Abstract
Papillary fibroelastoma (PFE) is a rare, slow-growing cardiac tumor. We encountered an 80-year-old man with PFE accidentally revealed by transthoracic echocardiography (TTE) to evaluate cardiac function before a non-cardiac operation. A 10-mm mass lesion adhered to the anterior papillary muscle of the left ventricle, which had not been detected with TTE performed nine months before. Emergency cardiac surgery to remove the mass was performed, and the mass was diagnosed as a PFE. The PFE grew to 10 mm in a maximum of 9 months; to our knowledge, this is the fastest growth of PFE in the left ventricle reported to date.
Learning objective
Papillary fibroelastoma (PFE) is a rare, slow-growing cardiac tumor. The surgical indication of PFE is sometimes controversial. The rapid growth of PFE might be considered as a criterion for surgery because this might result in the rapid progression of symptoms and complications.
Keywords: Papillary fibroelastoma, Left ventricular papillary muscle, Rapid growth
Introduction
Primary cardiac tumors are exceedingly rare, with a reported incidence of 0.021 % in autopsy cases. Approximately 8 % of these are papillary fibroelastoma (PFE) [1], [2]. In general, PFE is a slow-growing tumor that is dominantly attached to cardiac valves. We encountered a male patient with PFE in the left ventricle, which grew to 10 mm in a maximum of 9 months.
Case report
The patient was an 80-year-old man who was referred to the otorhinolaryngology department of our hospital for parathyroidectomy for hyperparathyroidism. He was diagnosed with angina pectoris and underwent a percutaneous coronary intervention for a proximal segment of the left anterior descending artery at age 75 years.
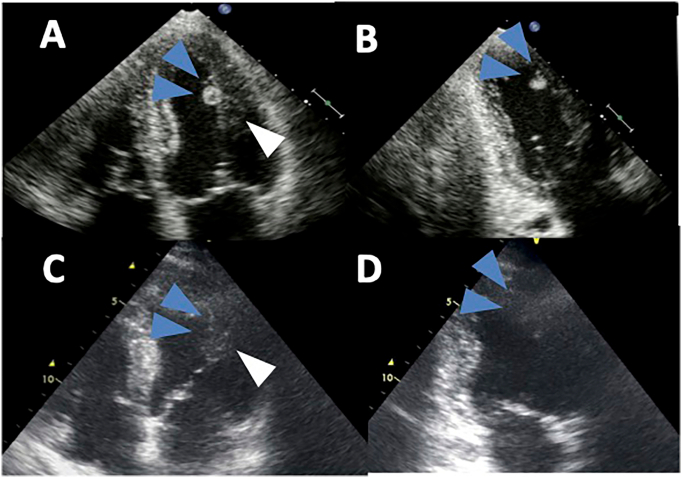
Transthoracic echocardiography (TTE) as part of the preoperative cardiac assessment revealed a 10-mm mass lesion attached to the anterior papillary muscle of the left ventricle (Fig. 1A, B, Video 1), which had not been observed 9 months previously (Fig. 1C, D). The mass was pedunculated and mobile with an irregular surface.
Fig. 1.
Transthoracic echocardiography on admission and nine months before the operation. Transthoracic echocardiography revealed a mobile 10-mm mass attached to the anterior papillary muscle of the left ventricle (white arrow head) in four-chamber view (A) and two-chamber view (B), which had not been observed in either view 9 months before (C, D).
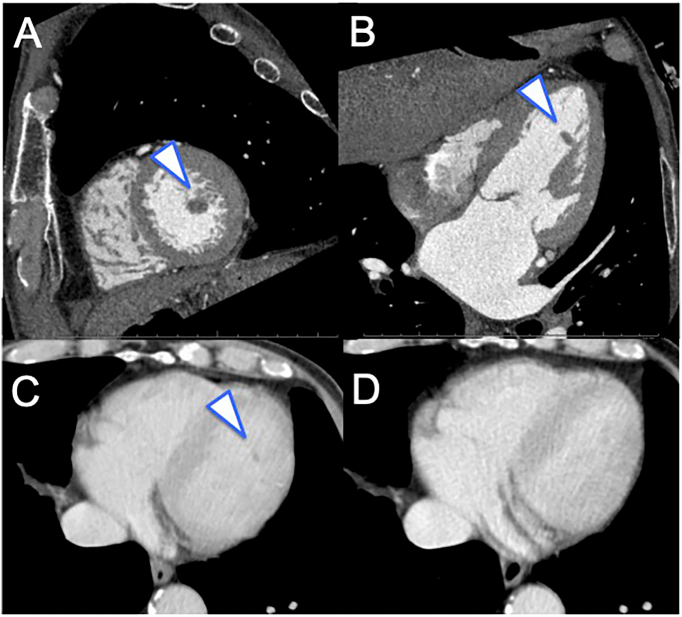
Physical examination was as follows: body temperature 36.7 °C, heart rate 58 beats/min, and blood pressure 154/76 mmHg. Cardiac murmur was not detected. The chest radiograph was normal while the electrocardiogram showed a complete right bundle branch block and left axis deviation. Laboratory tests revealed no increased inflammatory response or coagulation abnormality, and a C-reactive protein level of 0.02 mg/l, white blood cell count of 4400/μl, and D-dimer level of 0.6 μg/ml. No bacterium was detected in blood cultures. Cardiac contrast computed tomography (CT) showed a 10-mm mass on the left ventricular anterior papillary muscle (Fig. 2A, B), and no significant stenosis of coronary arteries was suspected. A retrospective review of past CT data showed the presence of the mass in the left ventricle lumen in contrast CT without electrocardiographic (ECG) gating with a slice thickness of 5 mm performed almost one month before (Fig. 2C, Video 2A), but not in contrast CT without ECG gating with a slice thickness of 1 mm performed under the same protocol seven months before (Fig. 2D, Video 2B).
Fig. 2.
Electrocardiography (ECG) gated computed tomography (CT) after admission and CT without ECG gating one month and seven months before the operation. ECG gated CT with a slice thickness of 0.5 mm revealed a pedunculated mass attached to the anterior papillary muscle of the left ventricle in a short axial image (A) and four-chamber view (B). The papillary fibroelastoma was detected as a mass in the left ventricle lumen on CT without ECG gating with a slice thickness of 5 mm, which was performed almost one month before the operation (C); however, it was not detected in contrast CT without ECG gating with a slice thickness of 1 mm performed under the same protocol seven months before the operation (D).
Considering the risk of embolism, emergency cardiac surgery to remove the mass was performed. The surgery was performed via a median sternotomy approach under cardiopulmonary bypass. The tumor of the left ventricle was resected via the mitral valve via an incision through the left atrium. The 10 × 6 × 5 mm mass was resected, with no thrombus attached. Pathological examination revealed papillary lesions with a vitreous interstitium covered with endothelial cells (Fig. 3), and the mass was diagnosed as PFE. The postoperative course was uneventful, and the patient was discharged eight days after the operation. No recurrence was observed in TTE two years later.
Fig. 3.
Intraoperative findings and postoperative pathological examination of the mass. The mass attached to anterior papillary muscle, was finally resected. (A) The resected mass showed papillary lesions with vitreous stroma, and the surface layer was covered with endothelial cells. (B) Hematoxylin-eosin stain, ×10 (C) hematoxylin-eosin stain, ×30.
Discussion
In general, PFE is recognized as a slow-growing tumor. A previous study reported that the average growth rate of PFE was 0.5 ± 0.9 mm/year [3]. In our present case, PFE had not been observed in the previous TTE performed nine months before. Given a reported sensitivity and specificity of TTE for the detection of PFE ≥2 mm of 88.9 % and 87.8 %, respectively, it is unlikely that the PFE had been overlooked at that time [4], suggesting in turn the high possibility that it had grown to 10 mm in a maximum of 9 months. Currently, no factors are available to predict the growth rate of PFE [3], and the determinants of growth are unclear. A few reports have described the rapid development of PFE on valve tissue, one of which was due to thrombus attachment [5], [6]. In our present case, however, no thrombus was attached to the resected mass, and its size of 10 mm was similar to that on evaluation of preoperative imaging. We, therefore, consider that the tumor grew relatively rapidly. PFE is a rare lesion and PFE in the left ventricle is rarer still. Moreover, no report has yet described a fast-growing PFE in the left ventricle. We, therefore, consider that this case is unique.
The etiology of PFE has been discussed in various ways, including hamartomas, acquired reactive lesions, and the possibility of gene mutations [7], but no conclusive answer has yet been obtained. The possibility that PFE may also be produced by endocardial damage due to intracardiac turbulence or hyperplasia due to fibrin deposition has also been proposed, which would suggest that PFE can occur anywhere in the heart [8]. The majority of cases occur on the valve tissue, and cases occurring in the left ventricle are rare [9]. The association between tumor position and growth rate is still unknown.
Patients with PFE are at risk of cerebral embolic events, angina, intestinal ischemia, heart failure due to valvular dysfunction, and sudden death. When PFE occurs in the left ventricle, as in this case, left ventricular obstruction may occur. The current management of PFE is surgical resection for symptomatic patients but controversy remains, especially regarding asymptomatic patients. Tumor mobility has been reported to predict a poor prognosis [9], and so might be a criterion for surgery in asymptomatic patients. An effect of tumor growth rate on the risk of embolic events has not been proved [3]; nevertheless, we consider that rapid growth should be recognized as a criterion for surgery because this might result in the rapid progression of symptoms and complications.
Conclusions
We experienced a male with PFE on the left ventricular anterior papillary muscle which had not been detected by TTE nine months previously. The PFE was resected as an emergency. The postoperative course was uneventful, and no recurrence was observed on TTE two years later.
The following are the supplementary data related to this article.
Transthoracic echocardiography (TTE) revealed a mobile 10-mm mass attached to the anterior papillary muscle of the left ventricle.
The papillary fibroelastoma was detected as a mass with a lower computed tomography (CT) value in the left ventricular lumen on contrast CT without electrocardiography (ECG) gating performed almost one month before the operation (A), but was not detected on contrast CT without ECG gating performed under the same protocol seven months before the operation (B).
Declaration of competing interest
The authors declare that there is no conflict of interest.
References
- 1.Reynen K. Frequency of primary tumors of the heart. Am J Cardiol. 1996;77:107. doi: 10.1016/s0002-9149(97)89149-7. [DOI] [PubMed] [Google Scholar]
- 2.Burke A., Virmani R. Atlas of tumor pathology. vol. 16. Armed Forces Institute of Pathology; Washington DC: 1996. Tumors of the heart and great vessels; pp. 1–11. (3rd series). [Google Scholar]
- 3.Kurmann R.D., El-Am E.A., Sorour A.A., Lee A.T., Scott C.G., Bois M.C., Maleszewski J.J., Klarich K.W. Papillary fibroelastoma growth: a retrospective follow-up study of patients with pathology-proven papillary fibroelastoma. J Am Coll Cardiol. 2021;77:2154–2155. doi: 10.1016/j.jacc.2021.02.027. [DOI] [PubMed] [Google Scholar]
- 4.Sun J.P., Asher C.R., Yang X.S., Cheng G.G., Scalia G.M., Massed A.G., Griffin B.P., Ratliff N.B., Stewart W.J., Thomas J.D. Clinical and echocardiographic characteristics of papillary fibroelastomas: a retrospective and prospective study in 162 patients. Circulation. 2001;103:2687–2693. doi: 10.1161/01.cir.103.22.2687. [DOI] [PubMed] [Google Scholar]
- 5.Kondoh H., Yamada T., Yoshida A., Arima R., Hatsuoka S., Shintani H. Rapid development of a papillary fibroelastoma in the aortic valve: report of a case. Surg Today. 2009;39:713–716. doi: 10.1007/s00595-008-3980-7. [DOI] [PubMed] [Google Scholar]
- 6.Joffe I.I., Jacobs L.E., Owen A.N., Ian I., Ioli A., Kotler M.N. Rapid development of a papillary fibroelastoma with associated thrombus: the role of transthoracic and transesophageal echocardiography. Echocardiography. 1997;14:287–292. doi: 10.1111/j.1540-8175.1997.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 7.Wittersheim M., Heydt C., Hoffmann F., Büttner R. KRAS mutation in papillary fibroelastoma: a true cardiac neoplasm? J Pathol Clin Res. 2017;3:100–104. doi: 10.1002/cjp2.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaian D.M., Labib S.B., Chang G. Cardiac papillary fibroelastoma. Ann Thorac Surg. 1995;59:538–541. doi: 10.1016/0003-4975(94)00857-4. [DOI] [PubMed] [Google Scholar]
- 9.Gowda R.M., Khan I.A., Nair C.K., Mehta N.J., Vasavada B.C., Sacchi T.J. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J. 2003;146:404–410. doi: 10.1016/S0002-8703(03)00249-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transthoracic echocardiography (TTE) revealed a mobile 10-mm mass attached to the anterior papillary muscle of the left ventricle.
The papillary fibroelastoma was detected as a mass with a lower computed tomography (CT) value in the left ventricular lumen on contrast CT without electrocardiography (ECG) gating performed almost one month before the operation (A), but was not detected on contrast CT without ECG gating performed under the same protocol seven months before the operation (B).