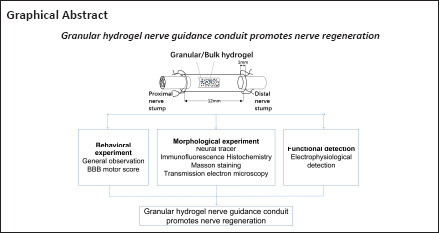
Key Words: functional recovery, granular hydrogel, hyaluronic acid, myelin sheath, nerve conduit, nerve regeneration, peripheral nerve regeneration, sciatic nerve injury, tissue engineering, transection injury
Abstract
A hyaluronic acid granular hydrogel can promote neuronal and astrocyte colony formation and axonal extension in vitro, suggesting that the hydrogel can simulate an extracellular matrix structure to promote neural regeneration. However, in vivo experiments have not been conducted. In this study, we transplanted a hyaluronic acid granular hydrogel nerve guidance conduit to repair a 10-mm long sciatic nerve gap. The Basso, Beattie, and Bresnahan locomotor rating scale, sciatic nerve compound muscle action potential recording, Fluoro-Gold retrograde tracing, growth related protein 43/S100 immunofluorescence staining, transmission electron microscopy, gastrocnemius muscle dry/wet weight ratio, and Masson’s trichrome staining results showed that the nerve guidance conduit exhibited similar regeneration of sciatic nerve axons and myelin sheath, and recovery of the electrophysiological function and motor function as autologous nerve transplantation. The conduit results were superior to those of a bulk hydrogel or silicone tube transplant. These findings suggest that tissue-engineered nerve conduits containing hyaluronic acid granular hydrogels effectively promote the morphological and functional recovery of the injured sciatic nerve. The nerve conduits have the potential as a material for repairing peripheral nerve defects.
Introduction
Peripheral nerve injury (PNI) occurs in 1.2–2.8% of patients that have experienced traumatic events such as trauma, joint dislocation, or surgical trauma (Li et al., 2016a; Carvalho et al., 2019). A PNI may impair motor and sensory functions (Yi et al., 2019). Tension-free suturing of the peripheral nerve membrane via microsurgery is the current treatment method; in cases where end-to-end suturing is not possible, autologous nerve grafting is preferred (López-Cebral et al., 2017). Autologous nerve grafting is typically used to repair long injuries, and the technique is an essential component of clinical nerve repair (Gu et al., 2014). However, the need for a second operation and limitations of the donor site hinder the clinical application of autologous nerve grafts (Tao et al., 2019). While it is challenging to repair, regenerate, and recover function after central nerve injury, it is possible to use autologous nerve graft substitutes to repair a PNI.
The development of nerve tissue engineering with new biomaterials, the incorporation of neurotrophic factors, and cell transplantation has provided hope for nerve repair and regeneration (Thoenen, 1995; He et al., 2015). Scaffolds comprising biological materials can simulate the extracellular matrix (ECM) and serve as structural support for endogenous and seeded cells. Thus, the selection of biomaterials, natural or synthetic, becomes a critical factor in nerve tissue engineering. Natural materials include ECM components (e.g., hyaluronic acid, fibrin, and laminin), chitosan, acellular tissue, and silk protein (Fornasari et al., 2020; Yan et al., 2022). Although natural materials have biocompatibility and are suitable for cell adhesion and growth, materials from nonhuman resources can trigger immune responses and further damage host tissues. Other disadvantages of materials from nonhuman resources are their heterogeneity and, thus, low reproducibility and repeatability, as well as difficulties in chemical modification uniformity (Vijayavenkataraman, 2020). Synthetic materials are polymers produced under controlled conditions that can be further divided into degradable and nondegradable materials (Houshyar et al., 2019). The main advantages include their facile modification according to need, presumably less immune response, low toxicity, and minimal risk of pathogenic infection (Keane and Badylak, 2015). The main disadvantage of synthetic materials is poor biocompatibility, although this may be improved by chemical and biological functionalization (Magaz et al., 2018).
Nerve guidance conduits with a specific internal structure made of synthetic or natural materials are widely used to overcome the limitations of autologous nerve transplantation methods (Yan et al., 2022). Recently, nerve guidance conduits containing hydrogels have shown promise in tissue engineering by simulating the cell microenvironment, promoting cell-cell interactions, and the exchange of biological factors and waste (George et al., 2020). For example, an aligned chitosan fiber hydrogel visibly promoted the proliferation of Schwann cells and the secretion of neurotrophic factors, in addition to repairing nerve sciatic injuries and blood vessel penetration in the early stage of an injury (Rao et al., 2020). In another study, a decellularized neural matrix hydrogel combined with glial-derived neurotrophic factor visibly enhanced nerve injury repair and relevant functional recovery (Qiu et al., 2020). Vascular endothelial growth factor and the widely used nerve growth factor have also been added to a porcine decellularized neural matrix hydrogel to greatly improve the motor functional recovery of nerve damage in rats (Li et al., 2021).
Scaffolds based on hyaluronic acid granular hydrogels and human-induced pluripotent stem cells were used to support three dimensional (3D) neural networks in vitro. The granular scaffold sustained longer axon extension, more axon-bearing cells, and longer axon extensions than a bulk hydrogel scaffold after seven days. Long-term cultivation of therapeutically relevant human induced pluripotent stem cell-derived nerve cells in hyaluronic acid granular hydrogels supported neuron and astrocyte colonies, as well as high-level axon extension (Hsu et al., 2022). This approach provides a facile method for obtaining a tissue-relevant culture of granular hydrogels using biocompatible hydrogels. Although the granular hydrogel construct showed promising results in long-term culture, its effects on nerve regeneration in an animal model have not been demonstrated. In this study, we combined the previously developed 3D nerve conduit based on hyaluronic acid granular hydrogels (Lin et al., 2021; Hsu et al., 2022) for PNI repair. The therapeutic effects of the conduit filled with hyaluronic acid granular hydrogels were examined by repairing a 10-mm sciatic nerve defect in a Sprague-Dawley model.
Methods
Animals
Male animals were used as the experimental model (Li et al., 2020; Yang et al., 2020; Onode et al., 2021). Thirty adult specific-pathogen-free Sprague-Dawley rats (240–260 g) aged 8–10 weeks were used. The rats were purchased from the Laboratory Animal Center of Air Force Military Medical University (Xi’an, China; license No. SCXK (Shaan) 2019-001). The rats were placed in a standard 12-hour light/dark cycle environment (temperature 22–26°C; humidity 55–60%). Experimental plans restricted the use of animals, and the experimental methodology was approved by the Animal Care and Use Committee at Air Force Military Medical University (approval No. IACUC-20210229) on February 5, 2021. All experiments were designed and reported in accordance with Animal Research: Reporting of In Vivo Experiments (ARRIVE) Guidelines (Percie du Sert et al., 2020).
Pentobarbital sodium (Sinopharm, Shanghai, China) was used to anesthetize the animals (35 mg/kg body weight; intraperitoneal injection). A median incision was made at the posterior part of the left thigh to expose and a section of the sciatic nerve was removed. Both the proximal and distal ends were sutured 1 mm into the conduits, leaving a 10-mm defect between the nerve ends. The animals were randomly separated into five groups: 1) autologous nerve group: the severed sciatic nerve was rotated 180°, and then the severed end was sutured; 2) silicone tube group: after injury, the defect was bridged with an empty silicone tube (length of 12 mm; inner diameter of 1 mm, Pureshi, Shanghai, China); 3) bulk hydrogel group: the defect was bridged with an empty silicone tube (length of 12 mm) and the bulk hydrogel (9 µL) was injected into the lumen of the tube; 4) granular hydrogel group: the defect was bridged with an empty silicone tube (length of 12 mm) and the granular hydrogel (9 µL) was injected into the lumen of the tube; and 5) chitosan conduit group (positive control): chitosan conduit (length of 12 mm; inner diameter of 1 mm, Capital Medical University, Beijing, China) (Li et al., 2009) was used to bridge the defect. There were six rats in each group. Pentobarbital sodium was used to euthanize the animals (100 mg/kg body weight; intraperitoneal injection) 16 weeks after surgery. The experimental procedures and the corresponding number of animals are shown in Figure 1.
Figure 1.
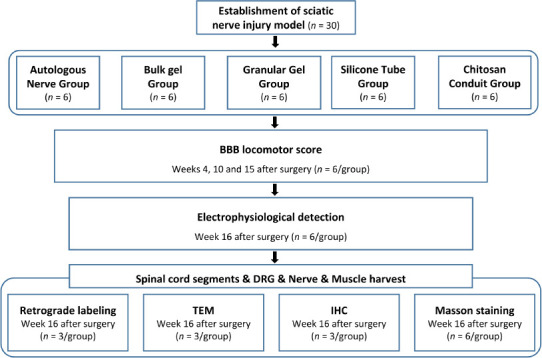
Flow chart of the experimental procedures and animal use.
BBB: Basso, Beattie, and Bresnahan score; DRG: dorsal root ganglion; IHC: immunohistochemistry; TEM: transmission electron microscopy.
Preparation of bulk and granular hydrogels
Bulk hydrogels were fabricated using the HyStem Cell Culture Scaffold Kit (Cat# HYS020, Sigma, St. Louis, MO, USA) containing a HyStem monomer, Extralink crosslinker, and degassed water. A 1 mL syringe was used to add 0.5 mL degassed water (4°C) into a bottle of HyStem (a structure-modified hyaluronan), and the bottle was placed on a shaker to fully dissolve the thiol-modified hyaluronan. Stroke-physiological saline solution (0.5 mL) was injected into the HyStem bottle and mixed well. Extralink crosslinker (12.4 µL), brain-derived neurotrophic factor (BDNF; Cat# 450-02, PeproTech, Rocky Hill, NJ, USA), and laminin (Cat# L2020-1MG, Sigma) were added to the HyStem bottle and mixed well. The final concentrations of BDNF and laminin in the solution were 2 µg/mL. The bulk hydrogel was formed at room temperature. The bulk hydrogel was collected using a syringe (Scientific Laboratory Supplies) and further crosslinked at 4°C for 1 hour.
Granular hydrogels were prepared by passing the bulk hydrogel through a 3D printed Toolkit (Hsu et al., 2022). The custom 3D printed Toolkit was assembled as previously described using a 40-µm pore size filter membrane (Cat# 352340, Scientific Laboratory Supplies) and 1 mL syringes (Scientific Laboratory Supplies). First, 350 µL of stroke-physiological saline solution and 16.8 µL of Extralink were added to a 1.5 mL centrifuge tube; all materials were mixed homogenously. Bulk hydrogel (350 µL), BDNF, and laminin were then added (the final concentrations of BDNF and laminin were 2 µg/mL). The solution was collected in a syringe and mixed thoroughly using the 3D printed Toolkit to fabricate the granular hydrogel by passing the bulk hydrogel three times through the filter membrane at room temperature. The granular hydrogel was then subjected to secondary crosslinking at room temperature for 30 minutes (Hsu et al., 2022).
Basso, Beattie, and Bresnahan locomotor score
The motor function recovery of the affected limb was evaluated using the Basso, Beattie, and Bresnahan (BBB) score (Basso et al., 1995; Schiaveto de Souza et al., 2004; Haidar et al., 2020). The specific method is as follows. Animals were placed in an open field. Two trained observers then recorded and scored the animal’s limb movement, paw placement, gait, and coordination characteristics. There were three parts of the score, and each part had a range of 0–7 points for a potential total score of 0–21. The higher the score, the better the recovery of the motor function of the animal. The first part assessed the joint activity of animal hind limbs, the second part assessed the gait and coordination function of hind limbs, and the third part assessed the fine movement of the claws. BBB scores were recorded 4, 10, and 15 weeks after surgery to determine the level of motor recovery in rats of each group. This experiment was conducted in strict accordance with the double-blinded principle.
Electrophysiological detection
Sixteen weeks after surgery, the compound muscle action potential (CMAP) in the autologous nerve, granular hydrogel, chitosan conduit, and bulk hydrogel groups was recorded to assess the electrophysiological recovery of the sciatic nerves of rats. CMAP was not measured in the silicone tube group because nerve regeneration was not observed. Anesthesia was applied to rats to expose their sciatic nerves. MadLab-8T (Zhongshi Technology, Beijing, China), a bioinformatics medical signal acquisition and processing system, was used. The recording electrode (concentric electrode; Kedoubc, Suzhou, China) was positioned in the belly of the gastrocnemius muscle, and the stimulating electrodes (concentric needle electrode; Kedoubc) were positioned 3 mm away from the proximal end of the autologous nerve or conduits (current stimulation, 5 mA, 1 Hz), and the CMAP was recorded.
Retrograde labeling
Three rats per group were selected for Fluoro-Gold (FG) injection to assess the neuron regeneration after rat sciatic nerve repair. Sixteen weeks after surgery, the sciatic nerve was exposed, and the injection point was identified 5 mm from the distal portion of the conduit or autologous nerve. After gently squeezing the injection area with vascular forceps, 3 µL 4% (v/v) FG (Cat# 80014, Biotium, San Francisco, CA, USA) in stroke-physiological saline solution was slowly injected into the trunk of the sciatic nerve, and the needle removal was delayed for 3 minutes. Five days later, the animals were perfused through the heart with 0.01 M phosphate buffered saline (PBS; pH 7.4) and 4% (w/v) paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4) for termination. The L4–L6 spinal cord segments with dorsal root ganglion (DRG), sciatic nerves on both sides of the animals, and the gastrocnemius muscle were removed after anesthesia. After dehydration, the collected samples were embedded in optimal cutting temperature compound (OCT) (Sakura, Torrance, CA, USA); 20-µm (spinal cord segments) and 10-µm (DRG) thick sections were acquired with a cryostat (CM1950; Leica, Wetzlar, Germany). The tissue sections were mounted on clean adherent slides, washed with PBS, dried naturally, and covered with a glass slip using 2.5% (w/v) triethylene diamine in glycerol and 0.01 M PBS (pH 7.4) (volume ratio: 1:1). Each obtained spinal cord sample was sectioned, and one of every five sections was used for counting. Ten sections per rat were taken for counting the FG-labeled motoneurons in the spinal anterior horn; three randomly selected sections from DRG sections of each rat were used for counting the FG-labeled sensory neurons in the DRG. An epifluorescence microscope (BX-60, Olympus, Tokyo, Japan) was used to observe and measure the number of positive neurons.
Transmission electron microscopy
To assess the ultrastructural recovery of regenerated axons 16 weeks after surgery, animals were anesthetized by perfusion of the heart with 0.01 M PBS (pH 7.4) followed by 2% (w/v) paraformaldehyde and 1.25% (w/v) glutaraldehyde in 0.1 M PB (pH 7.4) to harvest the regenerated sciatic nerves in the conduits. Following fixation with 2% glutaraldehyde overnight, 1% osmium tetroxide acid was used to fix and stain the tissues for 2 hours. The conduits were rinsed with gradient alcohol and embedded with an epoxy resin system (SPI-CHEM, Beijing, China) including dodecenyl succinic anhydride, epoxy resin monomer, nadic methyl anhydride, and 2-4-6-tris(dimethylaminomethyl)-phenol. Sections (70-nm thick) were created using an ultramicrotome (EM UC-7, Leica). The sections were mounted on a 100-mesh copper grid and placed in uranium dioxide acetate for 10 minutes. The sections were then washed in 0.1 M PB, immersed in lead nitrate solution for 10 minutes, and then washed again with 0.1 M PB. Finally, the sections were observed with a transmission electron microscope (TEM, JEM-1400, JEOL, Tokyo, Japan) equipped with a CCD camera (Olympus Veleta) and the associated software (ITEM 5.2, EMSIS, Munster, Germany). The distal ends of the sciatic nerves within the conduits were evaluated by TEM. For quantification, five random fields of each section were selected for imaging, and the following methods were used to evaluate regeneration. Quantification of myelin regeneration was performed by calculating the G-ratio of the diameter of axons and the diameter of nerve fibers. The morphological evaluation was performed by unbiased experimenters.
Immunohistochemistry
To assess the recovery of the sciatic nerve 16 weeks after surgery, the sciatic nerves on both sides were extracted and fixed, first with 4% (w/v) paraformaldehyde in 0.1 M PB (pH 7.4) for 12 hours, followed by 30% (w/v) sucrose in 0.1 M PB (pH 7.4). After dehydration, the sciatic nerves were embedded in OCT, and then sectioned by a cryostat into 15-µm thick slices. The sections were attached to clean adherent slides, dried naturally, and then washed using 0.01 M PBS. The sciatic nerves in the middle of the conduits were evaluated with immunohistochemistry. The sections were incubated with 10% normal donkey serum (Cat# 566460, Sigma) for 30 minutes at 20°C followed by the primary antibody mouse anti-S100 IgG (1:200, Abcam, Cambridgeshire, UK, Cat# ab4066, RRID: AB_304258) and rabbit anti-growth associated protein 43 (GAP43) IgG (1:200, Abcam, Cat# ab16053, RRID: AB_443303) overnight at 4°C. After washing, the sections were incubated in Alexa Fluor 594 donkey anti-rabbit IgG (1:200, Invitrogen, Carlsbad, CA, USA, Cat#A21207, RRID: AB_141637) and Alexa Fluor 488 donkey anti-mouse IgG (1:200, Invitrogen, Cat# A21202, RRID: AB_141607) at room temperature for 6 hours. A solution of 0.01 M PBS containing 1% normal donkey serum, 0.3% (v/v) Triton X-100, 0.02% (w/v) sodium azide, and 0.12% (w/v) carrageenan was used for the antibodies. All sections were examined and recorded with a confocal laser microscope (FV1000, Olympus) using FLUOVIEW software (FV10-ASW 4.2, Olympus).
Wet weight ratio of the gastrocnemius muscle
After perfusion, each rat’s intact gastrocnemius muscle was removed to assess the wet weight ratio of the gastrocnemius muscle 16 weeks after surgery. The residual blood on the muscle was removed using absorbent paper. The wet muscle weight was measured with an electronic balance (JY-3001, Minqiao, Shanghai, China) using the following formula: Gastrocnemius muscle wet weight ratio (%) = (wet muscle weight of the injury side/wet muscle weight of the normal side) × 100.
Masson’s staining
The gastrocnemius muscles of all groups were subject to Masson’s staining to assess the recovery of the muscle after sciatic nerve injury and 16 weeks after surgery. The gastrocnemius muscle was fixed with 4% (w/v) paraformaldehyde in 0.1 M PB (pH 7.4) overnight and then dehydrated with 30% (w/v) sucrose in 0.1 M PB (pH 7.4). After embedding in OCT, the muscle tissue was sectioned into 50-µm thick sections using a cryostat. All sections were mounted on clean adherent slides and dried naturally. Muscle fibers of the gastrocnemius muscle were stained by Masson’s staining (Leagene, Beijing, China). A microscope (BX-60, Olympus) was used to record the atrophy and recovery of the muscle fibers. Five fields (200× magnification) were randomly selected from each slice and ImageJ was used to analyze the images. The percentage of muscle fiber area was calculated using the atrophy and recovery of the muscle: Percentage of muscle fiber area = (area of the muscle fiber in each field/total area of the tissue) × 100.
Statistical analysis
No statistical methods were used to determine sample sizes; however, our sample sizes were similar to those reported in previous publications (Li et al., 2016b; Meder et al., 2021; Zaminy et al., 2021). No animals were excluded in the analysis. The evaluators were blinded to the assignments. Data analysis was conducted using GraphPad Prism 8.0.2 (GraphPad Software, San Diego, CA, USA, www.graphpad.com) and plotted as the mean ± standard deviation (SD). One-way analysis of variance followed by Tukey’s post hoc test was used for comparing data among multiple groups. A value of P < 0.05 was considered statistically significant.
Results
The granular hydrogel nerve guidance conduit improves the motor function recovery of rats with sciatic nerve injury
The BBB score reveals the recovery of motor function (Haidar et al., 2020). Four weeks after surgery, all rats in each group exhibited small movements of the hip and knee joints and large movements of the ankle joints of the affected limb. Ten weeks after surgery, all rats in the granular hydrogel group exhibited frequent plantar weight-bearing movements and consistent fore and hind coordination. The granular hydrogel group showed significantly higher BBB scores than the autologous nerve (P = 0.0009), chitosan conduit (P < 0.0001), bulk hydrogel (P < 0.0001), and silicone tube groups (P < 0.0001). Fifteen weeks after surgery, all rats in the autologous nerve, granular hydrogel, and chitosan conduit groups exhibited continuous plantar stepping and fore and hind limb coordination, with no or occasional toe gaps during advancement, in comparison to the bulk hydrogel and silicone tube groups. The BBB score of the granular hydrogel group was not significantly different from the autologous nerve group (P = 0.9974) or chitosan conduit group (P = 0.8632); however, the granular hydrogel group had significantly greater BBB scores than the bulk hydrogel group (P < 0.0001) and silicone tube group (P < 0.0001; Figure 2). These results indicated that the treatment with the granular hydrogel was comparable to that with autologous nerve 16 weeks after surgery, and the granular hydrogel group had a faster recovery of the rat nerve motor function 10 weeks after surgery.
Figure 2.
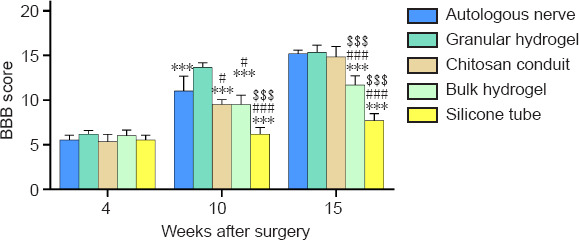
Effect of the granular hydrogel nerve guidance conduit on the BBB locomotor scale in rats with sciatic nerve injury.
The BBB locomotor scale was applied 4, 10, and 15 weeks after surgery. The higher the score, the better the motor function recovery of the animal. The scores of each group increased over time, and the difference in scores between the groups changed significantly. Data are shown as mean ± SD (n = 6). ***P < 0.001, vs. granular hydrogel group; #P < 0.05, ###P < 0.001, vs. autologous nerve group; $$$P < 0.001, vs. chitosan conduit group (one-way analysis of variance followed by Tukey’s post hoc test). BBB: Basso, Beattie, and Bresnahan score.
The granular hydrogel nerve guidance conduit improves the electrophysiological recovery of a rat sciatic nerve injury
CMAP recordings were used to assess the recovery of the electrophysiological properties of sciatic nerves repaired using autologous nerve, granular gel, chitosan conduit, and bulk gel. As shown in Additional Figure 1 (372.7KB, tif) , the CMAP of normal animals was 1.86 ± 0.07 mV (n = 3). The peak amplitudes of the CMAP waveforms of the granular hydrogel and autologous nerve groups were the greatest, followed by the chitosan conduit group, and then the bulk hydrogel group (Figure 3A). The CMAP amplitude of the granular hydrogel group was not significantly different from the autologous nerve group (P = 0.8105; Figure 3B). The granular hydrogel group exhibited a significantly greater CMAP amplitude than the chitosan conduit group (P < 0.0001) and bulk hydrogel group (P < 0.0001; Figure 3B). The electrophysiological function of the sciatic nerves was better in the granular hydrogel group than in the chitosan conduit and bulk hydrogel groups. The sciatic nerves in the silicone tube group showed no regeneration. Therefore, the axons, myelin, and CMAP were not examined in the silicone tube group.
Figure 3.
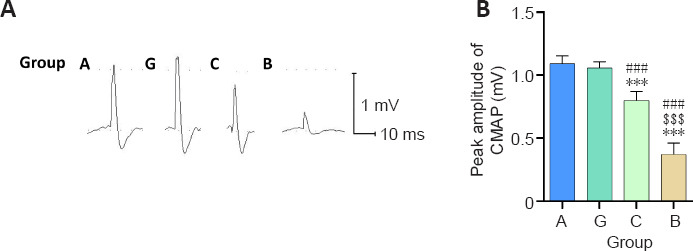
Effect of the granular hydrogel nerve guidance conduit on the CMAP of the operation side of the sciatic nerves in rats 16 weeks after surgery.
(A) The CMAP waveforms of the autologous nerve, granular hydrogel, chitosan conduit, and bulk gel conduit groups were recorded in the proximal nerve portion of the stimulation. The peak amplitudes of the CMAP waveforms in the autologous nerve and granular hydrogel groups were the greatest, followed by the chitosan conduit group and bulk hydrogel group. (B) Peak amplitude of CMAP. Data are shown as the mean ± SD (n = 6). ***P < 0.001, vs. Group G; ###P < 0.001, vs. Group A; $$$P < 0.001, vs. Group C (one-way analysis of variance followed by Tukey’s post hoc test). CMAP: Compound muscle action potential; Group A: autologous nerve group; Group B: bulk hydrogel group; Group C: chitosan conduit group; Group G: granular hydrogel group.
The granular hydrogel nerve guidance conduit improves the neuron regeneration after rat sciatic nerve repair
The numbers of motoneurons in the spinal anterior horn and sensory neurons in the DRG were examined with FG retrograde labeling. As shown in Figures 4 and 5, the granular hydrogel and autologous nerve groups showed no significant differences in the number of labeled motoneurons and sensory neurons (P = 0.9989 and P = 0.5625), while the granular hydrogel group showed more labeled neurons than the bulk hydrogel group (P < 0.0001 and P < 0.0001) and chitosan conduit group (P = 0.0015 and P = 0.0114).
Figure 4.
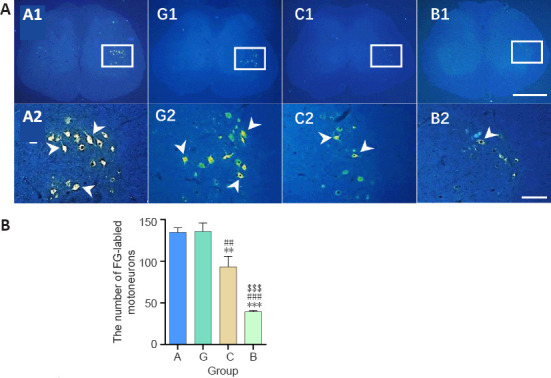
Effect of the granular hydrogel nerve guidance conduit on the FG-labeled motoneurons in the spinal anterior horn of rats with sciatic nerve injury 16 weeks after surgery.
(A) The distribution of the FG-labeled motoneurons (arrowheads) in the spinal anterior horn. Groups G (G1 and G2) and A (A1 and A2) showed more labeled neurons than Groups C (C1 and C2) and B (B1 and B2). A2, G2, C2, and B2 are the zoomed-in fields of A1, G1, C1, and B1, respectively. Scale bars: 1 mm (upper), 200 µm (lower). (B) The number of FG-labeled motoneurons on 10 spinal cord slices from each animal. Data are shown as the mean ± SD (n = 3). **P < 0.01, ***P < 0.001, vs. Group G; ##P < 0.01, ###P < 0.001, vs. Group A; $$$P < 0.001, vs. Group C (one-way analysis of variance followed by Tukey’s post hoc test). FG: Fluoro-gold; Group A: autologous nerve group; Group B: bulk hydrogel group; Group C: chitosan conduit group; Group G: granular hydrogel group.
Figure 5.
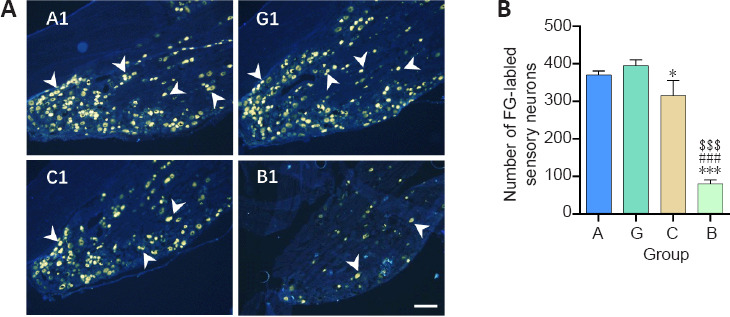
Effect of the granular hydrogel nerve guidance conduit on the FG-labeled sensory neurons in the DRG of rats with sciatic nerve injury 16 weeks after surgery.
(A) The distribution of the FG-labeled sensory neurons (arrowheads) in DRG. Groups G (G1) and A (A1) showed more labeled neurons than Groups C (C1) and B (B1). Scale bar: 200 µm. (B) The number of FG-labeled sensory neurons on three dorsal root ganglion slices from each animal. Data are shown as the mean ± SD (n = 3). *P < 0.05, ***P < 0.001, vs. Group G; ###P < 0.001, vs. Group A; $$$P < 0.001, vs. Group C (one-way analysis of variance followed by Tukey’s post hoc test). FG: Fluoro-gold; Group A: autologous nerve group; Group B: bulk hydrogel group; Group C: chitosan conduit group; Group G: granular hydrogel group.
The granular hydrogel nerve guidance conduit improves the ultrastructural recovery of regenerated axons in rats with sciatic nerve injury
Myelinated nerve fibers in the sciatic nerve were examined with TEM 16 weeks after surgery. The diameters of the nerve fibers in the granular hydrogel and autologous nerve groups were visibly larger, and the distribution of myelin sheaths was more compact and uniform than the chitosan conduit and bulk hydrogel groups, as observed in Figure 6A. In Figure 6B, the diameter of the axons of the granular hydrogel group did not significantly differ from that of the autologous nerve group (P = 0.9896) or chitosan conduit group (P = 0.3928); however the granular hydrogel group was significantly larger than the bulk hydrogel group (P = 0.0034). The diameter of the myelinated fibers of the granular hydrogel group did not significantly differ from the autologous nerve group (P = 0.5234), and was significantly greater than the chitosan conduit group (P = 0.0126) and bulk hydrogel group (P = 0.0001; Figure 6C). The G-ratio value of the regenerated nerves of the granular hydrogel group did not significantly differ from the autologous nerve group (P = 0.3112); however, the granule hydrogel group was significantly less than the chitosan conduit group (P = 0.0042) and bulk hydrogel group (P = 0.0022; Figure 6D). These results indicated that the myelin sheath of the sciatic nerve in the granular hydrogel group was significantly thicker than that in the chitosan conduit and bulk hydrogel groups and was similar to that in the autologous nerve group.
Figure 6.
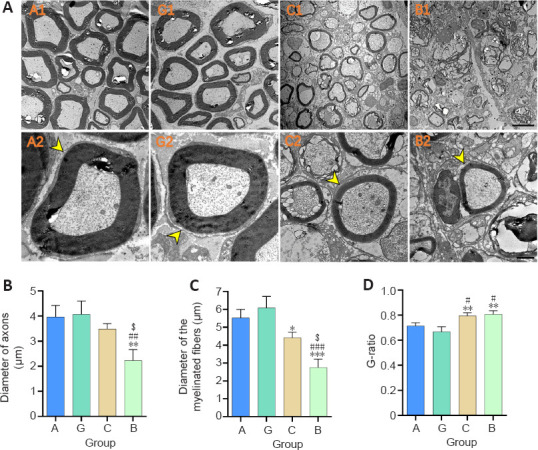
Effect of the granular hydrogel nerve guidance conduit on the morphology analysis of regenerated nerves in rats with sciatic nerve injury 16 weeks after surgery.
(A) The ultrastructure of the regenerated nerves (arrowheads). The diameters of nerve fibers in Groups A (A1 and A2) and G (G1 and G2) were visibly larger and the distribution of myelin sheaths was more compact and uniform than Groups C (C1 and C2) and B (B1 and B2). Scale bars: 5 µm (upper), 1 µm (lower). (B–D) Quantitative results of the diameter of axons (B), the diameter of myelinated fibers (C), and the G-ratio (D) in the distal portion of regenerated nerves. Data are shown as the mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, vs. Group G; #P < 0.05, ##P < 0.01, ###P < 0.001, vs. Group A; $P < 0.05, vs. Group C (one-way analysis of variance followed by Tukey’s post hoc test). Group A: autologous nerve group; Group B: bulk hydrogel group; Group C: chitosan conduit group; Group G: granular hydrogel group.
The granular hydrogel nerve guidance conduit improves the recovery of the sciatic nerve
Sixteen weeks after surgery, the sciatic nerve was examined by immunochemical staining with anti-S100 and anti-GAP43. GAP43 is an axon membrane protein that is related to neuronal regeneration and growth (Chen et al., 2012). S100 protein is widely distributed in Schwann cells, astrocytes, and oligodendrocytes (Schlaepfer and Micko, 1978). Immunofluorescence histochemical staining of a normal sciatic nerve illustrating the positively labeled axons and myelin sheaths is shown in Additional Figure 2 (329.2KB, tif) . As shown in Figure 7A, the regenerated myelin sheaths in the granular hydrogel and autologous nerve groups were relatively round or oval and the nerve fibers in the granular hydrogel group had a larger diameter than the chitosan conduit and bulk hydrogel groups. As shown in Figure 7B, the granular hydrogel group showed no significant difference in the number of GAP43-labeled axons from the autologous nerve group (P = 0.9013) and chitosan conduit group (P = 0.1211). The granular hydrogel group exhibited significantly more GAP43-labeled axons than the bulk hydrogel group (P = 0.0099). The results also clearly revealed a greater diameter of the regenerated nerve in the granular hydrogel group than those in the chitosan conduit and bulk hydrogel groups.
Figure 7.
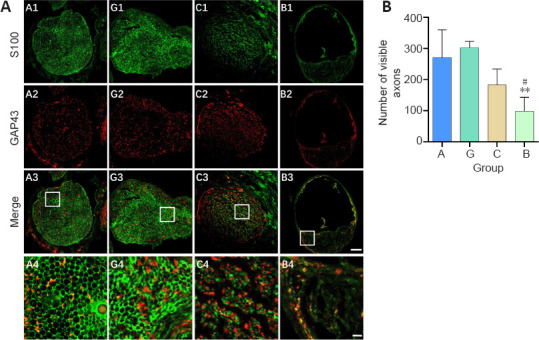
Effect of the granular hydrogel nerve guidance conduit on the regenerated nerves in rats with sciatic nerve injury 16 weeks after the surgery.
(A) The expression of S100 (green, Alexa Fluor 488) and GAP43 (red, Alexa Fluor 594) in the transverse sections of regenerated nerves. Visibly more myelin sheaths and axons were regenerated in Groups G (G1–G4) and A (A1–A4), followed by Groups C (C1–C4) and B (B1–B4). Scale bars: 100 µm (upper three rows), 20 µm (lowest row). A4, G4, C4, and B4 are the zoomed-in fields of A3, G3, C3, and B3, respectively. (B) Quantification of the number of visible axons. Data are shown as the mean ± SD (n = 3). **P < 0.01, vs. Group G; #P < 0.05, vs. Group A (one-way analysis of variance followed by Tukey’s post hoc test). GAP43: Growth associated protein 43; Group A: autologous nerve group; Group B: bulk hydrogel group; Group C: chitosan conduit group; Group G: granular hydrogel group.
The granular hydrogel nerve guidance conduit improves the recovery of gastrocnemius muscles after sciatic nerve injury
Results of the gastrocnemius muscle recovery 16 weeks after surgery are illustrated in Figure 8. The injured side (left) of the gastrocnemius muscle of each rat was obviously smaller than the normal side (right). As shown in Figure 8A, the gastrocnemius muscle of the surgical side was visibly smaller than that of the normal side and the granular hydrogel and autologous nerve groups had smaller gaps between the muscle fibers. The wet weight ratio of the granular hydrogel group showed no significant difference from that of the autologous nerve group (P = 0.9536) and chitosan conduit group (P = 0.4540); however, its wet weight ratio was significantly greater than that of the bulk hydrogel group (P < 0.0001) and silicone tube group (P < 0.0001). Furthermore, the area ratio of the gastrocnemius muscle fibers in the granular hydrogel group did not significantly differ from the autologous nerve group (P = 0.7917); however, its area ratio was significantly greater than the bulk hydrogel (P < 0.0001), chitosan conduit (P < 0.0001), and silicone tube groups (P < 0.0001; Figure 8B and C).
Figure 8.
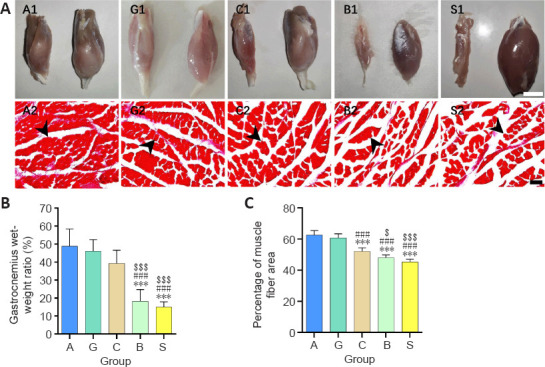
Effect of the granular hydrogel nerve guidance conduit on the gastrocnemius muscle atrophy in rats with sciatic nerve injury 16 weeks after surgery.
(A) Gross appearance of the gastrocnemius muscles on the injured (left) and normal (right) sides (A1, G1, C1, B1, and S1). Masson’s staining of gastrocnemius muscles in each group (A2, G2, C2, B2, and S2). The gastrocnemius muscle of the injured side was visibly smaller than that of the normal side and Groups A and G showed more muscle fibers and less spacing between muscle fibers in the same view size field. Arrowheads indicate the positive muscle fibers. Scale bars: 1 cm (upper) and 100 µm (lower). (B and C) Comparisons of the gastrocnemius wet weight ratio (B) and the average percentage of the muscle fiber area (C). Data are shown as the mean ± SD (n = 6). ***P < 0.001, vs. Group G; ###P < 0.001, vs. Group A; $P < 0.05, $$$P < 0.001, vs. Group C (one-way analysis of variance followed by Tukey’s post hoc test). Group A: autologous nerve group; Group B: bulk hydrogel group; Group C: chitosan conduit group; Group G: granular hydrogel group.
Discussion
Peripheral nerve regeneration in vivo is dependent on macrophage infiltration (Luk et al., 2003). The damaged nerve fibers break down into fragments in the early stages of injury (George et al., 1995). After differentiation, macrophages begin to clear neuronal debris and stimulate Schwann cells to secret cytokines, which in turn recruit macrophages and monocytes (Gaudet et al., 2011). Concurrently, Schwann cells proliferate and release ECM components (Chen et al., 2007). The activated macrophages, proliferated Schwann cells, and secreted ECM act in concert to create a favorable microenvironment for the proximal growth of axonal injury and myelin regeneration. The endogenous repair process of nerve fibers in response to nerve injury is characterized by Wallerian degeneration, including biochemical and morphological modifications to accommodate nerve regeneration. Autonomic and complete regeneration cannot be achieved in injuries with a gap larger than 5 mm (Zheng et al., 2021). Therefore, external support is required to facilitate nerve repair and regeneration in certain cases.
Various methods have been developed to integrate specific structural cues in external scaffolds for peripheral nerve repair, including surface-modified scaffolds (Hu et al., 2012), micronanofiber conduits (Xie et al., 2014), and 3D channel scaffolds (Biazar, 2011). Surface modification has drawbacks because it merely offers a 2D microenvironment for regeneration without sufficient guidance to induce nerve fiber growth or migration in a certain direction. Micronanofiber conduits may not be perfectly aligned at macroscopic distances in a regular manner and the intervals among the fibers may also differ from those of natural neural tissues. Nevertheless, a 3D neural scaffold with an internal structure that can mimic the ECM while guiding the directional growth and migration of nerve fibers can be advantageous.
Hsu et al. (2022) developed a customized 3D printed toolset using granular hydrogels produced by extruding hyaluronic acid hydrogel through nylon fabric, forming a cell scaffold that can promote neuronal differentiation and high-level neurite extension. Granular hydrogels have three main advantages: permeability for nutrient and waste exchange, enhanced cell-cell interactions and connectivity, and injectability (Hsu et al., 2022). In our research, the effect of conduits filled with granular hydrogels was examined for the promotion of sciatic nerve repair. Transplantation of a previously developed 3D nerve conduit with hyaluronic acid granular hydrogels into the sciatic nerve defects was examined to evaluate a new therapeutic option for nerve regeneration.
In this study, chitosan conduits were also examined in parallel with the hyaluronic acid granular and bulk hydrogels. Chitosan, a polysaccharide derived from animal crustaceans, can be artificially modified to regulate its degradation rate in the body. Moreover, it has been applied in several biomedical settings (Gonzalez-Perez et al., 2015). The main advantages of chitosan are biocompatibility, controlled biodegradation rate, and the ability to inhibit bacterial growth (Farhadihosseinabadi et al., 2019). Researchers have developed a chitosan hydrogel tissue engineering conduit. The conduit can significantly promote the proliferation and differentiation of neurons while reducing the formation of glial scars (Zheng et al., 2020). In addition, chitosan conduits can effectively repair PNI and improve motor function in rats (Wang et al., 2012). The control group of chitosan conduits can serve as an additional biomaterial control to compare their effectiveness in nerve repair with the experimental granular and bulk hyaluronic acid hydrogel conduits.
Hyaluronic acid is the main ECM component in the nervous system. Hyaluronic acid has a remarkable effect on neural activities (Sherman et al., 2015). A modified hyaluronic acid-laminin-hydrogel was developed as an intraluminal filling material for hollow nerve conduits to repair nerve injury (Huang et al., 2021). Four months after surgery, all animals in the autologous nerve group exhibited recovery of the electrophysiological and motor functions and the modified hyaluronic acid-laminin-hydrogel improved the regenerative effect of the hollow nerve conduit. Similar to the results in previous studies, our hyaluronic acid granular hydrogel conduit showed electrophysiological and motor functions of the rat sciatic nerves that were similar to rats in the autologous nerve group, indicating the hyaluronic acid granular hydrogel in this study can improve nerve injury repair. In another study, a hyaluronic acid and collagen hydrogel fabricated by magnetic particle templating was used to mimic the microstructure of the natural nerve basal lamina (Lacko et al., 2020). The hydrogel had better axonal regeneration than the normal hydrogel control. These findings were also observed when comparing our granular hydrogel group with the bulk hydrogel group, highlighting the importance of the 3D internal structures in all aspects of axonal regeneration.
In this study, laminin and BDNF were supplemented in the hyaluronic acid hydrogel conduits to promote nerve repair and regeneration. Laminin, one of the main ECM components in the peripheral nerve basement membrane, is a structural protein that activates cell β1-integrin receptors (Dodla and Bellamkonda, 2008). The regeneration of PNI is typically determined by the expansion of the damaged axon. Schwann cells then dedifferentiate and release laminin to promote axon growth during nerve repair (Chen and Strickland, 2003). BDNF is also a stimulating factor for neuron regeneration (Karagyaur et al., 2015). By incorporating laminin and BDNF into the hydrogel nerve conduit, the scaffold can better mimic the in vivo microenvironment to improve nerve recovery. While laminin and BDNF were added to both granular and bulk hydrogels, the internal structure of the granular hydrogel was more suitable than that of the bulk hydrogel for supporting cell behavior and functional recovery. The BDNF-free granular hydrogel group was not considered in the experimental design, which is a limitation of this study.
The BBB motor score can be applied to different types of PNI models (Dinh et al., 2009; Haidar et al., 2020). In this study, the motor function of each group recovered to different levels four weeks after the operation. On week 15, the BBB scores of the granular hydrogel group showed no significant difference from the autologous nerve and chitosan conduit groups; however, the group had significantly higher scores than the bulk hydrogel and silicone tube groups. This suggests that the sciatic nerve regeneration and motor function recovery of the granular hydrogel group was superior to the bulk gel and silicone tube controls, and the recovery of the motor function was equivalent to that of the autologous grafts. In general, a higher CMAP amplitude indicates more regenerated nerve fibers (Katiyar et al., 2020). Therefore, in comparison to the bulk hydrogel and chitosan conduit groups, the granular hydrogel group had the best electrophysiological recovery of the sciatic nerve, with more nerve fibers regenerating. In addition, nerve repair was further proven by detecting the atrophy recovery of the innervated muscles (Li et al., 2020). The gastrocnemius wet weight ratio and Masson’s staining showed that the granular hydrogel conduits can successfully promote the repair and motor function recovery of the sciatic nerve injury.
Axoplasmic transport is a phenomenon of material transport between the neuron body, axon, and its terminals (Guo et al., 2020). Generally, anterograde transport is defined as a transfer from the neuron body to its terminals, whereas retrograde transport is defined as transfer from the axon or its terminals to the neuron body. FG retrograde tracing was used in this study to evaluate the recovery of the axonal transport in sciatic nerves (Zeng et al., 2014). More positive motor and sensory neurons were observed in the granular hydrogel group, indicating that the granular hydrogel conduit can help injured nerve fibers regenerate and improve axon transport function.
After peripheral nerve repair, the expression of S100 in Schwann cells increases, further promoting myelination (Zhang et al., 2008). Immunofluorescence histochemical staining indicated that the number of GAP43-positive axons in the granular hydrogel group was greater than in the bulk hydrogel. Granular hydrogels provide an optimal internal structure for Schwann cell regeneration and axon extension, further promoting nerve regeneration by forming more myelin sheaths. The granular hydrogel group had significantly more myelinated fibers and a thicker myelin sheath than the bulk hydrogel. Therefore, the granular hydrogel conduit outperforms the bulk hydrogel groups in terms of supporting nerve regeneration. A 3D hierarchically aligned fibrin nanofiber hydrogel was applied to promote the regeneration of an injured sciatic nerve in a rat model. The electrophysiological results with their developed graft were better than that of hollow chitosan tubes and bulk macrofibrin nanofiber hydrogels (Du et al., 2017). These results were similar to the results of our study. However, the aligned fibrin nanofiber hydrogel group’s regenerated myelin sheath was noticeably thinner than that of the autologous nerve group (Du et al., 2017). In our investigation, the thickness of the myelin sheath of the hyaluronic acid granular hydrogel group was comparable to that of the autologous nerve group. Yang et al. (2020) developed a self-assembled nanofiber hydrogel based on laminin mimetic peptide and BDNF mimetic peptide for repairing rat sciatic nerve defects. The nanofiber hydrogel can significantly improve axonal regeneration and enhance myelin regeneration and motor function recovery. The TEM images of the myelin sheath in Yang et al.’s study (Yang et al., 2020) were similar to this study and the G-ratio in the nanofiber hydrogel group was similar to that of the granular hydrogel group in this research. Therefore, the hyaluronic acid granular hydrogel is effective in promoting nerve regeneration and has potential for neural tissue engineering applications.
During nerve regeneration, an impenetrable gelatinous scar formed around neurons can hinder the regeneration of neurons. In our study, the hyaluronic acid granular hydrogel conduits can still effectively promote nerve regeneration. The mechanism of such nerve repair may result from the optimal 3D internal structure that can mimic the in vivo cell microenvironment, particularly when supplemented by biological factors such as laminin and BDNF. The scaffold can facilitate cell signaling and transport of trophic factors while being able to support neuronal regeneration and cell migration with a physical and structural support. The crosslinked matrices in the granular hydrogel may also allow slower release of the supplemented bioactive factors, thus with prolonged neurotrophic effects in a local region. The relatively simple manufacturing process of the granular hydrogel nerve conduit and results shown in this study have demonstrated the potential of this scaffold for clinical applications.
In conclusion, our study showed that a granular hydrogel nerve conduit can promote the regeneration of axons, the myelin sheath of an injured sciatic nerve, and motor function recovery in a rat model. In addition, the granular hydrogel nerve conduit is comparable to autologous nerve repair in function and recovery of a PNI and this conduit can achieve the nerve repair while eliminating the risks of a second surgery and damage to donor sites that can occur in autologous nerve recovery. The limitations of this study include that the regenerative effect of the granular hydrogel nerve conduit in the CNS injury and how it affects nerve regeneration at the molecular level have not been studied. Hyaluronic acid, as the main component of the granular hydrogel nerve conduit, is biocompatible and can support cell behavior. The interconnected 3D structure creates a permissive environment for nutrient/bio-factor transport and axonal extension, thereby promoting the regeneration of nerve fibers. The developed granular hydrogel nerve conduit may serve as an ideal material for peripheral nerve repair and regeneration.
Additional files:
Additional Figure 1 (372.7KB, tif) : The CMAP recordings in the normal animals.
The CMAP recordings in the normal animals.
(A) The CMAP waveforms of the normal animals. (B) Histograms of the peak amplitude of CMAP in the normal animals. Data are shown as the mean ± SD (n = 3). CMAP: Compound muscle action potential.
Additional Figure 2 (329.2KB, tif) : Immunofluorescence histochemical staining of no-primary antibody and normal sciatic nerve.
Immunofluorescence histochemical staining of no-primary antibody and normal sciatic nerve.
(A1-3) No positive results were observed in immunofluorescence histochemical staining of no-primary antibodies. (B1-3) Immunofluorescence histochemical staining of normal sciatic nerve showed the positively labeled axons and myelin sheaths. Scale bars: 50 μm. GAP43: Growth associated protein 43.
Additional file 1: Open peer review reports 1 (101KB, pdf) and 2 (101.9KB, pdf) .
Acknowledgments:
We would like to thank professor Xiao-Guang Li (School of Basic Medical Sciences, Capital Medical University, Beijing, China) for providing their chitosan conduits.
Footnotes
Conflicts of interest: The authors declare no conflict of interest.
Availability of data and materials: All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open peer reviewers: Xavier Navarro, Universitat Autonoma de Barcelona, Spain; Hanin Abdel-Haq, Istituto Superiore di Sanita, Italy.
P-Reviewers: Navarro X, Abdel-Haq H; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Yu J, Song LP; T-Editor: Jia Y
References
- 1.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 2.Biazar E. Design of porous alginate conduit using a freeze drying technique for neural engineering. Orient J Chem. 2011;27:1029–1032. [Google Scholar]
- 3.Carvalho CR, Oliveira JM, Reis RL. Modern trends for peripheral nerve repair and regeneration:beyond the hollow nerve guidance conduit. Front Bioeng Biotechnol. 2019;7:337. doi: 10.3389/fbioe.2019.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y, Zhao C, Zhang C, Luo L, Yu G. Influence of chronic intermittent hypoxia on growth associated protein 43 expression in the hippocampus of young rats. Neural Regen Res. 2012;7:1241–1246. doi: 10.3969/j.issn.1673-5374.2012.16.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen ZL, Strickland S. Laminin gamma1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. J Cell Biol. 2003;163:889–899. doi: 10.1083/jcb.200307068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen ZL, Yu WM, Strickland S. Peripheral regeneration. Annu Rev Neurosci. 2007;30:209–233. doi: 10.1146/annurev.neuro.30.051606.094337. [DOI] [PubMed] [Google Scholar]
- 7.Dinh P, Hazel A, Palispis W, Suryadevara S, Gupta R. Functional assessment after sciatic nerve injury in a rat model. Microsurgery. 2009;29:644–649. doi: 10.1002/micr.20685. [DOI] [PubMed] [Google Scholar]
- 8.Dodla MC, Bellamkonda RV. Differences between the effect of anisotropic and isotropic laminin and nerve growth factor presenting scaffolds on nerve regeneration across long peripheral nerve gaps. Biomaterials. 2008;29:33–46. doi: 10.1016/j.biomaterials.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du J, Liu J, Yao S, Mao H, Peng J, Sun X, Cao Z, Yang Y, Xiao B, Wang Y, Tang P, Wang X. Prompt peripheral nerve regeneration induced by a hierarchically aligned fibrin nanofiber hydrogel. Acta Biomater. 2017;55:296–309. doi: 10.1016/j.actbio.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Farhadihosseinabadi B, Zarebkohan A, Eftekhary M, Heiat M, Moosazadeh Moghaddam M, Gholipourmalekabadi M. Crosstalk between chitosan and cell signaling pathways. Cell Mol Life Sci. 2019;76:2697–2718. doi: 10.1007/s00018-019-03107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fornasari BE, Carta G, Gambarotta G, Raimondo S. Natural-based biomaterials for peripheral nerve injury repair. Front Bioeng Biotechnol. 2020;8:554257. doi: 10.3389/fbioe.2020.554257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration:gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 2011;8:110. doi: 10.1186/1742-2094-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.George EB, Glass JD, Griffin JW. Axotomy-induced axonal degeneration is mediated by calcium influx through ion-specific channels. J Neurosci. 1995;15:6445–6452. doi: 10.1523/JNEUROSCI.15-10-06445.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.George J, Hsu CC, Nguyen LTB, Ye H, Cui Z. Neural tissue engineering with structured hydrogels in CNS models and therapies. Biotechnol Adv. 2020;42:107370. doi: 10.1016/j.biotechadv.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Perez F, Cobianchi S, Geuna S, Barwig C, Freier T, Udina E, Navarro X. Tubulization with chitosan guides for the repair of long gap peripheral nerve injury in the rat. Microsurgery. 2015;35:300–308. doi: 10.1002/micr.22362. [DOI] [PubMed] [Google Scholar]
- 16.Gu X, Ding F, Williams DF. Neural tissue engineering options for peripheral nerve regeneration. Biomaterials. 2014;35:6143–6156. doi: 10.1016/j.biomaterials.2014.04.064. [DOI] [PubMed] [Google Scholar]
- 17.Guo W, Stoklund Dittlau K, Van Den Bosch L. Axonal transport defects and neurodegeneration:Molecular mechanisms and therapeutic implications. Semin Cell Dev Biol. 2020;99:133–150. doi: 10.1016/j.semcdb.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Haidar MK, Timur SS, Kazanci A, Turkoglu OF, Gürsoy RN, Nemutlu E, Sargon MF, Bodur E, Gök M, Ulubayram K, Öner L, Eroğlu H. Composite nanofibers incorporating alpha lipoic acid and atorvastatin provide neuroprotection after peripheral nerve injury in rats. Eur J Pharm Biopharm. 2020;153:1–13. doi: 10.1016/j.ejpb.2020.05.032. [DOI] [PubMed] [Google Scholar]
- 19.He B, Zhu Q, Chai Y, Ding X, Tang J, Gu L, Xiang J, Yang Y, Zhu J, Liu X. Safety and efficacy evaluation of a human acellular nerve graft as a digital nerve scaffold:a prospective, multicentre controlled clinical trial. J Tissue Eng Regen Med. 2015;9:286–295. doi: 10.1002/term.1707. [DOI] [PubMed] [Google Scholar]
- 20.Houshyar S, Bhattacharyya A, Shanks R. Peripheral nerve conduit:materials and structures. ACS Chem Neurosci. 2019;10:3349–3365. doi: 10.1021/acschemneuro.9b00203. [DOI] [PubMed] [Google Scholar]
- 21.Hsu CC, George JH, Waller S, Besnard C, Nagel DA, Hill EJ, Coleman MD, Korsunsky AM, Cui Z, Ye H. Increased connectivity of hiPSC-derived neural networks in multiphase granular hydrogel scaffolds. Bioact Mater. 2022;9:358–372. doi: 10.1016/j.bioactmat.2021.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu C, Uchida T, Tercero C, Ikeda S, Ooe K, Fukuda T, Arai F, Negoro M, Kwon G. Development of biodegradable scaffolds based on magnetically guided assembly of magnetic sugar particles. J Biotechnol. 2012;159:90–98. doi: 10.1016/j.jbiotec.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Huang Z, Kankowski S, Ertekin E, Almog M, Nevo Z, Rochkind S, Haastert-Talini K. Modified hyaluronic acid-laminin-hydrogel as luminal filler for clinically approved hollow nerve guides in a rat critical defect size model. Int J Mol Sci. 2021;22:6554. doi: 10.3390/ijms22126554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karagyaur M, Dyikanov D, Makarevich P, Semina E, Stambolsky D, Plekhanova O, Kalinina N, Tkachuk V. Non-viral transfer of BDNF and uPA stimulates peripheral nerve regeneration. Biomed Pharmacother. 2015;74:63–70. doi: 10.1016/j.biopha.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Katiyar KS, Struzyna LA, Morand JP, Burrell JC, Clements B, Laimo FA, Browne KD, Kohn J, Ali Z, Ledebur HC, Smith DH, Cullen DK. Tissue engineered axon tracts serve as living scaffolds to accelerate axonal regeneration and functional recovery following peripheral nerve injury in rats. Front Bioeng Biotechnol. 2020;8:492. doi: 10.3389/fbioe.2020.00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keane TJ, Badylak SF. The host response to allogeneic and xenogeneic biological scaffold materials. J Tissue Eng Regen Med. 2015;9:504–511. doi: 10.1002/term.1874. [DOI] [PubMed] [Google Scholar]
- 27.Lacko CS, Singh I, Wall MA, Garcia AR, Porvasnik SL, Rinaldi C, Schmidt CE. Magnetic particle templating of hydrogels:engineering naturally derived hydrogel scaffolds with 3D aligned microarchitecture for nerve repair. J Neural Eng. 2020;17:016057. doi: 10.1088/1741-2552/ab4a22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li BB, Yin YX, Yan QJ, Wang XY, Li SP. A novel bioactive nerve conduit for the repair of peripheral nerve injury. Neural Regen Res. 2016a;11:150–155. doi: 10.4103/1673-5374.175062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li BH, Yang K, Wang X. Biodegradable magnesium wire promotes regeneration of compressed sciatic nerves. Neural Regen Res. 2016b;11:2012–2017. doi: 10.4103/1673-5374.197146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li R, Xu J, Rao Z, Deng R, Xu Y, Qiu S, Long H, Zhu Q, Liu X, Bai Y, Quan D. Facilitate angiogenesis and neurogenesis by growth factors integrated decellularized matrix hydrogel. Tissue Eng Part A. 2021;27:771–787. doi: 10.1089/ten.TEA.2020.0227. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Yang Z, Zhang A. The effect of neurotrophin-3/chitosan carriers on the proliferation and differentiation of neural stem cells. Biomaterials. 2009;30:4978–4985. doi: 10.1016/j.biomaterials.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Yang W, Xie H, Wang J, Zhang L, Wang Z, Wang L. CNT/sericin conductive nerve guidance conduit promotes functional recovery of transected peripheral nerve injury in a rat model. ACS Appl Mater Interfaces. 2020;12:36860–36872. doi: 10.1021/acsami.0c08457. [DOI] [PubMed] [Google Scholar]
- 33.Lin C, Ekblad-Nordberg Å, Michaëlsson J, Götherström C, Hsu CC, Ye H, Johansson J, Rising A, Sundström E, Åkesson E. In vitro study of human immune responses to hyaluronic acid hydrogels, recombinant spidroins and human neural progenitor cells of relevance to spinal cord injury repair. Cells. 2021;10:1713. doi: 10.3390/cells10071713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.López-Cebral R, Silva-Correia J, Reis RL, Silva TH, Oliveira JM. Peripheral nerve injury:current challenges, conventional treatment approaches, and new trends in biomaterials-based regenerative strategies. ACS Biomater Sci Eng. 2017;3:3098–3122. doi: 10.1021/acsbiomaterials.7b00655. [DOI] [PubMed] [Google Scholar]
- 35.Luk HW, Noble LJ, Werb Z. Macrophages contribute to the maintenance of stable regenerating neurites following peripheral nerve injury. J Neurosci Res. 2003;73:644–658. doi: 10.1002/jnr.10701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magaz A, Faroni A, Gough JE, Reid AJ, Li X, Blaker JJ. Bioactive silk-based nerve guidance conduits for augmenting peripheral nerve repair. Adv Healthc Mater. 2018;7:e1800308. doi: 10.1002/adhm.201800308. [DOI] [PubMed] [Google Scholar]
- 37.Meder T, Prest T, Skillen C, Marchal L, Yupanqui VT, Soletti L, Gardner P, Cheetham J, Brown BN. Nerve-specific extracellular matrix hydrogel promotes functional regeneration following nerve gap injury. NPJ Regen Med. 2021;6:69. doi: 10.1038/s41536-021-00174-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Onode E, Uemura T, Takamatsu K, Yokoi T, Shintani K, Hama S, Miyashima Y, Okada M, Nakamura H. Bioabsorbable nerve conduits three-dimensionally coated with human induced pluripotent stem cell-derived neural stem/progenitor cells promote peripheral nerve regeneration in rats. Sci Rep. 2021;11:4204. doi: 10.1038/s41598-021-83385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, et al. The ARRIVE guidelines 2.0:Updated guidelines for reporting animal research. PLoS Biol. 2020;18:e3000410. doi: 10.1371/journal.pbio.3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qiu S, Rao Z, He F, Wang T, Xu Y, Du Z, Yao Z, Lin T, Yan L, Quan D, Zhu Q, Liu X. Decellularized nerve matrix hydrogel and glial-derived neurotrophic factor modifications assisted nerve repair with decellularized nerve matrix scaffolds. J Tissue Eng Regen Med. 2020;14:931–943. doi: 10.1002/term.3050. [DOI] [PubMed] [Google Scholar]
- 41.Rao F, Wang Y, Zhang D, Lu C, Cao Z, Sui J, Wu M, Zhang Y, Pi W, Wang B, Kou Y, Wang X, Zhang P, Jiang B. Aligned chitosan nanofiber hydrogel grafted with peptides mimicking bioactive brain-derived neurotrophic factor and vascular endothelial growth factor repair long-distance sciatic nerve defects in rats. Theranostics. 2020;10:1590–1603. doi: 10.7150/thno.36272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schiaveto de Souza A, da Silva CA, Del Bel EA. Methodological evaluation to analyze functional recovery after sciatic nerve injury. J Neurotrauma. 2004;21:627–635. doi: 10.1089/089771504774129955. [DOI] [PubMed] [Google Scholar]
- 43.Schlaepfer WW, Micko S. Chemical and structural changes of neurofilaments in transected rat sciatic nerve. J Cell Biol. 1978;78:369–378. doi: 10.1083/jcb.78.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sherman LS, Matsumoto S, Su W, Srivastava T, Back SA. Hyaluronan synthesis, catabolism, and signaling in neurodegenerative diseases. Int J Cell Biol. 20152015:368584. doi: 10.1155/2015/368584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tao J, Zhang J, Du T, Xu X, Deng X, Chen S, Liu J, Chen Y, Liu X, Xiong M, Luo Y, Cheng H, Mao J, Cardon L, Gou M, Wei Y. Rapid 3D printing of functional nanoparticle-enhanced conduits for effective nerve repair. Acta Biomater. 2019;90:49–59. doi: 10.1016/j.actbio.2019.03.047. [DOI] [PubMed] [Google Scholar]
- 46.Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 47.Vijayavenkataraman S. Nerve guide conduits for peripheral nerve injury repair:a review on design, materials and fabrication methods. Acta Biomater. 2020;106:54–69. doi: 10.1016/j.actbio.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 48.Wang XL, Yang CY, Li XG, Zhang AF. Experimental study on sciatic nerve injury repairing by artificial nerve graft in rats. Zhongguo Kangfu Lilun yu Shijian. 2012;18:535–538. [Google Scholar]
- 49.Xie J, MacEwan MR, Liu W, Jesuraj N, Li X, Hunter D, Xia Y. Nerve guidance conduits based on double-layered scaffolds of electrospun nanofibers for repairing the peripheral nervous system. ACS Appl Mater Interfaces. 2014;6:9472–9480. doi: 10.1021/am5018557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yan Y, Yao R, Zhao J, Chen K, Duan L, Wang T, Zhang S, Guan J, Zheng Z, Wang X, Liu Z, Li Y, Li G. Implantable nerve guidance conduits:Material combinations, multi-functional strategies and advanced engineering innovations. Bioact Mater. 2022;11:57–76. doi: 10.1016/j.bioactmat.2021.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang S, Wang C, Zhu J, Lu C, Li H, Chen F, Lu J, Zhang Z, Yan X, Zhao H, Sun X, Zhao L, Liang J, Wang Y, Peng J, Wang X. Self-assembling peptide hydrogels functionalized with LN- and BDNF- mimicking epitopes synergistically enhance peripheral nerve regeneration. Theranostics. 2020;10:8227–8249. doi: 10.7150/thno.44276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yi S, Xu L, Gu X. Scaffolds for peripheral nerve repair and reconstruction. Exp Neurol. 2019;319:112761. doi: 10.1016/j.expneurol.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 53.Zaminy A, Sayad-Fathi S, Kasmaie FM, Jahromi Z, Zendedel A. Decellularized peripheral nerve grafts by a modified protocol for repair of rat sciatic nerve injury. Neural Regen Res. 2021;16:1086–1092. doi: 10.4103/1673-5374.300449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeng W, Rong M, Hu X, Xiao W, Qi F, Huang J, Luo Z. Incorporation of chitosan microspheres into collagen-chitosan scaffolds for the controlled release of nerve growth factor. PLoS One. 2014;9:e101300. doi: 10.1371/journal.pone.0101300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang AF, Ou XC, Yang CY, Liu YJ, Lu Q, Li XG. Repair of sciatic nerve gap of rats with chitosan tube combined with basic fibroblast growth factor. Zhongguo Linchuang Lilun yu Shijian. 2008;14:1133–1135. [Google Scholar]
- 56.Zheng F, Li R, He Q, Koral K, Tao J, Fan L, Xiang R, Ma J, Wang N, Yin Y, Huang Z, Xu P, Xu H. The electrostimulation and scar inhibition effect of chitosan/oxidized hydroxyethyl cellulose/reduced graphene oxide/asiaticoside liposome based hydrogel on peripheral nerve regeneration in vitro. Mater Sci Eng C Mater Biol Appl. 2020;109:110560. doi: 10.1016/j.msec.2019.110560. [DOI] [PubMed] [Google Scholar]
- 57.Zheng M, Guo J, Li Q, Yang J, Han Y, Yang H, Yu M, Zhong L, Lu D, Li L, Sun L. Syntheses and characterization of anti-thrombotic and anti-oxidative Gastrodin-modified polyurethane for vascular tissue engineering. Bioact Mater. 2021;6:404–419. doi: 10.1016/j.bioactmat.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The CMAP recordings in the normal animals.
(A) The CMAP waveforms of the normal animals. (B) Histograms of the peak amplitude of CMAP in the normal animals. Data are shown as the mean ± SD (n = 3). CMAP: Compound muscle action potential.
Immunofluorescence histochemical staining of no-primary antibody and normal sciatic nerve.
(A1-3) No positive results were observed in immunofluorescence histochemical staining of no-primary antibodies. (B1-3) Immunofluorescence histochemical staining of normal sciatic nerve showed the positively labeled axons and myelin sheaths. Scale bars: 50 μm. GAP43: Growth associated protein 43.


