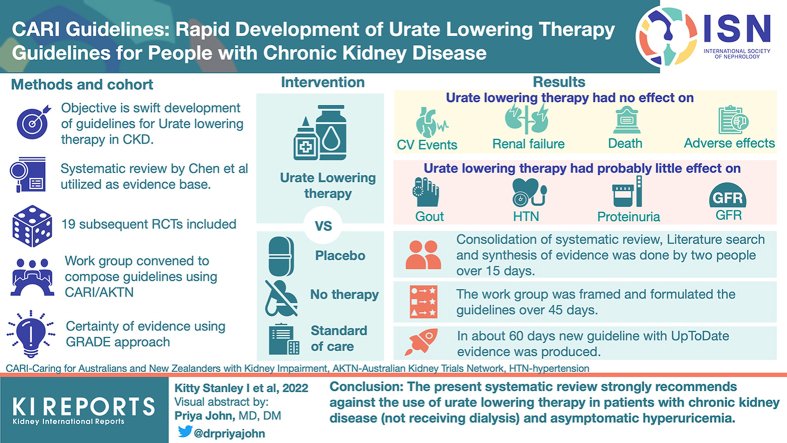
Abstract
Introduction
The slow transformation of new research findings into clinical guidelines is a barrier to providing evidence-based care. The Caring for Australians and New Zealanders with Kidney Impairment (CARI) guidelines are developing models to improve guideline production, one methodology involves more functional concordance between trial groups, such as the Australian Kidney Trials Network (AKTN) and CARI. The objective of this project was to rapidly produce an evidence-based guideline on urate-lowering therapy in patients with chronic kidney disease (CKD), in response to new clinical trial publications on the topic by the AKTN.
Methods
To produce a guideline as rapidly as possible, an existing systematic review was utilized as the evidence base, and then updated with the inclusion of clinical trials that had been published subsequently. A Work Group was convened to review the evidence and compose an appropriate guideline using CARI/GRADE methodology. The group met 3 times over 45 days to formulate the guideline.
Results
The result was a strong recommendation against the use urate-lowering therapies in individuals with CKD (not receiving dialysis) and asymptomatic hyperuricemia. The process of identifying an appropriate existing systematic review, updating the literature search, and synthesizing the evidence, was done by 2 individuals over 15 days. The Work Group was formulated and composed the guideline over 45 days. In all, a new guideline incorporating the most up-to-date evidence was formulated in 60 days.
Conclusion
This method of guideline development represents a potentially new way of releasing guidelines that encapsulates all available evidence in a time-efficient manner.
Key words: chronic kidney disease, clinical practice guideline, guideline development, hyperuricemia
Graphical abstract
Introduction
It is estimated that 40,000 new clinical trials and systematic reviews are published every year, and this number is only growing.1 Current methods of guideline development are time and labor intensive, and they are not keeping up with rapidly expanding medical literature2; it currently takes an average of 17 years for clinical research to be translated into guidelines and subsequently adapted into practice.3 CKD represents a major public health concern, and key to slowing disease progression is the timely application of appropriate medical therapies, which can attenuate decline in kidney function.4,5 The current lag in the dissemination of new research findings and their subsequent implementation into practice hinders clinicians’ abilities to do this.6
Serum urate levels are known to be increased in the presence of reduced glomerular filtration and therefore reduced urate excretion.7 Elevated serum urate, without symptoms (asymptomatic hyperuricemia) has been associated with more rapid progression of CKD7 and worse outcomes including kidney failure, cardiovascular events, and death.8,9 Nevertheless, the causal role that elevated serum urate levels may have in relation to CKD progression, and the effect that urate-lowering therapies may have on slowing CKD progression remained unclear. To address this clinical question the AKTN published the Controlled trial of slowing of kidney disease progression from the inhibition of xanthine oxidase. This large, randomized placebo-controlled trial aimed to evaluate the impact of allopurinol therapy on kidney function in participants with CKD stage 3 to 4 over 24 months.10 To support clinical decision making regarding the role of urate-lowering therapy in preventing CKD progression, the decision was made to create an updated guideline on serum urate lowering therapies in CKD in collaboration with AKTN and CARI guideline developers in June 2021. Relevant historical guidelines, such as the 2012 CARI guideline on early CKD, recommend against the use of urate-lowering therapy for patients with CKD stage 1 to 3 and asymptomatic hyperuricemia.11 The updated guideline’s development was to pilot a new methodology focusing on reducing development time, with the Controlled trial of slowing of kidney disease progression from the inhibition of xanthine oxidase trial (published in 2020) triggering the update to demonstrate a proof-of-concept model of updating clinical practice guidelines rapidly in response to newly published clinical trials. To do this, we aimed to identify an existing systematic review and meta-analysis, update this with results of recently published clinical trials, and produce an updated guideline within 60 days. This time frame was an internal target, rationalized on the basis of the number of personnel available, the release date of the most up-to-date comprehensive literature review, and expected time frames for each stage’s completion.
Methods
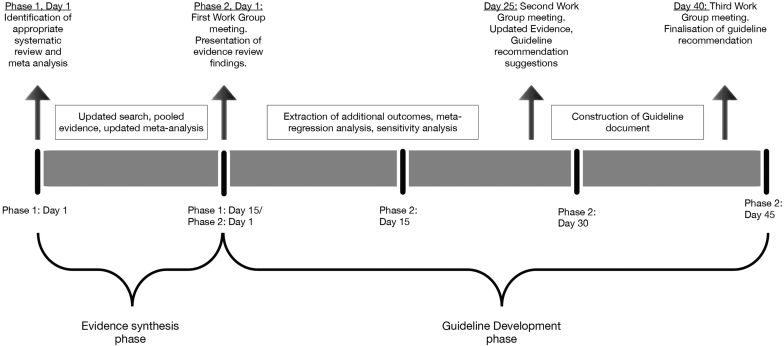
An overview of the guideline’s development methods is detailed in Figure 1.
Figure 1.
Timeline of guideline development process
Evidence Review
This guideline’s development methodology focused on optimizing processes to synthesize evidence rapidly. Two reviewers (IKS and DJT) located, analyzed, and synthesized appropriate evidence for the guideline over 15 days. To achieve this work in this time frame, a published systematic review and meta-analysis was located to serve as the underlying evidence base for the guideline. A review by Chen et al.12 was identified by AKTN and CARI Guidelines which fulfilled the following criteria:
-
•
Appropriate criteria (Population: included people with CKD; Intervention: urate-lowering therapy; Comparator: placebo and/or standard of care; Outcomes: clinical outcomes i.e., death, cardiovascular events, kidney failure, adverse events; Methods: randomized controlled trial)
-
•
Published within the last year
-
•
A full search strategy reported
-
•
Characteristics of included studies detailed in the review
-
•
Risk of Bias assessment with relevant critical appraisal tool (i.e., Cochrane Risk of Bias) undertaken and reported for all included studies
-
•
Meta-analysis undertaken with appropriate statistical methods and forest plots reported
Chen et al.12 compared the effects of urate-lowering therapies on cardiovascular and kidney outcomes. See Supplementary Table S1 for the critical appraisal using AMSTAR2 of Chen et al.12,13 The review included a total of 28 randomized controlled trials which were screened by 2 reviewers (IKS and DJT) to assess their relevance for inclusion in the guideline’s development according to the following criteria:
-
•
At least 66% of study participants with CKD
-
•
Evaluating any urate-lowering therapy
-
•
Compared with placebo, no therapy, or standard of care
-
•
Duration over 6 months
-
•
Reporting on major adverse cardiovascular events (MACEs), death, kidney failure, or side effects of therapy
Studies which did not meet these criteria were not considered for the guideline. Any differences between reviewers were resolved through discussion. In July 2021 the search from Chen et al.12 was rerun in Cochrane Central, MEDLINE and Embase, to capture any relevant studies published since the review’s search date of June 2020 (see Supplementary Table S2 for search strategy). The identified citations’ titles, abstracts and full texts were screened for relevance in the guideline by the reviewers and any differences were resolved through consensus.
To fully utilize this review and maximize time savings, CARI contacted the review’s authors and were granted access to raw extracted data for further analysis and assessment. The primary outcomes from Chen et al.12 were death, MACE, kidney failure, and side effects. Outcomes from the additional studies identified in the updated literature search were pooled with the results from Chen et al.12 to form the basis of the guideline evidence review. All analyses were conducted using Review Manager (RevMan) version 5.414 and meta-regression and publication bias assessed using R (4.2.1).15
Work Group Guideline Development
After the process of data synthesis was completed, a Work Group was convened to review the updated evidence and formulate the guideline. This group met 3 times to discuss the findings of the evidence review and write guideline recommendations over 45 days. The Work Group included relevant expertise such as nephrologists, rheumatologists, guideline methodologists, a pharmacist, and consumers. Members who had been involved with the 2012 CARI Guideline on the management of early CKD were invited and an expression of interest was circulate via Australian and New Zealand Society of Nephrology, and Renal Society of Australasia. Before the convening of the Work Group, all potential members were required to complete conflict of interest disclosure forms. Conflicts were managed and reported in accordance with the National Health and medical Research Council recommendations on identifying and managing conflicts of interest.16 Relevant and ongoing conflicts were identified and disclosed, where relevant the Work Group member did not participate in formation of recommendations.
Consideration was given to patient preferences and values regarding the benefits and harms of therapy throughout the guideline development process. Two of the Work Group members (both listed as coauthors) were consumers with CKD and experiences of using serum-urate lowering medication, 1 from Aotearoa New Zealand and 1 from Australia, who provided insights into the lived experience of CKD and advised on patient important factors, such as cost. Published scientific literature on the values and preferences of patients prescribed urate-lowering therapy were identified, reviewed and considered in the development of the guideline recommendations.17
The certainty of the evidence was assessed independently by 2 authors (IKS and DJT) using GRADE18 and summary of findings tables developed using GRADEpro.19 The Work Group utilized the GRADE evidence to decision framework while developing the guideline, which supports evidence-based decision making for guidelines in a transparent and structured way.20 The framework considers criteria such as the balance of benefits and harms, the certainty of evidence, costs, feasibility, and potential outcomes of different options. Cost and feasibility considerations were discussed within the Work Group, including the availability of therapies under the Australian Pharmaceutical Benefits Scheme or the New Zealand Pharmac, and the subsequent out of pocket costs for patients. Evaluation of equity was also to be considered in the guideline’s development; the GRADE equity framework was utilized along with the PROGRESS PLUS checklist to assist this assessment.21,22 This framework considered equity characteristics which may stratify health outcomes including race and ethnicity, place of residence, socioeconomic status, and gender.
Results
Findings of the Evidence Review
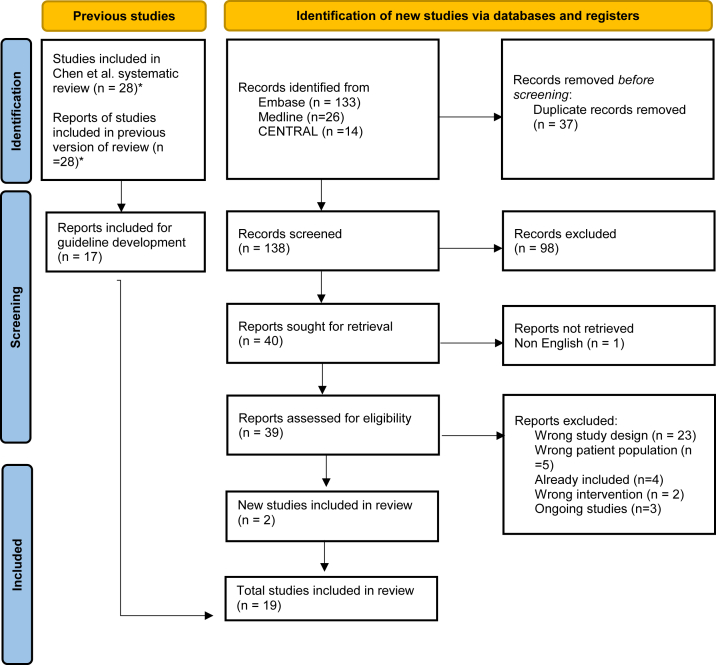
The updated literature search resulted in a total of 19 studies for consideration in the guideline.10,23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40 Prisma diagram of included studies is shown in Figure 2.
Figure 2.
PRIMSA diagram. ∗11 studies excluded from Chen et al12 systematic review because of not fulfilling guideline inclusion criteria of at least 66% of participants having CKD.
The characteristics of studies included in the guideline are shown in Table 1. Of the 19 studies considered in the guideline, 9 examined febuxostat compared with placebo, standard of care, or no therapy (1 study also with verinurad) at an average dosage of 40 mg per day and up to 80 mg per day, with an observational period of 24 weeks to 24 months. Eight studies compared allopurinol with placebo, standard of care or no therapy at dosages between 100 and 400 mg per day and observed from 6 months to 36 months. In addition, 1 study of topiroxostat and 1 study of pegloticase were identified and considered. The randomized controlled trials included participants of mean age of 60.4 (±9.5) years old, with stage 1 to 4 CKD (mean estimated glomerular filtration rate [eGFR] 49.66 ± 17.89 ml/min per 1.73 m2), and mean serum urate level of 7.96 (±1.12) mg/dl. The median trial duration was 44 weeks and the mean was 67 weeks.
Table 1.
Characteristic of included studies
| Study | Inclusion criteria | No (% with CKD) | Treatment | Follow up |
|---|---|---|---|---|
| Beddhu et al.23 | Diabetes, eGFR 30 to 60 ml/min/m2 or >60 ml/min/m2 with proteinuria or albuminuria. Serum urate ≥5.5 mg/dl in men and ≥4.6 mg/dl in women with no history of gout | 80 (100) | Febuxostat 80 mg/d | 24 wk |
| FEATHER40 | CKD stage 3, serum urate 7-10 mg/dl with no history of gout | 441 (100) | Febuxostat 10-40 mg/d | 108 wk |
| FREED39 | Serum urate 7-9 mg/dl and 1 risk factor for cerebral or cardio-renovascular disease (such as eGFR 30-60 ml/min/m2) and no symptomatic gout within the last year | 1070 (66) | Febuxostat 10-40 mg/d | 36 mo |
| Goicoechea et al.25 | eGFR 15-60 ml/min/m2 and serum urate ≥6 mg/dl | 113 (100) | Allopurinol 100 mg/d | 84 mo |
| Golmohammadi et al.24 | eGFR 15-60 ml/min/m2, and serum urate ≥6 mg/dl with no history of gout | 196 (100) | Allopurinol 100 mg/d | 12 mo |
| Kao et al.26 | CKD stage 3 with left ventricular hypertrophy. Mean serum urate was 0.43 mmol/l | 53 (100) | Allopurinol 300 mg/d | 9 mo |
| Liu et al.27 | Diabetic nephropathy (mean eGFR 90 ml/min/m2), serum urate 7–8 mg/dl | 152 (100) | Allopurinol initially 100 mg/d and adjusted per response | 3 yr |
| Mukri et al.28 | Diabetic nephropathy CKD stage 3–4 and asymptomatic hyperuricemia ≥400 μmol/l | 93 (100) | Febuxostat 40 mg/d | 6 mo |
| PERL29 | Type 1 diabetes and eGFR 40-99.9 ml/min/m2, serum urate 4.5 mg/dl | 530 (100) | Allopurinol 100–400 mg/d | 164 wk |
| CKD-FIX10 | CKD stage 3-4 with no history of gout. No specific urate level inclusion criteria (mean 8.2 mg/dl) | 369 (100) | Allopurinol 100–300 mg/d | 104 wk |
| Saag et al.30 | eGFR 15–50 ml/min/m2, serum urate ≥7 mg/dl with a history of gout (but no tophaceous gout) | 95 (100) | Febuxostat 30 mg twice daily or 40–80 mg/daily | 12 mo |
| Shi et al.32 | Nephropathy with eGFR ≥30 ml/min/m2, urate ≥7 in men or ≥6 mg/dl in women | 40 | Allopurinol 100–300 mg/d | 6 mo |
| Sircar et al.33 | eGFR 15–60 ml/min/m2, serum urate ≥7 mg/dl with no history of gout | 93 (100) | Febuxostat 40 mg/d | 6 mo |
| Siu et al.31 | Kidney disease as proteinuria >0.5g or SCr >1.53 mg/dl and serum urate ≥7.60 mg/dl | 50 (100) | Allopurinol 100–300 mg/d | 12 mo |
| Stack et al.34 | eGFR >30 ml/min/m2 and serum uric acid >6 mg/dl with no history of gout | 60 | Febuxostat 80 mg/d with Verinurad 9 mg | 12 wk |
| UPWARD35 | Diabetic kidney disease, eGFR ≥30 ml/min/m2, gout or hyperuricemia included | 65 (100) | Topiroxostat 40–160 mg/d | 28 wk |
| Wen et al.36 | Diabetic kidney disease eGFR 30-59 ml/min/m2, serum urate >6 mg/dl | 65 (100) | Febuxostat 20-60 mg/d | 24 wk |
| Yood et al.37 | CKD stage 3–4, serum urate ≥8 mg/dl and symptomatic gout | 103 (100) | Pegloticase 8 mg every 2–4 weeks | 86 wk |
| Dalbeth et al.38 | Serum urate ≥7 mg/dl and a history of gout | 314 (72) | Febuxostat 40–80 mg/d | 24 mo |
CKD-FIX 2020, controlled trial of slowing of kidney disease progression from the inhibition of xanthine oxidase; eGFR, estimated glomerular filtration rate; FREED 2019, Febuxostat for cerebral and caRdiorenovascular Events PrEvEntion StuDy; FEATHER 2018, Febuxostat versus Placebo Randomized Controlled Trial Regarding Reduced Renal Function in Patients With Hyperuricemia Complicated by Chronic Kidney Disease Stage 3; HbA1c, hemoglobin A1C; PERL, preventing early renal function loss; UPWARD 2018, uric acid-lowering and renoprotective effects of topiroxostat, a selective xanthine oxidoreductase inhibitor, in patients with diabetic nephropathy and hyperuricemia: a randomized, double-blind, placebo-controlled, parallel-group study.
The findings of the updated literature review are summarized in Table 2. Compared with placebo, no therapy or standard of care, urate-lowering therapy probably had little or no effect on MACE and adverse events and may have had little or no effects on death and kidney failure. Nevertheless, urate-lowering therapy probably reduced the incidence of gout attacks and may have slightly improved annual eGFR change and blood pressure.
Table 2.
Summary of findings table
| Outcome | Study results and measurements | Absolute effect estimates |
Certainty of the Evidence (Quality of evidence) | Plain language summary | |
|---|---|---|---|---|---|
| Placebo/ Standard of care/ no treatment | Urate-lowering therapy | ||||
| Major cardiovascular events | Relative risk: 0.83 (CI 95% 0.63–1.1) Based on data from 2977 patients in 8 studiesaFollow up Mean 32 months |
113 per 1000 | 94 per 1000 | Moderate Because of serious risk of biasb |
Urate-lowering therapy probably has little or no difference on major cardiovascular events |
| Difference: 19 fewer per 1000 (CI 95% 42 fewer-11 more) | |||||
| Death | Relative risk: 1.06 (CI 95% 0.77–1.48) Based on data from 3019 patients in 8 studiesc Follow up Mean 32 months |
48 per 1000 | 51 per 1000 | Low Because of serious risk of bias, Because of serious imprecisiond |
Urate-lowering therapy may have little or no difference on death |
| Difference: 3 more per 1000 (CI 95% 11 fewer-23 more) | |||||
| Kidney failure | Relative risk: 0.89 (CI 95% 0.56– 1.41) Based on data from 2610 patients in 6 studiese Follow up Mean 37 months |
2 per 1000 | 2 per 1000 | Low Because of serious risk of bias, Because of serious inconsistencyf |
Urate-lowering therapy may have little or no difference on kidney failure |
| Difference: 0 fewer per 1000 (CI 95% 1 fewer-1 more) | |||||
| Adverse events | Relative risk: 1.03 (CI 95% 0.93–1.14) Based on data from 3349 patients in 13 studiesg Follow up Mean 23 months |
402 per 1000 | 414 per 1000 | Moderate Because of serious risk of biash |
Urate-lowering therapy probably has little or no difference on adverse events |
| Difference: 12 more per 1000 (CI 95% 28 fewer-56 more) | |||||
| Annual eGFR | Measured by: Scale: based on data from 3583 patients in 17 studiesi Follow up Mean 22 months |
Mean | Mean | Low Because of serious risk of bias, Because of serious inconsistencyj |
Urate-lowering therapy may improve eGFR slightly |
| Difference: MD 1.37 higher (CI 95% 0.48 higher-2.26 higher) | |||||
| Systolic blood pressure | Measured by: Scale: based on data from 2185 patients in 12 studiesk |
Mean | Mean | Moderate Because of serious risk of biasl |
Urate-lowering therapy probably improves systolic blood pressure |
| Difference: MD 3.45 lower (CI 95% 6.10 lower-0.80 lower) | |||||
| Diastolic blood pressure | Measured by: Scale: based on data from 2185 patients in 12 studiesm |
Mean | Mean | Moderate Because of serious risk of biasn |
Urate-lowering therapy probably improves diastolic blood pressure |
| Difference: MD 2.02 lower (CI 95% 3.25 lower-0.78 lower) | |||||
| Proteinuria | Measured by: Scale: based on data from 110 patients in 2 studieso |
Mean | Mean | Low Because of serious risk of bias, Because of serious imprecisionp |
Urate-lowering therapy may have little or no effect on proteinuria |
| Difference: MD 0.10 lower (CI 95% 0.89 lower-0.69 higher) | |||||
| Urinary albumin excretion ratio | Measured by: Scale: based on data from 682 patients in 2 studiesq |
Mean | Mean | Low Because of serious risk of bias, Because of serious imprecisionr |
Urate-lowering therapy may have little or no effect on UAER |
| Difference: MD 1.34 lower (CI 95% 13.93 lower–11.25 higher) | |||||
| Urinary albumin creatinine ratio | Measured by: Scale: based on data from 514 patients in 3 studiess |
Mean | Mean | Low Because of serious risk of bias, Because of serious imprecisiont |
Urate-lowering therapy may have little or no effect on UACR |
| Difference: MD 8.05 lower (CI 95% 29.39 lower–13.30 higher) | |||||
| Gout Attacks | Relative risk: 0.4 (CI 95% 0.17 –0.94) Based on data from 1074 participants in 6 studies Follow up Mean 18 months |
156 per 1000 | 62 per 1000 | Moderate Because of serious risk of biasu |
Urate-lowering therapy probably decreases gout attacks |
| Difference: 94 fewer per 1000 (CI 95% 129 fewer–9 fewer) | |||||
CI, confidence interval; MD, mean difference.
Population: people with CKD and hyperuricemia.
Intervention: urate-lowering therapy.
Comparator: placebo/standard of care/no treatment.
Systematic review12 with included studies: FREED Study,39 Mukri,28 PERL,29 Saag et al.,30 Dalbeth et al.,38 CKD-Fix Study,10 FEATHER,40 Goicoechea et al.25 Baseline/comparator Control arm of reference used for intervention.
Risk of Bias: serious. Inadequate/lack of blinding of participants and personnel, resulting in potential for performance bias, Selective outcome reporting.
Systematic review12 with included studies: Mukri,28 PERL,29 Saag et al.,30 Dalbeth et al.,38 CKD-Fix Study,10 FEATHER,40 Goicoechea et al.,25 FREED Study39 Baseline/comparator Control arm of reference used for intervention.
Risk of Bias: serious. Selective outcome reporting; Imprecision: serious. Wide confidence intervals.
Systematic review12 with included studies: PERL,29 Sircar et al.,33 FREED Study,39 CKD-Fix Study,10 FEATHER,40 Goicoechea et al.25 Baseline/comparator primary study. Supporting references [4].
Risk of Bias: serious. Selective outcome reporting; Inconsistency: serious. The magnitude of statistical heterogeneity was high, with I2: 58%.
Systematic review12 with included studies: Goicoechea et al.,25 Mukri,28 PERL,29 Saag et al.,30 Sircar et al.,33 Beddhu et al.,23 CKD-Fix Study,10 FEATHER,40 Kao et al.,26 Stack et al.,34 Wen et al.,36 Dalbeth et al.,38 FREED Study39 Baseline/comparator Control arm of reference used for intervention.
Risk of Bias: serious. Selective outcome reporting.
Systematic review12 with included studies: Sircar et al.,33 Mukri,28 Stack et al.,34 Shi et al.,32 Beddhu et al.,23 UPWARD,35 Kao et al.,26 Yood et al.,37 Wen et al.,36 PERL,29 FREED Study,39 Golmohammadi et al.,24 Saag et al.,30 Liu et al.,27 Goicoechea et al.,25 CKD-Fix Study,10 FEATHER40 Baseline/comparator Control arm of reference used for intervention.
Risk of Bias: serious. Inadequate concealment of allocation during randomization process, resulting in potential for selection bias, Inadequate/lack of blinding of outcome assessors, resulting in potential for detection bias; Inconsistency: serious. Point estimates vary widely, the magnitude of statistical heterogeneity was high, with I2: 74%.;
Systematic review.12 Baseline/comparator Control arm of reference used for intervention.
Risk of Bias: serious. Inadequate concealment of allocation during randomization process, resulting in potential for selection bias, Inadequate/lack of blinding of outcome assessors, resulting in potential for detection bias.
Systematic review.12 Baseline/comparator Control arm of reference used for intervention.
Risk of Bias: serious. Inadequate concealment of allocation during randomization process, resulting in potential for selection bias, Inadequate/lack of blinding of outcome assessors, resulting in potential for detection bias.
Systematic review12 Baseline/comparator Control arm of reference used for intervention.
Risk of Bias: serious. Inadequate concealment of allocation during randomization process, resulting in potential for selection bias, Inadequate/lack of blinding of outcome assessors, resulting in potential for detection bias; Imprecision: serious. Low number of patients.
Systematic review12 with included studies: PERL,29 Liu et al.27 Baseline/comparator Control arm of reference used for intervention.
Risk of Bias: serious. Inadequate concealment of allocation during randomization process, resulting in potential for selection bias, Inadequate/lack of blinding of outcome assessors, resulting in potential for detection bias; Imprecision: serious. Low number of patients.
Systematic review12 Baseline/comparator Control arm of reference used for intervention.
Risk of Bias: serious. Inadequate concealment of allocation during randomization process, resulting in potential for selection bias, Inadequate/lack of blinding of outcome assessors, resulting in potential for detection bias; Imprecision: serious. Low number of patients.
Risk of Bias: serious. Inadequate sequence generation/ generation of comparable groups, resulting in potential for selection bias, Inadequate concealment of allocation during randomization process, resulting in potential for selection bias.
Work Group Meetings
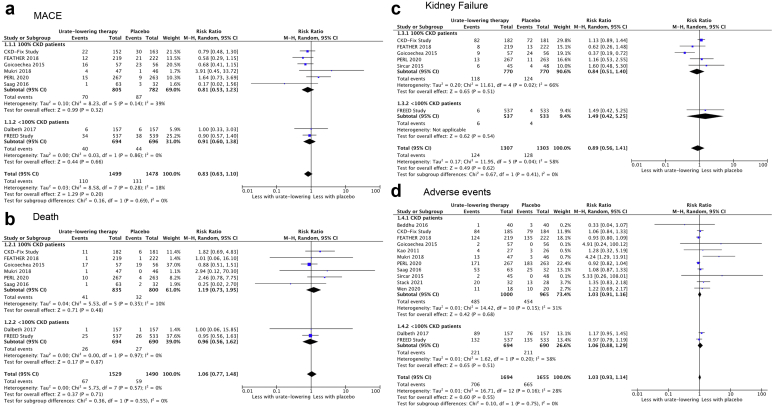
During the first of the Work Group’s meetings, CARI Guideline members reported the characteristics of included studies and discussed their appropriateness for consideration in the guideline’s development. Updated results of the primary outcomes from Chen et al12 (death, MACE, kidney failure, side effects) were reported to the Work Group (Figure 3). Clinicians and consumers in the Work Group discussed which additional outcomes were important for clinical decision making. These included the following:
-
•
eGFR change
-
•
Incidence of gout flares
-
•
A breakdown of specific side effects (particularly skin reactions and liver function)
Figure 3.
Meta-analysis forest plots of (a) MACE, (b) Death (c) Kidney Failure, (d) Adverse Events. CI, confidence interval; CKD, chronic kidney disease; CKD-FIX 2020, controlled trial of slowing of kidney disease progression from the inhibition of xanthine oxidase; dr, degrees of freedom; FEATHER 2018, febuxostat versus placebo randomized controlled trial regarding reduced renal function in patients with hyperuricemia complicated by chronic kidney disease stage 3; FREED 2019, febuxostat for cerebral and cardiorenovascular events PrEvEntion StuDy; MACE, major adverse cardiovascular events; PERL, preventing early renal function loss.
And important covariates requiring sensitivity analysis to explore heterogeneity were identified as:
-
•
Urate-lowering agent used (allopurinol, febuxostat, or other)
-
•
Duration of study (<12 months or >12 months)
-
•
Study gender composition (40%–60% male or >60% male)
-
•
Whether the study population excluded participants with a history of gout
-
•
The proportion of people with CKD in the included studies
-
•
Baseline eGFR
-
•
Baseline serum urate and exclusion of studies without hyperuricemia
-
•
PROGRESS PLUS characteristics
After this first meeting, 25 days were allocated for the CARI Guidelines (IKS and DJT) to carry out the relevant action points, extracting additional outcomes and performing sensitivity analyses. Presence of hyperuricemia, gout exclusion, and trial duration subgroup analyses, and eGFR and serum urate sensitivity analyses are provided in Supplementary Figures S1 and S2 (most of these outcomes had <10 studies thus are hypothesis generating). Following completion of these, the second Work Group meeting was held, during which the additional extracted outcomes and the results of sensitivity analyses results were reported to the group.
The impact of representation issues within the studies, which may have affected the guideline’s generalizability, were discussed. Specifically, of the 19 studies, only 6 had between 40% and 60% women, compared with 11 with <40% female participants. There was also poor reporting on the ethnicity of included participants. This was of particular concern to the Work Group because of the increased risk of allopurinol hypersensitivity in certain populations, such as Han Chinese.41 In addition, Controlled trial of slowing of kidney disease progression from the inhibition of xanthine oxidase was the only study to report inclusion of Aboriginal and/or Torres Strait Islander Peoples and Māori. In this second meeting, the Work Group also began drafting recommendations.
Based on the discussions at this meeting, 15 days were spent by CARI Guidelines (IKS and DJT) composing the first full draft of the guideline. This was circulated to the Work Group members for review, before being discussed in more detail during the third and final Work Group meeting. Thereafter, consensus was reached on the content of the guideline and a final draft was composed, with 5 more working days left for completion of the document and wider dissemination for peer review and consultation.
Guideline Recommendation
The full guideline is available online through the CARI website (https://www.cariguidelines.org/guidelines/chronic-kidney-disease/early-chronic-kidney-disease/medical-therapies-to-reduce-chronic-kidney-disease-progression-and-cardiovascular-risk-uric-acid-lowering-agents/) and MagicApp (https://app.magicapp.org/#/guideline/LqR80n). Based on current evidence, we recommend against the use of urate-lowering therapy in people with CKD (not receiving dialysis) and asymptomatic hyperuricaemia (Strong recommendation, low certainty of the evidence)
Rationale
The clinical importance of the annual eGFR change and blood pressure improvements were considered low by the Work Group and did not translate into improved hard endpoints such as death or kidney failure. Work Group members with lived experience of CKD also expressed concerns regarding long-term medication use, potential side-effects and increasing pill burden. These concerns were mirrored in available research; there has been limited research describing the perspectives and experiences of patients with CKD with asymptomatic hyperuricaemia. Nevertheless, a thematic synthesis of 20 qualitative studies41 examining the attitudes and values of patients with gout illustrated that patients were reluctant to continue medication, because of concerns regarding long-term side-effects. These patients were motivated to proceed with medication to maintain normal life and avoid painful flares. The transferability of these findings to patients with CKD and asymptomatic hyperuricemia is unclear.
The overall certainty of the evidence for the recommendation was low (Summary of Findings, Table 2). Evidence was downgraded due to high risk of bias, high inconsistency, and high imprecision. The outcomes of MACE, side effects, systolic blood pressure, and diastolic blood pressure, had moderate certainty of evidence; evidence certainty was downgraded 1 level for each outcome because of serious risk of bias. Death, kidney failure, urinary albumin creatinine ratio, urinary albumin excretion, and eGFR change were all downgraded to a low certainty of evidence because of high risk of bias for all outcomes, and high imprecision from wide confidence intervals or few events and participants, or high inconsistency because of high heterogeneity of the included studies.
Discussion
Overall, the completion of an up-to-date guideline on the role of urate-lowering therapy in people with CKD took 60 days, consisting of 15 days for synthesizing and updating the evidence, and 45 days for development of the guideline with a Work Group. Traditional processes for guideline development are generally far more time consuming;2 CARI Guidelines have traditionally taken 12 to 18 months to be developed, thus the time saving benefits were significant.
The methods used for this guideline’s development were streamlined and time efficient, primarily achieved through identification of an appropriate systematic review to be used as the evidence base. For these methods to be duplicated in the development of further guidelines, there must be an appropriate review available to be updated. The review’s search strategy and inclusion criteria must ensure that all studies relevant for the guideline’s development have been captured, and individual data from included randomized controlled trials must be reported (i.e., forest plots). The review utilized for this guideline had slightly broader population inclusion criteria than required, because it included studies in any adult participants (with or without CKD). To account for this, only studies where at least 66% of participants had CKD were extracted from the review and used for the guideline.
For this guideline, the availability of such as recent review may reduce the time required to update the literature search, as a search was only required to be carried out from July 2020 and an appropriate search strategy was already developed. An older review, with an appropriate search strategy and inclusion criteria, could also be used, however more time may need to be allocated to updating of the literature review.
Data analysis was reduced through utilization of the meta-analysis conducted by the review’s authors. Potential issues with data transparency were accounted for by obtaining raw data from the original authors’ meta-analysis for interrogation. Ideally, to further minimize data extraction, the systematic review’s outcomes should align with those relevant for clinical decision making. The outcomes explored by Chen et al.,12 namely kidney failure, cardiovascular disease, and mortality have been identified as core outcomes for patients with CKD.42 Any other additional important outcomes for clinicians or consumers in the Work Group were extracted from each included study during the 45-day development process.
This method requires a committed team of consumers and clinicians in the Work Group who are able to attend regular meetings and review the work over a 45-day period. In addition, literature searching, evidence synthesis, Work Group meeting preparation, and the guideline’s writing were primarily carried out by CARI Guidelines staff (IKS and DJT) who need to be available and able to manage the workload during the development period.
Recent publications have demonstrated the need for clinical decision makers to access constantly updated, critically appraised, and summarized evidence.43 Key issues in historical guideline development, that reduce access to evidence have been identified by the Australian Living Evidence Consortium, and include ineffectiveness of evidence synthesis methods, and publication overload;44 this method of guideline development addresses these issues. Another new method to improve guideline development, is Living Evidence and Living Guidelines. These utilize modern techniques such as machine learning and data synthesis to facilitate real-time updating of literature which maintains evidence-based guidelines.45 This approach has been successfully implemented during the COVID-19 global pandemic by the National COVID-19 Clinical Evidence Taskforce in Australia. Guidelines were maintained in a rapidly changing landscape with a high demand for up-to-date guidelines.46 Living methods are particularly useful in clinical areas with ongoing development where ongoing change to best practice may be expected. The decision was made to not make this a living guideline because major developments from upcoming publication or ongoing studies were not expected. Currently, to our knowledge CARI has been the only group to produce a living guideline in kidney disease.47 For this living guideline, a Cochrane systematic review was updated,48 which took at least 3 months and is currently undergoing editorial processes before publication. The approach of updating a recently published review, may decrease inefficiencies in evidence synthesis processes and improve the updating of clinical practice guidelines on management of kidney disease in near-real time. This method may be implemented and adapted for the production of future living guidelines.
This improved method of guideline development has the potential to allow for accurate kidney disease guidelines to be produced rapidly. Furthermore, of importance is the accessibility of these guidelines, and to facilitate and encourage shared decision making, an effective guideline should be easily accessible for clinicians and consumers. To achieve this, this guideline is fully accessible and available online through MagicApp, a web-based collaborative platform. In addition to consumer summaries of the guidelines, content are planned to be developed in partnership with people with lived experience of kidney disease.
The translation of new research findings into clinical practice is of key importance to improving how we provide quality patient care. Being able to integrate new research rapidly and accurately into guidelines maximizes its utility to inform clinical practice. This guideline on urate-lowering therapies in CKD was able to be updated with current evidence and the results of new clinical trials within 60 days. Further, more substantial trials with large populations and longer durations are needed to substantiate the certainty of evidence of urate-lowering therapy in CKD management.
Although the method used for this guideline’s development has limitations and may not always be applicable, it demonstrates the ability to rapidly update guidelines making trials results easily accessible.
Disclosure
RKSP has received consultancy/honorarium from AstraZeneca, NovoNordisk, Sanofi-Genzyme and support for travel from Novartis. ND has received consultancy fees and speaking honoraria from Astra Zeneca, Abbvie, Janssen, Horizon, Dyve Biosciences, PK Med, JW Pharmaceutical Corporation, Selecta, and Arthrosi, and research funding from Astra Zeneca and Amgen. ND is an investigator of the CKD-FIX Trial. DWJ has received consultancy/honorarium from Baxter Healthcare, Fresenius Medical Care, Astra Zeneca, AWAK, Ono and Bayer; has received support for travel from Amgen, and research funding for his institution from Baxter Healthcare, Fresenius Medical Care. DWJ is the Deputy Chair of the Australasian Kidney Trials Network and investigator of the CKD-FIX Trial. RK has received consultancy/honorarium from Baxter Healthcare; travel support from Amgen and Baxter Healthcare; and received funding for investigator-initiated research from Baxter Healthcare. DJT, VC, IKS, JK, and NDT declared no competing interests.
Acknowledgments
This guideline was funded by the National Health and Medical Research Program Grant ((APP1092957)–BEAT-CKD, and the Australian Government Department of Health, Public Health and Chronic Disease Kidney Disease (4-E85UZWV). Full guideline available from: https://www.cariguidelines.org/guidelines/chronic-kidney-disease/early-chronic-kidney-disease/medical-therapies-to-reduce-chronic-kidney-disease-progression-and-cardiovascular-risk-uric-acid-lowering-agents/. We want to acknowledge the CARI Guidelines Steering Committee and AKTN Steering Committee for their support
CARI Guidelines Steering Committee
Rathika Krishnasamy (Chair)
Vincent Lee (Deputy Chair)
Jane Boag
Helen Coolican
Jonathan Craig
Vanessa Cullen
Min Jun
Kelly Lambert
Thu Nguyen
Carla Scuderi
Emily See
Andrea K Viecelli
AKTN Steering Committee
Carmel M Hawley (Co-chair)
David W Johnson (Co-chair)
Matthew Roberts (Scientific Committee Chair)
Michael Collins (Scientific Committee Deputy Chair)
Yeoungjee Cho
Rathika Krishnasamy
Andrea K Viecelli
Donna Reidlinger
Elaine Pascoe
Laura Robison
Charani Kiriwandeniya
Julie Varghese
Footnotes
Figure S1. Sensitivity analysis of major adverse cardiovascular events (panel A and B) and eGFR (panel C and D).
Figure S2. Subgroup analysis of trial duration (panel A-D), hyperuricemia (panel E-I) and gout (panel J-M).
Table S1. AMSTAR2 critical appraisal of Chen et al.12
Table S2. Search strategy
Appendix
Members of the CARI Guidelines Steering Committee are Listed Below
Rathika Krishnasamy (Chair)
Vincent Lee (Deputy Chair)
Jane Boag, Federation University
Helen Coolican
Jonathan Craig
Vanessa Cullen
Min Jun
Kelly Lambert
Thu Nguyen
Carla Scuderi
Emily See
Andrea K Viecelli
Supplementary Material
Figure S1. Sensitivity analysis of major adverse cardiovascular events (panel A and B) and eGFR (panel C and D).
Figure S2. Subgroup analysis of trial duration (panel A-D), hyperuricemia (panel E-I) and gout (panel J-M).
Table S1. AMSTAR2 critical appraisal of Chen et al.12
Table S2. Search strategy
Reference
- 1.Niforatos J.D., Weaver M., Johansen M.E. Assessment of publication trends of systematic reviews and randomized clinical trials, 1995 to 2017. JAMA Intern Med. 2019;179:1593–1594. doi: 10.1001/jamainternmed.2019.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenfeld R.M., Shiffman R.N. Clinical practice guideline development manual: A quality-driven approach for translating evidence into action. Otolaryngol Head Neck Surg. 2009;140(Suppl 1):1–43. doi: 10.1016/j.otohns.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris Z.S., Wooding S., Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med. 2011;104:510–520. doi: 10.1258/jrsm.2011.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill N.R., Fatoba S.T., Oke J.L., et al. Global prevalence of chronic kidney disease-a systematic review and meta-analysis. PloS One. 2016;11 doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson D.W. Evidence-based guide to slowing the progression of early renal insufficiency. Int Med J. 2004;34:50–57. doi: 10.1111/j.1444-0903.2004.t01-6-.x. [DOI] [PubMed] [Google Scholar]
- 6.Clark E., Donovan E.F., Schoettker P. From outdated to updated, keeping clinical guidelines valid. Int J Qual Health Care. 2006;18:165–166. doi: 10.1093/intqhc/mzl007. [DOI] [PubMed] [Google Scholar]
- 7.Weiner D.E., Tighiouart H., Elsayed E.F., et al. Uric acid and incident kidney disease in the community. J Am Soc Nephrol. 2008;19:1204–1211. doi: 10.1681/ASN.2007101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu C.-y., Iribarren C., McCulloch C.E., et al. Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med. 2009;169:342–350. doi: 10.1001/archinternmed.2008.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turgut FMD, Kasapoğlu BMD, Kanbay MMD. Uric acid, cardiovascular mortality, and long-term outcomes in CKD. Am J Kidney Dis. 2009;54:582-582. Doi:10.1053/j.ajkd.2009.06.024 [DOI] [PubMed]
- 10.Badve S.V., Pascoe E.M., Tiku A., et al. Effects of allopurinol on the progression of chronic kidney disease. N Engl J Med. 2020;382:2504–2513. doi: 10.1056/NEJMoa1915833. [DOI] [PubMed] [Google Scholar]
- 11.Johnson D.W., Atai E., Chan M., et al. KHA-CARI Guideline: early chronic kidney disease: detection, prevention and management. Nephrology (Carlton) 2013;18:340–350. doi: 10.1111/nep.12052. [DOI] [PubMed] [Google Scholar]
- 12.Chen Q., Wang Z., Zhou J., et al. Effect of urate-lowering therapy on cardiovascular and kidney outcomes a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2020;15:1576–1586. doi: 10.2215/CJN.05190420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shea B.J., Reeves B.C., Wells G., et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Review manager (RevMan) Version 5.4. The Cochrane Collaboration; Published 2020. https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/revman-non-cochrane-reviews [Google Scholar]
- 15.R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing. The R Foundation; Vienna, Austria: 2022. http://www.R-project.org/https://intro2r.com/citing-r.html [Google Scholar]
- 16.National Health and Medical Research Council Guidelines for Guidelines: identifying and managing conflicts of interest. https://www.nhmrc.gov.au/guidelinesforguidelines/plan/identifying-and-managing-conflicts-interest Published 2018.
- 17.Rai S.K., Choi H.K., Choi S.H.J., et al. Key barriers to gout care: a systematic review and thematic synthesis of qualitative studies. Rheumatol (Oxf Engl) 2018;57:1282–1292. doi: 10.1093/rheumatology/kex530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balshem H., Helfand M., Schünemann H.J., et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 19.GRADEpro guideline development tool. McMaster University and Evidence Prime; 2022. https://www.gradepro.org/terms/cite [Google Scholar]
- 20.Alonso-Coello P., Oxman A.D., Moberg J., et al. GRADE evidence to decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: clinical practice guidelines. BMJ. 2016;353 doi: 10.1136/bmj.i2089. [DOI] [PubMed] [Google Scholar]
- 21.Teufer B., Nußbaumer-Streit B., Ebenberger A., et al. GRADE equity Guidelines 1: considering health equity in GRADE guideline development-introduction and rationale. Z Evid Fortbild Qual Gesundhwes. 2019;146:53–59. doi: 10.1016/j.zefq.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 22.O’Neill J., Tabish H., Welch V., et al. Applying an equity lens to interventions: using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. J Clin Epidemiol. 2014;67:56–64. doi: 10.1016/j.jclinepi.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Beddhu S., Filipowicz R., Wang B., et al. A randomized controlled trial of the effects of febuxostat therapy on Adipokines and markers of kidney fibrosis in asymptomatic hyperuricemic patients with diabetic nephropathy. Can J Kidney Health Dis. 2016;3 doi: 10.1177/2054358116675343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golmohammadi S., Almasi A., Manouchehri M., et al. Allopurinol against progression of chronic kidney disease. Iran J Kidney Dis. 2017;11:286–293. [PubMed] [Google Scholar]
- 25.Goicoechea M.M.D.P., Garcia de Vinuesa S.M.D., Verdalles U.M.D., et al. Allopurinol and progression of CKD and cardiovascular events: long-term follow-up of a randomized clinical trial. Am J Kidney Dis. 2015;65:543–549. doi: 10.1053/j.ajkd.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Kao M.P., Ang D.S., Gandy S.J., et al. Allopurinol benefits left ventricular mass and endothelial dysfunction in chronic kidney disease. J Am Soc Nephrol. 2011;22:1382–1389. doi: 10.1681/ASN.2010111185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu P., Chen Y., Wang B., et al. Allopurinol treatment improves renal function in patients with type 2 diabetes and asymptomatic hyperuricemia: 3-year randomized parallel-controlled study. Clin Endocrinol (Oxf) 2015;83:475–482. doi: 10.1111/cen.12673. [DOI] [PubMed] [Google Scholar]
- 28.Mukri M.N.A., Kong W.-Y., Mustafar R., et al. Role of febuxostat in retarding progression of diabetic kidney disease with asymptomatic hyperuricemia: a 6-months open-label, randomized controlled trial. Excli J. 2018;17:563–575. doi: 10.17179/excli2018-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doria A., Galecki A.T., Spino C., et al. Serum urate lowering with allopurinol and kidney function in type 1 diabetes. N Engl J Med. 2020;382:2493–2503. doi: 10.1056/NEJMoa1916624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saag K.G., Whelton A., Becker M.A., et al. Impact of febuxostat on renal function in gout patients with moderate-to-severe renal impairment. Arthritis Rheumatol. 2016;68:2035–2043. doi: 10.1002/art.39654. [DOI] [PubMed] [Google Scholar]
- 31.Siu Y.P., Leung K.T., Tong M.K., Kwan T.H. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47:51–59. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Shi Y., Chen W., Jalal D., et al. Clinical outcome of hyperuricemia in IgA nephropathy: a retrospective cohort study and randomized controlled trial. Kidney Blood Press Res. 2012;35:153–160. doi: 10.1159/000331453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sircar D., Chatterjee S., Waikhom R., et al. Efficacy of febuxostat for slowing the GFR decline in patients with CKD and asymptomatic hyperuricemia: a 6-month, double-blind, randomized, placebo-controlled trial. Am J Kidney Dis. 2015;66:945–950. doi: 10.1053/j.ajkd.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 34.Stack A.G., Dronamraju N., Parkinson J., et al. Effect of intensive urate lowering with combined verinurad and febuxostat on albuminuria in patients with type 2 diabetes: a randomized trial. Am J Kidney Dis. 2021;77:481–489. doi: 10.1053/j.ajkd.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wada T., Hosoya T., Honda D., et al. Uric acid-lowering and renoprotective effects of topiroxostat, a selective xanthine oxidoreductase inhibitor, in patients with diabetic nephropathy and hyperuricemia: a randomized, double-blind, placebo-controlled, parallel-group study (UPWARD study) Clin Exp Nephrol. 2018;22:860–870. doi: 10.1007/s10157-018-1530-1. [DOI] [PubMed] [Google Scholar]
- 36.Wen H., Yongling Z., Shuying Z., et al. Effect of febuxostat on renal function in patients from South China with CKD3 diabetic nephropathy. Braz J Nephrol. 2020;42:393–399. doi: 10.1590/2175-8239-jbn-2019-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yood R.A., Ottery F.D., Irish W., Wolfson M. Effect of pegloticase on renal function in patients with chronic kidney disease: a post hoc subgroup analysis of 2 randomized, placebo-controlled, phase 3 clinical trials. BMC Res Notes. 2014;7:54. doi: 10.1186/1756-0500-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dalbeth N., Saag K.G., Palmer W.E., et al. Effects of febuxostat in early gout: a randomized, double-blind, placebo-controlled study. Arthritis Rheumatol. 2017;69:2386–2395. doi: 10.1002/art.40233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kojima S., Matsui K., Hiramitsu S., et al. Febuxostat for cerebral and CaRdiorenovascular events PrEvEntion StuDy. Eur Heart J. 2019;40:1778–1786. doi: 10.1093/eurheartj/ehz119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimura K., Hosoya T., Uchida S., et al. Febuxostat therapy for patients with Stage 3 CKD and asymptomatic hyperuricemia: a randomized trial. Am J Kidney Dis. 2018;72:798–810. doi: 10.1053/j.ajkd.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 41.Stamp L.K., Barclay M.L. How to prevent allopurinol hypersensitivity reactions? Rheumatol (Oxf Engl) 2018;57(Suppl 1):i35–i41. doi: 10.1093/rheumatology/kex422. [DOI] [PubMed] [Google Scholar]
- 42.González A.M., Gutman T., Lopez-Vargas P., et al. Patient and caregiver priorities for outcomes in CKD: a multinational nominal group technique study. Am J Kidney Dis. 2020;76:679–689. doi: 10.1053/j.ajkd.2020.03.022. [DOI] [PubMed] [Google Scholar]
- 43.Elliott J., Lawrence R., Minx J.C., et al. Decision makers need constantly updated evidence synthesis. Nature. 2021;600:383–385. doi: 10.1038/d41586-021-03690-1. [DOI] [PubMed] [Google Scholar]
- 44.Report on the outcomes of a living evidence consortium planning meeting. Living evidence for Australian health care. Published 2018. https://australia.cochrane.org/sites/australia.cochrane.org/files/uploads/living_evidence_consortium_planning_mtg_report_final_for_web.pdf
- 45.El Mikati I.K., Khabsa J., Harb T., et al. A frame work for the development of living practice guidelines in health care. Ann Intern Med. 2022;175:1154–1160. doi: 10.7326/M22-0514. [DOI] [PubMed] [Google Scholar]
- 46.How we develop recommendations. National COVID-19 clinical evidence task force. https://covid19evidence.net.au/more-about-the-guidelines/
- 47.CARI living guideline lipid work group Management of cholesterol-lowering therapy for people with chronic kidney disease. CARI Guidelines. https://www.cariguidelines.org/guidelines/chronic-kidney-disease/early-chronic-kidney-disease/medical-therapies-to-reduce-chronic-kidney-disease-progression-and-cardiovascular-risk-lipid-lowering-therapy/ [DOI] [PubMed]
- 48.Palmer S.C., Navaneethan S.D., Craig J.C., et al. HMG CoA Reductase Inhibitors (statins) for people with chronic kidney disease not requiring dialysis. Cochrane Database Syst Rev. 2014;5 doi: 10.1002/14651858.CD007784.pub2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.