Abstract
There is accumulating evidence for a role of immunoglobulin G (IgG) in protection against malarial infection and disease. Only IgG1 and IgG3 are considered cytophilic and protective against P. falciparum, whereas IgG2 and IgG4 were thought to be neither and even to block protective mechanisms. However, no clear pattern of association between isotypes and protection has so far emerged. We analyzed the isotypic distribution of the IgG response to conserved epitopes and P. falciparum blood-stage extract in 283 malaria-exposed individuals whose occurrence of infection and malaria attack had been monitored for about 1 year. Logistic regression analyses showed that, at the end of the season of transmission, high levels of IgG2 to RESA and to MSP2 epitopes were associated with low risk of infection. Indeed, IgG2 is able to bind FcγRIIA in individuals possessing the H131 allele, and we showed that 70% of the study subjects had this allele. Also, high specific IgG4 levels were associated with an enhanced risk of infection and with a high risk of malaria attack. Moreover, specific IgG2 and IgG3 levels, as well as the IgG2/IgG4 and IgG3/IgG4 ratios, increased with the age of subjects, in parallel with the protection against infection and disease. IgG4 likely competes with cytophilic antibodies for antigen recognition and may therefore block cytotoxicity mediated by antibody-activated effector cells. In conclusion, these results favor a protective role of IgG3 and IgG2, which may activate effector cells through FcγRIIA, and provide evidence for a blocking role of IgG4 in malarial infection and disease.
There is growing evidence for the protective role of IgG in Plasmodium falciparum infection. Passive transfers of immunoglobulin G (IgG) have provided protection against the P. falciparum blood stage in South American monkeys (15, 16) and in humans (4, 9). Furthermore, human antibodies (Ab) efficiently inhibit in vitro P. falciparum merozoite proliferation (4) and mediate opsonization of infected erythrocytes (16). Cytophilic Ab are currently thought to be protective, while noncytophilic Ab against the same epitopes may block the protective activity of cytophilic ones (4, 5, 16). In areas where malaria is endemic, cytophilic IgG1 or IgG3 isotype has been associated with either lower parasitemia (38) or lower risk of malaria attack (1, 39). However, the association was not detected in all immunoepidemiological studies, and it may depend on parasite strains, on the parasite antigens (Ags) used in the analysis, and on the host genetic background. In particular, IgG3 directed to RESA, MSP1, and exoantigens was not associated with protection in Madagascar and Papua New Guinea (38), whereas IgG3 to P. falciparum blood-stage extract (P. falciparum extract) was associated with clinical protection in Senegal (1). Similarly, the levels of IgG1 to P. falciparum extract, RESA, and MSP1 were higher in nonprotected subjects than in protected subjects (14), whereas IgG1 to exoantigens was associated with clinical protection (8). No clear pattern of association between isotypes and protection against malaria has so far emerged.
Several asexual blood stage Ags may be the target of protective immunoglobulin; some of them were included in vaccine trials in humans (24). In particular, RESA, MSP1, and MSP2 are of major interest because they were the targets of protective immunity in experimental models (10, 23, 37) and because they are recognized by naturally acquired Ab (31, 36, 40). These Ags present polymorphic and conserved B-cell epitopes (11, 20, 31) and are therefore potential targets of strain-specific and conserved immune responses. Although the relative contributions of such immune responses are still under debate, modeling studies indicated that the slow accumulation of immune responses against poorly immunogenic conserved determinants better explains the development of the age-dependent protection (17).
The aim of the present study was to investigate, in a population of 283 individuals living in an endemic area in Burkina Faso, the protective effect of IgG subclasses directed against RESA, MSP1, and MSP2 conserved epitopes, and P. falciparum extract. We evaluated the influence of age on the levels of cytophilic and noncytophilic IgG, and we examined the relationship between the pattern of IgG isotype and the risks of infection and malaria attack.
MATERIALS AND METHODS
Study area, subjects, and plasma samples.
The study population lived for more than 20 years in an urban district of Bobo-Dioulasso (Burkina Faso). The population structure and the area of parasite exposure were described extensively elsewhere (32, 41). Informed consent for multiple immunoparasitological and clinical surveys was obtained individually from all participants. The Medical Authority of Burkina Faso approved the study protocol. Blood samples were taken from 283 individuals by venipuncture in July 1994 (n = 211) at the end of the dry season (P1) and in December 1994 (n = 248) at the end of the rainy season (P2). In the study area, the parasite transmission was detectable only during the rainy season; the mean number of infected bites per person was 30 in all capture sites of the district (August to October). The malaria transmission was therefore seasonal and homogeneous in the study area (41).
Parasitological and clinical data.
Each subject was visited 14 times from April 1994 to December 1994. The mean number of parasitemia data per subject was 9.4 ± 3.2. Fingerprint peripheral blood was taken from all subjects; thick and thin blood films were prepared according to standard procedures. Parasite determination and numeration were established as described previously (41); only the asexual forms were retained to determine parasitemia in the absence of malaria attack. Parasitemia was much lower from April to July than from August to December (32); for instance, the geometric mean of parasitemia was about fivefold lower at P1 than at P2. In the analysis, individuals were classified as infected or noninfected. Seventy percent of the individuals were infected at least once during the study.
Active case detection of malaria attack was done once a week during the season of malaria transmission. All subjects filled out a questionnaire about symptoms that had occurred during the week. The questionnaire was established with the assistance of pediatric physicians of the hospital Souro Sanou of Bobo-Dioulasso. The axillary temperature was recorded for every subject who complained of illness during the visit or during the previous days. For patients with fever, a thick blood film was analyzed immediately.
A diagnosis of malaria attack was based on P. falciparum parasitemia, fever (axillary temperature of >37.5°C), and clinical symptoms (headache, aching, vomiting, or diarrhea in the children); in these cases no threshold of parasitemia was used. In the absence of classical symptoms of malaria, and once others pathologies could not be eliminated, only children with more than 5,000 parasites per μl and adults with more than 2,000 parasites per μl were considered as having had a malaria attack. Successive malaria attacks separated by less than 3 weeks were not considered a new ones. One hundred and one malaria attacks were recorded from August to December in 63 subjects, and no malaria attack was recorded from April to July. In the study population, 22.3% of the subjects (63 of 283) experienced at least one malaria attack.
According to the recommendation of the Centre National de Lutte Contre le Paludisme of Burkina Faso, each episode of illness was treated with 25 mg of chloroquine per kg for 3 days or until recovery. Parasitemia was checked at the end of the treatment.
P. falciparum blood-stage extract and peptides.
P. falciparum W2 (Southeast Asian) was maintained and synchronized as previously described (22). When the parasitemia reached 10%, schizont-infected red blood cells (RBC) were treated with 0.15% saponin. Isolated schizonts were sonicated on ice in phosphate-buffered saline (PBS) containing protease inhibitors (50 μM phenylmethylsulfonyl fluoride; aprotinin, 50 μg/ml; 1 μM pepstatin; leupeptin, 20 μg/ml; α2-macroglobulin, 10 μg/ml). Sonicates were centrifuged, and the supernatants were filtered through a 0.22-μm (pore-size) membrane. These P. falciparum crude extract were separated into aliquots and stored at −70°C until use.
Five synthetic peptides corresponding to highly conserved B-cell epitopes were used: (i) the epitope (EENV)4 of the C terminal part of RESA (31), which is immunodominant and to which antibodies were associated with resistance to clinical malaria (35); (ii) the epitope (KLYQAQYDLSF) represents the 277- to 287-amino acid sequence of the N-terminus conserved part of MSP1 (30), to which antibodies were significantly associated with clinical protection (36); (iii) the epitope (KAASNTFINNA) represents the 27- to 34-amino-acid sequence of the N-terminus conserved region of MSP2; (iv) the epitope MSP2-Ct1 (AAAQHGHMHGS) represents the 199- to 206-amino-acid sequence of the C-terminus conserved region of MSP2; and (v) the epitope (AAANTSDSQKE) represents the 213- to 220-amino-acid sequence of the C-terminus conserved region of MSP2 (20).
Determination of specific IgG levels.
Specific levels of IgG1, IgG2, IgG3, IgG4, and IgG were measured by enzyme-linked immunosorbent assay (ELISA). Plates (Nunc) were coated either with 1 μg of P. falciparum extract per ml in sodium carbonate buffer (100 mM, pH 9.6) or with 10 μg of peptides per ml conjugated to glutaraldehyde-activated poly-l-lysine (3). Plates were saturated with 3% bovine serum albumin in PBS. Serum dilutions were incubated for 16 h at 4°C (1:20 for IgG2 and IgG4, 1:100 for IgG1 and IgG3, and 1:400 for IgG). The following monoclonal antibodies were used: anti-IgG1 (clone 8c/6-39; The Binding Site), IgG2 and IgG3 (clone HP 6002 and HP 6050; Clinisciences), and IgG4 (clone RJ4; Immunotech). Total IgG were detected by using a goat F(ab′)2 anti-human IgG (Jackson Laboratories). The anti-IgG1 and IgG were conjugated to alkaline phosphatase, the anti-IgG2 and IgG3 were biotinylated, and the anti-IgG4 was unlabeled. F(ab′)2 anti-mouse IgG conjugated to alkaline phosphatase was used for IgG4 detection. Signal amplification was performed for IgG2 and IgG3 detection by using streptavidine and biotinylated alkaline phosphatase (Pierce); the sensitivity of the assay was 30-fold higher than that of the assay with the same monoclonal antibodies conjugated to alkaline phosphatase. After 2 h of incubation at room temperature, enzymatic activities were revealed by use of p-nitrophenyl phosphate (Sigma) at 1 mg/ml in Tris buffer (pH 9.6). The optical densities were read at 405 nm with a DIAS automatic plate reader (Dynex Technology).
Thirty negative reference sera were used to determine the detection threshold; competition experiments with IgG1, IgG2, IgG3, and IgG4 purified myeloma were performed to check the specificity and sensitivity of ELISAs. No cross-reaction was observed. Two-hundred samples from the study subjects were pooled, and the pool was used to draw standard curves. We titrated 50 samples, and we checked that the curves were parallel to the standard curve for each isotype and each antigen. All tests were done in duplicate; antibody levels were calculated by using the standard curve and were expressed as arbitrary units.
Determination of FcγRIIA H/R131 polymorphism.
FcγRIIA H/R131 polymorphism was determined by using an allele-specific restriction enzyme digestion method (19). The PCR conditions were modified by treatment as follows: one cycle of 5 min at 96°C; 35 cycles of 92°C for 40 s, 55°C for 30 s, and 72°C for 10 s; and one cycle of 10 min at 72°C. The PCR product of the H131 allele contains a BstUI site in the 3′ region, and the PCR product of R131 allele contains two BstUI sites, which are located in the 3′ and 5′ regions. Finally, digestion products were analyzed by electrophoresis on a 3% agarose gel stained by ethidium bromide. The DNA from the U937, K562, and Jurkat cell lines were used as a reference. The genotypes of the U937, K562, and Jurkat cell lines are, respectively, RR, HR, and HH.
Statistical analyses.
The influence of age on IgG isotypes was evaluated using Spearman's rank correlation; the age was considered as a continuous variable. Individual antibody levels were compared by using the paired student's t test; we applied a logarithmic transformation based on log(1 + IgG) to allow for zero values. The association between the pattern of isotype distribution and the risk of malaria attack or infection was tested by using logistic regression. Age was considered a continuous covariate. IgG isotype levels were categorized into quintile groups numbered 1 to 5 from the lowest to the highest quintile. The logit of the probability P of infection during the study period can be expressed in the form of linear function of age and IgG isotype quintile as follows: log(P/1 − P) = βo + βaAge + β1IgG1 + β2IgG2 + β3IgG3 + β4IgG4, where βo is constant and βa, β1, β2, β3, and β4 are the regression coefficient. Similarly, the logistic model of malaria attack included age, age square, IgG isotype quintile, and hemoglobin genotype. The coding scheme of hemoglobin genotype was 0 (AA) or 1 (AS, AC, and CC). For each antigen, we started with all isotypes as covariates, and we eliminated nonsignificant covariates through the likelihood ratio criterion. Interactions between isotypes were also tested. The odds of malaria attack and infection between the lowest and the highest quintile were exp(4βi). The goodness-of-fit of the model was tested by the Hosmer-Lemeshow statistic (18); a significant test indicated that the model poorly fitted the data. Computations were performed by using the SPSS software (SPSS, Boulogne, France). Only terms significant at the 5% level were retained.
RESULTS
Recognition of P. falciparum blood-stage antigens by human IgG antibodies and influence of age on isotype distribution.
We evaluated the levels of Ab to RESA, MSP1, and MSP2 peptides, and P. falciparum blood-stage extract (P. falciparum extract) at periods P1 (July) and P2 (December) before and at the end of the transmission time, respectively. At period P1, we detected neither transmission nor malaria attack, and parasitemia was low. At period P2, the geometric mean of parasitemia was fivefold higher than at P1, and numerous malaria attacks were recorded.
IgG directed against RESA, MSP1, and MSP2 peptides and IgG directed against P. falciparum extract were detected in all of the individuals. Furthermore, the levels of anti-P. falciparum extract IgG and the levels of anti-RESA, MSP1, and MSP2 IgG were correlated (P < 0.0001). Most of the individuals had anti-P. falciparum extract and anti-peptide Ab of each IgG subclass. Anti-P. falciparum extract IgG1, IgG2, IgG3, and IgG4 were detected in 98.6, 79.6, 99.5, and 95.7% of individuals, respectively, at P1. At P2, similar percentages were obtained. Prevalence of anti-RESA, MSP1, and MSP2 IgG subclasses was slightly lower than the prevalence of anti-P. falciparum extract IgG subclasses. Most of the individuals had all IgG subclasses directed to RESA, MSP1, or MSP2 epitopes, indicating that the IgG subclasses may compete for the binding to these epitopes.
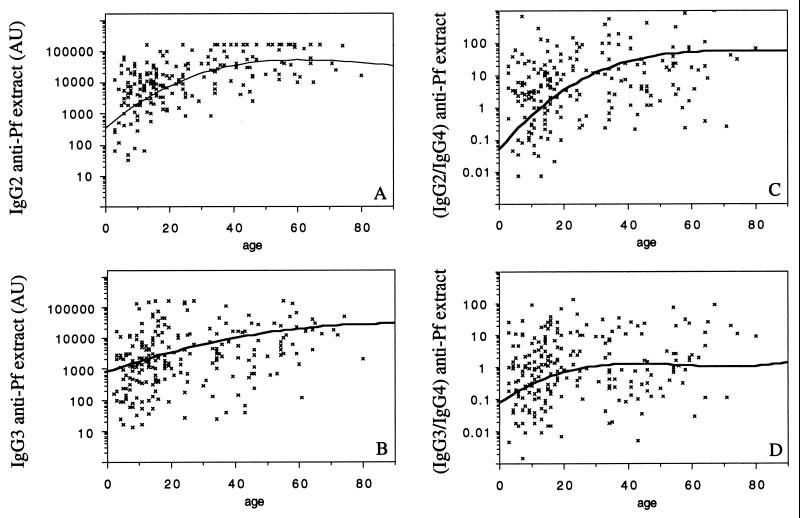
Upon comparing the Ab levels for each subject between P1 and P2, we found that anti-P. falciparum extract IgG2, IgG3, and IgG4 levels were higher at P2 than at P1 (Table 1). Anti-RESA, MSP1, and MSP2-Ct IgG2 and IgG4 levels were also increased at P2 (Table 1). Age had a significant effect on anti-P. falciparum extract IgG levels of all subclasses at P1 and P2 (Table 2 and Fig. 1A and B). Moreover, age was positively correlated with anti-RESA, MSP1, and MSP2 IgG2 and IgG3 levels at P1 and P2 (Table 2). Since IgG4 may block the protective effect of cytophilic IgG, we evaluated the influence of age on the IgG1/IgG4, IgG2/IgG4, and IgG3/IgG4 ratios; the IgG2/IgG4 and IgG3/IgG4 ratios strongly correlated with age at P2 (Table 2 and Fig. 1C and D).
TABLE 1.
Variations in Ab levels before (P1) and at the end (P2) of the season of transmission
| Isotype | Ab levels to antigena
|
|||||
|---|---|---|---|---|---|---|
| Peptide RESA | Peptide MSP1-Nt | Peptide MSP2-Nt | Peptide MSP2-Ct1 | Peptide MSP2-Ct2 | Extract | |
| IgG1 | NS | −8.7 (<10−4) | −6.8 (<10−4) | −12.8 (<10−4) | −11.3 (<10−4) | NS |
| IgG2 | 15.8 (<10−4) | 6.3 (<10−4) | 17.5 (<10−4) | 7.0 (<10−4) | 11.1 (<10−4) | 2.7 (0.008) |
| IgG3 | NS | −2.7 (0.007) | NS | −9.3 (<10−4) | 2.0 (0.045) | 7.6 (<10−4) |
| IgG4 | 11.3 (<10−4) | 3.4 (0.001) | NS | 4.2 (<10−4) | 3.8 (<10−3) | 7.0 (<10−4) |
| IgG | 2.9 (0.004) | −5.6 (<10−4) | NS | 2.7 (0.007) | −10.5 (<10−4) | 16.9 (<10−4) |
Variations in Ab levels were evaluated using paired t test; the t values are given, and the P value is shown in parentheses. NS, not significant. Positive t values indicate an increase in antibody levels between P1 and P2.
TABLE 2.
Ab responses to P. falciparum blood-stage epitopes correlated with age
| Isotype(s) | Sampleb | Ab levels to antigena
|
|||||
|---|---|---|---|---|---|---|---|
| Peptide RESA | Peptide MSP1-Nt | Peptide MSP2-Nt | Peptide MSP2-Ct1 | Peptide MSP2-Ct2 | Extract | ||
| IgG1 | P1 | NS | NS | NS | NS | NS | 0.28 (<10−4) |
| P2 | NS | NS | NS | 0.20 (0.002) | NS | 0.16 (0.015) | |
| IgG2 | P1 | 0.51 (<10−4) | 0.43 (<10−4) | 0.42 (<10−4) | 0.39 (<10−4) | 0.40 (<10−4) | 0.59 (<10−4) |
| P2 | 0.56 (<10−4) | 0.57 (<10−4) | 0.58 (<10−4) | 0.61 (<10−4) | 0.63 (<10−4) | 0.65 (<10−4) | |
| IgG3 | P1 | 0.24 (0.001) | 0.29 (<10−4) | 0.30 (<10−4) | 0.31 (<10−4) | 0.17 (0.016) | 0.54 (<10−4) |
| P2 | 0.22 (0.001) | 0.22 (0.001) | 0.23 (<10−3) | 0.20 (0.002) | 0.24 (<10−3) | 0.40 (<10−4) | |
| IgG4 | P1 | NS | NS | NS | NS | NS | 0.20 (0.004) |
| P2 | NS | NS | −0.14 (0.030) | NS | NS | 0.19 (0.004) | |
| IgG | P1 | 0.15 (0.029) | 0.16 (0.025) | NS | NS | NS | 0.47 (<10−4) |
| P2 | 0.38 (<10−4) | 0.24 (<10−4) | 0.35 (<10−4) | 0.27 (<10−4) | 0.29 (<10−4) | 0.41 (<10−4) | |
| IgG1/IgG4 | P1 | NS | NS | NS | NS | NS | NS |
| P2 | 0.14 (0.029) | NS | 0.21 (0.001) | 0.20 (0.002) | 0.15 (0.020) | NS | |
| IgG2/IgG4 | P1 | 0.16 (0.024) | NS | NS | NS | 0.18 (0.011) | 0.50 (<10−4) |
| P2 | 0.44 (<10−4) | 0.38 (<10−4) | 0.46 (<10−4) | 0.48 (<10−4) | 0.50 (<10−4) | 0.46 (<10−4) | |
| IgG3/IgG4 | P1 | NS | NS | NS | NS | 0.15 (0.032) | 0.33 (<10−4) |
| P2 | 0.25 (<10−4) | 0.21 (0.001) | 0.29 (<10−4) | 0.25 (<10−4) | 0.29 (<10−4) | 0.26 (<10−4) | |
Results are expressed as Spearman's correlation coefficient. The levels of significance are given in parentheses. NS, not significant.
Blood samples were taken before the season of transmission (P1) and at the end of the season of transmission (P2).
FIG. 1.
Anti-P. falciparum extract IgG subclass levels versus age in sera from 248 subjects at period P2. Ab levels are expressed as arbitrary units; curves predicted by polynomial regression analysis are shown. (A to D) Anti-P. falciparum extract IgG2 (A), anti-P. falciparum extract IgG3 (B), IgG2/IgG4 (C), and IgG3/IgG4 (D) ratios by age. AU, arbitrary units.
The variation of Ab levels between P1 and P2 also depended on age. The increase in anti-P. falciparum extract, anti-RESA, MSP1, and MSP2 IgG2 from adult sera (age, >20) was higher than the increase in IgG2 from young people (age, <20) (P < 0.016). In contrast, the increase in anti-P. falciparum extract and anti-RESA, MSP2-Ct IgG4 from adult sera (age, >20) was lower than the increase in IgG4 in children and young adults (age, <20) (P < 0.05).
Isotype responses associated with risk of malaria attack.
During the study, 63 subjects developed at least one malaria attack and 220 had none. At times P1 and P2, these 220 individuals presented higher anti-P. falciparum extract and anti-RESA, MSP1, and MSP2 IgG2 levels than did nonprotected individuals (P < 0.02). Similarly, these individuals had higher anti-P. falciparum extract and anti-RESA, MSP1, and MSP2 IgG3 levels than did nonprotected individuals at P1 (P < 0.02). In contrast, the anti-P. falciparum extract and anti-RESA, MSP1, and MSP2 IgG4 levels were lower in individuals who did not develop a malaria attack than in nonprotected individuals at P2 (P < 0.02).
As age negatively correlated with the occurrence of malaria attack, we took into account the effect of age through logistic regression. At P2, high anti-P. falciparum extract, anti-RESA, and MSP2 IgG4 levels were associated with enhanced risk of malaria attack (Table 3). The odds between the lowest (IgG4 = 1) and the highest (IgG4 = 5) quintiles were between 2.0 and 2.9 (Table 3). The values of the Hosmer-Lemeshow goodness-of-fit statistic were not significant (P > 0.74), indicating that the models including age, age square, hemoglobin genotype, and IgG4 levels fit quite well. At P1, before the time of transmission, we observed a negative correlation between anti-MSP2-Ct2 IgG3 levels and the risk of malaria attack (P = 0.034). The odds between the lowest (IgG3 = 1) and the highest (IgG3 = 5) anti-MSP2-Ct2 IgG3 quintile was 0.35 (95% confidence interval [CI], 0.12 to 0.94).
TABLE 3.
Specific IgG4 levels and malaria attack
| Modela | Slope | SE | Pb | Odd ratioc (95% CI) |
|---|---|---|---|---|
| IgG4 anti-RESA | 0.250 | 0.093 | 0.006 | 2.7 (1.3–5.6) |
| IgG4 anti-MSP1-Nt | 0.179 | 0.110 | NS | |
| IgG4 anti-MSP2-Nt | 0.216 | 0.091 | 0.016 | 2.3 (1.2–4.8) |
| IgG4 anti-MSP2-Ct1 | 0.222 | 0.091 | 0.013 | 2.4 (1.2–5.0) |
| IgG4 anti-MSP2-Ct2 | 0.175 | 0.089 | 0.048 | 2.0 (1.0–4.0) |
| IgG4 anti-extract | 0.268 | 0.108 | 0.011 | 2.9 (1.2–6.8) |
Age and hemoglobin genotypes were taken into account in each model. Six models were analyzed and corresponded to the Ags used. Ab levels were evaluated in sera taken at the end of the season of transmission (P2).
The P value was calculated by using the likelihood ratio criterion.
The odds between the lowest (IgG4 = 1) and the highest quintile (IgG4 = 5) are shown.
Isotype responses associated with risk of infection.
To ascertain whether some isotype responses to blood-stage epitopes were associated with the risk of infection, we used logistic regression models that took into account the effect of age. At P2, anti-RESA and MSP2 IgG2 levels were negatively correlated with the risk of infection (Table 4). The results of the Hosmer-Lemeshow test indicated that the models fit the data (P > 0.31). The odds between the lowest (IgG2 = 1) and the highest (IgG2 = 5) anti-RESA, MSP2-Nt, and MSP2-Ct1 IgG2 quintiles were between 0.26 and 0.34. We also observed a negative trend for the correlation between the risk of infection and the levels of IgG2 directed to the other Ags. Since the FcγRIIA H/R131 polymorphism alters the affinity of the receptor for IgG2, we analyzed the distribution of FcγRIIA H/R131 in the population. The frequency of the H131 allele was 0.43; 15, 55, and 30% of the individuals were HH, HR, and RR, respectively. Seventy percent of the individuals had, therefore, the FcγRIIA H131 allele, the product of which efficiently binds IgG2. We further evidenced a negative correlation between anti-RESA and MSP2 IgG2 levels and the risk of infection when selecting individuals bearing the FcγRIIA-H131 allele (P < 0.05). Nevertheless, we did not detect significant correlation between IgG2 levels and the risk of infection when selecting RR individuals.
TABLE 4.
Specific IgG2 levels and risk of infection
| Modela | Slope | SE | Pb | Odds ratioc (95% CI) |
|---|---|---|---|---|
| IgG2 anti-RESA | −0.337 | 0.130 | 0.010 | 0.26 (0.09–0.72) |
| IgG2 anti-MSP1-Nt | −0.127 | 0.124 | NS (0.304) | |
| IgG2 anti-MSP2-Nt | −0.272 | 0.128 | 0.033 | 0.34 (0.12–0.92) |
| IgG2 anti-MSP2-Ct1 | −0.286 | 0.128 | 0.025 | 0.32 (0.12–0.87) |
| IgG2 anti-MSP2-Ct2 | −0.237 | 0.130 | NS (0.060) | |
| IgG2 anti-extract | −0.152 | 0.133 | NS (0.252) |
Age was taken into account in each model. Ab levels were evaluated in sera taken at the end of the season of transmission.
The P value was calculated by using the likelihood ratio criterion.
The odds between the lowest (IgG2 = 1) and the highest quintile (IgG2 = 5) are shown.
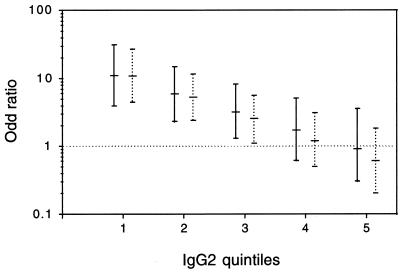
Since the noncytophilic IgG4 isotype was correlated with the risk of malaria attack, we tested the correlation of IgG4 with the risk of infection in the presence of age, IgG2, and the interaction between IgG2 and IgG4. As shown by the results of the Hosmer-Lemeshow test, the models fit well. For example, the test of the goodness-of-fit of the model, including Ab levels, to P. falciparum extract yielded a P value of 0.32. There was a positive correlation between risk of infection and anti-P. falciparum extract and anti-RESA, MSP1, and MSP2 IgG4 at P2 (P < 0.0001). Since interaction between IgG2 and IgG4 was significant at P2 (P < 0.0001), the odds ratio depended on IgG2 levels (Table 5 and Fig. 2). When IgG2 levels were low (IgG2 = 1), the odds of infection between IgG4 quintiles 1 and 5 were between 9.8 and 13.5, depending on the Ags at P2 (Table 5). The odds were no longer significant when IgG2 levels were high (IgG2 = 4 and IgG2 = 5). At P1, we also evidenced a positive correlation between risk of infection and anti-P. falciparum extract and anti-peptides IgG4 (P < 0.0001). The odds of infection between IgG4 quintiles were also found to depend on IgG2 levels. For example, the odds between the anti-P. falciparum extract IgG4 quintiles 1 and 5 were 10.9 (95% CI, 3.8 to 31.2) and 0.9 (95% CI, 0.3 to 3.5) when IgG2 levels were low (IgG2 = 1) and high (IgG2 = 5), respectively (Fig. 2).
TABLE 5.
Specific IgG4 levels and risk of infection
| Modela | Odds ratiob (95% CI) for IgG2 quintile:
|
||
|---|---|---|---|
| IgG2 = 1 | IgG2 = 2 | IgG2 = 3 | |
| IgG4 anti-RESA | 13.5 (5.4–34.3) | 5.9 (2.7–13.0) | 2.6 (1.2–5.6) |
| IgG4 anti-MSP1-Nt | 12.6 (4.6–34.4) | 4.7 (1.9–11.4) | NS |
| IgG4 anti-MSP2-Nt | 10.3 (4.4–23.9) | 4.6 (2.2–9.4) | NS |
| IgG4 anti-MSP2-Ct1 | 9.8 (4.1–23.2) | 3.6 (1.7–7.7) | NS |
| IgG4 anti-MSP2-Ct2 | 12.3 (5.0–30.3) | 4.6 (2.2–9.9) | NS |
| IgG4 anti-extract | 10.9 (4.4–27.0) | 5.2 (2.4–11.4) | 2.5 (1.1–5.5) |
Age was taken into account in each model. Ab levels were evaluated in sera taken at the end of the season of transmission.
The odds between the lowest (IgG4 = 1) and the highest quintile (IgG4 = 5) are shown for three IgG2 quintiles (IgG2 = 1, 2, and 3). The odds were not significant for the other IgG2 quintiles (IgG2 = 4 and IgG2 = 5).
FIG. 2.
Isotype responses and risk of infection: odds ratio for IgG4 in the presence of interaction with IgG2 at P1 (solid bar) and P2 (dotted bar). The odds between the lowest IgG4 quintile (IgG4 = 1) and the highest IgG4 quintile (IgG4 = 5) are shown with 95% CI values for each IgG2 quintile. The odds were no longer significant when IgG2 levels were high (IgG2 = 4 and IgG2 = 5).
DISCUSSION
Cytophilic IgG acts in cooperation with cells in parasite-killing effector responses such as opsonization and Ab-dependent cellular inhibition. The cytophilic IgG are believed to be protective against the P. falciparum infection, and the noncytophilic IgG that recognize the same epitopes are thought to block the effector mechanisms (4, 5). In this study we have evaluated the anti-P. falciparum IgG response of subjects whose parasitemia and occurrence of malaria attack had been carefully monitored for about 1 year. To overcome the confounding effects of Ag polymorphism, we deliberately focused on conserved protein sequences commonly expressed by parasites. Accordingly, we used a P. falciparum strain from Asia to prepare P. falciparum extract. We showed that IgG1, IgG2, IgG3, and IgG4 from most of the study subjects recognize conserved B-cell epitopes from RESA, MSP1, and MSP2. Similarly, Dubois et al. (14) reported that IgG to the central repeat of RESA often belongs to the four subclasses, and Groux and Gysin (16) demonstrated a competition effect between the four IgG subclasses. This clearly indicates that cytophilic and noncytophilic IgG compete for the binding to epitopes and is consistent with the role of an isotype imbalance in the resistance and/or susceptibility to malaria infection (5).
We showed here that age had a strong influence on parasite-specific IgG2 and IgG3 levels but age hardly any on IgG1 and IgG4. Similarly, Aribot et al. (1) reported that age was the major factor associated with the specific distribution pattern; the main changes detectable were those affecting the parasite-specific IgM, IgG2, and IgG3. In our study, IgG2 and IgG3 directed against the conserved epitopes dramatically increased with age. This is consistent with an accumulation of immune responses against poorly immunogenic conserved determinants, an accumulation that may explain the development of the age-dependent protection (12). However, the development of protective immunity may also be due to the gradual acquisition of specific immunity to most of the parasite strains circulating in the population (25). Alternatively, intrinsic immune factors that change with age independently of the cumulative effects of repeated exposure may govern the degree of naturally acquired immunity (2).
Parasite-specific IgG2 levels were shown to be higher at the end of the transmission time (P2) than before it (P1). This increase was even higher in the older individuals, who have progressively developed an efficient protective immunity. Moreover, anti-RESA and MSP2 IgG2 were shown at P2 to inversely correlate with risk of infection, suggesting that IgG2 is involved in protection against P. falciparum. Similarly, Deloron et al. (13) showed an association between high IgG2 levels and low risk of acquiring P. falciparum infection from birth to 6 months of age. Yet IgG2 was described as a blocking isotype; in vitro, IgG2 blocks opsonization, phagocytosis, and antibody-dependent cellular inhibition (5, 16), and IgG2 does not bind to FcγRIIA, which is involved in the production of tumor necrosis factor alpha and in P. falciparum killing (6). Conversely, IgG2 in cooperation with activated eosinophils kills Schistosoma mansoni schistosomula (21). The polymorphism of the gene encoding FcγRIIA was recently described, and this likely explains such conflicting results. The product of the allele H131, whose frequency was 46% in a Caucasian population (28), was found to bind IgG2 (29) and to be activated by IgG2 for opsonization of RBC by monocytes (27). Note that the low-affinity receptor FcγRIIA but not the high-affinity FcγRI was involved in growth inhibition of P. falciparum (6). This may be explained by a higher turnover of IgG bound on FcγRIIA, and it emphasizes the role of IgG isotypes that bind to FcγRIIA of effector cells. In our population, the H131 allele frequency was 43%, and 70% of the individuals had the H131 allele, the product of which binds IgG2. Furthermore, a new mutation that confers IgG2 binding properties to the R131 allelic form of FcγRIIA was recently described (27). This clearly shows that IgG2 must not be considered a noncytophilic isotype, especially in African people exposed to P. falciparum, and suggests that IgG2 is involved in the protection against malaria infection.
Furthermore, we found that anti-P. falciparum extract, anti-RESA, anti-MSP1, and anti-MSP2 IgG4 levels were positively correlated with the risk of infection when we took into account the interaction between IgG2 and IgG4. The correlation depended on IgG2 levels and was stronger when IgG2 levels were low. This suggests that IgG4 counteracts IgG2-dependent cellular cytotoxicity mediated by monocytes or other effector cells. During the season of malaria transmission, IgG4 levels were also correlated with risk of malaria attack, but higher IgG2 levels were not associated with a lower risk of malaria attack. Before the time of malaria transmission and malaria attacks, high levels of IgG3 directed to the conserved MSP2-Ct2 epitope were associated with a low risk of malaria attack. This is consistent with previous works, which evidenced a negative association between risk of malaria attack and IgG3 to recombinant MSP2 (39). Taken together, these results strengthen the hypothesis of a blocking role of IgG4 and suggest that, in addition to IgG3, IgG2 may be involved in protection against P. falciparum. In the same way, we noted a wide variation of the IgG2/IgG4 and IgG3/IgG4 ratios and found a strong positive correlation between age and these ratios during the rainy season. The facts that IgG4 levels strongly increased during the transmission season and that the increase was higher in the younger than in the older people also favor a blocking role of IgG4. In vitro, IgG4 was shown to inhibit the IgG1- and IgG3-mediated opsonization of infected erythrocytes (16). Furthermore, IgG from nonprotected individuals, which did not inhibit P. falciparum growth in ADCI assays, decreased the ADCI effect of IgG from immune African adults (5). In S. mansoni infection, IgG4 blocks antibody-dependent protective mechanisms (33) and, especially, inhibits eosinophil-mediated killing of schistosomula (21). Similarly, in malaria infection, IgG4 may block cytophilic antibody-dependent cellular cytotoxicity mediated by monocytes. Nevertheless, we cannot exclude that this isotype may be an indicator of a specific cytokine response responsible for disease or protection. This is consistent with our recent results showing a genetic linkage of parasitemia to chromosome 5q31-q33 (34), which contains genes encoding cytokines involved in isotype switching toward IgG4 and in proliferation, differentiation, and activation of immune system cells (7, 26). We propose here that genes located on chromosome 5q31-q33 influence various immunological parameters involved in the protection or disease. These include the production of IgG4 that may block protective antibody-dependent mechanisms.
The isotypic analyses in various areas where the disease is endemic do not reveal a clear pattern of relationship between parasitemia, malaria attack, and isotype distribution. This may be due to parasite and host genetic factors, to the immunoassay used, or to the design of the field study. For instance, in our study, we evaluated the risk of infection for 1 year, while the relationship between parasitemia and antibody responses is generally evaluated at the time of bleeding. Moreover, the pattern of association likely changes during the year. In particular, we observed seasonal alterations of IgG2 and IgG4, and we showed associations between IgG2 and risk of infection on the one hand and between IgG4 and risks of both infection and malaria attack on the other at P2 only, during the season of high parasitemia and malaria attacks. We cannot exclude that the P2 observations may only reflect the effect of prior malarial infections; alternatively, the P2 observations may reflect the protective immune response after antigenic stimulation. In spite of the apparent discrepancies between the studies, it should be stressed that numerous reports favor a critical role for antibody-cell cooperation mechanisms in the defense against the P. falciparum blood stage (4, 5, 16, 38). Several investigators have emphasized the role of cytophilic IgG3 isotype in protection (1, 39).
Our results suggest that IgG3 or IgG2-dependent immunity develops slowly. We found that high anti-RESA and MSP2 epitopes IgG2 levels were associated with low risk of infection, and we suggest that IgG2 acts as a cytophilic isotype and contributes to parasite clearance. Our main results are the associations of high IgG4 levels and high risk of infection and malaria attack; we suggest that IgG4 may block the protective effect of cytophilic Ab. These various findings contribute to a better understanding of the role of IgG isotype in protective mechanisms and may provide new insights into the development of malaria control strategies, especially vaccination.
ACKNOWLEDGMENTS
We thank all volunteer families of Bobo-Dioulasso for contribution and the entomological team of Centre Muraz (Bobo-Dioulasso) for valuable technical assistance. We thank the medical authority of Burkina Faso Ministère de la Santé Ouagadougou and Direction Provinciale de la Santé, Bobo-Dioulasso, for encouragement during this work. We thank Alain Bourgois and Alfred S. Traoré for helpful advice and for critical reading of the manuscript.
This work was supported by research grants from the French Ministry of Coopération and Développement and from the AUPELF-UREF LAF 303. C.A. is supported by a studentship from the French Ministry of Research and Technology, and Y.T. is supported by a studentship from AUPELF-UREF.
REFERENCES
- 1.Aribot G, Rogier C, Sarthou J L, Trape J F, Balde A T, Druilhe P, Roussilhon C. Pattern of immunoglobulin isotype response to Plasmodium falciparum blood-stage antigens in individuals living in a holoendemic area of Senegal (Dielmo, West Africa) Am J Trop Med Hyg. 1996;54:449–457. doi: 10.4269/ajtmh.1996.54.449. [DOI] [PubMed] [Google Scholar]
- 2.Baird J K. Host age as a determinant of naturally acquired immunity to Plasmodium falciparum. Parasitol Today. 1995;11:105–113. doi: 10.1016/0169-4758(95)80167-7. [DOI] [PubMed] [Google Scholar]
- 3.Ball J M, Henry N L, Montelaro R C, Newman M J. A versatile synthetic peptide-based ELISA for identifying antibody epitopes. J Immunol Methods. 1994;171:37–44. doi: 10.1016/0022-1759(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 4.Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T, Druilhe P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med. 1990;172:1633–1641. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouharoun-Tayoun H, Druilhe P. Plasmodium falciparum malaria: evidence for an isotype imbalance which may be responsible for delayed acquisition of protective immunity. Infect Immun. 1992;60:1473–1481. doi: 10.1128/iai.60.4.1473-1481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouharoun-Tayoun H, Oeuvray C, Lunel F, Druilhe P. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J Exp Med. 1995;182:409–418. doi: 10.1084/jem.182.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chomarat P, Banchereau J. Interleukin-4 and interleukin-13: their similarities and discrepancies. Int Rev Immunol. 1998;17:1–52. doi: 10.3109/08830189809084486. [DOI] [PubMed] [Google Scholar]
- 8.Chumpitazi B F, Lepers J P, Simon J, Deloron P. IgG1 and IgG2 antibody responses to Plasmodium falciparum exoantigens correlate inversely and positively, respectively, to the number of malaria attacks. FEMS Immunol Med Microbiol. 1996;14:151–158. doi: 10.1111/j.1574-695X.1996.tb00282.x. [DOI] [PubMed] [Google Scholar]
- 9.Cohen S, McGregor A, Carrington S. Gamma globulin and acquired immunity to malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 10.Collins W E, Anders R F, Pappaioanou M, Campbell G H, Brown G V, Kemp D J, Coppel R L, Skinner J C, Andrysiak P M, Favaloro J M, Corcoran L M, Broderson J R, Mitchell G F, Campbell C C. Immunization of Aotus monkeys with recombinant proteins of an erythrocyte surface antigen of Plasmodium falciparum. Nature. 1986;323:259–262. doi: 10.1038/323259a0. [DOI] [PubMed] [Google Scholar]
- 11.Cooper J A. Merozoite surface antigen-1 of Plasmodium. Parasitol Today. 1993;9:50–54. doi: 10.1016/0169-4758(93)90031-a. [DOI] [PubMed] [Google Scholar]
- 12.Day K P, Marsh K. Naturally acquired immunity to Plasmodium falciparum. Immunol Today. 1991;12:68–71. doi: 10.1016/s0167-5699(05)80020-9. [DOI] [PubMed] [Google Scholar]
- 13.Deloron P, Dubois B, Le Hesran J Y, Riche D, Fievet N, Cornet M, Ringwald P, Cot M. Isotypic analysis of maternally transmitted Plasmodium falciparum-specific antibodies in Cameroon, and relationship with risk of P. falciparum infection. Clin Exp Immunol. 1997;110:212–218. doi: 10.1111/j.1365-2249.1997.tb08319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubois B, Deloron P, Astagneau P, Chougnet C, Lepers J P. Isotypic analysis of Plasmodium falciparum-specific antibodies and their relation to protection in Madagascar. Infect Immun. 1993;61:4498–4500. doi: 10.1128/iai.61.10.4498-4500.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fandeur T, Dubois P, Gysin J, Dedet J P, Da Silva L P. In vitro and in vivo studies on protective and inhibitory antibodies against Plasmodium falciparum in the Saimiri monkey. J Immunol. 1984;132:432–437. [PubMed] [Google Scholar]
- 16.Groux H, Gysin J. Opsonization as an effector mechanism in human protection against asexual blood stages of Plasmodium falciparum: functional role of IgG subclasses. Res Immunol. 1990;141:529–542. doi: 10.1016/0923-2494(90)90021-p. [DOI] [PubMed] [Google Scholar]
- 17.Gupta G, Day K P. A theoretical framework for the immunoepidemiology of Plasmodium falciparum malaria. Parasite Immunol. 1994;16:361–370. doi: 10.1111/j.1365-3024.1994.tb00361.x. [DOI] [PubMed] [Google Scholar]
- 18.Hosmer D W, Lemeshow S. Applied logistic regression. New York, N.Y: John Wiley & Sons; 1989. Assessing the fit of the model; pp. 135–175. [Google Scholar]
- 19.Jiang X M, Arepally G, Poncz M, McKenzie S E. Rapid detection of the Fc gamma RIIA-H/R 131 ligand-binding polymorphism using an allele-specific restriction enzyme digestion (ASRED) J Immunol Methods. 1996;199:55–59. doi: 10.1016/s0022-1759(96)00164-0. [DOI] [PubMed] [Google Scholar]
- 20.Jones G L, Edmundson H M, Lord R, Spencer L, Mollard R, Saul A J. Immunological fine structure of the variable and constant regions of a polymorphic malarial surface antigen from Plasmodium falciparum. Mol Biochem Parasitol. 1991;48:1–9. doi: 10.1016/0166-6851(91)90158-3. [DOI] [PubMed] [Google Scholar]
- 21.Khalife J, Dunne D W, Richardson B A, Mazza G, Thorne K J, Capron A, Butterworth A E. Functional role of human IgG subclasses in eosinophil-mediated killing of schistosomula of Schistosoma mansoni. J Immunol. 1989;142:4422–4427. [PubMed] [Google Scholar]
- 22.Lambros C, Vanderberg J P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 23.Ling I T, Ogun S A, Holder A A. Immunization against malaria with a recombinant protein. Parasite Immunol. 1994;16:63–67. doi: 10.1111/j.1365-3024.1994.tb00324.x. [DOI] [PubMed] [Google Scholar]
- 24.Long C A. Immunity to blood stages of malaria. Curr Opin Immunol. 1993;5:548–556. doi: 10.1016/0952-7915(93)90036-r. [DOI] [PubMed] [Google Scholar]
- 25.Marsh K, Howard R J. Antigens induced on erythrocytes by P. falciparum: expression of diverse and conserved determinants. Science. 1986;231:150–153. doi: 10.1126/science.2417315. [DOI] [PubMed] [Google Scholar]
- 26.Metcalf D. Control of granulocytes and macrophages: molecular, cellular, and clinical aspects. Science. 1991;254:529–533. doi: 10.1126/science.1948028. [DOI] [PubMed] [Google Scholar]
- 27.Norris C F, Pricop L, Millard S S, Taylor S M, Surrey S, Schwartz E, Salmon J E, McKenzie S E. A naturally occurring mutation in Fc gamma RIIA: a Q to K127 change confers unique IgG binding properties to the R131 allelic form of the receptor. Blood. 1998;91:656–662. [PubMed] [Google Scholar]
- 28.Osborne J M, Chacko G W, Brandt J T, Anderson C L. Ethnic variation in frequency of an allelic polymorphism of human Fc gamma RIIA determined with allele specific oligonucleotide probes. J Immunol Methods. 1994;173:207–217. doi: 10.1016/0022-1759(94)90299-2. [DOI] [PubMed] [Google Scholar]
- 29.Parren P W, Warmerdam P A, Boeije L C, Arts J, Westerdaal N A, Vlug A, Capel P J, Aarden L A, Van de Winkel J G. On the interaction of IgG subclasses with the low affinity Fc gamma RIIa (CD32) on human monocytes, neutrophils, and platelets. Analysis of a functional polymorphism to human IgG2. J Clin Investig. 1992;90:1537–1546. doi: 10.1172/JCI116022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patarroyo M E, Romero P, Torres M L, Clavijo P, Moreno A, Martinez A, Rodriguez R, Guzman F, Cabezas E. Induction of protective immunity against experimental infection with malaria using synthetic peptides. Nature. 1987;328:629–632. doi: 10.1038/328629a0. [DOI] [PubMed] [Google Scholar]
- 31.Perlmann H, Perlmann P, Berzins K, Wahlin B, Troye-Blomberg M, Hagstedt M, Andersson I, Hogh B, Petersen E, Bjorkman A. Dissection of the human antibody response to the malaria antigen Pf155/RESA into epitope-specific components. Immunol Rev. 1989;112:115–132. doi: 10.1111/j.1600-065x.1989.tb00555.x. [DOI] [PubMed] [Google Scholar]
- 32.Rihet P, Abel L, Traore Y, Traore-Leroux T, Aucan C, Fumoux F. Human malaria: segregation analysis of blood infection levels in a suburban area and a rural area in Burkina Faso. Genet Epidemiol. 1998;15:435–450. doi: 10.1002/(SICI)1098-2272(1998)15:5<435::AID-GEPI1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 33.Rihet P, Demeure C E, Ouattara M, Bourgois A, Carvalho D, Abel L, Dessein A J. Proceedings of the 15th European Congress of Allergology and Clinical Immunology. 1992. Immunological mechanisms of human resistance to schistosome infection; the protective effect of immunoglobulin E is balanced by a blocking action of immunoglobulin G4; pp. 83–91. Paris. Advances in Allergology and Clinical Immunology, EAACI, Stockholm, Sweden. [Google Scholar]
- 34.Rihet P, Traore Y, Abel L, Aucan C, Traore-Leroux T, Fumoux F. Malaria in humans: Plasmodium falciparum blood infection levels are linked to chromosome 5q31-q33. Am J Hum Genet. 1998;63:498–505. doi: 10.1086/301967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riley E M, Allen S J, Troye B M, Bennett S, Perlmann H, Andersson G, Smedman L, Perlmann P, Greenwood B M. Association between immune recognition of the malaria vaccine candidate antigen Pf155/RESA and resistance to clinical disease: a prospective study in a malaria-endemic region of west Africa. Trans R Soc Trop Med Hyg. 1991;85:436–443. doi: 10.1016/0035-9203(91)90207-f. [DOI] [PubMed] [Google Scholar]
- 36.Riley E M, Allen S J, Wheeler J G, Blackman M J, Bennett S, Takacs B, Schonfeld H J, Holder A A, Greenwood B M. Naturally acquired cellular and humoral immune responses to the major merozoite surface antigen (PfMSP1) of Plasmodium falciparum are associated with reduced malaria morbidity. Parasite Immunol. 1992;14:321–337. doi: 10.1111/j.1365-3024.1992.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 37.Saul A, Lord R, Jones G L, Spencer L. Protective immunization with invariant peptides of the Plasmodium falciparum antigen MSA2. J Immunol. 1992;148:208–211. [PubMed] [Google Scholar]
- 38.Shi Y P, Sayed U, Qari S H, Roberts J M, Udhayakumar V, Oloo A J, Hawley W A, Kaslow D C, Nahlen B L, Lal A A. Natural immune response to the C-terminal 19-kilodalton domain of Plasmodium falciparum merozoite surface protein 1. Infect Immun. 1996;64:2716–2723. doi: 10.1128/iai.64.7.2716-2723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor R R, Allen S J, Greenwood B M, Riley E M. IgG3 antibodies to Plasmodium falciparum merozoite surface protein 2 (MSP2): increasing prevalence with age and association with clinical immunity to malaria. Am J Trop Med Hyg. 1998;58:406–413. doi: 10.4269/ajtmh.1998.58.406. [DOI] [PubMed] [Google Scholar]
- 40.Taylor R R, Smith D B, Robinson V J, McBride J S, Riley E M. Human antibody response to Plasmodium falciparum merozoite surface protein 2 is serogroup specific and predominantly of the immunoglobulin G3 subclass. Infect Immun. 1995;63:4382–4388. doi: 10.1128/iai.63.11.4382-4388.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Traore Y, Rihet P, Traore L T, Aucan C, Gazin P, Coosemans M, Smith A, Abel L, Tall F, Nacro B, Traore A, Fumoux F. Analyse des facteurs génétiques contrôlant l'infection palustre che l'homme. Sante. 1999;9:53–59. [PubMed] [Google Scholar]