Abstract
Diabetes is the most common cause of kidney failure worldwide. Patients with diabetes and chronic kidney disease (CKD) are also at markedly higher risk of cardiovascular disease, particularly heart failure (HF), and death. Through the processes of gluconeogenesis and glucose reabsorption, the kidney plays a central role in glucose homeostasis. Insulin resistance is an early alteration observed in CKD, worsened by the frequent presence of hypertension, obesity, and ongoing chronic inflammation, and oxidative stress. Management of diabetes in moderate to severe CKD warrants special consideration because of changes in glucose and insulin homeostasis and altered metabolism of glucose-lowering therapies. Kidney failure and initiation of kidney replacement therapy by dialysis adds to management complexity by further limiting therapeutic options, and predisposing individuals to hypoglycemia and hyperglycemia. Glycemic goals should be individualized, considering CKD severity, presence of macrovascular and microvascular complications, and life expectancy. A general hemoglobin A1c (HbA1c) goal of approximately 7% may be appropriate in earlier stages of CKD, with more relaxed targets often appropriate in later stages. Use of sodium glucose cotransporter2 (SGLT2) inhibitors and glucagon like peptide-1 receptor agonists (GLP-1RAs) meaningfully improves kidney and heart outcomes for patients with diabetes and CKD, irrespective of HbA1c targets, and are now part of guideline-directed medical therapy in this high-risk population. Delivery of optimal care for patients with diabetes and CKD will require collaboration across health care specialties and disciplines.
Keywords: GLP-1 receptor agonists, heart failure, insulin, kidney failure, metformin, SGLT-2 inhibitors
Approximately 40% of patients with type 2 diabetes (T2D) and 30% of those with type 1 diabetes develop CKD characterized by albuminuria, low glomerular filtration rate (GFR), or both.1 CKD in diabetes, also known as diabetic kidney disease, is the leading cause of kidney failure worldwide.2,3 In 2021 it was estimated that 537 million, or 1 in 10 adults worldwide, lived with diabetes, the majority living in low-income and middle-income countries.4 Projections from the International Diabetes Federation estimate that by 2045 the number of people with diabetes will rise to 783 million.4 From 1990 to 2019, all countries and regions have shown upward trends in CKD prevalence in diabetes, with the highest burden exhibited in Southeast Asia, China, the United States, and India.3 Consequently, the rates of kidney failure driven by diabetes are expected to double by the year 2030.2 The excess risk for all-cause and cardiovascular death in diabetes is largely attributable to the presence of CKD. For example, the 10-year mortality rate was reported at 11.5% among individuals with diabetes and no kidney disease, and 31% among diabetic individuals with CKD.5 Indeed, a diagnosis of CKD in diabetes greatly increases risk for cardiovascular events, hospitalization, and death.6, 7, 8 This risk is extraordinarily high in patients with diabetes treated for kidney failure by maintenance dialysis. In this population, the annual risk of death among 25-year-old to 34-year-old persons is increased 500-fold to 1000-fold and corresponds to that of the general population older than 80 years of age.9 Furthermore, access to kidney replacement therapy in middle-income and low-income countries is reported to be as low as 9% to 16%, resulting in more than 2.2 million deaths worldwide in 2010.10,11
Initially approved as glucose-lowering therapies, SGLT2 inhibitors and GLP-1RAs, offer new treatment modalities that are transforming the CKD therapeutic landscape. Both classes of medications improve modifiable risk factors for CKD, including hyperglycemia, blood pressure, and body weight. Furthermore, a growing body of evidence shows that agents from these medication classes reduce risks of CKD onset and progression in diabetes, hospital admissions for HF, atherosclerotic cardiovascular disease events, and death.12,13
In this review, we describe the role of the kidney in glucose and insulin homeostasis as a foundation for understanding optimal management of diabetes in CKD, with an emphasis on multidisciplinary models of care delivery. Herein, we outline the considerations for use of glucose-lowering agents in the setting of CKD, and present pathogenic mechanism and clinical evidence supporting use of SGLT2 inhibitors and GLP-1RAs for kidney and heart protection.
Kidney and Glucose Homeostasis
Normal Conditions
The kidney regulates blood glucose by gluconeogenesis and reabsorption of glucose from the glomerular filtrate.14 In the postabsorptive state (>12 hours of fasting), the kidneys provide about 20% to 25% of circulating glucose via gluconeogenesis.15, 16, 17 In contrast to the liver, gluconeogenesis in the kidney increases by approximately 2-fold and accounts for around 60% of endogenous glucose release in the postprandial state.18 In normal kidneys, the poorly perfused medulla is the site of considerable glycolysis, whereas the cortex is the preferred site for gluconeogenesis.19 The kidneys, primarily the medulla, consume approximately 10% of all glucose utilized by the body.18,20
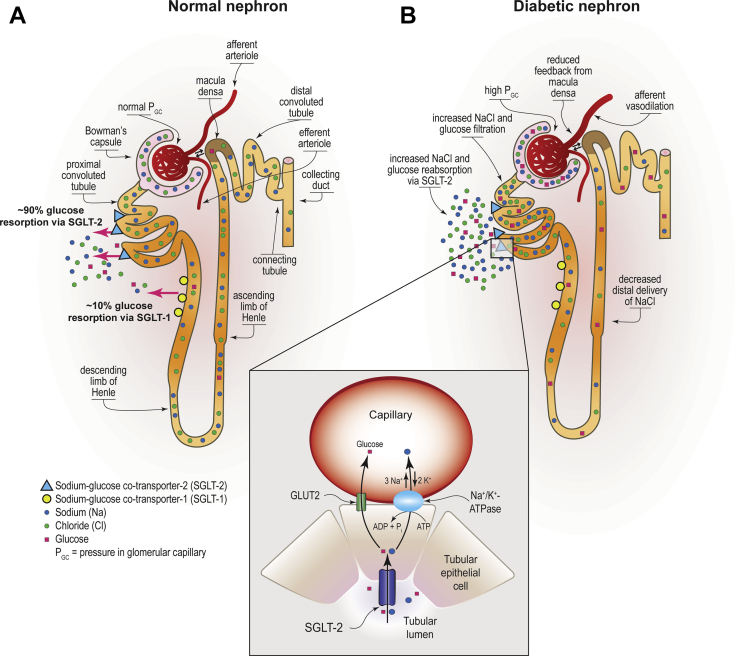
Under normoglycemic and moderately hyperglycemic conditions, nearly all filtered glucose undergoes reabsorption via cells of the proximal tubule. Glucose reabsorption is achieved via apically located SGLT2 and SGLT1 transporters, and through facilitative glucose transporters located on the basolateral side of the tubular cells.21 The mechanism of glucose reabsorption and the complementary role of SGLT1 and SGTL2 was fully understood only in the late 1980s and early 1990s when cotransporters were identified, cloned, and structurally characterized.22, 23, 24, 25 Reabsorption of >90% of filtered glucose is achieved via low-affinity, high-capacity SGLT2 expressed almost exclusively in the proximal convoluted tubule. Filtered glucose that escapes reabsorption by SGLT2 (∼10%) is subsequently resorbed by high-affinity, low-capacity SGLT1 located in epithelial cells of the straight descending proximal tubule.26, 27, 28, 29, 30 Glucose reabsorption is a principal contribution of the kidney to maintain glucose homeostasis and a major energy-requiring process.31 In order to provide the energy required for this process, glucose transport across the apical cell membrane is coupled to the electrochemical gradient generated by active sodium/potassium transport by adenosine triphosphatase. (Figure 1).32, 33, 34
Figure 1.
Glucose reabsorption by SGLT1 and SGLT2 in normal and diabetic kidney. (a) Glucose reabsorption by sodium/glucose cotransporter 2 (SGLT2) is a low-affinity, high-capacity glucose trans=porter, is expressed apically in epithelial cells of the proximal convoluted tubule and accomplishes reabsorption of approximately 90% of filtered glucose. Approximately 10% of glucose that escapes reabsorption by SGLT2 is subsequently resorbed by high-affinity low-capacity SGLT1, which is expressed apically in epithelial cells of the straight descending proximal tubule. To provide the energy required to drive glucose transport against its concentration gradient, both SGLT2 and SGLT1 couple glucose transport across the apical cell membrane to the electrochemical gradient generated by active sodium-potassium transport via the Na+/K+-ATPase pump located on the basolateral membrane of the proximal tubule. Na+/K+-ATPase pump drives Na+ exchange for K+ removing Na+ out of cell, generating adenosine in this process. Adenosine acts in paracrine manner to constrict afferent arteriola maintaining glomerular hemodynamics. As the intracellular concentration of Na+ decreases, this ion moves passively with glucose from the tubular lumen to the intracellular space via SGLT2 and SLGT1. Once in the cellular lumen, the passively concentration gradient translocates reabsorbed glucose into the interstitial space via glucose cotransporters. (b) In the diabetic nephron increased proximal tubular reabsorption of glucose and sodium chloride by SGLT1 and SGLT2 leads to decreased solute delivery to the macula densa in the distal convoluted tubule. As a result of reduced concentration of sodium chloride, adenosine triphosphatase activity at the macula densa is reduced with a consequent reduction in adenosine generation. Reduced production of adenosine leads to afferent arteriolar vasodilation that drives glomerular hyperperfusion and hyperfiltration. ADP, adenosine diphosphate; ATP, adenosine triphosphate; ATPase, adenosine triphosphatse; GC, glomerular capillary; K+, pottassium; Na+, sodium; NaCl, sodium chloride. Reprinted with permission from Alicic RZ, Johnson EJ, Tuttle KR. SGLT2 inhibition for the prevention and treatment of diabetic kidney disease: a review. Am J Kidney Dis. 2018;72:267–277.
Diabetes
In patients with T2D, fasting gluconeogenesis in the kidney is substantially greater than hepatic gluconeogenesis.35 Likewise, postprandial kidney glucose release is greater in people with T2D when compared to people with normal glucose tolerance, primarily because of impaired suppression of endogenous glucose production.36 Increased uptake of glucose in the proximal tubule has been observed in people with diabetes, reflecting enhanced reabsorptive transport capacity in diabetes.37 Indeed, the tubular threshold for glucosuria in diabetes is increased to a serum glucose level of approximately 200 to 240 mg/dl (11.1−13.3 mmol/l), in contrast to approximately 180 mg/dl (∼10 mmol/l) in persons without diabetes.38, 39, 40 The change in resorptive capacity in diabetes is related to increased activity and expression of SGLT and glucose transporters.39,41,42 As a result of increased glucose and sodium chloride uptake in the proximal tubule, solute delivery to the macula densa in the distal convoluted tubule is reduced. This change significantly contributes to a vasoactive imbalance between the afferent (dilated) and efferent (constricted) arterioles, resulting in glomerular hyperfiltration (Figure 1).38,43,44
CKD
Even in the absence of diabetes, CKD is associated with disordered glucoregulatory mechanisms and insulin metabolism.45, 46, 47 Insulin resistance is an important complication of CKD, closely correlated with severity of disease.47, 48, 49, 50 Insulin resistance is further exacerbated by comorbidities common in CKD (e.g., hypertension and obesity), accumulation of uremic toxins (e.g., pseudouridine), increased levels of proinflammatory cytokines (e.g., interleukin-6 and tumor necrosis factor-α), adipocytokines (e.g., leptin), poor physical fitness, loss of muscle mass, and vitamin D deficiency.51, 52, 53, 54, 55, 56 Compared to healthy individuals, patients with moderate to severe CKD demonstrate lower insulin sensitivity and insulin clearance.48, 49, 50,57 In a study of patients with a mean estimated GFR of approximately 38 ml/min per 1.73 m2, the relationship between insulin sensitivity and estimated GFR was attenuated after adjustment for physical activity, dietary features, fat, and fat-free mass.50 Incretin-mediated gastrointestinal excretion and kidney-mediated glucose excretion are less than in healthy controls.44,58 In addition, progressive loss of nephron mass in CKD results in reduced gluconeogenesis.59,60
Glycemic Management in CKD
Glycemic Targets and Monitoring
Extensive evidence from landmark studies has demonstrated that glycemic control, measured by HbA1c, resulted in decreased risk of “nephropathy” in patients with type 1 diabetes and T2D. In the Diabetes Control and Complications Trial, intensive glycemic control compared to conventional therapy (HbA1c 7.2% vs. 9.1%, P < 0.001), resulted in a 34% adjusted risk reduction for microalbuminuria development.61 In patients with T2D, a long-term follow up of the UK Prospective Diabetes Study also showed that for every 1% reduction in HbA1c, there was a 21% reduction in any diabetes-related complications, 21% reduction in death related to diabetes, 14% reduction in all-cause mortality, and a 37% reduction in microvascular complications.62 There are no large randomized trials evaluating outcomes associated with intensive glycemic control in patients treated by maintenance dialysis, but a meta-analysis of 10 observational studies, including approximately 84,000 patients treated by hemodialysis described an increase in mortality risk with HbA1c ≥8.5% or ≤5.4% compared to an HbA1c between 6.5 and 7.4%.63
Clinical practice guidelines recommended a personalized HbA1c target.64,65 For patients with early-stage CKD, in which prevention of disease progression is the main goal, recommended HbA1c target is <6.5% to 7%, particularly for those with low comorbidity and hypoglycemic burden, and longer life-expectancy. For patients with advanced CKD, HbA1c levels <7.5% to 8% may be preferred, particularly for those with multiple comorbidities and higher hypoglycemia risk (Table 1).64,65 Notably, it is well established that HbA1c accuracy decreases as estimated GFR declines, with poor HbA1c precision in patients with advanced CKD.64,66 Alternative glycemic markers such as fructosamine and glycated albumin have been proposed as short-term or intermediate markers of glycemic control, estimating average glucose from the prior 2 to 4 weeks.66 Nevertheless, these assays are biased in patients with hypoalbuminemia, a common kidney failure complication.66 Some studies have shown that glycated albumin correlates with mortality in patients with kidney failure treated by dialysis.67, 68, 69
Table 1.
| ADA HbA1c targets | Personalized patient selection | KDIGO HbA1c targets | Personalized patient selection | Testing frequency |
|---|---|---|---|---|
| <8% |
|
7%–8% |
|
|
| <7% |
|
∼7% |
|
|
| <6.5% |
|
ADA, American Diabetes Association; CGM, continuous glucose monitoring; CKD, chronic kidney disease; GMI, glucose management indicator; HbA1c, glycated hemoglobin A1c; KDIGO, Kidney Disease Improving Global Outcomes.
Blood glucose monitoring using capillary point-of-care devices is recommended in patients with CKD, particularly for those in whom HbA1c is not reliable.66 Caution is recommended with interpretating results from meters using glucose dehydrogenase pyrroloquinoline quinone in patients on peritoneal dialysis with icodextrin dialysate, because it can cause falsely higher glucose values.66 Recent developments in diabetes technology, including continuous glucose monitors (CGMs), provide an alternative to glycemic control markers in patients with advanced CKD. CGM sensors are minimally invasive, with a small cannula inserted in the subcutaneous tissue that measures interstitial tissue glucose every 5 to 15 minutes.66 Real-time CGM values are now available, providing a comprehensive report of glycemic excursions, beyond single glucose values in time (i.e., fasting glucose), and providing glucose patterns, rate of glucose change, and better evaluation of asymptomatic and nocturnal hypoglycemic events.70 New CGM-derived glycemic control metrics have been developed, such as time-in-target range, time-below range, time-above range, and the “Glucose Management Indicator.” Glucose management indicator is a metric to estimate HbA1c, as derived from CGM values, that can be reported to patients as an alternative to HbA1c.71 For most patients, target time in range (time-in-target range 70−180 mg/dl or 3.9−10 mmol/l) is >70%, but for older patients or patients with high risk for hypoglycemia, a time-in-target range >50% with <10% of TAR (>250 mg/dl or 13.9 mmol/l) may be acceptable, aiming to minimize hypoglycemia (i.e., time-below range <1%).72 Though CGM is a promising technology, there are limited studies in patients with CKD.66
Glucose-Lowering Therapies
Current guidelines recommend SGLT2 inhibitor as first-line therapy for most patients with T2D and CKD, and use of metformin is optional.64,73 Additional glucose-lowering agents can then be added as needed to meet individualized glycemic targets based on consideration of patient-specific and medication-specific factors.13,64,73 A summary of dosing for select glucose-lowering agents based on estimated GFR is shown in Table 2.13,74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89
Table 2.
Recommended dosing of additional noninsulin glucose-lowering agents by eGFR13,74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88
| Medication | Labeled dosing recommendations by eGFR (ml/min/1.73 m2) |
|---|---|
| SGLT2 inhibitors | |
| Canagliflozin |
|
|
|
|
|
|
|
| Dapagliflozin |
|
|
|
|
|
|
|
| Empagliflozin |
|
|
|
|
|
|
|
| Ertugliflozin |
|
|
|
|
|
| GLP-1 receptor agonists | |
| Dulaglutide |
|
|
|
| Exenatide | Twice-daily Product: |
|
|
|
|
|
|
| Once-weekly product: | |
|
|
|
|
| Liraglutide |
|
|
|
| Lixisenatide |
|
|
|
|
|
|
|
| Semaglutide | No dose adjustment required |
| DPP-4 inhibitors | |
| Alogliptin |
|
|
|
|
|
| Linagliptin | No dose adjustment required |
| Saxagliptin |
|
|
|
| Sitagliptin |
|
|
|
|
|
| Sulfonylureas (Second generation) | |
| Glimepiride |
|
|
|
| Glipizide |
|
|
|
| Glyburide | Use not recommended |
| Thiazolidinediones | |
| Pioglitazone | No dose adjustment required |
| Alpha-glucosidase inhibitors | |
| Acarbose | eGFR <30: use not recommended |
| Miglitol | eGFR <25: use not recommended |
CKD, chronic kidney disease; CrCl, creatinine clearance; CV, cardiovascular; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; GI, gastrointestinal; HF, heart failure; SGLT2, sodium glucose cotransporter-2; T2D, type 2 diabetes.
SGLT2 Inhibitors
SGLT2 inhibitors are recommended for most patients with T2D and CKD with an estimated GFR ≥20 ml/min per 1.73 m2 (Table 2).73 The glucose-lowering effect of SGLT2 inhibitors is blunted as kidney function declines, so this recommendation is primarily driven by the desire for kidney and heart protection with SGLT2 inhibitors in the setting of T2D and CKD. SGLT2 inhibitors should be dose adjusted based on estimated GFR, thus kidney function must be monitored during use. Contrary to the initial concerns of increased risk of acute kidney injury, SGLT2 inhibitor use has been shown to reduce risk of acute kidney injury.90
GLP-1RAs
GLP-1RAs are effective and safe antihyperglycemic agents for patients with T2D and CKD (Table 2). The need for dose adjustment based on kidney function varies for agents within the GLP-1RA class. For patients with T2D and CKD requiring additional glucose lowering beyond recommended first-line treatment with an SGLT2 inhibitor, Kidney Disease: Improving Global Outcomes (KDIGO) preferentially recommends the addition of a long-acting GLP-1RA.64 This recommendation is based in part on favorable secondary kidney outcomes showing benefits on reduction in albuminuria and likely preservation of estimated GFR with GLP-1RA treatment.64 In contrast to SGLT2 inhibitors, however, the glucose-lowering benefits of GLP-1RAs are preserved in advanced CKD and have demonstrated efficacy and safety in large clinical trials down to an estimated GFR of 15 ml/min per 1.73 m2.64
Metformin
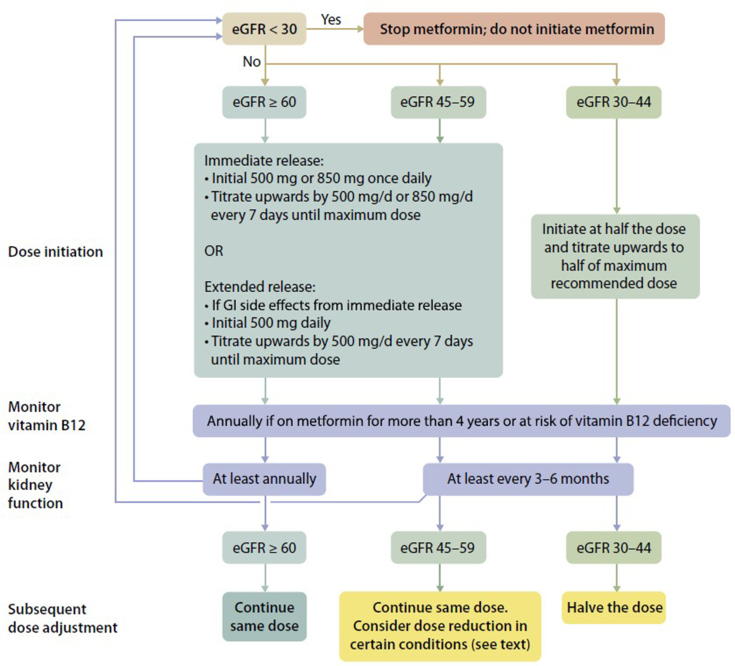
Metformin may be used for glycemic control in patients with T2D and CKD who have an estimated GFR ≥30 ml/min per 1.73 m2 (Table 2).64 Metformin is not metabolized and is excreted unchanged in urine.91 The label for metformin, therefore, includes a boxed warning for increased risk of lactic acidosis in patients with CKD due to impaired metformin excretion.92 Despite this boxed warning, evidence suggests that overall risk for metformin-associated lactic acidosis is low.93 Estimated GFR should be monitored at least annually in patients taking metformin, with the frequency of monitoring increased to every 3 to 6 months once the estimated GFR falls below 60 ml/min per 1.73m2.64 KDIGO further recommends that the dose of metformin be reduced to 1000 mg daily in patients with an estimated GFR between 30 to 45 ml/min per 1.73 m2, and that a reduction be considered in patients with an eGFR of 45 to 59 ml/min per 1.73 m2 if they have a comorbidity that may place them at increased risk for hypoperfusion or hypoxemia (Figure 2).64
Figure 2.
Suggested approach in dosing metformin based on the level of kidney function. eGFR, estimated glomerular filtration rate; GI, gastrointestinal. Reprinted with permission from de Boer IH, Caramori L, Chan JCN, et al. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020;98:S1–S115.
Insulin
Though evidence supporting use of noninsulin agents in T2D and CKD is mounting, insulin remains a main therapeutic option.89 In patients with advanced CKD (estimated GFR <30 ml/min per 1.73 m2), use of many other glucose-lowering agents is restricted. Intensive insulin therapy can help to achieve HbA1c targets in the setting of progressive insulin resistance and unreliable gastric absorption (e.g., delayed gastric emptying due to gastroparesis).94 Exogenous insulin is primarily metabolized by the kidney (30%–80%). Prandial insulin analogs (e.g., lispro, aspart, glulisine) are associated with a lower risk of hypoglycemia and better postprandial glucose control compared to regular human insulin. There is no need for dose adjustment for aspart insulin across different stages of estimated GFR (e.g., ˂60 ml/min per 1.73 m2, 60–80 ml/min per 1.73 m2, ˃90 mlmin per 1.73 m2).95 Among basal insulins, insulin degludec does not require dose adjustment, whereas insulin glargine and insulin detemir should generally be reduced by approximately 30% in patients with estimated GFR ˂60 ml/min per 1.73 m2.95,96 Total daily insulin dose requirements typically decrease by approximately 25% when estimated GFR falls below 60 ml/min per 1.73 m2 (Table 3).97 Ultimately, empiric insulin adjustments should be considered to prevent hypoglycemia with subsequent titration to meet individualized glycemic goals as informed by glucose monitoring (e.g., blood glucose monitoring or CGM).
Table 3.
Recommendations for empiric dosing and titration of insulins in CKD97
| eGFR | % Reduction of TDD | Insulin dose (units/kg/d) |
|
|---|---|---|---|
| T1D | T2D | ||
| >60 ml/min/1.73 m2 | No reduction | 1.0 | 0.5 |
| 60–15 ml/min/1.73 m2 | 25% | 0.75 | 0.3–0.4 |
| <15 ml/min/1.73 m2 | 50% | 0.5 | 0.25 |
eGFR, estimated glomerular filtration rate; T1D, type 1 diabetes mellitus; T2D, type 2 diabetes mellitus; TDD, total daily dose.
Dipeptidyl Peptidase-4 (DPP-4) Inhibitors
All DPP-4 inhibitors are labeled for use in patients with T2D and CKD.85, 86, 87, 88 Alogliptin, saxagliptin, and sitagliptin require dose modification based on estimated GFR. Linagliptin, however, is eliminated primarily through the enterohepatic system and does not require dose adjustment based on kidney function.86 Though several cardiovascular outcome trials with agents from the DPP-4 inhibitor class showed attenuation of progression of albuminuria as a secondary outcome, DPP-4 inhibitors have not shown a benefit on estimated GFR decline or cardiovascular outcomes, and so are primarily used in T2D and CKD for glucose lowering.98 Though DPP-4 inhibitors can be used at various stages of CKD, it should be noted that they are not recommended for use in combination with a GLP-1RA due to overlapping mechanism of action.99
Sulfonylureas
Sulfonylureas are effective glucose-lowering agents but carry a significant risk for hypoglycemia in patients with T2D and CKD.89 Second generation sulfonylureas are available generically and provide an option for patients who lack access to newer, costlier glucose-lowering agents. Among the sulfonylureas, glyburide is not recommended for use in the setting of CKD (estimated GFR <60 ml/min per 1.73 m2) because it is extensively metabolized in the liver to several active metabolites and increase risk of hypoglycemia.89,100 If used, it is prudent to initiate glimepiride or glipizide at the lowest dose and titrate conservatively in patients with CKD to avoid treatment-emergent hypoglycemia.13
Thiazolidinediones
Pioglitazone is extensively metabolized by the liver and dosing adjustments are not required in CKD.101 Additional potential benefits of pioglitazone use in CKD include oral administration and low hypoglycemia risk.64 Some data suggests that thiazolidinediones may convey benefits in patients with T2D and CKD through improvements in the lipid profile and possibly reductions in albuminuria.102 These findings are not substantiated with large clinical trials. Factors contributing to the limited use in CKD include side effects such as fluid retention, HF, and increased fracture risk.13
Alpha-Glucosidase Inhibitors
Acarbose and miglitol are minimally absorbed from the gastrointestinal tract, but plasma drug levels can increase in patients with CKD.103 Therefore, use is cautioned in patients with estimated GFR <30 ml/min per 1.73 m2.13 Alpha-glucosidase inhibitors preferentially target postprandial glucose excursions and may be useful in select patients who are able to tolerate the gastrointestinal side effects (e.g., flatulence, diarrhea) commonly experienced with use, yet are associated with relatively modest effects on HbA1c and are not recommended for use in end-stage kidney disease.
Special Considerations for Dialysis
Patients treated by hemodialysis are especially prone to frequent episodes of hypoglycemia and hyperglycemia, with both extremes closely related to morbidity and mortality.63,104 Hypoglycemic episodes, frequently asymptomatic, are driven by reduced gluconeogenesis, weight loss, anorexia, improved insulin resistance with initiation of dialysis, and removal of glucose during the hemodialysis sessions especially with use of glucose free-dialysate.60,105,106 Occurrence of hypoglycemia during the dialysis session induces secretion of counter-regulatory hormones (e.g., glucagon, cortisol) which leads to rebound hyperglycemia.60 Absorption of insulin onto dialysis membranes during hemodialysis sessions may reduce circulating insulin and exacerbate hyperglycemia.106,107 However, total insulin requirements decrease by approxmately 50% when estimated GFR falls below 10 ml/min per 1.73 m2.60 To prevent and detect episodes of asymptomatic hypoglycemia it is recommended to provide thorough education on blood glucose monitoring or CGM, especially on dialysis days.60
Few studies have demonstrated the safety and efficacy of other glucose-lowering agents in patients receiving dialysis. Because the clearance of GLP-1RAs is not primarily via kidney metabolism, but rather proteolytic degradation, there is no dose adjustment needed for liraglutide, semaglutide and dulaglutide.66 Available pilot studies demonstrated that gastrointestinal side effects may occur at lower doses, indicating the importance of careful dose adjustment, preference for low to intermediate doses, and close monitoring.108 Because the thiazolidinediones (pioglitazone and rosiglitazone) are completely metabolized by the liver, no dose adjustments are needed for patients on dialysis.
Glucose-Lowering Agents for Kidney and Heart Protection
Use of glucose-lowering agents with evidence of kidney and cardiovascular disease risk reduction is now standard-of-care for guideline-directed medical therapy.64,73,109 Based on compelling evidence from large clinical trials, agents from the SGLT2 inhibitor and GLP-1RA classes are recommended by major guideline forming organizations for kidney and heart protection irrespective of glycemic control.64,73,109, 110, 111
SGLT2 Inhibitors
Following the observation that SGLT2 inhibitors improved primary cardiovascular and secondary HF and kidney disease outcomes in large cardiovascular outcome trials,112, 113, 114, 115, 116 dedicated kidney outcome trials confirmed the benefits of SGLT2 inhibition in patients with CKD (Table 4).117, 118, 119, 120 The first dedicated SGLT2 inhibitor kidney outcome trial to demonstrate benefit was the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation, which enrolled participants with T2D and CKD.117 The Dapagliflozin and Prevention of Adverse Outcomes in CKD trial confirmed benefit on kidney and cardiovascular outcomes with SGLT2 inhibition.118 Treatment with the SGLT2/SGLT1 inhibitor sotagliflozin was associated with a reduction in the primary composite outcome of deaths from cardiovascular causes and hospitalizations and urgent visits for HF, but no significant difference in kidney related outcomes were observed in the Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients with Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk trial.120 However, the trial ended early because of lack of support from the sponsor.120 Though the results are not yet available, it was recently announced that the Study of Heart and Kidney Protection with Empagliflozin (EMPA-KIDNEY) trial was stopped early due to evidence of clear, positive efficacy.119 Secondary analyses of large cardiovascular outcome trials with SGLT2 inhibitors show that these kidney and heart protective benefits are consistent across levels of baseline albuminuria, estimated GFR, and glycemia.93,121 SGLT2 inhibitor trials have also demonstrated consistent benefits on kidney and HF outcomes in patients with CKD with and without T2D.122, 123, 124, 125, 126 Comprehensive meta-analysis of close to 22,000 participants of 5 large HF trials (Empagliflozin in Heart Failure With a Preserved Ejection Fraction [EMPEROR -Preserved], Dapagliflozin in Heart Failure With Mildly Reduced or Preserved Ejection Ffraction [DELIVER], Cardiovascular and Renal Outcomes With Empagliflozin in Heart Failure ([EMPEROR-Reduced], Dapagliflozin in Patients With Heart Failure and Reduced Ejection Fraction [DAPA-HF], and Effect of Sotagliflozin on Cardiovascular Events in Patients with Type 2 Diabetes Post Worsening Heart Failure [SOLOIST-WHF]) showed that SGLT2 inhibitors reduced risk of cardiovascular death and hospitalizations for HF across the spectrum of HF.127 Current guidelines recommend use of SGLT2 inhibitors to improve HF outcomes, irrespective of background T2D.128 A summary of dedicated HF trials with SGLT2 inhibitors in provided in Table 5.122, 123, 124, 125, 126 Importantly, the benefits of SGLT2 inhibition were realized on background angiotensin-converting enzyme inhibitor or angiotensin receptor blocker therapy and other HF therapies indicating that SGLT2 inhibitors address residual kidney and cardiovascular risks.117, 118, 119 SGLT2 inhibition is recommended for initiation in patients with an estimated GFR ≥20 ml/min per 1.73 m2 and can be continued until the patient is initiated on kidney replacement therapy.73 Notably, the joint consensus statement from the American Diabetes Association and KDIGO recommends the use of SGLT2 inhibitors in most patients with T2D and CKD irrespective of albuminuria.73 This recommendation is based on combined evidence from major SGLT2 inhibitor outcome trials suggesting that kidney and heart benefits are consistent irrespective of baseline albuminuria, including patients with normal levels of albumin excretion.90
Table 4.
| Study | CREDENCE (N = 4401) | DAPA-CKD (N = 4304) | EMPA-KIDNEY (N = 6609) | SCORED (N = 10,584) |
|---|---|---|---|---|
| Agent | Canagliflozin | Dapagliflozin | Empagliflozin | Sotagliflozin |
| Median follow-up | 2.6 yr | 2.4 yr | Trial stopped in March 2022 due to efficacy | 16 mo (stopped early due to lack of funding) |
| Diabetes-related inclusion criteria | T2D | T2D or non-T2D | T2D or non-T2D | T2D |
| Inclusion criteria | eGFR ≥30 to <90 ml/min/1.73 m2 UACR: ≥300 to <5000 mg/g |
eGFR ≥25 to <75 ml/min/1.73 m2 UACR: ≥200–5000 mg/g |
eGFR ≥20 to <45 ml/min/1.73 m2 OR eGFR ≥45 to <90 ml/min/1.73 m2 with UACR ≥ 200 mg/g |
eGFR ≥25 to <60 ml/min/1.73 m2 UACR ≥30 mg/g Additional CV risk factors |
| Baseline eGFR | 56 ml/min/1.73 m2 | 43 ml/min/1.73 m2 | 37.5 ml/min/1.73 m2 | 44.4 ml/min/1.73 m2 |
| Median Baseline UACR | 927 mg/g | 949 mg/g | 412 mg/g | 74 mg/g |
| Kidney outcome(s) | Primary outcome ESKD (dialysis, transplantation, or sustained eGFR <15 ml/min/1.73 m2), doubling of SCr, or death from renal causes: HR: 0.70 (CI: 0.59–0.82) |
Primary outcome ≥50% decrease in eGFR, ESKD, or death from renal or cardiovascular causes: HR: 0.61 (CI:0.51–0.72) |
Primary outcomea ≥40% decrease in eGFR, ESKD, sustained decline in eGFR to <10 ml/min/1.73 m2 or cardiovascular death |
Secondary outcome No significant difference in >50% decline in the eGFR, long-term dialysis, kidney transplantation, or a sustained eGFR <15 ml/ min/1.73 m2 > 30 d |
| Cardiovascular outcome(s) | Secondary outcomes Reduction of composite of CV death or hospitalization for HF: HR 0.69 (CI: 0.57–0.83) Reduction in hospitalizations for HF: HR 0.61(CI: 0.47–0.80) |
Secondary outcome Reduction of composite of CV death or hospitalization for HF: 0.71 (CI: 0.55–0.92) |
Secondary outcomea Time to CV death or first hospitalization for HF Time to CV death Time to CV death or ESKD |
Primary outcome Reduction of composite of CV deaths, and hospitalization and urgent visits for HF: HR 0.74 (CI: 0.63–0.88)) Secondary outcome Hospitalizations and urgent visits for heart failure. HR 0.67 (CI: 0.55–0.82) |
CREDENCE, Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation; CV, cardiovascular; CI, confidence interval; DAPA-CKD, dapagliflozin and prevention of adverse outcomes; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; EMPA-KIDNEY, The Study of Heart and Kidney Protection With Empagliflozin; HF, heart failure; HR, hazard ratio; SCr, serum creatinine; SCORED, Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients With Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk; T2D, type 2 diabetes; UACR, urinary albumin-to-creatinine ratio.
Full release of EMPA-KIDNEY trial findings pending. CV risk factors: hospitalization for HF, Left ventricular hypertrophy; elevated NT-proBNP, coronary artery calcium, troponin, high sensitivity c-reactive protein, body mass index ≥ 35 kg/m2, LDL >130 mg/dl or HDL <40 mg/dl for men or <50 mg/dl for women on maximally-tolerated statin therapy, current smoking, systolic blood pressure >140 mm Hg and diastolic blood pressure >90 mm Hg, family history of premature coronary heart disease.
Table 5.
| Study | EMPEROR-reduced (N = 3730) | EMPEROR-preserved (N = 5988) | DAPA-HF (N = 4744) | SOLOIST-WHF (N = 1222) | DELIVER (N = 6263) |
|---|---|---|---|---|---|
| Agent | Empagliflozin | Empagliflozin | Dapagliflozin | Sotagliflozin | Dapagliflozin |
| Diabetes related criteria | T2D or non-T2D (50% with T2D) | T2D or non-T2D (49% with T2D) | T2D or non-T2D (45% with T2D) | T2D | T2D or non-T2D (∼45% with T2D) |
| Study population | LVEF ≤40% NYHA class II–IV HF eGFR ≥20 ml/min/1.73 m2 |
LVEF >40% NYHA class II–IV HF Elevated NT-proBNP level 50% with eGFR < 60 ml/min/1.73 m2 |
LVEF ≤40% NYHA class II–IV HF eGFR ≥30 ml/min/1.73 m2 |
Recent hospitalization for HF with IV diuretics eGFR > 30 ml/min/1.73m2 |
LVEF >40% Evidence of structural heart disease Elevated BNP eGFR 61 ±19 ml/min/1.73 m2 |
| Cardiovascular outcome(s) (no difference between diabetic and nondiabetic) |
Primary outcome Reduction in the composite of CV death or hospitalization for HF: HR: 0.75 (CI: 0.65–0.86) Reduction in hospitalization for HF: HR: 0.70 (CI: 0.58–0.85) |
Primary outcome Reduction in the composite of CV death or hospitalization for HF: HR 0.79 (CI: 0.69–0.90) Reduction in hospitalization for HF: HR: 0.71 (CI: 0.60–0.83) Reduction of CV death: HR: 0.91 (CI: 0.76–1.09) |
Primary outcome Improvement in the composite of worsening HF or death from CV causes. HR: 0.74 (CI: 0.65–0.85) |
Primary outcome Reduction in CV deaths and hospitalization and ED visits for HF: HR 0.67 (CI: 0.52–0.85) Secondary outcome Reduction in hospitalizations and urgent visits: HR 0.64 (CI: 0.49–0.83) |
Primary outcome Reduction in the composite of CV death and HF events (hospitalizations and urgent HF visits: HF: HR: 0.82 (CI: 0.73–0.92) Secondary outcome (s) HF events: HR 0.79 (CI: 0.69–0.91) CV death: HR 0.88 (CI: 0.74–1.05) |
| Kidney outcome(s) | Secondary outcome Decrease in the rate of decline in eGFR over the course of the study. Between-group difference of 1.73 ml/min/1.73 m2 (95% CI: 1.10–2.37) |
Secondary outcome Decrease in the rate of decline in eGFR over the course of the study: 1.25 ml/min/1.73 m2 per year in empagliflozin vs. 2.62 ml/min/1.73 m2 per year in placebo group |
Secondary outcome No significant benefit in worsening kidney function. |
Secondary outcome No significant mean change in eGFR |
Safety outcome No difference in any kidney serious adverse event or adverse event that led to discontinuation of dapagliflozin or placebo (2.3 % vs. 2.5 % in dapagliflozin and placebo groups respectively) |
AF, atrial fibrillation; CI, confidence interval; CV, cardiovascular; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; HR, hazard ratio; HF, heart failure; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; NT-proBNP, N-terminal–pro hormone B-type natriuretic peptide; SCr, serum creatinine; SOLOSIST- WHF, Sotagliflozin in Patients With Diabetes and Recent Worsening Heart Failure; T2D, type 2 diabetes; UACR, urinary albumin-to-creatinine ratio.
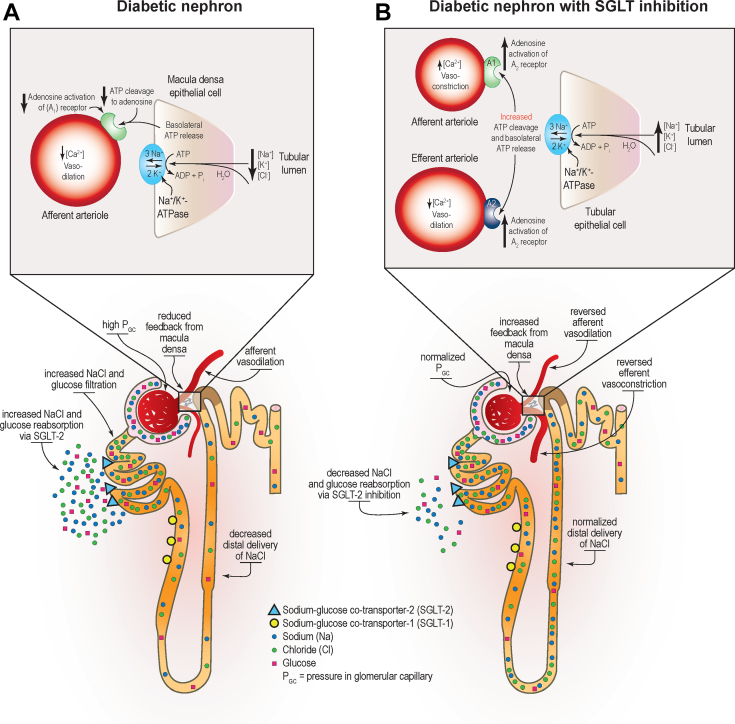
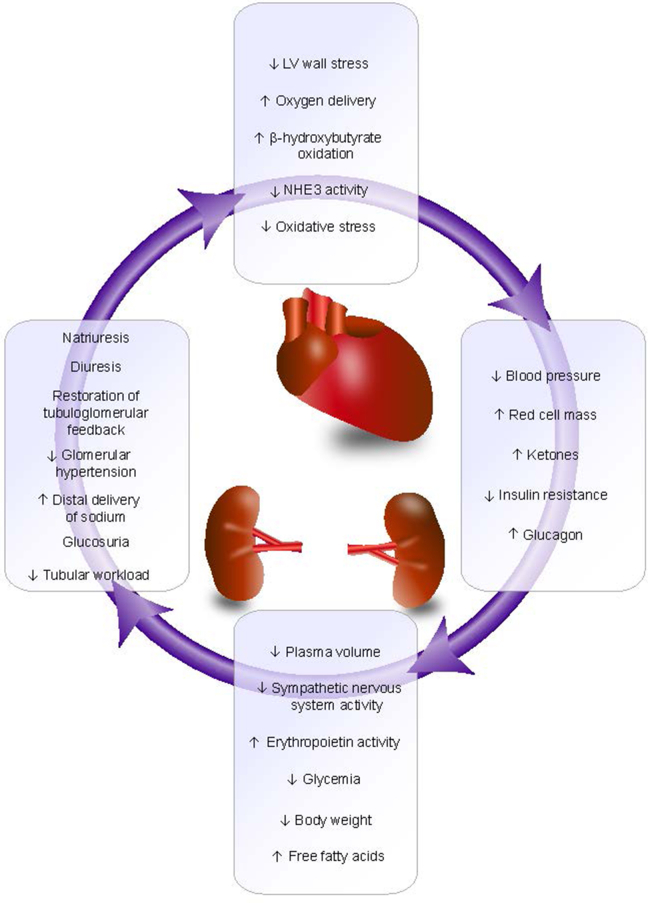
A principal mechanism by which SGLT2 inhibitors convey kidney protection is believed to be reduced glomerular hyperfiltration via restoration of solute delivery to the macula densa and subsequent restoration of tubuloglomerular feedback (Figure 3).34 Other effects that may contribute to kidney and heart protection include a diuretic effect because glycosuria leads to osmotic diuresis, altered fuel metabolism through transition from carbohydrate utilization to ketogenesis, and modest reductions in systolic blood pressure and body weight (Figure 4).129
Figure 3.
Effect of diabetes and sodium-glucose cotransporter 2 (SGLT2) inhibition on nephron hemodynamics. (a) In the diabetic nephron, overexpression and compensatory upregulation of the activity of SGLT2 glucose and sodium reabsorption in the proximal convoluted tubule results in decreased delivery of solutes to the macula densa. The resulting reduction in solute and water transport into the tubular epithelial cells reduces Na+/K+-ATPase pump activity and ATP release from the basolateral membrane of tubular epithelial cells, which in turn reduces adenosine production and activation of the A1 receptor expressed in the afferent arteriole with a net effect of vasodilation. (b) In the diabetic nephron with SGLT inhibition, lessening SGLT2-driven sodium-coupled glucose transport in the proximal convoluted tubule normalizes solute delivery to the macula densa, increasing solute and water reabsorption and increasing basolateral release of ATP from the tubular epithelium. The resulting increase in adenosine activation of the A1 adenosine receptor reverses afferent arteriole vasodilation associated with diabetic kidney disease. Resolution of arterial vasodilation helps normalize glomerular hemodynamics. ADP, adenosine diphosphate; ATP, adenosine triphosphate; ATPase, adenosine triphosphatse; GC, glomerular capillary; K+, pottassium; Na+, sodium; NaCl, sodium chloride. Reprinted with permission from Alicic RZ, Johnson EJ, Tuttle KR. SGLT2 Inhibition for the prevention and treatment of diabetic kidney disease: a review. Am J Kidney Dis. 2018; 72:267–277.
Figure 4.
SGLT2 inhibitor-mediated kidney and heart protection. LV, left ventricular; NHE3, sodium-hydrogen exchanger 3. Reproduced courtesy of Emily J. Cox, PhD; original graphic 2020 E.J. Cox.
GLP-1RAs
Data on CKD onset and progression with GLP-1RAs are limited to secondary kidney outcomes from select cardiovascular outcomes trials and glycemic lowering trials.130, 131, 132, 133 In the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results trial, liraglutide treatment was associated with a lower rate for the secondary composite outcome of new-onset severely increased albuminuria, persistent doubling of serum creatinine, kidney failure, or death due to kidney disease compared to placebo.130 This finding is supported by several other studies in patients with T2D reporting a reduction in albuminuria with liraglutide.134 Similar benefits on secondary kidney composite outcomes were seen in cardiovascular outcomes trials completed with injectable semaglutide and dulaglutide (Table 6).130, 131, 132, 133 A signal for kidney benefit with dulaglutide was also observed in the “A Study Comparing Dulaglutide With Insulin Glargine on Glycemic Control in Participants With Type 2 Diabetes and Moderate or Severe Chronic Kidney Disease” trial.132 A Randomized, Open-Label, Parallel-Arm Study Comparing the Effect of Once-weekly Dulaglutide With Insulin Glargine on Glycemic Control in Patients With Type 2 Diabetes and Moderate or Severe Chronic Kidney Disease (AWARD-7) trialenrolled individuals with moderate-to-severe CKD (mean estimated GFR 38 ml/min per 1.73 m2). Participants receiving treatment with dulaglutide experienced less estiamted GFR decline when compared to treatment with insulin glargine. Importantly, estimated GFR decline was markedly reduced in the participants with macroalbuminuria, a group at high risk for rapid progression to kidney failure (Table 6).132
Table 6.
| Study | LEADER (n = 9340) | SUSTAIN-6 (n = 3297) | AWARD-7 (n = 577) | REWIND (n = 9901) |
|---|---|---|---|---|
| Agent | Liraglutide | Semaglutide | Dulaglutide | Dulaglutide |
| Median follow-up (yr) | 3.8 | 2.1 | 52 wk | 5.4 |
| Prior CVD (%) | 81 | 60 | NA | 31 |
| Mean baseline A1C (%) | 8.7 | 8.7 | 7.5–10.5 | 7.3 |
| Kidney function at baseline (ml/min/1.73 m2) | 21% eGFR 30–59 2% eGFR < 30 |
25% eGFR 30–59 3% eGFR < 30 |
26% eGFR 45–60 35% eGFR 30–44 31% eGFR < 30 46% UACR > 300 mg/g |
21.8% eGFR < 60 34.5% UACR > 30 mg/g |
| Primary outcomeb | 3-point MACE 0.87 (0.78–0.97) |
3-point MACE 0.74 (0.58–0.95) |
HbA1C change from baseline to 26-weeks:
|
3-point MACE 0.88 (0.79–0.99) |
| Secondary outcomes | Lower incidence of composite outcome (new onset albuminuria, doubling of sCr and CrCl <45 ml/min, need for KRT, death due to renal causes) 1.5 events/100 patient/year in liraglutide vs. 1.9 events events/100 patient/year in placebo group (P = 0.003) |
Lower incidence of new or worsening nephropathy: 3.8% in semaglutide vs. 6.1% in placebo group (P = 0.005) Lower rate of new onset macroalbuminuria: 2.5% in semaglutide vs. 4.9% in placebo |
eGFR decline (ml/min/1.73 m2): • 3.3 insulin glargine • 0.7 duaglutide 0.75 mga and 1.5 mga • aP < 0.05 compared with glargine eGFR decline (ml/min/1.73 m2) in UACR > 300 mg/g group: • 5.5 insulin glargine • 0.7 dulaglutide 0.75 mga • 0.5 dulaglutide 1.5 mga • aP < 0.05 compared with glargine UACR reduction: • 13% insulin glargine • 29% dulaglutide 1.5 mga • aP < 0.05 compared with glargine |
Lower incidence of the composite endpoint (new onset macroalbuminuria, ≥30% decline in eGFR, or need for chronic KRT); 17% in dulaglutide vs. 20% in placebo group (P < 0.001) |
| Worsening nephropathyb | 0.78 (0.67–0.92) | 0.64 (0.46–0.88) | - | 0.85 (0.77–0.93) |
A1C, glycated hemoglobin; AWARD, X; CI, confidence interval; eGFR, glomerular filtration rate; GLP-1RA, glucagon-like peptide-1 receptor agonist; HR, hazard ratio; KRT, kidney replacement therapy; LEADER, A Long-term, Multi-centre, International, Randomised Double-blind, Placebo-controlled Trial to Determine Liraglutide Effects on Cardiovascular Events; MACE, major adverse cardiovascular events; REWIND, The Effect of Dulaglutide on Major Cardiovascular Events in Patients With Type 2 Diabetes: Researching Cardiovascular Events With a Weekly INcretin in Diabetes; SUSTAIN, A Long-term, Randomised, Double-blind, Placebo-controlled, Multinational, Multi-centre Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes; UACR, urine to albumin creatinine ratio.
Statistically significant.
Outcome data represented as HR and 95% CI.
A pooled analysis of 12,637 participants from the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results and A Long-term, Randomised, Double-blind, Placebo-controlled, Multinational, Multi-centre Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6) trials showed that compared with placebo, semaglutide and liraglutide lowered albuminuria from baseline to 2 years after randomization by 24%.135 Both agents demonstrated slowing of estimated GFR decline and risks of 40% and 50% estimated GFR reductions from baseline versus placebo. The likelihood of slowing estimated GFR decline was greater in patients with estimated GFR levels of 30 to 60 ml/min per 1.73 m2 compared to estimated GFR >60 ml/min per 1.73 m2.135
The proposed mechanisms by which GLP-1RAs reduce the risk of macroalbuminuria and slow estimated GFR decline include antioxidant, antiinflammatory, and antifibrotic effects in the diabetic kidney promoted by GLP-1 signaling.98 Furthermore, GLP-1RAs produce modest reductions in blood pressure.130,131,136, 137, 138 GLP-1RAs also promote substantial weight loss (often >5 kg) compared with other glucose-lowering therapies in T2D.139,140 Notably, the weight loss effect is preserved in patients with CKD.132,141
KDIGO preferentially recommends addition of a long-acting GLP-1RA (with proven cardiovascular benefits) in patients with T2D and CKD who need additional glucose lowering despite recommended first-line therapy with metformin plus a SGLT2 inhibitor.64 The American Diabetes Association recommends use of long-acting GLP-1RAs interchangeably with SGLT2 inhibitors in patients with estimated GFR <60 ml/min per 1.73 m2 or in those with albuminuria who cannot take SGLT2 inhibitors.109 Two ongoing trials, the Effect of Semaglutide Versus Placebo on the Progression of Renal Impairment in Subjects With Type 2 Diabetes and CKD (FLOW, NCT03819153) and Renal Mode of Action of Semaglutide in Patients With Type 2 Diabetes and CKD study (REMODEL, NCT04865770) are testing the effects of injectable semaglutide on a primary kidney disease outcome and investigating mechanistic outcomes of kidney inflammation, perfusion, and oxygenation by magnetic resonance imaging and kidney biopsies, respectively.142,143
Safety and Risk Mitigation for Glucose-Lowering Agents
An important aspect of managing patients with T2D and CKD is educating patients and caregivers about potential side effects and risks of therapy and how to best mitigate risks (Table 7).64,73 In general, monitoring for changes in estimated GFR to guide dosing of many glucose-lowering therapies is important to ensure medication use safety.89 For example, this is especially important with metformin to protect against metformin-associated lactic acidosis.64 Because of the association with dose and treatment duration-related B12 deficiency with metformin use,144 B12 monitoring is also recommended in patients treated with metformin for over 4 years.64 Risk mitigation strategies for SGLT2 inhibitors may include, but are not limited to, hygiene counseling to avoid genital mycotic infections in women and men, provision of sick day rules and insulin administration guidelines to reduce risk of SGLT2 inhibitor-associated diabetic ketoacidosis, and proactive reduction of diuretics as needed for patients at risk for hypovolemia and orthostasis.64 SGLT2 inhibitor initiation is associated with a reversible decline in estimated GFR of 3 to 5 ml/min per 1.73 m2. Following an initial “eGFR dip,” kidney function will generally return toward baseline in several weeks and then stabilize during SGLT2 inhibitor therapy.145,146 Among participants in the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation trial, the initial decline in estimated GFR (up to 30%) did not influence subsequent estimated GFR, except in those with baseline estimated GFR of 45 to 59 ml/min per 1.73 m2 in whom the initial dip was actually associated with a slower rate of estimated GFR decline.147 In an analysis of approximately 6700 participants enrolled in the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients trial, a decrease of >10% from baseline estimated GFR was found to be associated with more advanced CKD and diuretic use, and did not impact cardiovascular or kidney outcomes.146 Therefore, the initial “eGFR dip” generally does not necessitate drug discontinuation.64 Because SGLT2 inhibition results in an initial natriuresis, it may be reasonable to reduce the dose of background diuretics in patients at risk for hypovolemia.64 To minimize gastrointestinal adverse events, GLP-1RAs should be initiated at the lowest dose and titrated slowly per patient response.64
Table 7.
| Medication class | Consideration | Monitoring and/or risk mitigation strategies |
|---|---|---|
| Metformin |
|
|
|
|
|
| SGLT2 inhibitors |
|
|
|
|
|
|
|
|
|
|
|
| GLP1 receptor agonists |
|
|
|
|
eGFR, estimated glomerular filtration rate; GI, gastrointestinal; GLP1, glucagon-like peptide-1; SGLT2, sodium-glucose cotransporter-2.
Because SGLT2 inhibitors and GLP-1RAs are recommended for use in T2D and CKD for kidney and heart protection irrespective of glycemic control, they may be initiated in patients not requiring additional glucose lowering. In this situation, considering adjustment of background hypoglycemic agents is appropriate to mitigate hypoglycemia risk.64 In patients on an insulin regimen who have an HbA1c ≤8.0%, consideration should be given to lowering basal insulin by approximately 20% when starting a glucose-lowering agent for kidney and heart protection.148,149 For those on background sulfonylurea therapy, the sulfonylurea dose may be reduced by approximately 50% or discontinued upon initiation of the SGLT2 inhibitor or GLP-1RA. For those with an HbA1c >8.0%, the SGLT2 inhibitor or GLP-1RA can usually be initiated without adjustment of background glucose-lowering therapies. In those with a history of severe hypoglycemia or hypoglycemia unawareness, additional caution is appropriate.
Optimizing Outcomes in Patients with T2D and CKD: Multidisciplinary Models of Care
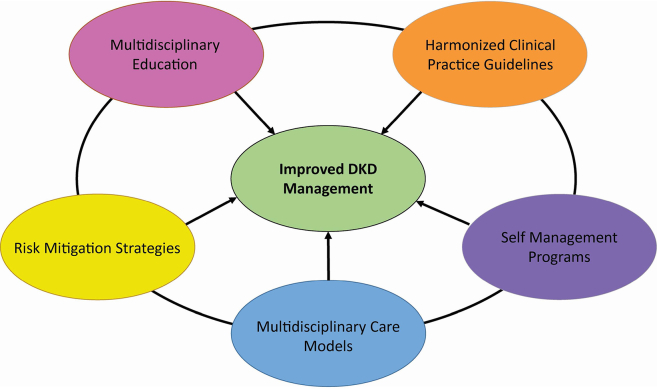
With the establishment of SGLT2 inhibitors and GLP-RAs as agents that improve kidney and cardiovascular outcomes, it is now critical to optimize use of these life-saving therapies.150 KDIGO recommends a team-based integrated care approach delivered by multidisciplinary healthcare professionals to meet patient-centered goals, including attainment of guideline-directed glycemic, blood pressure, and lipid goals; use of kidney and heart protective agents, and ongoing self-management support.150,151 Multidisciplinary efforts to optimize treatment and outcomes in the setting of T2D and CKD is important when considering the historical underuse of recommended therapies, such as ACE inhibitors and ARBs, in this high-risk population (Table 8).150, 151, 152 Effective use of multidisciplinary teams has the potential to address many barriers to optimize care for patients with T2D and CKD (Figure 5).150,151
Table 8.
Opportunities for addressing barriers to optimized T2D and CKD care through use of multidisciplinary teams150,151
| Barrier/Gap | Utilize members of the multidisciplinary team (e.g., clinical pharmacists, nurses) to: |
|---|---|
| Underuse of recommended therapies (e.g., ACE inhibitors/ARBs, SGLT2 inhibitors, GLP1 receptor agonists, finerenone) and overuse of potentially nephrotoxic agents (e.g., PPIs, NSAIDs) |
|
| Access barriers and high costs of medications (e.g., SGLT2 inhibitors) |
|
| Need for longitudinal assessment of kidney function and other risk factors to direct care |
|
| Lack of coordinated care and effective communication among members of the healthcare team |
|
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CKD, chronic kidney disease; NSAIDs, nonsteroidal antiinflammatory drugs; PPI, proton pump inhibitor; SGLT2, sodium-glucose cotransporter 2; T2D, type 2 diabetes.
Figure 5.
Overcoming barriers to management of diabetic kidney disease. Overcoming key barriers in the management of DKD is important to improve DKD management and patient outcomes. Patient-level, provider-level, and system-level barriers lead to low disease awareness, suboptimal screening and identification, delayed implementation of kidney protective therapies, and poor patient outcomes. Initiatives and efforts to address these barriers may include multidisciplinary educational activities about DKD, harmonization of clinical practice guidelines, provision of diabetes self-management education programs that emphasize DKD-related education, development and utilization of multidisciplinary DKD care models, and educating patients about risk mitigation strategies to maximize medication use safety. DKD, diabetic kidney disease. Reprinted with permission from Neumiller JJ, Alicic RZ, Tuttle KR. Overcoming barriers to implementing new therapies for diabetic kidney disease: lessons learned. Adv Chronic Kidney Dis. 2021;28(4):318–327.
Conclusions
Recent therapeutic advances offer a unique opportunity to transform care of patients with diabetes and CKD. With therapies readily available and proven to save lives, kidneys, and hearts, it is imperative to address barriers to implementation150 In many countries, limited access to kidney replacement therapy equates progression to kidney failure and to high likelihood of death.11 Systematic transformative approaches are needed to increase use of therapies that will deliver optimal care for CKD and cardiovascular risk reduction in diabetes.
Disclosure
RZA reports consulting fees from Boehringer Ingelheim, grant research funding support from the Centers for Disease Control and Prevention, and Goldfinch Bio, the Kidney Company, Bayer pharmaceuticals outside the submitted work. JJN reports personal fees and other support from Bayer AG, personal fees from Sanofi, personal fees from Novo Nordisk, and personal fees from Dexcom outside of the submitted work. KRT reports fees from Eli Lilly, Boehringer Ingelheim, Gilead, Astra Zeneca, Goldfinch Bio, Novo Nordisk, Bayer, and Travere for research and other support regarding diabetes and CKD. RJG reports research support to Emory University for investigator-initiated studies from Novo Nordisk, Dexcom, and Eli Lilly; and consulting fees from Sanofi, Eli Lilly, Boehringer, Pfizer, Merck and Weight Watchers, outside of this work.
References
- 1.United States renal data system. 2021 USRDS annual data report. Epidemiology of Kidney Disease in the United States: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
- 2.Saeedi P., Petersohn I., Salpea P., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. 9th edition. [DOI] [PubMed] [Google Scholar]
- 3.Deng Y., Li N., Wu Y., et al. Global, regional, and national burden of diabetes-related chronic kidney disease from 1990 to 2019. Front Endocrinol (Lausanne) 2021;12:672350. doi: 10.3389/fendo.2021.672350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.IDF Diabetes Atlas International Diabetes Federation. https://www.diabetesatlas.org 10th edition. Published 2021.
- 5.Afkarian M., Sachs M.C., Kestenbaum B., et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24:302–308. doi: 10.1681/ASN.2012070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soriano C.L., Johansson S., Stefansson B., Rodríguez L.A. Cardiovascular events and all-cause mortality in a cohort of 57,946 patients with type 2 diabetes: associations with renal function and cardiovascular risk factors. Cardiovasc Diabetol. 2015;14:38. doi: 10.1186/s12933-015-0204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scirica B.M., Mosenzon O., Bhatt D.L., et al. Cardiovascular outcomes according to urinary albumin and kidney disease in patients with type 2 diabetes at high cardiovascular risk: observations from the SAVOR-TIMI 53 trial. JAMA Cardiol. 2018;3:155–163. doi: 10.1001/jamacardio.2017.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daratha K.B., Short R.A., Corbett C.F., et al. Risks of subsequent hospitalization and death in patients with kidney disease. Clin J Am Soc Nephrol. 2012;7:409–416. doi: 10.2215/CJN.05070511. [DOI] [PubMed] [Google Scholar]
- 9.Jankowski J., Floege J., Fliser D., et al. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143:1157–1172. doi: 10.1161/CIRCULATIONAHA.120.050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crews D.C., Bello A.K., Saadi G., et al. Burden, access, and disparities in kidney disease. Kidney Int. 2019;95:242–248. doi: 10.1016/j.kint.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Liyanage T., Ninomiya T., Jha V., et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 12.Brown E., Heerspink H.J.L., Cuthbertson D.J., Wilding J.P.H. SGLT2 inhibitors and GLP-1 receptor agonists: established and emerging indications. Lancet. 2021;398:262–276. doi: 10.1016/S0140-6736(21)00536-5. [DOI] [PubMed] [Google Scholar]
- 13.Tuttle K.R., Bakris G.L., Bilous R.W., et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Am J Kidney Dis. 2014;64:510–533. doi: 10.1053/j.ajkd.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Gerich J.E. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med. 2010;27:136–142. doi: 10.1111/j.1464-5491.2009.02894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landau B.R., Wahren J., Chandramouli V., et al. Contributions of gluconeogenesis to glucose production in the fasted state. J Clin Invest. 1996;98:378–385. doi: 10.1172/JCI118803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stumvoll M., Meyer C., Mitrakou A., et al. Renal glucose production and utilization: new aspects in humans. Diabetologia. 1997;40:749–757. doi: 10.1007/s001250050745. [DOI] [PubMed] [Google Scholar]
- 17.Mather A., Pollock C. Glucose handling by the kidney. Kidney Int Suppl. 2011;120:S1–S6. doi: 10.1038/ki.2010.509. [DOI] [PubMed] [Google Scholar]
- 18.Meyer C., Dostou J.M., Welle S.L., Gerich J.E. Role of human liver, kidney, and skeletal muscle in postprandial glucose homeostasis. Am J Physiol Endocrinol Metab. 2002;282:E419–E427. doi: 10.1152/ajpendo.00032.2001. [DOI] [PubMed] [Google Scholar]
- 19.Gronda E., Jessup M., Iacoviello M., et al. Glucose metabolism in the kidney: neurohormonal activation and heart failure development. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.018889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerich J.E. Physiology of glucose homeostasis. Diabetes Obes Metab. 2000;2:345–350. doi: 10.1046/j.1463-1326.2000.00085.x. [DOI] [PubMed] [Google Scholar]
- 21.Alsahli M., Gerich J.E. Renal glucose metabolism in normal physiological conditions and in diabetes. Diabetes Res Clin Pract. 2017;133:1–9. doi: 10.1016/j.diabres.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 22.Miller D., Bilhler I. In: Membrane Transport and Metabolism A Symposium. Kleinzeller A., Kotyk A., editors. Czech Academy of Sciences and Academic Press; 1960. The restrictions on possible mechanisms of intestinal transport of sugars; pp. 439–464. [Google Scholar]
- 23.Turk E., Martin M.G., Wright E.M. Structure of the human Na+/glucose cotransporter gene SGLT1. J Biol Chem. 1994;269:15204–15209. doi: 10.1016/S0021-9258(17)36592-4. [DOI] [PubMed] [Google Scholar]
- 24.Hediger M.A., Coady M.J., Ideda T.S., Wright E.M. Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. Nature. 1987;330:379–381. doi: 10.1038/330379a0. [DOI] [PubMed] [Google Scholar]
- 25.Hediger M.A., Budarf M.L., Emanuel B.S., et al. Assignment of the human intestinal Na+/glucose cotransporter gene (SGLT1) to the q11.2----qter region of chromosome 22. Genomics. 1989;4:297–300. doi: 10.1016/0888-7543(89)90333-9. [DOI] [PubMed] [Google Scholar]
- 26.DeFronzo R.A., Hompesch M., Kasichayanula S., et al. Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care. 2013;36:3169–3176. doi: 10.2337/dc13-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu Y., Griffen S.C., Boulton D.W., Leil T.A. Use of systems pharmacology modeling to elucidate the operating characteristics of SGLT1 and SGLT2 in renal glucose reabsorption in humans. Front Pharmacol. 2014;5:274. doi: 10.3389/fphar.2014.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorboulev V., Schurmann A., Vallon V., et al. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes. 2012;61:187–196. doi: 10.2337/db11-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hummel C.S., Lu C., Liu J., et al. Structural selectivity of human SGLT inhibitors. Am J Physiol Cell Physiol. 2012;302:C373–C382. doi: 10.1152/ajpcell.00328.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright E.M., Hirayama B.A., Loo D.F. Active sugar transport in health and disease. J Intern Med. 2007;261:32–43. doi: 10.1111/j.1365-2796.2006.01746.x. [DOI] [PubMed] [Google Scholar]
- 31.Brenner B.M. 7th ed. WB Saunders Company; 2004. Brenner & Rector’s the Kidney. [Google Scholar]
- 32.Wright E.M., Loo D.D.F., Hirayama B.A. Biology of human sodium glucose transporters. Physiol Rev. 2011;91:733–794. doi: 10.1152/physrev.00055.2009. [DOI] [PubMed] [Google Scholar]
- 33.DeFronzo R.A. The effect of insulin on renal sodium metabolism. A review with clinical implications. Diabetologia. 1981;21:165–171. doi: 10.1007/BF00252649. [DOI] [PubMed] [Google Scholar]
- 34.Alicic R.Z., Johnson E.J., Tuttle K.R. SGLT2 Inhibition for the prevention and treatment of diabetic kidney disease: a review. Am J Kidney Dis. 2018;72:267–277. doi: 10.1053/j.ajkd.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 35.Meyer C., Stumvoll M., Nadkarni V., et al. Abnormal renal and hepatic glucose metabolism in type 2 diabetes mellitus. J Clin Invest. 1998;102:619–624. doi: 10.1172/JCI2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer C., Woerle H.J., Dostou J.M., et al. Abnormal renal, hepatic, and muscle glucose metabolism following glucose ingestion in type 2 diabetes. Am J Physiol Endocrinol Metab. 2004;287:E1049–E1056. doi: 10.1152/ajpendo.00041.2004. [DOI] [PubMed] [Google Scholar]
- 37.Mogensen C.E. Maximum tubular reabsorption capacity for glucose and renal hemodynamics during rapid hypertonic glucose infusion in normal and diabetic subjects. Scand J Clin Lab Investig. 1971;28:101–109. doi: 10.3109/00365517109090668. [DOI] [PubMed] [Google Scholar]
- 38.Pollock C.A., Lawrence J.R., Field M.J. Tubular sodium handling and tubuloglomerular feedback in experimental diabetes mellitus. Am J Physiol. 1991;260:F946–F952. doi: 10.1152/ajprenal.1991.260.6.F946. [DOI] [PubMed] [Google Scholar]
- 39.Vestri S., Okamoto M.M., de Freitas H.S., et al. Changes in sodium or glucose filtration rate modulate expression of glucose transporters in renal proximal tubular cells of rat. J Membr Biol. 2001;182:105–112. doi: 10.1007/s00232-001-0036-y. [DOI] [PubMed] [Google Scholar]
- 40.Rahmoune H., Thompson P.W., Ward J.M., et al. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes. 2005;54:3427–3434. doi: 10.2337/diabetes.54.12.3427. [DOI] [PubMed] [Google Scholar]
- 41.DeFronzo R.A., Davidson J.A., Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab. 2012;14:5–14. doi: 10.1111/j.1463-1326.2011.01511.x. [DOI] [PubMed] [Google Scholar]
- 42.Umino H., Hasegawa K., Minakuchi H., et al. High basolateral glucose increases sodium-glucose cotransporter 2 and reduces Sirtuin-1 in renal tubules through glucose transporter-2 detection [Sci Rep] Sci Rep. 2018;8:6791. doi: 10.1038/s41598-018-25054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tuttle K.R. Back to the future: glomerular hyperfiltration and the diabetic kidney. Diabetes. 2017;66:14–16. doi: 10.2337/dbi16-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alicic R.Z., Neumiller J.J., Johnson E.J., et al. Sodium-glucose cotransporter 2 inhibition and diabetic kidney disease. Diabetes. 2019;68:248–257. doi: 10.2337/dbi18-0007. [DOI] [PubMed] [Google Scholar]
- 45.Hornum M., Clausen P., Kjaergaard J., et al. Pre-diabetes and arterial stiffness in uraemic patients. Nephrol Dial Transplant. 2010;25:1218–1225. doi: 10.1093/ndt/gfp558. [DOI] [PubMed] [Google Scholar]
- 46.Eldin W.S., Ragheb A., Klassen J., Shoker A. Evidence for increased risk of prediabetes in the uremic patient. Nephron Clin Pract. 2008;108:c47–c55. doi: 10.1159/000112529. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi S., Maesato K., Moriya H., et al. Insulin resistance in patients with chronic kidney disease. Am J Kidney Dis. 2005;45:275–280. doi: 10.1053/j.ajkd.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 48.Pham H., Robinson-Cohen C., Biggs M.L., et al. Chronic kidney disease, insulin resistance, and incident diabetes in older adults. Clin J Am Soc Nephrol. 2012;7:588–594. doi: 10.2215/CJN.11861111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeFronzo R.A., Alvestrand A., Smith D., et al. Insulin resistance in uremia. J Clin Invest. 1981;67:563–568. doi: 10.1172/JCI110067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Boer I.H., Zelnick L., Afkarian M., et al. Impaired glucose and insulin homeostasis in moderate-severe CKD. J Am Soc Nephrol. 2016;27:2861–2871. doi: 10.1681/ASN.2015070756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Henry S.L., Barzel B., Wood-Bradley R.J., et al. Developmental origins of obesity-related hypertension. Clin Exp Pharmacol Physiol. 2012;39:799–806. doi: 10.1111/j.1440-1681.2011.05579.x. [DOI] [PubMed] [Google Scholar]
- 52.Mancusi C., Izzo R., di Gioia G., et al. Insulin resistance the hinge between hypertension and type 2 diabetes. High Blood Press Cardiovasc Prev. 2020;27:515–526. doi: 10.1007/s40292-020-00408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eidmak I., Feldt-Rasmussen B., Kanstrup I.L., et al. Insulin resistance and hyperinsulinemia in mild to moderate progressive chronic renal failure and its association with aerobic work capacity. Diabetologia. 1995;38:565–572. doi: 10.1007/BF00400725. [DOI] [PubMed] [Google Scholar]
- 54.Martinez Cantarin M.P., Whitaker-Menezes D., Lin Z., Falkner B. Uremia induces adipose tissue inflammation and muscle mitochondrial dysfunction. Nephrol Dial Transplant. 2017;32:943–951. doi: 10.1093/ndt/gfx050. [DOI] [PubMed] [Google Scholar]
- 55.Stenvinkel P., Ketteler M., Johnson R.J., et al. IL-10, IL-6, and TNF-α: central factors in the altered cytokine network of uremia—the good, the bad, and the ugly. Kidney Int. 2005;67:1216–1233. doi: 10.1111/j.1523-1755.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- 56.Kim J.C., Kalantar-Zadeh K., Kopple J.D. Frailty and protein-energy wasting in elderly patients with end-stage kidney disease. J Am Soc Nephrol. 2013;24:337–351. doi: 10.1681/ASN.2012010047. [DOI] [PubMed] [Google Scholar]
- 57.Fliser D., Pacini G., Engelleiter R., et al. Insulin resistance and hyperinsulinemia are already present in patients with incipient renal disease. Kidney Int. 1998;53:1343–1347. doi: 10.1046/j.1523-1755.1998.00898.x. [DOI] [PubMed] [Google Scholar]
- 58.Idorn T., Knop F.K., Jørgensen M., et al. Gastrointestinal factors contribute to glucometabolic disturbances in nondiabetic patients with end-stage renal disease. Kidney Int. 2013;83:915–923. doi: 10.1038/ki.2012.460. [DOI] [PubMed] [Google Scholar]
- 59.Arem R. Hypoglycemia associated with renal failure. Endocrinol Metab Clin North Am. 1989;18:103–121. doi: 10.1016/S0889-8529(18)30391-8. [DOI] [PubMed] [Google Scholar]
- 60.Abe M., Kalantar-Zadeh K. Haemodialysis-induced hypolycaemia an glycaemic disarrays. Nat Rev Nephrol. 2015;11:302–313. doi: 10.1038/nrneph.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.The diabetes control and complications (DCCT) research group Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int. 1995;47:1703–1720. doi: 10.1038/ki.1995.236. [DOI] [PubMed] [Google Scholar]
- 62.Stratton I.M., Adler A.I., Neil H.A., et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hill C.J., Maxwell A.P., Cardwell C.R., et al. Glycated hemoglobin and risk of death in diabetic patients treated with hemodialysis: a meta-analysis. Am J Kidney Dis. 2014;63:84–94. doi: 10.1053/j.ajkd.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 64.Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020;98:S1–S115. doi: 10.1016/j.kint.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 65.American Diabetes Association Professional Practice Committee. Draznin B., Aroda V.R., et al. Glycemic targets: standards of Medical Care in Diabetes −2022. Diabetes Care. 2022;45(Suppl 1):S83–S96. doi: 10.2337/dc22-S006. [DOI] [PubMed] [Google Scholar]
- 66.Galindo R.J., Beck R.W., Scioscia M.F., et al. Glycemic monitoring and management in advanced chronic kidney disease. Endocr Rev. 2020;41:756–774. doi: 10.1210/endrev/bnaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peacock T.P., Shihabi Z.K., Bleyer A.J., et al. Comparison of glycated albumin and hemoglobin A(1c) levels in diabetic subjects on hemodialysis. Kidney Int. 2008;73:1062–1068. doi: 10.1038/ki.2008.25. [DOI] [PubMed] [Google Scholar]
- 68.Rooney M.R., Daya N., Tang O., et al. Glycated albumin and risk of mortality in the US adult population. Clin Chem. 2022;68:422–430. doi: 10.1093/clinchem/hvab232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Copur S., Siriopol D., Afsar B., et al. Serum glycated albumin predicts all-cause mortality in dialysis patients with diabetes mellitus: meta-analysis and systematic review of a predictive biomarker. Acta Diabetol. 2021;58:81–91. doi: 10.1007/s00592-020-01581-x. [DOI] [PubMed] [Google Scholar]
- 70.Galindo R.J., Aleppo G. Continuous glucose monitoring: the achievement of 100 years of innovation in diabetes technology. Diabetes Res Clin Pract. 2020;170:108502. doi: 10.1016/j.diabres.2020.108502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bergenstal R.M., Beck R.W., Close K.L., et al. Glucose management indicator (GMI): A new term for estimating A1C from continuous glucose monitoring. Diabetes Care. 2018;41:2275–2280. doi: 10.2337/dc18-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Battelino T., Danne T., Bergenstal R.M., et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care. 2019;42:1593–1603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Boer I.H., Khunti K., Sadusky T., et al. Diabetes management in chronic kidney disease: A consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Care. 2022:dci220027. doi: 10.2337/dci22-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Invokana (Canagliflozin) Janssen Pharmaceuticals, Inc; 2020. [Google Scholar]
- 75.Farxiga (dapagliflozin) [Prescribing Information] AstraZeneca Pharmaceuticals LP; 2021. [Google Scholar]
- 76.Jardiance (Empagliflozin) [Prescribing Information] Boehringer Ingelheim Pharmaceuticals, Inc; 2022. [Google Scholar]
- 77.Steglatro (Ertugliflozin) [Prescribing Information] Merck & Co., Inc; 2021. [Google Scholar]
- 78.Trulicity (Dulaglutide) Injection [Prescribing Information] Eli Lilly and Company; 2021. [Google Scholar]
- 79.Byetta (exenatide) injection [Prescribing Information] AstraZeneca Pharmaceuticals LP; 2021. [Google Scholar]
- 80.Bydureon (exenatide extended-release) injection [Prescribing Information] AstraZeneca Pharmaceuticals LP; 2021. [Google Scholar]
- 81.Victoza (Liraglutide) Injection [Prescribing Information] Novo Nordisk, Inc; 2021. [Google Scholar]
- 82.Adlyxin (Lixisenatide) Injection [Prescribing Information] Sanofi-aventis U.S., LLC; 2021. [Google Scholar]
- 83.Ozempic (Semaglutide) Injection [Prescribing Information] Novo Nordisk, Inc; NJ: 2022. [Google Scholar]
- 84.Rybelsus (Semaglutide) Tablets [Prescribing Information] Novo Nordisk, Inc; 2021. [Google Scholar]
- 85.Nesina (alogliptin) [Prescribing Information] Takeda Pharm Am Inc Lexington, MA; MA: 2022. [Google Scholar]
- 86.Tradjenta (Linagliptin) [Prescribing Information] Boehringer Ingelheim Pharmaceuticals, Inc; 2022. [Google Scholar]
- 87.Onglyza (saxagliptin) [Prescribing Information] AstraZeneca Pharmaceuticals LP; 2019. [Google Scholar]
- 88.Januvia (Sitagliptin) [Prescribing Information] Merck & Co., Inc; 2021. [Google Scholar]
- 89.Neumiller J.J., Alicic R.Z., Tuttle K.R. Therapeutic considerations for antihyperglycemic agents in diabetic kidney disease. J Am Soc Neprhol. 2017;28:2263–2274. doi: 10.1681/ASN.2016121372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Neuen B.L., Young T., Heerspink H.J.L., et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019;7:845–854. doi: 10.1016/S2213-8587(19)30256-6. [DOI] [PubMed] [Google Scholar]
- 91.Graham G.G., Punt J., Arora M., et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50:81–98. doi: 10.2165/11534750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 92.Glucophage (metformin) [Prescribing Information] Bristol-Myers Squibb Company; 2018. [Google Scholar]
- 93.Inzucchi S.E., Lipska K.J., Mayo H., et al. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA. 2014;312:2668–2675. doi: 10.1001/jama.2014.15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sampanis C.H. Management of hyperglycemia in patients with diabetes mellitus and chronic renal failure. Hippokratia. 2008;12:22–27. [PMC free article] [PubMed] [Google Scholar]
- 95.Kulozik F., Hasslacher C. Insulin requirements in patients with diabetes and declining kidney function: differences between insulin analogues and human insulin? Ther Adv Endocrinol Metab. 2013;4:113–121. doi: 10.1177/2042018813501188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kiss I., Arold G., Roepstorff C., et al. Insulin degludec: pharmacokinetics in patients with renal impairment. Clin Pharmacokinet. 2014;53:175–183. doi: 10.1007/s40262-013-0113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rajput R., Sinha B., Majumdar S., et al. Consensus statement on insulin therapy in chronic kidney disease. Diabetes Res Clin Pract. 2017;127:10–20. doi: 10.1016/j.diabres.2017.02.032. [DOI] [PubMed] [Google Scholar]
- 98.Alicic R.Z., Cox E.J., Neumiller J.J., Tuttle K.R. Incretin drugs in diabetic kidney disease: biological mechanisms and clinical evidence. Nat Rev Nephrol. 2021;17:227–244. doi: 10.1038/s41581-020-00367-2. [DOI] [PubMed] [Google Scholar]
- 99.Davies M.J., D’Alessio D.A., Fradkin J., et al. Management of hyperglycemia in type 2 diabetes 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;41:2669–2701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rydberg T., Jönsson A., Røder M., Melander A. Hypoglycemic activity of glyburide (glibenclamide) metabolites in humans. Diabetes Care. 1994;17:1026–1030. doi: 10.2337/diacare.17.9.1026. [DOI] [PubMed] [Google Scholar]
- 101.Actos (Pioglitazone) [Prescribing Information] Takeda Canada Inc; 2018. [Google Scholar]
- 102.Sarafidis P.A., Bakris G.L. Protection of the kidney by thiazolidinediones: an assessment from bench to bedside. Kidney Int. 2006;70:1223–1233. doi: 10.1038/sj.ki.5001620. [DOI] [PubMed] [Google Scholar]
- 103.Charpentier G., Riveline J.P., Varroud-Vial M. Management of drugs affecting blood glucose in diabetic patients with renal failure. Diabetes Metab. 2000;26(suppl 4):73–85. [PubMed] [Google Scholar]
- 104.Monnier L., Wojtusciszyn A., Colette C., Owens D. The contribution of glucose variability to asymptomatic hypoglycemia in patients with type 2 diabetes. Diabetes Technol Ther. 2011;13:813–818. doi: 10.1089/dia.2011.0049. [DOI] [PubMed] [Google Scholar]
- 105.Jackson M.A., Holland M.R., Nicholas J., et al. Hemodialysis-induced hypoglycemia in diabetic patients. Clin Nephrol. 2000;54:30–34. [PubMed] [Google Scholar]
- 106.Abe M., Kaizu K., Matsumoto K. Plasma insulin is removed by hemodialysis: evaluation of the relation between plasma insulin and glucose by using a dialysate with or without glucose. Ther Apher Dial. 2007;11:280–287. doi: 10.1111/j.1744-9987.2007.00491.x. [DOI] [PubMed] [Google Scholar]
- 107.Abe M., Okada K., Ikeda K., et al. Characterization of insulin adsorption behavior of dialyzer membranes used in hemodialysis. Artif Organs. 2011;35:398–403. doi: 10.1111/j.1525-1594.2010.01112.x. [DOI] [PubMed] [Google Scholar]
- 108.Hahr A.J., Molitch M.E. Management of diabetes mellitus in patients with CKD: core curriculum 2022. Am J Kidney Dis. 2022;79:728–736. doi: 10.1053/j.ajkd.2021.05.023. [DOI] [PubMed] [Google Scholar]
- 109.American Diabetes Association Professional Practice Committee. Draznin B., Aroda V.R., et al. Chronic kidney disease and risk management: standards of Medical Care in Diabetes−2022. Diabetes Care. 2022;45(suppl 1):S175–S184. doi: 10.2337/dc22-S011. [DOI] [PubMed] [Google Scholar]
- 110.American Diabetes Association Professional Practice Committee Cardiovascular disease and risk management: standards of Medical Care in Diabetes −2022. Diabetes Care. 2022;45(Suppl 1):S144–S174. doi: 10.2337/dc22-S010. [DOI] [PubMed] [Google Scholar]
- 111.Garber A.J., Handelsman Y., Grunberger G., et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm−2020 Executive Summary. Endocr Pract. 2020;26:107–139. doi: 10.4158/CS-2019-0472. [DOI] [PubMed] [Google Scholar]
- 112.Zinman B., Wanner C., Lachin J.M., et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 113.Neal B., Perkovic V., Mahaffey K.W., et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 114.Wiviott S.D., Raz I., Bonaca M.P., et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 115.Cannon C.P., Pratley R., Dagogo-Jack S., et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383:1425–1435. doi: 10.1056/NEJMoa2004967. [DOI] [PubMed] [Google Scholar]
- 116.Cherney D.Z.I., Charbonnel B., Cosentino F., et al. Effects of ertugliflozin on kidney composite outcomes, renal function and albuminuria in patients with type 2 diabetes mellitus: an analysis from the randomised VERTIS CV trial. Diabetologia. 2021;64:1256–1267. doi: 10.1007/s00125-021-05407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Perkovic V., Jardine M.J., Neal B., et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 118.Heerspink H.J.L., Stefánsson B.V., Correa-Rotter R., et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 119.EMPA-Kidney EMPA-Kidney Trial Stops Early due to Evidence of Efficacy. https://www.empakidney.org/news/empa-kidney-trial-stops-early-due-to-evidence-of-efficacy Published March 16, 2022.
- 120.Bhatt D.L., Szarek M., Pitt B., et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021;384:129–139. doi: 10.1056/NEJMoa2030186. [DOI] [PubMed] [Google Scholar]
- 121.Jardine M.J., Zhou Z., Mahaffey K.W., et al. Renal, cardiovascular, and safety outcomes of canagliflozin by baseline kidney function: a secondary analysis of the CREDENCE randomized trial. J Am Soc Nephrol. 2020;31:1128–1139. doi: 10.1681/ASN.2019111168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McMurray J.J.V., Solomon J.D., Inzucchi S.E., et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 123.Packer M., Anker S.D., Butler J., et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 124.Anker S.D., Butler J., Filippatos G., et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 125.Solomon S.D., McMurray J.J.V., Claggett B., et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089–1098. doi: 10.1056/NEJMoa2206286. [DOI] [PubMed] [Google Scholar]
- 126.Bhatt D.L., Szarek M., Steg P.G., et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384:117–128. doi: 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
- 127.Vaduganathan M., Docherty K.F., Claggett B.L., et al. SGLT2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet. 2022;400:757–767. doi: 10.11016/S0140-6736(22)01429-5. [DOI] [PubMed] [Google Scholar]
- 128.Heidenreich P.A., Bozkurt B., Aguilar D., et al. AHA/ACC/HFSA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. 2022;79(17):e263–e421. doi: 10.1016/j.jacc.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 129.Vasilakou D., Karagiannis T., Athanasiadou E., et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159:262–274. doi: 10.7326/0003-4819-159-4-201308200-00007. [DOI] [PubMed] [Google Scholar]
- 130.Marso S.P., Daniels G.H., Brown-Frandsen K., et al. LEADER trial investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Marso S.P., Bain S.C., Consoli A., et al. SUSTAIN-6 investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 132.Tuttle K.R., Lakshmanan M.C., Rayner B., et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6:605–617. doi: 10.1016/S2213-8587(18)30104-9. [DOI] [PubMed] [Google Scholar]
- 133.Gerstein H.C., Colhoun H.M., Dagenais G.R., et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomized placebo-controlled trial. Lancet. 2019;394:121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 134.Zavattaro M., Caputo M., Sama M.T., et al. One-year treatment with liraglutide improved renal function in patients with type 2 diabetes: a pilot prospective study. Endocrine. 2015;50:620–626. doi: 10.1007/s12020-014-0519-0. [DOI] [PubMed] [Google Scholar]
- 135.Shaman A.M., Bain S.C., Bakris G.L., et al. Effect of the glucagon-like peptide-1 receptor agonists semaglutide and liraglutide on kidney outcomes in patients with type 2 diabetes: pooled analysis of SUSTAIN 6 and LEADER. Circulation. 2022;145:575–585. doi: 10.1161/CIRCULATIONAHA.121.055459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pfeffer M.A., Claggett B., Diaz R., et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 137.Holman R.R., Bethel M.A., Mentz R.J., et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228–1239. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pauza A.G., Thakkar P., Tasic T., et al. GLP1R attenuates sympathetic response to high glucose via carotid body inhibition. Circ Res. 2022;130:694–707. doi: 10.1161/CIRCRESAHA.121.319874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vilsbøll T., Christensen M., Junker A.E., et al. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344:d7771. doi: 10.1136/bmj.d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Potts J.E., Gray L.J., Brady E.M., et al. The effect of glucagon-like peptide 1 receptor agonists on weight loss in type 2 diabetes: a systematic review and mixed treatment comparison meta-analysis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0126769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Davies M.J., Bain S.C., Atkin S.L., et al. Efficacy and safety of liraglutide versus placebo as add-on to glucose-lowering therapy in patients with type 2 diabetes and moderate renal impairment (LIRARENAL): a randomized clinical trial. Diabetes Care. 2016;39:222–230. doi: 10.2337/dc14-2883. [DOI] [PubMed] [Google Scholar]
- 142.A research study to see how semaglutide works compared to placebo in people with type 2 diabetes and chronic kidney disease (FLOW). US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT03819153?term=semaglutide+FLOW&draw=2&rank=1
- 143.A research study to find out how semaglutide works in the kidneys compared to placebo US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT04865770
- 144.Aroda V.R., Edelstein S.L., Goldberg R.B., et al. Long-term metformin use and vitamin B12 deficiency in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab. 2016;101:1754–1761. doi: 10.1210/jc.2015-3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nespoux J., Vallon V. SGLT2 inhibition and kidney protection. Clin Sci (Lond) 2018;132:1329–1339. doi: 10.1042/CS20171298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kraus B.J., Weir M.R., Bakris G.L., et al. Characterization and implications of the initial estimated glomerular filtration rate ‘dip’ upon sodium-glucose cotransporter-2 inhibition with empagliflozin in the EMPA-REG OUTCOME trial. Kidney Int. 2021;99:750–762. doi: 10.1016/j.kint.2020.10.031. [DOI] [PubMed] [Google Scholar]
- 147.Oshima M., Jardine M.J., Agarwal R., et al. Insights from CREDENCE trial indicate an acute drop in estimated glomerular filtration rate during treatment with canagliflozin with implications for clinical practice. Kidney Int. 2021;99:999–1009. doi: 10.1016/j.kint.2020.10.042. [DOI] [PubMed] [Google Scholar]
- 148.Carris N.W., Talor J.R., Gums J.G. Combining a GLP-1 receptor agonist and basal insulin: study evidence and practical considerations. Drugs. 2014;74:2141–2152. doi: 10.1007/s40265-014-0325-2. [DOI] [PubMed] [Google Scholar]
- 149.Gomez-Peralta F., Abreu C., Lecube A., et al. Practical approach to initiating SGLT2 inhibitors in type 2 diabetes. Diabetes Ther. 2017;8:953–962. doi: 10.1007/s13300-017-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Neumiller J.J., Alicic R.Z., Tuttle K.R. Overcoming barriers to implementing new therapies for diabetic kidney disease: lessons learned. Adv Chronic Kidney Dis. 2021;28:318–327. doi: 10.1053/j.ackd.2021.02.001. [DOI] [PubMed] [Google Scholar]
- 151.Neumiller J.J., Shubrook J.H., Manley T., et al. Optimizing use of SGLT2 inhibitors and other evidence-based therapies to improve outcomes in patients with type 2 diabetes and chronic kidney disease: an opportunity for pharmacists. Am J Health Syst Pharm. 2022;79:e65–e70. doi: 10.1093/ajhp/zxab271. [DOI] [PubMed] [Google Scholar]
- 152.Tuttle K.R., Alicic R.Z., Duru O.K., et al. Clinical characteristics of and risk factors for chronic kidney disease among adults and children: an analysis of the CURE-CKD registry. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.18169. [DOI] [PMC free article] [PubMed] [Google Scholar]