Cornelis S, Declercq W, Decoster E, Denecker G, Depuydt B, De Valck D, De Wilde G, Goossens V, Grooten J, Haegeman G, Heyninck K, Penning L, Plaisance S, Vancompernolle K, Van Criekinge W, Vandenabeele P, Vanden Berghe W, Van de Craen M, Vandevoorde V, Vercammen D. TNF-induced intracellular signalling leading to gene induction or to cytotoxicity by necrosis or by apoptosis. J Inflamm. 1996;47:67–75. [PubMed] [Google Scholar]
- 23.Flynn J L, Goldstein M M, Chan J, Triebold K J, Pfeffer K, Lowenstein C J, Schreiber R, Mak T W, Bloom B R. Tumor necrosis factor-α is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 24.Gordon C, Wofsy D. Effects of recombinant murine tumor necrosis factor-α on immune function. J Immunol. 1990;144:1753–1758. [PubMed] [Google Scholar]
- 25.Green L, Wagner D, Glogowski J, Skipper P, Wishnok J, Tannenbaum S. Analysis of nitrate, nitrite and (15N) nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 26.Gripenberg-Lerche C, Toivanen P. Yersinia associated arthritis in SHR rats: effect of the microbial status of the host. Ann Rheum Dis. 1993;52:223–228. doi: 10.1136/ard.52.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsieh C, Macatonia S, Ogarra A, Murphy K. Pathogen-induced Th1 phenotype development in CD4+ alpha-beta-TCR transgenic T-cells is macrophage dependent. Int Immunol. 1993;5:371. doi: 10.1093/intimm/5.4.371. [DOI] [PubMed] [Google Scholar]
- 28.Hu-Li J, Ohara J, Watyson C, Tsang W, Paul W E. Derivation of a T cell line that is highly responsive to IL-4 and IL-2 (CT.4R) and of an IL-2 hyporesponsive mutant of that line (CT.4S) J Immunol. 1989;142:800–807. [PubMed] [Google Scholar]
- 29.Ledru E, Lecoeur H, Garcia S, Debord T, Gougeon M. Differential susceptibility to activation-induced apoptosis among peripheral Th1 subsets: correlation with Bcl-2 expression and consequences for AIDS pathogenesis. J Immunol. 1998;160:3194–3206. [PubMed] [Google Scholar]
- 30.Leist M, Ganter F, Jilg S, Wendel A. Activation of the p55-kDa TNF receptor is necessary and sufficient for TNF-induced liver failure, hepatocyte apoptosis, and nitrite release. J Immunol. 1995;154:1307. [PubMed] [Google Scholar]
- 31.Lopes M, da Veiga V, Santos A, Fonseca M, DosReis G. Activation-induced CD4+ T cell death by apoptosis in experimental Chagas' disease. J Immunol. 1995;154:744–752. [PubMed] [Google Scholar]
- 32.Mills S, Boland A, Sory M-P, van der Smissen P, Kerbourch C, Finlay B, Cornelis G. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc Natl Acad Sci USA. 1997;94:12638–12643. doi: 10.1073/pnas.94.23.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monack D, Mecsas J, Bouley D, Falkow S. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J Exp Med. 1998;188:2127–2137. doi: 10.1084/jem.188.11.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mosmann T, Moore K. The role of IL-10 in cross-regulation of Th1 and Th2 responses. Immunol Today. 1991;12:A49–A53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- 35.Nakajima R, Brubaker R R. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect Immun. 1993;61:23–31. doi: 10.1128/iai.61.1.23-31.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakajima R, Motin V L, Brubaker R R. Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunization. Infect Immun. 1995;63:3021–3029. doi: 10.1128/iai.63.8.3021-3029.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nashleanas M, Kanaly S, Scott P. Control of Leishmania major infection in mice lacking TNF receptors. J Immunol. 1998;160:5506–5513. [PubMed] [Google Scholar]
- 38.Pfeffer K, Matsuyama Y, Kundig T M, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi P S, Krönke M, Mak T M. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 39.Portnoy D. Innate immunity to a facultative intracellular bacterial pathogen. Curr Opin Immunol. 1992;4:20–24. doi: 10.1016/0952-7915(92)90118-x. [DOI] [PubMed] [Google Scholar]
- 40.Rabinovitch A, Suarez-Pinzon W, Sorensen O, Rajotte R, Power R. TNF-α down-regulates type 1 cytokines and prolongs survival of syngeneic islet grafts in nonobese diabetic mice. J Immunol. 1997;159:6298–6303. [PubMed] [Google Scholar]
- 41.Roggenkamp A, Neuberger H R, Flugel A, Schmoll T, Heesemann J. Substitution of two histidine residues in Yad A protein of Yersinia enterocolitica abrogates collagen binding, cell adherence and mouse virulence. Mol Microbiol. 1995;16:1207–1219. doi: 10.1111/j.1365-2958.1995.tb02343.x. [DOI] [PubMed] [Google Scholar]
- 42.Rosat J, MacDonald H, Louis J. A role for gamma delta+ T cells during experimental infection of mice with Leishmania major. J Immunol. 1993;150:550–555. [PubMed] [Google Scholar]
- 43.Ruckdeschel K, Harb S, Roggenkamp A, Hornef M, Zumbihl R, Kšhler S, Heesemann J, Rouot B. Yersinia enterocolitica impaires activation of transcription factor NF-kB: involvement in the induction of programmed cell death and in the suppression of the macrophage tumor necrosis factor α production. J Exp Med. 1998;187:1069–1079. doi: 10.1084/jem.187.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarin A, Conan-Cibotti M, Henkart P. Cytotoxic effect of TNF and lymphotoxin on T lymphoblasts. J Immunol. 1995;155:3716–3718. [PubMed] [Google Scholar]
- 45.Scharton T, Scott P. Natural killer cells are a source of interferon γ that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993;178:567–577. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt A, Röllinghoff M, Beuscher H U. Suppression of TNF by V antigen of Yersinia spp. involves activated T cells. Eur J Immunol. 1999;29:1149–1157. doi: 10.1002/(sici)1521-4141(199904)29:04<1149::aid-immu1149>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 47.Tartaglia L, Rothe M, Hu Y, Goeddel D. Tumor necrosis factor's cytotoxic activity is signaled by the p55 TNF-α receptor. Cell. 1993;73:213–216. doi: 10.1016/0092-8674(93)90222-c. [DOI] [PubMed] [Google Scholar]
- 48.Vieira L Q, Goldschmidt M, Nashleanas M, Pfeffer K, Mak T, Scott P. Mice lacking the TNF receptor p55 fail to resolve lesions caused by infection with Leishmania major, but control parasite replication. J Immunol. 1996;157:827–835. [PubMed] [Google Scholar]
- 49.Wong G, Goeddel D. Fas antigen and p55 TNF receptor signal apoptosis through distinct pathways. J Immunol. 1994;152:1751. [PubMed] [Google Scholar]
- 50.Wong G, Goeddle D. Fas antigen and p55 TNF receptor signal apoptosis through distinct pathways. J Immunol. 1994;152:1751–1755. [PubMed] [Google Scholar]
- 51.Yap G, Scharton-Kersten T, Charest H, Sher A. Decreased resistance of TNF receptor p55- and p75-deficient mice to chronic toxoplasmosis despite normal activation of inducible nitric oxide synthase in vivo. J Immunol. 1998;160:1340–1345. [PubMed] [Google Scholar]
- 52.Zhao Y-X, Abdelnour A, Holmdahl R, Tarkowski A. Mice with the xid B cell defect are less susceptible to developing Staphylococcus aureus-induced arthritis. J Immunol. 1995;155:2067–2076. [PubMed] [Google Scholar]
- 53.Zhao Y-X, Abdelnour A, Ljungdahl A, Olsson T, Tarkowski A. Patterns of interferon-γ mRNA expression in toxic shock syndrome toxin-1 expanded Vβ11+ T lymphocytes. Cell Immunol. 1995;161:28–33. doi: 10.1006/cimm.1995.1005. [DOI] [PubMed] [Google Scholar]
- 54.Zhao Y-X, Zhang H, Chiu B, Payne U, Inman R D. Tumor necrosis factor receptor p55 controls the severity of arthritis in experimental Yersinia enterocolitica infection. Arthritis Rheum. 1999;42:1662–1672. doi: 10.1002/1529-0131(199908)42:8<1662::AID-ANR15>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]