Abstract
Lipomatous hypertrophy of the interatrial septum is a rare benign condition characterized by adipocyte hyperplasia with fat infiltration between the myocardial fibers in the interatrial septum. Although lipomatous hypertrophy does not occur only in the interatrial septum, its location in the interventricular septum is extremely rare. A 45-year-old woman with no medical or family history of cardiac disease presented with an episode of syncope. Transthoracic echocardiography revealed an echogenic mass in the interventricular septum and no outflow obstruction. The mass-like area showed fat tissue-specific features on computed tomography and magnetic resonance imaging, and furthermore, it showed late gadolinium enhancement. We diagnosed it as lipomatous hypertrophy of the interventricular septum. An implantable loop recorder documented paroxysmal complete atrioventricular block with presyncope. A permanent dual-chamber pacemaker was implanted. This is the first reported case of lipomatous hypertrophy of the interventricular septum treated with a pacemaker for complete atrioventricular block with syncope. We have described the case and the treatment strategy in detail.
Learning objective
To understand lipomatous hypertrophy, a rare disorder, and its characteristics and differences between lipomatous hypertrophy and cardiac adipose tumors on computed tomography and magnetic resonance imaging. To learn about the appropriate treatment and clinical management of this benign condition and treat symptomatic patients.
Keywords: Atrioventricular block, Cardiac pacemaker, Cardiac magnetic resonance, Late gadolinium enhancement, Lipomatous hypertrophy
Introduction
Lipomatous hypertrophy of the interatrial septum is a rare benign condition characterized by adipocyte hyperplasia with fat infiltration between the myocardial fibers in the interatrial septum. These lesions are not capsulated and usually feature a transverse diameter of the interatrial septum exceeding 2 cm on computed tomography (CT) [1]. Although lipomatous hypertrophy does not occur only in the interatrial septum, its location in the interventricular septum is extremely rare [2], [3]. Lipomatous hypertrophy of the interatrial septum causes a variety of atrial arrhythmias, such as frequent premature atrial complexes, multifocal atrial tachycardia, wandering atrial pacemaker, atrial fibrillation, sick sinus syndrome, and sudden death [4]. Multimodality imaging is useful for diagnosing lipomatous hypertrophy and differentiating it from other cardiac tumors. We discuss the diagnosis and treatment strategy of lipomatous hypertrophy.
Case report
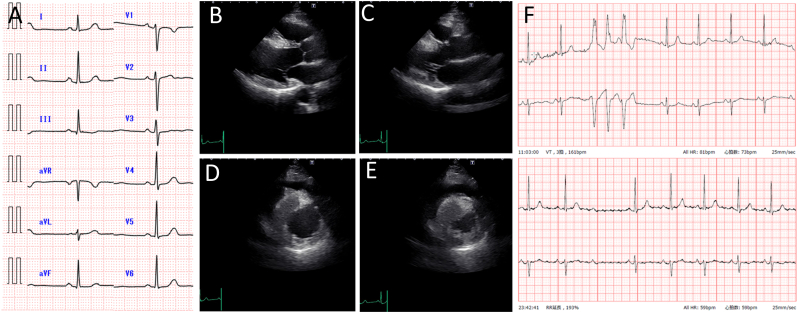
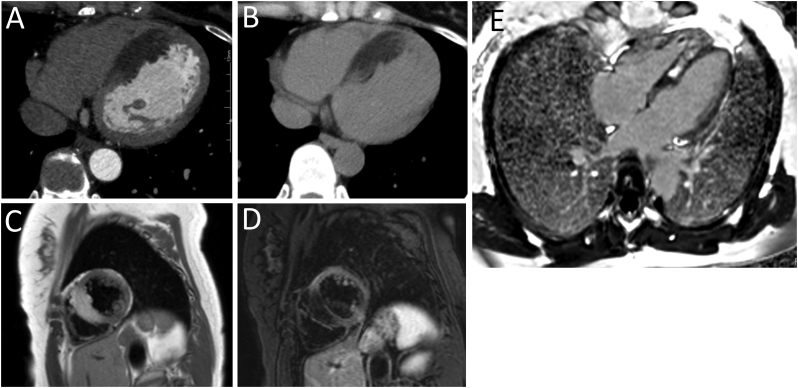
A 45-year-old woman presented with an episode of syncope. The patient also noticed presyncope 3 months before the visit. The patient had no medical or family history of cardiac disease. Her height was 159.0 cm and her weight was 58.6 kg. Her body mass index was 23.2. On physical examination, the patient's blood pressure was normal and heart rhythm was regular, and no heart murmurs were detected. Electrocardiography showed normal sinus rhythm and no evidence of a conduction disorder (Fig. 1A). Transthoracic echocardiography revealed an increased thickness and an echogenic mass measuring 1.7 × 3.7 cm in the interventricular septum (Fig. 1B-E). The patient's ejection fraction was normal and, no left ventricular outflow obstruction was noted. Twenty-four-hour Holter monitoring documented triplet ventricular tachycardia once and a lack of only one QRS wave as a Mobitz type II atrioventricular (AV) block (Fig. 1F). The plasma B-type natriuretic peptide level was 6.0 pg/mL. CT and magnetic resonance imaging (MRI) were performed to image cardiomyopathy and cardiac tumors and to rule out coronary artery disease that causes Adams-Stokes attacks. Multidetector CT revealed a normal coronary and unencapsulated inhomogeneous mass-like area without contrast enhancement in the interventricular septum (Fig. 2A). The CT value of the area was −66-46 Hounsfield units, confirming the presence of fatty tissue (Fig. 2B). Cardiac MRI showed high intensity on T1-weighted images of the interventricular septum and part of the right ventricular apical wall (Fig. 2C). These areas showed signal loss in the fat suppression sequence (Fig. 2D). A late gadolinium-enhanced image revealed a hyperintense signal in the central part of the interventricular septum (Fig. 2E). Based on these imaging features, the mass within the interventricular septum was diagnosed as lipomatous hypertrophy of the interventricular septum. To detect arrhythmias for the repeated episodes of syncope, we placed an implantable loop recorder. Thereafter, a long pause of approximately 5 s with presyncope caused by paroxysmal complete AV block was documented. We considered it difficult to resolve the complete AV block even with surgical resection; therefore, a permanent dual-chamber pacemaker was implanted. The patient had no symptoms after pacemaker implantation over a one-year follow-up period.
Fig. 1.
(A) Electrocardiography at presentation showed normal sinus rhythm. Transthoracic echocardiogram of an echogenic mass measuring 1.7 × 3.7 cm in the interventricular septum; (B) long-axis view, end-diastolic phase; (C) long-axis view, systolic phase; (D) short-axis view, end-diastolic phase; and (E) short-axis view, systolic phase. (F) Twenty-four-hour Holter monitoring documented triplet ventricular tachycardia and a Mobitz type II atrioventricular block.
Fig. 2.
Computed tomography. (A) Multidetector row computed tomography with contrast, axial transverse view showing no contrast enhancement in the interventricular septum. (B) Plain computed tomography, axial transverse view showing a negative value (minimal −66 Hounsfield units) in several lesions in the interventricular septum. Cardiac magnetic resonance imaging. (C) T1-weighted imaging, short-axial view showing a hyperintense signal from the interventricular septum. (D) T2-weighted imaging with fat suppression, short-axial view showing suppression of the signal from the interventricular septum. (E) Late gadolinium-enhanced cardiac magnetic resonance T1-weighted imaging, axial view showing late enhancement in the central part of the interventricular septum.
Discussion
To our knowledge, this is the first reported case of lipomatous hypertrophy of the interventricular septum, which was treated with a pacemaker due to a paroxysmal complete AV block with syncope. If a less mobile intracavitary, intramyocardial, or pericardial echogenic mass is seen on transthoracic echocardiography at a screening, CT and MRI examinations are performed for further evaluation of the mass. If a cardiac tumor is suspected, its etiology can be determined by considering the age of the presentation, epidemiologic likelihood, location of the tumor, and tissue characterization with noninvasive imaging modalities, especially MRI. MRI can assess morphology, dimensions, location, extension, homogeneity, presence of infiltration in the surrounding tissues, and signal characteristics that can help with histopathological characterization (fat infiltration, necrosis, hemorrhage, calcification, vascularity, etc.). Using this approach and integrating the clinical data, an accurate diagnosis and treatment strategy is usually possible without the need for percutaneous or open surgical biopsy [5]. Primary cardiac tumors containing fat tissue include benign to malignant tumors such as lipomatous hypertrophy, lipoma, hemangioma, liposarcoma, and any sarcomas (Table 1). In this case, noninvasive cardiac imaging did not show either heterogeneous features or contrast enhancement on CT. Thus, lipoma or lipomatous hypertrophy was indicated as a differential diagnosis. Cardiac lipoma presents as homogenous encapsulated masses of fat attenuation (<−50 Hounsfield units) without contrast enhancement on CT. Cardiac MRI shows high signal intensity on T1-weighted images, slightly less hyperintensity on T2-weighted images, and signal suppression on fat-saturated images. In addition, lipomas do not demonstrate any enhancement after contrast material administration, including delayed enhanced sequences [6]. Lipomatous hypertrophy shows adipose tissue-specific features on CT and MRI, whereas lipomatous hypertrophy is not encapsulated or homogeneous. Although there is a case report of lipomatous hypertrophy of the interventricular septum that showed late gadolinium enhancement in the affected area, there is a lack of data on the findings of gadolinium enhancement in lipomatous hypertrophy [7]. Therefore, our case also offers valuable data about late gadolinium enhancement allowing differentiation between lipoma and lipomatous hypertrophy. The use of positron emission tomography with fluorodeoxyglucose (FDG) is reported due to assessing malignancy and the extent of FDG uptake by tumors is useful for differentiation between benign and malignant cardiac tumors. Lipomatous hypertrophy, unlike lipoma, has been reported to increase FDG uptake because of the presence of fetal brown fat [8]. It may be confusing that it could be a malignant tumor.
Table 1.
Typical imaging characteristics and findings of cardiac adipose tumor on CT and cardiac MRI.
| Tumor | Most common location | Enhanced CT | Imaging sequence of cardiac MRI |
FDG-PET | |||
|---|---|---|---|---|---|---|---|
| T1-Weighted | T2-weighted | Fat saturation | Delayed enhancement | ||||
| Benign tumor | |||||||
| Lipomatous Hypertrophy | Interatrial septum | None | High | High | Signal dropout | – | Increased uptake |
| Lipoma | Any chamber | None | High | High | Signal dropout | None | No uptake |
| Hemangioma | Any chamber | Strong enhancement | Isointense (heterogeneous) |
High | No change | None | No uptake |
| Malignant tumor | |||||||
| Liposarcoma | Left or right atrium | Mild enhancement | Isointense (heterogeneous) |
– | Signal dropout | – | Increased uptake |
CT, computed tomography; FDG-PET, fluorodeoxyglucose positron emission tomography; MRI, magnetic resonance imaging.
There are no guidelines for the treatment of cardiac lipoma or lipomatous hypertrophy. Surgical resection is selected for symptomatic patients depending on outflow tract obstruction, heart failure due to tumor compression, or severe arrhythmia. In general, it is more difficult to excise lipomatous hypertrophy than cardiac lipoma. Pacemaker placement is considered for the treatment of AV conduction disorder in typical lipomatous hypertrophy of the interatrial septum [4]. However, it is unclear whether similar management is appropriate for lipomatous hypertrophy of the interventricular septum. For other primary cardiac tumors, resection does not resolve the AV block. A patient with cardiac fibroma presenting with an AV block required permanent pacemaker implantation despite resection [9]. In some cases of cystic tumor of the AV node, also known as mesothelioma of the AV node, partial excision was attempted to avoid injury to the conduction system and close follow-up was needed to detect AV node dysfunction [10].
In our patient, echocardiography showed an echogenic mass, and CT and MRI findings were compatible with lipomatous hypertrophy of the interventricular septum. Lipomatous hypertrophy is a benign condition associated with a long-term prognosis; however, it is associated with a risk of sudden death. Our patient presented with a paroxysmal complete AV block; however, triplet ventricular tachycardia was documented only once. Except for syncope due to the paroxysmal complete AV block, our patient had no outflow obstruction or heart failure; therefore, no symptoms were observed. There was no confirmation of whether resection would be an effective option to improve the AV block and avoid pacemaker implantation. Thus, we decided to perform permanent pacemaker implantation, not implantable cardioverter defibrillator instead and continue close follow-up.
The presence of an echogenic mass or increased thickness of the interventricular septum might be secondary to lipoma, lipomatous hypertrophy, or other cardiac tumors. Therefore, it is important to perform multimodality cardiac imaging to enable a tissue-specific diagnosis and the detection of life-threatening arrhythmias. Unless surgical resection is definitely necessary and effective, careful follow-up with a pacemaker is a good treatment strategy.
Declaration of competing interest
The authors declare that there is no conflict of interest.
References
- 1.Heyer C.M., Kagel T., Lemburg S.P., Bauer T.T., Nicolas V. Lipomatous hypertrophy of the interatrial septum: a prospective study of incidence, imaging findings, and clinical symptoms. Chest. 2003;124:2068–2073. doi: 10.1378/chest.124.6.2068. [DOI] [PubMed] [Google Scholar]
- 2.Rocha R.V., Butany J., Cusimano R.J. Adipose tumors of the heart. J Card Surg. 2018;33:432–437. doi: 10.1111/jocs.13763. [DOI] [PubMed] [Google Scholar]
- 3.Vaidya Y.P., Green G.R. Lipomatous hypertrophy of the interventricular septum. J Card Surg. 2020;35:1740–1742. doi: 10.1111/jocs.14681. [DOI] [PubMed] [Google Scholar]
- 4.Jayaprakash S. Clinical presentations, diagnosis, and management of arrhythmias associated with cardiac tumors. J Arrhythm. 2018;34:384–393. doi: 10.1002/joa3.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tyebally S., Chen D., Bhattacharyya S., Mughrabi A., Hussain Z., Manisty C., Westwood M., Ghosh A.K., Guha A. Cardiac tumors: JACC CardioOncology state-of-the-art review. JACC CardioOncol. 2020;2:293–311. doi: 10.1016/j.jaccao.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Donnell D.H., Abbara S., Chaithiraphan V., Yared K., Killeen R.P., Cury R.C., Dodd J.D. Cardiac tumors: optimal cardiac MR sequences and spectrum of imaging appearances. AJR Am J Roentgenol. 2009;193:377–387. doi: 10.2214/AJR.08.1895. [DOI] [PubMed] [Google Scholar]
- 7.Stephant E., Barthelet M., Leroux P.Y., Revel D. Images in cardiovascular medicine. Lipomatous hypertrophy of the interventricular septum: echocardiography, cardiac magnetic resonance, and multidetector computerized tomography imaging. Circulation. 2008;118:e71–e72. doi: 10.1161/CIRCULATIONAHA.107.746529. [DOI] [PubMed] [Google Scholar]
- 8.Fan C.M., Fischman A.J., Kwek B.H., Abbara S., Aquino S.L. Lipomatous hypertrophy of the interatrial septum: increased uptake on FDG PET. AJR Am J Roentgenol. 2005;184:339–342. doi: 10.2214/ajr.184.1.01840339. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y., Sun J., Chen W., Peng Y., An Q. Third-degree atrioventricular block in an adult with a giant cardiac fibroma. Circulation. 2013;127:e522–e524. doi: 10.1161/CIRCULATIONAHA.112.131417. [DOI] [PubMed] [Google Scholar]
- 10.Luc J.G.Y., Phan K., Tchantchaleishvili V. Cystic tumor of the atrioventricular node: a review of the literature. J Thorac Dis. 2017;9:3313–3318. doi: 10.21037/jtd.2017.08.101. [DOI] [PMC free article] [PubMed] [Google Scholar]