Abstract
Introduction
Currently, no consensus on optimal renal replacement modality has been reached for end-stage renal disease (ESRD) patients complicated with hemophilia. They may require infusion of coagulation factors during each hemodialysis session. In comparison, peritoneal dialysis (PD) might be preferred considering that coagulation replacement is only required for catheter placement. However, limited data on the safety and efficacy of PD for treating ESRD patients with hemophilia were reported.
Methods
This is a single-center retrospective cohort study. ESRD patients diagnosed with hemophilia under PD in Peking Union Medical College Hospital from January 1, 1996 to December 31, 2021 were included and followed-up with every month. Their baseline clinical data, catheter insertion procedure, coagulation factor replacement, complications, and outcome were analyzed and compared with general PD patients.
Results
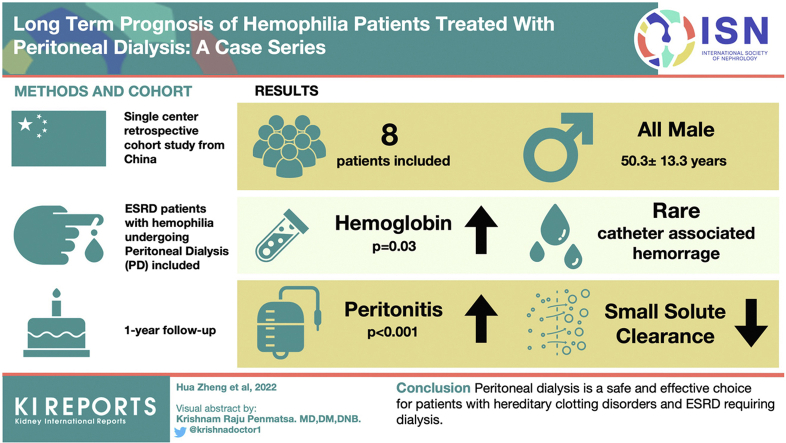
In total, 8 patients diagnosed with hemophilia were included, all-male, with a mean age of 50.3±13.3 years old. Two were acquired hemophilia A, whereas the rest were hereditary hemophilia A (HHA). Seven patients experienced significant hemoglobin (Hgb) increment after PD. Peritoneal hemorrhage only consisted of a small portion of all hemorrhage. Patients with hemophilia seemed to have lower small solute clearance despite higher baseline peritoneal permeability, and appeared to have increased peritonitis rate than other male PD patients, yet this study is not powered to prove this.
Conclusion
PD is a safe and effective choice for patients with hemophilia and ESRD requiring dialysis. More studies are required to evaluate this certain rare group of patients.
Keywords: end-stage kidney disease, hemophilia, peritoneal dialysis, peritoneal hemorrhage, peritonitis
Graphical abstract
Hemophilia is the most common coagulation factor deficiency disease, is hereditary or acquired, and caused by factor inhibitors. Hemophilia A is caused by deficiency of coagulation factor VIII (F VIII), whereas hemophilia B and C patients lack factor IX and factor XI, respectively. Patients with severe hemophilia suffer from spontaneous bleeding, possibly hemophiliac arthroplasty, and require routine prophylactic coagulation factor infusion.1
Patients with hemophilia appear to have a higher incidence of chronic kidney disease (CKD) than the general population.1 Kulkarni et al.2 reported that the rate of CKD was 4.7 per 1000 among hemophilia patients, and the estimated rate of CKD in the general male population was 2.9 per 1000 in the US. Potential etiologies leading to renal dysfunction in these patients may include HIV infection, hepatitis C virus (HCV) infection,1 and urinary obstruction due to blood clots.2
With rising life expectancy,3 an increasing number of hemophilia patients with CKD progressed to ESRD and required renal replacement treatment. Until now, the consensus on optimal dialysis modality for these patients was not reached. Hemodialysis (HD) is the most common modality in ESRD patients worldwide.4 However, coagulation factor deficiency in hemophilia patients obliges coagulation factor administration during each HD session. The anticoagulation and potential significant blood pressure fluctuation complicate the hemostasis issue. For PD patients, coagulation replacement therapy was only required for peritoneal catheter placement. Less blood pressure variability also theoretically reduces hemorrhage risk. However, there has been limited data about the safety and efficacy of PD in treating ESRD patients with hemophilia. In this study, we report a single-center case series of 8 ESRD patients with hemophilia treated with PD. Their clinical characteristics, catheter insertion procedure, coagulation factor replacement, complications, outcome of PD, and hemophilia treatment were analyzed.
Methods
Study Design and Participants
This is a single-center, retrospective cohort study. The ESRD patients with acquired or hereditary hemophilia who received PD treatment in Peking Union Medical College Hospital from January 1, 1996 to December 31, 2021 were included. Their baseline data were compared with other male PD patients in our center. This study was approved by Peking Union Medical College Hospital ethical review committee [ZS-1152]. Given the nonintrusive nature of the research, the written consent was waived.
Baseline Data Collection
The clinical data, including demographic information, primary renal disease, hemophilia diagnosis, and comorbidity were extracted from the medical record. The baseline laboratory data, including Hgb levels, serum creatinine (Cr) levels, F VIII activity, and inhibitors, activated partial thromboplastin clotting time, and estimated glomerular filtration rate calculated based on the Chronic Kidney Disease Epidemiology formula were also collected (Tables 1 and 2).5 The severity classification was based on practice guidelines from the International Society on Thrombosis and Hemostasis.6
Table 1.
Baseline characteristics of 8 ESRD patients with hemophilia
| Patient # | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Gender | M | M | M | M | M | M | M | M |
| Dialysis age | 44 | 28 | 31 | 55 | 67 | 59 | 56 | 61 |
| Causes of renal failure | HCV-GN | Obstruction | GN | DN | DN, ATN | DN | Ectopic kidney, Obstruction, NS | DN |
| Hemophilia type | Severe HHA | Severe HHA | Mild HHA | Moderate HHA | AHA | Severe HHA | Severe HHA | AHA |
| Other comorbidity | HCV, THPT | Acute myelitis, HTN | HTN, HCV (cured) |
HTN | AF, HF | HCV | HTN, HF | CG, HTN |
| ESRD complication | Overload | data unavailable | Overload, Uremia, HF | None | Overload, HF | Overload HK |
Overload, HF | Uremia |
| Known CKD duration | 4 yr | 2 yr | 10 yr | 5 yr | 9 yr | 5 yr | 2 wka | 4 yr |
| Urea (mmol/l) | 20.7 | 56.6 | 37.6 | 29.2 | 28.0 | 36.2 | 37.6 | 41.2 |
| SCr (μmol/l) | 1592 | 2153 | 1283 | 645 | 600 | 868 | 946 | 743 |
| HD before PD | 3 mo | <2 wk | None | None | None | None | < 2 wk | None |
| Notable concurrent treatmentsb | None | None | None | Vitrectomy | Bortezomib | None | None | Rituximab+ bortezomib |
AHA, acquired hemophilia type A; CG, cryoglobulinemia; dx, diagnosis; DN, diabetic nephropathy; GN, glomerulonephritis; HCV, hepatitis C virus; HD, hemodialysis or continue renal replacement therapy; HF, heart failure; HK, hyperkalemia; HHA, hereditary hemophilia type A; HTN, hypertension; M, male; NS, nephrotic syndrome; PD, peritoneal dialysis; THPT, tertiary hyperparathyroidism.
The judgment of chronic nature of the patient’s kidney disease was based on bilateral renal atrophy.
Refers to important treatment within 2 weeks after catheter placement.
Table 2.
Perioperative management and initial dialysis of 8 patients
| Patient # | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Days per hemorrhage episode before | Unknown | Unknown | NAa | NAa | NA | 127 | 77 | NA |
| Baseline FV III (%) | 1.4 | Data unavailable | 12.2 | 4.6 | 13.2 | 2.6 | 1.8 | 34 |
| Baseline APTT | 87.1s | 101s | 73.1s | 51.5s | 72.7s | 53.5s | 93.5s | 40.0s |
| F VIII Inhibitor (BU/ml) | 0 | 0 | 0 | 0 | 2 BU/ml | 0 | 0 | 0.7 BU/ml |
| F VIII target on operational day (%) | 80 | 80 | 80 | 50 | None | 80 | 80 | None |
| Perioperative complication | Data unavailable | None | D2 Peritonitis D10 blood-tinged effluent. |
D3 Minor tunnel exit bleeding | D2 blood-tinged effluent | None | D9 knee | Tunnel exit infection |
| PD type | Elective | Elective | Elective | Elective | Elective | Elective | Emergent | Elective |
| Dialysis prescription 1 week within PD initiation | 1.5%×2 sets+ 2.5%×2 sets +dwell | 1.5%×4 sets+ 2.5%×1 set +dwell | 1.5%×3 sets+ 2.5%×2 sets+dwell | 1.5%×3 sets | 1.5%×3sets | 1.5%×4 sets×1.5l×3h | 1.5%×4 sets×2l×1h→3h | 1.5%×4 sets×2l×3h |
| CCr l/w 1.73 m2 2 months after PD initiation | 58.6 | 41.33 | 72.53 | 90.0 | 65.1 | 72.1 | 60.0 | 76.5 |
| KT/V 2 months after PD initiation | 1.51 | 2.27 | 1.69 | 2.30 | 1.58 | 1.84 | 1.61 | 2.21 |
| NPCR g/kd·d 2 months after PD initiation | 0.89 | 0.57 | 0.81 | 0.92 | 0.56 | 0.80 | 0.56 | 0.79 |
| rGFR ml/min 2 months after PD initiation | 1.69 | 0.08 | 4.44 | 6.02 | 2.56 | 4.50 | 2.64 | 5.22 |
| UF (ml/d) 2 months after PD initiation | −350 | 750 | 0 | −350 | 260 | 200 | 1050 | 700 |
| Urine (ml/d) 2 months after PD initiation | 800 | 0 | 2600 | 2300 | 620 | 1050 | 1050 | 1600 |
| Baseline Cr 4h D/P 6 months after PD initiation | 0.74 (HA) | 0.77 (HA) | 0.71 (HA) | 0.85 (H) | 0.84 (H) | 0.85 (H) | b | 0.89 (H) |
APTT, Activated partial thromboplastin clotting time; CCr, creatinine clearance; F VIII, coagulation factor VIII; HA, High-average; H, High; HD, hemodialysis or continue renal replacement therapy; Kt/V, urea clearance index; nPCR, normalized protein catabolic rate; PD, peritoneal dialysis; rGFR, residual renal glomerular filtration rate; UF, ultrafiltration.
No significant hemorrhage event was noticed in the previous year.
PET postponed due to peritonitis.
Catheter Insertion
All patients were inserted with the swan-neck silicone rubber catheter under regional anesthesia with 2 Dacron cuffs and coiled tips through blind surgical procedure. The incision allowing passage of the catheter were made as small as possible. Special care was used for minimal trauma and to avoid collateral damage and excessive bleeding. Catheter flow tests were performed right after surgery.
Perioperative Coagulation Factor Supplement
The perioperative supplement plan generally followed the World Federation of Hemophilia guideline7 for severe HHA patients. More specifically, F VIII activity was set for 40% to approximately 80% for postoperative day 0 to day 3, 30% to 60% for day 4 to day 6, and 20% to 40% for day 7 to day 14, which was also consistent with later published Chinese Society of Hematology Guideline8 for moderate surgery. In the event of minor hemoperitoneum causing nonsignificant Hgb fluctuation, an additional 20 IU/kg F VIII infusion was given. However, the individual plan was additionally determined by the following: (i) independent judgment of hemorrhage risk based on previous bleeding events by different hematologists, and (ii) F VIII affordability of the patient. F VIII pharmacokinetic study was also conducted before surgery to determine F VIII half-life whenever possible in HHA patients, which guided the supplement infusion frequency in case of emergent major bleeding. Activated partial thromboplastin clotting time, F VIII activity, inhibitors, and Hgb level were monitored perioperatively. If Hgb remained stable and no obvious hemorrhage was observed at the end of 2 weeks (“perioperative period”), we considered no further need for excessive perioperative supplement. The patients then received regular hemophiliac prophylactic replacement.
Perioperative Dialysis Management
The catheter was routinely flushed on days 2, 7, and 14 postoperatively, and might increase to 3 times a day in the events of peritoneal hemorrhage to avoid obstruction. To avoid hemorrhage caused by rapid dialysate current, the fresh bag in the double-bag Y-set system was kept 60 cm to approximately 80 cm above the patient. Patients were also encouraged to keep a supine position whenever possible to minimize abdominal pressure for the first 2 weeks of PD. Measures like strict dialysate temperature control (37 °C), and special care to avoid stretching or abrasion were taken as well.
Follow-ups and Outcomes
Following the clinical routine, all PD patients in our center visited the clinic for the monthly laboratory examination. Their clinical status, comorbidity, complications of infection, and hemorrhage were documented. The urea clearance index (Kt/V), Cr clearance, and residual renal glomerular filtration rate were measured bimonthly. The peritoneal equilibration test was performed every 6 months. Patients with hemophilia were also followed by the hematologists. The primary endpoint was defined as death. Complications, including, peritonitis,9 and mechanical complications of PD were recorded. In terms of hemorrhage episodes, it was collected through monthly self-reports. Hemoperitoneum was simply defined as self-reported blood-tinged effluent. Average peritonitis rates were calculated as total follow-up months divided by the number of peritonitis episodes.
Statistical Analysis
Continuous variables with normal distribution were expressed as mean ± SD and compared by t-tests. The skewed distributed variables were presented as the median and interquartile range, analyzed by Mann-Whitney U tests. Categorical data were presented as percentages and compared by chi square test for statistical significance testing. Cox proportional regression model was performed with peritonitis survival. All statistical analyses were conducted by SPSS, version 19.0. (SPSS Inc., Chicago, IL, USA).
Results
Baseline Characteristics
Until December 31, 2021, our PD program enrolled 947 patients. Among them, 8 hemophilia patients were included in this study. All these patients were male, with a mean age of 50.3±13.3 years old at the initiation of dialysis. Six of them were diagnosed with hereditary hemophilia and the remaining 2 had acquired hemophilia A. As for severity assessment, 4 of the 6 HHA patients were classified as severe, and the other 2 patients were moderate and mild (Table 1).
The causes of ESRD in these patients were various, including diabetic nephropathy (50%, patient 4, 5, 6, 8), postrenal obstruction (patient 2 and 7), and glomerulonephritis (patient 3). Patient 5 was also considered to have acute tubular necrosis due to hypovolemic and cardiogenic shock. Patient 7 suffered from retroperitoneal hematoma, complicated with ectopic kidney and nephrotic syndrome of unknown etiology. The median time from CKD diagnosis to PD was 4.5 years (interquarline range 11.5, 97.25) (Table 1). Baseline median Cr right before dialysis was 907 (interquarline range 669.5, 1515) μmol/l whereas median urea was 36.9 (interquarline range 28.3, 40.3) mmol/l (Table 1). Their serum Cr and urea levels were significantly higher than general male PD patients (Table 3). No significant difference in baseline age, body mass index, Hgb, white blood cell counts, platelets, albumin, electrolytes, and estimated glomerular filtration rate was found between male PD patients with hemophilia and those without (Table 3).
Table 3.
Demographic characteristics and baseline laboratory data of PD patients with or without hemophilia
| Groups | General male PD patients, N = 288 | Patients with hemophilia, N = 8 | P value |
|---|---|---|---|
| Age (yr) | 56.6±16.9 | 49.8±15.0 | 0.24 |
| BMI (kg/m2) | 23.1±2.9 | 25.8±4.2 | 0.12 |
| Hemoglobin (g/l) | 90.8±16.9 | 83.7±19.4 | 0.47 |
| WBC (109/l) | 7±2.8 | 6.7±1.8 | 0.65 |
| Blood platelets (109/l) | 187.5±72.1 | 159.3±68.3 | 0.29 |
| Albumin (g/l) | 34.3±6.1 | 33.4±3.2 | 0.46 |
| LDL (mmol/l) | 2.8±1 | 2.2±1.1 | 0.21 |
| K (mmol/l) | 4.5±0.7 | 4.4±0.6 | 0.43 |
| Ca (mmol/l) | 2.3±0.3 | 2.2±0.2 | 0.17 |
| P (mmol/l) | 1.6±0.6 | 1.7±0.7 | 0.64 |
| Uric acid (umol/l) | 474±137 | 565.3±106.8 | 0.04 |
| Creatinine (umol/l) | 694 (552, 871) | 907 (669.5, 1515) | 0.02 |
| Urea (umol/l) | 25.9 (19.5, 32.5) | 36.9 (28.3, 40.3) | 0.02 |
| eGFR (ml/min/1.73 m2) | 6.7±0.2 | 5.4±0.8 | 0.15 |
| PTH (pg/ml) | 153 (37, 328) | 347(111,400) | 0.11 |
BMI, body mass index; Ca, serum total calcium; eGFR, estimated glomerular filtration rate; K, serum potassium; LDL, low-density lipoprotein; PD, peritoneal dialysis; P, serum phosphorus; PTH, parathyroid hormone; WBC, blood white blood cell counts.
Values for continuous variables given as mean ± SD or median (25th percentile, 75th percentile).
Catheter Insertion Procedure
Catheter insertion procedure was associated with <5 ml hemorrhage for all patients. No significant complication was noted.
Perioperative Coagulation Management
All hereditary hemophilia patients tested negative for F VIII inhibitors before catheterization. For 5 patients (patient 1, 2, 3, 6, and 7), the targeted factor activity before surgery was set as 80%. Patient 4 did not have previous major bleeding history and was only diagnosed with HA before vitrectomy preop workup. The patient had no noticeable bleeding in vitrectomy when F VIII activity target was 50%, and thus the same target was set for catheter placement. The detailed perioperative supplements information was included in Supplementary Table S1. All HHA patients but patient 4 conducted F VIII pharmacokinetic study.
Both acquired HA patients received immunosuppressive treatment with satisfactory inhibitors reduction before catheterization. Patient 5 received 4 courses of bortezomib treatment. Then his F VIII inhibitor dropped from 153.6 to 2.0 BU/ml, activated partial thromboplastin clotting time improved from 114 seconds to 72.7 seconds, and F VIII improved from 0.1% to 13.2% before the placement. He was infused with prothrombin complex concentrate 30 U/kg q8h on operation day and postoperative day 1, 30 u/kg q12h on day 2 to 3, and 20 u/kg QD on day 4 to 7. Patient 7 underwent 1 course of rituximab and bortezomib before the surgery. F VIII inhibitor dropped from 120.0 to 0.7 BU/ml, whereas activated partial thromboplastin clotting time improved from 63.8 seconds to 40 seconds and F VIII improved from 1.2% to 34.0%. No additional supplementation of coagulation product was used perioperatively (Table 2).
Perioperative Complication
One HHA patient (patient 3) and 1 acquired HA patient (patient 5) showed blood-tinged effluent on postoperation day 2. Patient 3 recovered with the enhancement of coagulation factor infusion whereas hemorrhage in patient 5 spontaneously eased after the start of PD (Table 2). All patients showed stable Hgb levels during the 2 postoperative weeks (see raw data in Supplementary Table S2).
Initiation of PD
Patient 7 received urgent-start PD due to heart failure (New York Heart Association functional classification IV) on postop day 3, starting with automated PD 800 ml/set×100 min/set×10 sets×2 days and gradually transited to continuous ambulatory PD (CAPD) 1.5 l/set×4 hours×4 sets/day in 2 weeks. The other 7 patients started PD 2 weeks after catheterization. All temporary HD or other renal replacement therapy stopped after PD initiation (Table 2), then transited to CAPD in 2 weeks. First clearance evaluation (2 months after dialysis) showed that HA patients had lower mean Kt/V (Kt/V, 1.88±0.33 vs. 2.31±0.65, P = 0.01), lower Cr clearance (Cr clearance, 67.0±14.4 vs. 85.1±31.2 L/w·1.73 m2, P = 0.01) and lower nPCR (0.74 ±0.15 vs. 0.91±0.23 g/kd·d, P = 0.02) than general male PD patients in our center. Meanwhile, no significant difference was found for residual renal glomerular filtration rate (3.54 [1.91, 5.04] vs. 3.35 [1.68, 5.57] ml/min, P = 0.82), ultrafiltration (230 [−263, 737] vs. 450 [23.75, 877.5] ml, P = 0.30) and urine volume (1050 [665, 2125] vs. 850 [500, 1400] ml, P = 0.53). The ratio between 4-hour Cr dialysate and plasma (Cr 4h D/P) in peritoneal equilibration test 6 months after the start of PD was significantly higher than the general male PD patients in our center (0.81±0.07 vs. 0.68±0.11, P = 0.01).
Hemophiliac Outcomes
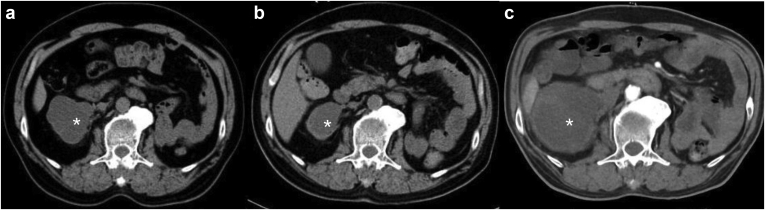
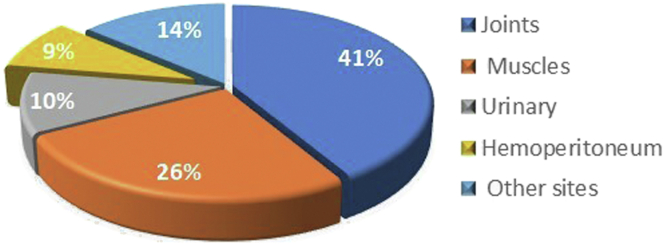
Seven patients demonstrated significantly increased 6-month average Hgb levels after PD (average increment 25.6g/l, P = 0.03). Only patient 7 showed significant Hgb decrement, possibly due to an enlarged retroperitoneal hematoma (Figure 1). Two of the 4 severe HHA patients (patients 6 and 7) received increased F VIII infusions after initiation of PD (Table 4). Among the total 58 episodes of bleeding reported before this study (Figure 2), most of the events were joint hemorrhage (52%), muscle bleeding (16%), and urinary bleeding (10%). Of the 5 (8.6%, once every 71 months on average frequency) episodes of peritoneal hemorrhage, 1 major hemorrhage episode (1.7%) was associated with hypovolemic shock and the rest were merely blood-tinged without significant Hgb reduction.
Figure 1.
Suspected retroperitoneum hematoma (shown as∗). (a) 16 months before PD, size 6.6×5.5×9.5 cm. (b) Same month of catheter placement, size 3.5×5.2×6.5 cm. (c) 7 months after PD, size 7.3×7.3×11 cm.
Table 4.
Follow-ups on complications and outcome of PD
| Patient # | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| PD modality | CAPD | CAPD→APD | CAPD | CAPD | CAPD | CAPD | CAPD | CAPD |
| Outcome | Continue | Continue | Continue | Transferred | Deceased | Continue | Continue | Continue |
| Total follow-ups (mo) | 159 | 131 | 86 | 7 | 13 | 17 | 21 | 6 |
| 6-month average F VIII infusion before PD | Data unavailable | Data unavailable | 0 | 0 | NAa | 108 IU/d | 94 IU/d | NAa |
| 6-month average F VIII infusion after PD | 33 IU/d | 68 IU/d | 0 | 0 | NAa | 266 IU/d | 301 IU/d | NAa |
| Days per hemorrhage episode 1 year before PD | Data unavailable | Data unavailable | NAb | NAb | NAc | 127 | 77 | NAc |
| Days per hemorrhage after PDd | 167 | 165 | 2395 | NAb | NAc | 76 | 90 | NAc |
| Hemoperitoneum after PD | 0 | 2 | 1 | 0 | 0 | 0 | 2 | Data unavailable |
| 6-month average Hgb before PD (g/l) | 105 | 78 | 81 | 85 | 78 | 94 | 89 | 67 |
| 6-month average Hgb after PD (g/l)e | 111 | 111 | 147 | 112 | 112.5 | 118 | 78 | 112 |
| Average peritonitis rates (mo) | 159 | 33 | 17 | 7 | 13 | 17 | 21 | Data unavailable |
| Average peritonitis rates of corresponding controlf (mo) | 77 | 56 | 71 | 69 | 68 | 69 | 69 | 68 |
| Tunnel exit infection (counts) | 4 | 4 | 4 | 1 | 3 | 0 | 2 | 0 |
| Most recent CCr l/w 1.73 m2 | 62.4 | 52.01 | 37.5 | 97.3 | 51.2 | 94.1 | 61.8 | 76.6 |
| Most recent KT/V | 1.93 | 1.77 | 1.50 | 2.14 | 1.61 | 2.71 | 1.65 | 1.97 |
| Most recent nPCR g/kd.d | 0.90 | 0.57 | 0.93 | 0.83 | 0.64 | 0.95 | 0.78 | 0.95 |
| Most recent rGFR ml/min | 0 | 0 | 0 | 5.85 | 1.63 | 6.18 | 0.78 | 3.05 |
| Most recent UF (ml/d) | 900 | 1200 | 2400 | 500 | 1050 | 180 | 450 | −200 |
| Most recent Urine (ml/d) | 0 | 0 | 0 | 1250 | 550 | 1350 | 350 | 1500 |
APD, automated peritoneal dialysis; CAPD, continuous ambulatory peritoneal dialysis; F VIII, coagulation factor III; Hgb, hemoglobin; PD, peritoneal dialysis; UF, ultrafiltration.
Acquired HA.
No significant hemorrhage event was noticed in the previous year.
Incomparable due to improvement in F VIII inhibitors.
One year or longest possible follow-up duration, whichever shorter.
Or longest possible follow-up duration, whichever shorter.
Compared to patients of the same age group in general male PD patients.
Figure 2.
The location of bleeding episodes reported after initiation of peritoneal dialysis.
PD Complications and Outcomes
The median follow-up was 15 (10.8, 92.3) months till February 1, 2022. None of the patients experienced technical failure or were transferred to HD or renal transplant. Patient 5 achieved complete remission for his acquired hemophilia A after 6 courses of bortezomib treatment. He died of pneumonitis after a period of rituximab therapy for the acquired hemophiliac relapse in the thirteenth month of PD. Patient 4 moved to another center in the seventh month because of personal reasons (Table 4). Patient 2 switched to automated PD due to insufficient small solute clearance in the fifth year, while the rest of the patients continued with CAPD. The average peritonitis rate of the 8 patients was every 29.7 months, much shorter than every 77.0 months of the general PD patients (Table 4), whereas 93% of the total 14 episodes (Supplementary Table S3) happened in the first 3 years of PD and 2 patients (Patient 2 and 3) suffered 63% of the episodes. Hemophilia was a risk factor in Cox regression for peritonitis in male patients (Relative risk = 4.4, 95% confidence interval 1.9–10.0, P < 0.001), though the size of this cohort was not powered to support cofounding factor adjustment. Details on peritonitis in PD patients and a summary of bacteriology and risk factors in PD patients in our center were listed in the supplementary material.
Discussion
To our limited knowledge, this study might be the largest case series presenting data of ESRD patients with hemophilia treated with PD. Both successful HD10, 11, 12, 13, 14, 15 and PD10,16, 17, 18 cases were reported before, with unique advantages of each technique. HD provided more intensive care but increased the risk of bleeding exposure and required regular administration of coagulation factor in concomitance with each HD process.2 The PD patients only need extra factor infusion perioperatively with routine safe dialysis at home; it was especially beneficial for patients who suffered from hemophiliac arthropathy with restricted mobility. Therefore, some literature recommended PD to be the optimal choice for ESRD patients with hemophilia and other hereditary clotting disorders.17 Nevertheless, other literature addressed the possible limitations of PD, including the potential risk of PD-related complications, such as hemoperitoneum, peritonitis, and peritoneal catheter infection;1 and especially limitation of its efficacy in complicated HIV-positive subjects and decompensated HCV patients with ascites.
For primary renal causes of our ESRD patients, besides general common etiology like diabetic nephropathy,19 HCV infection and urinary obstruction from hematoma were notable. HCV infection was not rare in elderly HHAs who were prone to frequent blood product infusion back in days when HCV testing was not a standard procedure. Obstruction due to hematoma might be another hemophilia-specific cause of ESRD though not proven significant in hemophilia CKD patients in Europe.1 In general, we should pay more attention to viral infection and obstruction in the etiology of CKD patients with hemophilia.
Surprisingly, we noticed that patients with hemophilia had lower small solute clearance compared to other male patients despite higher peritoneal permeability. It might be because of the shorter dwell time to avoid hemorrhage at starting dialysis (Figure 2). During the CAPD process, hemophiliac arthropathy and insufficient family assistance might lead to shorter exchanges. Considering that the increased Kt/V or Cr clearance did not contribute to survival in recent studies,20 lower Kt/V was not the critical limitation for PD. As a proven risk factor of the technique survival,20 the ultrafiltration was sufficient in these patients with hemophilia.
Our data showed that catheterization and initiation of PD was a safe process for patients with hemophilia. Even in urgent start dialysis, major perioperative hemorrhage could be avoided. During follow-ups, stable Hgb could be anticipated if monthly clinic visits, routine monitoring, in-time F VIII infusions, and erythropoietin and iron supplement were guaranteed (Supplementary Table S4). Contrary to our anticipation, hemoperitoneum was unusual in these patients. It was difficult to confirm whether the bleeding was spontaneous or related to catheter insertion, because both procedures (invasion, abrasion by catheter, volume-expansion caused by dialysate) and hemophilia could contribute to peritoneal hemorrhage. In this study, joints and muscles still contributed to most hemorrhage episodes, similar to general patients with hemophilia.21
Potential disturbance over hemostasis was another concern in PD patients. Some were worried that F VIII might leak into the dialysate, and others reported that PD facilitates regional fibrin consumption,22 which could both worsen coagulation. Nonetheless, F VIII has a size of 200 to 300 Kd,23 making it extremely difficult to pass through the peritoneal membrane. In addition, previous literature showed that PD patients had higher, rather than lower, plasma fibrinogen concentration, tissue factors, and low-grade coagulation system than HD patients or healthy controls, indicating a more prothrombotic profile in these patients.24, 25, 26 In pediatric PD patients, F VIII, VII, and von Willebrand factor activity were even higher than in healthy controls.27 Until now, no evidence supports that PD might deteriorate hemophilia treatment. However, special vigilance for hemorrhage is still necessary, especially in patients with previous retroperitoneal hematoma as demonstrated by patient 7.
Our data showed that patients with hemophilia might have a higher peritonitis rate than other PD patients. Though other risk factors for peritonitis in our center (Tenckhoff catheter, CAD, see in Supplementary Table S5) were similar between hemophilia and general male PD patients (Table 3), this study was not powered enough to determine if hemophilia was an independent risk factor. Two patients (patient 2 and 3) contributed 63% of all peritonitis episodes, which might prompt suspicion that “outlier” individuals skewed the average frequency in a small sample size cohort. Nonetheless, the rest of the 6 patients still had higher peritonitis frequency than the general male PD patients in our center (per 38 months vs. 77 months). The potential risk factors for higher peritonitis frequency were poor self-care capacity because of hemophiliac arthropathy (patient 1, 2, 6, and 7) or myelitis (patient 2), the immunosuppressant therapy for acquired hemophilia (patient 5 and 8), secondary infection of hematoma, and less support from poor financial status (patient 3). The more frequent tunnel exit incidence paralleled the peritonitis for similar reasons. Multiple manual access in CAPD may theoretically contribute to higher rates compared with automated PD.8 Nevertheless, the automated PD ratio was similar between patients with hemophilia and without (12.5% vs. 14.3%) in our center (n = 553), indicating that there might be other contributing factors causing peritonitis.
Our study has several limitations. The sample size was quite small due to the scarcity of patients. It restricted additional parameter adjustment in statistical analysis. More studies were required to evaluate whether dialysis inadequacy, higher peritoneal permeability, uncommon peritoneal hemorrhage, and higher rate of peritonitis were typical for these patients. Due to the limited data, the perioperative coagulation replacement was exploratory and not standardized. We are working on developing a standard protocol in hopes of offering more evidence for future management of these patients. Finally, all patients were type A, thereby limiting the experience to be extended to type B, type C, and von Willebrand disease.
In conclusion, we reported a case series of ESRD hemophilia patients treated with PD. Our data showed that PD was a safe and effective modality of choice for ESRD hemophilia patients. Besides proper perioperative coagulation factor supplement, a close monitoring of Hgb, local symptoms, and potential secondary infection or hemorrhage over existing hematoma should be warranted.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work was partially supported by grants from the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CIFMS 2020-I2M-C&T-A-001,2021-1-I2M-003 to C.L. 2021-I2M-C&T-B-011 to X.P.); Capital’s Funds for Health Improvement and Research (CFH 2020-2-4018 to C.L.); National Natural Scientific Foundation of China (82170709, 81970607 to C.L.); Beijing Natural Science Foundation (L202035 to C.L.); The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Table S1. Perioperative coagulation replacement for ESRD patient with hemophilia.
Table S2. Hgb (g/l) measurement during first 14 days after PD catheter insertion.
Table S3. Peritonitis in patients with hemophilia.
Table S4. Anemia-supportive medication and average serum iron and ferritin within first 6 months after PD initiation.
Table S5. Risk factor for peritonitis in single variable logistic regression in general PD patients (N = 553).
Contributor Information
Peng Xia, Email: xiapeng@pumch.cn.
Limeng Chen, Email: chenlimeng@pumch.cn.
Supplementary Material
Table S1. Perioperative coagulation replacement for ESRD patient with hemophilia.
Table S2. Hgb (g/l) measurement during first 14 days after PD catheter insertion.
Table S3. Peritonitis in patients with hemophilia.
Table S4. Anemia-supportive medication and average serum iron and ferritin within first 6 months after PD initiation.
Table S5. Risk factor for peritonitis in single variable logistic regression in general PD patients (N = 553).
References
- 1.Esposito P., Rampino T., Gregorini M., et al. Renal diseases in haemophilic patients: pathogenesis and clinical management. Eur J Haematol. 2013;91:287–294. doi: 10.1111/ejh.12134. [DOI] [PubMed] [Google Scholar]
- 2.Kulkarni R., Soucie J.M., Evatt B. Hemophilia Surveillance System Project Investigators. Renal disease among males with haemophilia. Haemophilia. 2003;9:703–710. doi: 10.1046/j.1351-8216.2003.00821.x. [DOI] [PubMed] [Google Scholar]
- 3.Darby S.C., Kan S.W., Spooner R.J., et al. Mortality rates, life expectancy, and causes of death in people with hemophilia A or B in the United Kingdom who were not infected with HIV. Blood. 2007;110:815–825. doi: 10.1182/blood-2006-10-050435. [DOI] [PubMed] [Google Scholar]
- 4.Li P.K., Chow K.M., Van de Luijtgaarden M.W., et al. Changes in the worldwide epidemiology of peritoneal dialysis. Nat Rev Nephrol. 2017;13:90–103. doi: 10.1038/nrneph.2016.181. [DOI] [PubMed] [Google Scholar]
- 5.Inker L.A., Eneanya N.D., Coresh J., et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385:1737–1749. doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanchette V.S., Key N.S., Ljung L.R., et al. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12:1935–1939. doi: 10.1111/jth.12672. [DOI] [PubMed] [Google Scholar]
- 7.Srivastava A., Brewer A.K., Mauser-Bunschoten E.P., et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19:e1–e47. doi: 10.1111/j.1365-2516.2012.02909.x. [DOI] [PubMed] [Google Scholar]
- 8.Thrombosis, Hemostasis Group Chinese Society of Hematology, Chinese Medical Association/Hemophilia Treatment Center Collaborative Network of China. Chinese guidelines on the treatment of hemophilia (version 2020) Zhonghua Xue Ye Xue Za Zhi. 2020;41:265–271. doi: 10.3760/cma.j.issn.0253-2727.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piraino B., Bernardini J., Brown E., et al. ISPD position statement on reducing the risks of peritoneal dialysis-related infections. Perit Dial Int. 2011;31:614–630. doi: 10.3747/pdi.2011.00057. [DOI] [PubMed] [Google Scholar]
- 10.Kato N., Chin-Kanasaki M., Tanaka Y., et al. Successful renal replacement therapy for a patient with severe hemophilia after surgical treatment of intracranial hemorrhage and hydrocephalus. Case Rep Nephrol. 2011;2011:824709. doi: 10.1155/2011/824709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishii H., Miyoshi C., Hirai K., et al. Induction of hemodialysis with an arteriovenous fistula in a patient with hemophilia A. CEN Case Rep. 2020;9:225–231. doi: 10.1007/s13730-020-00461-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amberker D., Li T.T., Rampure R. Successful use of arterio-venous graft for hemodialysis in patient with hemophilia. Hemodial Int. 2018;22(suppl 2):S88–S91. doi: 10.1111/hdi.12706. [DOI] [PubMed] [Google Scholar]
- 13.Gopalakrishnan N., Usha T., Thopalan B., et al. Hemodialysis in a patient with severe hemophilia A and factor VIII inhibitor. Hemodial Int. 2016;20:E11–E13. doi: 10.1111/hdi.12429. [DOI] [PubMed] [Google Scholar]
- 14.Roy-Chaudhury P., Manfro R.C. Renal replacement therapy for hemophiliacs. Int J Artif Organs. 1997;20:241–243. doi: 10.1177/039139889702000501. [DOI] [PubMed] [Google Scholar]
- 15.Sakan S., Bandić D., Perić M., et al. Renal replacement therapy in a polytraumatized patient with hemophilia. Acta Med Croat. 2012;66:247–250. [PubMed] [Google Scholar]
- 16.Xie Y., Yan Z. Operation of huge pseudoaneurysm with low- dose coagulant factor 8 replacement therapy on a severe hemophilia A patient with uremia: a case report. Zhonghua Xue Ye Xue Za Zhi. 2015;36:764. doi: 10.3760/cma.j.issn.0253-2727.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bajo M.A., del Peso G., Jiménez V., et al. Peritoneal dialysis is the therapy of choice for end-stage renal disease patients with hereditary clotting disorders. Adv Perit Dial. 2000;16:170–173. [PubMed] [Google Scholar]
- 18.Solak Y., Turkmen K., Atalay H., Turk S. Successful peritoneal dialysis in a hemophilia A patient with factor VIII inhibitor. Perit Dial Int. 2010;30:114–116. doi: 10.3747/pdi.2009.00011. [DOI] [PubMed] [Google Scholar]
- 19.Yang C., Wang H., Zhao X., et al. CKD in China: evolving spectrum and public health implications. Am J Kidney Dis. 2020;76:258–264. doi: 10.1053/j.ajkd.2019.05.032. [DOI] [PubMed] [Google Scholar]
- 20.Boudville N., de Moraes T.P. 2005 Guidelines on targets for solute and fluid removal in adults being treated with chronic peritoneal dialysis: 2019 Update of the literature and revision of recommendations. Perit Dial Int. 2020;40:254–260. doi: 10.1177/0896860819898307. [DOI] [PubMed] [Google Scholar]
- 21.Aviña-Zubieta J.A., Galindo-Rodriguez G., Lavalle C. Rheumatic manifestations of hematologic disorders. Curr Opin Rheumatol. 1998;10:86–90. doi: 10.1097/00002281-199801000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Homma S., Masunaga Y., Kurosu M., et al. Changes in peritoneal coagulation and fibrinolysis after discontinuation of chronic peritoneal dialysis. Perit Dial Int. 2002;22:178–183. doi: 10.1177/089686080202200203. [DOI] [PubMed] [Google Scholar]
- 23.Chavin S.I. Factor VIII: structure and function in blood clotting. Am J Hematol. 1984;16:297–306. doi: 10.1002/ajh.2830160312. [DOI] [PubMed] [Google Scholar]
- 24.Alwakeel J., Gader A.M., Hurieb S., et al. Coagulation inhibitors and fibrinolytic parameters in patients on peritoneal dialysis and haemodialysis. Int Urol Nephrol. 1996;28:255–261. doi: 10.1007/BF02550871. [DOI] [PubMed] [Google Scholar]
- 25.Preloznik Zupan I., Sabovic M., Salobir B., Buturovic Ponikvar J. Characterization of the pro-thrombotic state in CAPD patients. Ren Fail. 2008;30:597–602. doi: 10.1080/08860220802132130. [DOI] [PubMed] [Google Scholar]
- 26.Gries E., Kopp J., Thomae U., Kuhlmann H. Relation of intraperitoneal and intravascular coagulation and fibrinolysis related antigens in peritoneal dialysis. Thromb Haemost. 1990;63:356–360. doi: 10.1055/s-0038-1645046. [DOI] [PubMed] [Google Scholar]
- 27.Jones C.L., Andrew M., Eddy A., et al. Coagulation abnormalities in chronic peritoneal dialysis. Pediatr Nephrol. 1990;4:152–155. doi: 10.1007/BF00858827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.