Abstract
Introduction
Secretion of solutes by the proximal tubules represents an intrinsic kidney function not directly reflected by the glomerular filtration rate (GFR). The early loss of secretory clearance may reflect unrecognized kidney dysfunction, portending future disease progression.
Methods
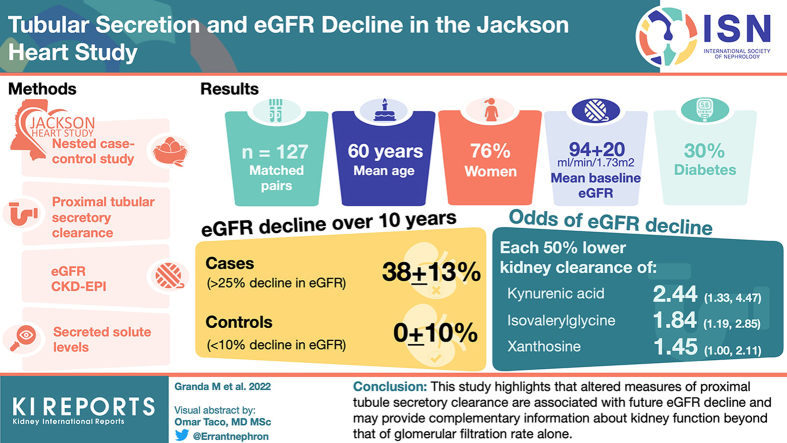
We designed a nested case-control study within the Jackson Heart Study (JHS), a prospective study of African American adults in Mississippi, to associate baseline differences in proximal tubular secretion of 5 endogenously produced solutes with future estimated glomerular rate (eGFR) decline. We matched 127 pairs by creatinine-eGFR, age, diabetes, and sex among the patients who provided a 24-hour urine collection; cases had a ≥25% decline in eGFR compared to <10% in controls over 10 years of follow-up. We measured baseline plasma and urine concentrations of secretory solutes using liquid chromatography-mass spectrometry to determine the odds ratio of kidney disease progression.
Results
Mean age was 60 years; 76% were women; 30% had diabetes; mean baseline eGFR was 94±20 ml/min per 1.73 m2. The eGFR decline over 10 years was 38±13% in cases and 0±10% in controls. After adjustment for the matching variables plus albuminuria, systolic blood pressure, body mass index, and smoking, each 50% lower kidney clearance of isovalerylglycine, kynurenic acid, and xanthosine were associated with 1.4 to 2.2 greater odds of eGFR decline. Kynurenic acid exhibited the strongest association; each 50% lower clearance of this secretory solute was associated with 2.20-fold higher odds of eGFR decline (95% confidence interval [CI] 1.32–3.67).
Conclusion
We found that in this community-based study of adults without significant kidney disease, lower proximal tubular secretory solute clearance is associated with future eGFR decline.
Keywords: CKD progression, eGFR decline, Jackson Heart Study, proximal tubules, secretory clearance, tubular secretion
Graphical abstract
See Commentary on Page 2558
Chronic kidney disease (CKD) affects nearly 50 million Americans and is associated with declines in cardiovascular and metabolic health, lower quality of life, and increased healthcare expenditures, particularly upon progression to end stage kidney disease.1, 2, 3 Though CKD is a heterogeneous entity, established risk factors for the disease and its progression include hypertension, diabetes, and obesity. Due to a combination of socioeconomic, healthcare access, and biologic factors, racial and ethnic minorities are disproportionately affected by CKD.4
The detection and monitoring of CKD is performed clinically by estimation GFR, which quantifies only a single dimension of kidney function, lacks sensitivity for detecting early disease, and may be less reliable in non-White populations.5 The addition of albuminuria to CKD screening improves detection of early disease; however neither albuminuria nor GFR directly assesses the functional capacity of the kidney tubules, which are the primary site for eliminating endogenously produced protein-bound toxins and prescribed medications, synthesizing amino acids and glucose, and maintaining homeostasis.6 Tubular functions may provide unique prognostic information to existing measures of glomerular kidney function because tubulointerstitial fibrosis represents the final common pathway of progressive kidney disease.7
We previously described methods to estimate tubular secretory clearance based on measurements of endogenous secretory solutes in plasma and urine. We demonstrated that lower tubular secretory solute clearance is associated with progression of CKD in persons with established kidney disease, and predicts the kidney elimination of prescribed medications.8, 9, 10 Herein, we evaluated tubular secretory clearance in a community-based cohort of African American adults without clinically significant kidney disease. We tested the hypothesis that lower secretory clearance is associated with kidney function decline over 10 years of follow-up.
Methods
Data Source, Study Population, Case-Control Definition, and Matching
We conducted a case-control study nested within the JHS, a prospective study of cardiovascular disease and its risk factors among African American adults residing in Jackson, Mississippi, USA. From 2000 to 2004, the JHS recruited 5306 participants aged 21years to 84 years from the Jackson, Mississipi area, including Hinds, Madison, and Rankin counties. JHS investigators collected detailed medical information and biosamples at baseline (exam 1) and subsequent exams conducted from 2005 to 2008 (exam 2) and from 2009 to 2013 (exam 3).11 Serum creatinine was measured at exam 1 (baseline) and exam 3 (10-year exam) only. JHS participants provided written informed consent, and the study protocol was approved by institutional review boards of the University of Mississippi Medical Center, Jackson State University, and Tougaloo College.
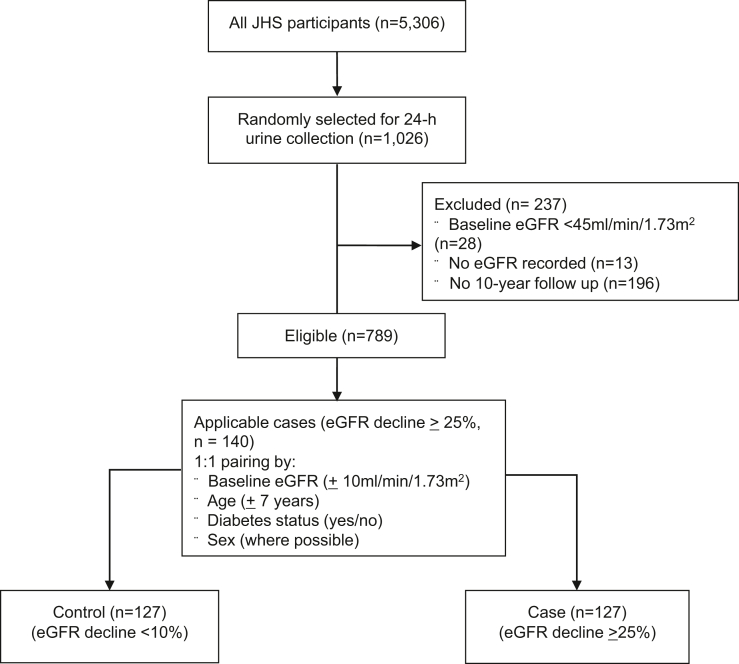
To estimate secretory solute clearance, we focused on the random subset of 1026 JHS participants who provided a 24-hour urine collection within 1 week of the baseline exam. Plasma and urine were collected on the same day. From this group, we excluded 28 participants with an eGFR <45 ml/min per 1.73 m2, 13 with missing eGFR records, and 196 who did not return for the 10-year follow-up visit, leaving 789 participants for case-control selection. We then defined cases by a 25% decline in eGFR from baseline to the 10-year follow-up exam (n = 159 participants). For each case participant, we attempted to find 1 control participant with a <10% decline in eGFR and individually matched by baseline eGFR ( 10 ml/min per 1.73 m2), age ( 7 years), diabetes status (yes vs. no), and sex (where possible). A total of 127 suitable controls were identified yielding 127 case-control pairs for analysis (Figure 1).
Figure 1.
Consort diagram of enrollment and pairing. Enrollment from all JHS participants. Only patients that were randomly selected for 24-hour urine collection were eligible. Patients without a baseline or 10-year eGFR recording were excluded, as were any patients with eGFR <45ml/min/1.73 m2, leaving 789 eligible patients. Pairs were created by matching baseline eGFR, age, diabetes, and, where possible, sex. eGFR, estimated glomerular filtration rate; JHS, Jackson Heart Study.
Measurements of Secretory Solute Clearance
We measured plasma and urine concentrations of endogenous solutes previously selected based on the literature evidence supporting tubular secretion as the primary kidney mode of elimination and our experience from previous studies.8,12 We did not measure other common tubular markers such as KIM-1 or NGAL because these are more reflective of tubular injury than function. Due to the relatively small size of this study, we selected a limited panel with 5 secretory solutes for analysis, namely cinnamoylglycine, isovalerylglycine, kynurenic acid, p-cresol sulfate, and xanthosine. The following solutes exhibited strong collinearity and so were not presented: we did not assess indoxyl sulfate due to the high correlation with p-cresol sulfate, we did not assess tiglyglycine due to high correlation with isovalerylglycine. Pyridoxic acid was not included due to unsatisfactory assay calibration in these samples.
Plasma samples were precipitated in organic solvent and extracted using solid-phase extraction (Phree phospholipid removal plate; Phenomenex, Torrance, CA). Urine samples underwent 2 parallel solid-phase extractions (HLB or MCX μElution plates; Waters, Milford, MA). Dried extracts were reconstituted in 80μl 5% acetonitrile/0.2% formic acid in H2O and passed through a large-pore filter plate (MSBVN1210; Millipore). The resulting extracts were analyzed using liquid chromatography with quantitative tandem mass spectrometry (Shimadzu and Sciez). Labeled internal standards were used to reduce sample-specific matrix effects and single-point external calibration was used to determine concentrations and reduce between-batch variability. Within-batch coefficients of variation for individual solutes in plasma and urine ranged from 3.9% to 11.1%.
We calculated the kidney clearance of each endogenous solute as:
where Ux represents the solute concentration in the 24-hour urine sample, Px represents the solute concentration in plasma, and V represents the 24-hour urine volume, expressed in ml/min. We also calculated a summary secretion score to reflect the scaled average of the secretory clearances by first scaling each log-transformed clearance to a common 0 to 100 scale and then calculating the mean.8,12 Absolute clearance values were preferred over fractional excretions to avoid underestimating secretory changes of solutes when normalized to creatinine clearance given the partial secretion of creatinine. However we also replicated the model using fractional excretion of each solute in the Supplementary Material.
Measurements of Other Study Data
Serum creatinine measurements at baseline were corrected to isotope-dilution mass spectrometry standards. Exam 3 creatinine measurements were measured on instruments calibrated to isotope-dilution mass spectrometry. Creatinine assays were performed by the JHS on the Vitros 950 Ortho-Clinical Diagnosis analyzer (Raritan, New Jersey) utilizing a peroxidase method.13 For determining eGFR decline and matching we used the 2009 Chronic Kidney Disease Epidemiology Collaboration creatinine equation because serum cystatin C concentrations were not available for all participants at both visits. In sensitivity analyses, we adjusted for baseline eGFR using the 2012 Chronic Kidney Disease Epidemiology Collaboration creatinine and cystatin equation by incorporating baseline serum concentrations of cystatin C.14 Race coefficients were not used. Repeating the original urine creatinine measurements by JHS (Vitros 950 enzymatic method, Ortho-Clinical Diagnosis, Raritan, New Jersey) revealed strong correlation (R2 = 0.96) to those measured on a DxC analyzer utilizing the Jaffe method (Beckman-Coulter, Brea, California). JHS investigators recorded blood pressures as the average of 2 readings from the Random-Zero machine during the baseline study visit. The presence of diabetes was determined by a fasting glucose >126 mg/dl, hemoglobin A1c ≥ 6.5%, or use of a diabetes medication in the previous 2 weeks. Albuminuria was measured from the 24-hour urine specimen collected at the baseline visit.
Statistical Analysis
We summarized baseline participant characteristics by case-control status. We described solute clearances by their median and interquartile range. We used conditional logistic regression to estimate the association of each solute clearance at baseline with case status (eGFR decline) and linear regression with inverse probability weighting to estimate the association of solute clearance at baseline with albuminuria at 10 years. Secretory clearances were scaled to report each result as associations per 50% lower clearance or a 10-point lower summary secretion score. Conditional regression models were adjusted for the following matching variables: age, baseline eGFR, diabetes, A1c, and sex (model 1). A second model added adjustment for log-transformed 24-hour urine albumin, systolic blood pressure, body mass index, and current smoking (model 2). We modeled continuous variables linearly except for A1c, body mass index, and systolic blood pressure, which were modeled with cubic splines. Sensitivity analyses repeated the main analysis after truncating extreme measured clearances and examined the effect of further adjustment for renin-angiotensin aldosterone system inhibitor use, diuretic use, and HbA1c (Supplementary Table S1, model 3). A 2-sided P value of 0.05 was used to declare statistical significance, and odds ratios were reported with their corresponding 95% CIs. A Bonferroni correction for multiple comparisons was applied for a significant P value of 0.01. We repeated this analysis using fractional excretion of secretory solutes (Supplementary Table S2). Analyses were performed using R 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study Population and Patient Characteristics
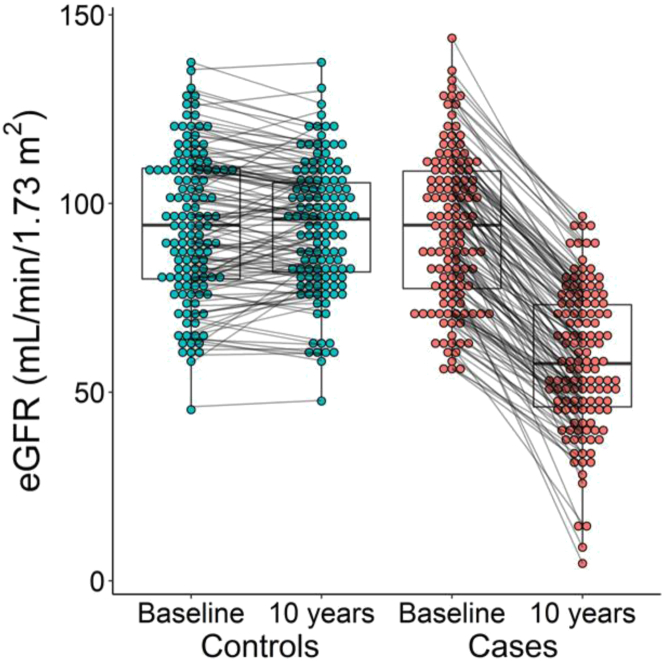
Among the 127 case-control pairs, the mean age was 61 10 years, 76% were female, 30% had diabetes, and the mean baseline eGFR was 94 20 ml/min per 1.73 m2 (Table 1). The mean decline in eGFR was 38 13% in case participants and 0 10% in control participants (Figure 2), with mean absolute differences in eGFR of 34.5 12 versus 0 9.5 ml/min per 1.73 m2 respectively. Case and control participants were similar with respect to the matching variables by design, as well as baseline albuminuria, anthropometric characteristics, and achieved education level. The prevalence of cardiovascular diseases was modestly higher among the cases compared with controls.
Table 1.
Demographic characteristics of the case-control sample
| Characteristic | Controls (N = 127) | Cases (N = 127) |
|---|---|---|
| Age (yr)a | 60.3 (10.2) | 60.4 (10.5) |
| eGFR (CKD-EPI) (ml/min/1.73 m2)a | 94.5 (19.3) | 93.4 (20.1) |
| Diabetesa | 40 (31) | 39 (31) |
| Malea | 35 (28) | 27 (21) |
| Education | ||
| Less than high school | 22 (17) | 29 (23) |
| High school graduate/GED | 19 (15) | 25 (20) |
| Vocational school, trade school, or college | 86 (68) | 73 (57) |
| Insurance | ||
| Private and public | 35 (28) | 34 (27) |
| Private only | 57 (45) | 54 (43) |
| Public only | 25 (20) | 28 (22) |
| Uninsured | 10 (8) | 10 (8) |
| Height (cm) | 167.7 (9.4) | 167.9 (8.0) |
| Weight (kg) | 90.0 (17.9) | 92.8 (21.0) |
| BMI (kg/m2) | 32.0 (6.1) | 32.9 (6.7) |
| Current smoking | 14 (11) | 18 (14) |
| Systolic blood pressure (mm Hg) | 127.9 (14.7) | 129.8 (17.5) |
| Diastolic blood pressure (mm Hg) | 74.2 (7.9) | 74.0 (8.9) |
| History of CVD | 8 (6) | 17 (13) |
| History of MI | 4 (3) | 7 (6) |
| History of stroke | 2 (2) | 6 (5) |
| Atrial fibrillation detected on ECG | 1 (1) | 1 (1) |
| Antihypertensives | 85 (67) | 96 (76) |
| RAASi | 37 (29) | 46 (36) |
| Beta blockers | 17 (13) | 18 (14) |
| Calcium channel blockers | 27 (21) | 31 (24) |
| Diuretics | 62 (49) | 57 (45) |
| Urine albumin (mg/24h), median (IQR), 24 hour urine) | 5.9 (3.9 – 10.3) | 6.0 (4.3 – 12.8) |
| Microalbuminuria, 24 hour urineb | 7 (6) | 14 (11) |
BMI, body mass index; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; ECG, electrocardiogram; GED, general education development; IQR, interquartile range; MI, myocardial infarction; RAASi, renin-angiotensin aldosterone system inhibitor.
Entries are mean (SD) for continuous variables or N (%) for categorical variables, except as noted.
Cases and controls were matched on age (±7 years), eGFR (±10 ml/min/1.73 m2), and diabetes status; cases and controls were further matched on sex, when possible.
Matching variable.
Microalbuminuria defined as urine albumin to creatinine ratio 30 mg/g.
Figure 2.
10-year changes in eGFR among case and controls. Baseline and 10-year eGFR among cases and controls. Each paired set of points represent the same patient at baseline and after 10 years. eGFR, estimated glomerular filtration rate.
Association of Secretory Solute Clearance With eGFR Decline
The median baseline kidney clearances of isovalerylglycine, kynurenic acid, p-cresol sulfate, and xanthosine, but not cinnamoylglycine, were lower in cases compared with controls (Table 2). After adjustment for the following matching variables: age, baseline eGFR, diabetes, and sex; lower 24-hour clearances of isovalerylglycine, kynurenic acid, and xanthosine, as well as the summary secretion score were associated with higher odds of eGFR decline. After further adjustment for baseline albuminuria, systolic blood pressure, body mass index, and current smoking, lower kidney clearance of these solutes were associated with estimated odds ratios of 1.4 to 2.2 for eGFR decline. Kynurenic acid clearance exhibited the strongest association as follows: each 50% lower kynurenic acid clearance was associated with 2.2-fold higher odds of eGFR decline (95% CI 1.3–3.7). Associations were statistically significant at the nominal P < 0.05 level for isovalerylglycine, kynurenic acid, xanthosine, and the summary secretion score; the associations for kynurenic acid, isovalerylglycine, and the summary score were significant at the multiple comparisons Bonferroni corrected <0.01 level. Study findings were nearly identical in sensitivity analyses truncating 7 extreme values of secretory clearances (Supplementary Table S3). Xanthosine reaches statistical significance when adjusted instead by cystatin C-eGFR (Supplementary Table S4), and the summary secretion score reaches significance at the multiple comparisons Bonferroni corrected <0.01 level with inclusion of diuretic or renin-angiotensin aldosterone system inhibitor use (Supplementary Table S1, model 3). A repeat analysis using the fractional excretion of secretory solutes did not meaningfully change our findings (Supplementary Table S2).
Table 2.
Associations of secretory solute clearance with eGFR decline
| Solute | Median (IQR) clearance (ml/min) |
Model 1 |
Model 2 |
|||
|---|---|---|---|---|---|---|
| Controls | Cases | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Cinnamoylglycine | 151.8 (94.9–309.0) | 170.7 (95.5–289.2) | 0.89 (0.72, 1.10) | 0.29 | 0.88 (0.71, 1.09) | 0.25 |
| Isovalerylglycine | 715.8 (521.5–1221.0) | 632.5 (445.0–997.3) | 1.69 (1.14, 2.52) | 0.0091b | 1.91 (1.23, 2.96) | 0.0038b |
| Kynurenic acid | 403.4 (273.7–588.3) | 324.3 (243.1–456.9) | 1.93 (1.18, 3.16) | 0.0086b | 2.26 (1.33, 3.87) | 0.0028b |
| P-cresol sulfate | 34.9 (24.4–51.6) | 30.7 (20.5–48.0) | 1.12 (0.87, 1.45) | 0.38 | 1.22 (0.92, 1.62) | 0.16 |
| Xanthosine | 68.2 (47.4–106.2) | 63.1 (40.7–88.8) | 1.39 (1.00, 1.93) | 0.052 | 1.48 (1.02, 2.13) | 0.037a |
| Summary score | 50.8 (43.6–57.0) | 47.2 (39.6–53.4) | 1.43 (1.06, 1.92) | 0.019a | 1.57 (1.13, 2.18) | 0.0076b |
CI, confidence interval; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; HbA1c, hemoglobin A1c; IQR, interquartile range; OR, odds ratio; SBP, systolic blood pressure.
Odds ratios are per 50% lower secretory clearance for individual solutes and per 10-point decrement for summary score. Model 1 adjusts for age, baseline estimated glomerular filtration rate (2012 CKD-EPI creatinine), diabetes, flexibly- modeled HbA1c, and sex; model 2 further adjusts for log-transformed 24-hour urine albumin, SBP, body mass index, and current smoking.
Significant at P < 0.05.
Significant at P < 0.01 accounting for multiple comparisons.
Association of Secretory Solute Clearance With Changes in Albuminuria
The median baseline urine albumin-to-creatinine ratio was 6.0 mg/g among case participants and 5.9 mg/g among controls. Over 10 years of follow-up, urinary albumin-to-creatinine ratio increased by a median of 62% (interquartile range −4% to 228%) among case participants and 45% (interquartile range 1%–179%) among controls. After full adjustment, lower kidney clearances of secretory solutes were associated with numerically larger changes in urinary albumin-to-creatinine ratio; however, these associations did not meet statistical significance (Table 3). A 10-point lower summary secretion score, which combines all 5 solutes, was associated with an estimated 15% greater increase in urinary albumin-to-creatinine ratio (95% CI 2%–30% greater).
Table 3.
Associations of secretory solute clearance with albuminuria
| Solute | Model 1 |
Model 2 |
|||
|---|---|---|---|---|---|
| Relative difference in uACR (95% CI) | P-value | Relative difference in uACR (95% CI) | P-value | ||
| Cinnamoylglycine | 0.99 (0.88, 1.12) | 0.89 | 1.00 (0.89, 1.12) | 0.96 | |
| Isovalerylglycine | 1.11 (0.95, 1.30) | 0.19 | 1.15 (0.99, 1.34) | 0.07 | |
| Kynurenic acid | 1.18 (0.98, 1.42) | 0.08 | 1.18 (0.98, 1.42) | 0.08 | |
| P-cresol sulfate | 1.08 (0.96, 1.21) | 0.19 | 1.10 (0.98, 1.23) | 0.09 | |
| Xanthosine | 1.13 (1.00, 1.28) | 0.05 | 1.11 (0.98, 1.26) | 0.09 | |
| Summary score | 1.12 (1.00, 1.25) | 0.06 | 1.13 (1.00, 1.27) | 0.046a | |
CI, confidence interval; SBP, systolic blood pressure; uACR, urine albumin-to-creatinine ratio.
Entries are the ratio in geometric mean 10-year log-transformed urine albumin-to-creatinine ratio per 50% lower secretory clearance for individual solutes and per 10 point decrement for summary score. Model 1 adjusts for age, baseline estimated glomerular filtration rate, baseline log urine albumin-to-creatinine ratio, diabetes, flexibly modeled A1c, and sex; model 2 further adjusts for SBP, body mass index, and current smoking.
Significant at P < 0.05.
Discussion
We found that lower kidney clearances of secretory solutes were associated with a greater risk of kidney function decline over 10 years in a community-based study of African American adults. These associations persisted after adjustment for baseline eGFR and albuminuria, the currently established metrics of kidney function. Associations for the secretory solutes under investigation were in the same direction, except for cinnamoylglycine. These findings support the hypothesis that measurements of tubular secretory clearance provide complementary information to existing glomerular-based measures of kidney function.
The kidney specific and energy dependent processes necessary for tubular secretory clearance suggest that this kidney function may serve as a potential marker of early disease. The secretion of organic solutes into the urine requires cellular uptake via specialized transporters on the basolateral surface of proximal tubules, transport across the cell, and extrusion against a concentration gradient. Dysfunction in any of these steps may result in reduced secretory clearance. In contrast, GFR is typically maintained or even increased in early kidney disease via hemodynamic autoregulatory mechanisms. Combined with the difficulty in estimating normal to near-normal GFR values using plasma markers, it is unsurprising that early kidney disease is not readily detected by changes in eGFR.
Identifying early kidney disease in at-risk patients represents an important first step toward implementing therapeutic strategies to prevent kidney function decline. With the emergence of new and potentially early treatment options for CKD, such as sodium glucose cotransporter 2 inhibitors, which block tubular glucose reabsorption, it is more important than ever to detect incipient kidney disease before the onset of irreversible tubulointerstitial fibrosis. This study offers an opportunity to rethink current standards for evaluating kidney function, particularly in a population that is at greatest risk for end stage kidney disease.
The results of this study complement our previous findings in a larger prospective CKD cohort. Among 3416 participants in the Chronic Renal Insufficiency Cohort study who already had impaired eGFR at baseline, lower kidney clearances of isovalerylglycine, kynurenic acid, as well as pyridoxic acid, cinnamoylglycine, indoxyl sulfate, and xanthosine were associated with kidney disease progression after controlling for eGFR, albuminuria, and other potential confounding factors.8 Compared to our prior study, participants had highly preserved eGFR at baseline, no known kidney disease, and were followed for 4 additional years.
One strength of this study is evaluation of persons who were generally free of clinically significant kidney disease at baseline but with a high prevalence of risk factors. Additional strengths include 24-hour urine collections to determine secretory solute clearance, clear separation of cases and controls with respect to kidney function decline, and standardized methods for data collection in JHS. Several important limitations should be mentioned. First, sample size was modest as a result of the rarity of CKD progressors (N = 140) in the underlying cohort of 789 eligible JHS participants selected for 24-hour urine collection, and limitations imposed after subsequent matching of controls. Consequently, wide CIs for the associations under investigation leave uncertainty as to the true size of these associations in the underlying population (Supplementary Table S5). The cohort that was selected was female-predominant which is not representative of the CKD population at large; we have previously shown higher secretory clearance rates in males, but there is no systematic difference after adjusting for height and weight.15 Because pairs were sex-matched and adjusted in analysis we do not anticipate any major effect on our findings despite differences in our cohort from that of the JHS at large.
Second, we estimated secretory solute clearance based on endogenous substrates of tubular secretory transporters. The protein binding of cinnamoylglycine, kynurenic acid, and p-cresol sulfate are 95%, suggesting that glomerular filtration plays only a small role in their kidney clearance.8 Isovalerylglycine and xanthosine are less protein bound, but the total kidney clearance of these solutes substantially exceeds GFR. In the absence of a singular gold-standard test for determining secretory solute clearance, estimates of this kidney function based on endogenous solutes are subject to error due to imprecision in urine collection timing and biologic fluctuation of plasma concentrations.15,16 The use of kidney clearances should negate the effect of production; however, the plasma or urine values can be differentially affected by factors such as diet, medications, and comorbidities. The resulting mismatch may push concentrations outside of measurable ranges or result in extreme values unrelated to loss of secretory clearance. Improvements in the methods to estimate secretory clearance are needed if such measurements are to move beyond research settings.
Third, though minimal. the partial secretion of creatinine may introduce error in the matching process because patients were selected by changes in creatinine-based GFR estimation. Altered secretion of creatinine over time may affect estimates of GFR leading to inappropriate matching; however, the effect would likely be minimal because creatinine is predominantly filtered. Evolution of the methods used to calibrate and measure serum creatinine during the 10-year study period may also affect relative changes in GFR, although these differences would likely be uniform throughout all participants, which would not affect our findings.
It bears mentioning the differences among the solutes themselves and their association with GFR decline. Though these solutes are substrates of the organic anion transporter 1/3, they do not necessarily demonstrate similar affinities for these transporters, or share identical protein binding characteristics or cellular export pathways. Moreover, these solutes may be differentially prone to competitive inhibition by uremic toxins or drugs. Consequently, a decline in their clearance may not occur linearly with that of overall tubular secretion. Multiple solutes were utilized to generate a summary secretion score to better distribute the pros and cons of each solute. Though the fractional excretion of secretory solutes was believed to minimize changes in tubular secretion due to the normalization with creatinine clearance (which is also partially secreted), the results of this study did not change meaningfully when the analysis was repeated using fractional excretion.
In summary, this study highlights the potential utility of proximal tubular secretory clearance in providing complementary information about kidney function beyond that of glomerular-based measures. Glomerular and tubular kidney functions may decline at differing rates across individuals depending on the underlying pathophysiologic processes in the kidneys; identifying these clinical scenarios is an important future direction of this area of research. With continued focus on new tools to quantify tubular kidney functions, these measurements may emerge as a new tool in the arsenal of early detection and prevention of kidney disease.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors wish to thank the staffs and participants of the JHS.
Funding
This project was funded via the National Insitute of Health grant R01DK107931. Funding for the primary author was provided by the University of Washington training grant via NIH T32DK007467-37. The Jackson Heart Study is supported and conducted in collaboration with Jackson State University (HHSN268201800013I), Tougaloo College (HHSN268201800014I), the Mississippi State Department of Health (HHSN268201800015I) and the University of Mississippi Medical Center (HHSN268201800010I, HHSN268201800011I and HHSN268201800012I) contracts from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities.
Author Contributions
BRK designed the study. MLG and BRK conducted background literature search. AH performed solute measurements. MLG, DKP, LRZ, and BRK analyzed data. MLG, and BRK drafted the manuscript, with assistance from LRZ in creating figures. All authors revised and approved the final version of the manuscript.
Disclaimers
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Footnotes
Table S1. Associations of secretory solute clearance with eGFR decline, including further adjustment Model 3, including smoking, RAAS inhibitors, and diuretics.
Table S2. Associations of secretory fractional excretion with eGFR decline
Table S3. Associations of secretory solute clearance with eGFR decline after truncating extreme solute clearance values.
Table S4. Associations of secretory solute clearance with cystatin C-eGFR decline.
Table S5. Comparison of case-control population to all Jackson Heart Study participants that completed 24-hour urine collection.
Supplementary Material
Table S1. Associations of secretory solute clearance with eGFR decline, including further adjustment model 3, including smoking, RAAS inhibitors, and diuretics
Table S2. Associations of secretory fractional excretion with eGFR decline
Table S3. Associations of secretory solute clearance with eGFR decline after truncating extreme solute clearance values
Table S4. Associations of secretory solute clearance with cystatin C-eGFR decline
Table S5. Comparison of case-control population to all Jackson Heart Study participants that completed 24-hour urine collection
References
- 1.Chronic Kidney Disease Surveillance System Prevalence of CKD in the U.S. Population 1999–2004 vs. 2005–2010 vs. 2020 Target by year and progress measure. Centers for Disease Control and Prevention. https://nccd.cdc.gov/CKD/detail.aspx?QNum=Q633#refreshPosition
- 2.Abdel-Kader K., Fischer G.S., Johnston J.R., Gu C., Moore C.G., Unruh M.L. Characterizing pre-dialysis care in the era of eGFR reporting: a cohort study. BMC Nephrol. 2011;12:12. doi: 10.1186/1471-2369-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afkarian M., Sachs M.C., Kestenbaum B., et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24:302–308. doi: 10.1681/ASN.2012070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afkarian M., Katz R., Bansal N., et al. Diabetes, kidney disease, and cardiovascular outcomes in the Jackson Heart study. Clin J Am Soc Nephrol. 2016;11:1384–1391. doi: 10.2215/CJN.13111215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levey A.S., Eckardt K.U., Tsukamoto Y., et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 6.Levey A.S., Becker C., Inker L.A. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic review. JAMA. 2015;313:837–846. doi: 10.1001/jama.2015.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeisberg M., Neilson E.G. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol. 2010;21:1819–1834. doi: 10.1681/ASN.2010080793. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y., Zelnick L.R., Wang K., et al. Kidney clearance of secretory solutes is associated with progression of CKD: the CRIC study. J Am Soc Nephrol. 2020;31:817–827. doi: 10.1681/ASN.2019080811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y., Zelnick L.R., Wang K., et al. Association of tubular solute clearances with the glomerular filtration rate and complications of chronic kidney disease: the chronic renal insufficiency cohort study. Nephrol Dial Transplant. 2020;36:1271–1281. doi: 10.1093/ndt/gfaa057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang K., Kestenbaum B. Proximal tubular secretory clearance: a neglected partner of kidney function. Clin J Am Soc Nephrol. 2018;13:1291–1296. doi: 10.2215/CJN.12001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wyatt S.B., Diekelmann N., Henderson F., et al. A community-driven model of research participation: the Jackson Heart Study participant recruitment and retention study. Ethn Dis. 2003;13:438–455. [PubMed] [Google Scholar]
- 12.Chen Y., Zelnick L.R., Hoofnagle A.N., et al. Prediction of kidney drug clearance: a comparison of tubular secretory clearance and glomerular filtration rate. J Am Soc Nephrol. 2021;32:459–468. doi: 10.1681/ASN.2020060833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young B.A., Katz R., Boulware L.E., et al. Risk factors for rapid kidney function decline among African Americans: the Jackson Heart Study (JHS) Am J Kidney Dis. 2016;68:229–239. doi: 10.1053/j.ajkd.2016.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suchy-Dicey A.M., Laha T., Hoofnagle A., et al. Tubular secretion in CKD. J Am Soc Nephrol. 2016;27:2148–2155. doi: 10.1681/ASN.2014121193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGuire B.B., Bhanji Y., Sharma V., et al. Predicting patients with inadequate 24- or 48-hour urine collections at time of metabolic stone evaluation. J Endourol. 2015;29:730–735. doi: 10.1089/end.2014.0544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.