Abstract
Introduction
Granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA), 2 major clinicopathologic variants of antineutrophil cytoplasmic autoantibody (ANCA) vasculitides, are mostly associated with proteinase 3 (PR3)-ANCA and myeloperoxidase (MPO)-ANCA, respectively. Less is known regarding the uncommon forms of ANCA vasculitis, PR3-ANCA MPA and MPO-ANCA GPA.
Methods
In this cohort study we detailed the clinical presentation and outcome of patients with PR3-ANCA MPA and MPO-ANCA GPA from the Glomerular Disease Collaborative Network (GDCN) inception cohort. Baseline clinical manifestations, relapses, end-stage kidney disease (ESKD), and survival were compared within MPA cases by PR3-ANCA (n = 116) versus MPO-ANCA (n = 173) and within GPA cases by PR3-ANCA (n = 108) versus MPO-ANCA (n = 43). Fisher’s exact test and Wilcoxon two sample test were used for comparisons. Proportional hazards models were used to evaluate the development of relapses, ESKD, and death.
Results
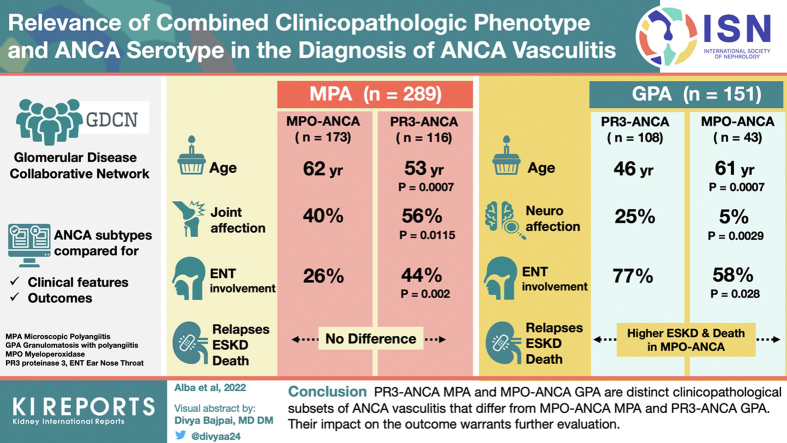
Patients with PR3-ANCA MPA were younger (53 years vs. 62 years, P = 0.0007) and had increased prevalence of joint involvement (56% vs. 40%, P = 0.0115) and ear, nose, and throat (ENT) involvement (44% vs. 26%, P = 0.002) than MPO-ANCA MPA. Relapses, ESKD, and survival were similar between both MPA subsets. Within the GPA group, patients with MPO-ANCA GPA were older (61 years vs. 46 years, P = 0.0007) and more likely female (56% vs. 35%, P = 0.027) than PR3-ANCA GPA patients. MPO-ANCA GPA was also characterized by less prevalent ENT manifestations (58% vs. 77%, P = 0.028) and neurologic manifestations (5% vs. 25%, P = 0.0029), and increased ESKD and mortality.
Conclusions
PR3-ANCA MPA and MPO-ANCA GPA are clinicopathologically distinct subsets of ANCA vasculitis that differ from MPO-ANCA MPA and PR3-ANCA GPA. Although the impact of these differences on the clinical management and outcome warrants further evaluation, these results support the recommendation of including both the phenotypic diagnosis and ANCA serotype in the diagnosis of ANCA vasculitis.
Keywords: ANCA, granulomatosis with polyangiitis, microscopic polyangiitis, MPO, PR3, vasculitis
Graphical abstract
ANCAs bind to specific antigens in the cytoplasmic granules of neutrophils and lysosomes of monocytes. The 2 main autoantigen targets recognized by ANCA are myeloperoxidase and proteinase 3.1 ANCA vasculitis, the most frequent primary small-vessel vasculitis in adults, comprises a distinctive group of multiorgan system diseases characterized by necrotizing vasculitis, with few or no immune deposits, that predominantly affects capillaries, venules, arterioles, and small arteries.1
ANCA vasculitis has 3 major clinicopathologic variants, namely GPA, MPA, and eosinophilic granulomatosis with polyangiitis (EGPA).1 GPA is characterized by vasculitis in addition to extravascular necrotizing granulomatous inflammation, usually involving the respiratory tract. Eosinophilic granulomatosis with polyangiitis has asthma, eosinophilia, and granulomatous inflammation in addition to vasculitis. MPA shares vasculitic clinical and pathological features with the other ANCA vasculitides but lacks granulomatous inflammation, asthma, or eosinophilia.1
Although either ANCA specificity can occur in any clinicopathologic phenotype, GPA is primarily associated with PR3-ANCA (69%−88% of cases) in White populations, whereas antibodies against MPO are predominantly detected in MPA (47%−96%).2, 3, 4, 5, 6, 7, 8, 9 In contrast, MPO-ANCA are the most common type of ANCA identified in China and Japan, irrespective of clinical diagnosis.10
Clinical, epidemiologic, genetic, and experimental evidence suggest that ANCA serotype may determine distinct but overlapping clinical and pathologic features of ANCA vasculitis.11 Previous studies have shown that the ANCA antigen specificity is predictive of significant differences in both clinical manifestations and outcome of affected patients as follows: PR3-ANCA is associated with a higher prevalence of ophthalmologic and ENT symptoms, increased risk of relapse, and better response to rituximab-based induction therapy.3,11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 In contrast, MPO-ANCA is associated with an elevated frequency of kidney involvement and development of chronic kidney disease and pulmonary fibrosis.3,11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 Further, candidate gene approaches and large genome-wide association studies have revealed that ANCA vasculitis has a heterogenic genetic background, with PR3-ANCA vasculitis associated with polymorphisms in the human leukocyte antigen-DP region, SERPINA1, and PRTN3 genes; and MPO-ANCA disease associated with distinct human leukocyte antigen-DQ variants.24,25
Though a large number of studies have demonstrated that MPO-ANCA and PR3-ANCA vasculitis have substantial clinical differences,3,11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 scarce evidence exists on the categorization of differences based on serotype within a specific clinicopathologic category. Thus, data on the less common subgroups PR3-ANCA MPA and MPO-ANCA GPA is limited. In North American and European cohorts, PR3-ANCA is found in 0% to 28% of MPA patients7, 8, 9, 10,26 whereas MPO-ANCA rarely occurs in GPA (2%−9%).2, 3, 4,7,27, 28, 29, 30 The characterization of particular ANCA vasculitis subsets based on phenotypic characteristics and ANCA serotype could be of relevance to improve patient care and prognosis. Anticipating different propensities for specific organ manifestations and complications, or predicting treatment response and long-term course, could result in more individualized targeted therapy. In addition, this information could facilitate the design of clinical trials, epidemiological studies, or translational research aimed to obtain a better understanding of ANCA vasculitis pathophysiology.
In the present study we tested the hypothesis that ANCA specificity determines distinctive subsets in MPA and GPA showing that PR3-ANCA MPA and MPO-ANCA GPA were associated with distinct clinical characteristics and outcome; thus, allowing their differentiation from the more traditional MPO-ANCA MPA and PR3-ANCA GPA. To this purpose, we analyzed the initial organ involvement and long-term outcome, including relapse rate, ESKD, and death in these specific subgroups in a large inception cohort of ANCA vasculitis patients. Our results potentially challenge the concept of categorization of ANCA vasculitis patients exclusively based on ANCA specificity or clinicopathologic phenotype and support the relevance of including both in the diagnosis of ANCA vasculitis.
Methods
Study Population
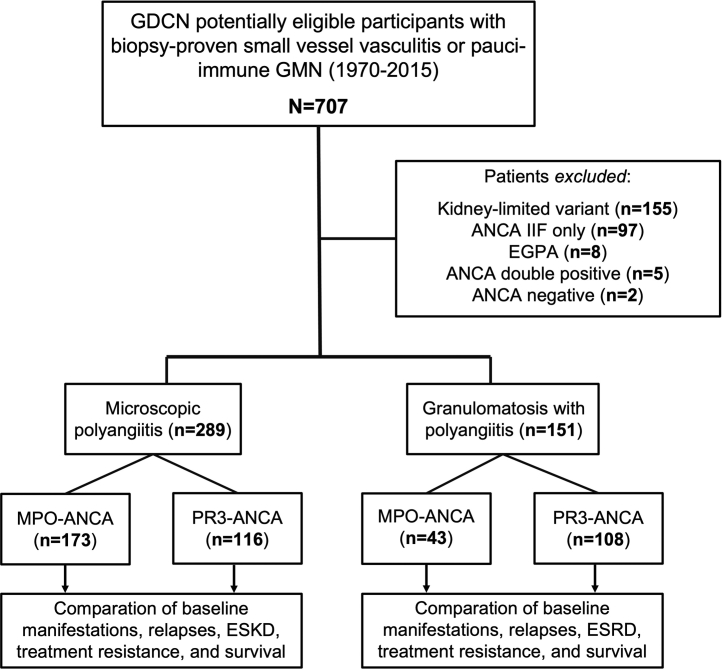
Clinical data from subjects enrolled in the GDCN ANCA inception cohort were analyzed in this study. The GDCN is a longitudinal, glomerular disease patient registry and biobank repository that has been ongoing for more than 35 years with patients primarily from southeastern United States. The GDCN primarily identifies patients diagnosed by a renal biopsy evaluated by the University of North Carolina nephropathology service. This includes patients evaluated and followed at University of North Carolina hospitals, which constitute approximately 60% of the GDCN cohort, and from private practice nephrologists. Methods for identification and enrollment of patients have been previously reported.20 Subjects were eligible for this retrospective cohort study based on the following criteria: (i) had biopsy-proven small vessel vasculitis or pauci-immune glomerulonephritis diagnosed between June 1970 and December 2015 and (ii) had a positive ANCA test with specificity to PR3 or MPO detected by enzyme-linked immunosorbent assay. Participants with eosinophilic granulomatosis with polyangiitis, those with ANCA determined only by immunofluorescence microscopy (c-ANCA or p-ANCA), and patients with kidney-limited vasculitis (pauci-immune necrotizing crescentic glomerulonephritis without overt signs of systemic vasculitis)1 were excluded, as were cases with double positive ANCA (Figure 1). The study was approved by the University of North Carolina Institutional Review Board.
Figure 1.
Flow diagram of the study cohort. ANCA, Antineutrophil cytoplasmic autoantibodies; eosinophilic granulomatosis with polyangiitis, eosinophilic granulomatosis with polyangiitis; ESKD, end-stage kidney disease; GDCN, glomerular disease collaborative network; GMN, glomerulonephritis; IIF, indirect immunofluorescence; MPO, myeloperoxidase; PR3, proteinase 3.
Definitions and Outcome Measures
Clinicopathologic phenotype (GPA and MPA) was established on an ongoing basis according to the Chapel Hill Consensus Conference nomenclature.1,31 Disease classification is rendered at a specific moment and may change over time as disease develops, for example, from MPA to GPA phenotype. Therefore, patients included in the GDCN cohort are prospectively followed from the time of diagnosis and records are reviewed approximately annually to maintain updated information on phenotypes and outcomes. GPA was defined by the presence of necrotizing granulomatous inflammation in any tissue by histology, and/or imaging showing pulmonary noninfectious nodules or cavities and/or invasive bony disease, mass lesions in the paranasal sinuses, pansinusitis and/or subglottic stenosis in the upper respiratory tract whereas MPA was considered to be present in patients with biopsy-proven small-vessel vasculitis without evidence of granulomatous inflammation or asthma.5,20,32 Disease subsets were categorized within each major clinical phenotype based on ANCA reactivity, (e.g., PR3-ANCA MPA or MPO-ANCA MPA). Baseline clinical manifestations, initial response to treatment, and long-term outcome were compared between those with MPO-ANCA and PR3-ANCA within the same clinical phenotype (Figure 1). Clinical manifestations and outcome were recorded by review of medical records. Initial organ involvement included the presence of upper and lower respiratory tract disease; muscle and joint manifestations; cutaneous vasculitis; and eye, peripheral nervous system, gastrointestinal, and kidney involvement, as previously described.5,20,32 Radiology reports from pulmonary and paranasal sinuses evaluations made by conventional radiography or computed tomography scan were included. Peak serum creatinine level was defined as the highest measurement at the time of diagnosis before the start of treatment. Kidney histopathology parameters such as glomerular necrosis, glomerular sclerosis, or interstitial fibrosis, scored semiquantitatively from 0 (none) to 4 (severe), were recorded when biopsy reports were available.
Primary clinical outcomes included relapse rate, ESKD, treatment resistance, and patient survival. Definitions of remission, relapse, and treatment resistance have been previously described.33 Briefly, remission was considered as the absence of dysmorphic red blood cells and red blood cell casts on microscopic urinalysis and no evidence of vasculitic lesions or manifestations in any organ. The term remission on therapy was used when remission was maintained with immunosuppressive drugs or prednisone at a dose of >7.5 mg per day. Treatment resistance was recorded when persistence or new appearance of any extrarenal manifestation of vasculitis despite immunosuppressive therapy and/or progressive decline in kidney function in the setting of active urinary sediment were present; this was determined at least 1 month after the start of treatment. Relapse was defined as the reactivation of vasculitis in any organ system occurring after a period of remission that was severe enough to warrant an adjustment in therapy. Persistent proteinuria alone was not considered indicative of active disease. ESKD was defined as the need for permanent kidney replacement therapy or transplantation. Death from any cause was noted.
Standard remission induction therapy typically included 3 daily pulses of methylprednisolone (7 mg/kg of body weight per day) followed by daily oral prednisone (1 mg/kg/day for the first month), which was tapered over 3 to 4 months. Cyclophosphamide was administered by intravenous pulse (0.5 to 1 g/m2 per month) or orally (1 to 2 mg/kg per day). Rituximab was used at a dose of 375 mg/m2 per week for 4 weeksor 1 gm q14d x2. Plasmapheresis was added for patients with severe kidney failure or diffuse alveolar hemorrhage.
Statistical Analysis
Descriptive statistics are presented as median with interquartile range for continuous variables and as percentage for qualitative data. Comparisons between ANCA subgroups were performed by using Fisher’s exact tests for qualitative variables and Wilcoxon two-sample test for continuous measures. Kaplan Meier estimates with Log-Rank test were used for comparing ESKD, patient survival, and relapses curves between ANCA subgroups; P values were calculated and adjusted by the Šidák correction for multiple comparisons of Kaplan Meier curves. Proportional hazards models were used to evaluate hazard ratios (HRs) for the development of relapses, ESKD, and death after controlling for age, sex, lung and ENT involvement, maximum creatinine at diagnosis, and induction therapy with cyclophosphamide. Analyses were performed using SAS statistical software, version 9.4 (SAS Institute, Inc., Cary, North Carolina, USA).
Results
A total of 707 patients with ANCA vasculitis were identified as eligible for this study. Of those, 440 cases fulfilled the inclusion criteria and were included in the final analysis (Figure 1).
MPA
The analyzed sample consisted of 289 MPA patients, 47% female, with a median age at diagnosis of 59 (interquartile range 39–71) years. MPO-ANCA and PR3-ANCA were positive in 59% and 41% of the study population, respectively. Demographic characteristics and a detailed description of major organ involvement are shown in Table 1.
Table 1.
Detailed baseline clinical manifestations among microscopic with polyangiitis patients according to ANCA specificity (n = 289)
| Variable | PR3-ANCA MPA (n = 116) | MPO-ANCA MPA (n = 173) | P valuea |
|---|---|---|---|
| Age at diagnosis, median (IQR), yr | 53 (39–65) | 62 (50–71) | 0.0007 |
| Female, no. (%) | 48 (41) | 90 (52) | 0.0925 |
| Initial manifestations | |||
| Lung involvement, no. (%) | 67 (58) | 99 (57) | 1.0000 |
| Pulmonary infiltrates, no. (%) | 47 (41) | 62 (36) | 0.4584 |
| Pulmonary nodules, no. (%) | 0 (0) | 0 (0) | NA |
| Pulmonary cavitations, no. (%) | 0 (0) | 0 (0) | NA |
| ENT involvement, no. (%) | 51 (44) | 45 (26) | 0.0021 |
| Clinical sinusitis, no. (%) | 32 (28) | 28 (16) | 0.026 |
| Radiology sinusitis, no. (%) | 9 (8) | 5 (3) | 0.0905 |
| Nose/oral ulcers, no. (%) | 11 (9) | 10 (6) | 0.2543 |
| Otitis media, no. (%) | 14 (12) | 9 (5) | 0.0452 |
| Articular involvement, no. (%) | 65 (56) | 70 (40) | 0.0115 |
| Gastrointestinal involvement, no. (%) | 14 (12) | 21 (12) | 1.0000 |
| Nervous system involvement, no. (%) | 20 (17) | 23 (13) | 0.4005 |
| Skin involvement, no. (%) | 32 (28) | 45 (26) | 0.7872 |
| Eye involvement, no. (%) | 9 (8) | 8 (5) | 0.3118 |
| Kidney involvement, no. (%) | 111 (96) | 172 (99) | 0.04 |
| Peak entry creatinine, median (IQR), mg/dl | 3 (2–6) | 4 (2–6) | 0.1283 |
| Acute dialysis at disease onset, no. (%) | 15 (13) | 17 (10) | 0.4473 |
| Histological parameters on kidney biopsy | |||
| bCrescents percentage, median (IQR) | 40 (20–80) | 31 (15–60) | 0.2169 |
| cGlomerular necrosis, median (IQR) | 2 (1–2) | 1 (1–2) | 0.0027 |
| dGlomerular crescents, median (IQR) | 2 (1–4) | 2 (1–3) | 0.0559 |
| eGlomerular sclerosis, median (IQR) | 1 (1–2) | 1 (1–2) | 0.0471 |
| fInterstitial sclerosis, median (IQR) | 1 (1–2) | 2 (1–2) | 0.0314 |
| gCrescent sclerosis, median (IQR) | 1 (0–1) | 1 (0–2) | 0.0206 |
| Number of affected organs, median (IQR) | 3 (2–4) | 3 (2–4) | 0.003 |
| Induction therapy | |||
| Methylprednisolone bolus, no. (%) | 78 (67) | 121 (70) | 0.6977 |
| Prednisone, no. (%) | 105 (91) | 161 (93) | 0.5077 |
| Cyclophosphamide, no. (%) | 98 (84) | 144 (83) | 0.8713 |
| Prednisone and cyclophosphamide, no. (%) | 112 (97) | 168 (97) | 1.0000 |
| Plasma exchange, no. (%) | 31 (27) | 39 (23) | 0.484 |
| Rituximab, no. (%) | 4 (3) | 12 (7) | 0.2948 |
| Response to initial induction therapy | |||
| Complete remission off therapy, no. (%) | 41 (38) | 82 (50) | 0.1287 |
| Remission sustained with therapy, no. (%) | 46 (43) | 54 (33) | |
| Therapy resistant, no. (%) | 17 (16) | 27 (16) |
ANCA, antineutrophil cytoplasmic autoantibody; ENT, ear, nose and throat; IQR, interquartile range; MPA, microscopic polyangiitis; MPO, myeloperoxidase; NA, not applicable; PR3, proteinase 3.
P-values were calculated using Fisher exact test for categorical variables and Wilcoxon two sample test for continuous variables.Number of available kidney biopsy reports for MPO-MPA and PR3-MPA:
135 and 91.
104 and 70.
96 and 65.
109 and 73.
93 and 65.
107 and 71, respectively.
Baseline Organ Involvement and Response to Treatment According to ANCA Specificity
Comparison of PR3-ANCA-positive MPA (n = 116) with the most common MPO-ANCA MPA subset (n = 173) disclosed several differences. PR3-ANCA patients were significantly younger and had a higher frequency of articular and ENT manifestations such as sinusitis and otitis media) (Table 1). MPA individuals with anti-PR3 ANCA had less conspicuous glomerular and interstitial sclerosis but increased glomerular necrosis. No significant differences were found regarding induction immunosuppressive therapy or initial response to treatment (Table 1).
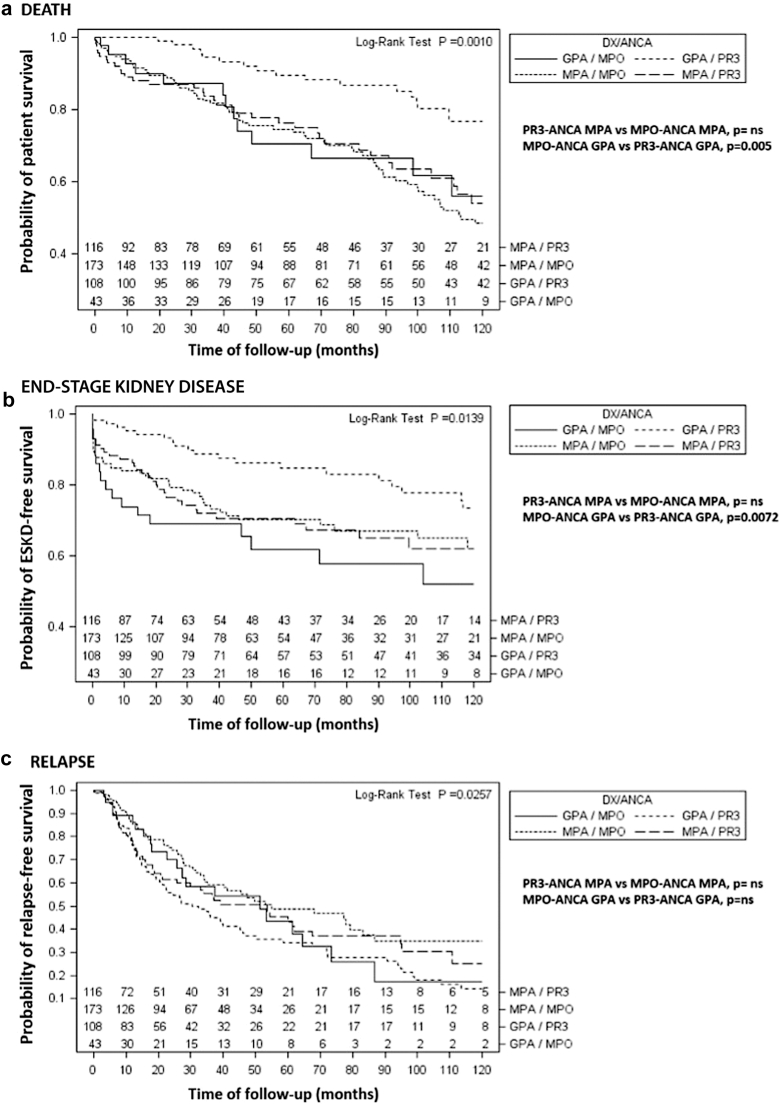
Clinical Outcomes of Interest
Anti-MPO and anti-PR3 ANCA MPA patients had similar frequencies of relapses, ESKD, and survival rate (Table 2, Figure 2). Multivariable analysis (Table 3) showed that ANCA serotype was not associated with any of the selected outcomes of interest. In contrast, lung involvement (HR 2.21, 95% confidence interval [CI] 1.35−3.59), peak creatinine at diagnosis (HR 1.23, 95% CI 1.18−1.29), and use of cyclophosphamide (HR 0.45, 95% CI 0.25−0.82) predicted ESKD; whereas age at disease diagnosis (HR 1.03, 95% CI 1.01−1.05), pulmonary disease (HR 1.96, 95% CI 1.26−3.06), maximum level of creatinine (HR 1.07, 95% CI 1.02−1.13), and use of cyclophosphamide (HR 0.49, 95% CI 0.30−0.80) influenced patient survival. None of the analyzed variables was associated with disease flares.
Table 2.
Long-term outcome of microscopic with polyangiitis and granulomatosis with polyangiitis patients according to ANCA specificity
| Outcome | Microscopic with polyangiitis |
Granulomatosis with polyangiitis |
||||
|---|---|---|---|---|---|---|
| PR3-ANCA (n = 116) | MPO-ANCA (n = 173) | P valuea | MPO-ANCA (n = 43) | PR3-ANCA (n = 108) | P valuea | |
| Follow-up, months, median (IQR) | 35 (10–84) | 37 (13–75) | 0.8575 | 38 (14–102) | 61 (24–131) | 0.0298 |
| Relapse, no. (%) | 51 (44) | 63 (36) | 0.2203 | 20 (47) | 72 (67) | 0.0270 |
| Number of relapses | ||||||
| 0 | 61 (53) | 107 (62) | 0.1366 | 21 (49) | 31 (29) | 0.0721 |
| 1 | 29 (25) | 42 (24) | 8 (19) | 30 (28) | ||
| >1 | 26 (22) | 24 (14) | 14 (33) | 47 (44) | ||
| End-stage kidney disease, no. (%) | 33 (28) | 47(27) | 0.8934 | 17 (40) | 20 (19) | 0.0111 |
| Dead, no. (%) | 35 (30) | 63 (36) | 0.3111 | 13 (30) | 17 (16) | 0.0687 |
ANCA, antineutrophil cytoplasmic autoantibody; IQR, interquartile range; MPO, myeloperoxidase; PR3, proteinase 3.
P-values were calculated using Fisher exact test for categorical variables and Wilcoxon two sample test for continuous variables.
Figure 2.
(a) Probability of survival without death, (b) end-stage kidney disease, and (c) relapse among MPA and GPA patients according to ANCA specificity. Log-rank test for comparison between MPO and PR3 positive patients within each major clinicopathologic phenotype is shown. The numbers represent the number of patients being followed in each subgroup during 120 months. P-values were adjusted for multiple comparisons with the Šidák correction. ∗Significant statistical comparisons between MPA versus GPA include: (a) Death, PR3-ANCA MPA vs. PR3-ANCA GPA, P = 0.0121; MPO-ANCA MPA vs. PR3-ANCA GPA, P = 0.001 and (b) Relapses: MPO-ANCA MPA vs. PR3-ANCA GPA, P = 0.0139. ANCA, antineutrophil cytoplasmic autoantibody; Dx, diagnosis; GPA, granulomatosis with polyangiitis; MPA, microscopic polyangiitis; MPO, myeloperoxidase; ns, nonsignificant; PR3, proteinase 3.
Table 3.
Proportional hazards models for relapse, ESKD development, and mortality among MPA and GPA patients according to ANCA specificity
|
Microscopic polyangiitis (PR3-ANCA MPA vs. MPO-ANCA MPA) | ||
|---|---|---|
| Variables | ||
| HRs of relapse | HR (95% CI) | P value |
| ANCA specificity (PR3 vs. MPO) | 1.32 (0.88–1.97) | 0.1748 |
| Age | 0.99 (0.98–1.00) | 0.234 |
| Sex (Female vs. Male) | 1.42 (0.96–2.09) | 0.0819 |
| Lung involvement (Yes vs. No) | 1.09 (0.74–1.61) | 0.6695 |
| Upper respiratory tract involvement (Yes vs. No) | 1.08 (0.71–1.62) | 0.7269 |
| Maximum creatinine at diagnosis | 0.97 (0.90–1.04) | 0.4022 |
| Cyclophosphamide (Yes vs. No) | 0.71 (0.42–1.22) | 0.2197 |
| HRs of ESKD | ||
| ANCA specificity (PR3 vs. MPO) | 1.01 (0.63–1.62) | 0.9637 |
| Age | 1.00 (0.99–1.02) | 0.7902 |
| Sex (Female vs. Male) | 1.11 (0.71–1.75) | 0.6392 |
| Lung involvement (Yes vs. No) | 2.21 (1.35–3.59) | 0.0015 |
| Upper respiratory tract involvement (Yes vs. No) | 1.00 (0.60–1.67) | 0.9937 |
| Maximum creatinine at diagnosis | 1.23 (1.18–1.29) | <0.0001 |
| Cyclophosphamide (Yes vs. No) | 0.45 (0.25–0.82) | 0.0095 |
| HRs of death | ||
| ANCA specificity (PR3 vs. MPO) | 1.13 (0.74–1.74) | 0.5714 |
| Age | 1.03 (1.01–1.05) | 0.0001 |
| Sex (Female vs. Male) | 0.93 (0.62–1.40) | 0.729 |
| Lung involvement (Yes vs. No) | 1.96 (1.26–3.06) | 0.0028 |
| Upper respiratory tract involvement (Yes vs. No) | 1.29 (0.82–2.03) | 0.2712 |
| Maximum creatinine at diagnosis | 1.07 (1.02–1.13) | 0.0102 |
| Cyclophosphamide (Yes vs. No) | 0.49 (0.30–0.80) | 0.0039 |
|
Granulomatosis with polyangiitis (MPO-ANCA GPA vs. PR3-ANCA GPA) | ||
|---|---|---|
| HRs of Relapse | HR (95% CI) | P value |
| ANCA specificity (PR3 vs. MPO) | 0.65 (0.24–1.75) | 0.3887 |
| Age | 1.01 (0.99–1.04) | 0.3326 |
| Sex (Female vs. Male) | 0.54 (0.17–1.65) | 0.2775 |
| Lung involvement (Yes vs. No) | 2.41 (0.60–9.71) | 0.2174 |
| Upper respiratory tract involvement (Yes vs. No) | 0.53 (0.20–1.41) | 0.2018 |
| Maximum creatinine at diagnosis | 1.31 (1.12–1.52) | 0.0005 |
| Cyclophosphamide (Yes vs. No) | 0.38 (0.07–2.15) | 0.2768 |
| HRs of ESKD | ||
| ANCA specificity (PR3 vs. MPO) | 0.50 (0.24–1.03) | 0.0593 |
| Age | 1.02 (1.00–1.04) | 0.0675 |
| Sex (Female vs. Male) | 0.51 (0.23–1.12) | 0.0911 |
| Lung involvement (Yes vs. No) | 2.74 (0.83–9.03) | 0.0976 |
| Upper respiratory tract involvement (Yes vs. No) | 0.43 (0.19–0.97) | 0.0417 |
| Maximum creatinine at diagnosis | 1.44 (1.30–1.59) | <0.0001 |
| Cyclophosphamide (Yes vs. No) | 0.22 (0.05–0.96) | 0.0443 |
| HRs ofdeath | ||
| ANCA specificity (PR3 vs. MPO) | 0.83 (0.37–1.85) | 0.6454 |
| Age | 1.07 (1.04–1.10) | <0.0001 |
| Sex (Female vs. Male) | 1.23 (0.55–2.74) | 0.6181 |
| Lung involvement (Yes vs. No) | 0.79 (0.24–2.63) | 0.6993 |
| Upper respiratory tract involvement (Yes vs. No) | 1.45 (0.62–3.35) | 0.3906 |
| Maximum creatinine at diagnosis | 1.12 (1.00–1.26) | 0.0573 |
| Cyclophosphamide (Yes vs. No) | 1.12 (0.20–6.43) | 0.8995 |
ANCA, antineutrophil cytoplasmic autoantibody; CI, confidence interval; ESKD, end-stage kidney disease; GPA, granulomatosis with polyangiitis; HR, hazard ratio; MPA, microscopic polyangiitis; MPO, myeloperoxidase; PR3, proteinase 3.HRs and 95% CIs are shown.
Although a comparison between PR3-ANCA MPA and PR3-ANCA GPA was not the main objective of this study (for detailed characteristics see Supplementary Tables S1 and S2), PR3-ANCA MPA patients were older and had a significantly lower prevalence of pulmonary and ENT involvement, but higher frequency and severity of kidney disease than PR3-ANCA GPA patients. In addition, PR3-ANCA MPA was associated with lower prevalence of relapses and higher mortality rate than PR3-ANCA GPA. Moreover, as previously reported,34,35 MPA patients showed higher degrees of kidney involvement than those with GPA, with a stepwise increase in rates of frequency in our GDCN cohort with classic PR3-ANCA GPA having the lowest frequency (83%), followed by MPO-ANCA GPA (93%), PR3-ANCA MPA (96%), and MPO-ANCA MPA, which exhibited the highest prevalence (99%).
GPA
A total of 151 GPA patients, 41% female, with a median age at diagnosis of 48 (interquartile range 34.5−63) years were analyzed. MPO-ANCA and PR3-ANCA tested positive in 29% and 71% of the cases, respectively.
Baseline Organ Involvement and Response to Treatment According to ANCA Specificity
Among patients with GPA, classification on the basis of major ANCA antigen specificity showed that compared with the more common PR3-ANCA-GPA subset (n = 108), the less common MPO-ANCA patients (n = 43) were significantly older at the time of diagnosis and were more likely to be female (Table 4). Clinical manifestations that characterized MPO-ANCA GPA included a lower prevalence of ENT and nervous system symptoms (Table 4). Regarding kidney disease, we found a similar prevalence in both ANCA serotypes; however, score for glomerular sclerosis was significantly higher in biopsy specimens of MPO-ANCA GPA individuals (Table 4). Globally, the number of affected organs at the time of diagnosis was lower in MPO-ANCA patients (median 3 vs. 4 in PR3-ANCA GPA, P = 0.01). As for induction therapy, cyclophosphamide was used more frequently in the PR3-ANCA subgroup, probably as a consequence of the more widespread extrarenal involvement usually associated with this ANCA serotype. More patients in the MPO-ANCA GPA group were considered resistant to initial treatment (Table 4).
Table 4.
Detailed baseline clinical manifestations among granulomatosis with polyangiitis patients according to ANCA specificity (n = 151)
| Baseline clinical manifestation | MPO-ANCA GPA (n = 43) | PR3-ANCA GPA (n = 108) | P valuea |
|---|---|---|---|
| Age at diagnosis, median (IQR), yr | 61 (44–72) | 46 (30–61) | 0.0007 |
| Female, no. (%) | 24 (56) | 38 (35) | 0.0275 |
| Granulomatosis, no. (%) | 13 (30) | 44 (41) | 0.2672 |
| Initial manifestations | |||
| Lung involvement, no. (%) | 34 (79) | 86 (80) | 1.0000 |
| Pulmonary infiltrates, no. (%) | 20 (47) | 40 (37) | 0.3571 |
| Pulmonary nodules, no. (%) | 22 (51) | 46 (43) | 0.3688 |
| Pulmonary cavitations, no. (%) | 6 (14) | 28 (26) | 0.1338 |
| ENT involvement, no. (%) | 25 (58) | 83 (77) | 0.0281 |
| Clinical sinusitis, no. (%) | 14 (33) | 55 (51) | 0.0475 |
| Radiology sinusitis, no. (%) | 9 (21) | 34 (31) | 0.2336 |
| Nose/oral ulcers, no. (%) | 8 (19) | 23 (21) | 0.8251 |
| Otitis media, no. (%) | 6 (14) | 28 (26) | 0.1338 |
| Bone erosion/destruction, no. (%) | 4 (9) | 11(10) | 1.0000 |
| Subglottic stenosis, no. (%) | 3 (7) | 9 (8) | 1.0000 |
| Articular involvement, no. (%) | 18 (42) | 61 (56) | 0.1483 |
| Gastrointestinal involvement, no. (%) | 7 (16) | 8 (7) | 0.1309 |
| Nervous system involvement, no. (%) | 2 (5) | 27 (25) | 0.0029 |
| Skin involvement, no. (%) | 8 (19) | 36 (33) | 0.0780 |
| Eye involvement, no. (%) | 5 (12) | 17 (16) | 0.6160 |
| Kidney involvement, no. (%) | 40 (93) | 90 (83) | 0.1909 |
| Peak entry creatinine, median (IQR), mg/dl | 3 (1–6) | 2 (1–4) | 0.0839 |
| Acute dialysis at disease onset, no. (%) | 5 (12) | 13 (12) | 1.0000 |
| Histological parameters on kidney biopsy | |||
| bCrescents percentage, median (IQR) | 25 (20–58) | 30 (8–55) | 0.1788 |
| cGlomerular necrosis, median (IQR) | 2 (1–3) | 2 (1–2) | 0.267 |
| dGlomerular crescents, median (IQR) | 2 (1–4) | 1 (1–2) | 0.0569 |
| eGlomerular sclerosis, median (IQR) | 2 (1–2) | 1 (0–2) | 0.0126 |
| fInterstitial sclerosis, median (IQR) | 2 (1–2) | 1 (1–2) | 0.0846 |
| gCrescent sclerosis, median (IQR) | 1 (0–2) | 0 (0–2) | 0.0595 |
| Number of affected organs, median (IRQ) | 3 (2–4) | 4 (3–5) | 0.0109 |
| Induction therapy | |||
| Methylprednisolone bolus, no. (%) | 33 (77) | 69 (64) | 0.1772 |
| Prednisone, no. (%) | 41 (95) | 105 (97) | 0.6234 |
| Cyclophosphamide, no. (%) | 34 (79) | 98 (91) | 0.0001 |
| Prednisone and cyclophosphamide, no. (%) | 42 (98) | 108 (100) | 0.2848 |
| Plasma exchange, no. (%) | 11 (26) | 20 (19) | 0.3742 |
| Rituximab, no. (%) | 3 (7) | 17 (16) | 0.1899 |
| Response to initial induction therapy | |||
| Complete remission off therapy, no. (%) | 16 (38) | 38 (37) | 0.0160 |
| Remission sustained with therapy, no. (%) | 16 (38) | 57 (56) | |
| Therapy resistant, no. (%) | 9 (21) | 7 (7) |
ANCA, antineutrophil cytoplasmic autoantibody; ENT, ear, nose and throat; IQR, interquartile range; MPO, myeloperoxidase; PR3, proteinase 3.
P-values were calculated using Fisher exact test for categorical variables and Wilcoxon two sample test for continuous variables. Number of available kidney biopsy reports for MPO-GPA and PR3-GPA:
32 and 56,
19 and 45,
17 and 46,
20 and 48,
18 and 46,
20 and 48, respectively.
Clinical Outcomes of Interest
The anti-MPO ANCA subgroup progressed more frequently to ESKD and showed an increased mortality than those with PR3-ANCA-positive antibodies (Table 2 and Figure 2). Proportional hazards models (Table 3) showed that maximum creatinine level at diagnosis (HR 1.31, 95% CI 1.12−1.52) predicted relapses; whereas ENT involvement (HR 0.43, 95% CI 0.19−0.97), peak creatinine at diagnosis (HR 1.44, 95% CI 1.30−1.59), and cyclophosphamide administration (HR 0.22, 95%CI 0.05-0.96) significantly influenced ESKD development. Age at diagnosis (HR 1.07, 95% CI 1.04−1.10) was the only predictor variable of patient survival.
Supplementary Tables S2 and S3 show the comparison between MPO-ANCA GPA and the MPO-ANCA MPA. MPO-ANCA GPA patients exhibited a higher prevalence of ENT and pulmonary involvement, had a lower frequency of kidney involvement and relapsed multiple times more frequently than MPO-ANCA MPA subjects. Overall, patients with GPA had higher degrees of lung involvement compared to MPA34,35 with frequencies of approximately 80% versus 60%, respectively. Similarly, ENT disease was predominantly found in GPA irrespective of the ANCA serotype, that is, 58% for MPO-ANCA and 77% for PR3-ANCA versus 26% and 44% for MPO-ANCA and PR3-ANCA patients with MPA, respectively.
Discussion
The present study is a detailed analysis of one of the largest cohorts of PR3-ANCA MPA ever described and one of the very few that investigated not only the spectrum of organ involvement but also the prognosis of the 2 less common subsets of ANCA vasculitis PR3-ANCA MPA and MPO-ANCA GPA. Our results demonstrated that in comparison with MPO-ANCA MPA cases, PR3-ANCA individuals were significantly younger, had a higher frequency of articular involvement and ENT symptoms and exhibited a lower prevalence of chronic histopathologic kidney lesions. Second, we contribute to the characterization of MPO-ANCA GPA (Table 5) by showing that, in comparison with PR3-ANCA GPA vasculitis, these patients were usually older and female, with lower frequencies of ENT and nervous system involvement but higher prevalence of glomerular sclerosis on kidney biopsies. More importantly, MPO-ANCA GPA progressed more frequently to ESKD and had a higher mortality than those with PR3-ANCA GPA. These data contribute to the limited information regarding the most uncommon subsets of ANCA disease and suggest that categorization of ANCA vasculitis by combining both phenotype and ANCA serotype, rather than the classical dichotomy of GPA/MPA or PR3-ANCA/MPO-ANCA, might better identify distinct subgroups with clinical and prognostic relevance.
Table 5.
Characteristics of MPO-ANCA GPA patients in diverse international patient series
| Feature | MPO-ANCA GPA vs. PR3-ANCA GPA | MPO-ANCA GPA vs. MPO-ANCA MPA | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| USA36 | Germany37 | Germany7 | China38 | France39 | Japan28 | Japan29 | Japan30 | Italy40 | France41a | USA36 | Germany37 | Germany7 | China38 | France39 | Italy40 | |
| MPO-ANCA GPA (n) | 33 | 59 | 7 | 124 | 20 | 17 | 6 | 8 | 23 | 119 | ||||||
| Center | Clinical trials | Rheumatology | Rheumatology | Nephrology | Internal medicine/Nephrology | Rheumatology | Respiratory | Rheumatology | Rheumatology | Multicentre | ||||||
| Classification criteria | ACR | EMA algorithm | ACR CHCC |
CHCC EMA algorithm |
CHCC EMA algorithm |
EMA algorithm | EMA algorithm | EMA algorithm | EMA algorithm | ACR | ||||||
| Age | = | Matched | NS | = | = | ↑ | = | ↑ | = | ↑ | ↓ | ↓ | NS | = | = | ↓ |
| Female | ↑ | Matched | ↑ | ↑ | ↑ | ↑ | ↑ | = | = | ↑ | = | ↑ | NS | = | ↑ | = |
| Manifestations | ||||||||||||||||
| Lung | = | ↓ | = | = | = | NS | = | = | = | = | = | = | NS | = | = | = |
| Kidney | = | ↓ | ↓ | = | = | = | = | = | = | ↑ | ↓ | ↓ | ↓ | = | = | ↓ |
| ENT | = | = | = | = | = | ↑ | = | ↓ | = | = | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| Skin | = | NS | NS | ↓ | = | NS | = | = | = | ↓ | = | NS | NS | = | = | = |
| Joints | NS | NS | NS | ↓ | = | NS | = | NS | = | ↓ | NS | NS | NS | = | = | = |
| Eye | = | = | ↓ | ↓ | NS | = | = | ↑ | = | ↓ | = | = | NS | ↑ | = | = |
| Gastrointestinal | = | = | NS | = | NS | NS | NS | NS | = | = | = | = | NS | = | NS | = |
| Heart | = | = | NS | NS | NS | = | NS | NS | NS | = | = | = | NS | NS | NS | NS |
| Nervous system | = | = | ↓ | = | = | ↑ | = | = | = | = | = | ↓ | NS | = | = | = |
| Outcome | ||||||||||||||||
| Relapses | = | = | NS | = | = | = | ↓ | NS | ↑ | = | ↑ | NS | NS | = | ↑ | NS |
| ESKD | NS | ↓ | NS | ↓ | = | ↓ | NS | NS | = | NS | NS | ↓ | NS | ↑ | = | = |
| Mortality | NS | NS | NS | NS | ↓ | = | NS | NS | ↑ | ↓ | NS | NS | NS | NS | ↓ | NS |
ACR, 1990 American College of Rheumatology classification criteria; ANCA, antineutrophil cytoplasmic autoantibody; CHCC, Chapel Hill Consensus Conference; Downward arrows, decreased frequency; ENT, ear, nose and throat; EMA, European Medicines Agency; ESKD, end-stage kidney disease; MPA, microscopic polyangiitis; MPO, myeloperoxidase; NS, not specified; PR3, proteinase 3; Upward arrows, increased frequency.
Differences based on significant statistical differences reported in each study.
In this particular study, 41 comparison included MPO-ANCA GPA vs. PR3-ANCA + ANCA-negative GPA patients.
How to best subclassify patients with ANCA vasculitis has been a long-standing debate. Traditional classification of clinicopathologic diagnoses of GPA, MPA, or eosinophilic granulomatosis with polyangiitis or more recently according to ANCA specificity have demonstrated that ANCA subclasses may be associated with different geographic distribution, unique genetic background, a particular cytokine profile, and distinct organ predilection and outcome.15,22,42,43 A novel alternative approach that has been proposed for improving classification and diagnosis of ANCA vasculitis is the use of cluster analysis, a powerful exploratory technique, or using an artificial neural network,44, 45, 46 although confirmation of biological and clinical relevance of the resulted subclasses of ANCA vasculitis are still needed.
MPO-ANCA antibodies have been associated with female predominance, older age at diagnosis, pulmonary fibrosis, more frequent and more severe kidney disease, and worse outcome, (i.e., common progression to ESKD), frequent failure to obtain complete remission, and in some studies, higher mortality.3,11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 In agreement, we found than MPO-ANCA patients were predominantly female and older (61 years for MPO-ANCA GPA and 62 years for MPO-ANCA MPA) than PR3-ANCA cases (46 years and 53 years for GPA and MPA, respectively). Similarly, our results are in line with previous studies showing that patients with PR3-ANCA vasculitis usually have a higher prevalence of lung cavities, destructive ENT lesions, ocular disease, and nervous system involvement (17% and 25% in PR3-ANCA GPA and MPA, respectively vs. 5% and 13% in MPO-ANCA GPA and MPA, in our series). PR3-ANCA patients also show more widespread extrarenal organ disease, more frequent relapses, and better response to induction with rituximab.3,11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23
Of relevance, these differences have led some authors to suggest that classification of patients exclusively according to ANCA specificity could be sufficient for the diagnosis and treatment of ANCA vasculitides.47 Nevertheless, evidence from the present study and other recent publications suggest that stratification according to clinicopathologic phenotype in combination with ANCA serotype may provide additional and complementary information useful in the management of these systemic autoimmune diseases.37,38
MPA has been traditionally associated with MPO-ANCA, because only 0% to 28% of patients tested positive for PR3-ANCA in former series.7, 8, 9, 10,26 Among MPA patients, PR3-ANCA patients are rare in clinical practice; thus, only 1 previous study has described the characteristics of this ANCA subset.39 In this publication,39 the only difference in clinical presentation between MPA patients with PR3-ANCA (n = 13) and MPO-ANCA (n = 43) antibodies was a lower prevalence of lung involvement in the PR3-ANCA subset. In contrast, our analysis of 116 patients shows that in comparison with the traditional MPO-ANCA MPA, PR3-ANCA subjects were younger, suffered from ENT symptoms and joint involvement more frequently, and had a higher prevalence of glomerular necrosis (but lower glomerular sclerosis). Though these patients behave similarly to the prototypical MPA cases described in other cohorts where major clinical features include kidney (79%−98% of cases) and pulmonary disease (25%−53%)7, 8, 9, 10,26; the younger age at diagnosis and the increased prevalence of ENT symptoms more closely resembled that reported in GPA and PR3-ANCA vasculitis.14, 15, 16,18 In previous studies, upper respiratory tract involvement was described only in 0% to 20% of MPA patients,7, 8, 9, 10,26,39,48 which markedly contrast with the 44% observed in our population. Although ENT manifestations such as sinusitis, otitis media, epistaxis, or rhinitis have never been emphasized as part of MPA phenotype, available information shows that these symptoms may be observed both at initial presentation and during relapses48; and in contrast to GPA, lesions tend to be mild and not destructive in nature.9,48 Our findings are consistent with previous publications showing that PR3-ANCA usually has more active histopathologic kidney lesions such as glomerular necrosis, whereas MPO-ANCA has more chronic glomerular injury.16,49,50
Despite differences in initial presentation, our group and others39 were unable to show significant differences in the outcome of MPA patients based on their ANCA reactivity. This could be probably related to the fact that 2 of our clinical outcomes of interest, ESKD and mortality, are heavily influenced by kidney involvement,32 which was highly prevalent in our population. Interestingly, as in other series,20,32 our multivariable analysis showed that use of cyclophosphamide was associated with a better renal survival and patient survival in MPA, although this finding should be taken cautiously, because our study did not specifically compare the effect of cyclophosphamide with other treatment modalities (e.g., rituximab) and the total number of patients receiving other adjunctive therapies was small. Still, PR3-ANCA-positive MPA seems to represent a particular ANCA subset as suggested not only by its differences with MPO-ANCA MPA cases but also by its differentiation with PR3-ANCA GPA patients (i.e., older age at diagnosis, lower prevalence of lung and ENT involvement, higher proportion of patients with kidney disease, and lower relapse rate), which is in line with differences reported in previous publications.39
In addition to the PR3-ANCA-postive MPA subset, we also analyzed the characteristics of patients diagnosed as GPA who were positive for MPO-ANCA, a particular group that has been recently systematically studied in several cohorts36, 37, 38, 39, 40, 41 and formerly in small case-series7,28, 29, 30 (Table 5). Our results show that MPO-ANCA GPA patients were older than PR3-ANCA GPA individuals and that women predominate in this group. In agreement with these findings, MPO-ANCA cases have been reported to be older in earlier series of GPA patients28,30,41 and in studies comparing ANCA specificity (irrespective of the clinical phenotype).14,15 Also consistent with our observations, sex differences with a clear predominance of female patients have been reported consistently in studies detailing the characteristics of MPO-ANCA GPA vasculitis.7,28, 29, 30,36,38,39,41
Regarding clinical manifestations, we found that ENT and nervous system involvement were less common in MPO-ANCA GPA than in PR3-ANCA GPA. In agreement, ENT manifestations as well as peripheral neuropathy were reported less frequently in MPO-ANCA GPA cases in some studies7,30 but not in others,36, 37, 38,40,41 some of which have reported an interesting association of anti-MPO GPA patients and increased prevalence of subglottic stenosis.37,40 In addition to different organ involvement, other distinctive characteristics of MPO-ANCA GPA include more sclerotic lesions in initial kidney biopsies, higher prevalence of ESKD, and increased mortality. Although limited information is available on the prognosis of patients with GPA and MPO-ANCA, previous publications have found a similar or even lower prevalence of ESKD in the this subset,28,37,38 which is surprising considering that kidney involvement is usually more frequent and more severe in vasculitis patients with positive MPO-ANCA antibodies.11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 In fact, MPO-ANCA serotype has been consistently associated with more chronic renal damage and higher progression to ESKD.16,49,50 With regard of the survival of this particular subgroup, a previous study reported a higher mortality rate when compared with PR3-ANCA GPA, which is in line with our observation.40 Lastly, although PR3-ANCA have been associated with increased relapse rate, we and other authors36, 37, 38, 39,41 observed a comparable frequency in MPO-ANCA and PR3-ANCA GPA groups.
It is important to note that although ANCA serotype seems to influence GPA phenotype in most reports (Table 5),7,28, 29, 30,36, 37, 38, 39,41 for example with differences in demographics and organ involvement, results from these former studies remain inconsistent.47,51 Though clinical manifestations of MPO-ANCA and PR3-ANCA GPA patients remained similar in a monocentric retrospective study (n = 20)39 and in a pooled analysis of 33 patients enrolled in 2 large randomized clinical trials,36 a case-control study of 59 patients from a single center in Germany37 reported a lower prevalence of lung and kidney disease with higher frequency of limited vasculitis; whereas in a Chinese report that included 124 cases, MPO-ANCA GPA was associated with less articular, cutaneous, and ocular involvement as well as less severe kidney disease.38 In addition, a large multicenter study from France showed an increased frequency of kidney disease and lower prevalence of ocular disease, arthralgia, and palpable purpura in 119 GPA patients with positive MPO-ANCA.41 Importantly, in these previous studies the outcome of both serotypes within GPA patients were similar, which is in contrast to our observation that MPO-ANCA GPA progressed more frequently to ESKD and had a higher mortality than PR3-ANCA GPA.
Discrepancies in these findings are probably explained by differences47 in geographic and ethnic factors, because some studies reported on the characteristics of Caucasian and others reported on Asian populations; the effect of selection criteria, because some publications derived from randomized clinical trials whereas others included patients from observational cohorts; and some series derived from nephrology units in contrast to rheumatology or internal medicine departments; and/or differences in treatment protocols, because glucocorticoid and immunosuppressive therapies were not uniformly used. In addition, differences in length of follow-up, classification schemes, and disease definitions across the reported studies also contributed to these discrepancies. For example, clinicopathologic phenotype was either assigned on the basis of the 1990 American College of Rheumatology criteria, the Chapel Hill Consensus Conference nomenclature, or the European Medicines Agency algorithm; all of which have inherent limitations. The newly released 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for ANCA vasculitis should reduce uncertainties concerning the classification of patients in future studies.52,53
Our data and that reported from other cohorts illustrates how ANCA specificity may confer differences in GPA and MPA phenotype and outcome.36, 37, 38, 39,41 How ANCA serotype influence or dictate clinical phenotype is currently unknown, although there may be multiple interrelated pathogenic mechanisms. The nature and entrance portal of triggering antigens may vary across serotype and phenotype, for example by bacteria (PR3-ANCA), silica (MPO-ANCA), or drugs. Differences may also be driven by genetic abnormalities, overexpression of MPO and PR3 in specific tissues, and/or MPO-induced or PR3-induced inflammatory effect and damage on the endothelium. The ability of the antigenic stimuli to be distributed through the body may differ, as could alterations of natural inhibitors such as alpha-1 antitrypsin and ceruloplasmin. The presence or absence of concomitant synergistic inflammation such as upper respiratory tract infections could also be influential. Another consideration is that the characteristics of the pathogenic ANCA autoantibodies, such as differences in the cytokine pattern induced by each antibody type or differences in ANCA activating capacity effector response, may differ; with PR3-ANCA vasculitis eliciting a more extended immune dysregulation involving antigen-presenting cells and MPO-ANCA antibodies inducing a more profound proinflammatory response.11,25,51,54, 55, 56, 57, 58
Strengths of the present study include the evaluation of the largest cohort of PR3-ANCA MPA patients as well as one of the largest cohorts of MPO-ANCA GPA. In addition, this is one of the few analyses that have examined 3 different clinical outcomes relevant for daily clinical practice, namely relapses, ESKD, and death in 2 rare ANCA subsets. Finally, because patients included in the GDCN cohort are followed closely over time, phenotypes and outcomes were kept updated.
Our study also has certain limitations. The GDCN is primarily composed of nephrologists and the cohort was, for the most part, identified by renal biopsies; therefore, patients without renal involvement may be underrepresented. Although prevalence of renal involvement in ANCA has been reported in 75% to 90% of patients,59 and thus our population seems to represent the overall majority of patients with ANCA vasculitis, we cannot exclude that subtle differences of uncommon disease manifestations that might be overlooked. For example, MPO-ANCA-associated pulmonary fibrosis, a recently and well documented manifestation of ANCA vasculitis was rarely reported and not consistently evaluated. In addition, the high rate of kidney involvement, a well-known prognostic factor, could have influenced our capacity to identify very small differences in ESKD development, particularly among those with MPA, of which more than 95% of patients in each subgroup had renal manifestations. Other limitations inherent to community-based cohort studies conducted over a long period of time should be considered. Over the more than 3 decades in this study there were changes in treatment protocols and general variations in patient care patterns, and early tests for ANCA were not standardized across the entire cohort (although there is good concordance between earlier ANCA test methods and the currently available kits60); also, adoption of novel diagnostic imaging techniques (e.g., high resolution CT-scans), which could increase the prevalence of certain pulmonary manifestations. Further, comparison of our results with selected series28,37, 41 was hindering by the fact that major clinical phenotypes were established according to the Chapel Hill Consensus Conference nomenclature in the present study, whereas the 1990 American College of Rheumatology criteria or the European Medicines Agency system was used by other investigators. Finally, we did not systematically assess general symptoms such as fever or weight loss, manifestations commonly described at early phases of GPA and MPA. Although kidney biopsies were performed in almost all patients, histopathological score of selected lesions were performed only in a limited number of cases with available information.
In spite of these limitations, the importance of stratifying ANCA vasculitis patients on the basis of clinical diagnosis and ANCA type could conceivably lead to the individualization of induction and long-term treatment plans, thus potentially avoiding both cumulative organ damage due to recurrent flares and unnecessary chronic exposure to systemic corticosteroids and immunosuppressive drugs in patients whose predicted prognosis is better. However, the current evidence is limited, and specific recommendations cannot be done for the management of specific ANCA subsets. Further studies among larger populations are needed to confirm and refine clinical care.
Conclusion
In conclusion, our results suggest that PR3-ANCA MPA and MPO-ANCA GPA have distinct clinical manifestations and prognosis and support the Chapel Hill Consensus Conference nomenclature recommendation of including both the phenotypic diagnosis and ANCA serotype in the diagnosis of ANCA vasculitis patients. Further studies are needed to investigate the pathogenic mechanisms responsible for the effect of serotype on disease phenotype and response to therapy.
Disclosure
All the authors declared no competing interests.
Acknowledgments
MA Alba was supported by the Biomedicine International Training Research programme for Excellent clinician-Scientists (BITRECS), IDIBAPS, Barcelona, Spain.
This work was supported in part by federal grant P01 DK058335, ANCA Glomerulonephritis: from Molecules to Man (Principal Investigator: R.J. Falk) from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Table S1. Comparison of baseline clinical manifestations and long-term outcome between PR3-ANCA MPA and PR3-ANCA GPA patients.
Table S2. Proportional hazards models for relapse, ESKD development, and mortality in microscopic polyangiitis (MPA) and granulomatosis with polyangiitis (GPA) subtypes.
Table S3. Comparison of baseline clinical manifestations and long-term outcome between MPO-ANCA GPA and MPO-ANCA MPA patients.
STROBE Statement.
Supplementary Material
Table S1. Comparison of baseline clinical manifestations and long-term outcome between PR3-ANCA MPA and PR3-ANCA GPA patients
Table S2. Proportional hazards models for relapse, ESKD development, and mortality in microscopic polyangiitis (MPA) and granulomatosis with polyangiitis (GPA) subtypes
Table S3. Comparison of baseline clinical manifestations and long-term outcome between MPO-ANCA GPA and MPO-ANCA MPA patients
STROBE Statement.
References
- 1.Jennette J.C., Falk R.J., Bacon P.A., et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 2.JU Holle, Gross W.L., Latza U., et al. Improved outcome in 445 patients with Wegener’s granulomatosis in a German vasculitis center over four decades. Arthritis Rheum. 2011;63:257–266. doi: 10.1002/art.27763. [DOI] [PubMed] [Google Scholar]
- 3.Flossmann O., Berden A., de Groot K., et al. Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis. 2011;70:488–494. doi: 10.1136/ard.2010.137778. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman G.S., Kerr G.S., Leavitt R.Y., et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992;116:488–498. doi: 10.7326/0003-4819-116-6-488. [DOI] [PubMed] [Google Scholar]
- 5.Nachman P.H., Hogan S.L., Jennette J.C., Falk R.J. Treatment response and relapse in antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol. 1996;7:33–39. doi: 10.1681/ASN.V7133. [DOI] [PubMed] [Google Scholar]
- 6.Westman K.W., Bygren P.G., Olsson H., et al. Relapse rate, renal survival, and cancer morbidity in patients with Wegener’s granulomatosis or microscopic polyangiitis with renal involvement. J Am Soc Nephrol. 1998;9:842–852. doi: 10.1681/ASN.V95842. [DOI] [PubMed] [Google Scholar]
- 7.Schonermarck U., Lamprecht P., Csernok E., Gross W.L. Prevalence and spectrum of rheumatic diseases associated with proteinase 3-antineutrophil cytoplasmic antibodies (ANCA) and myeloperoxidase-ANCA. Rheumatology (Oxford) 2001;40:178–184. doi: 10.1093/rheumatology/40.2.178. [DOI] [PubMed] [Google Scholar]
- 8.Schirmer J.H., Wright M.N., Vonthein R., et al. Clinical presentation and long-term outcome of 144 patients with microscopic polyangiitis in a monocentric German cohort. Rheumatology (Oxford) 2016;55:71–79. doi: 10.1093/rheumatology/kev286. [DOI] [PubMed] [Google Scholar]
- 9.Guillevin L., Durand-Gasselin B., Cevallos R., et al. Microscopic polyangiitis: clinical and laboratory findings in eighty-five patients. Arthritis Rheum. 1999;42:421–430. doi: 10.1002/1529-0131(199904)42:3<421::AID-ANR5>3.0. CO;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Furuta S., Chaudhry A.N., Hamano Y., et al. Comparison of phenotype and outcome in microscopic polyangiitis between Europe and Japan. J Rheumatol. 2014;41:325–333. doi: 10.3899/jrheum.130602. [DOI] [PubMed] [Google Scholar]
- 11.Lionaki S., Blyth E.R., Hogan S.L., et al. Classification of antineutrophil cytoplasmic autoantibody vasculitides: the role of antineutrophil cytoplasmic autoantibody specificity for myeloperoxidase or proteinase 3 in disease recognition and prognosis. Arthritis Rheum. 2012;64:3452–3462. doi: 10.1002/art.34562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldschmeding R., Tervaert J.W., Gans R.O., et al. Different immunological specificities and disease associations of c-ANCA and p-ANCA. Neth J Med. 1990;36:114–116. [PubMed] [Google Scholar]
- 13.Tervaert J.W., Goldschmeding R., Elema J.D., et al. Autoantibodies against myeloid lysosomal enzymes in crescentic glomerulonephritis. Kidney Int. 1990;37:799–806. doi: 10.1038/ki.1990.48. [DOI] [PubMed] [Google Scholar]
- 14.Franssen C., Gans R., Kallenberg C., et al. Disease spectrum of patients with antineutrophil cytoplasmic autoantibodies of defined specificity: distinct differences between patients with anti-proteinase 3 and anti-myeloperoxidase autoantibodies. J Intern Med. 1998;244:209–216. doi: 10.1046/j.1365-2796.1998.00357.x. [DOI] [PubMed] [Google Scholar]
- 15.Unizony S., Villarreal M., Miloslavsky E.M., et al. Clinical outcomes of treatment of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis based on ANCA type. Ann Rheum Dis. 2016;75:1166–1169. doi: 10.1136/annrheumdis-2015-208073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kristensen T., Gregersen J.W., Krag S.R., Ivarsen P. The relation between histopathological classification and renal outcome, ANCA subtype and treatment regimens in ANCA-associated vasculitis. Clin Exp Rheumatol. 2016;34(Suppl 97):S105–S110. [PubMed] [Google Scholar]
- 17.Geffriaud-Ricouard C., Noel L.H., Chauveau D., et al. Clinical spectrum associated with ANCA of defined antigen specificities in 98 selected patients. Clin Nephrol. 1993;39:125–136. [PubMed] [Google Scholar]
- 18.Mohammad A.J., Segelmark M. A population-based study showing better renal prognosis for proteinase 3 antineutrophil cytoplasmic antibody (ANCA)-associated nephritis versus myeloperoxidase ANCA-associated nephritis. J Rheumatol. 2014;41:1366–1373. doi: 10.3899/jrheum.131038. [DOI] [PubMed] [Google Scholar]
- 19.Hauer H.A., Bajema I.M., van Houwelingen H.C., et al. Renal histology in ANCA-associated vasculitis: differences between diagnostic and serologic subgroups. Kidney Int. 2002;61:80–89. doi: 10.1046/j.1523-1755.2002.00089.x. [DOI] [PubMed] [Google Scholar]
- 20.Hogan S.L., Falk R.J., Chin H., et al. Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann Intern Med. 2005;143:621–631. doi: 10.7326/0003-4819-143-9-200511010-00005. [DOI] [PubMed] [Google Scholar]
- 21.Mukhtyar C., Flossmann O., Hellmich B., et al. Outcomes from studies of antineutrophil cytoplasm antibody associated vasculitis: a systematic review by the European League Against Rheumatism systemic vasculitis task force. Ann Rheum Dis. 2008;67:1004–1010. doi: 10.1136/ard.2007.071936. [DOI] [PubMed] [Google Scholar]
- 22.Walsh M., Flossmann O., Berden A., et al. Risk factors for relapse of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2012;64:542–548. doi: 10.1002/art.33361. [DOI] [PubMed] [Google Scholar]
- 23.Alba M.A., Flores-Suarez L.F., Henderson A.G., et al. Interstital lung disease in ANCA vasculitis. Autoimmun Rev. 2017;16:722–729. doi: 10.1016/j.autrev.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyons P.A., Rayner T.F., Trivedi S., et al. Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med. 2012;367:214–223. doi: 10.1056/NEJMoa1108735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merkel P.A., Xie G., Monach P.A., et al. Identification of functional and expression polymorphisms associated with risk for antineutrophil cytoplasmic autoantibody-associated vasculitis. Arthritis Rheumatol. 2017;69:1054–1066. doi: 10.1002/art.40034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savage C.O., Winearls C.G., Evans D.J., et al. Microscopic polyarteritis: presentation, pathology and prognosis. Q J Med. 1985;56:467–483. [PubMed] [Google Scholar]
- 27.Finkielman J.D., Lee A.S., Hummel A.M., et al. ANCA are detectable in nearly all patients with active severe Wegener’s granulomatosis. Am J Med. 2007;120:643.e649. doi: 10.1016/j.amjmed.2006.08.016. 614. [DOI] [PubMed] [Google Scholar]
- 28.Ono N., Niiro H., Ueda A., et al. Characteristics of MPO-ANCA-positive granulomatosis with polyangiitis: a retrospective multi-center study in Japan. Rheumatol Int. 2015;35:555–559. doi: 10.1007/s00296-014-3106-z. [DOI] [PubMed] [Google Scholar]
- 29.Ikeda S., Arita M., Misaki K., et al. Comparative investigation of respiratory tract involvement in granulomatosis with polyangiitis between PR3-ANCA positive and MPO-ANCA positive cases: a retrospective cohort study. BMC Pulm Med. 2015;15:78. doi: 10.1186/s12890-015-0068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsuchida Y., Shibuya M., Shoda H., et al. Characteristics of granulomatosis with polyangiitis patients in Japan. Mod Rheumatol. 2015;25:219–223. doi: 10.3109/14397595.2014.937475. [DOI] [PubMed] [Google Scholar]
- 31.Jennette J.C., Falk R.J., Andrassy K., et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–192. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 32.Hogan S.L., Nachman P.H., Wilkman A.S., et al. Prognostic markers in patients with antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol. 1996;7:23–32. doi: 10.1681/ASN.V7123. [DOI] [PubMed] [Google Scholar]
- 33.Falk R.J., Hogan S., Carey T.S., Jennette J.C. Clinical course of anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and systemic vasculitis. The Glomerular Disease Collaborative Network. Ann Intern Med. 1990;113:656–663. doi: 10.7326/0003-4819-113-9-656. [DOI] [PubMed] [Google Scholar]
- 34.Jennette J.C., Falk R.J. Small-vessel vasculitis. N Engl J Med. 1997;337:1512–1523. doi: 10.1056/NEJM199711203372106. [DOI] [PubMed] [Google Scholar]
- 35.Seo P., Stone J.H. The antineutrophil cytoplasmic antibody-associated vasculitides. Am J Med. 2004;117:39–50. doi: 10.1016/j.amjmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 36.Miloslavsky E.M., Lu N., Unizony S., et al. Myeloperoxidase-antineutrophil cytoplasmic antibody (ANCA)-positive and ANCA-negative patients with granulomatosis with polyangiitis (Wegener’s): distinct patient subsets. Arthritis Rheumatol. 2016;68:2945–2952. doi: 10.1002/art.39812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schirmer J.H., Wright M.N., Herrmann K., et al. Myeloperoxidase-antineutrophil cytoplasmic antibody (ANCA)-positive granulomatosis with polyangiitis (Wegener’s) is a clinically distinct subset of ANCA-associated vasculitis: a retrospective analysis of 315 patients from a German vasculitis referral center. Arthritis Rheumatol. 2016;68:2953–2963. doi: 10.1002/art.39786. [DOI] [PubMed] [Google Scholar]
- 38.Chang D.Y., Li Z.Y., Chen M., Zhao M.H. Myeloperoxidase-ANCA-positive granulomatosis with polyangiitis is a distinct subset of ANCA-associated vasculitis: A retrospective analysis of 455 patients from a single center in China. Semin Arthritis Rheum. 2018;48:701–706. doi: 10.1016/j.semarthrit.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Deshayes S., Martin Silva N., Khoy K., et al. Clinical impact of subgrouping ANCA-associated vasculitis according to antibody specificity beyond the clinicopathological classification. Rheumatology (Oxford) 2019;58:1731–1739. doi: 10.1093/rheumatology/kez016. [DOI] [PubMed] [Google Scholar]
- 40.Monti S., Felicetti M., Delvino P., et al. Anti-neutrophil cytoplasmic antibody specificity determines a different clinical subset in granulomatosis with polyangiitis. Clin Exp Rheumatol. 2021;39(Suppl 129):107–113. doi: 10.55563/clinexprheumatol/50919f. [DOI] [PubMed] [Google Scholar]
- 41.Puechal X., Iudici M., Pagnoux C., et al. Comparative study of granulomatosis with polyangiitis subsets according to ANCA status: data from the French Vasculitis Study Group Registry. RMD Open. 2022;8 doi: 10.1136/rmdopen-2021-002160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cornec D., Cornec-Le Gall E., Fervenza F.C., Specks U. ANCA-associated vasculitis - clinical utility of using ANCA specificity to classify patients. Nat Rev Rheumatol. 2016;12:570–579. doi: 10.1038/nrrheum.2016.123. [DOI] [PubMed] [Google Scholar]
- 43.Berti A., Warner R., Johnson K., et al. Brief report: circulating cytokine profiles and antineutrophil cytoplasmic antibody specificity in patients with antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 2018;70:1114–1121. doi: 10.1002/art.40471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahr A., Katsahian S., Varet H., et al. Revisiting the classification of clinical phenotypes of anti-neutrophil cytoplasmic antibody-associated vasculitis: a cluster analysis. Ann Rheum Dis. 2013;72:1003–1010. doi: 10.1136/annrheumdis-2012-201750. [DOI] [PubMed] [Google Scholar]
- 45.Wojcik K., Biedron G., Wawrzycka-Adamczyk K., et al. Subphenotypes of ANCA-associated vasculitis identified by latent class analysis. Clin Exp Rheumatol. 2021;39(Suppl 129):62–68. doi: 10.55563/clinexprheumatol/d01o72. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe H., Sada K.E., Harigai M., et al. Exploratory classification of clinical phenotypes in Japanese patients with antineutrophil cytoplasmic antibody-associated vasculitis using cluster analysis. Sci Rep. 2021;11:5223. doi: 10.1038/s41598-021-84627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pagnoux C., Springer J. Editorial: classifying antineutrophil cytoplasmic antibody (ANCA)-associated vasculitides according to ANCA type or phenotypic diagnosis: salt or pepper? Arthritis Rheumatol. 2016;68:2837–2840. doi: 10.1002/art.39860. [DOI] [PubMed] [Google Scholar]
- 48.Samson M., Puechal X., Devilliers H., et al. Long-term follow-up of a randomized trial on 118 patients with polyarteritis nodosa or microscopic polyangiitis without poor-prognosis factors. Autoimmun Rev. 2014;13:197–205. doi: 10.1016/j.autrev.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 49.Franssen C.F., Gans R.O., Arends B., et al. Differences between anti-myeloperoxidase- and anti-proteinase 3-associated renal disease. Kidney Int. 1995;47:193–199. doi: 10.1038/ki.1995.23. [DOI] [PubMed] [Google Scholar]
- 50.Quintana L.F., Perez N.S., De Sousa E., et al. ANCA serotype and histopathological classification for the prediction of renal outcome in ANCA-associated glomerulonephritis. Nephrol Dial Transplant. 2014;29:1764–1769. doi: 10.1093/ndt/gfu084. [DOI] [PubMed] [Google Scholar]
- 51.Fordham S., Mukhtyar C. Are PR3 positive and MPO positive GPA the same disease? Int J Rheum Dis. 2018;22(Suppl 1):86–89. doi: 10.1111/1756-185X.13278. [DOI] [PubMed] [Google Scholar]
- 52.Robson J.C., Grayson P.C., Ponte C., et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for granulomatosis with polyangiitis. Ann Rheum Dis. 2022;81:315–320. doi: 10.1136/annrheumdis-2021-221795. [DOI] [PubMed] [Google Scholar]
- 53.Suppiah R., Robson J.C., Grayson P.C., et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for microscopic polyangiitis. Ann Rheum Dis. 2022;81:321–326. doi: 10.1136/annrheumdis-2021-221796. [DOI] [PubMed] [Google Scholar]
- 54.Harper L., Radford D., Plant T., et al. IgG from myeloperoxidase-antineutrophil cytoplasmic antibody-positive patients stimulates greater activation of primed neutrophils than IgG from proteinase 3-antineutrophil cytosplasmic antibody-positive patients. Arthritis Rheum. 2001;44:921–930. doi: 10.1002/1529-0131(200104)44:4<921::AID-ANR149>3.0. CO;2-4. [DOI] [PubMed] [Google Scholar]
- 55.O’Brien E.C., White C.A., Wyse J., et al. Pro-inflammatory stimulation of monocytes by ANCA is linked to changes in cellular metabolism. Front Med (Lausanne) 2020;7:553. doi: 10.3389/fmed.2020.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Popa E.R., Franssen C.F., Limburg P.C., et al. In vitro cytokine production and proliferation of T cells from patients with anti-proteinase 3- and antimyeloperoxidase-associated vasculitis, in response to proteinase 3 and myeloperoxidase. Arthritis Rheum. 2002;46:1894–1904. doi: 10.1002/art.10384. [DOI] [PubMed] [Google Scholar]
- 57.Griffin S.V., Chapman P.T., Lianos E.A., Lockwood C.M. The inhibition of myeloperoxidase by ceruloplasmin can be reversed by anti-myeloperoxidase antibodies. Kidney Int. 1999;55:917–925. doi: 10.1046/j.1523-1755.1999.055003917.x. [DOI] [PubMed] [Google Scholar]
- 58.Rahmattulla C., Mooyaart A.L., van Hooven D., et al. Genetic variants in ANCA-associated vasculitis: a meta-analysis. Ann Rheum Dis. 2016;75:1687–1692. doi: 10.1136/annrheumdis-2015-207601. [DOI] [PubMed] [Google Scholar]
- 59.Franssen C.F., Stegeman C.A., Kallenberg C.G., et al. Antiproteinase 3- and antimyeloperoxidase-associated vasculitis. Kidney Int. 2000;57:2195–2206. doi: 10.1046/j.1523-1755.2000.00080.x. [DOI] [PubMed] [Google Scholar]
- 60.Lim L.C., Taylor J.G., 3rd, Schmitz J.L., et al. Diagnostic usefulness of antineutrophil cytoplasmic autoantibody serology. Comparative evaluation of commercial indirect fluorescent antibody kits and enzyme immunoassay kits. Am J Clin Pathol. 1999;111:363–369. doi: 10.1093/ajcp/111.3.363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.