Abstract
Killing of gram-positive bacteria by mammalian group IIA phospholipases A2 (PLA2) requires the catalytic activity of the enzyme. However, nearly complete degradation of the phospholipids can occur with little effect on bacterial viability, suggesting that PLA2-treated bacteria can biosynthetically replace phospholipids that are lost due to PLA2 action. In the presence of albumin, phospholipid degradation products are quantitatively sequestered extracellularly. In the absence of albumin, the bacteria retain and substantially reutilize the phospholipid breakdown products and survive an otherwise lethal dose of PLA2. PLA2-treated bacteria also continue to incorporate sodium [2-14C]acetate into phospholipids, suggesting that the bacteria are attempting to repair the damaged membranes by de novo synthesis of phospholipids. To determine whether PLA2 action also triggers activation of bacterial lipolytic enzymes, the effects of nisin and PLA2 on the degradation of S. aureus lipids were compared. In contrast to nisin treatment, PLA2 treatment does not stimulate endogenous phospholipase activity in S. aureus. These findings show that S. aureus responds to PLA2 attack by continued phospholipid (re)synthesis by both de novo and salvage pathways. The fate of PLA2-treated S. aureus therefore appears to depend on the relative rates of phospholipid degradation and synthesis.
Diverse defense mechanisms have been developed by multicellular organisms to deal with invasion and subsequent infection by microbes. The inflammatory reaction elicited in response to microbial invasion is composed of both cellular (8, 12, 15, 19) and extracellular (33) antimicrobial components. Previous work in this and other laboratories showed that a 14-kDa secretory group IIA phospholipase A2 (PLA2) is largely responsible for the potent antimicrobial activity found against many species of gram-positive bacteria, including Staphylococcus aureus, in acute inflammatory fluids (33), the plasma of animals with septicemia (31), and tears (25). This PLA2 exerts its antibacterial effects by first binding to the bacterial cell wall, traversing the thick peptidoglycan layer, and rapidly hydrolyzing the membrane phospholipids (32). While PLA2-mediated degradation of bacterial phospholipids is required for its bactericidal action, previous results with an autolysis-deficient strain of S. aureus, Lyt−, showed that degradation alone is not sufficient for bacterial killing (10).
During normal growth, the membrane phospholipids of S. aureus are actively turned over. Phosphatidylglycerol (PG), the major phospholipid of S. aureus (14, 28), is degraded and resynthesized up to three times during one bacterial doubling (9). PG is degraded by a PLC-like action to form diacylglycerol and glycerophosphate; the latter is used in the synthesis of lipoteichoic acid (9). A majority of the diacylglycerol (∼85%) is rephosphorylated to generate phosphatidic acid (9). Phosphatidic acid is then reconverted to PG by the addition of glycerol. In addition, a small fraction of PG (15 to 20%) is converted to the amino acid-substituted form, lysyl-PG (13). Efficient recycling of phospholipids by S. aureus may indicate that these bacteria are well equipped to tolerate a substantial amount of PLA2-mediated phospholipid degradation.
Based on the increased resistance of the Lyt− strain of S. aureus to PLA2-mediated killing, coupled with the efficient recycling of S. aureus phospholipids during growth, we hypothesized that under certain conditions (e.g., low dose of PLA2), S. aureus may be able to tolerate PLA2-induced membrane damage by (re)synthesis of membrane phospholipids. In this study we examined the ability of S. aureus to repair the membrane damage caused by PLA2, as well as the contribution of bacterial phospholipolytic enzymes to PLA2-mediated phospholipid degradation. Our findings show that S. aureus continues to synthesize phospholipids during PLA2 treatment by the de novo pathway and, in the absence of albumin, also by utilizing the lyso-PG generated by PLA2 to resynthesize PG. We also show that the action of the exogenous PLA2 does not activate bacterial phospholipases.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study included S. aureus RN450 (8325-4) (24), a laboratory strain provided by B. Kreiswirth (Public Health Research Institute, New York, N.Y.) and S. aureus Lyt−, an isogenic mutant of strain RN450 deficient in the two major autolysins of S. aureus (20), provided by R. K. Jayaswal (Illinois State University, Normal, Ill.). The bacteria were grown overnight at 37°C, washed once in sterile physiological saline (Baxter Healthcare Corp., Deerfield, Ill.), and then subcultured for 2.5 to 3 h in fresh tryptic soy broth (TSB; Difco Laboratories, Detroit, Mich.) at a starting concentration of 1.5 × 107 bacteria/ml. After being harvested, the bacteria were sedimented by centrifugation at 3,000 × g for 5 min and resuspended in sterile physiological saline (Baxter Healthcare Corp.) to a concentration of 109 bacterial/ml. The bacteria were used within 30 min of harvesting.
Purification of rabbit AF PLA2.
Rabbit group IIA PLA2 was purified from ascitic fluid (AF) by ion exchange chromatography and reversed-phase high-pressure liquid chromatography as previously described (11, 32, 35).
Radiolabeling of S. aureus lipids during growth.
The lipids of mid-log-phase S. aureus were radiolabeled during subculture in medium supplemented with 1 μCi of [1-14C]oleic acid (NEN Life Sciences, Boston, Mass.) per ml and 0.1% bovine serum albumin (BSA) as previously described (32). Bacteria were harvested by centrifugation and were incubated in medium without [1-14C]oleate at 37°C for 20 min to chase cell-associated, unesterified precursor fatty acid into ester positions and washed as described previously (32). A sample of 106 S. aureus cells contains ∼3,000 cpm incorporated into bacterial lipids, approximately 70 to 80% of which are phospholipids (mainly PG) and are susceptible to PLA2 attack (32).
S. aureus RN450 was subcultured for 4 h in tryptic soy broth supplemented with 10 μCi [U-14C]lysine (NEN Life Sciences) per ml and 0.1% BSA to specifically label lysyl-PG. After incubation at 37°C for 20 min in medium without [U-14C]lysine, the lipids were extracted under acidified conditions (13). The chloroform phase, which contained all the labeled lysyl-PG, was stored at 4°C. Approximately 1% of the added [U-14C]lysine was associated with the phospholipids.
Assay of phospholipid degradation.
We have previously established that 1% albumin added to the incubation mixture is sufficient to complex and sequester in the extracellular medium all phospholipid breakdown products generated by PLA2 action (7, 10, 32). This addition of albumin was used, therefore, as the means of measuring phospholipid degradation products (free fatty acids and lysophospholipids) formed during PLA2 treatment of S. aureus, by simply counting albumin-complexed radiolabeled products in the supernatant after sedimentation of the bacteria, including remaining intact phospholipids (11,000 × g for 4 min) (7, 10). To confirm that the released material corresponds to phospholipid degradation, the lipids were extracted and resolved by thin-layer chromatography (TLC) as previously described (32).
Assays of bactericidal activity.
Bactericidal activity was measured as the effect of purified rabbit AF group IIA PLA2 on bacterial colony-forming ability. Typical incubation mixtures contained 107 bacteria/ml in RPMI 1640 (Biowhittaker, Walkersville, Md.) supplemented with 10 mM HEPES (pH 7.4), 1 mM calcium chloride, and either 0, 0.3, or 1% (wt/vol) BSA. Incubations were carried out at 37°C for up to 3 h. After incubation, aliquots of bacterial suspensions were serially diluted in sterile physiological saline and plated in 5 ml of molten (50°C) tryptic soy agar (Difco). Bacterial viability was measured after 18 to 24 h of incubation at 37°C.
Extraction of bacterial (phospho)lipids.
The lysyl moiety of PG is labile and dissociates from PG during phospholipid extraction using the Bligh and Dyer method (2). To recover lysyl-PG in the extracted phospholipids, the phospholipids of S. aureus were extracted under acidified conditions as described by Gould and Lennarz with slight modifications (13). Briefly, S. aureus suspended in RPMI 1640 or saline was extracted with 20 sample volumes of chloroform-methanol (2:1, vol/vol) and 0.13 sample volume of 2 N HCl. The sample was vortexed to create a single phase and incubated for 90 mins at 37°C with gentle agitation or overnight at room temperature. After addition of acidified physiological saline, pH 2.0 (8.3 sample volumes), the sample was vortexed for 30 s and briefly centrifuged (70 × g for 5 min) to facilitate separation of the phases. The aqueous phase was removed, and the remaining chloroform phase was washed by addition of methanol (6.6 original sample volumes) plus acidified saline (8.3 sample volumes) to the chloroform. The wash and spin were repeated, and the aqueous phases were pooled. Small aliquots of each phase were used to measure the counts partitioning into each by liquid scintillation counting. Virtually all the PG, lysyl-PG, and lyso-PG partitioned into the chloroform phase.
TLC analysis of bacterial (phospho)lipids.
To identify the degradation products formed during treatment of [1-14C]oleate-labeled S. aureus with PLA2 or the lantibiotic nisin (1) (Sigma Chemical Co., St. Louis, Mo.), the phospholipids were resolved by TLC. The chloroform phase containing the extracted phospholipids was dried under nitrogen (N-Evap; Organomation, South Berlin, Mass.) and resuspended in 30 μl of chloroform-methanol (2:1, vol/vol). The samples were applied to TLC plates (Analtech, Newark, Del.) in small aliquots. The sample tubes were washed with 10 μl of chloroform-methanol to ensure full application of the samples. All samples were run with the appropriate standards to aid in the identification of the lipid species, using one of the following solvent systems: (i) chloroform-methanol-H2O-glacial acetic acid (65:25:4:1, by volume) (To separate PG, lysyl-PG, lyso-PG, and phosphatidic acid, Silica Gel G plates [Analtech] were developed for 90 to 100 min in this solvent; to further separate lysyl-PG from the degradation product lyso-PG, the plates were dried and developed a second time.) or (ii) petroleum ether-diethyl ether-glacial acetic acid (70:30:1, by volume) (To separate diacylglycerols from free fatty acids, which can run together in the solvent system described above, Silica Gel G plates were developed for 45 to 50 min in this solvent; the free fatty acids run at the solvent front, the diacylglycerols run in two bands approximately halfway up the plate, and the intact phospholipids and lyso compounds remain at the origin.).
Quantitation of radiolabeled lipids resolved by TLC.
After development, radiolabeled species on the TLC plates were visualized using an Ambis 1000 radioanalytic imager (Ambis Inc., San Diego, Calif.). The radioactivity associated with each (phospho)lipid species was determined using the Ambis quantitation program and expressed as the percent counts per minute in each spot relative to the total counts per minute in the sample lane.
Measurements of phospholipid synthesis.
To assay phospholipid synthesis by S. aureus, the bacteria were incubated at 37°C, with shaking, alone or with a low dose of PLA2 in RPMI 1640 supplemented with 10 mM HEPES (pH 7.4), 03.% BSA, and 5 μCi of sodium [2-14C] acetate (NEN) per ml. At various times, samples were plated for viability and the lipids were extracted under acidified conditions. A portion of the chloroform phase was dried under nitrogen and counted using a liquid scintillation counter. The radiolabeled lipids recovered in the chloroform phase were further defined by TLC analysis as described above.
To measure the ability of S. aureus to use lyso-PG for the resynthesis of PG, [1-14C]oleate-labeled S. aureus was incubated at 37°C, with shaking, with sublethal doses of PLA2 in RPMI 1640 with or without 1% BSA and 10 mM HEPES (pH 7.4). At the indicated times, the lipids of the samples were extracted as described above and the lipids in the chloroform phase were resolved by TLC.
RESULTS
Nearly complete membrane degradation can occur with minimal effects on bacterial viability.
In preliminary experiments, the dose response of S. aureus RN450 Lyt+ and Lyt− to purified rabbit AF group IIA PLA2 was examined in RPMI 1640 incubated with increasing concentrations of albumin (data not shown). A concentration of PLA2 and albumin was selected for each strain that caused maximum degradation of prelabeled bacterial phospholipids with minimal effects on bacterial viability (Fig. 1). A threefold-higher dose of PLA2 could be used against the Lyt− strain because that strain tolerates more PLA2-triggered phospholipid degradation (10).
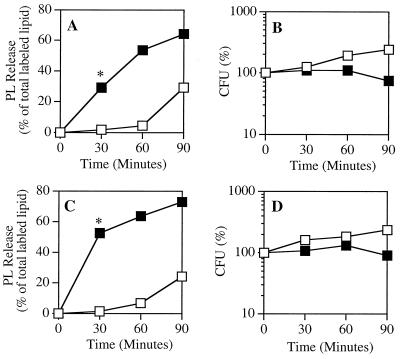
FIG. 1.
Viability and phospholipid hydrolysis of S. aureus treated with a sublethal dose of PLA2. [1-14C]oleate-labeled S. aureus RN450 Lyt+ (A and B) and Lyt− (C and D) were treated with 50 ng (Lyt+) or 150 ng (Lyt−) of PLA2 per ml in RPMI- 1640 supplemented with 0.3% BSA, 10 mM HEPES (pH 7.4), and 1 mM CaCl2. At the indicated times, samples were removed to measure phospholipid degradation (A and C) and viability (B and D), as described in Materials and Methods. The results represent the mean and standard error of the mean of three or more independent determinations (∗, P ≤ 0.01 by Student's t test). The error bars are masked by the symbols. Open squares, bacteria alone; solid squares, bacteria plus PLA2.
At later time points, chloroform-soluble counts were released into the supernatant of the bacteria incubated without PLA2 (Fig. 1A and B). The supernatants of these samples were extracted, and the (phospho)lipid species were resolved by TLC. PG was the dominant lipid, and only minor amounts of other species were found. No lyso-PG was detected (data not shown). The extracellular release of these (phospho)lipid products presumably reflects shedding of envelope constituents during normal bacterial growth and division. In contrast, essentially all radiolabeled species released into the supernatant of PLA2-treated bacteria were phospholipid breakdown products (e.g., lyso-PG).
Membrane repair in S. aureus: de novo synthesis.
The survival of S. aureus for at least 60 min despite the nearly quantitative degradation of the prelabeled phospholipids suggested that these strains of S. aureus can compensate for the PLA2-mediated membrane damage by performing continued synthesis of phospholipids. To test this hypothesis, we compared the incorporation of sodium [2-14C]acetate by PLA2-treated and untreated bacteria. Acetate incorporation was used as a measure of de novo phospholipid synthesis (for reviews, see references ;[9] and ;[23]).
The majority of the acetate was incorporated during the first 30 min. After that time, control bacteria incorporated acetate into chloroform-soluble material at a rate roughly reflecting the rate of growth (doubling time, 60 to 75 min) (Fig. 2). During the first 30 min, [14C]acetate incorporation by both the PLA2-treated Lyt+ and Lyt− bacteria was the same as or greater than that by the control bacteria (Fig. 2). At later time points, little further accumulation of chloroform-soluble counts occurred in the PLA2-treated, growth-arrested Lyt− strain. The chloroform-soluble counts in the PLA2-treated Lyt+ strain reproducibly decreased. This may be attributable to incomplete recovery of bacterial membrane lipids, since the Lyt+ bacteria underwent lysis during longer incubation with PLA2 (10).
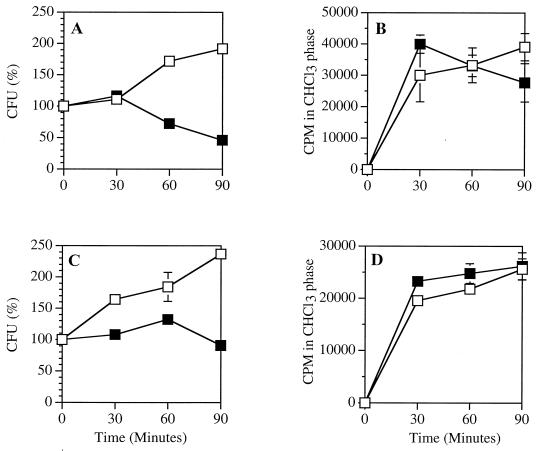
FIG. 2.
Effect of PLA2 treatment on acetate incorporation by S. aureus. S. aureus RN450 Lyt+ (A and B) and Lyt− (C and D) (107/ml) were incubated in RPMI 1640 supplemented with 0.3% BSA, 10 mM HEPES (pH 7.4), 1 mM CaCl2, and sodium [2-14C] acetate (5 μCi/ml) with or without 50 ng (Lyt+) or 150 ng (Lyt−) of PLA2 per ml. At the indicated times, samples were removed to measure viability (A and C) and the lipids were extracted to measure sodium [2-14C]acetate incorporation into chloroform-soluble material (B and D), as described in Materials and Methods. The results represent the mean and standard error of the mean of three independent determinations. Some error bars are masked by the symbols. Open squares, control; solid squares, PLA2.
To confirm that the [2-14C]acetate incorporated by the bacteria was used for phospholipid synthesis, a portion of the extracted, radiolabeled material recovered in the chloroform phase was resolved by TLC (data not shown). In control (no PLA2) bacteria, the acetate was incorporated into PG, as well as the various intermediates of normal phospholipid turnover during cell growth (data not shown). In the PLA2-treated bacteria, a radiolabeled spot migrating with lyso-PG also appeared, indicating the continued degradation of the newly synthesized phospholipids by PLA2.
Salvage pathway.
The above results showed that synthesis of membrane phospholipids continued or even increased via the de novo pathway during PLA2 treatment of S. aureus. This is the only pathway for phospholipase synthesis in S. aureus documented in the literature. The pathway for membrane phospholipid synthesis is considered to be similar in most species of bacteria (17). Other species of bacteria (e.g., Escherichia coli) also possess a salvage pathway for phospholipid synthesis. In the salvage pathway, lyso compounds are either directly reacylated or further degraded by endogenous (bacterial) enzymes before reutilization (5). Because PLA2 treatment of [1-14C]oleate-labeled S. aureus generates a large quantity of radiolabeled lyso-PG, these conditions provided a convenient setting to determine if a salvage pathway for phospholipid synthesis exists in S. aureus.
Albumin (1%), added to the incubation medium, quantitatively complexes and sequesters the phospholipid degradation products lyso-PG and free fatty acid from the bacterial membrane in the supernatant (7, 10), thus preventing their reutilization for phospholipid synthesis. To determine if S. aureus could reutilize lyso-PG and if this limited the bactericidal effects of PLA2 treatment, S. aureus RN450 Lyt+ and Lyt− were treated with PLA2 in the presence and absence of albumin.
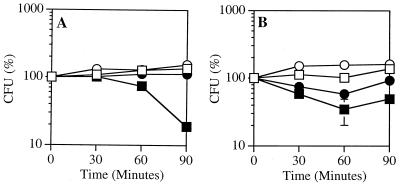
Under these conditions, killing of S. aureus Lyt+ by PLA2 depended on the presence of albumin in the assay medium. In the absence of albumin, bacterial growth was arrested but the bacteria were not killed (Fig. 3A). Differences in the viability of S. aureus Lyt− treated with PLA2 with and without albumin were less dramatic, since PLA2-triggered killing was limited even in the presence of albumin (Fig. 3B). These results suggest that the presence of albumin enhances PLA2-mediated killing of S. aureus by blocking reutilization of phospholipid breakdown products for resynthesis of phospholipids.
FIG. 3.
S. aureus is less susceptible to PLA2-mediated killing in albumin-free medium. [1-14C]oleate-labeled S. aureus RN450 Lyt+ (A) and Lyt− (B) were incubated in RPMI 1640 supplemented with 10 mM HEPES (pH 7.4), and 1 mM CaCl2, with or without 1% BSA and 50 ng (Lyt+) or 150 ng (Lyt−) of PLA2 per ml. At the indicated times, aliquots of the samples were removed to measure viability, as described in Materials and Methods. The results represent the mean and standard error of the mean of three independent determinations (at the 90-min time point, P ≤ 0.01 by Student's t test). Some error bars are masked by the symbols. Open squares, bacteria plus BSA; open circles, bacteria with no BSA; solid squares, Bacteria plus BSA plus PLA2; solid circles, bacteria with no BSA plus PLA2.
To further test this hypothesis, the amounts of radiolabeled PG and lyso-PG in Lyt+ and Lyt− S. aureus were measured during the course of PLA2 treatment with and without albumin in the assay medium (Table 1). In the Lyt+ strain, in the presence of albumin, the nearly complete loss of prelabeled PG during the 90-min incubation was matched by a corresponding accumulation of labeled lyso-PG. The accumulation of labeled lyso-PG in the first 30 min was nearly the same in albumin-free medium as in the presence of albumin, but it did not increase thereafter, while labeled PG levels declined only moderately. Similar effects of albumin were seen in the Lyt− strain, except that the higher dose of PLA2 caused more rapid and extensive phospholipid degradation. These findings strongly suggest that in the absence of albumin, the bacteria efficiently reutilize lyso-PG to resynthesize PG, thereby blunting the antibacterial effects of PLA2.
TABLE 1.
Percentage of radiolabeled PG and lyso-PG in S. aureus treated with PLA2 in medium with and without albumin
| Strain | Time (min) | % of PG and LPG in S. aureus witha:
|
|||
|---|---|---|---|---|---|
| 1% BSA
|
No BSA
|
||||
| PG | LPG | PG | LPG | ||
| Lyt+ | 0 | 68.4 ± 3.2 | 1.9 ± 0.8 | 74.5 ± 7.3 | 0 ± 0 |
| 30 | 55.4 ± 3.1 | 14.9 ± 2.5 | 64.3 ± 4.6 | 15.7 ± 1.9 | |
| 60 | 20.6 ± 1.4 | 51.9 ± 2.9 | 58.9 ± 4.1 | 16.7 ± 4.6 | |
| 90 | 2.9 ± 0.3 | 68.3 ± 0.7 | 48.6 ± 6.5 | 14.7 ± 1.8 | |
| Lyt− | 0 | 79.8 ± 3.6 | 8.8 ± 1.5 | 65.2 ± 5.7 | 7.8 ± 1.5 |
| 30 | 16.2 ± 0.4 | 57.2 ± 3.8 | 36.9 ± 0.9 | 29.9 ± 2.7 | |
| 60 | 10.9 ± 0.7 | 62.2 ± 6.4 | 32.3 ± 2.7 | 26.9 ± 1.5 | |
| 90 | 3.8 ± 1.5 | 63.6 ± 4.6 | 32.7 ± 6.9 | 23.8 ± 3.3 | |
Values represent the mean ± standard error of the mean (three independent samples) of [1-14C]oleate-labeled PG and lyso-PG in chloroform-soluble counts resolved by TLC after incubation of Lyt+ (50 ng/ml) or Lyt− (150 ng/ml) (107/ml) with PLA2 in RPMI 1640 supplemented with 10 mM HEPES (pH 7.4) and 1 mM CaCl2 with and without BSA.
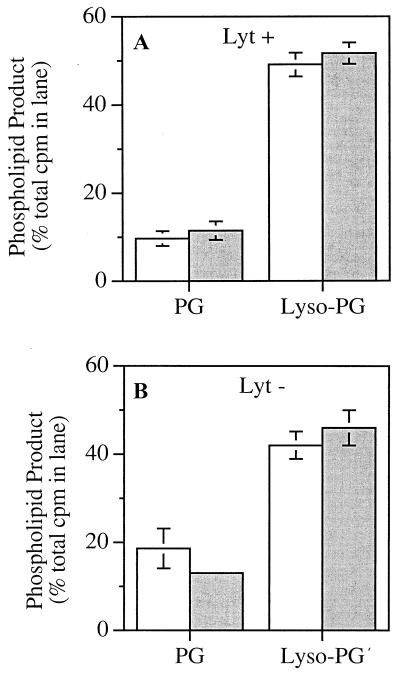
Because the phospholipid degradation products are less efficiently removed from the site of PLA2 action in the absence of albumin, we considered the possibility that the catalytic activity of PLA2 in albumin-free medium was subject to product inhibition. To determine if the reduced accumulation of lyso-PG was due to decreased catalytic activity of PLA2, the phospholipids of [1-14C]oleate-labeled S. aureus were extracted and treated in the presence and absence of albumin with a dose of PLA2 comparable to that used against intact bacteria (10). In contrast to the effects on the intact bacteria (Table 1), degradation of radiolabeled PG and a corresponding accumulation of radiolabeled lyso-PG during PLA2 treatment were essentially the same in the presence and absence of albumin (Fig. 4). Thus, we conclude that the decreased accumulation of lyso-PG in bacteria treated with PLA2 in the absence of albumin reflects the ability of the PLA2-treated bacteria to reutilize their phospholipid breakdown products for resynthesis of phospholipids.
FIG. 4.
PLA2 degradation of extracted S. aureus phospholipids in the presence and absence of BSA. [1-14C]oleate-labeled phospholipids extracted from S. aureus RN450 Lyt+ (A) and Lyt− (B) (4 × 107/ml) were resuspended by sonication in RPMI 1640 supplemented with 10 mM HEPES (pH 7.4) and 1 mM CaCl2 and incubated at 37°C for 60 min with 300 ng of PLA2 per ml with and without 1% BSA. (The higher dose of PLA2 matches the increase in phospholipid substrate extracted from 4 × 107 bacteria/ml compared to 1 × 107 bacteria/ml used in experiments with intact bacteria.) The samples were reextracted and resolved by TLC as described in Materials and Methods. The results represent the mean and standard error of the mean of three experiments. Open bars, 1% albumin; shaded bars, no albumin.
Activation of bacterial phospholipolytic enzymes during PLA2 treatment.
Bacterial killing by PLA2 requires extensive bacterial phospholipid degradation and catalytically active enzyme (32). However, it is possible, as in other species of bacteria (27, 34, 35), that membrane perturbation caused by PLA2 treatment includes activation of endogenous (bacterial) enzymes, including phospholipases, that might aid PLA2 in the destruction of the bacterial membrane. To explore this possibility, we made use of a membrane-active lantibiotic, nisin, which has no endogenous phospholipase activity (1), to compare the products of phospholipid degradation produced by a nonphospholipolytic agent (nisin) and PLA2.
During nisin treatment, there is an initial accumulation of diglycerides (Fig. 5A) followed by a later accumulation of phosphatidic acid and free fatty acids. The appearance of diglycerides is consistent with activation of a PLC-like activity, as well as a deacylating activity that generates free fatty acid. The late accumulation of phosphatidic acid may reflect the phosphorylation of the accumulated diglycerides or possibly activation of a PLD-like activity. Alternatively, these products would also be formed if nisin blocked the normally efficient recycling of PG, resulting in accumulation of diglycerides and phosphatidic acid.
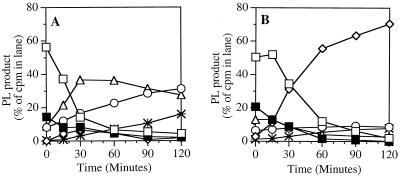
FIG. 5.
(Phospho)lipid degradation products produced during nisin or PLA2 treatment of S. aureus. [1-14C]oleate-labeled S. aureus RN450 (Lyt+) (108/ml) was incubated at 37°C in RPMI 1640 supplemented with 1% BSA, 10 mM HEPES (pH 7.4), and 1 mM CaCl2 with nisin (10 μg/ml) (A) or PLA2 (500 ng/ml) (B). At the indicated times, samples were extracted and the phospholipid species were resolved by TLC, as described in Materials and Methods. The results represent the mean of two experiments. Open squares, PG; solid squares, lysyl-PG; open circles, phosphatidic acid; open diamonds, lyso-PG; open triangles, diglycerides; stars, free fatty acids.
The products formed during PLA2 treatment are very different (Fig. 5B). PG is rapidly degraded, and there is a corresponding accumulation of labeled lyso-PG. Both the nonsubstituted and the lysyl-substituted form of PG are degraded during PLA2 treatment. There is no increase in diglycerides or phosphatidic acid, indicating that PLA2 does not trigger a PLC-like or PLD-like activity. Taken together, these results indicate that bacterial PLC or PLD enzymes do not contribute to PLA2-mediated phospholipid degradation.
DISCUSSION
It has been shown previously that killing of S. aureus by mammalian group IIA PLA2 is accompanied by degradation of nearly all bacterial phospholipids (10, 32). It would be expected that such extensive membrane breakdown would have catastrophic effects on cell integrity and viability. However, an S. aureus strain deficient in autolysins (Lyt−) largely survives PLA2 treatment (10), suggesting that phospholipid degradation is not necessarily coupled to irreversible bacterial damage in the absence of most autolysins (10). Similarly, S. aureus Lyt+ treated with a low dose of PLA2 can survive nearly complete phospholipid degradation (Fig. 1A and B). Thus, the ability of these strains to survive attack by PLA2 implies that the bacteria can replenish the degraded phospholipids by synthesis of new phospholipids, reducing the net loss of membrane phospholipids.
The results of this study show that the response of S. aureus to low PLA2 doses included de novo synthesis of phospholipids (Fig. 2). In both the Lyt+ and Lyt− strains, the control and PLA2-treated bacteria incorporated a comparable amount of acetate into the phospholipids, despite the lack of growth by the PLA2-treated bacteria. This is consistent with the view that the loss of membrane phospholipids caused by PLA2 leads to continued de novo phospholipid synthesis. In the absence of new membrane synthesis associated with bacterial growth, it is likely that the acetate incorporation by PLA2-treated bacteria reflects an attempt to repair the PLA2-mediated membrane damage by replacing the lost phospholipids.
In addition, S. aureus can replenish phospholipids by reacylating lyso-PG (Table 1). This mechanism for reusing lyso-PG has not been described previously in S. aureus. Since lyso compounds (3, 16) and free fatty acids (6, 22, 29) are toxic for gram-positive bacteria, it is reasonable to hypothesize that S. aureus has a mechanism for reuse of lyso compounds and free fatty acids. In other bacteria (e.g., E. coli), there are two pathways by which lyso-PG is reused. The first involves direct reacylation of the lyso compound by an acyltransferase (4, 26, 30). In the second pathway, the lyso compound is further degraded by a lysophospholipase and the resulting breakdown products are used to synthesize new phospholipids (5). The exact biochemical mechanism for reutilization of lyso-PG by S. aureus remains to be defined.
The presence or absence of albumin in the incubation medium during PLA2 treatment of S. aureus has a profound effect on both the net degradation of bacterial phospholipids and survival. Thus, when albumin is present, the products of PLA2-mediated hydrolysis (free fatty acids and lysophospholipids) are quantitatively sequestered extracellularly (7, 32), preventing their reutilization for phospholipid synthesis and thereby maximizing net phospholipid loss, membrane damage, and bacterial killing. Apparently, under these conditions, continued de novo synthesis is insufficient to counteract the continued action of PLA2 (Table 1). In contrast, when albumin is omitted from the medium, the products of hydrolysis are retained by the cell and are available for resynthesis of phospholipids by reacylation of lyso-PG to PG (Table 1). Under these conditions, bacterial killing is markedly diminished, reflecting much reduced net phospholipolysis and consequent membrane damage.
It is unlikely that there are sites in the mammalian host that are completely free of albumin during a bacterial infection. Plasma proteins, including albumin, translocate from the circulation to the site of infection (18, 21). The concentration of albumin affects the ability of the bacteria to survive PLA2-mediated phospholipid degradation and killing. A dose of PLA2 that was lethal for S. aureus Lyt+ when incubated in medium containing 1% albumin (Fig. 3A) was not lethal for this same strain when incubated in medium containing 0.3% albumin (Fig. 1B). This suggests that there may be sites in the body where S. aureus is more protected from PLA2-mediated killing because of a limiting concentration of albumin and/or PLA2.
Albumin may have other effects on the antibacterial action of PLA2, including enhancing its catalytic activity by reducing product inhibition or by altering its interaction with the target bacteria. However, albumin had no effect on the catalytic efficiency of PLA2 toward extracted S. aureus phospholipids (Fig. 4). Although not measured directly, albumin does not appear to affect the initial binding of PLA2 to S. aureus, because phospholipid degradation during the first 30 min was the same with or without albumin in the medium (Table 1).
In conclusion, the accumulation of lysophospholipids during treatment of S. aureus with a mammalian inflammatory group IIA PLA2 has enabled us to demonstrate for the first time in S. aureus the operation of a salvage pathway that permitted efficient recycling of the degradation products of PLA2 action, providing these microorganisms with a biochemically inexpensive means of counteracting the membrane-damaging effects of the enzyme. The relative contributions of direct reacylation of lyso-PG, the further breakdown of lyso-PG, and stimulation of de novo synthesis of phospholipids by S. aureus in its defense against attack by host PLA2 remain to be determined.
ACKNOWLEDGMENTS
We thank Barry Kreiswirth and Radheshyam Jayaswal for providing the bacterial strains used in this study.
This work was supported by U.S. Public Health Service grant AI-18571 and a Ford Foundation Predoctoral Fellowship (to A.K.F.-W.).
REFERENCES
- 1.Bierbaum G, Sahl H G. Induction of autolysis of staphylococci by the basic peptide antibiotics Pep 5 and nisin and their influence on the activity of autolytic enzymes. Arch Microbiol. 1985;141:249–254. doi: 10.1007/BF00408067. [DOI] [PubMed] [Google Scholar]
- 2.Bligh E S, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 3.Coonrod J D, Yoneda K. Detection and partial characterization of antibacterial factor(s) in alveolar lining material of rats. J Clin Investig. 1983;71:129–141. doi: 10.1172/JCI110741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper C L, Hsu L, Jackowski S, Rock C O. 2-Acylglycerolphosphoethanolamine acyltransferase/acyl-acyl carrier protein synthetase is a membrane-associated acyl carrier protein binding protein. J Biol Chem. 1989;264:7384–7389. [PubMed] [Google Scholar]
- 5.Cronan J E, Rock C O. Biosynthesis of membrane lipids. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 2. Washington, D.C.: American Society for Microbiology; 1997. pp. 612–636. [Google Scholar]
- 6.Dye E S, Kapral F A. Characterization of a bactericidal lipid developing within staphylococcal abscesses. Infect Immun. 1981;32:98–104. doi: 10.1128/iai.32.1.98-104.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elsbach P, Weiss J. Utilization of labeled Escherichia coli as phospholipase substrate. Methods Enzymol. 1991;197:24–31. doi: 10.1016/0076-6879(91)97130-q. [DOI] [PubMed] [Google Scholar]
- 8.Elsbach P, Weiss J, Levy O. Oxygen-independent antimicrobial systems of phagocytes. In: Gallin J I, Snyderman R, editors. Inflammation: basic principles and clinical correlates. 3rd ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 1999. pp. 801–818. [Google Scholar]
- 9.Fischer W. Lipoteichoic acids and lipoglycans. In: Ghuysen J-M, Hackenbeck R, editors. Bacterial cell wall. Amsterdam, The Netherlands: Elsevier Science Publishing; 1994. pp. 199–215. [Google Scholar]
- 10.Foreman-Wykert A K, Weinrauch Y, Elsbach P, Weiss J. Cell-wall determinants of the bactericidal action of group IIA phospholipase A2 against gram-positive bacteria. J Clin Investig. 1999;103:715–721. doi: 10.1172/JCI5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forst S, Weiss J, Elsbach P, Maraganore J M, Reardon I, Heinrikson R L. Structural and functional properties of a phospholipase A2 purified from an inflammatory exudate. Biochemistry. 1986;25:8381–8385. doi: 10.1021/bi00374a008. [DOI] [PubMed] [Google Scholar]
- 12.Ganz T, Lehrer R I. Antimicrobial peptides of leukocytes. Curr Opin Hematol. 1997;4:53–58. doi: 10.1097/00062752-199704010-00009. [DOI] [PubMed] [Google Scholar]
- 13.Gould R M, Lennarz W J. Metabolism of phosphatidylglycerol and lysyl phosphatidylglycerol in Staphylococcus aureus. J Bacteriol. 1970;104:1135–1144. doi: 10.1128/jb.104.3.1135-1144.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanemasa Y, Yoshioka T, Hayashi H. Alteration of the phospholipid composition of Staphylococcus aureus cultured in medium containing NaCl. Biochim Biophys Acta. 1972;280:444–450. [PubMed] [Google Scholar]
- 15.Klebanoff S J. Oxygen metabolites from phagocytes. In: Gallin J I, Snyderman R, editors. Inflammation: basic principles and clinical correlates. 3rd ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 1999. pp. 721–768. [Google Scholar]
- 16.Kondo E, Kanai K. Mechanism of bactericidal activity of lysolecithin and its biological implication. Jpn J Med Sci Biol. 1985;38:181–194. doi: 10.7883/yoken1952.38.181. [DOI] [PubMed] [Google Scholar]
- 17.Langley K E, Yaffe M P, Kennedy E P. Biosynthesis of phospholipids in Bacillus megaterium. J Bacteriol. 1979;140:996–1007. doi: 10.1128/jb.140.3.996-1007.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsson K, Tornling G, Gavhed D, Muller-Suur C, Palmberg L. Inhalation of cold air increases the number of inflammatory cells in the lungs in healthy subjects. Eur Respir J. 1998;12:825–830. doi: 10.1183/09031936.98.12040825. [DOI] [PubMed] [Google Scholar]
- 19.Levy O. Antibiotic proteins of polymorphonuclear leukocytes. Eur J Haematol. 1996;56:263–277. doi: 10.1111/j.1600-0609.1996.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 20.Mani N, Tobin P, Jayaswal R K. Isolation and characterization of autolysis-defective mutants of Staphylococcus aureus created by Tn917-lacZ mutagenesis. J Bacteriol. 1993;175:1493–1499. doi: 10.1128/jb.175.5.1493-1499.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meduri G U, Kohler G, Headley S, Tolley E, Stentz F, Postlethwaite A. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest. 1995;108:1303–1314. doi: 10.1378/chest.108.5.1303. [DOI] [PubMed] [Google Scholar]
- 22.Miller S J, Aly R, Shinefeld H R, Elias P M. In vitro and in vivo antistaphylococcal activity of human stratum corneum lipids. Arch Dermatol. 1988;124:209–215. [PubMed] [Google Scholar]
- 23.Navarre W W, Schneewind O. Surface proteins of gram-positive bacteria and mechanism of their targeting to the cell wall envelope. Microbiol Mol Biol Rev. 1999;63:174–229. doi: 10.1128/mmbr.63.1.174-229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967;33:155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- 25.Qu X D, Lehrer R I. Secretory phospholipase A2 is the principal bactericide for staphylococci and other gram-positive bacteria in human tears. Infect Immun. 1998;66:2791–2797. doi: 10.1128/iai.66.6.2791-2797.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ray T K, Cronan J., Jr Activation of long chain fatty acids with acyl carrier protein: demonstration of a new enzyme, acyl-acyl carrier protein synthetase, in Escherichia coli. Proc Natl Acad Sci USA. 1976;73:4374–4378. doi: 10.1073/pnas.73.12.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scandella C J, Kornberg A. A membrane-bound phospholipase A1 purified from Escherichia coli. Biochemistry. 1971;10:4447–4456. doi: 10.1021/bi00800a015. [DOI] [PubMed] [Google Scholar]
- 28.Short S A, White D C. Metabolism of phosphatidylglycerol, lysylphosphatidylglycerol, and cardiolipin of Staphylococcus aureus. J Bacteriol. 1971;108:219–226. doi: 10.1128/jb.108.1.219-226.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shryock T R, Kapral F A. The production of bactericidal fatty acids from glycerides in staphylococcal abscesses. J Med Microbiol. 1992;36:288–292. doi: 10.1099/00222615-36-4-288. [DOI] [PubMed] [Google Scholar]
- 30.Vos M M, op den Kamp J A, Beckerdite-Quagliata S, Elsbach P. Acylation of monoacylglycerophosphoethanolamine in the inner and outer membranes of the envelope of an Escherichia coli K12 strain and its phospholipase A-deficient mutant. Biochim Biophys Acta. 1978;508:165–173. doi: 10.1016/0005-2736(78)90198-0. [DOI] [PubMed] [Google Scholar]
- 31.Weinrauch Y, Abad C, Liang N-S, Lowry S F, Weiss J. Mobilization of potent plasma bactericidal activity during systemic bacterial challenge: role of group IIA phospholipiase A2. Infect Immun. 1998;102:633–638. doi: 10.1172/JCI3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinrauch Y, Elsbach P, Madsen L M, Foreman A, Weiss J. The potent anti-Staphylococcus aureus activity of a sterile rabbit inflammatory fluid is due to a 14-kD phospholipase A2. J Clin Investig. 1996;97:250–257. doi: 10.1172/JCI118399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinrauch Y, Foreman A, Shu C, Zarember K, Levy O, Elsbach P, Weiss J. Extracellular accumulation of potently microbicidal bactericidal/permeability-increasing protein and p15s in an evolving sterile rabbit peritoneal inflammatory exudate. J Clin Investig. 1995;95:1916–1924. doi: 10.1172/JCI117873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss J, Franson R C, Beckerdite S, Schmeidler K, Elsbach P. Partial characterization and purification of a rabbit granulocyte factor that increases permeability of Escherichia coli. J Clin Investig. 1975;55:33–42. doi: 10.1172/JCI107915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright G W, Ooi C E, Weiss J, Elsbach P. Purification of a cellular (granulocyte) and an extracellular (serum) phospholipase A2 that participate in the destruction of Escherichia coli in a rabbit inflammatory exudate. J Biol Chem. 1990;265:6675–6681. [PubMed] [Google Scholar]