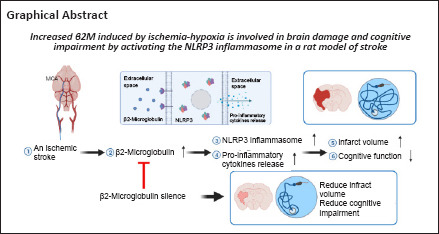
Key Words: cognitive impairment, cognitive improvement, glial activation, infarct volume, ischemia, middle cerebral artery occlusion, neuroinflammation, NLRP3 inflammasome, stroke, β2 microglobulin
Abstract
β2-Microglobulin (β2M), a component of the major histocompatibility complex class I molecule, is associated with aging-related cognitive impairment and Alzheimer’s disease. Although upregulation of β2M is considered to be highly related to ischemic stroke, the specific role and underlying mechanistic action of β2M are poorly understood. In this study, we established a rat model of focal cerebral ischemia by occlusion of the middle cerebral artery. We found that β2M levels in the cerebral spinal fluid, serum, and brain tissue were significantly increased in the acute period but gradually decreased during the recovery period. RNA interference was used to inhibit β2M expression in the acute period of cerebral stroke. Tissue staining with 2,3,5-triphenyltetrazolium chloride and evaluation of cognitive function using the Morris water maze test demonstrated that decreased β2M expression in the ischemic penumbra reduced infarct volume and alleviated cognitive deficits, respectively. Notably, glial cell, caspase-1 (p20), and Nod-like receptor pyrin domain containing 3 (NLRP3) inflammasome activation as well as production of the inflammatory cytokines interleukin-1β, interleukin-6, and tumor necrosis factor-α were also effectively inhibited by β2M silencing. These findings suggest that β2M participates in brain injury and cognitive impairment in a rat model of ischemic stroke through activation of neuroinflammation associated with the NLRP3 inflammasome.
Introduction
Stroke is known to cause approximately 5.5 million deaths per year worldwide with more than 80 million stroke survivors suffering from cognitive and/or physical disabilities to varying degrees (Chamorro et al., 2021; Duncan et al., 2021). For managing stroke survivors, strategies focus on reducing disability by rapid reperfusion with intravenous thrombolytic agents or endovascular thrombectomy. With the expanded time window for intravenous thrombolysis from 4.5 hours up to 9 hours (Ma et al., 2012), approximately 15% of patients with stroke may be suitable for blood clot removal (Chia et al., 2016). Confronted with the challenges of stroke treatment, the underlying pathophysiology and mechanisms leading to ischemic insult have been illuminated comprehensively. For example, excitotoxicity, inflammation, glial activation, oxidative stress, and free radical or pro-inflammatory cytokine-mediated cytotoxicity have been reported to be involved in stroke pathology (Kuriakose and Xiao, 2020).
Recent studies have shown that β2-microglobulin (β2M), an approximately 12 kDa protein, affects multiple pathogenic conditions, such as kidney disease, some forms of cancer, and Alzheimer’s disease (Xie and Yi, 2003; Luton et al., 2013; Prizment et al., 2016). As a subunit of the major histocompatibility complex (MHC) class I molecule, β2M separates from MHC class I and regulates brain development, synaptic plasticity, and neurobehavior without independence from the canonical immune function (Huh et al., 2000; Boulanger and Shatz, 2004; Lee et al., 2014). Notably, soluble β2M in the cerebral spinal fluid (CSF) of patients with Alzheimer’s disease was higher than that in healthy controls (Carrette et al., 2003), and β2M may be implicated in cognitive impairment (Corlin et al., 2005). Moreover, accumulation of β2M in the serum has adverse effects on cognitive function and hippocampal neurogenesis in mice (Smith et al., 2015). A previous study performed correlation analyses of serum β2M and major adverse cardiovascular events and showed that β2M from patients with prevalent asymptomatic carotid atherosclerosis was independently and significantly associated with major adverse cardiovascular events (Amighi et al., 2011). Additionally, serum β2M levels were increased in patients with acute ischemic stroke (Qun et al., 2019) and positively associated with a poor outcome at 3 months (Hu et al., 2020). Numerous studies have shown that the pathology of ischemic stroke is accompanied by neuroinflammation controlled by chemokines, cytokines, and reactive oxygen species (Maida et al., 2020). Although these clinical data demonstrated that β2M is an important marker of inflammation and is highly associated with stroke patients, the role of β2M in ischemic stroke and its mechanism remain unknown. In this study, we established a rat model of stroke by middle cerebral artery occlusion (MCAO) and investigated the role of β2M in Nod-like receptor pyrin domain containing 3 (NLRP3) inflammation activation and cognitive impairment in model rats.
Methods
Animals and stroke model
This work was approved by the Animal Research Ethics Committee of Xuzhou Medical University, China (approval No. 201902W017) on June 12, 2019. All experiments were designed and reported according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines (Percie du Sert et al., 2020). To exclude estrogen interference (Zhang et al., 2018b; Guo et al., 2020; Lu et al., 2020), 12-week-old male Sprague-Dawley rats (specific pathogen-free level, 220–250 g) from the Experimental Animal Center of Xuzhou Medical University (license No. SYXK [Su] 2005-0018) were used. The rats were fed a normal diet and allowed to adapt to the environment for 1 week using a 12-hour light/dark cycle at 24°C. Then, they were randomly divided into sham, MCAO, β2M shRNA lentivirus-treated MCAO (β2M RNAi), and scramble control lentivirus-treated (SC) groups. The number of rats in each experiment is listed in Additional Table 1. Rats were anesthetized with 2.5% isoflurane (Shandong Keyuan Pharmaceutical Co.; Jinan, China) (in oxygen) inhalation during the study.
Additional Table 1.
The number of animals or samples in every experiment
| Experimental methods | Number of animals/samples each group (n) |
|---|---|
| Morris water maze | 12 |
| HE staining | 4 |
| TTC staining | 6-8 |
| Immunofluorescent staining | 4 |
| Western blot | 4 |
| ELISA | 9 |
ELISA: Enzyme-linked immunosorbent assay; HE: hematoxylin-eosin; TTC: triphenyl-2,3,5-tetrazoliumchloride.
The MCAO model was established by the intraluminal filament technique as previously described (Yao et al., 2009). The right external carotid artery was separated, a 19 mm filament (0.26 mm diameter; Guangzhou Jialing Biotechnology Limited; Guangzhou Province, China) was inserted into the right internal carotid artery via the external carotid artery stump, and the wound was sutured. For reperfusion, the filament was removed after 90 minutes of occlusion. The modified neurological severity scores (mNSS) test was used to evaluate neurological deficits at 24 hours after MCAO. The mNSS test includes motor, sensory, balance, and reflex tests. The total score is graded from 0 (normal) to 14 (maximum), and a higher score represents more severe injury (Li et al., 2000). In this study, rats with MCAO that showed no or incomplete forelimb placing with rotational asymmetry were included in this study. The filament was not inserted into the artery of sham rats. A flow chart of this study design is shown in Figure 1.
Figure 1.
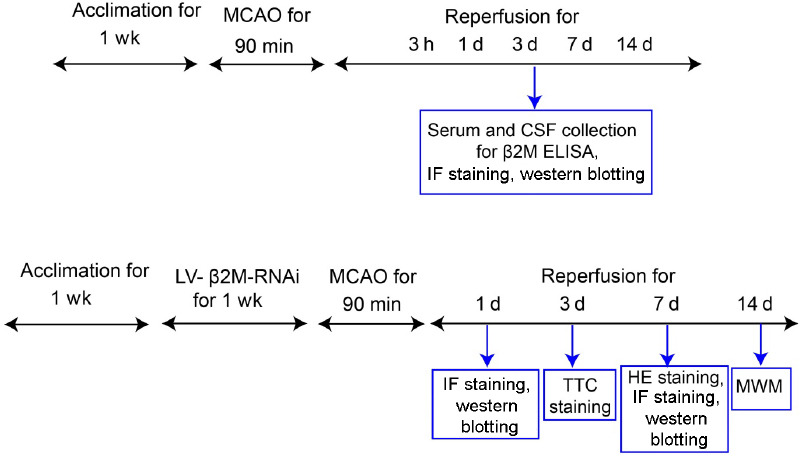
Flow chart of the study design.
CSF: Cerebral spinal fluid; ELISA: enzyme-linked immunosorbent assay; HE: hematoxylin-eosin; IF: immunofluorescent staining; LV: lentivirus; MCAO: middle cerebral artery occlusion; MWM: Morris water maze; RNAi: RNA interference; TTC: triphenyl-2,3,5-tetrazoliumchloride; β2M: β2 microglobulin.
Lentiviral production and stereotaxic surgery
The shRNA target sequence 5'-ACC GAT GTA TAT GCT TGC A-3' for rat β2M and the control sequence 5'-TTC TCC GAA CGT GTC ACG T-3' were amplified and inserted into GV118 vectors to form β2M or SC recombinant plasmids. The recombinant and lentiviral helper plasmids were used to co-transfect HEK293 T cells for 8 hours, the medium was removed, and fresh medium was added. Cells were cultured for another 36 hours, and the lentiviruses were harvested, filtered, and concentrated. The final titer of the LV-β2M-RNAi was 3 × 108 infectious units/mL. Lentiviral production was conducted by Shanghai Genechem Co. (Shanghai, China). For stereotactic injection of lentivirus into the brain, rats were anesthetized, positioned, and a small hole was created in the skull. A total of 1 μL lentivirus (0.2 μL/minute) was injected into the brain site relative to the bregma (anterior-posterior, +2.5 mm; medial-lateral, ±1.7 mm; dorsal-ventral, –2.5 mm) using a Hamilton syringe needle (33 gauge; Hamilton; Bonaduz, Switzerland). The needle was removed after 10 minutes.
Morris water maze test
As previously described (Fan et al., 2017), the rats were subjected to the Morris water maze test at 4 weeks after surgery to assess cognitive function. Briefly, each rat was trained to find the submerged escape platform each day for 4 days. On day 5, the rats swam in the water tank without the platform for 1 minute. The escape latency, swimming speed, and the number of crossings over the previous platform area were captured on day 5 using a video system (RWD Life Science; Shenzhen, China).
Hematoxylin-eosin staining
At 7 days after MCAO, rats were perfused, and brain samples were collected, paraffin-embedded, and sectioned using a microtome (Leica Microsystems; Nussloch, Baden-Wurttemberg, Germany). To observe pathological changes, 5-μm coronal sections were stained with hematoxylin-eosin in accordance with the manufacturer’s instructions (Beyotime Biotechnology Inc.; Shanghai, China). Briefly, the samples were dewaxed with xylene, washed using a 100%, 95%, 80%, and 75% ethanol series, then stained with hematoxylin for approximately 10 minutes. After washing, the samples were immersed in hydrochloric acid/ethanol for 30 seconds and then washed with tap water. After staining with eosin solution for 2 minutes, the sections were washed and dehydrated with graded ethanol and sealed with neutral resin.
Immunofluorescent staining
As previously reported (Fan et al., 2017), rats were transcardially perfused with 0.9% NaCl solution at 3 hours, and 1, 3, 7, or 14 days after MCAO. After post-fixing in 4% paraformaldehyde for several hours, the brains were placed in 15% sucrose in phosphate buffer overnight and then in 30% sucrose until water was replaced completely. Serial cryosections (20 μm) were prepared on poly-lysine-coated slides for immunofluorescent staining. For western blots, the ischemic penumbra of each brain was separated, weighed, and then homogenized in cold lysis buffer with phenylmethylsulfonyl fluoride solution and protease and phosphatase inhibitors [1:3 (w:v)]. After sonicating, the homogenates were centrifuged, and the supernatants were aliquoted and maintained in an ultra-cold storage freezer (ThermoFisher Scientific; Waltham, MA, USA).
After blocking in 3% normal donkey serum (Abbkine Scientific Co., Ltd., Wuhan, China) for 1 hour at room temperature, cryosections were incubated with one of the following primary antibodies overnight at 4°C: anti-β2M (mouse; 1:100; Santa Cruz Biotechnology, Dallas, TX, USA, Cat# sc-32241, RRID: AB_626750), anti-glial fibrillary acidic protein (GFAP; marker for astrocytes; rabbit; 1:500; Proteintech, Cat# 16825-1-AP, RRID: AB_2109646; Chicago, IL, USA), anti-NeuN (marker for neurons; rabbit; Cell Signal Technology, Boston, MA, USA, Cat# 12943S, RRID: AB_2630395), and anti-ionized calcium-binding adaptor molecule 1 (IBA-1; marker for microglia; rabbit; FUJIFILM Wako Shibayagi, Osaka, Japan, Cat# 016-20001, RRID: AB_839506). The sections were washed several times and incubated with the IFKine™ Green donkey anti-mouse IgG (H+L) (1:200; Abbkine, Cat# A24211, RRID: AB_2904244) or IFKine™ red donkey anti-rabbit IgG (H+L) (1:200; Abbkine, Cat# A24421, RRID: AB_2904243) for 2 hours at room temperature. The sections were washed and incubated with 4′,6-diamidino-2-phenylindole (Beyotime Biotechnology Inc.) to label the nuclei. Images were captured using a Zeiss Axioskop 40 microscope (Carl Zeiss, Oberkochen, Germany). The numbers of positive cells were quantified using Image-Pro Plus software.
Western blotting analysis
For protein analysis, brain tissue protein was extracted using tissue lysis buffer supplemented with proteinase inhibitors. After the protein concentration was determined, the lysates were separated on 10% or 15% polyacrylamide gels by electrophoresis. After electrophoresis, the blotting membrane (0.45 μm nitrocellulose) was trimmed to fit the size of the gel. For electroblotting, the membrane, filter papers, and blotting pads were soaked in transfer buffer. The current strength was set at 350 mA and time for western blotting was approximately 70–120 minutes according to the protein molecular weight. After transferring proteins to the membranes, the membranes were incubated with one of the following antibodies for at least 12 hours at 4°C: anti-β2M (described above), anti-NLRP3 (rabbit; 1:1000; Abcam, Cat# ab263899, RRID: AB_2889890), anti-apoptosis-associated speck-like protein containing a CARD (ASC; mouse; 1:500; Santa Cruz Biotechnology, Cat# sc-514414, RRID: AB_2737351), anti-interleukin-6 (IL-6; mouse; 1:2000; Santa Cruz Biotechnology, Cat# sc-57315, RRID: AB_2127596), anti-IBA-1 (described above), anti-tumor necrosis factor alpha (TNFα; mouse; 1:500; Santa Cruz Biotechnology Cat# sc-28318, RRID: AB_628370), anti-caspase-1(p20) (mouse; 1:500; Santa Cruz Biotechnology, Cat# sc-398715, RRID: AB_2819181), anti-GFAP (described above), anti-interleukin-1β (IL-1β; rabbit; 1:1000; Cell Signal Technology, Cat# 83186, RRID: AB_2800010), and anti-β-actin (mouse; 1:1000; Santa Cruz Biotechnology, Cat# sc-8432, RRID: AB_626630). After washing thoroughly, the membranes were incubated with horseradish peroxidase-conjugated anti-rabbit (1:1000; Santa Cruz Biotechnology, Cat# sc-2313, RRID: AB_641181) or horseradish peroxidase-conjugated anti-mouse secondary antibodies (1:1000; Santa Cruz Biotechnology, Cat# sc-2314, RRID: AB_641170) at room temperature for 2 hours. The protein bands were scanned using the Odyssey infrared imager (LI-COR, Lincoln, NE, USA) and quantified using the optical density values.
Enzyme-linked immunosorbent assay
At 3 hours, and 1, 3, 7, and 14 days after MCAO, venous blood was collected from the rats into serum tubes from the angular vein using a blood collection needle (Roche; Basel, Switzerland). The blood was coagulated for 30 minutes at 25°C. After centrifugation, the serum was collected and stored at –70°C. CSF was collected according to a minimally-invasive method (Simats et al., 2018). Briefly, the rats were anesthetized and properly positioned on a stereotaxic apparatus (RWD Life Science). A needle (25 gauge), which was connected to a collection tube, was vertically and slowly inserted into the cisterna magna. To avoid blood contamination, the tube was clipped when the collection was almost finished, and the needle was withdrawn slowly. β2M concentrations in sera and CSF were measured by an enzyme-linked immunosorbent assay kit (Dakewe Biotech Co., Ltd., Wuhan, China) following the manufacturer’s protocol.
2,3,5-Triphenyl tetrazolium chloride staining
Rat brain slices were stained with 2,3,5-triphenyl tetrazolium chloride in accordance with the manufacturer’s protocol. At 3 days after MCAO, the brains were collected and serial slices (2 mm thick) were prepared. The slices were stained in 2% triphenyl-2,3,5-tetrazolium chloride solution (Sigma-Aldrich, St. Louis, MO, USA) for 30 minutes and washed completely with double-distilled water. The infarct areas were photographed and measured using ImageJ software (National Institutes of Health; Bethesda, MD, USA) (Schneider et al., 2012).
Statistical analysis
According to pilot studies and a previous research study (Yao et al., 2009), we chose a proper hypothesis and simplified the studies by using numeric results for testing two proportions and the Z-test with unpooled variance for sample size calculation. Statistical analyses were performed with GraphPad Prism version 8.0.0 for Windows (GraphPad Software, San Diego, CA, USA, www.graphpad.com) by observers blinded to the experimental design. Two-way analysis of variance was used when the subjects are subjected to repeated measures, and one-way analysis of variance followed by Tukey’s honestly significant difference post hoc test was used to test for more independent groups. Data are presented as means ± standard error of the mean (SEM). P-values < 0.05 were considered statistically significant.
Results
The β2M levels are increased in the serum, CSF, and brain tissue in the rat stroke model
We performed enzyme-linked immunosorbent assays, immunofluorescent staining, and western blotting to determine whether ischemic-hypoxic brain injury resulted in changes in β2M levels in sera, CSF, and brain tissue at 3 hours and 1, 3, 7, and 14 days post-MCAO. CSF (P < 0.0001 at 3 hours, P = 0.024 at 1 day) and serum β2M (P < 0.0001 at 3 hours, P = 0.016 at 1 day, P = 0.010 at 3 days, P = 0.0071 at 7 days) levels were significantly elevated in the model rats, peaked at 3 hours after MCAO, and gradually returned to normal levels at 3 and 14 days, respectively (Figure 2A and B). In addition, the protein expression of β2M (P = 0.02 at 3 hours, P < 0.0001 at 1 day, P = 0.0179 at 3 days; Figure 2C and D) and β2M-positive cell numbers (P < 0.0001 at 3 hours, P < 0.0001 at 1 day, P < 0.0001 at 3 days, P < 0.0001 at 7 days, P = 0.0035 at 14 days; Figure 2E and F) were also markedly increased in the ischemic penumbra. As shown in Figure 2G, β2M was mainly expressed in NeuN-positive neurons. Our results suggest that ischemic stroke leads to an enhancement of β2M expression in rats.
Figure 2.
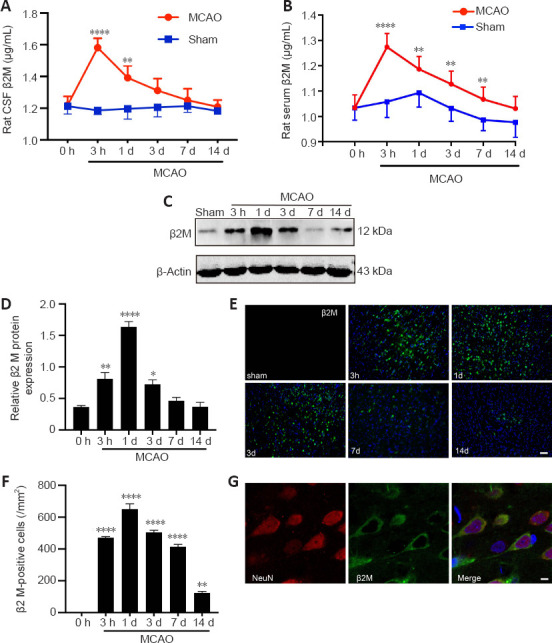
Cerebral stroke leads to an increase in β2M in rats.
(A, B) β2M in the CSF (A) and serum (B) was detected using ELISA. (C, D) Protein levels of β2M in the ischemic penumbra were quantified. β2M was significantly increased after ischemia. (E, F) Immunofluorescent photomicrographs of β2M (IFKine™ green) and quantitative analysis showed that the number of β2M-positive cells in the ischemic penumbra was also increased in ischemic rats. Data are presented as means ± SEM (n = 4). *P < 0.05, **P < 0.01, ****P < 0.0001, vs. 0-hour group (two-way analysis of variance followed by Bonferroni’s multiple comparisons test for ELISA, one-way analysis of variance followed by Tukey’s honestly significant difference post hoc test for the other data). (G) Immunofluorescent photomicrographs show β2M (IFKine™ green) and NeuN (IFKine™ red) double-labeled cells. Scale bars: 50 μm in E, 2 μm in G. CSF: Cerebral spinal fluid; ELISA: enzyme-linked immunosorbent assay; β2M: β2 microglobulin.
Lentiviral-mediated RNA interference silences β2M expression effectively in the rat stroke model
To explore the role of β2M in neurological function and neuroinflammation of MCAO rats, RNA interference was used to silence the β2M gene. Both the number of β2M-positive cells (P = 0.0011; Figure 3A and B) and β2M protein levels (P = 0.0002; Figure 3C) were decreased in the ischemic penumbra of the MCAO rats after β2M shRNA treatment compared with those in the SC shRNA-treated rats.
Figure 3.
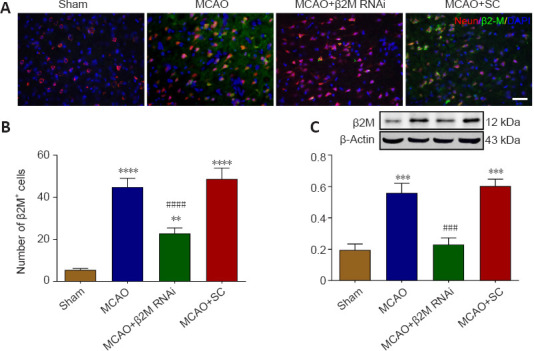
β2M is silenced by lentivirus-mediated RNA interference.
(A, B) β2M (IFKine™ green) and NeuN (IFKine™ red) immunofluorescent staining (A) and quantitative analysis (B) of β2M-positive cells. The number of β2M-positive cells was markedly decreased in the MCAO rats after β2M knockdown. Scale bar in A: 50 μm. (C) β2M protein expression levels using western blot analysis. Data are presented as means ± SEM (n = 4). **P < 0.01, ***P < 0.001, ****P < 0.0001, vs. sham group; ###P < 0.001, ####P < 0.0001, vs. MCAO + SC group (one-way analysis of variance followed by Tukey’s honestly significant difference post hoc test). MCAO: Middle cerebral artery occlusion; RNAi: RNA interference; SC: scramble control; β2M: β2 microglobulin.
β2M knockdown decreases the infarct volume and improved cognitive deficits in the rat stroke model
Cerebral infarct volume of the MCAO rats was decreased by β2M knockdown as observed with triphenyl-2,3,5-tetrazolium chloride staining (P = 0.033, Figure 4). During the 4-day training portion of the Morris water maze test, MCAO rats showed longer escape latency (P < 0.0001 on day 3 and P = 0.0001 on day 4), and β2M knockdown significantly decreased the latency period compared with that in the SC group on day 4 (P = 0.0399; Figure 5A and B). On day 5, the swimming time for MCAO rats in the target quadrant was less than that of sham rats (P = 0.0005), whereas β2M knockdown increased the swimming time compared with that for the SC lentivirus-treated rats (P = 0.0445; Figure 5C). Moreover, β2M knockdown significantly increased the number of crossings over the original platform location (P = 0.0462; Figure 5D) without affecting the swimming speed (Figure 5E). These results indicated that inhibition of β2M expression improved the cognitive function of MCAO rats.
Figure 4.
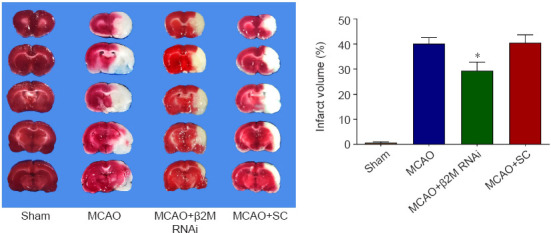
β2M knockdown decreases the infarct volume in rats after MCAO.
Cerebral infarct volume at 72 hours after MCAO was measured by TTC staining. β2M inhibition decreased the infarct volume compared with that in the SC-treated rats. Data are presented as means ± SEM (n = 6–8). *P < 0.05, vs. MCAO + SC group (one-way analysis of variance followed by Tukey’s honestly significant difference post hoc test). MCAO: Middle cerebral artery occlusion; RNAi: RNA interference; SC: scramble control; TTC: triphenyl-2,3,5-tetrazoliumchloride; β2M: β2 microglobulin.
Figure 5.
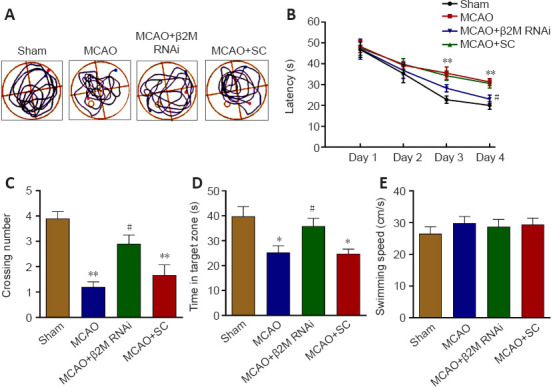
β2M knockdown alleviates the cognitive deficits in MCAO rats.
The MWM test was used to examine cognitive function. (A) Images of swimming paths from the starting location to the hidden platform. (B) The latency was decreased in rats after β2M knockdown in the training test. (C–E) Both the number of crossings and the time spent in the target quadrant were increased in the rats after β2M knockdown. However, the swimming speed was not altered among the groups on day 5. Data are presented as means ± SEM (n = 12). *P < 0.05, **P < 0.01, vs. sham group; #P < 0.05, vs. MCAO + SC group (two-way analysis of variance followed by Bonferroni’s multiple comparisons test). MCAO: Middle cerebral artery occlusion; MWM: Morris water maze; RNAi: RNA interference; SC: scramble control; β2M: β2 microglobulin.
Knockdown of β2M inhibits glial activation induced by ischemia-hypoxia
Representative images of hematoxylin-eosin staining in Figure 6A show normal morphology of neuronal cells in the cerebral cortex of sham rats. However, cell loss, cells showing nuclear condensation with dark staining, and apparent inflammatory cell infiltration were detected in the lateral ischemic brains of MCAO rats. β2M knockdown alleviated hypoxia-ischemia–induced cell death and inflammatory cell infiltration. Glial cell activation was detected by GFAP and IBA-1 immunofluorescent staining (Figure 6B and C). Compared with those in the sham group, ischemia increased the number of GFAP+ (all P < 0.0001) and IBA-1+ (MCAO vs. sham, P < 0.0001; MCAO+ β2M RNAi vs. sham, P = 0.0048; MCAO+ SC vs. sham, P < 0.0001) cells. However, inhibition of β2M markedly decreased the numbers of astrocytes (P < 0.0001) and microglial cells (P = 0.0323; Figure 6D and E). Furthermore, the protein levels of GFAP (P = 0.0006) and IBA-1 (P = 0.0088) increased after MCAO compared with those in the sham group. However, β2M knockdown decreased the levels of GFAP (P = 0.0073) and IBA-1 (P = 0.0164) compared with those in the SC group (Figure 6F and G).
Figure 6.
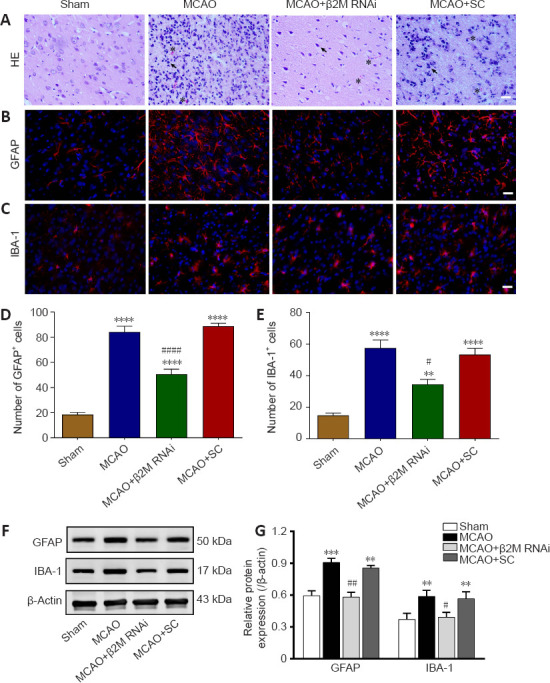
β2M knockdown inhibits glial activation after cerebral ischemia.
(A) Images of HE stained ischemic penumbra slices with apparent cell nuclear condensation (arrowheads) and inflammatory cell infiltration (asterisks) from rats after MCAO. β2M inhibition alleviated inflammatory cell infiltration. Scale bars: 100 μm. (B, C) Images of (B) GFAP (IFKine™ red) and (C) IBA-1 (IFKine™ red) immunofluorescent staining of the ischemic penumbra. Knockdown of β2M decreased the numbers of GFAP- and IBA-1-positive cells. Scale bars: 50 μm. (D, E) Quantitative analysis of the number of (D) GFAP- and (E) IBA-1-positive cells. (F, G) GFAP and IBA-1 protein expression and quantification by western blot analysis. Data are presented as means ± SEM (n = 4). **P < 0.01, ***P < 0.001, ****P < 0.0001, vs. sham group; #P < 0.05, ##P < 0.01, vs. MCAO + SC group (one-way analysis of variance followed by Tukey’s honestly significant difference post hoc test). GFAP: Glial fibrillary acidic protein; HE; hematoxylin-eosin; IBA-1: ionized calcium-binding adaptor molecule 1; MCAO: middle cerebral artery occlusion; RNAi: RNA interference; SC: scramble control; β2M: β2 microglobulin.
Inhibition of β2M alleviates NLRP3 inflammasome activation and neuroinflammation in the rat stroke model
Western blot analysis showed that protein levels of NLRP3 and ASC (an NLRP3 inflammasome constituent) were notably increased (P < 0.0001 for NLRP3; P < 0.0001 for ASC) in ischemic cerebral cortexes of the MCAO model rats compared with those of sham rats (Figure 7A and B). In addition, caspase-1 (p20) (P = 0.0004), IL-1β (P = 0.0003), IL-6 (P < 0.0001), and TNFα (P = 0.0021) were significantly upregulated in the MCAO rats, indicating that ischemia-hypoxia induced NLRP3 inflammasome activation in the brain. However, the NLRP3 (P = 0.0003), ASC (P = 0.001), caspase-1 (p20) (P = 0.0047), IL-1β (P = 0.0014), IL-6 (P < 0.0001), and TNFα (P = 0.0004) levels were downregulated by β2M knockdown compared with those in the MCAO rat brains. SC lentivirus treatment did not reverse the outcomes of the model rats (Figure 7A–C).
Figure 7.
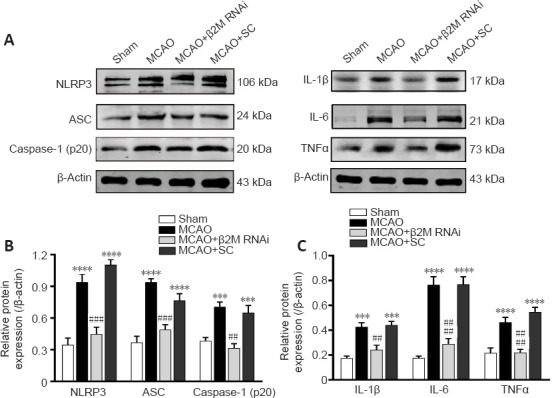
Silence of β2M reduces NLRP3 inflammasome activation and pro-inflammatory cytokine production in ischemic cerebral cortexes.
(A) NLRP3, ASC, caspase-1 (p20), IL-1β, IL-6 and TNFα proteins were detected using western blotting. (B, C) Quantitative results of protein levels normalized to β-actin. Data are presented as means ± SEM (n = 4). ***P < 0.01, ****P < 0.001, vs. sham group; ##P < 0.01, ###P < 0.001, ####P < 0.0001, vs. MCAO + SC group (one-way analysis of variance followed by Tukey’s honestly significant difference post hoc test). ASC: Apoptosis-associated speck-like protein containing a CARD; IL-1β: interleukin-1β; IL-6: interleukin-6; MCAO: middle cerebral artery occlusion; NLRP3: Nod-like receptor pyrin domain containing 3; RNAi: RNA interference; SC: scramble control; TNFa: tumor necrosis factor alpha; β2M: β2 microglobulin.
Discussion
Studies have indicated that neuroinflammation plays a significant role in cerebral ischemia and may cause neural damage by promoting cell death or induce beneficial stimulation of remedial action. Here, we investigated whether β2M is involved in the pathophysiology of ischemic stroke by activating the NLRP3 inflammasome and pro-inflammatory cytokine production. Our results demonstrated that ischemia-hypoxia caused a significant increase in β2M and NLRP3 inflammasome activation; β2M knockdown inhibited NLRP3 inflammasome activation and inflammatory reactions. Furthermore, silencing β2M resulted in smaller infarct size and improved cognitive function. Our study established the detrimental effects of β2M in ischemic stroke.
Accumulating evidence suggests that β2M, a light chain of approximately 100 amino acids that is co-expressed with MHC class I molecules (Zijlstra et al., 1990), is closely related to neurological disorders. Serum β2M was significantly increased in schizophrenia patients compared with healthy controls, and the level of β2M was positively correlated with the total psychopathology score (Chittiprol et al., 2009). In addition, soluble β2M in the CSF was elevated in patients with dementia (McArthur et al., 1992), Parkinson’s disease (Carrette et al., 2003), and Alzheimer’s disease (Carrette et al., 2003; Zhang et al., 2008). Moreover, high levels of β2M resulted in cognitive function impairment and neurogenesis inhibition (Smith et al., 2015) and directly induced depressive-like behaviors in rats (Zhang et al., 2018a). In recent years, clinical studies have demonstrated that increased serum β2M is highly associated with ischemic stroke (Rist et al., 2017; Hu et al., 2019; Qun et al., 2019). Using a cerebral ischemia model, we revealed a dramatically time-dependent increase in the levels of β2M in serum, CSF, and brain tissue of rats. β2M was secreted by most nucleated cells, especially inflammatory cells. Ischemic stroke is accompanied by destruction of the blood-brain barrier. We infer that β2M is mainly secreted by inflammatory cells, which leads to abnormally high levels of serum and CSF β2M in the acute stage of ischemia. However, in the brain parenchyma, β2M transcription, translation, and secretion were delayed in neuronal cells. Thus, the level of β2M expression peaked on day 1 in the brain but peaked at 3 hours in the CSF and serum after MCAO. These observations suggest that β2M abnormalities may be potentially associated with the pathogenesis of ischemic stroke.
Previous studies provided evidence that the β2M level is negatively associated with cognitive performance (Smith et al., 2015; Gao et al., 2018; Chen et al., 2019). To address the role of β2M in cognitive impairment induced by ischemia-hypoxia, we reduced the level of β2M using shRNA cloned into a lentivirus vector. The Morris water maze test confirmed the detrimental effects of β2M on learning and memory function. Moreover, we observed that β2M knockdown reduced the infarct volume of MCAO rats, suggesting that increased β2M after MCAO has a detrimental effect on cognition and brain function. However, the mechanisms of β2M involvement in cognitive impairment remain unclear.
More recent studies have demonstrated that the NLRP3 inflammasome is a critical mediator of inflammatory responses after stroke. Inhibiting NLRP3 considerably prevented a cascade of inflammatory reactions and attenuated neuronal damage after ischemia/ reperfusion injury in animal models (Deroide et al., 2013; Yang et al., 2014; Han et al., 2020), suggesting that the NLRP3 inflammasome is a potential therapeutic intervention target for ischemic stroke (Feng et al., 2020). It has been reported that β2M is a marker of low grade inflammation (Raikou and Kyriaki, 2015; Wu et al., 2017). Therefore, we determined whether β2M was associated with a corresponding change in inflammation within the brain. As expected, β2M silencing reversed NLRP3 inflammasome activation, pro-inflammatory cytokines production, and glial activation, suggesting that β2M is involved in the neuroinflammatory reaction after stroke.
Pro-inflammatory cytokines promote the progression of harmful neuroinflammation after cerebral ischemia. As one of the first cytokines to be produced, TNFα increases in the cerebral parenchyma following MCAO (Liu et al., 1994) and triggers secondary inflammatory reactions (Murakami et al., 2005). Following cerebral ischemia, IL-1β binds to its receptor and activates the nuclear factor-κB pathway (Liao et al., 2015). IL-1β knockout significantly alleviated cerebral damage of mice after MCAO (Ohtaki et al., 2003); however, administration of IL-1β exacerbated cerebral ischemic injury (Yamasaki et al., 1995). Following cerebral ischemia, IL-6 increased markedly in serum within several hours and persisted for up to 90 days (Herrmann et al., 2003). Because neuroinflammation definitively and adversely affects cognitive function (Kulesh et al., 2016), we propose that β2M inhibition protects the brain, to a certain extent, against ischemic damage by reducing inflammation.
In conclusion, increased β2M plays a role in neuroinflammation and cognitive impairment after ischemic stroke. Inhibition of β2M is beneficial for improving repair of cerebral ischemic injury and cognitive impairment, and the mechanism is related to the reduction of NLRP3 inflammasome activation and inflammatory cytokine production. There were some deficiencies in this study. For example, we did not add recombinant β2M back to the knockdown rats to examine whether the phenotype was rescued. We also did not observe whether the outcomes were worse if β2M was overexpressed or exogenously administered to the rats. Clarification of these issues will require further investigations.
Additional file:
Additional Table 1: The number of animals or samples in every experiment.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81771337 (to RQY).
Conflicts of interest: The authors declare that they have no conflicts of interest.
Availability of data and materials: All data generated or analyzed during this study are included in this published article and its supplementary information files.
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Yu J, Song LP; T-Editor: Jia Y
References
- 1.Amighi J, Hoke M, Mlekusch W, Schlager O, Exner M, Haumer M, Pernicka E, Koppensteiner R, Minar E, Rumpold H, Schillinger M, Wagner O. Beta 2 microglobulin and the risk for cardiovascular events in patients with asymptomatic carotid atherosclerosis. Stroke. 2011;42:1826–1833. doi: 10.1161/STROKEAHA.110.600312. [DOI] [PubMed] [Google Scholar]
- 2.Boulanger LM, Shatz CJ. Immune signalling in neural development, synaptic plasticity and disease. Nat Rev Neurosci. 2004;5:521–531. doi: 10.1038/nrn1428. [DOI] [PubMed] [Google Scholar]
- 3.Carrette O, Demalte I, Scherl A, Yalkinoglu O, Corthals G, Burkhard P, Hochstrasser DF, Sanchez JC. A panel of cerebrospinal fluid potential biomarkers for the diagnosis of Alzheimer's disease. Proteomics. 2003;3:1486–1494. doi: 10.1002/pmic.200300470. [DOI] [PubMed] [Google Scholar]
- 4.Chamorro Á, Lo EH, Renú A, van Leyen K, Lyden PD. The future of neuroprotection in stroke. J Neurol Neurosurg Psychiatry. 2021;92:129–135. doi: 10.1136/jnnp-2020-324283. [DOI] [PubMed] [Google Scholar]
- 5.Chen SM, Yi YL, Zeng D, Tang YY, Kang X, Zhang P, Zou W, Tang XQ. Hydrogen sulfide attenuates β2-microglobulin-induced cognitive dysfunction:involving recovery of hippocampal autophagic flux. Front Behav Neurosci. 2019;13:244. doi: 10.3389/fnbeh.2019.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chia NH, Leyden JM, Newbury J, Jannes J, Kleinig TJ. Determining the number of ischemic strokes potentially eligible for endovascular thrombectomy:a population-based study. Stroke. 2016;47:1377–1380. doi: 10.1161/STROKEAHA.116.013165. [DOI] [PubMed] [Google Scholar]
- 7.Chittiprol S, Venkatasubramanian G, Neelakantachar N, Allha N, Shetty KT, Gangadhar BN. Beta2-microglobulin abnormalities in antipsychotic-naïve schizophrenia:evidence for immune pathogenesis. Brain Behav Immun. 2009;23:189–192. doi: 10.1016/j.bbi.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Corlin DB, Sen JW, Ladefoged S, Lund GB, Nissen MH, Heegaard NH. Quantification of cleaved beta2-microglobulin in serum from patients undergoing chronic hemodialysis. Clin Chem. 2005;51:1177–1184. doi: 10.1373/clinchem.2005.049544. [DOI] [PubMed] [Google Scholar]
- 9.Deroide N, Li X, Lerouet D, Van VréE, Baker L, Harrison J, Poittevin M, Masters L, Nih L, Margaill I, Iwakura Y, Ryffel B, Pocard M, Tedgui A, Kubis N, Mallat Z. MFGE8 inhibits inflammasome-induced IL-1βproduction and limits postischemic cerebral injury. J Clin Invest. 2013;123:1176–1181. doi: 10.1172/JCI65167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duncan PW, Bushnell C, Sissine M, Coleman S, Lutz BJ, Johnson AM, Radman M, Pvru Bettger J, Zorowitz RD, Stein J. Comprehensive stroke care and outcomes:time for a paradigm shift. Stroke. 2021;52:385–393. doi: 10.1161/STROKEAHA.120.029678. [DOI] [PubMed] [Google Scholar]
- 11.Fan HB, Chen LX, Qu XB, Ren CL, Wu XX, Dong FX, Zhang BL, Gao DS, Yao RQ. Transplanted miR-219-overexpressing oligodendrocyte precursor cells promoted remyelination and improved functional recovery in a chronic demyelinated model. Sci Rep. 2017;7:41407. doi: 10.1038/srep41407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng YS, Tan ZX, Wang MM, Xing Y, Dong F, Zhang F. Inhibition of NLRP3 inflammasome:a prospective target for the treatment of ischemic stroke. Front Cell Neurosci. 2020;14:155. doi: 10.3389/fncel.2020.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao R, Li G, Yang R, Yuan H, Zhang S. Hippocampal β2-microglobulin mediates sepsis-induced cognitive impairment. Mol Med Rep. 2018;17:7813–7820. doi: 10.3892/mmr.2018.8858. [DOI] [PubMed] [Google Scholar]
- 14.Guo H, Yang J, Liu M, Wang L, Hou W, Zhang L, Ma Y. Selective activation of estrogen receptor βalleviates cerebral ischemia neuroinflammatory injury. Brain Res. 20201726:146536. doi: 10.1016/j.brainres.2019.146536. [DOI] [PubMed] [Google Scholar]
- 15.Han D, Wang J, Wen L, Sun M, Liu H, Gao Y. Vinpocetine attenuates ischemic stroke through inhibiting NLRP3 inflammasome expression in mice. J Cardiovasc Pharmacol. 2020;77:208–216. doi: 10.1097/FJC.0000000000000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrmann O, Tarabin V, Suzuki S, Attigah N, Coserea I, Schneider A, Vogel J, Prinz S, Schwab S, Monyer H, Brombacher F, Schwaninger M. Regulation of body temperature and neuroprotection by endogenous interleukin-6 in cerebral ischemia. J Cereb Blood Flow Metab. 2003;23:406–415. doi: 10.1097/01.WCB.0000055177.50448.FA. [DOI] [PubMed] [Google Scholar]
- 17.Hu FY, Wu J, Tang Q, Zhang J, Chen Z, Wang X, Liu Q, Wang J, Ge W, Qun S. Serum β2-microglobulin is closely associated with the recurrence risk and 3-month outcome of acute ischemic stroke. Front Neurol. 2019;10:1334. doi: 10.3389/fneur.2019.01334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulesh AA, Kuklina EM, Shestakov VV. The relationship between serum and liquor IL-1β, IL-6, TNFα, IL-10 levels and clinical, cognitive and functional characteristics in acute ischemic stroke. Klin Med (Mosk) 2016;94:657–662. [PubMed] [Google Scholar]
- 20.Kuriakose D, Xiao Z. Pathophysiology and treatment of stroke:present status and future perspectives. Int J Mol Sci. 2020;21:7609. doi: 10.3390/ijms21207609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee H, Brott BK, Kirkby LA, Adelson JD, Cheng S, Feller MB, Datwani A, Shatz CJ. Synapse elimination and learning rules co-regulated by MHC class I H2-Db. Nature. 2014;509:195–200. doi: 10.1038/nature13154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Chopp M, Chen J, Wang L, Gautam SC, Xu YX, Zhang Z. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab. 2000;9:1311–1319. doi: 10.1097/00004647-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Liao Z, Xiao HT, Zhang Y, Tong RS, Zhang LJ, Bian Y, He X. IL-1β:a key modulator in asthmatic airway smooth muscle hyper-reactivity. Expert Rev Respir Med. 2015;9:429–436. doi: 10.1586/17476348.2015.1063422. [DOI] [PubMed] [Google Scholar]
- 24.Liu T, Clark RK, McDonnell PC, Young PR, White RF, Barone FC, Feuerstein GZ. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke. 1994;25:1481–1488. doi: 10.1161/01.str.25.7.1481. [DOI] [PubMed] [Google Scholar]
- 25.Lu Y, Sareddy GR, Wang J, Zhang Q, Tang FL, Pratap UP, Tekmal RR, Vadlamudi RK, Brann DW. Neuron-derived estrogen is critical for astrocyte activation and neuroprotection of the ischemic brain. J Neurosci. 2020;40:7355–7374. doi: 10.1523/JNEUROSCI.0115-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luton D, Delezoide AL, Leguy MC, Gobeaux C, Vuillard E, Grangé G, Guibourdenche J. Foetal serum but not urinary β2-microglobulin correlates with histological injury to the kidney. Clin Biochem. 2013;46:1607–1610. doi: 10.1016/j.clinbiochem.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 27.Ma H, Parsons MW, Christensen S, Campbell BC, Churilov L, Connelly A, Yan B, Bladin C, Phan T, Barber AP, Read S, Hankey GJ, Markus R, Wijeratne T, Grimley R, Mahant N, Kleinig T, Sturm J, Lee A, Blacker D, et al. A multicentre, randomized, double-blinded, placebo-controlled Phase III study to investigate EXtending the time for Thrombolysis in Emergency Neurological Deficits (EXTEND) Int J Stroke. 2012;7:74–80. doi: 10.1111/j.1747-4949.2011.00730.x. [DOI] [PubMed] [Google Scholar]
- 28.Maida CD, Norrito RL, Daidone M, Tuttolomondo A, Pinto A. Neuroinflammatory mechanisms in ischemic stroke:Focus on cardioembolic stroke, background, and therapeutic approaches. Int J Mol Sci. 2020;18:6454. doi: 10.3390/ijms21186454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McArthur JC, Nance-Sproson TE, Griffin DE, Hoover D, Selnes OA, Miller EN, Margolick JB, Cohen BA, Farzadegan H, Saah A. The diagnostic utility of elevation in cerebrospinal fluid beta 2-microglobulin in HIV-1 dementia. Multicenter AIDS Cohort Study. Neurology. 1992;42:1707–1712. doi: 10.1212/wnl.42.9.1707. [DOI] [PubMed] [Google Scholar]
- 30.Murakami Y, Saito K, Hara A, Zhu Y, Sudo K, Niwa M, Fujii H, Wada H, Ishiguro H, Mori H, Seishima M. Increases in tumor necrosis factor-alpha following transient global cerebral ischemia do not contribute to neuron death in mouse hippocampus. J Neurochem. 2005;93:1616–1622. doi: 10.1111/j.1471-4159.2005.03163.x. [DOI] [PubMed] [Google Scholar]
- 31.Ohtaki H, Takaki A, Yin L, Dohi K, Nakamachi T, Matsunaga M, Horai R, Asano M, Iwakura Y, Shioda S. Suppression of oxidative stress after transient focal ischemia in interleukin-1 knock out mice. Acta Neurochir Suppl. 2003;86:191–194. doi: 10.1007/978-3-7091-0651-8_41. [DOI] [PubMed] [Google Scholar]
- 32.Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, et al. The ARRIVE guidelines 2.0:Updated guidelines for reporting animal research. PLoS Biol. 2020;18:e3000410. doi: 10.1371/journal.pbio.3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prizment AE, Linabery AM, Lutsey PL, Selvin E, Nelson HH, Folsom AR, Church TR, Drake CG, Platz EA, Joshu C. Circulating beta-2 microglobulin and risk of cancer:the Atherosclerosis Risk In Communities study (ARIC) Cancer Epidemiol Biomarkers Prev. 2016;25:657–664. doi: 10.1158/1055-9965.EPI-15-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qun S, Hu F, Wang G, Wu J, Tang Q, Zhang J, Chen Z, Wang X, Liu Q, Ge W. Serum beta2-microglobulin levels are highly associated with the risk of acute ischemic stroke. Sci Rep. 2019;9:6883. doi: 10.1038/s41598-019-43370-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raikou VD, Kyriaki D. The relationship between glycemic control, beta2-microglobulin and inflammation in patients on maintenance dialysis treatment. J Diabetes Metab Disord. 2015;14:34. doi: 10.1186/s40200-015-0162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rist PM, Jiménez MC, Rexrode KM. Prospective association between β(2)-microglobulin levels and ischemic stroke risk among women. Neurology. 2017;88:2176–2182. doi: 10.1212/WNL.0000000000004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ:25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simats A, García-Berrocoso T, Ramiro L, Giralt D, Gill N, Penalba A, Bustamante A, Rosell A, Montaner J. Characterization of the rat cerebrospinal fluid proteome following acute cerebral ischemia using an aptamer-based proteomic technology. Sci Rep. 2018;8:7899. doi: 10.1038/s41598-018-26237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith LK, He Y, Park JS, Bieri G, Snethlage CE, Lin K, Gontier G, Wabl R, Plambeck KE, Udeochu J, Wheatley EG, Bouchard J, Eggel A, Narasimha R, Grant JL, Luo J, Wyss-Coray T, Villeda SA. β2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat Med. 2015;21:932–937. doi: 10.1038/nm.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu HC, Lee LC, Wang WJ. Associations among Serum Beta 2 Microglobulin, malnutrition, inflammation, and advanced cardiovascular event in patients with chronic kidney disease. J Clin Lab Anal. 2017;31:e22056. doi: 10.1002/jcla.22056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie J, Yi Q. Beta2-microglobulin as a potential initiator of inflammatory responses. Trends Immunol. 2003;24:228–229. doi: 10.1016/s1471-4906(03)00076-0. author reply 229-230. [DOI] [PubMed] [Google Scholar]
- 42.Yamasaki Y, Matsuura N, Shozuhara H, Onodera H, Itoyama Y, Kogure K. Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke. 1995;26:676–680. doi: 10.1161/01.str.26.4.676. discussion 681. [DOI] [PubMed] [Google Scholar]
- 43.Yang F, Wang Z, Wei X, Han H, Meng X, Zhang Y, Shi W, Li F, Xin T, Pang Q, Yi F. NLRP3 deficiency ameliorates neurovascular damage in experimental ischemic stroke. J Cereb Blood Flow Metab. 2014;34:660–667. doi: 10.1038/jcbfm.2013.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao RQ, Zhang L, Wang W, Li L. Cornel iridoid glycoside promotes neurogenesis and angiogenesis and improves neurological function after focal cerebral ischemia in rats. Brain Res Bull. 2009;79:69–76. doi: 10.1016/j.brainresbull.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 45.Zhang J, Sokal I, Peskind ER, Quinn JF, Jankovic J, Kenney C, Chung KA, Millard SP, Nutt JG, Montine TJ. CSF multianalyte profile distinguishes Alzheimer and Parkinson diseases. Am J Clin Pathol. 2008;129:526–529. doi: 10.1309/W01Y0B808EMEH12L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang P, Zeng D, Yi YL, Tang YY, Zou W, Yang XF, Wang CY, Tang XQ. β2-microglobulin induces depressive- and anxiety-like behaviors in rat. PLoS One. 2018a;13:e0198027. doi: 10.1371/journal.pone.0198027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Z, Qin P, Deng Y, Ma Z, Guo H, Guo H, Hou Y, Wang S, Zou W, Sun Y, Ma Y, Hou W. The novel estrogenic receptor GPR30 alleviates ischemic injury by inhibiting TLR4-mediated microglial inflammation. J Neuroinflammation. 2018b;15:206. doi: 10.1186/s12974-018-1246-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zijlstra M, Bix M, Simister NE, Loring JM, Raulet DH, Jaenisch R. Beta 2-microglobulin deficient mice lack CD4-8+cytolytic T cells. Nature. 1990;344:742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]


