Abstract
Background & Aims:
Use of antibiotics affects the composition of the microbiome and might affect development of colorectal polyps, which are precursors to colorectal cancer.
Methods:
We performed a nested case–control study in Sweden of 45,744 patients with a colorectal polyp (cases) in the nationwide gastrointestinal ESPRESSO histopathology cohort, using unaffected full siblings as controls (n=93,307). Polyps were classified by morphology SnoMed codes into conventional adenomas and serrated polyps. Through linkage to the Prescribed Drug Register, we assessed use and cumulative dispensations of antibiotic until one year prior to polyp diagnosis for cases and their sibling controls.
Results:
During a median study period of 6.9 years, compared with non-users, users of antibiotics (28,884 cases [63.1%] and 53,222 sibling controls [57.0%]) had a higher risk of colorectal polyps. Risk increased with higher number of dispensations (odds ratio [OR] for ≥ 6 dispensations, 1.33; 95% CI, 1.25–1.43) (Ptrend<.0001). We observed a stronger association with polyps for broad-spectrum antibiotics (odds ratio [OR], 1.23; 95% CI, 1.18–1.29) than for narrow-spectrum antibiotics (OR, 1.05; 95% CI, 1.01–1.10), and for tetracyclines and quinolones (OR, 1.21) than penicillin and other classes (ORs ranged from 1.04 to 1.16). The findings remained robust with several sensitivity analyses, including use of a 2-year lead-in period for antibiotic assessment and correction for misclassification in controls. Use of broad-spectrum antibiotics was more strongly associated with risk of serrated polyps (OR, 1.29; 95% CI, 1.21–1.38) compared with risk of conventional adenomas (OR, 1.17; 95% CI, 1.11–1.24). We found no differences in risk of colon vs rectal polyps with antibiotic use (Pheterogeneity>0.10). We found stronger associations for younger (<50 years) vs older adults (≥50 years) for users of quinolones, sulfonamides, trimethoprim, and cephalosporins (Pinteraction<0.001).
Conclusions:
In a nationwide case–control study in Sweden, after accounting for hereditary and early life environmental factors, antibiotic use was associated with increased risk of colorectal polyps. Our findings indicate a role for intestinal dysbiosis in early stages of colorectal carcinogenesis.
Keywords: anti-aerobic, anaerobic, bacteria, sessile serrated polyp
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer death worldwide.1 The overall death rate of CRC has been decreasing at the global level (−13.5% from 1990 to 2017),2 likely driven by the increasing use of CRC screening tests. However, substantial increases in CRC mortality have been seen in less developed regions, particularly south Asia and central Latin America (20.4%). Moreover, an increasing trend of early-onset CRC that occurs in adults younger than 50 years has been noted across several regions.3 Although lifestyle factors, such as smoking, Western diet, and obesity, have an established role in CRC and may contribute to 40–60% of CRC cases and deaths in many countries,3, 4 identifying other modifiable risk factors for CRC remains a priority to improve prevention efforts.
Increasing evidence suggests a role of the gut microbiota in initiation of CRC.5 Compositional shifts in the gut microbiota have been observed in patients with CRC precursors, namely colorectal polyps, compared to healthy individuals.6, 7 In support of the role of dysbiosis in CRC, exposure to antibiotics have been associated with increased risk of CRC in several studies.8–13 However, the findings remain inconsistent, particularly for specific classes of antibiotics and the differences by tumor subsite. Moreover, it remains largely unknown whether antibiotics play a role in the early stage of CRC development.10
Therefore, to better understand the role of antibiotics in the development of CRC precursors, we performed a nationwide nested case-control study in Sweden among patients with a colorectal polyp and their unaffected full sibling controls. We used sibling controls to reduce confounding by hereditary and shared environmental factors.
Materials and methods
Study population
Sweden has a public health care system with universal coverage. Individual-level data from various national registries were linked based on the unique personal identity number assigned at birth to all Swedish residents.14 Participants with polyps were drawn from the ESPRESSO study (Epidemiology Strengthened by histoPathology Reports in Sweden) that included gastrointestinal (GI) biopsies from all 28 pathology departments in Sweden between 1965 and 2017.15 In ESPRESSO, histopathologic findings were defined by codes of topography and morphology (a Swedish modification of the Systematized Nomenclature of Medicine [SnoMed] coding system). We used topography codes of T67 (for colon) and T68 (for rectum) in combination with SnoMed codes to identify colorectal polyps.16 For conventional adenomas, we used the SnoMed codes of M82100 (tubular adenoma), M82630 (tubulovillous adenoma), and M82611 (villous adenoma). Serrated polyps included hyperplastic polyps and sessile serrated polyps (SSPs). We used the SnoMed code of M72040 for hyperplastic polyps, and used a combination of SnoMed codes (M82160, M82130, and M72041) and free text search for SSPs. The accuracy of polyp identification has been described in our previous studies.16–18
Information on antibiotic use was derived from the Swedish Prescribed Drug Register, which has collected information on all medications prescribed to the entire Swedish population since July 1, 2005.19 To ensure that we had at least one-year information on antibiotic use, we identified participants with the first diagnosis of colorectal polyps aged at least 18 years in ESPRESSO since July 1, 2006. To minimize the influence of hereditary predisposition and shared early life exposures, we selected unaffected full siblings of participants with polyps as the control group. We excluded individuals who had a history of CRC. To reduce the influence of missed cancers at the time of endoscopy, we also excluded individuals who had a diagnosis of CRC within the first 6 months after the diagnosis of polyps for cases and their sibling controls. After further excluding individuals with inflammatory bowel disease (IBD), unspecified histology of polyps, and erroneous records on the date of diagnosis, and those without sibling controls, a total of 45,744 polyp cases and 93,307 matched sibling controls were included in the study (see flowchart in Figure 1). The mean number of sibling controls per case was 2.0 (standard deviation [SD], 1.4). The study was approved by the Stockholm Ethics Review Board. Informed consent was waived by the board since the study was strictly register-based.20
Figure 1.
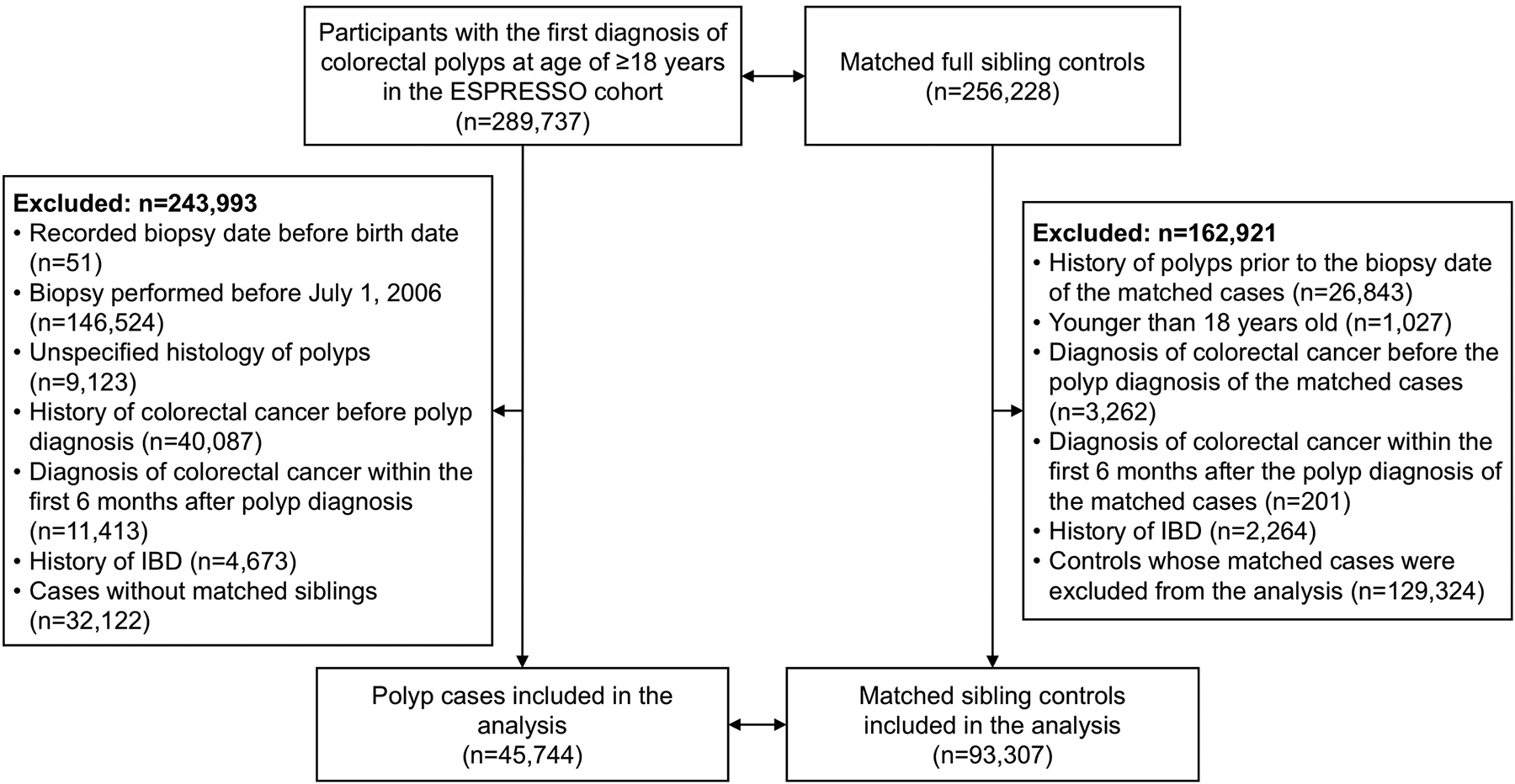
Flowchart of participant selection
Abbreviations: ESPRESSO, Epidemiology Strengthened by histoPathology Reports in Sweden; IBD, inflammatory bowel disease.
Exposure and covariate assessment
We identified antibiotic use from the register using the World Health Organization (WHO) Anatomical Therapeutic Chemical (ATC) codes for the therapeutic subgroup of antibacterials approved for systemic usage. To minimize reverse causality, we assessed the total number of antibiotic dispensations up to one year prior to the diagnosis of polyps for cases and their sibling controls. We examined antibiotics according to class (penicillin, cephalosporins, macrolides, fluoroquinolones, tetracyclines, sulfonamides, and others) and categorization (anti-anaerobic and anti-aerobic; narrow and broad-spectrum; Supplementary Table 1). Details of covariate assessment are described in the Supplementary Methods.
Statistical analysis
We calculated means (SD) for continuous variables and percentages for categorical variables among cases and controls. We used conditional logistic regression to calculate the odds ratio (OR) and 95% confidence interval (CI) of colorectal polyps according to use of antibiotics (yes, no) and cumulative dispensations (0, 1, 2, 3–5, and ≥6). We considered two models: model 1 was adjusted for age (continuous), sex (binary), and year of birth (continuous); and model 2 was further adjusted for income levels (quintiles), education (9 years or less, 10–12 years, >12 years, missing), total number of prior clinic visits (quintiles), Charlson comorbidity score (continuous), and major comorbidities with a prevalence of at least 1% (all binary, including diabetes, cardiovascular disease, non-colorectal cancer, liver disease, chronic pulmonary disease, connective tissue disease, and peptic ulcer disease). Ptrend was calculated using the number of dispensations as a continuous variable. We also conducted several sensitivity analyses. First, to minimize confounding by utilization of endoscopic exams, we assessed use of endoscopic exams at the time of polyp diagnosis for cases and their matched sibling controls using the established procedure codes in the patient registries21 and included this variable in the multivariable model. Second, given the potential confounding effect by smoking and that patients with chronic pulmonary disease are frequently prescribed with broad-spectrum antibiotics, particularly tetracyclines, we performed a sensitivity analysis by excluding individuals with a history of chronic pulmonary disease. Third, because diverticulitis relates to antibiotic prescription, inflammation in the colorectum and possibly polyp diagnostics, we excluded individuals with diverticular disease in another sensitivity analysis. Fourth, to minimize reverse causality, we excluded antibiotic dispensations within 2 years of polyp diagnosis. Finally, because polyps are common and sibling controls may have undetected polyps due to lack of national screening programs in Sweden, we performed two additional analyses to account for outcome misclassification in sibling controls using the method proposed by Fox et al22 and to assess the relationship between antibiotic use and polyp risk according to the likelihood of outcome misclassification in sibling controls (details are described in the Supplementary Materials). All these sensitivity analyses were adjusted for the same set of covariates as in the primary analysis.
Furthermore, we examined the risk of polyps according to type of antibiotics and major individual classes of antibiotics. We performed subgroup analyses according to histology (conventional adenomas and serrated polyps) and sublocation (colon and rectum) of polyps, and calculate the P for heterogeneity using the contrast test method.23 For conventional adenomas, we further examined the associations according to the most advanced histology a patient had (the precedence order being villous adenoma [n=397], tubulovillous adenoma [n=7,784], and tubular adenoma [n=15,716]). Given the speculated role of the gut microbiota in early-onset CRC, we conducted a secondary analysis by stratifying according to age. We calculated P for interaction using Wald test for the product term between age and antibiotic use.
We used SAS 9.4 for the analyses. All statistical tests were two-sided with the significance level of 0.05.
Results
Table 1 shows the basic characteristics of participants. The mean age was 60.6 (SD, 11.8) years for cases and 60.8 (11.8) years for sibling controls. During a median follow-up of 6.9 years, 28,884 cases (63.1%) and 53,222 sibling controls (57.0%) had at least one dispensation of antibiotics. Among antibiotic users in cases, the interval between the first recorded dispensation and polyp diagnosis ranged from 1.0 to 12.8 years (median, 5.0 years; interquartile range, 3.0–7.2 years). There were more users for narrow- than broad-spectrum antibiotics (47% vs. 34% in controls) and for anti-aerobic than anti-anaerobic antibiotics (56% vs. 8% in controls). Common classes of antibiotics included penicillin, tetracyclines, and quinolones. Cases had a higher number of clinic visits and comorbidities than sibling controls.
Table 1.
Basic characteristics of study participants
| Cases (n=45,744) | Sibling Controls (n=93,307) | |
|---|---|---|
| Age, year, mean (SD) | 60.6 (11.8) | 60.8 (11.8) |
| <50 years | 7,884 (17.2) | 15,837 (17.0) |
| ≥50, <60 years | 9,047 (19.8) | 22,007 (23.6) |
| ≥60, <70 years | 18,330 (40.1) | 34,300 (36.8) |
| ≥70 years | 10,483 (22.9) | 21,163 (22.7) |
| Women, n (%) | 23,564 (51.5) | 45,851 (49.1) |
| Birth year, mean (SD) | 1951 (12) | 1951 (12) |
| Year of diagnosis, n (%) | ||
| 2006–2007 | 4,481 (9.8) | - |
| 2008–2010 | 12,316 (26.9) | - |
| 2011–2013 | 14,050 (30.7) | - |
| ≥2014 | 14,897 (32.6) | - |
| Total number of prior clinic visit, mean (SD) | 15.5 (16.3) | 8.8 (12.9) |
| Antibiotics dispensations, n (%) | ||
| No | 16,860 (36.9) | 40,085 (43.0) |
| 1 | 8,094 (17.7) | 17,583 (18.8) |
| 2 | 5,629 (12.3) | 10,970 (11.8) |
| 3–5 | 8,563 (18.7) | 14,719 (15.8) |
| ≥6 | 6,598 (14.4) | 9,950 (10.7) |
| Use of narrow-spectrum antibiotics, n (%) | 23,465 (51.3) | 43,453 (46.6) |
| Use of broad-spectrum antibiotics, n (%) | 19,094 (41.7) | 31,788 (34.1) |
| Use of anti-aerobic antibiotics, n (%) | 28,437 (62.2) | 52,267 (56.0) |
| Use of anti-anaerobic antibiotics, n (%) | 4,330 (9.5) | 7,382 (7.9) |
| Use of antibiotics by class, n (%) | ||
| Penicillin | 22,414 (49.0) | 41,419 (44.4) |
| Tetracyclines | 11,670 (25.5) | 18,578 (19.9) |
| Quinolones | 6,701 (14.7) | 10,672 (11.4) |
| Macrolides | 5,262 (11.5) | 8,700 (9.3) |
| Sulfonamides and trimethoprim | 3,854 (8.4) | 6,278 (6.7) |
| Cephalosporins and other non-penicillin beta-lactams | 2,680 (5.9) | 4,586 (4.9) |
| Other classes | 2,959 (6.5) | 5,124 (5.5) |
| Charlson comorbidity score, mean (SD)* | 0.8 (1.1) | 0.5 (1.0) |
| Individual comorbidity, n (%)* | ||
| Myocardial Infarction | 1,967 (4.3) | 3,883 (4.2) |
| Congestive Heart Failure | 1,347 (2.9) | 2,114 (2.3) |
| Peripheral Vascular Disease | 1,198 (2.6) | 1,275 (1.4) |
| Cerebrovascular Disease | 2,655 (5.8) | 4,791 (5.1) |
| Dementia | 137 (0.3) | 402 (0.4) |
| Chronic Pulmonary Disease | 4,246 (9.3) | 5,208 (5.6) |
| Connective Tissue Disease-Rheumatic Disease | 1,355 (3.0) | 1,205 (1.3) |
| Peptic Ulcer Disease | 1,352 (3.0) | 1,224 (1.3) |
| Mild Liver Disease | 852 (1.9) | 1,012 (1.1) |
| Diabetes without complications | 3,092 (6.8) | 3,608 (3.9) |
| Diabetes with complications | 1,099 (2.4) | 724 (0.8) |
| Paraplegia and Hemiplegia | 264 (0.6) | 467 (0.5) |
| Renal Disease | 714 (1.6) | 782 (0.8) |
| Cancer | 14,006 (30.6) | 18,690 (20.0) |
| Moderate or Severe Liver Disease | 259 (0.6) | 339 (0.4) |
| Metastatic Carcinoma | 428 (0.9) | 1,870 (2.0) |
| AIDS/HIV | 34 (0.1) | 51 (0.1) |
The complete list of ICD codes for comorbidities is presented in Supplementary Table 2.
Table 2 shows the association of antibiotic use with risk of colorectal polyps. Compared with non-users, users of antibiotics had a higher risk of colorectal polyps. The OR (95% CI) was 1.31 (1.26–1.36) in the model adjusted for age, sex and birth year only; and attenuated to 1.08 (1.04–1.13) after further adjustment for other covariates. The change appeared to be due to a cumulative effect of multiple covariates rather than driven by individual covariates. A stronger association was found for broad-spectrum (multivariable OR=1.23, 95% CI=1.18–1.29) compared with narrow-spectrum antibiotics (1.05, 1.01–1.10), and for tetracyclines (1.21, 1.16–1.27) and quinolones (1.21, 1.14–1.28) compared with penicillin (1.04, 1.00–1.09) and other classes.
Table 2.
Association between use of antibiotics and risk of colorectal polyps
| Nonusers | Users | OR (95% CI) | P value† | ||||
|---|---|---|---|---|---|---|---|
| No. of cases | No. of sibling controls | No. of cases | No. of sibling controls | Model 1* | Model 2† | ||
| All antibiotics | 16,860 | 40,085 | 28,884 | 53,222 | 1.31 (1.26–1.36) | 1.08 (1.04–1.13) | 0.0003 |
| Narrow-spectrum antibiotics | 22,279 | 49,854 | 23,465 | 43,453 | 1.23 (1.18–1.27) | 1.05 (1.01–1.10) | 0.01 |
| Broad-spectrum antibiotics | 26,650 | 61,519 | 19,094 | 31,788 | 1.40 (1.35–1.46) | 1.23 (1.18–1.29) | <0.0001 |
| Anti-aerobic antibiotics | 17,307 | 41,040 | 28,437 | 52,267 | 1.30 (1.25–1.35) | 1.08 (1.04–1.13) | 0.0003 |
| Anti-anaerobic antibiotics | 41,414 | 85,925 | 4,330 | 7,382 | 1.23 (1.16–1.31) | 1.10 (1.03–1.18) | 0.006 |
| Penicillin | 23,330 | 51,888 | 22,414 | 41,419 | 1.21 (1.17–1.26) | 1.04 (1.00–1.09) | 0.04 |
| Tetracyclines | 34,074 | 74,729 | 11,670 | 18,578 | 1.36 (1.30–1.42) | 1.21 (1.16–1.27) | <0.0001 |
| Quinolones | 39,043 | 82,635 | 6,701 | 10,672 | 1.36 (1.29–1.44) | 1.21 (1.14–1.28) | <0.0001 |
| Macrolides | 40,482 | 84,607 | 5,262 | 8,700 | 1.28 (1.21–1.36) | 1.14 (1.08–1.22) | <0.0001 |
| Sulfonamides and trimethoprim | 41,890 | 87,029 | 3,854 | 6,278 | 1.26 (1.18–1.35) | 1.16 (1.08–1.24) | <0.0001 |
| Cephalosporins and other non-penicillin beta-lactams | 43,064 | 88,721 | 2,680 | 4,586 | 1.24 (1.15–1.34) | 1.13 (1.04–1.23) | 0.005 |
| Other classes | 42,785 | 88,183 | 2,959 | 5,124 | 1.15 (1.06–1.24) | 1.07 (0.99–1.16) | 0.10 |
Conditional logistic regression was used with adjustment for age (continuous), sex (binary), and year of birth (continuous).
Multivariable model was further adjusted for income levels (quintiles), education (9 years or less, 10–12 years, >12 years, missing), total number of prior clinic visits (quintiles), Charlson comorbidity score (continuous), and major comorbidities (all binary, including diabetes, cardiovascular disease, non-colorectal cancer, liver disease, chronic pulmonary disease, connective tissue disease, and peptic ulcer disease).
We conducted a sensitivity analysis by accounting for potential misclassification of polyp status in controls (Supplementary Table 3). The associations of total and different classes of antibiotics with polyp risk were all strengthened, with the OR ranged from 1.72 to 2.14. The OR was 1.84 (95% CI, 1.17–2.95) for any antibiotics and 2.13 (95% CI, 1.33–3.56) for broad-spectrum antibiotics. In the sensitivity analysis further adjusted for use of prior endoscopic exams (8.9% in cases and 0.9% in controls), similar results were observed (OR for any antibiotics=1.07, 95% CI=1.02–1.12; OR for broad-spectrum antibiotics=1.21, 95% CI=1.16–1.27). Moreover, the results did not essentially change, after excluding individuals with a history of chronic pulmonary disease who were more likely to use tetracyclines and other broad-spectrum antibiotics (OR for broad-spectrum antibiotics=1.18, 95% CI=1.13–1.24; OR for tetracyclines=1.16, 95% CI=1.10–1.22), excluding individuals with diverticular disease (OR for broad-spectrum antibiotics=1.18, 95% CI=1.13–1.23), and excluding antibiotic dispensations within 2 years of polyp diagnosis (OR for broad-spectrum antibiotics=1.15, 95% CI=1.08–1.22).
When polyps were classified into conventional adenomas and serrated polyps, antibiotic use showed a generally stronger association with serrated polyps than conventional adenomas (Figure 2A). The difference was particularly prominent for broad-spectrum antibiotics (OR for serrated polyps=1.29, 95% CI=1.21–1.38; OR for conventional adenomas=1.17, 95% CI=1.11–1.24; Pheterogeneity=0.04) and anti-anaerobic antibiotics (OR for serrated polyps=1.20, 95% CI=1.08–1.34; OR for conventional adenomas=1.02, 95% CI=0.93–1.12; Pheterogeneity=0.03). No differences were observed for the association of antibiotics with colon or rectal polyps (Figure 2B). When conventional adenomas were further classified by histology (Supplementary Figure 1), we found that most of the antibiotic groups tended to be more strongly associated with higher risk of villous adenomas than tubulovillous or tubular adenomas, although the confidence interval for villous adenomas were wide due to the limited case number (n=397).
Figure 2.
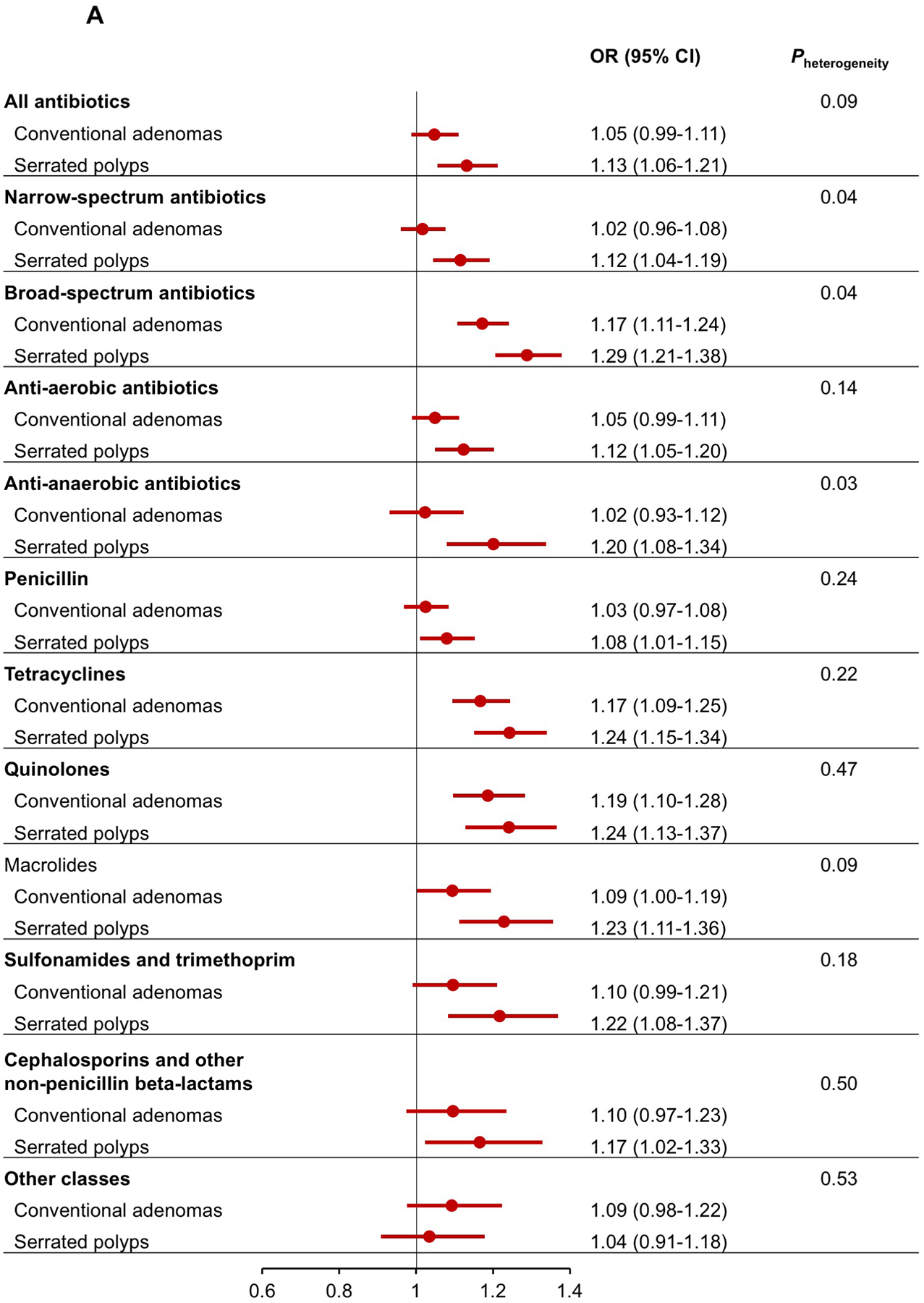
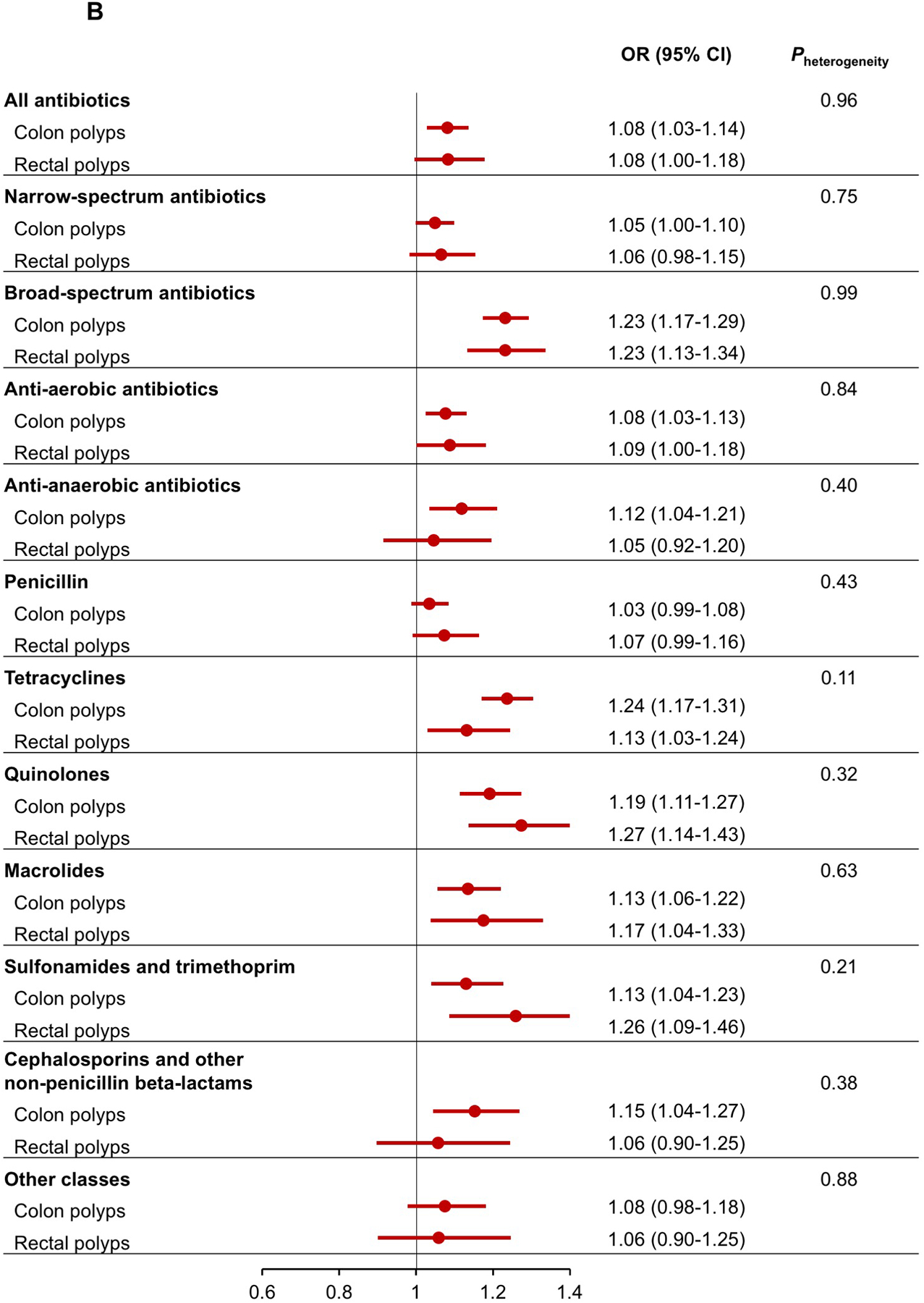
Multivariable association between antibiotic use and risk of conventional adenomas and serrated polyps (A), and colon and rectal polyps (B).
A dose-response relationship was observed when antibiotic use was assessed by cumulative dispensations (Ptrend<0.0001, Table 3). The increased risk of polyps did not emerge until 3–5 dispensations of antibiotics (OR=1.19, 95% CI=1.12–1.26) and became higher with at least 6 dispensations (OR=1.33, 95% CI=1.25–1.43). Again, the associations appeared stronger for broad-spectrum than narrow-spectrum antibiotics. A statistically significant trend was found for anti-anaerobic but not anti-aerobic antibiotics, although the number of cases with more than two dispensations of anti-anaerobic antibiotics was small (n=572). The risk of polyps also increased with more dispensations of penicillin, tetracyclines, and quinolones (Ptrend<0.0001), but not with other classes.
Table 3.
Association between cumulative dispensations of antibiotics and risk of colorectal polyps
| No prior use | 1 prior dispensation | 2 prior dispensations | 3–5 prior dispensations | ≥6 prior dispensations | P trend | |
|---|---|---|---|---|---|---|
| All antibiotics | ||||||
| No. of cases | 16,860 | 8,094 | 5,629 | 8,563 | 6,598 | |
| No. of sibling controls | 40,085 | 17,583 | 10,970 | 14,719 | 9,950 | |
| OR (95% CI)* | 1 (ref) | 0.95 (0.90–1.01) | 1.05 (0.98–1.12) | 1.19 (1.12–1.26) | 1.33 (1.25–1.43) | <0.0001 |
| Narrow-spectrum antibiotics | ||||||
| No. of cases | 22,279 | 9,482 | 5,267 | 5,982 | 2,734 | |
| No. of sibling controls | 49,854 | 19,115 | 9,906 | 10,152 | 4,280 | |
| OR (95% CI)* | 1 (ref) | 1.00 (0.95–1.05) | 1.02 (0.96–1.09) | 1.17 (1.10–1.25) | 1.19 (1.09–1.30) | 0.03 |
| Broad-spectrum antibiotics | ||||||
| No. of cases | 26,650 | 8,184 | 4,196 | 4,424 | 2,290 | |
| No. of sibling controls | 61,519 | 15,269 | 6,714 | 6,543 | 3,262 | |
| OR (95% CI)* | 1 (ref) | 1.12 (1.06–1.18) | 1.32 (1.23–1.42) | 1.37 (1.28–1.47) | 1.38 (1.25–1.52) | <0.0001 |
| Anti-aerobic antibiotics | ||||||
| No. of cases | 17,307 | 8,271 | 5,679 | 8,386 | 6,101 | |
| No. of sibling controls | 41,040 | 17,904 | 10,938 | 14,289 | 9,136 | |
| OR (95% CI)* | 1 (ref) | 0.95 (0.90–1.01) | 1.05 (0.98–1.11) | 1.19 (1.13–1.26) | 1.33 (1.24–1.43) | <0.0001 |
| Anti-anaerobic antibiotics | ||||||
| No. of cases | 41,414 | 2,965 | 793 | 439 | 133 | |
| No. of sibling controls | 85,925 | 5,189 | 1,229 | 728 | 236 | |
| OR (95% CI)* | 1 (ref) | 1.08 (1.00–1.17) | 1.20 (1.03–1.39) | 1.18 (0.97–1.44) | 0.76 (0.53–1.10) | 0.36 |
| Penicillin | ||||||
| No. of cases | 23,330 | 9,487 | 5,186 | 5,633 | 2,108 | |
| No. of sibling controls | 51,888 | 19,149 | 9,660 | 9,383 | 3,227 | |
| OR (95% CI)* | 1 (ref) | 0.98 (0.93–1.03) | 1.06 (0.99–1.13) | 1.14 (1.07–1.22) | 1.23 (1.11–1.36) | <0.0001 |
| Tetracyclines | ||||||
| No. of cases | 34,074 | 6,685 | 2,423 | 1,899 | 663 | |
| No. of sibling controls | 74,729 | 11,554 | 3,509 | 2,589 | 926 | |
| OR (95% CI)* | 1 (ref) | 1.13 (1.07–1.19) | 1.33 (1.22–1.46) | 1.37 (1.24–1.52) | 1.43 (1.20–1.69) | <0.0001 |
| Quinolones | ||||||
| No. of cases | 39,043 | 3,902 | 1,443 | 1,049 | 307 | |
| No. of sibling controls | 82,635 | 6,354 | 2,211 | 1,607 | 500 | |
| OR (95% CI)* | 1 (ref) | 1.20 (1.11–1.29) | 1.32 (1.18–1.49) | 1.16 (1.01–1.32) | 1.12 (0.88–1.42) | <0.0001 |
| Macrolides | ||||||
| No. of cases | 40,482 | 3,453 | 961 | 630 | 218 | |
| No. of sibling controls | 84,607 | 5,903 | 1,506 | 949 | 342 | |
| OR (95% CI)* | 1 (ref) | 1.12 (1.04–1.21) | 1.15 (1.00–1.32) | 1.33 (1.12–1.57) | 1.01 (0.76–1.36) | 0.08 |
| Sulfonamides and trimethoprim | ||||||
| No. of cases | 41,890 | 2,617 | 657 | 434 | 146 | |
| No. of sibling controls | 87,029 | 4,159 | 1,109 | 751 | 259 | |
| OR (95% CI)* | 1 (ref) | 1.19 (1.10–1.30) | 1.09 (0.92–1.28) | 1.09 (0.89–1.34) | 1.06 (0.76–1.48) | 0.31 |
| Cephalosporins and other non-penicillin beta-lactams | ||||||
| No. of cases | 43,064 | 2,045 | 408 | 176 | 51 | |
| No. of sibling controls | 88,721 | 3,541 | 663 | 317 | 65 | |
| OR (95% CI)* | 1 (ref) | 1.17 (1.07–1.29) | 1.01 (0.82–1.25) | 0.89 (0.64–1.22) | 1.11 (0.57–2.18) | 0.09 |
| Other classes | ||||||
| No. of cases | 42,785 | 1,786 | 502 | 415 | 256 | |
| No. of sibling controls | 88,183 | 3,098 | 862 | 725 | 439 | |
| OR (95% CI)* | 1 (ref) | 1.11 (1.00–1.23) | 1.10 (0.91–1.33) | 1.04 (0.84–1.27) | 0.83 (0.64–1.08) | 0.20 |
Multivariable conditional logistic regression model was adjusted for age (continuous), sex (binary), year of birth (continuous), income levels (quintiles), education (9 years or less, 10–12 years, >12 years, missing), total number of prior clinic visits (quintiles), Charlson comorbidity score (continuous), and major comorbidities (all binary, including diabetes, cardiovascular disease, non-colorectal cancer, liver disease, chronic pulmonary disease, connective tissue disease, and peptic ulcer disease).
In the stratified analysis by age, although the P for interaction was less than 0.05 for overall antibiotic use and several individual categories of antibiotics, most of the differences in ORs were fairly modest, except for a particularly stronger association with increased risk of polyps in younger than older adults for quinolones, sulfonamides and trimethoprim, and cephalosporins (Pinteraction<0.001, Supplementary Figure 2). For example, the OR (95% CI) for quinolones was 1.33 (1.08–1.62) in adults aged <50 years and 1.16 (1.01–1.33) in ≥70 years; the OR for sulfonamides and trimethoprim was 1.37 (1.08–1.75) in <50 years and 1.10 (0.94–1.29) in ≥70 years.
Discussion
In this nationwide case-control study, even after accounting for hereditary and early life environmental factors, we found that patients with a colorectal polyp were more likely to have been exposed to antibiotics than their unaffected siblings. The risk elevation of colorectal polyps associated with antibiotic use was higher for broad-spectrum compared to narrow-spectrum antibiotics, and for tetracyclines and quinolones compared to penicillin and other classes. The associations became stronger after accounting for potential misclassification in the control group due to the unknown polyp status. Our findings complement earlier reports of an association between antibiotics and increase risk of CRC and provide novel evidence for the role of the gut dysbiosis in early development of colorectal neoplasia.
Exposure to antibiotics induces pervasive changes in the gut microbial community. Although the overall community can be largely restored within 4 weeks after antibiotic treatment, the recovery varies between individuals and classes of antibiotics and is often incomplete.24, 25 Particularly, repeated exposures to antibiotics can lead to a persistent regime shift.24 A recent study in the UK reported that use of antibiotics, primarily anti-aerobic antibiotics, was associated with an increased risk of colon cancer, but a lower risk of rectal cancer.13 In contrast, anti-anaerobic, but not anti-aerobic, antibiotics were associated with a higher risk of both colon and rectal cancer in a case-control study in patients with type 2 diabetes in Taiwan.12 Increasing data suggest that the pro-CRC effect of gut dysbiosis starts early in carcinogenesis. Patients with colorectal polyps have already demonstrated substantial shifts in the composition of the gut microbiota.6, 7 However, existing epidemiologic evidence is largely cross-sectional and unable to address whether microbial alterations are a cause or consequence of colorectal carcinogenesis.5 Moreover, given the long latency period of CRC and that infection can be associated with the clinical presentation of CRC, short-term studies using CRC as the endpoint are prone to reverse causality.
Therefore, to shed light on the role of gut microbiota in initiation of colorectal carcinogenesis, we focused on generally asymptomatic CRC precursors and examined antibiotic exposures at a median of 5 years prior to diagnosis of colorectal polyps. By further excluding polyp cases diagnosed within one year after exposure assessment, we minimized any influence of reverse causality. In line with our findings, prior evidence in CRC also suggest an early-acting effect of antibiotics. Two studies found that use of antibiotics more than 10 years before the cancer diagnosis were more strongly associated with increased CRC risk, compared to antibiotic use within 10 years before CRC diagnosis.8, 13 For conventional adenomas, we found that antibiotics tended to be more strongly associated with villous adenomas than tubular and tubulovillous adenomas, indicating potential role of antibiotics in the malignant transformation of precancers.
Furthermore, we found a stronger association for broad-spectrum than narrow-spectrum antibiotics. This is not unexpected, since antibiotics with broad-spectrum activity can influence a wide variety of microbial populations and produce long-lasting effects on the microbiota.26 Among the broad-spectrum antibiotics, we found a particularly strong association for tetracycline and quinolone family of antibiotics with increased risk of both colon and rectal polyps. These findings contrast with a recent study that observed a beneficial association of tetracyclines with rectal cancer.13 While there is some evidence for the anti-inflammatory effects of tetracyclines, doxycycline, the most commonly used agent within the tetracycline family in our study population, increased colonic tumor multiplicity and promoted metastasis in an in vivo study.27 Moreover, exposure to tetracyclines has been associated with increased risk of other GI disorders, such as IBD and irritable bowel syndrome.28, 29 As for quinolones, these drugs are commonly prescribed for GI infections and have been associated with higher CRC risk in several prior studies.8, 9, 11
CRC is a heterogeneous disease. Conventional adenomas and serrated polyps represent two distinct groups of precursors for the adenoma-carcinoma pathway and the serrated pathway, respectively.3 Compared to other CRCs, serrated CRCs are more likely to have BRAF mutation and microsatellite instability. Some risk factors, such as smoking and obesity, have been more strongly associated with serrated polyps than conventional adenomas.30 Positivity of F. nucleatum, one of the most studied pro-CRC microbes, has been associated with clinicopathological and molecular features of serrated neoplasia.5 Despite these data, no study has yet examined whether antibiotic use was differentially associated with adenomatous versus serrated colorectal neoplasia. In the current study, we found that antibiotic use was more strongly associated with increased risk of serrated polyps than conventional adenomas (for 11 out of the 12 studied antibiotic categories, Figure 2A), suggesting a particularly important role of gut dysbiosis in the serrated pathway of colorectal neoplasia. Further studies assessing CRC by molecular features will be important to better understand the mechanisms through which gut microbiota influences CRC.
Strengths of our study include the use of a large, nationally representative sample, a validated approach for polyp ascertainment, and linkage of the national drug registry that has virtually complete information on all drug dispensations, including antibiotics. Moreover, our use of full sibling controls eliminated any confounding effect of ethnicity and hereditary predisposition and minimized the confounding by shared environmental factors.
Our study also has several limitations. First, residual confounding cannot be excluded, particularly because we lacked data on indications for antibiotic use and major CRC risk factors, such as smoking and adiposity status. However, our observations for the dose-response relationship and differences in the associations across classes of antibiotics that have common indications suggest that confounding by indication is unlikely to fully explain our findings. Furthermore, we adjusted for the Charlson comorbidity score and several major individual comorbidities that are strongly associated with smoking and obesity. Second, the Prescribed Drug Register does not contain antibiotics data from inpatient care or use of parenteral antibiotics. However, more than 75% of parenteral antibiotics in Sweden consisted of benzylpenicillin31 that has a limited impact on the microbiota.32 Third, because the Drug Register did not start until 2005, we were unable to assess use of antibiotics in the remote past. However, this is less of a concern for studies of colorectal polyps, which represent the very early alterations in the continuum of colorectal carcinogenesis. Fourth, the polyp status of sibling controls is largely unknown due to the lack of endoscopic screening programs. However, any misclassification in the control group could only have attenuated our effect estimates, as demonstrated in our sensitivity analysis. Finally, the prescription patterns may differ between countries and thus the generalizability of our findings needs to be confirmed.
In conclusion, antibiotic use was associated with increased risk of colorectal polyps. The association was stronger for broad-spectrum antibiotics and tetracyclines and quinolones. A greater risk elevation associated with antibiotics was also noted for serrated polyps compared to conventional adenomas. Our findings support a role of the gut dysbiosis in early colorectal carcinogenesis.
Supplementary Material
Need to Know.
Background:
Use of antibiotics affects the composition of the microbiome and might affect development of colorectal polyps, precursors to colorectal cancer
Findings:
A nationwide case–control study of 45,744 adults with a colorectal polyp and 93,307 unaffected full sibling controls found an 8% increase in risk of colorectal polyps associated with users of any antibiotics and a 33% increase in risk associated with at least 6 dispensations of antibiotics. Antibiotic use was more strongly associated with higher risk of serrated polyps than conventional adenomas.
Implications for patient care:
Intestinal dysbiosis might contribute to early stages of colorectal carcinogenesis. Strategies should be developed to reduce these effects of antibiotics and prevent colorectal cancer.
Funding statement:
The work was supported by the U.S. National Institutes of Health (NIH, R01 CA202704 and R01 CA137178 to ATC, R00 CA215314 to MS), American Cancer Society (MRSG-17-220-01-NEC to MS), and Union for International Cancer Control (Yamagiwa-Yoshida Award YY2/17/554363 to MS). ATC is a Stuart and Suzanne Steele MGH Research Scholar. None of the authors are employed by NIH.
The study sponsors had no role in the study design; data collection; data analysis; and interpretation of data; writing of the report; and the decision to submit the paper for publication.
The corresponding author (JFL) had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Conflicts of interest:
Dr. Chan reports grants from Bayer Pharma AG, personal fees from Pfizer Inc., personal fees from Janssen Pharmaceuticals, personal fees from Boeringher Ingelheim, outside the submitted work. Dr Ludvigsson coordinates a study on behalf of the Swedish IBD quality register (SWIBREG) and that study has received funding from Janssen corporation.
Abbreviations:
- CI
confidence interval
- CRC
colorectal cancer
- ESPRESSO
Epidemiology Strengthened by histoPathology Reports in Sweden
- GI
gastrointestinal
- IBD
inflammatory bowel disease
- OR
odds ratio
- SD
standard deviation
- SnoMed
Systematized Nomenclature of Medicine
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.Collaborators GBDCC. The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4(12):913–33. Epub 2019/10/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16(12):713–32. Epub 2019/08/29. [DOI] [PubMed] [Google Scholar]
- 4.Song M, Giovannucci E. Preventable Incidence and Mortality of Carcinoma Associated With Lifestyle Factors Among White Adults in the United States. JAMA Oncol. 2016;2(9):1154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song M, Chan AT, Sun J. Influence of the Gut Microbiome, Diet, and Environment on Risk of Colorectal Cancer. Gastroenterology. 2020;158(2):322–40. Epub 2019/10/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakatsu G, Li X, Zhou H, et al. Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat Commun. 2015;6:8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters BA, Dominianni C, Shapiro JA, et al. The gut microbiota in conventional and serrated precursors of colorectal cancer. Microbiome. 2016;4(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armstrong D, Dregan A, Ashworth M, et al. The association between colorectal cancer and prior antibiotic prescriptions: case control study. Br J Cancer. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boursi B, Haynes K, Mamtani R, et al. Impact of antibiotic exposure on the risk of colorectal cancer. Pharmacoepidemiol Drug Saf. 2015;24(5):534–42. [DOI] [PubMed] [Google Scholar]
- 10.Cao Y, Wu K, Mehta R, et al. Long-term use of antibiotics and risk of colorectal adenoma. Gut. 2018;67(4):672–8. Epub 2017/04/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dik VK, van Oijen MG, Smeets HM, et al. Frequent Use of Antibiotics Is Associated with Colorectal Cancer Risk: Results of a Nested Case-Control Study. Dig Dis Sci. 2016;61(1):255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang JL, Chang CH, Lin JW, et al. Infection, antibiotic therapy and risk of colorectal cancer: a nationwide nested case-control study in patients with Type 2 diabetes mellitus. Int J Cancer. 2014;135(4):956–67. Epub 2014/01/29. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Haines C, Watson AJM, et al. Oral antibiotic use and risk of colorectal cancer in the United Kingdom, 1989–2012: a matched case-control study. Gut. 2019;68(11):1971–8. Epub 2019/08/21. [DOI] [PubMed] [Google Scholar]
- 14.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, et al. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–67. Epub 2009/06/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ludvigsson JF, Lashkariani M. Cohort profile: ESPRESSO (Epidemiology Strengthened by histoPathology Reports in Sweden). Clin Epidemiol. 2019;11:101–14. Epub 2019/01/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song M, Emilsson L, Bozorg SR, et al. Risk of colorectal cancer incidence and mortality after polypectomy: a Swedish record-linkage study. Lancet Gastroenterol Hepatol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emilsson L, Loberg M, Bretthauer M, et al. Colorectal cancer death after adenoma removal in Scandinavia. Scand J Gastroenterol. 2017;52(12):1377–84. Epub 2017/09/15. [DOI] [PubMed] [Google Scholar]
- 18.Bozorg SR, Song M, Emilsson L, et al. Validation of Serrated Polyps (SPs) in Swedish Pathology Registers. BMC Gastroenterology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wettermark B, Hammar N, Fored CM, et al. The new Swedish Prescribed Drug Register--opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16(7):726–35. [DOI] [PubMed] [Google Scholar]
- 20.Ludvigsson JF, Haberg SE, Knudsen GP, et al. Ethical aspects of registry-based research in the Nordic countries. Clinical epidemiology. 2015;7:491–508. Epub 2015/12/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludvigsson JF, Lebwohl B, Ekbom A, et al. Outcomes of Pregnancies for Women Undergoing Endoscopy While They Were Pregnant: A Nationwide Cohort Study. Gastroenterology. 2017;152(3):554–63 e9. Epub 2016/10/25. [DOI] [PubMed] [Google Scholar]
- 22.Fox MP, Lash TL, Greenland S. A method to automate probabilistic sensitivity analyses of misclassified binary variables. Int J Epidemiol. 2005;34(6):1370–6. Epub 2005/09/21. [DOI] [PubMed] [Google Scholar]
- 23.Wang M, Spiegelman D, Kuchiba A, et al. Statistical methods for studying disease subtype heterogeneity. Stat Med. 2016;35(5):782–800. Epub 2015/12/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A. 2011;108 Suppl 1:4554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dethlefsen L, Huse S, Sogin ML, et al. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6(11):e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willing BP, Russell SL, Finlay BB. Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat Rev Microbiol. 2011;9(4):233–43. Epub 2011/03/02. [DOI] [PubMed] [Google Scholar]
- 27.Nanda N, Dhawan DK, Bhatia A, et al. Doxycycline Promotes Carcinogenesis & Metastasis via Chronic Inflammatory Pathway: An In Vivo Approach. PLoS One. 2016;11(3):e0151539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Margolis DJ, Fanelli M, Hoffstad O, et al. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am J Gastroenterol. 2010;105(12):2610–6. [DOI] [PubMed] [Google Scholar]
- 29.Villarreal AA, Aberger FJ, Benrud R, et al. Use of broad-spectrum antibiotics and the development of irritable bowel syndrome. WMJ. 2012;111(1):17–20. [PubMed] [Google Scholar]
- 30.He X, Wu K, Ogino S, et al. Association Between Risk Factors for Colorectal Cancer and Risk of Serrated Polyps and Conventional Adenomas. Gastroenterology. 2018;155(2):355–73 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jankovic N, Geelen A, Winkels RM, et al. Adherence to the WCRF/AICR Dietary Recommendations for Cancer Prevention and Risk of Cancer in Elderly from Europe and the United States: A Meta-Analysis within the CHANCES Project. Cancer Epidemiol Biomarkers Prev. 2017;26(1):136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Huang Y, Zhou Y, et al. Antibiotic administration routes significantly influence the levels of antibiotic resistance in gut microbiota. Antimicrob Agents Chemother. 2013;57(8):3659–66. Epub 2013/05/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


