Human immunodeficiency virus-1 (HIV) reservoirs in the human brain: Antiretroviral therapy (ART) effectively decreases active HIV replication to undetectable levels. Therefore, it greatly improves the quality of life for people living with HIV (PLWH). However, except for a few exceptional cases after stem-cell transplantation from CCR5Δ32 mutation donors, such as in Berlin and London patients, there is no cure for HIV, due to the latent HIV reservoirs harbored in the long-lived HIV permissive cells. HIV quickly rebounds upon the disruption of ART, causing the life-long burden of ART for PLWH in order to control viral replication. Similar to peripheral blood, HIV establishes reservoirs in the brain very early in infection (Putatunda et al., 2019), which is associated with HIV-associated neurocognitive disorders despite ART in PLWH, potentially caused directly by residual HIV replication or indirectly by neuroinflammation. Animal model studies suggest that brain myeloid cells (BMCs) are latently infected and contribute to viral reservoirs in the central nervous system (CNS) (Honeycutt et al., 2016; Avalos et al., 2017). To achieve a cure for HIV, latently infected replication-competent HIV hosted in both periphery and CNS must be targeted by viral eradication strategies.
It remains to be determined which cell types serve as stable and replication-competent HIV reservoirs in the brain. HIV DNA or RNA was detectable in BMCs, astrocytes, pericytes, neural stem cells, oligodendrocytes, and CD4+ T cells (Honeycutt et al., 2016; Bertrand et al., 2019; Putatunda et al., 2019). As one of the major immune cells, the long-lived and self-renewable resident microglia act as the first line of defense against viral infection in the brain (Ajami et al., 2007). BMCs, especially microglia, express CCR5 and low levels of CD4 (Gumbs et al., 2022), which can be infected by HIV for productive HIV infection, allowing persistent HIV infection and serving as bona fide HIV reservoirs in the CNS. Blood-borne monocyte-derived macrophages (MDMs) constitute 5–10% of total BMCs (Ginhoux et al., 2010). MDMs originate from perivascular regions and are terminally differentiated and frequently replenished by hematopoietic progenitors. However, with a short lifespan (~ a few months) and a lack of self-renewal potential, it is unclear whether MDMs can support long-lasting HIV infection in the brain, a prerequisite feature for any HIV reservoir. While astrocytes were infected by HIV to support HIV egress from the CNS to the periphery in an astrocyte-implanted mouse model (Lutgen et al., 2020), it remains to be determined whether astrocytes can be directly infected by HIV to serve as a replication-competent HIV reservoir in the brain. Most recently, blood-brain barrier pericytes were reported as a unique source of HIV infection and possibly established HIV latency in the brain (Bertrand et al., 2019). It remains to be defined whether pericytes are long-lived with self-renewal potential to serve as another source of viral reservoirs in the human brain. Together, BMCs, especially microglia, are one of the major HIV reservoirs in the brain. HIV curative strategies to target the microglia reservoir would permit ART-free remission and prevent neuronal dysfunction, neuroinflammation, and HIV-associated neurocognitive disorder progression in the CNS. For example, it has been shown that microglia activation and HIV-associated neuronal damage are linked to HIV-associated neurocognitive disorders in PLWH (Putatunda et al., 2019). Therefore, the development of tools to specifically target HIV in microglia is the first step to achieving HIV cure in the CNS.
Brain HIV cure: “Shock and kill” has been proposed as one of the major strategies for the cure of HIV. It originated from the idea that: 1) Latent HIV does not efficiently express HIV components or its viral particles via blocks of HIV transcription and/or translation, hiding it from the compromised immune surveillance in PLWH. 2) If latent HIV can be disrupted to express itself by latency reversal, i.e. proteins, RNA, or viral particles, HIV reservoirs could be eradicated following killing strategies, including enhanced immune responses, neutralizing antibodies, or direct killing. However, it has been shown that HIV components may induce neuronal injury or other side effects in the CNS (Proust et al., 2020). Therefore, alternative tools are being developed to avoid this caveat without latency reversal, among which is gene editing to directly knock out the HIV genome for a cure for HIV (Yin et al., 2017).
While initially it was developed in the model of HIV latently infected CD4+ T cells, recent studies have shown that the HIV genome can be disrupted by CRISPR/Cas gene editing in the brain in vivo although its efficiency and specificity are unclear (Yin et al., 2017). Two aspects of technologies have to be improved for gene editing as a therapeutic approach in the CNS: 1) Efficient delivery of the gene-editing system into the brain and 2) Specific target of the gene-editing system into the reservoir cells harboring HIV proviruses. Fortunately, state-of-the-art technologies have been applied to discover unique genes that are exclusively expressed in the CNS subset cells. One of such genes is HexB, which is uniquely expressed in the brain microglia but not MDMs or other neural cells (Masuda et al., 2020), even under pathological conditions (Masuda et al., 2020). This property makes HexB gene promoter an excellent candidate for microglia-specific targeting for the therapeutic of CNS injury. We recently characterized a small-sized version of HexB (134 bp) gene promoter (Shah et al., 2022), which drives its target gene expression largely in microglia but not astrocytes. Interestingly, a 135 bp version of CD68 gene promoter was also identified in this study, which similarly induces gene expression in microglia over astrocytes (Shah et al., 2022). These small-sized microglia-specific promoters are the candidate elements ideal for the packaging capacity and transduction efficiency of adeno-associated virus (AAV), which is one of the best viral delivery systems for human gene therapy. Future work will also determine whether or not it has transcription activity in non-microglia cell types in the brain in vivo.
As mentioned above, it is essential to have a tool to efficiently deliver gene editor into the human brain so that HIV proviruses in the microglia can be targeted in vivo. One way is to develop an microglia-tropic AAV serotype that can effectively cross the blood-brain barrier to facilitate the delivery of gene editor into cellular HIV reservoirs in the brain (Rosario et al., 2016). Investigation is underway to discover such an AAV serotype with the currently available AAV serotypes. Combination of microglia-tropic AAV with an effective promoter to selectively express the gene editor in the brain microglia may further improve microglia specificity to reduce the side effects during gene editing. Lastly, the obstacle of blood-brain barrier penetration needs to be overcome in order to design an AAV serotype to target microglia, the major cellular reservoir of HIV in the CNS (Honeycutt et al., 2016; Alvarez-Carbonell et al., 2019).
The remaining hurdle for the brain HIV cure study is the appropriate model of human brain HIV reservoirs to test such a gene-editing tool. HIV-infected humanized mice and simian immunodeficiency virus-infected rhesus macaques are able to resemble active HIV infection, suppression of HIV replication by ART and establishment of HIV latency in PLWH (Honeycutt et al., 2016; Avalos et al., 2017). However, both models are expensive and require high standards for facilities to house the animals. There are emerging cerebral organoid models, a three-dimensional cell culture system that recapitulates the development of human brain suitable for HIV cure study. Nevertheless, most of these organoid models lack microglia, where exogenous microglia has to be incorporated into such organoid models to develop into mini-brains during organoid maturation. Most recently, native microglia-containing organoid (MCO) models were developed to allow productive HIV infection via its co-receptor CCR5 (Bodnar et al., 2021; Gumbs et al., 2022). However, it is unclear whether HIV latency can be established, whether microglia is the major cellular reservoir in the CNS and whether a proper gene-editing strategy can be developed in MCO. Currently, there is a lack of appropriate in vitro cellular models of HIV infection and latency in microglia. Microglia could be directly isolated from fresh brain of PLWH. Unfortunately, the resources are greatly limited as isolation, culture, and maintenance of microglia from brain tissues are tremendous challenges for ex vivo studies. In collaboration with the “Last Gift” cohort, we are establishing a protocol to isolate highly pure BMCs from post-mortem brains from PLWH receiving long-term ART. This novel technology may provide us with a physiologically relevant platform, enabling us to closely study HIV brain reservoirs.
Summary and future perspectives: We are at the infant stage to characterize HIV reservoirs and develop the cure strategies for HIV in the CNS (Figure 1). It is uncertain whether HIV reservoirs in the brain can be eradicated. Despite that, studies of stable viral reservoirs in the CNS and therapeutic tools developed to attack such a reservoir will help us better understand viral CNS infection, persistence, and neuroinflammation, which may be useful for us to fight against other CNS viral infections in the future.
Figure 1.
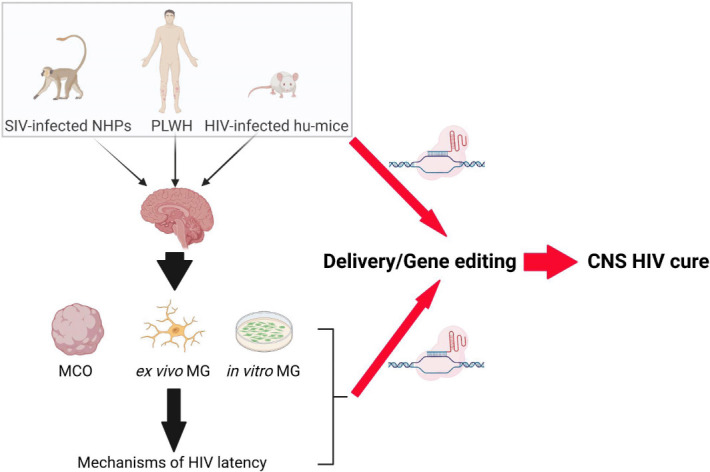
Road map to CNS HIV cure by gene editing.
Post-mortem brain tissues from ART-suppressed PLWH, SIV-infected NHPs and HIV-infected humanized mice (hu-mice) are the appropriate models to study HIV reservoirs in vivo. HIV-infected MCO and primary MG are necessary to complement the ex vivo and in vivo models to study MG reservoirs in vitro. These models can be utilized to study MG targeted delivery and the design of gene editing strategies for the eradication of HIV reservoirs in the human brain in vivo. CNS: Central nervous system; HIV: human immunodeficiency virus-1; MCO: MG-containing organoid; MG: microglia; NHP: Non-human primate; PLWH: people living with HIV; SIV: simian immunodeficiency virus.
We apologize for not being able to cite all the excellent publications due to the limitation of citations.
GJ was supported by Qura Therapeutics, R01DA055491, UM1 AI164567, P30AI50410 and R21MH128034.
Additional file: Open peer review report 1 (79.4KB, pdf) .
Footnotes
Availability of data and materials: All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open peer reviewer: Mengmeng Jin, Rutgers University - Busch Campus, USA
P-Reviewer: Jin M; C-Editors: Zhao M, Liu WJ, Wang Lu; T-Editor: Jia Y
References
- 1.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Carbonell D, Ye F, Ramanath N, Garcia-Mesa Y, Knapp PE, Hauser KF, Karn J. Cross-talk between microglia and neurons regulates HIV latency. PLoS Pathog. 2019;15:e1008249. doi: 10.1371/journal.ppat.1008249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avalos CR, Abreu CM, Queen SE, Li M, Price S, Shirk EN, Engle EL, Forsyth E, Bullock BT, Mac Gabhann F, Wietgrefe SW, Haase AT, Zink MC, Mankowski JL, Clements JE, Gama L. Brain macrophages in simian immunodeficiency virus-infected, antiretroviral-suppressed macaques:a functional latent reservoir. mBio. 2017;8:e01186–17. doi: 10.1128/mBio.01186-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertrand L, Cho HJ, Toborek M. Blood-brain barrier pericytes as a target for HIV-1 infection. Brain. 2019;142:502–511. doi: 10.1093/brain/awy339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodnar B, Zhang Y, Liu J, Lin Y, Wang P, Wei Z, Saribas S, Zhu Y, Li F, Wang X, Yang W, Li Q, Ho WZ, Hu W. Novel scalable and simplified system to generate microglia-containing cerebral organoids from human induced pluripotent stem cells. Front Cell Neurosci. 2021;15:682272. doi: 10.3389/fncel.2021.682272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gumbs SBH, Berdenis van Berlekom A, Kubler R, Schipper PJ, Gharu L, Boks MP, Ormel PR, Wensing AMJ, de Witte LD, Nijhuis M. Characterization of HIV-1 infection in microglia-containing human cerebral organoids. Viruses. 2022;14:829. doi: 10.3390/v14040829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honeycutt JB, Wahl A, Baker C, Spagnuolo RA, Foster J, Zakharova O, Wietgrefe S, Caro-Vegas C, Madden V, Sharpe G, Haase AT, Eron JJ, Garcia JV. Macrophages sustain HIV replication in vivo independently of T cells. J Clin Invest. 2016;126:1353–1366. doi: 10.1172/JCI84456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lutgen V, Narasipura SD, Barbian HJ, Richards M, Wallace J, Razmpour R, Buzhdygan T, Ramirez SH, Prevedel L, Eugenin EA, Al-Harthi L. HIV infects astrocytes in vivo and egresses from the brain to the periphery. PLoS Pathog. 2020;16:e1008381. doi: 10.1371/journal.ppat.1008381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masuda T, Amann L, Sankowski R, Staszewski O, Lenz M, P DE, Snaidero N, Costa Jordao MJ, Bottcher C, Kierdorf K, Jung S, Priller J, Misgeld T, Vlachos A, Meyer-Luehmann M, Knobeloch KP, Prinz M. Novel Hexb-based tools for studying microglia in the CNS. Nat Immunol. 2020;21:802–815. doi: 10.1038/s41590-020-0707-4. [DOI] [PubMed] [Google Scholar]
- 11.Proust A, Barat C, Leboeuf M, Drouin J, Gagnon MT, Vanasse F, Tremblay MJ. HIV-1 infection and latency-reversing agents bryostatin-1 and JQ1 disrupt amyloid beta homeostasis in human astrocytes. Glia. 2020;68:2212–2227. doi: 10.1002/glia.23833. [DOI] [PubMed] [Google Scholar]
- 12.Putatunda R, Ho WZ, Hu W. HIV-1 and compromised adult neurogenesis:emerging evidence for a new paradigm of HAND persistence. AIDS Rev. 2019;21:11–22. doi: 10.24875/AIDSRev.19000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosario AM, Cruz PE, Ceballos-Diaz C, Strickland MR, Siemienski Z, Pardo M, Schob KL, Li A, Aslanidi GV, Srivastava A, Golde TE, Chakrabarty P. Microglia-specific targeting by novel capsid-modified AAV6 vectors. Mol Ther Methods Clin Dev. 2016;3:16026. doi: 10.1038/mtm.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah S, Wong LM, Ellis K, Bodnar B, Saribas S, Ting J, Wei Z, Tang Y, Wang X, Wang H, Ling B, Margolis DM, Garcia JV, Hu W, Jiang G. Microglia-specific promoter activities of HEXB gene. Front Cell Neurosci. 2022;16:808598. doi: 10.3389/fncel.2022.808598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin C, Zhang T, Qu X, Zhang Y, Putatunda R, Xiao X, Li F, Xiao W, Zhao H, Dai S, Qin X, Mo X, Young WB, Khalili K, Hu W. In vivo excision of HIV-1 provirus by saCas9 and multiplex single-guide RNAs in animal models. Mol Ther. 2017;25:1168–1186. doi: 10.1016/j.ymthe.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


