Fetal growth restriction (FGR) describes a fetus that has not grown to its expected biological potential in utero. FGR can result from maternal, fetal, or placental complications, though it is commonly caused by placental insufficiency. The prolonged hypoxic environment the FGR fetus is exposed to has detrimental effects on the newborn, which extends to adverse long-term neurological outcomes in a significant proportion of FGR infants (Malhotra et al., 2019). Unfortunately, there are currently no therapies to reduce the adverse neurological outcomes in FGR. Neuronal injury is evident in the FGR brain, and therefore understanding which neurons are lost and how they are lost will aid in the selection of treatment options for FGR.
Adverse neurodevelopmental outcomes are likely due to the disruption of key developmental processes in the fetal brain. Many mechanisms could mediate brain injury in FGR; however, inflammation may be a key mechanism associated with brain injury in FGR. A recent human study shows that increased inflammatory markers in FGR neonatal blood are associated with adverse neurological outcomes (Leviton et al., 2013). In a clinically relevant animal model of FGR, neuroinflammation is shown to exacerbate neuronal injury (Wixey et al., 2019a, b). Neuroinflammatory responses are evident through marked increases in activated microglia, reactive astrocytes, as well as increased expression of proinflammatory cytokines (interleukin-1β, tumor necrosis factor-α) and decreased levels of anti-inflammatory cytokines (interleukin-4 and -10) in the FGR piglet brain (Wixey et al., 2019a, b). Chronic inflammation deregulates the neuronal microenvironment to an injury-permissive state. Neuroinflammatory cytokines have been implicated in the dysregulation of neurotransmitter metabolism including the synthesis, release, and reuptake of serotonin (5-hydroxytrypamine [5-HT]).
Critical for fetal brain development, 5-HT is one of the most abundant and crucial neurotransmitter systems in the early mammalian brain. It is the first neurotransmitter to appear in the brain and is fundamental in both prenatal and postnatal brain development (Whitaker-Azmitia, 2020). Serotonergic neurons are localized in the dorsal or median raphé nuclei in the brainstem and project widely to innervate virtually all regions of the neonatal brain, coordinating mood, cognition, reward, learning, memory, and numerous physiological processes such as body temperature and breathing. Prenatal disruption to 5-HT can lead to an alteration of fetal brain development and an increased risk of psychiatric disorders during childhood and adulthood. Disruption to 5-HT signaling has been associated with several neurological disorders such as depression, anxiety, psychosis, and autism spectrum disorder (Ye et al., 2021); all evident in a significant proportion of FGR infants. 5-HT shapes neuronal microcircuitry, neuronal migration, and positioning during development as well playing an important role in cell survival, growth and differentiation and synaptogenesis (Daubert and Condron, 2010). In the developing brain, 5-HT levels are significantly higher to aid in the developmental role. Any disruptions in this process can have serious long-term consequences on the mature brain. Therefore, understanding if and how the serotoninergic system may be dysregulated within the FGR brain may hasten the selection of therapies for FGR.
We know neurons are injured in the FGR brain, however, whether 5-HT neurons are the predominant phenotype remains to be determined (Figure 1). Animal models of FGR are important to examine the evolution of brain injury and delve into neuronal phenotypes susceptible to this injury. Multiple animal species are used to model FGR brain injury including the rat, guinea pig, sheep, and pig; however, there are pros and cons for each. In human, FGR arises from multiple aetiologies and therefore it is difficult for animal models to precisely mimic this complex situation. Further, brain maturation varies for different animal species, for instance in the rat, brain growth spurt occurs postnatally and prenatally in the sheep. Whilst the piglet has a brain growth trajectory similar to the human around the time of birth (Chand et al., 2022). FGR occurs spontaneously in the piglet due to placental insufficiency unlike other FGR animal models requiring physical or nutritional interventions. FGR in the piglet is identified by lower birth weight (< 10th centile for cohort), however, can also include brain to body weight ratio and brain to liver weight ratio due to cardiac blood redistribution from peripheral organs towards vital organs such as the brain; termed asymmetric growth restriction. Asymmetric growth restriction occurs in 70–80% of human FGR cases. The piglet has a gyrencephalic brain with a gray to white matter ratio similar to the human newborn. The FGR piglet displays neuronal and white matter injury like the FGR human brain (Chand et al., 2022) demonstrating the piglet is a highly appropriate species to explore the effects of FGR on brain outcomes.
Figure 1.
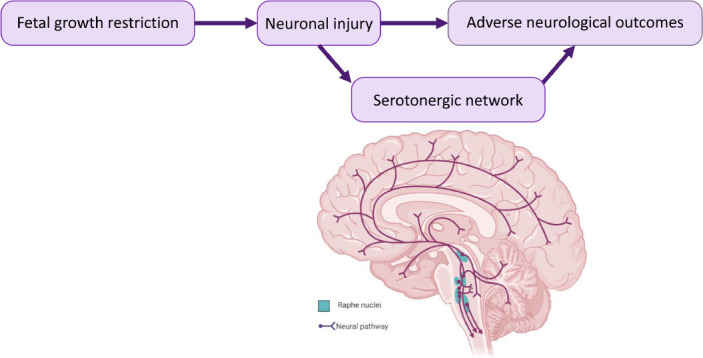
Potential for serotonin pathways to be involved in abnormal brain outcomes in fetal growth restriction.
Neuronal injury is evident in the fetal growth restriction (FGR) brain and has been associated with adverse neurological outcomes. Disruption to serotonin could be linked with these FGR-induced adverse neurological outcomes. Deep purple lines = serotonin projections; teal = Raphe nuclei.
As previously mentioned, abnormal levels of brain 5-HT are linked to neurodevelopment disorders in other neurological disorders, though whether these potential abnormalities arise antenatally or postnatally in FGR remain to be determined. Although no studies to date have examined levels of 5-HT in the FGR human newborn brain, evidence from limited FGR piglet and rat studies suggest there may be alterations to levels of 5-HT during fetal and postnatal development and thus, behavioral abnormalities may be related to these alterations. A study in FGR piglets showed higher concentrations of a serotonin metabolite in the hippocampus of male FGR term piglets (Vazquez-Gomez et al., 2016) with multiple FGR rat studies corroborating this finding reporting increased 5-HT activity levels in the FGR fetal and postnatal brain. An FGR rat study demonstrated an increase in 5-HT levels with a resultant reduction in the 5-HT transporter (SERT) immunoreactivity at postnatal day 3 (P3) and P7 (Medina-Aguirre et al, 2008). The authors state that increased availability of 5-HT in FGR may result from a dual mechanism; increased 5-HT synthesis and reduced uptake (Medina-Aguirre et al., 2008). In agreement with this reduction in serotonergic fiber immunoreactivity, a recent FGR rat study showed fewer 5-HT-positive neurons in the brainstem of FGR rat pups than controls during the nursing period (Hernández-Rodríguez et al., 2021). The authors also observed morphological differences in 5-HT neurons between controls and FGR group, demonstrating small neurons with little cytoplasm and smaller nuclei.
From the limited FGR piglet and rat studies, an increase in 5-HT brain levels with a concurrent reduction in 5-HT fiber networks and neurons is evident. However, most of the studies examine limited components of the 5-HT system. A comprehensive analysis of the entire 5-HT system, at multiple time points in multiple brain regions, in a large animal model of FGR would assist in elucidating the impact of FGR on this potentially vulnerable neuronal phenotype and will determine whether there is a functional disruption to this system. It has also been proposed placental insufficiency leads to altered placental 5-HT synthesis, yet it remains unclear the pathophysiology of placental 5-HT in FGR (Ranzil et al., 2019). Studies are evolving in this area but are currently inconclusive therefore, further investigation is required into whether disruption to 5-HT (placental or fetal/neonatal) could be linked to FGR-induced adverse neurological outcomes.
In conclusion, 5-HT is abundant in the developing brain and regulates a plethora of brain functions throughout life. Although the potential role 5-HT plays in FGR has not been thoroughly examined. Animal models of neonatal brain injury show inflammation is associated with 5-HT neuronal injury. Future work into the potential disruption to the 5-HT neuronal network and its association with inflammation in FGR would benefit this area of research.
As the developing brain exhibits plasticity and the potential for regeneration following injury, we should focus on understanding which neuronal populations are vulnerable to FGR conditions. This will allow for targeted treatments to reduce or even prevent adverse long-term neurological outcomes in FGR neonates. With inflammation being associated with poorer neurological outcomes in human FGR neonates, the consequences it has on the monoamine system needs to be explored. Understanding the role of 5-HT in development has made significant contributions to the study of developmental disabilities like hypoxia-ischemia, fetal alcohol syndrome and autism yet very little is available in the field of FGR. Given that 5-HT receptor expression changes dynamically across development, this system allows for the targeting of many different developmental time points, further expanding its potential value as a therapeutic target. With no current therapeutic intervention available for FGR neonates, it is now time to explore the ubiquitous nature of 5-HT within the developing FGR brain.
This work was supported by The University of Queensland Stimulus Fellowship (to JAW), and Queensland Children’s Hospital Foundation Grant (No. WIS0012021, to support KB).
Footnotes
Open peer reviewer: Thomas R. Wood, University of Washington, USA.
P-Reviewer: Wood TR; C-Editors: Zhao M, Zhao LJ, Wang Lu; T-Editor: Jia Y
References
- 1.Chand KK, Pannek K, Colditz PB, Wixey JA. Brain outcomes in runted piglets:a translational model of fetal growth restriction. Dev Neurosci. 2022 doi: 10.1159/000523995. doi:10.1159/000523995. [DOI] [PubMed] [Google Scholar]
- 2.Daubert EA, Condron BG. Serotonin:a regulator of neuronal morphology and circuitry. Trends Neurosci. 2010;33:424–434. doi: 10.1016/j.tins.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernández-Rodríguez J, Chagoya-Guzmán G, Mondragón-Herrera JA, Oca AB, Mercado-Camargo R, Manjarrez-Gutiérrez G. How intrauterine growth restriction due to nutritional stress changes the function of key proteins in brain serotonin metabolism during development. Bol Med Hosp Infant Mex. 2021;78:571–583. doi: 10.24875/BMHIM.20000334. [DOI] [PubMed] [Google Scholar]
- 4.Leviton A, Fichorova RN, O'Shea TM, Kuban K, Paneth N, Dammann O, Allred EN Investigators ES. Two-hit model of brain damage in the very preterm newborn:small for gestational age and postnatal systemic inflammation. Pediatr Res. 2013;73:362–370. doi: 10.1038/pr.2012.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malhotra A, Allison BJ, Castillo-Melendez M, Jenkin G, Polglase GR, Miller SL. Neonatal morbidities of fetal growth restriction:pathophysiology and impact. Front Endocrinol (Lausanne) 2019;10:55. doi: 10.3389/fendo.2019.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medina-Aguirre I, Gutiérrez-Ospina G, Hernández-Rodríguez J, Boyzo A, Manjarrez-Gutiérrez G. Development of 5-HT1B, SERT and thalamo-cortical afferents in early nutrionally restricted rats:An emerging explanation for delayed barrel formation. Int J Dev Neurosci. 2008;26:225–231. doi: 10.1016/j.ijdevneu.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Ranzil S, Walker DW, Borg AJ, Wallace EM, Ebeling PR, Murthi P. The relationship between the placental serotonin pathway and fetal growth restriction. Biochimie. 2019;161:80–87. doi: 10.1016/j.biochi.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Vazquez-Gomez M, Valent D, Garcia-Contreras C, Arroyo L, Ovilo C, Isabel B, Bassols A, Gonzalez-Bulnes A. Sex and intrauterine growth restriction modify brain neurotransmitters profile of newborn piglets. Int J Dev Neurosci. 2016;55:9–14. doi: 10.1016/j.ijdevneu.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Whitaker-Azmitia PM. Serotonin and development. In: Müller CP, Cunningham KA, editors. Handbook of behavioral neuroscience. Elsevier; 2020. pp. 413–435. [Google Scholar]
- 10.Wixey JA, Lee KM, Miller SM, Goasdoue K, Colditz PB, Bjorkman ST, Chand KK. Neuropathology in intrauterine growth restricted newborn piglets is associated with glial activation and proinflammatory status in the brain. J Neuroinflammation. 2019a;16:5. doi: 10.1186/s12974-018-1392-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wixey JA, Sukumar KR, Pretorius R, Lee KM, Colditz PB, Bjorkman ST, Chand KK. Ibuprofen treatment reduces the neuroinflammatory response and associated neuronal and white matter impairment in the growth restricted newborn. Front Physiol. 2019b;10:541. doi: 10.3389/fphys.2019.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye X, Shin BC, Baldauf C, Ganguly A, Ghosh S, Devaskar SU. Developing brain glucose transporters, serotonin, serotonin transporter, and oxytocin receptor expression in response to early-life hypocaloric and hypercaloric dietary, and air pollutant exposures. Dev Neurosci. 2021;43:27–42. doi: 10.1159/000514709. [DOI] [PMC free article] [PubMed] [Google Scholar]


