Abstract
Sleep is a critical part of our daily routine. It impacts every organ and system of our body, from the brain to the heart and from cellular metabolism to immune function. A consistent daily schedule of quality of sleep makes a world of difference to our health and well-being. Despite its importance, so many individuals have trouble sleeping well. Poor quality sleep has such a detrimental impact on many aspects of our lives; it affects our thinking, learning, memory, and movements. Further, and most poignantly, poor quality sleep over time increases the risk of developing a serious medical condition, including neurodegenerative disease. In this review, we focus on a potentially new non-pharmacological treatment that improves the quality of sleep. This treatment, called photobiomodulation, involves the application of very specific wavelengths of light to body tissues. In animal models, these wavelengths, when applied at night, have been reported to stimulate the removal of fluid and toxic waste-products from the brain; that is, they improve the brain’s inbuilt house-keeping function. We suggest that transcranial nocturnal photobiomodulation, by improving brain function at night, will help improve the health and well-being of many individuals, by enhancing the quality of their sleep.
Key Words: aquaporin 4, glymphhatic, infrared, non-pharmacological, red, sleep cap, transcranial, wakefulness
Introduction
The brain is considered to have two quite distinct operative states. The first is the state of wakefulness, the so-called “day-time brain”. In this state, the brain is in a conscious mode, being receptive to, and interactive with, the environment. It is engaged fully with the generation and orchestration of complex neural circuitry associated with the executive functions, such as focusing attention, being cognitively active, encoding memories, and undertaking skilled movements. Each of these brain functions deals with the many challenges and events faced by individuals daily.
The second is the state of sleep, the so-called “night-time brain”. In this state, the brain is in an unconscious, but arousable mode and is far less receptive to the environment (Rash and Born, 2013; Eugene and Masiak, 2015). Although the precise function of sleep remains a mystery, notwithstanding the considerable amount of time and effort invested by both scientists and philosophers over many centuries, there is recent evidence indicating that it is associated with a house-keeping function. This function involves the disposal of all the metabolic debris and waste-products that have accumulated in the brain during the day; there is a “cleaning of the house”, so to speak. These waste-products need to be cleared; otherwise, they accumulate and become toxic. The brain undertakes this house-keeping function largely by using a flow of fluid that sweeps across the spaces between its constituent neural cells, taking all the waste-products with it, draining ultimately into the venous system. This house-keeping function is not, in fact, too far removed from Aristotle’s original idea, all those centuries ago, that sleep serves to help filter, cleanse and refresh the body and brain (Iliff et al., 2012; Aspelund et al., 2015; Jessen et al., 2015; Loveau et al., 2015, 2017; Brodziak et al., 2018; Hablitz et al., 2020; Mestre et al., 2020; Nedergaard and Goldman, 2020; Reddy and van der Werf, 2020; Yan et al., 2021).
In the section that follows, we consider the sleeping brain and its house-keeping function. We will then focus on how the brain undertakes this house-keeping function, exploring the system and the mechanisms involved. Next, we discuss the method of photobiomodulation - the application of very specific wavelengths of light to body tissues - and its impact on the brain at night and during sleep. Finally, we will highlight the idea that photobiomodulation may form an effective, non-pharmacological treatment that improves the quality of sleep and hence the health and well-being of many individuals.
The Sleeping Brain and the House-Keeping Function
The house-keeping function of the brain has been considered to be reserved largely for the state of sleep (Iliff et al., 2012; Rash and Born, 2013; Aspelund et al., 2015; Eugene and Masiak, 2015; Jessen et al., 2015; Loveau et al., 2015, 2017; Brodziak et al., 2018; Hablitz et al., 2020; Mestre et al., 2020; Nedergaard and Goldman, 2020; Reddy and van der Werf, 2020; Yan et al., 2021). The brain cannot readily fulfill this house-keeping during wakefulness, while it is undertaking so many complex higher-order executive functions involving billions of precise synaptic connections. It would be counter-productive to have waves of fluid, filled with toxic waste-products, flowing through it simultaneously; some of these waste-products would include, for example, excess neurotransmitters (e.g., glutamate) that may well stimulate unwanted activity in surrounding synapses (Nedergaard and Goldman, 2020). In this context, the ascending neurotransmitter systems that are active during wakefulness (e.g., noradrenaline) tend to suppress the drainage and clearance of fluid within the brain; with a reduction of these neurotransmitter systems during sleep, the fluid clearance system of the brain is resumed (Constantinople and Bruno, 2011). It should be noted that day-time sleep (e.g., cat-nap or siesta) is generally much lighter than night-time sleep, presumably because it is not aligned with the house-keeping function (Iliff et al., 2012; Rash and Born, 2013; Aspelund et al., 2015; Eugene and Masiak, 2015; Jessen et al., 2015; Loveau et al., 2015, 2017; Brodziak et al., 2018; Hablitz et al., 2020; Mestre et al., 2020; Nedergaard and Goldman, 2020; Reddy and van der Werf, 2020; Yan et al., 2021).
There are two main types of sleep (Dijk, 2009; Rash and Born, 2013; Eugene and Masiak, 2015). The first type is referred to as slow-wave, non-rapid eye movement (nREM) sleep and is characterized by slow-wave, high-amplitude oscillations; it is very much reflective of a state of rest. This type of sleep can be divided into three main stages, namely stages 1, 2, and 3. Stage 1 is considered to be the transition between wakefulness and sleep; stage 2 is considered light sleep where muscle relaxation occurs, heart rate slows, and temperature drops; and stage 3 is deep sleep, characterized by the predominance of the slow-wave forms (e.g., δ and θ waves). The second main type of sleep is referred to as paradoxical, rapid eye movement (REM) and is characterized by fast-wave, low-amplitude oscillations (e.g., α and β waves). In this type of sleep, most of our dreams occur and the brain is just as active, if not more so than in the state of wakefulness; it is not at all in a state of rest! In general, during an eight-hour sleep period, the brain may cycle through the different types of sleep types multiple times; the cycle starts first with slow-wave nREM sleep, followed by REM sleep, and so on, alternating between the two during the night. Towards the end of the full sleep period, the periods of REM sleep generally become more intense (Dijk, 2009; Rash and Born, 2013; Eugene and Masiak, 2015).
If individuals are deprived of quality sleep - that is, adequate periods of slow-wave sleep (Dijk, 2009) - and the brain does not clear its waste-products effectively, then many negative consequences may develop; for example, during the day, individuals are generally less attentive, have a slower cognitive function and memory recall, and/or may have problems with motor functions. In essence, the higher-order executive brain functions are diminished (Iliff et al., 2012; Aspelund et al., 2015; Jessen et al., 2015; Loveau et al., 2015, 2017; Brodziak et al., 2018; Hablitz et al., 2020; Mestre et al., 2020; Nedergaard and Goldman, 2020; Reddy and van der Werf, 2020; Yan et al., 2021). In the case of memory, while the brain during wakefulness is optimized for encoding memories that occur during the day, the brain during sleep is optimized for consolidating these memories. This consolidation process can occur during both slow-wave nREM sleep, where memories are laid-down and developed into long-term memory, and paradoxical REM (i.e., dream) sleep, where these laid-down memories appear to be stabilized. With poor quality sleep, coupled with the poor clearance of waste-products, there is considerable disruption to the whole process of memory, namely the encoding, consolidation, and stabilization of long-term memories (Rash and Born, 2013).
The factors that contribute to poor quality sleep are extensive and varied. They may include the following; medications being used, food and water intake, stress levels, and environmental factors. It does not improve the older we get. In particular, those over 60 years generally have reduced periods of slow-wave sleep, resulting in shorter and lighter sleep patterns, interrupted more often with multiple awakenings. If sleep deprivation becomes chronic, then the consequences become even more severe. There is an increased risk of developing a range of serious medical conditions, including cardiovascular disease, hypertension, diabetes, obesity, psychiatric illnesses (e.g., depression), and neurodegenerative diseases (e.g., Alzheimer’s disease). Conversely, individuals who have consistent patterns of good quality sleep tend to live longer and suffer less disease later in life (Iliff et al., 2012; Aspelund et al., 2015; Eugene and Masiak, 2015; Jessen et al., 2015; Loveau et al., 2015, 2017; Brodziak et al., 2018; Hablitz et al., 2020; Mestre et al., 2020; Nedergaard and Goldman, 2020; Reddy and van der Werf 2020; Yan et al., 2021).
The Glymphatic System: the House-Keeper of the Sleeping Brain
What are the system and the mechanisms that underpin the clearance of fluid and waste-products from the brain? Who or what is the house-keeper? In the periphery, the lymphatic system forms an extensive system of vessels and nodes that clear all of the excess fluid and waste from the organs of the body, from the skin to the muscles, and from the heart to the intestines, disposing these contents into the venous system. In the brain, things are a little different. There are no distinct lymphatic vessels or well-defined structural pathways for fluid and waste clearance. Hence, it was thought that the brain must have in-built mechanisms of protein and cell recycling. In more recent times, however, rather than having a set of distinct lymphatic vessels and nodes, the brain has been shown to clear fluid and waste-products by a series of perivascular spaces in between the blood vessels and astrocytic glial cells (Figure 1A). This brain waste-disposal network has been referred to as the glymphatic system, a combination of the terms “glia” and “lymphatic” (Iliff et al., 2012).
Figure 1.
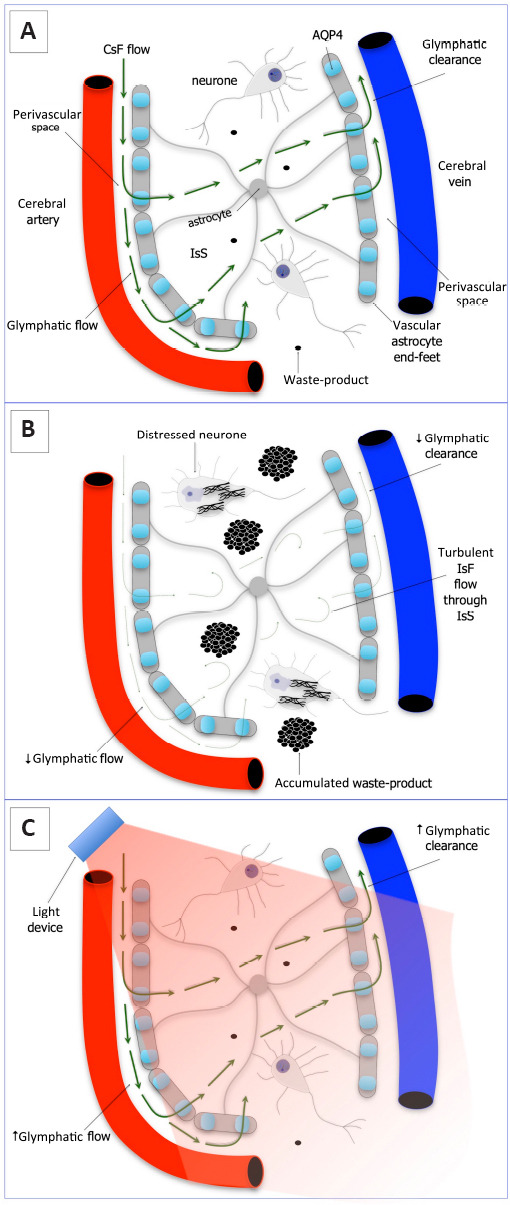
Schematic diagram of the glymphatic system of the brain.
(A) Normal: cerebrospinal fluid (CsF) flows into interstitial space (IsS) through the perivascular space and aquaporin 4 (AQP4) molecule on astrocytes. The interstitial fluid (IsF) is then cleared away into the venous system, together with any cellular waste-products (e.g., β-amyloid). This is the so-called glymphatic system. (B) Disease (or poor quality sleep): glymphatic flow and clearance are much reduced and the flow of IsF through IsS becomes turbulent. This leads to the accumulation of waste products in the IsS (e.g., that may lead to β-amyloid plaque formation), leading to distress and dysfunction in the surrounding neurons (eg, that may lead to the development of neurofibrillary tangles). (C) Photobiomodulation-treated: glymphatic flow and clearance are improved/restored and the surrounding neuropathology is reduced. We suggest that photobiomodulation may work to increase the permeability of the aquaporin-4 water channels on the astrocytes, thereby helping to increase the flow of fluid through the brain. Adapted from Louveau et al. (2017) and Brodziak et al. (2018).
In general, the mechanics and organization of the glymphatic system are as follows. Cerebrospinal fluid passes through the perivascular spaces surrounding the arteries and then into the interstitial space of the brain, via a water channel protein called aquaporin-4 located on the vascular end-feet of the astrocytes. The flow of fluid is polarized directionally, being driven largely by arterial pulsations, which push the fluid out of the interstitial space and then into the perivascular space surrounding the veins (Figure 1A; Iliff et al., 2012; Aspelund et al., 2015; Eugene and Masiak 2015; Jessen et al., 2015; Loveau et al., 2015, 2017; Brodziak et al., 2018; Hablitz et al., 2020; Mestre et al., 2020; Nedergaard and Goldman, 2020; Reddy and van der Werf, 2020; Yan et al., 2021). The fluid then leaves the brain via a series of lymphatic vessels within the meninges. These very delicate, meningeal lymphatic vessels drain subsequently into the cervical lymph nodes. This drainage is rather rapid; contrast agents introduced into the cerebrospinal fluid in the brain can be detected in the deep cervical lymph nodes within minutes. From there, the fluid and waste-products are emptied ultimately into the venous system (Aspelund et al., 2015; Loveau et al., 2015, 2017; Ahn et al., 2019; Mestre et al., 2020; Nedergaard and Goldman, 2020).
There are at least five additional features of the glymphatic system that are worthy of further comment. First, the system is under circadian control. The activity of aquaporin-4 has been reported to be greatest during sleep and least during wakefulness and a loss of aquaporin-4 from the system leads to an elimination of the sleep-wakefulness difference in glymphatic activity. The glymphatic flow correlates closely with the onset of slow-wave brain activity, characteristic of slow-wave, nREM sleep (see above). Conversely, glymphatic activity reduces considerably, almost stops, with a change to the faster wave patterns at the onset of wakefulness (and during REM sleep), together with the activation of the ascending neurotransmitter systems (see above). The glymphatic flow has been described to peak during the sleep phase of diurnal activity and then fall during the active phase, being independent of the light cycle (Iliff et al., 2012; Aspelund et al., 2015; Eugene and Masiak, 2015; Jessen et al., 2015; Loveau et al., 2015, 2017; Brodziak et al., 2018; Hablitz et al., 2020; Mestre et al., 2020; Nedergaard and Goldman, 2020; Reddy and van der Werf, 2020; Yan et al., 2021).
Second, there is a clear age-related decline in glymphatic activity, in both fluid inflow and clearance (Jessen et al., 2015; Nedergaard and Goldman, 2020; Reddy and van der Werf, 2020). When tracers are introduced into the cerebrospinal fluid of aged mice, the overall flow is very much lower compared to that in younger mice (reduced by ~85%); further, contrast clearance in the brains of older human subjects is much slower than in younger individuals. This decline in glymphatic activity with age is due largely to dysfunction of the aquaporin-4 water channels (Ahn et al., 2019), as well as an atrophy of the delicate meningeal lymphatic vessels (Kress et al., 2014).
Third, the activity of the glymphatic system is also very much reduced in disease, for example after subarachnoid hemorrhage or multiple micro-infarction, as well as in neurodegenerative diseases, such as Alzheimer’s disease (Figure 1B; Iliff et al., 2012; Jessen et al., 2015; Loveau et al., 2017; Nedergaard and Goldman, 2020; Reddy and van der Werf, 2020). In a mouse model of Alzheimer’s disease, there is reduced glymphatic influx, as well as less clearance of β-amyloid protein (Iliff et al., 2012; Jessen et al., 2015; Loveau et al., 2017; Mestre et al., 2020; Nedergaard and Goldman, 2020; Reddy and van der Werf, 2020). If there is an abnormal accumulation of this protein, presumably due to a lack of efficient clearance by the glymphatic system, then it becomes toxic; β-amyloid has long been associated with the development and progression of Alzheimer’s disease (Ballard et al., 2011; Nelson and Tabet, 2015; Scheletens et al., 2016; Crous-Bou et al., 2017). Further, pretreatment of wild-type mice with β-amyloid leads to a suppression of fluid tracer influx, indicating that β-amyloid aggregation feed-forwards and generates a further reduction of glymphatic activity (Nedergaard and Goldman, 2020). Finally, in a mouse model of tauopathy, there is a clear dysfunction of glymphatic activity, in the particular flow of cerebrospinal fluid and aquaporin-4 polarization, with the use of an aquaporin-4 inhibitor (Harrison et al., 2020).
Fourth, linking closely with the last feature, there are indications that there is a topographical pattern of glymphatic flow across the brain and that this pattern appears to correlate with the general topography of pathology in Alzheimer’s disease. When contrast agents are introduced into the cerebrospinal fluid, they travel alongside the large anterior and middle cerebral arteries and spread across the frontal and temporal lobes, as well as the hippocampus and entorhinal cortex. Within these brain regions, the contrast agents tend to stay trapped inside the tortuous interstitial spaces for prolonged periods (Ringstad et al., 2018; Shetty and Zanirati 2020); these same regions are the major sites of pathology in Alzheimer’s disease (Ballard et al., 2011; Nelson and Tabet, 2015; Scheletens et al., 2016; Crous-Bou et al., 2017). The stagnation and trapping of fluid within the brain have been suggested to encourage the aggregation of proteins in the interstitial space and to promote the onset of disease (Nedergaard and Goldman, 2020).
Finally, in addition to its waste elimination role, it has been suggested that the glymphatic system is involved in the distribution of non-waste products, for example, glucose and various types of neurotransmitters, across the brain (Jessen et al., 2015).
Evidence that Photobiomodulation Helps the Garbage Disposal System of the Sleeping Brain
When taking together the findings from all these previous studies, it is clear that good quality sleep results in an efficient clearance of fluid and waste from the brain by the glymphatic system, leading ultimately to a better state of overall health and well-being. It also reduces the risk of developing a range of serious medical conditions, including cardiovascular and neurodegenerative diseases. It stands to reason then, that a stimulation and maintenance of an efficient glymphatic system would lead to better quality sleep for individuals, together with improving their health and well-being, as well as helping to delay the onset of disease.
In this context, photobiomodulation, the application of red to near infrared light on body tissues, has been shown recently to improve the clearance of fluid and toxic substances from both the periphery and from the brain (Figure 1C; Yue et al., 2019; Zinchenko et al., 2019; Semyachkina-Glushkovskaya et al., 2020, 2021a, b; Salehpour et al., 2022). In the periphery, photobiomodulation (λ=1267 nm) prompts the clearance of fluid through the lymphatic system, through a relaxation of lymphatic vessels, presumably after a photobiomodulation-induced release of nitric oxide, together with an increase in the permeability of lymphatic endothelium (e.g., Semyachkina-Glushkovskaya et al., 2021a). In the brain, photobiomodulation leads to an improved clearance of experimentally-introduced substances, for example, gold nanorods and dextran, into the cerebrospinal fluid (Semyachkina-Glushkovskaya et al., 2020). Further, and most importantly, photobiomodulation has been shown to reduce β-amyloid brain accumulation and cognitive behavior of Alzheimer’s-induced mice more effectively during sleep, than during wakefulness (Semyachkina-Glushkovskaya et al., 2021b). It also reduces β-amyloid deposition in the interstitial space and stimulates the overall flow of interstitial fluid, as well as inducing the break-up of β-amyloid assemblies and activating enzymes that reduce β-amyloid aggregation (λ = 630 nm; Yue et al., 2019). In addition, photobiomodulation when applied to normal mice prompts a much faster clearing of β-amyloid from the lateral ventricle of the brain down to the deep cervical lymph nodes at night, than during the day (Semyachkina-Glushkovskaya et al., 2021b). Finally, photobiomodulation (λ = 1267 nm) has been shown to stimulate the clearance of fluid within the lymphatic vessels of the meninges; the efficacy of these delicate vessels is crucial in clearing β-amyloid away from the brain (Zinchenko et al., 2019).
The precise photobiomodulation-induced mechanisms that underpin the improved fluid clearance and disposal of waste-products of the sleeping brain are not clear. Several have been suggested, however. For example, the ability to break down protein aggregations and to stimulate vasodilation, at least within the meningeal lymphatic vessels, after a release of nitric oxide, may be a contributing factor (Yue et al., 2019; Zinchenko et al., 2019; Semyachkina-Glushkovskaya et al., 2020, 2021a,b; Salehpour et al., 2022). Photobiomodulation may also impact the composition of cerebrospinal fluid, by changing the structure of the water molecules, making the fluid less viscous and freer flowing (Salehpour et al., 2022). In addition, the heat generated from an extracranial photobiomodulation device (see below) may create a temperature gradient encouraging the flow of cerebrospinal fluid through the brain (Salehpour et al., 2022). It is likely that many other, currently unknown, mechanisms are at play also. In particular, the mechanisms behind the photobiomodulation-induced stimulation of the glymphatic system remain to be determined. We speculate, however, that photobiomodulation may work primarily to increase the permeability of the aquaporin-4 water channels on the astrocytes, thereby helping to increase the flow of fluid through the brain.
It should be noted that the range of wavelengths of light considered as “photobiomodulation” (ie ~ λ = 600–1000 nm) has not been shown to suppress the brain release of melatonin, the key hormone in maintaining circadian rhythm and sleep. In fact, these longer wavelengths have even been suggested to promote the release of melatonin, which can only be of benefit to a better night’s sleep (Yaeger et al., 2007). Further to this point, photobiomodulation has been reported to induce sleeping and prolong sleep duration in mice (Zhang et al., 2017). Blue light (λ = 380–500 nm), by contrast, has the reverse effect by suppressing melatonin release and prolonged night-time exposure results in a poor quality of sleep (Wahl et al., 2019).
The stage is set for the development of a photobiomodulation device that works at night, on the sleeping brain. Although many pharmacological interventions work to improve the length of sleep, many individuals either do not respond to the drugs available and/or prefer to use a non-pharmacological option. Such a non-pharmacological option, like photobiomodulation, would hence be of enormous benefit to many individuals across the wider community. Although there are a large number of extracranial “helmet” devices available on the market delivering photobiomodulation to the brain, for example, the well-red coronet (www.wellred.com.au) and vielight (www.vielight.com), these devices have been designed to work on the day-time brain, during wakefulness, but not at night-time, during sleep. The day-time devices serve to improve the executive functions in healthy individuals, together with those suffering from a neurodegenerative disease (e.g., Alzheimer’s or Parkinson’s disease), traumatic brain injury, or stroke (Hamblin, 2016; Mitrofanis, 2019). A night-time device may work on different systems, for example, stimulating the glymphatic system, helping improve sleep and overall well-being.
Conclusions
Sleep is such a critical part of our day and periods of poor quality sleep can affect severely our normal day-to-day functioning. When these periods of poor sleep become more long-term, there is an increased risk of developing a serious medical condition, like a cardiovascular or neurodegenerative disease. Recent studies in animal models have shown that photobiomodulation improves the function of the brain during sleep; that it stimulates the removal of toxic waste products into the venous system. We suggest that nocturnal photobiomodulation, by stimulating the function of the glymphatic system of the brain at night, will form an effective non-pharmacological treatment that helps improve the overall quality of sleep, and hence well-being and long-term health of many individuals.
Footnotes
Conflicts of interest: CH is director and co-founder of well-red coronet helmets. All other authors declare no conflict of interest.
Editor note: JM is an Editorial Board member of Neural Regeneration Research. He was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer review handled independently of this Editorial Board member and their research groups.
C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
References
- 1.Ahn JH, Cho H, Kim JH, Kim SH, Ham JS, Park I, Suh SH, Hong SP, Song JH, Hong YK, Jeong Y, Park SH, Koh GY. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature. 2019;572:62–66. doi: 10.1038/s41586-019-1419-5. [DOI] [PubMed] [Google Scholar]
- 2.Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212:991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer's disease. Lancet. 2011;377:1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 4.Brodziak A, Wolinska A, Rozyk-Myrta A. Significance of understanding function of glymphatic system to manage practical clinical problems of the elderly. ARC J Neurosci. 2018;3:15–20. [Google Scholar]
- 5.Constantinople CM, Bruno RM. Effects and mechanisms of wakefulness on local cortical networks. Neuron. 2011;69:1061–1068. doi: 10.1016/j.neuron.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crous-Bou M, Minguillón C, Gramunt N, Molinuevo JL. Alzheimer's disease prevention:from risk factors to early intervention. Alzheimers Res Ther. 2017;9:71. doi: 10.1186/s13195-017-0297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dijk DJ. Regulation and functional correlates of slow wave sleep. J Clin Sleep Med. 2009;5:S6–S15. [PMC free article] [PubMed] [Google Scholar]
- 8.Eugene AR, Masiak J. The neuroprotective aspects of sleep. MEDtube Sci. 2015;3:35–40. [PMC free article] [PubMed] [Google Scholar]
- 9.Hablitz LM, Plá V, Giannetto M, Vinitsky HS, Stæger FF, Metcalfe T, Nguyen R, Benrais A, Nedergaard M. Circadian control of brain glymphatic and lymphatic fluid flow. Nat Commun. 2020;11:4411. doi: 10.1038/s41467-020-18115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamblin MR. Shining light on the head:photobiomodulation for brain disorders. BBA Clin. 2016;6:113–124. doi: 10.1016/j.bbacli.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison IF, Ismail O, Machhada A, Colgan N, Ohene Y, Nahavandi P, Ahmed Z, Fisher A, Meftah S, Murray TK, Ottersen OP, Nagelhus EA, O'Neill MJ, Wells JA, Lythgoe MF. Impaired glymphatic function and clearance of tau in an Alzheimer's disease model. Brain. 2020;143:2576–2593. doi: 10.1093/brain/awaa179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jessen NA, Munk ASF, Lundgaard I, Nedergaard M. The glymphatic system:a beginner's guide. Neurochem Res. 2015;40:2583–2599. doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew JA, Plog BA, Ding F, Deane R, Nedergaard M. Impairment of paravascular clearance pathways in the aging brain:paravascular clearance. Ann Neurol. 2014;76:845–861. doi: 10.1002/ana.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louveau A, Plog BA, Antila S, Alitalo K, Nedergaard M, Kipnis J. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Invest. 2017;127:3210–3219. doi: 10.1172/JCI90603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mestre H, Mori Y, Nedergaard M. The brain's glymphatic system:current controversies. Trends Neurosci. 2020;43:458–466. doi: 10.1016/j.tins.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitrofanis J. Run in the light:exploring exercise and photobiomodulation in Parkinson's disease. San Raeael, CA: Morgan &Claypool Publishers.; 2019. [Google Scholar]
- 19.Nedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science. 2020;370:50–56. doi: 10.1126/science.abb8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson L, Tabet N. Slowing the progression of Alzheimer's disease;what works? Ageing Res Rev. 2015;23:193–209. doi: 10.1016/j.arr.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Rasch B, Born J. About sleep's role in memory. Physiol Rev. 2013;93:681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reddy OC, van der Werf YD. The sleeping brain:harnessing the power of the glymphatic system through lifestyle choices. Brain Sciences. 2020;10:868. doi: 10.3390/brainsci10110868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ringstad G, Valnes LM, Dale AM, Pripp AH, Vatnehol SAS, Emblem KE, Mardal KA, Eide PK. Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. JCI Insight. 2018;3:e121537. doi: 10.1172/jci.insight.121537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salehpour F, Khademi M, Bragin DE, DiDuro JO. Photobiomodulation therapy and the glymphatic system:promising applications for augmenting the brain lymphatic drainage system. IJMS. 2022;23:2975. doi: 10.3390/ijms23062975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheltens P, Blennow K, Breteler MMB, de Strooper B, Frisoni GB, Salloway S, Van der Flier WM. Alzheimer's disease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 26.Semyachkina-Glushkovskaya O, Abdurashitov A, Dubrovsky A, Klimova M, Agranovich I, Terskov A, Shirokov A, Vinnik V, Kuzmina A, Lezhnev N, Blokhina I, Shnitenkova A, Tuchin V, Rafailov E, Kurths J. Photobiomodulation of lymphatic drainage and clearance:perspective strategy for augmentation of meningeal lymphatic functions. Biomed Opt Express. 2020;11:725. doi: 10.1364/BOE.383390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semyachkina-Glushkovskaya O, Penzel T, Blokhina I, Khorovodov A, Fedosov I, Yu T, Karandin G, Evsukova A, Elovenko D, Adushkina V, Shirokov A, Dubrovskii A, Terskov A, Navolokin N, Tzoy M, Ageev V, Agranovich I, Telnova V, Tsven A, Kurths J. Night photostimulation of clearance of beta-amyloid from mouse brain:new strategies in preventing Alzheimer's disease. Cells. 2021a;10:3289. doi: 10.3390/cells10123289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Semyachkina-Glushkovskaya O, Postnov D, Lavrova A, Fedosov I, Borisova E, Nikolenko V, Penzel T, Kurths J, Tuchin V. Biophotonic strategies of measurement and stimulation of the cranial and the extracranial lymphatic drainage function. IEEE J Select Topics Quantum Electron. 2021b;27:1–13. [Google Scholar]
- 29.Shetty AK, Zanirati G. The interstitial system of the brain in health and disease. Aging Dis. 2020;11:200. doi: 10.14336/AD.2020.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wahl S, Engelhardt M, Schaupp P, Lappe C, Ivanov IV. The inner clock-blue light sets the human rhythm. J Biophotonics. 2019;12:e201900102. doi: 10.1002/jbio.201900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan T, Qiu Y, Yu X, Yang L. Glymphatic dysfunction:a bridge between sleep disturbance and mood disorders. Front Psychiatry. 2021;12:658340. doi: 10.3389/fpsyt.2021.658340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeager RL, Oleske DA, Sanders RA, Watkins JB 3rd, Eells JT, Henshel DS. Melatonin as a principal component of red light therapy. Med Hypotheses. 2007;69:372–376. doi: 10.1016/j.mehy.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 33.Yue X, Mei Y, Zhang Y, Tong Z, Cui D, Yang J, Wang A, Wang R, Fei X, Ai L, Di Y, Luo H, Li H, Luo W, Lu Y, Li R, Duan C, Gao G, Yang H, Sun B, et al. New insight into Alzheimer's disease:Light reverses Aβ-obstructed interstitial fluid flow and ameliorates memory decline in APP/PS1 mice. Alzheimers Dement. 2019;5:671–684. doi: 10.1016/j.trci.2019.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z, Wang HJ, Wang DR, Qu WM, Huang ZL. Red light at intensities above 10 lx alters sleep-wake behavior in mice. Light Sci Appl. 2017;6:e16231. doi: 10.1038/lsa.2016.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zinchenko E, Navolokin N, Shirokov A, Khlebtsov B, Dubrovsky A, Saranceva E, Abdurashitov A, Khorovodov A, Terskov A, Mamedova A, Klimova M, Agranovich I, Martinov D, Tuchin V, Semyachkina-Glushkovskaya O, Kurts J. Pilot study of transcranial photobiomodulation of lymphatic clearance of beta-amyloid from the mouse brain:breakthrough strategies for non-pharmacologic therapy of Alzheimer's disease. Biomed Opt Express. 2019;10:4003–4017. doi: 10.1364/BOE.10.004003. [DOI] [PMC free article] [PubMed] [Google Scholar]


