Abstract
Global and regional trends of population aging spotlight major public health concerns. As one of the most common adverse prognostic factors, advanced age is associated with a remarkable incidence risk of many non-communicable diseases, affecting major organ systems of the human body. Age-dependent factors and molecular processes can change the nervous system’s normal function and lead to neurodegenerative disorders. Oxidative stress results from of a shift toward reactive oxygen species (ROS) production in the equilibrium between ROS generation and the antioxidant defense system. Oxidative stress and neuroinflammation caused by Amyloid-ß protein deposition in the human brain are the most likely pathogenesis of Alzheimer’s disease (AD). Walnut extracts could reduce Amyloid-ß fibrillation and aggregation, indicating their beneficial effects on memory and cognition. Walnut can also improve movement disabilities in Parkinson’s disease due to their antioxidant and neuroprotective effect by reducing ROS and nitric oxide (NO) generation and suppressing oxidative stress. It is noteworthy that Walnut compounds have potential antiproliferative effects on Glioblastoma (the most aggressive primary cerebral neoplasm). This effective therapeutic agent can stimulate apoptosis of glioma cells in response to oxidative stress, concurrent with preventing angiogenesis and migration of tumor cells, improving the quality of life and life expectancy of patients with glioblastoma. Antioxidant Phenolic compounds of the Walnut kernel could explain the significant anti-convulsion ability of Walnut to provide good prevention and treatment for epileptic seizures. Moreover, the anti-inflammatory effect of Walnut oil could be beneficial in treating multiple sclerosis. In this study, we review the pharmaceutical properties of Walnut in age-related neurological disorders.
Keywords: Walnut, Juglans spp., Age-related neurological disorders
Highlights
-
•
Walnut exerts neuroprotective effects in several neurological disorders.
-
•
Walnut defibrillates fibrillar amyloid beta protein with vitamin E, ellagitannins, and urothilins in Alzheimer’s disease.
-
•
Walnut works as an antioxidant and reduce the activation monoamine oxidase-B enzyme in Parkinson’s disease.
-
•
Fatty acids found in the nut can protect the cells against exogenous toxins thus the risk of neuroinflammation.
-
•
The effects on memory can be considered as the result of a rise in production and reducing the destruction of acetylcholine.
1. Introduction
Among the wide range of noncontagious diseases, diseases caused by neurological disorders account for a large share of mortality and morbidity (Feigin et al., 2020, Anon, 2022). We know that subclinical changes that occur at the molecular level can affect the aging process. These subclinical changes involve telomere attrition, accumulation of mutations, and epigenetic alterations, which result in genomic instability. These changes, like the snowball effect, increase over time and lead to adverse changes in the central nervous system (CNS) such as degeneration of brain function, progressive neuronal loss, decreased level of neurotransmitters, and impaired vascular integrity which leads to infarction and microbleeds. In addition, impaired performance of the deoxyribonucleic acid (DNA) repair process can increase the likelihood of spontaneous mutagenesis, eventuating in age-related neoplasia. Besides, many elderlies suffer from malnutrition, leading to vitamin B12 and folic acid deficiencies. The lack of these two essential elements leads to homocysteine metabolic disorders that ultimately cause damage to the vascular system. All the factors mentioned above can lead to multiplying the risk of getting conditions related to the CNS in older people, such as Alzheimer’s disease (AD), epilepsy, stroke, and Parkinson's disease (PD) (Kowalska et al., 2017). We should consider that not only is there no pharmaceutical product to prevent age-related neurological disorders (ANDs), but also there are not many drugs available to treat this disease (Bhullar and Rupasinghe, 2013), and these available AND drugs have only a symptomatic effect (Berg et al., 2013).
For the reasons mentioned above, we can also consider herbal medicines and natural products. Herbal medicines and natural products contain various chemical elements, which have evolved to fortify the survival of organisms (Brahmachari, 2013). So, it is better to consider natural products to prevent ANDs. English Walnut is one of these natural products rich in alpha-linolenic acid, polyphenolics, linoleic acid, and micronutrients. The results of laboratory research have shown us that a Walnut diet can play a role in improving memory and cognitive level (Willis et al., 2009, Willis et al., 2008). This study aims to gather all available information about the pharmacotherapeutic potential of Walnuts in ANDs.
2. Walnut and its active constituents
Walnut is the name called for the seed of the Juglans plant. Interestingly, it has 21 species which are distinguished by some chemical makers such as tetralones, naphthoquinones, and diarylheptanoids. The Walnut's fruit has four principal parts: kernel, skin, shell, and green husk. According to Jahanban-Esfahlan et al. study, the green husk part is a reach source of some bioactive chemicals such as antioxidants, antimicrobial compounds, and other constituents like chlorogenic acid, caffeic acid, ferulic acid, sinapic acid, gallic acid, ellagic acid, protocatechuic acid, syringic acid, vanillic acid, and Catechin (Jahanban-Esfahlan et al., 2019, Habibie et al., 2019, Chatrabnous et al., 2018a).
The kernel part contains high levels of unsaturated fatty acids, digestible proteins, phenolic compounds, and dietary fiber. Additionally, this part is found in both fresh and dried manner, but fresh ones have a higher quality than dried ones (Pakrah et al., 2022). One of the active substances of Walnut, which is found in various parts is Juglone (the chemical formula: 5-hydroxy-1,4-naphthoquinone). It can inhibit the key enzymes of metabolic reactions and has anti-cancer properties. Here is a list of chemical constituents of Walnut (especially the husk part's contents):
Hydrolyzable Tannins, Naphthoquinones, Naphthoquinone Glycosides, Naphthalenes, α-Tetralones, α-Tetralones Glycosides, and α-Tetralone Dimers, Hydroxybenzoic Acids, Hydroxycinnamic Acids, Flavonoids, Diarylheptanoids, Ceramides, Alkanes, Steroids, Triterpenoids, Sesquiterpenes, Neolignans and other compounds such as minerals (Jahanban-Esfahlan et al., 2019, Chatrabnous et al., 2018b, Pakrah et al., 2021). These chemicals cause antioxidant, antimicrobial, antifungal, anti-cancer, and antiplatelet properties in Walnut (Jahanban-Esfahlan et al., 2019, Jahanbani et al., 2016).
Walnut is an excellent option for a healthy diet because it is a rich source of vitamins (folic acid, vitamin B5, vitamin B6, tocopherols), minerals (phosphorus, manganese, magnesium, zinc, potassium), omega-3 fatty acids, and phytochemicals (Vu et al., 2020).
Concerning Vu DC et al. study, the antioxidant activity of Walnut is significantly related to phenolic compounds which exist in the Walnut. These phenolic compounds are a group of phenolic acids, flavonoids, stilbenes, coumarins, and tannins. In addition, htis group causes anti-bacterial properties in Walnut. For example, gallic acid, ellagic acid, ferulic acid, and naringin can inhibit bacteria from growing. This process is done by destroying their plasma membrane or inhibiting their DNA from being duplicated (Vu et al., 2020). In addition, some phenolic compounds such as gallic acid, quercetin, quercetin glycosides, 3-methylquercetin, and ellagitannins are linked to causing anti-inflammatory activities. It is suggested that these compounds can suppress some inflammatory cytokines (Vu et al., 2020).
Antidiabetic activity of Walnut is due to the significant amount of linoleic acid, which is an unsaturated fat that affects insulin sensitivity, that can impact glycemic control. Another positive feature of Walnut is its anti-cancer activity which is related to the ϒ-tocopherol compound (Vu et al., 2020). About 400 compounds are found in various parts of Walnut, but the most available compounds are quinones, phenolics, triterpenoids, and diarylheptanoids. These constituents cause pharmacological activities, such as anti-tumor, immunoregulatory, anti-inflammatory, neuroprotective, antidiabetic, anti-viral, antimicrobial, and anti-melanogenesis activities (Luan et al., 2021).
Due to Junaidh et al. study, Walnut has anti-histaminic, bronchodilator, anti-fertility, analgesic, immunomodulatory, hepatoprotective, anti-ulcer, lipolytic, anti-hypertensive, neuroprotective, and insecticidal properties (Mohammed Junaidh et al., 2022). Furthermore, it has been proved that some protein compounds of Walnut have inhibitory effects on angiotensin-converting enzyme (ACE). This enzyme converts angiotensin I to angiotensin II. Angiotensin II causes contraction in blood vessels which increases the blood pressure. Therefore, inhibition of ACE can help with the treatment of high blood pressure (Jahanbani et al., 2018).
More and more studies indicate the relation between the existence of Walnut in diet and disease prevention and treatment. According to Zhi-Jing Ni et al. study (Ni et al., 2021) polyphenols in Walnut can decrease the risk of cardiovascular diseases due to their effect on the amount of low-density lipoprotein cholesterol. It's because of polyunsaturated fatty acids (PUFA) and linoleic acid in Walnut. In addition, previous studies have shown the effect of Walnut (as a reach source of PUFAs) on boosting brain's health and function. They have also demonstrated the inhibitory effects of Walnut compounds such as ellagic acid on cancer development and inflammation. Some surveys have reported that the long-term consumption of Walnut effectively prevents type 2 diabetes. A tremendous amount of Walnut's therapeutic effects are due to its antioxidative activity. Walnuts contain many unique and potent antioxidants such as quinone, tannin, neuraminic acid, and flavonol. Overall, Walnut is recommended as an excellent snack due to their contents. Its compounds can forbid inflammation and oxidative stress, which leads to the prevention of many diseases. From the information mentioned above, Walnut consumption can reduce the probability of cardiovascular diseases, depression, dementia, and type 2 diabetes. However, additional research is strongly recommended (Ni et al., 2021).
3. Methods
The data supporting this review article was collected by applying an advanced search strategy in google scholar, PubMed/Medline, and Scopus electronic databases. Filtering the search results to English Publications, we searched the following Keywords and MeSH terms filtered on title or abstract: ("Neurodegenerative Diseases"[Mesh]), ("Nervous System Diseases"[Mesh]), ("Glioblastoma"[Mesh]), ("Glioma"[Mesh]), ("Epilepsy"[Mesh]), ("Alzheimer Disease"[Mesh]), ("Parkinson Disease"[Mesh]), ("Multiple Sclerosis"[Mesh]), ("Neuroinflammatory Diseases"[Mesh]), ("Oxidative Stress"[Mesh]), ("Juglans"[Mesh]) and (Walnuts). After screening the titles and abstracts of the articles provided by our search strategy, 57 original articles were considered to review in this study (Fig. 1). This method was applied to retrieve documents published until August 2022. The search strategy of Google scholar, PubMed and Scopus are summarized in Table 1.
Fig. 1.
The PRISMA flow diagram of study.
Table 1.
Search strategies for Google Scholar, PubMed, and Scopus Databases.
| Search engine | Search Strategy | Additional filters |
|---|---|---|
| PubMed/MEDLINE | (("Neurodegenerative Diseases"[Mesh]) OR ("Nervous System Diseases"[Mesh]) OR ("Oxidative Stress"[Mesh]) OR ("Neuroinflammatory Diseases"[Mesh]) OR ("Glioblastoma"[Mesh]) OR ("Glioma"[Mesh]) OR ("Epilepsy"[Mesh]) OR (Seizure) OR ("Alzheimer Disease"[Mesh]) OR (Alzheimer’s disease) OR ("Parkinson Disease"[Mesh]) OR (Parkinson’s disease) OR ("Multiple Sclerosis"[Mesh]) OR (MS) OR (Neuroinflammation)) AND (("Juglans"[Mesh]) AND ((Walnuts [Title/Abstract]) OR (Walnut [Title/Abstract]))) | English, August 31, 2022 |
| Scopus | TITLE-ABS ((("Neurodegenerative Diseases") OR ("Nervous System Diseases") OR ("Oxidative Stress") OR ("Neuroinflammatory Diseases") OR ("Glioblastoma") OR ("Glioma") OR ("Epilepsy") OR ("Seizure") OR ("Alzheimer Disease") OR ("Alzheimer's disease") OR ("Parkinson Disease") OR ("Parkinson's disease") OR ("Multiple Sclerosis") OR ("MS") OR ("Neuroinflammation")) AND (("Juglans") OR ("Walnuts") OR ("Walnut"))) AND (LIMIT-TO (SUBJAREA,"MEDI")) AND ( LIMIT-TO ( LANGUAGE, “English")) | English, Medicine Subject area, August 31, 2022 |
| Google Scholar | #1: "neurodegenerative diseases" OR "Nervous System Diseases" OR "Oxidative Stress" OR "Neuroinflammatory Diseases" OR "Glioblastoma" OR "Glioma" OR "Epilepsy" OR "Seizure" #2: "Alzheimer Disease" OR "Alzheimer's disease" OR "Parkinson Disease" OR "Parkinson's disease" OR "Multiple Sclerosis" OR "MS" OR "Neuroinflammation" #3: ”Juglans” #4: “Walnuts” #5: “Walnut” #6: #1 AND #3 #7: #1 AND #4 #8: #1 AND #5 #9: #2 AND #3 #10: #2 AND #4 #11: #2 AND #5 #12: #6 OR #7 OR #8 OR #9 OR #10 OR #11 |
English, August 31, 2022 |
4. Investigated disorders
In this section, we examined the neurological diseases that Walnuts can play a role in improving.
4.1. Glioblastoma
Glioblastoma (GBM), comprised of astrocytes of the brain or spinal cord as the most malignant primary cerebral neoplasm, tends to rapid proliferation and progression (Chen et al., 2016). As the most aggressive type of astrocytoma, it makes up 50% of all gliomas and accounts for 2.5% of neoplasm-associated death in 2018 worldwide (Hanif et al., 2017, Rock et al., 2012). The rapid progression of GBM as one of the malignant primary brain neoplasms with a mean diagnosis age of 68–70 years could be significant (Ostrom et al., 2020). Recent studies indicate the effects of age-dependent factors influencing GBM (Kim et al., 2021). The poor prognosis of this type of astrocytoma concerning the current therapeutic approach In glioma eliminating tumor stem-like cells (TSCs) cannot be accomplished even by combined-modality therapy, indicating the principal reason for relapse and chemotherapy resistance of GBM (Qiang et al., 2009). Resistance of GBM to chemotherapy is considered one of the critical barriers to achieving effective anti-cancer treatment, showing the importance of investigating effective chemotherapeutic agents targeting GBM to improve quality of life and life expectancy for patients who have GBM (De Matteis et al., 2019, Naletova et al., 2019). Accumulated knowledge reveals that Pin1 protein, which has a critical role in malignant neoplasm formation, is overexpressed in GBM specimens, suggesting that Pin1 inhibitors should be scrutinized as novel therapeutic agents to improve clinical management of GBM (Atkinson et al., 2009, Yang et al., 2013).
Juglone (5-hydroxy-1,4-naphthoquinone) is a natural element extracted from Juglans nigra and its potential antiproliferative effects on GBM by suppressing the Pin1-mediated signaling pathway are significant (Wang et al., 2017). The processes of redox cycling and reactivity to nucleophiles have contributed to the cytotoxicity effect of naphthoquinones associated with apoptosis of glioma cells in response to oxidative stress. According to the study conducted by Pavan et al. (2017). to evaluate the antiproliferative effect of 2-(2,4-dihydroxy phenyl)− 8-hydroxy-1,4-naphthoquinone (DiNAF), a synthetic derivation of Juglone, on rat glioma cells. This substance and its properties are compared with the element of natural resources and a series of naphthoquinones. According to biological results and NMR experiments, DiNAF has the lowest tendency to undergo reduction; thus, compared to juglones and other derivatives, DiNAF reduced glioma cell viability due to its efficient proapoptotic effect. In addition, compared with Juglone, temozolomide, and paclitaxel (PTX), DiNAF represented a remarkably increased apoptosis induction in vitro and diminished cell viability in rat glioma. These observations suggest the potential therapeutic effects of DiNAF and its cytotoxic activity in the treatment process in the glioma (Pavan et al., 2017). In another research, Wang et al. (2017) evaluated the anti-cancer effects of Juglone on the U251 human glioma cell line and explored its prospective basic molecular processes. Apoptosis, angiogenesis, migration, cell survival, and molecular targets were examined. The results revealed that juglone significantly suppresses tumor cell viability and triggers apoptosis of cultured U251 cells, gradually attenuating glioma cell viability in a way that depends on concentration and time. Angiogenesis, as a prerequisite for migration of glioma, is inhibited by juglone, indicating that juglone manages the proliferation and migration of tumor cells. According to the findings of this study, juglone may prevent neoplasm formation by stimulating apoptosis, restricting migration of U251 glioma cells, and deranging angiogenesis.
Former studies revealed that reactive oxygen species (ROS) could activate the p38-MAPK pathway involved in apoptosis. In another study, Wu et al. (2017) assessed the cytotoxicity effect of juglones on TSCs. TSCs (in glioma) are composed of U87 and two primary cells (SHG62 and SHG66). According to the findings, juglone can dramatically prevent glioma (stem-like cells) growth by reducing cell viability, inducing apoptosis, and cell shrinkage, along with increasing caspase-9 cleavage in a dose-dependent manner. Meanwhile, juglone may be involved in ROS production and increased p38 phosphorylation (leading to activation of the p38-MAPK pathway in TSCs). Additionally, the cytotoxicity induced by juglone can get eliminated by ROS scavengers. Most notably, juglone as an effective anti-glioma therapeutic agent could markedly inhibit tumor proliferation in vivo and enhance the survival rate of glioma-carrying mice compared to controls. However, temozolomide showed a better cytotoxic result.
The standard therapy, based on temozolomide, induces several side effects, in particular, lymphocytopenia and neutropenia, which commonly triggers opportunistic infections. Genovese et al. (2020) studied the impact of Walnut septum extract on GBM cell survival and its anti-bacterial properties. The cytostatic task of the function of Walnut septum extract against the human GBM cell line (A172) was carefully studied, and from the results of these studies can be assumed that Walnut extract can reduce the proliferation and migration of GBM. The possible cytotoxic effects of Walnut on A172 cells and a non-cancerous cell line (HFF‑1) are evaluated using an mean transit time assay. A considerable reduction in HFF-1 cell viability is detected in response to the highest concentration of Walnut extracts. Although, a dose-dependent decrease in cancer cell viability is identified when GBM cells are exposed to higher doses of the Walnut septum extract. The caspase‑3 assay and cytofluorimetric analyses are performed to investigate the proapoptotic properties of the Walnut extract against GBM. The results showed that therapy with Walnut extract could increase early and late apoptosis of A172 cells. Examination of the anti-bacterial effects indicated the ability of Walnut extract to decrease Gram‑negative and Gram‑positive bacterial growth. Most of them are resistant to the antibiotic ciprofloxacin. More importantly, prediction of the activity spectrum for high-performance liquid chromatography detected compounds analysis suggested a range of activities for each substance involving antimutagenic, anti‑infective, antineoplastic, antiseptic, and cytostatic effects.
4.2. Epilepsy
Epilepsy is one of the leading neurological diseases of the CNS, affecting nearly 4% of individuals during their lifetime (Browne and Holmes, 2001, Thurman et al., 2018). The current therapeutic approach is based on pharmacological agents, effects on ion channels, and neurotransmitter receptors of nervous systems (Oyrer et al., 2018). Conventional antiepileptic drugs only suppress seizures and provide symptomatic treatment but cannot completely cure the disease (Macleod and Appleton, 2007). Despite the common antiepileptic drugs, approximately 30% of epileptic patients proceed to present seizures that are refractory to all therapeutic plans (Löscher, 2011, Shorvon, 2009). Given the current situation, research is needed in the field of novel and effective anti-convulsion therapeutic approaches to provide good prevention and treatment for epileptic seizures. Phenolic compounds in the Walnut kernel have antioxidant functions and can play an essential role in maintaining human health. Walnut kernel also has a neuroprotective function, which can be due to the elements they contain, such as melatonin, vitamin E, and folate. According to Shekaari et al (Asadi-Shekaari et al., 2012a). study, Walnut kernel extract administration considerably increased the Pentylenetetrazole (PTZ) dose for threshold seizure induction and reduced the severity of attacks to an acceptable amount. Flumazenil did not significantly reduce the anticonvulsant effect of Walnuts. On the other hand, a combination of diazepam and Walnut showed a synergic anticonvulsant effect. Based on available data, it is said that Walnut kernel can protect neurons against oxidative stress caused by seizures. Their study confirmed that Walnut has anticonvulsant effects and suggested that this effect may be mediated through mechanisms other than the Benzodiazepines pathway.
In another study, the walnut peptide extracts' anti-seizure property was evaluated in three different mouse seizure models including PTZ-induced clonic seizure, chemical kindling, and maximal electroshock. Walnut peptides (20 mg/Kg) were administered by intraperitoneal injection of mice 60 min before seizure induction in the three models. To delineate the mechanisms of walnut peptides' anti-seizure activity, they evaluated the impact of diazepam, flumazenil, and a nitric oxide synthase (NOS) inhibitor on this activity. Intraperitoneal administration of walnut peptides significantly increased the seizure threshold. Their results also demonstrated that walnut peptides exert their anti-seizure properties through the modulation of benzodiazepine receptors. Thus, walnut peptides may be considered a new anti-convulsion agent, which can reduce seizure occurrence and slow down seizure progression (Jahanbani et al., 2021).
Oxidative stress is associated with the appearance of epilepsy and seizure development. Accordingly, antioxidants that could decrease oxidative stress are considered in epilepsy treatment approaches (Delanty and MAJAon, 2000, Shekh-Ahmad et al., 2019). According to research, natural protein sources that have oxidant effects are abundant. among these, trees have a significant place. Many are used in traditional medicine because of their anti-convulsion effects (Yaro et al., 2018). Many herbal antioxidant products have anticonvulsant activity (Hsieh et al., 1999, Lian et al., 2005). Walnut kernels phenolic compounds have beneficial properties for human health, including antioxidant, neuroprotective, and anti-atherogenic effects. Moreover, it is confirmed that extreme generation of free radicals has been associated with the pathogenesis of epilepsy and some neurological disease, suggesting that antioxidants coupled with antiepileptic medicines can be effective for improving the management of seizures (Devi et al., 2008). Walnut is one of the substances that has the freest and the most fiber-bound antioxidants. Walnut kernel contains high oil levels which have polyunsaturated fatty acids. Walnuts have many valuable nutrients that are important for maintaining human health (such as omega 3, 6, and 9). Some studies revealed that increasing the use of Walnut kernels can delay the kindling process and reduce seizures caused by the amygdala (Harandi et al., 2013a, Harandi et al., 2013b).
NO can participate in the mechanisms involved in seizure progression and PTZ-induced seizure threshold (Bahramnjead et al., 2018, Bahremand et al., 2010). Elevated Levels of secondary products originating from lipid peroxidation have also been found to be significant in the cerebral cortex of rats with these types of seizures (Bashkatova et al., 2003). NOS inhibitors are capable of postponing or suppressing the occurrence of PTZ-induced clonic seizures (Osonoe et al., 1994). It is validated that Walnuts could diminish the generation of NO, tumor necrosis factor-α, and the expression of inducible NO synthase in BV-2 microglial cells stimulated by lipopolysaccharides. Asadi-Shekaari et al. (2012b) evaluated the efficacy of the anticonvulsant and neuroprotective effects of Walnut kernel on rat brain cortex. Adult male rats were divided into three groups: an experimental group (PTZ injection, fed with Walnut kernel), a control group (PTZ injection, fed with ordinary food), and a sham group (without PTZ injection, only for histological studies). The results revealed that Pretreatment with Walnut kernel is associated with an increased threshold of PTZ-induced seizure and decreased mortality rate in the experimental group compared with controls. Moreover, after PTZ administration, the architecture of cortical neurons in the Walnut kernel-treated group was almost intact. However, in the control group majority of the cortical neurons underwent significant changes involving cytoplasm darkening, mitochondrial swelling, and shrinkage of nuclei. Accordingly, Walnut pretreatment is associated with remarkable neuroprotective and anticonvulsant activities in PTZ-induced seizures, this indicates that Walnut kernel supplementation may be effective in PTZ-induced seizure prevention and its relevant neurodegeneration in male rats. In another study, Asadi-Shekaari et al. (2014) assessed the potential antiepileptic properties of Walnut kernel extract on PTZ-induced seizures in rats. They evaluated the effects of benzodiazepines and ethosuximide on these pathways. They divided male Wistar rats into eight groups. Seizures were induced by PTZ intravenous injection. Applying PTZ, animals were treated with Walnut kernel extract, with or without cotreatment with diazepam, or flumazenil. they observed that Walnut kernel extract administration could remarkably increase the PTZ dose needed to provoke the first myoclonic motion, decrease seizure grades’ severity, and reduce the mortality rate of rats to 0%. The application of flumazenil had no significant effects in lowering the anticonvulsant properties of Walnut kernel extract. The combination of Walnut kernel extract and diazepam exhibited a synergic antiepileptic activity; conversely, ethosuximide had no considerable impact on Walnut anti-seizure effects. Considering the low influence of flumazenil on Walnut kernel extract effects as an antagonist of benzodiazepine receptors and the synergistic effect of Walnuts and diazepam, it can also be said that the antiepileptic effects of Walnut is exerted through pathways other than benzodiazepine receptors. different ways could contribute to the antiepileptic effects of Walnuts. Various studies have been performed on the role of NO in the pathophysiology of disorders such as trauma, stroke, and seizures (Bashkatova et al., 2000, Wiesinger, 2001)., which could explain the mechanisms underlying the anticonvulsant effect of Walnuts. In a nutshell, the findings of this study indicated that Walnut kernel extract was effective at increasing the needed dose to the first myoclonic jerk, at reducing the severity of seizures, and the high efficacy of the extracts at preventing mortality was considerable, 100% of animals were protected (Asadi-Shekaari et al., 2014). The results are also supported by the study by Poulose et al. (2014).
4.3. Parkinson’s disease
Monoamine oxidase (MAO) is a catalytic enzyme that catalyzes the oxidative deamination of monoamines like dopamine. In Parkinson's disease (PD), MAO is highly expressed. MAO activity rises with age, which may lead to neuronal degeneration within brain as a result of oxidative stress. The gradual decline of dopaminergic neurons within the substantia nigra pars compacta (SNpc) is followed by a decrease in striatal dopamine as well as its metabolites in such a procedure. ROS produced by MAO-B can harm cells in the environment as well as in neurons (Tatton et al., 2003). Neurons are primarily liable to oxidative stress (Mallajosyula et al., 2008). Through PD model types, MAO-B levels doubled in the SNpc, which corresponds well with the proportion of selective dopaminergic cell death in the SNpc (Mallajosyula et al., 2008, Damier et al., 1996). MAO-B inhibitors have been shown to reduce dopamine breakdown and limit the generation of neurotoxic dopamine metabolites and ROS. As a result of its protective role, this type of drug is now employed in the symptomatic therapy of PD (Nagatsu and Sawada, 2006, Schapira, 2011, Weinreb et al., 2010). Choi et al. found that juglandis semen extract, a water-soluble extract of juglandis semen, has superior antioxidant properties and MAO-B has an inhibitory activity in vitro compared to extract of a ginkgo leaf, which is a common natural MAO-B inhibitor. In comparison to the control group, juglandis semen extract therapy decreased the MAO-B fluorescence intensity generated by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) poisoning. The in vivo capacity of juglandis semen extract to inhibit parkinsonian neurodegeneration was investigated in this study. Juglandis semen extract therapy reduced mesencephalic neuronal death in vitro by inhibiting oxidative and nitrosative stresses induced by 1-methyl-4-phenylpyridinium (MPP+). In mice susceptible to the neurotoxic action of MPTP, juglandis semen extract protected dopaminergic neurons and avoided the decrease of striatal dopamine and its metabolites (Choi et al., 2016).
Complex I, which is located in the mitochondrial electron transport chain, also named nicotinamide adenine dinucleotide-ubiquinone oxidoreductase, is an integrated complex that facilitates electron flow from nicotinamide adenine dinucleotide to ubiquinone. These enzymes cause a speed-limiting phase for the mitochondrial respiratory chain. As a result, it is vital in regulating oxidative phosphorylation, which is linked to the synthesis of adenosine triphosphate (Petrosillo et al., 2008). Complex I function is reduced in the substantia nigra of PD, yet stays normal in other neuronal areas (Schapira et al., 1990), which is consistent with Essa et al. (2015). research. When compared with control mice, MPTP dramatically increased MAO-B activity and decreased mitochondrial complex I performance in substantia nigra. Since passing the blood-brain barrier, accumulating MPTP is processed by MAO-B to MPP+ and absorbed by the dopamine transporter, which suppresses complex I of the mitochondrial respiratory chain. In addition, the current study found that Walnut may have mitochondrial protective properties through recovering complex-I function. Inside the substantia nigra of PD patients, post - mortem investigation showed higher rates of lipid peroxidation, Superoxide dismutase (SOD), and catalase (CAT) activity, as well as enhanced free iron amounts (Sian et al., 1994). The actions of glutathione (GSH) and its dependent enzyme, GSH peroxidase (GHS-Px), were reduced in the MPTP-induced group. Walnut treatment results in a significant increase in GSH content and GSH-Px activity. Because of its antioxidant effect, Walnut intake decreased the lipid peroxidation reactions, the actions of SOD, and CAT generated by MPTP in Essa et al. study (Essa et al., 2015).
Proline-directed protein kinases and phosphatases control bidirectional phosphorylation on Ser/Thr-Pro motifs (a crucial molecular shift in managing numerous cellular activities). For controlling cellular signaling, proline-directed kinases, as well as several Ser/Thr phosphatases, phosphorylate and dephosphorylate these patterns. Pin1 is one of the few peptidyl-prolyl isomerases that detect phosphorylated serine or threonine sites directly before proline (Ser(P)/Thr-Pro) in a set of proteins and catalyzes the isomerization of the peptide bond's cis/trans conformation (Joseph et al., 2003). Conformational regulation intervened by Pin-1 has been demonstrated in various studies to include a significant influence on cell proliferation, stress feedback, immunological function, germ cell formation, neuronal specialization, and survival. Pin 1 signaling imbalance is associated with a variety of diseases. Pin1 is highly expressed in differentiated cells and post-mitotic neurons, suggesting that this might have a function in the nervous system. Pin1 has been found in Lewy bodies in PD and has been demonstrated to aid in producing alpha-synuclein aggregates in a cellular model of alpha-synuclein clusters (Becker and Bonni, 2006, Ryo et al., 2006). Pin1 is substantially increased in cell culture, animal models of PD, and in human PD brains. According to Ghosh et al. (2013), Pin1 operates as a proapoptotic mediator in dopaminergic neuron atrophy, suggesting that knocking it out reduces apoptotic processes in PD cell culture models. MPP-induced Pin1 production was decreased in a PD cellular model when Pin1 activity was inhibited with the pharmacological antagonist juglone. In an experimental mouse model of PD, juglone therapy reduced Pin1 levels and preserved the nigrostriatal axis. To test the neuroprotective potential of juglone, a recognized suppressor of Pin1, they used a validated MPTP model of PD. Pin1 inhibitors include juglone (5-hydroxy-1,4-napthalenedione (C10H6O3)), the naphthoquinone discovered mainly in the roots, leaves, and bark of black Walnut trees. By modifying the thiol groups of molecules, juglone disables human Pin1's reaction rate irreversibly. We discovered that micromolar doses (1 M) of juglone inhibited Pin1 production which is generated by MPP, in their research. Juglone also preserved mesencephalic and striatal primary neurons and their neurites against MPP+ poisoning and greatly improved dopamine uptake in principle mesencephalic neurons. In the striatum of mice medicated with MPTP, juglone recovered behavioral activity and also dopamine and its metabolites concentrations. Numerous lines of the research reported in the study by Gosh et al. distinctly reveal up-regulation of Pin1 production in dopaminergic cell culture models, an animal model of PD, and the midbrain of PD patients. They further show that a pharmacological suppressor of Pin1 which prevents Pin1 production from being induced preserves dopaminergic neurons from neurotoxic stimuli (Ghosh et al., 2013).
Extraction of Walnut in aqueous form has a neuroprotective role. Utilizing a mouse model of PD, Walnut juice decreased ROS and NO generation, and limited the reduction of striatal dopamine and its metabolites, leading to a significant enhancement in PD motion abnormalities (Choi et al., 2016). Walnut has neuroprotective properties due to its capacity to suppress the MAO-B enzyme which raises oxidative stress in PD patients, as well as antioxidant and mitochondrial defensive properties (Essa et al., 2015).
Gallic acid, according to Sameri et al., helps reduce motor dysfunctions and enhances gamma wave power in rats with 6-hydroxydopamine-induced dopaminergic neurodegeneration and PD. They discovered that GA's positive impact is due to its antioxidant and free radical neutralizing properties (Sameri et al., 2011).
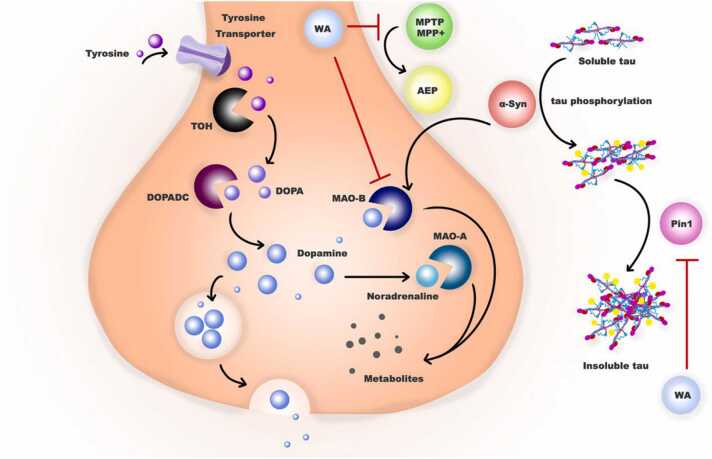
"Hormesis" is a modifying stress reaction in which a cell or organism develops tolerance to a harmful chemical after being exposed to a low dosage, and "neurohormesis" is hormesis inside the nervous system. Several neurohormetic phytochemicals interfere with the hormetic pathways, which is fascinating. Hormetic phytochemicals are valuable assets for the production of new neuroprotectants. Hormetic phytochemicals can trigger several transcription elements and regulate the adaptive stress reaction to various stressors. The stimulation of antioxidants and detoxifying enzymes implicated in the control of oxidative stress and cellular calcium homeostasis occurs often when the hormetic path is activated. Naphthoquinones, including juglone, are some of the known hormetic phytochemicals with strong hormetic stress reactions. The study conducted by Choi et al. (2012). showed that naphthazarin, a naphthoquinone derivative, has a protective effect on neurons in a PD model produced by MPTP and MPP1. Fig. 2 summarizes the impact of Walnut on PD.
Fig. 2.
Walnut function as a MAO-B inhibitor in Parkinson’s disease. Alpha-synuclein (α-Syn) aid tau protein phosphorylation, leading to formation of insoluble tau. Pin1 has also been found in Lewy bodies in Parkinson's disease and has been demonstrated to aid in the production of α-Syn aggregation. Monoamine oxidase (MAO)-A converts noradrenaline and MAO-B converts dopamine into its metabolites, resulting in neuronal disruptions. Walnut can inhibit MAO-B and Pin1 activity.
4.4. Multiple sclerosis
Multiple sclerosis (MS) is a long-term disease in which demyelination of the CNS and inflammation occurs. Women are affected twice more often as men by this disease beginning generally from the age of 20–40 years (Tullman, 2013). The exact cause of MS is not known yet. This disorder is known as a multi-factorial disease in which environmental and genetic components have an effect on susceptivity to disease. The immune system in patients with MS and its animal figure, determined as experimental autoimmune encephalomyelitis, attacks the myelin cover that encircles neurons’ axons in the central nervous system. Since there is no cure for some inflammatory disorders, such as MS, investigators and physicians have concentrated on the benefits of fruits, vegetables, and seeds for the reduction of manifestation. In that wise, Walnut is an anti-inflammatory nut. Walnut oil carries unsaturated fatty acids. Walnut oil is also exceedingly enhanced in omega-3 and omega-6 fatty acids, proteins, sterols, potassium, tocopherols, aliphatic alcohol, fiber, vitamin E, magnesium, triterpenes, and copper, each of which has anti-inflammatory and antioxidant properties (Leray et al., 2016, Polman et al., 2011).
Ganji et al. studied the result of using Walnut oil on encephalomyelitis. After Walnut oil therapy the severity of mice’s experimental autoimmune encephalomyelitis disorder was reduced, and the immune response shifted from destructive to regulatory. These discoveries recommend that Walnut oil is an effective treatment for MS. Walnut oil decreases the mean maximum seriousness of the sickness by lessening T-helper 1 activity. There is evidence that pro-inflammatory cytokines, like interferon-gamma (IFN-γ), released by T-helper 1 cells, participate in the pathogenesis of MS. Walnut oil also diminishes the usual maximum seriousness of the illness by boosting T-helper 2 cell responses. It has appeared that T-helper 2 cells can restrain T-helper 1 cell reactions through cytokine generation containing interleukin (IL)− 4, IL-5, IL-10, and tumor growth factor-β. There was a notable reduction in disorder severity, a hindrance to plaque formation, and modified cytokine production (Ganji et al., 2019).
4.5. Neurotoxicity
Neurotoxicity is caused by the direct or indirect effects of chemicals on the nervous system, nerve cells, or metabolic processes vital to the nervous system that impair the function of the human nervous system, harm growth, or damage the adult nervous system. Numerous studies have shown that the aging process can have an undeniable effect on neurotoxicity. It is also shown that diet and using a range of specific nutrients can slow or even stop the progression of this process. One nutrient that cannot be overlooked because of its unique effect on neurotoxicity is Walnut (Spencer and Lein, 2014). In a study conducted by Liu et al. (2019a), the antioxidant and anti-apoptotic effects of the Walnut kernel and Walnut seed coats on the diet of aging mice with learning and memory impairments treated with D-galactose were investigated. It is noteworthy that D-galactose impairs memory and cognition, causes signs of aging in mice with brain and liver damage, and moderates levels of hepatocyte apoptosis, necrosis, and inflammatory cell infiltration. It can also increase malondialdehyde (MDA) concentration and decrease total antioxidant (AOC) functions, total SOD, and GSH-Px activities. Adding Walnut kernels and Walnut seed coats to these mice’s feeding program causes extensive changes. Chemical analysis of these nutrients has shown that Walnut kernels contain a variety of substances with antioxidant properties (such as flavonoids, melatonin, and vitamin E), antioxidant unsaturated fatty acids, and polyphenols. Also, Walnut seed coats are rich in polyphenols. The result of research has shown that these nutrients reduce oxidative damage and inflammation, improve learning and memory, reduce the weight of mice, and return antioxidant enzyme concentration to normal levels. They also prevent damage to the hippocampus and liver in D-galactose-induced aging mice. Walnut seed coats reduced pathological changes in the liver and brain tissue and increased the number of round-shaped neurons and well-organized fibers in the hippocampus of experimental mice. The mechanism of action of Walnut seed coats may include the regulation of acetylcholinesterase action, the reform of histopathological changes, and the providing adenosine triphosphate energy to brain tissue. In the final results of the experiment, an increase in lots of hairs in mice and a decrease in the number of errors and jumping time of mice with the mentioned diet from the platform were also observed, the second said item is due to the increase in cognitive abilities of this group. Walnuts are generally considered as an anti-cancer, anti-inflammatory, blood purifier, and antioxidant nutrient and also as a treatment for a wide range of diseases in traditional medicine. In research conducted by Shabani et al. (2012), the ability of Walnut to protect against cisplatin-induced neurotoxicity was evaluated by observing behaviors related to the hippocampus and cerebellum of these mice. The purpose of this was to investigate the effect of Walnuts on pain. Extensive tissue damage and negative impact on cerebellar cortical neurons (especially Purkinje cells which have a significant impact on the control of motor activity) resulted in complications such as renal toxicity, neurotoxicity, ear toxicity, movement, balance problems, muscle weakness, and vomiting. It increased pain response delay and decreased exploratory behaviors and memory function. One of the ways to overcome the side effects of this drug in cancer patients is to intervene in their diet with a variety of nutrients as a moderator of toxicity of anti-cancer agents. Walnuts are also rich in omega-3 and alpha-linolenic acid, which boost cognitive function. It also has anti-inflammatory and antioxidant properties and a defensive role against cisplatin-induced neurotoxicity due to its various substances such as vitamin E, folate, melatonin, flavonoids, and polyphenols. Also, Walnut peel extract, with its antioxidant properties, is effective in modulating the effects of cisplatin on the liver and kidneys of male mice. Also, the final results of the research show that the movement balance and coordination of the muscles of mice treated with a diet containing Walnuts are different due to the richness of this nutrient from linoleic acid and α-Linolenic acid, the prevention of cisplatin-induced memory changes in them due to other diets. The inclusion of Walnuts in the diet of these mice modulates cisplatin-induced CNS changes.
Investigate the preventive and therapeutic effects of Walnut leaf extract on diabetic neuropathy. Intraperitoneal injection of STZ into diabetic rats showed sciatic nerve degeneration, more expression of caspase 3, cyclooxygenase-2 (COX-2), and inducible NOS (iNOS) were present in these rats after STZ injection. These effects are moderated after the administration of Juglans regia L. (GRL) leaf extract. Administration of this nutrient extract also reduced lipid peroxidation and pain response, besides improving the antioxidant status of the sciatic nerve in these rats. In conclusion, GRL leaf extract can improve the behavioral and structural parameters of diabetic neuropathy, fasting blood sugar, and hemoglobin A1c and increases glucose uptake by inhibiting protein tyrosine phosphatase 1B. STZ injection also caused thin, loose, irregular myelinated nerve fibers with the infiltration of Several inflammatory cells into the isolated nerve fibers. Administration of GRL leaf extract improved this effect, after which only the focal loss of myelin sheath and several scattered inflammatory cells were observed around nerve fibers.
Neurotoxicity can occur because of exposure to toxic substances or heavy metals like cadmium. A small amount of cadmium can have harmful effects on nerve cells and the brain. It can affect memory, and speed of learning, and lowers intelligence quotient levels. Further conditions may also develop (Batool et al., 2017). Batool et al. (2019) studied the protective effect of nuts such as Walnuts and almonds on the neurotoxicity of cadmium - a metal without physiological function in humans and a potent neurotoxin that is toxic to body tissues due to long half-life and accumulation even in shallow doses-on mice. Cadmium causes depression, anxiety, and decreased memory function by reducing the concentration of noradrenaline, dopamine, and serotonin in the brain and by significantly increasing the concentration of metabolites. Cadmium can also cause neurotoxicity by reducing neurotransmitters at synapses by blocking Ca2+-dependent intracellular signal pathways and inhibiting the integration of storage vesicles. Walnuts and almonds reduced the toxicity of this metal, increased the synthesis of neurotransmitters, and overcame nutritional deficiencies. Also, these nutrients reduced the degree of anxiety and depression in mice. According to the Batool et al. research, rats with the administration of cadmium escape slower than rats with a diet of Walnut and almond. Open field test checks the effect of cadmium besides Walnut and almond on memory functionality. It demonstrated that substances in Walnut and almonds can reduce the impact of cadmium on memory functionality.
Walnut as a nutrient has a wide range of different benefits. One of the compounds in this nutrient is ellagic acid, a polyphenolic and organic heterotricyclic compound. In a study by Ceci et al., strategies for providing ellagic acid as a nutrient with comprehensive therapeutic and pharmacological benefits were examined. The results illustrated the broad effects of ellagic acid on the recovery of chronic diseases associated with oxidative damage (such as cancers, cardiovascular, neurodegenerative disorders, etc.). In addition, it has anti-cancer, anti-inflammatory, anti-bacterial, and anti-viral properties. Also, ellagic acid is effective in wound healing by increasing blood coagulation, via activating the intrinsic cascade factor, XII. The compound has low water solubility, limited absorption, and low plasma half-life while increasing its solubility can increase the absorption of this nutrient. It is also effective in neutralizing active species by increasing the expression and activity of SOD, GSH-Px, GSH reductase, and CAT enzymes. Ellagic acid has an anti-tumor effect in organs such as the prostate, colon, pancreas, and ovary with different mechanisms such as inhibition of cell proliferation, angiogenesis, and invasion of the extracellular matrix. So, it can be considered as a chemopreventive or chemotherapeutic compound. Finally, this substance with anti-inflammatory traits can be effective in the treatment of chronic inflammatory diseases (such as contact dermatitis and pancreatitis).
The study of Ren et al. (2018) illustrated that the Manchurian Walnut hydrolyzed peptide is an antioxidative protein and can decrease oxidative stress and apoptosis and control neurotransmitter action. Manchurian Walnut hydrolyzed peptide can boost the functionality of memory and speed up learning and lessen the effects of oxidative stress. Oxidative stress can damage nerve cells and make some problems with memory and the brain’s functionality. Manchurian Walnut hydrolyzed peptide, as an antioxidative substance, can reduce the damage and help to improve. In this study, the mice taking some dose of Manchurian Walnut hydrolyzed peptide did the water maze test better and escaped faster. According to the high susceptibility of the brain to the degenerative changes caused by some species of free radicals and oxidative products, The effect of Walnut on these impairments is searchable. All of the examined mice were separated into two groups: saline and scopolamine-treated groups. The results showed that scopolamine-treated mice were confronted with cholinergic dysfunction and oxidative stress which were entirely eliminated by the usage of Walnut.
Feng et al. (2018) did some research about the dose-related effects of Walnut on memory. These investigations show that a high-Walnut-dose regimen will induce an increase in memory functions and recognition index and a decrease in transfer latency and escape latency. In another part of the research, the effect of Walnut on memory functions and the level of acetylcholine was investigated. Generally, the level of acetylcholine shows the rate of memory function. But, as the research results conducted, there is a more significant increase in the level of acetylcholine via using Walnut in saline and scopolamine-treated mice. In another part of the study, it was observed that Walnut is also effective in the relation between MDA level and memory retention. Any correlation between MDA level and memory retention was observed in saline and scopolamine-treated mice. Also, there was a negative correlation between MDA level with SOD, GSH-Px, and CAT activities in these mice.
Oxidative stress is one of the causes of cell death and is involved in neurodegenerative disorders such as AD, PD, or cerebral ischemia-reperfusion after stroke (González-Sarrías et al., 2017). Neurons consume large amounts of oxygen and produce hydrogen peroxide through their mitochondria. Since the central nervous system contains a high amount of neurons, its sensitivity to reactive oxygen species is also increased (Shichiri, 2014). Walnut hydrolysis protein has various peptides. One of these peptides, which has an EVSGPGLSPN sequence, has an antioxidant function and can play a role in protecting neurons by reducing ROS generation (Liu et al., 2019b). A study written by González-Sarrías et al. (2017) showed that dietary polyphenols can have health benefits. The results of experiments performed by this research group include the role of 3,4-dihydroxy phenyl propionic acid, 3,4-hydroxyphenyl acetic acid, gallic acid, gallic acid, and urolithins in reducing the amount of ROS and subsequently preventing apoptosis. Nutrients that contain polyphenols precursors of the molecules mentioned above can play a role in reducing oxidative stress and later cell death and preventing neurological disorders (examples of these nutrients are cocoa, tea, strawberries, Walnuts, and pomegranates) (González-Sarrías et al., 2017). Amelioration of oxidative stress was the main approach to boost neurodegenerative disorder. In the Liu et al. (2019b) study, one of the effective peptides in Walnuts was identified as EVSGPGLSPN)by sequencing(. This peptide has a unique property in reducing ROS and has an antioxidant effect. As a result, treatment with this peptide can play a role in reducing the amount of ROS and increasing cell viability. According to research, scopolamine can play a role in memory loss. Peptides in Manchurian Walnuts can prevent or reduce this memory impairment. Their data indicated the EVSGPGLSPN was qualified to protect PC12 cells from H2O2-induced damage. EVSGPGLSPN considerably inhibited ROS levels compared to the H2O2 injured group. As a result of this experiment, it can be said that peptide EVSGPGLSPN plays an essential role in cell defense against oxidative damage caused by H202. SOD, CAT, and GSH-Px are some of the most important antioxidant enzymes that work in the brain. Among the enzymes mentioned, SOD and CAT are involved in cell protection against damage caused by ROS. After exposure of PC12 cells to H2O2, the activities of SOD, CAT, and GSH-Px in the cells were greatly reduced (in comparison with control group). Although, treatment of these PC12 cells with EVSGPGLSPN peptide can significantly improve SOD’s action. Moreover, pretreatment with this peptide dramatically boosted the activity of CAT.
Enhanced accumulation of p-CREB in hippocampal CA1 neurons was shown previously to be along with increased neuroprotection in a mice model of vascular dementia (Hu et al., 2017). Macromolecules in cells and tissues (such as carbohydrates, lipids, proteins, and nucleic acids) can be disrupted by oxidative stress damage and ultimately interrupt tissue and organ function. Urolithins are a subset of dibenzo [b, d] pyran-6-ones (Djedjibegovic et al., 2020). Based on the results of experimental data (OARC test) it can be said that Urolithin A has a higher antioxidant activity than ellagitannins, and this result tells us that Urolithin can be an essential mediator to protect ellagitannins. It has been proven that oxidative stress can cause inflammatory activities. Since antioxidants play a role in eliminating ROS, they can also play a role in preventing provocative activities (Ishimoto et al., 2011). Due to the position of pomegranate, Walnuts, and berries in protecting the cardiovascular system, these nutrients are called “superfoods.”
The general characteristic of these foods is the high compounds of ellagitannins and urolithins. In a review from Djedjibegovic et al. (2020), some studies showed that urolithin B increased mitogen-activated protein kinase (MAPK) phosphorylation which protects the oxidative stress attacks and declined some factors that indicated their function to enhance the oxidative stress levels, such as Akt, c-Jun N-terminal kinase (JNK), and extracellular signal-related kinases (ERK) phosphorylation without affecting phospho-p38. The expressed mechanisms exhibit anti-inflammatory activity. Urolithin B also has an antioxidant role due to its inhibiting ROS generation in lipopolysaccharides (LPS). It seems that the mechanism of the antioxidant effect is inhibiting NADPH oxidase subunits, along with the positive regulation of heme oxygenase-1 (HO-1). For the neuroprotective function of urolithin A, the condition of cytochrome c, cleaved caspase-9, cleaved caspase-3, and cleaved poly (ADP-ribose) polymerase (PARP) were repressed, which validates that urolithin A reduces cell death which exposed to H202.
In summary, urolithin A can reduce the amount of ROS in cells, prevent pathways leading to apoptosis and cell death, and modulate the MAPK p-38 pathway (Kim et al., 2020). The results obtained from studies prove that urolithins can be used as a treatment to counteract oxidative stress and reduce tissue injuries. Recent research shows that urolithin metabolism has a unique cell activity (cell-specific), which changes its activity in vitro. This evidence has not yet been proven and needs further investigation (Djedjibegovic et al., 2020). The results are consistent with Poulose et al. (2014) and Fisher et al. (2017).
4.6. Neuroinflammation
Inflammation involves a set of local immune responses to trauma-induced injuries or infections caused by pathogens that can facilitate the destructive effects and angiogenesis in the affected area. On the other hand, inflammation in the brain, along with its necessity and positive impact, can destroy neurons. Inflammation of the brain affects all brain cells, including neurons, microglia, and macroglia. Inflammation is involved in many brain diseases such as AD, PD, and MS. High levels of inflammation and oxidative stress can lead to memory disorders. In cerebral inflammation, microglia in the CNS with excessive activity secretion of inflammatory mediators such as NO, prostaglandin E2, and IL-6 are involved, which ultimately leads to nerve damage. Walnut is one of the foods that, with its peptides, can modulate memory disorders and cognitive problems caused by inflammation (Wang et al., 2020). The results of previous research have shown us that a diet rich in nuts, vegetables, and fruits can affect a various range of diseases. Walnut has been proven to be a nutrient-rich in unsaturated and essential fatty acids such as alpha-linolenic and linoleic acid, which contribute to some cellular processes in the brain, such as activating microglia and thus producing cytotoxic mediators that are related to the development or progression of AND. Also, the effect of this nutrient on improving memory, cognition, and neurological outcomes associated with oxidative stress and inflammation has been proven (Shabab et al., 2017).
Willis et al. (2010) investigated the anti-inflammatory effects of Walnut, by generating methanolic extract of this nutrient and exposing microglia cells to it and studied its impact on activating these cells in contact with the bacterial cell wall (LPS). The result showed a reduction in the production of nitric oxide and nitric oxide synthase expression induction through desensitization of provocative actions of LPS as well as a drop rate production in tumor necrosis-alpha (TNF-α). In addition, they realized that Walnut extract makes internalization of the LPS receptor. The anti-inflammatory effects of Walnut depend on the activation of phospholipase D2 function. In the final results, they saw the impact of Walnut on modulating the microglia cells’ ability to respond to subsequent pro-inflammatory stimuli. Also, the fatty acids in this nutrient can have different impacts on molecular functions. For example, n-3 PUFA α-Linolenic acid suppresses nitric oxide production in macrophage cells, palmitic acid by stimulation of TLR4 signaling pathways, and oleic acid and linoleic acid fatty acids by inhibiting the stimulatory effects of LPS and saturated fatty acids in macrophages can affect molecular processes.
Findings from the research have shown that increasing the accumulation of misfolded/ damaged poly-ubiquitinated proteins has a significant effect on AND. In a different study, Poulose et al. (2013) investigated the molecular effects of Walnut on the homeostasis of these proteins in the process of aging, the diet of laboratory mice with a food content of 6% or 9% Walnuts was adjusted, which was followed by autophagy activation through inhibition of mTOR phosphorylation, upregulation of ATG7, and Beclin 1 and circulating MAP1BLC3. Autophagy with protection against age-related oxidative stress and inflammation, degradation of amyloid-β (Aβ) precursor protein in the process of AD, α-Synuclein in PD, and Htt in Huntington's disease might reduce the accumulation of misfolded/ damaged poly-ubiquitinated proteins. In a study by Wang et al. (2020), the neuroprotective effects of Walnut peptides on memory-impaired mice fed a diet containing LPS and anti-inflammatory mechanisms against LPS 6-induced inflammation in BV-2 cells were investigated. The study showed the effect of Walnut protein hydrolysate and 18 peptides with anti-inflammatory activity in LDS-activated BV-2 cells in improving LPS-induced memory disorders by making the inflammatory response and oxidative stress in the brain seem normal. LPF, GVYY, and APTLW can reduce the concentration of cytokines by decreasing the expression of mRNA and the content of pro-inflammatory mediators by decreasing the expression of related enzymes, thereby modulating brain inflammation. The study of Xu et al. (2018) showed that Urolithins positively effect neuroinflammation. Urolithins are produced by the metabolism of ellagitannins of microbes in the gut. Walnuts, pomegranates, and strawberries are rich-ellagitannin food. Many of ellagitannins and ellagic acid in pomegranate can prevent inflammation in the brain of AD mice. Urolithin A, Urolithin B, and Urolithin C are different kinds of Urolithin and have other effects. Urolithins can cross the blood-brain barrier and affect inflammations in CNS. NO is an essential molecule in the cause of neuroinflammation. Urolithin A and B reduce the amount of nitric oxide and prevent inflammations. Urolithin A and B decrease the number of iNOS and COX-2 proteins. These two proteins have a significant role in the production of nitric oxide and cause neuroinflammation. The study showed that Urolithin A and B reduce the expression of inflammatory genes and help to accelerate the healing of inflammation in the CNS.
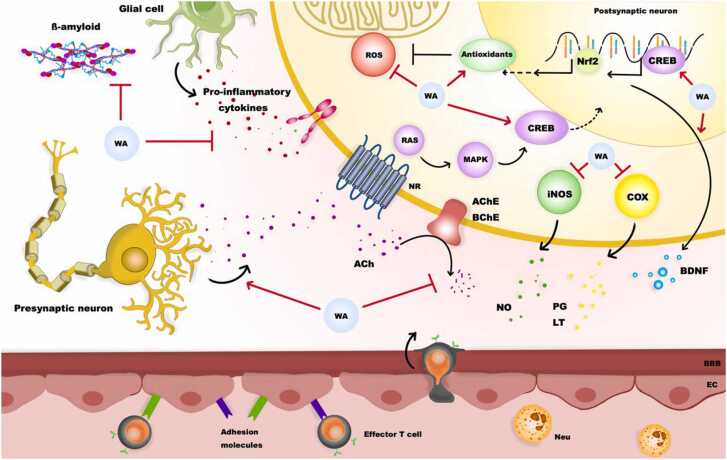
Neuroinflammation is related to cognitive decline and neurodegenerative disease. According to Fisher et al. (2021) study, Walnuts and blueberries have neuroprotective and anti-inflammatory effects. They tested the individual and potentially synergistic good symptoms of Walnut oil and blueberries on LPS-induced neuroinflammation in rat microglial cells by biomarkers measurement of inflammation: nitrite, iNOS, and COX-2. The tests showed that blueberry, Walnut oil, and Walnut oil/blueberry decreased LPS-induced nitrite, COX2, and iNOS relative to control. Blueberry was stronger in reducing nitrite generation than Walnut oil/blueberry, and both blueberries and Walnut oil/ blueberries were more substantial than Walnut oil. But, no notable differences between treatments for COX2 and iNOS expression were observed. All three treatments weakened LPS-induced nitrite, COX2, and iNOS in a concentration- and time-dependent manner. The reduction in neuroinflammation after all treatments indicates that adding blueberries and, or Walnuts to our diet can decrease the neurodegenerative effects of inflammation. However, the result suggests that blueberries and WO do not act synergistically to decline inflammation in microglia. Fig. 3 summarizes the impact of Walnut on Neuro inflammation.
Fig. 3.
Potential mechanisms of walnut in controlling the neuroinflammation. Neuroinflammation start with local immune responses, following recruitment of adaptive and innate immune cells to the brain. the anti-inflammatory effects of walnut (WA) on neurons include suppression of pro-inflammatory cytokines, ß-amyloid aggregation, Reactive oxygen spice (ROS), Acetylcholine esterase (AchE) activation, and inducible nitrous oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) formation. Walnut can also induce Nrf2, brain-derived neurotrophic factor (BDNF) and cAMP response element binding protein (CREB) expression.
4.7. Memory
Memory dysfunction is related to the whole gamut of neurologic issues that have an effect on brain functionality in disorders, some as low as epilepsy and some as dangerous as stroke. It has uprising clinical relevance as the people grow older and AD and other neurodegenerative diseases increase in prevalence (Matthews, 2015). The research of Lee et al. described urolithin B and its benefits. Urolithin B is the final product of ellagitannin metabolism. It prevents the production of nitric oxide and pro-inflammatory cytokines. Urolithin B is an antioxidative substance that reduces producing of reactive oxygen species.
On the other hand, Urolithin B increases the phosphorylation of adenosine monophosphate-activated protein kinase and stops the phosphorylation of JNK and ERK. Microglia is a defensive cell in CNS. It works in brain injury and inflammation and helps in apoptosis. Urolithin B forbids microglia activation after injection of LPS and prevents inflammation. Lipopolysaccharide cause memory deficiency and some other problems with memory function. The study of Kim et al. proved that proteins in Walnut could improve memory function and reduce the damage of inflammation in the blood-brain barrier and help to heal its cells faster. Aβ can cause some structural defects in the blood-brain barrier, and cause Inflammatory reactions and damage, and cut the strong connection between cells in the blood-brain barrier. Aβ can cause oxidative stress and destroy the central nervous system’s cells. Walnuts have active antioxidative substances and polyunsaturated fatty acids. It prevents the aggregation of fat in the liver. The result of a few tests shows that Walnut improves the speed of learning and decreases ROS in mitochondria. Also, proteins in Walnut increase the synthesis of Acetylcholine and reduce the rate of its destruction.
Memory dysfunction has various causes, such as aging, injuries like brain tumors, stroke, etc. Unfortunately, sometimes even drugs have side effects and can affect memory. Scopolamine treatment can block the central muscarinic acetylcholine receptor, impairing cognitive and memory function. It has been proved that Walnut protein hydrolysates can decrease the memory shortage induced by scopolamine. Wang et al. have evaluated the neuroprotective effects of Walnut hydrolysis protein against defects of memory caused by scopolamine in mice and zebrafish. Their survey indicated that oral consumption of Walnut hydrolysis protein not only causes notable betterment in behavioral performance such as memory and learning but also moderates the disorders of the cholinergic system and oxidative stress in the brains of mice.
Moreover, it can boost the transcription of some genes associated with antioxidant defense-related proteins and neurotrophic-related proteins such as brain-derived neurotrophic factor and cyclic adenosine monophosphate response element-binding protein (CREB). In addition, it was understood that FY and SGFDAE could, respectively, inhibit acetylcholinesterase (AChE) and Keap1, which cause an improvement of memory deficits induced by scopolamine.
On the other hand, scopolamine can decrease GSH-Px, SOD, and CAT activities in the mice brains which weakens the antioxidant defense system. Although, piracetam and Walnut hydrolysis protein administration can normalize the activities of these antioxidative enzymes to some extent except for CAT activity. Injection of scopolamine showed MDA, the lipid peroxidation product in mice brains. Interestingly, it is validated that Walnut hydrolysis protein can reduce MDA contents.
Some kinds of Walnut-derived peptides such as YVLLPSPK have had a significant neuroprotective result against scopolamine-induced cognitive deficits in mice. In this article, the main way in which YVLLPSPK works is investigated. According to the researchers, YVLLPSPK does not play any toxic role for mice and would not change any parts of their liver and kidney. According to Wu et al (Zhao et al., 2021)., mice induced with scopolamine had a deficit in cognitive and memory abilities. These deficiencies were relieved by using Walnut products. Also, these mice showed a longer escape latency. Another investigation designed by Li et al. found that mice induced with scopolamine had longer escape latency and crossing and target times in spatial probes. Also, Sheng et al. reported that having a defatted Walnut meal will decrease the escape latency in scopolamine-induced mice. According to all of these findings, YVLLPSPK, which has an essential role in Walnut antioxidant activity, has a significant correlation with escape latency and cognitive abilities in scopolamine-induced mice. Another part of YVLLPSPK is known to be used therapeutically to relieve escape latency and delayed neurological deficits in AD.
According to Maze et al (Liao et al., 2020)., Walnut has some effects on memory impairment in mice, such as preventing cholinergic function damage, inhibiting oxidative stress, and preventing histological changes of neurons in hippocampal regions. On successive days, the behaviors of mice were measured, and there had been a noticeable improvement in them. All of the databases showed that Walnut usage would improve the spatial memory of mice, such as swimming distance and escape latency. Also, Walnut can affect the AChE and choline acetyltransferase (ChAT) activity caused by scopolamine administration. The results showed that Walnut will cause an increase in the activity of AChE and also a decrease in ChAT. The effects of this material were not restricted to these articles and it also has some significant influences on T-SOD, GSH, and MDA levels in brain tissues. The investigations showed an apparent increase in MDA levels, unlike the T-SOD and GSH levels. Another efficacy of Walnut is remarkable changes in the count of live neurons in hippocampal regions in mice’s brains. It showed a noticeable increase in the count of the neurons (Liao et al., 2020).
Regarding the significant increase of neurodegenerative diseases in recent years, some products such as defatted Walnut meal protein hydrolysates have been used to treat these cases. In this article, the effect of defatted Walnut meal protein hydrolysates on neurotoxicity induced by D-galactose and aluminum chloride (AlCl3) in mice is being investigated thoroughly. The results showed that defatted Walnut meal protein hydrolysates could reduce oxidative stress, reserve cholinergic dysfunctions, and decrease the effects of D-galactose and AlCl3 in the brain of mice (Haider et al., 2018). According to one of the studies by Haider et al. (2018), mice treated with D-galactose and AlCl3 had a decreased ability for memory and learning, their neurons were contracted, and their density was reduced in the cortex region. As the research showed, the amount of SOD and GSH-Px were decreased in the brain tissue of these mice; in contrast, the amount of MDA was increased. Also, their cholinergic system was confronted with some disorders, so, there was an increase in the amount of AChE but a decrease in the amount of Ach and ChAT. Ach is correlative with cognitive improvement, and its decrease has neurotic dysfunction. It was found in further research that the amount of TNF-α and IL-1β was increased in the brain tissues of D-galactose and AlCl3 treated mice which were eliminated by defatted Walnut meal protein hydrolysates. Also, the more microglia cells activated, the fewer TNF-α and IL-1β remained in the tissue. Defatted Walnut meal protein hydrolysates is made structurally by amino acids. These are amino acids such as Lys and Asp that play a vital role as antioxidants. Also, because of its acidic amino structure, it is broken down by peptidase enzymes such as pepsin. It breaks defatted Walnut meal protein hydrolysates into small peptides, and then these molecules are again made into smaller portions, which can be absorbed quickly and be used for antioxidant activities (Haider et al., 2018).
In a 2-site randomized controlled trial on 708 free-living elders, Sala-Vila et al. discovered that compared to a control diet (abstention from Walnuts), nutritional supplementation with Walnuts at 15% of daily calories for two years did not affect cognitive function in cognitively healthy elderly. However, brain fMRI and post hoc analysis found that the intervention was successful in participants from the Barcelona location, who had poorer education and lower background status of dietary α-Linolenic acid (the −3 given by Walnuts) than the Loma Linda cohort (Sala-Vila et al., 2020a). Based on in vivo behavioral testing, Li et al. investigated the protective effects of Walnut protein hydrolysate on scopolamine-induced learning and memory impairments in mice. The biochemical results showed that the beneficial effects of Walnut hydrolysis protein on scopolamine-induced dementia mice might be attributable to a dramatically increased number of acetylcholine receptors and up-regulation of choline acetyltransferase mRNA expression (Li et al., 2017). In a study by Bishop et al. on 3632 US adults, they attempted to evaluate the relationship between whole Walnut consumption and cognitive change. They found an association between Walnut consumption and cognitive function in elderlies, but they did not discover that Walnut intake was preventative of age-related cognitive decline (Bishop and Zuniga, 2021).
4.8. Traumatic brain injury
Traumatic brain injury (TBI) can lead to brain edema due to blood-brain barrier (BBB) breakdown and increased endothelium permeability after TBI. Since brain edema increases intracranial pressure, it can cause nerve damage and higher mortality and morbidity rates in TBI patients (Ansari et al., 2016). Studies showed that Walnut kernel is rich in phenolic compounds with antioxidant and anti-inflammatory properties. Thus, Walnut kernel has positive effects on human health. Ansari et al. designed a study to investigate the impact of Walnut kernel feeding on brain edema and neuronal degeneration in male rats after TBI. The results showed brain water content, and intracranial pressure was lesser in the treatment group than in the control. According to these findings, Walnut kernel pretreatment can reduce pathological symptoms after TBI in male rats (Ansari et al., 2016).
Results showed that Walnut extract reduces TNF-α production in microglial cells, which are activated by LPS. Walnut extract and its component, ellagic acid, even showed anti-inflammatory results in human aorta endothelial cells and osteoblastic results in the cells. Moreover, a newer study showed that fatty acids have a role in inflammation response reduction in peritoneal macrophages (Papoutsi et al., 2008). In Ansari et al.'s study, the reduction of neuronal degeneration in the pretreatment group may be due to the anti-inflammatory constituents of Walnut kernel. Walnut kernel pretreatment reduced brain edema, improve neurological scores, and stopped increased neuronal degeneration post-TBI in male rats (Ansari et al., 2016).
Carbon tetrachloride (CCl4) is extensively used to induce hepatotoxicity in experimental animals. High doses of CCl4 lead to nonspecific toxicity, such as central nervous system depression and respiratory failure, with causes death. The free radicals generated from CCl4 and CCl4 harm the endoplasmic reticulum, which leads to the accumulation of lipids, reduced protein synthesis, and mixed-function oxidases activity (Weber et al., 2003, Recknagel et al., 1989, Ritesh et al., 2015). According to Aydın et al.'s (Aydın et al., 2015) study, Walnut is rich in nutrients with cholesterol-free contents, substitutes for animal proteins, and high concentrations of unsaturated fatty acids. It is rich in linoleic acid (18:3, n-3) and linolenic acid (18:2, n-6), polyunsaturated fatty acids that are essential for the body. It is metabolized to haloalkane free radicals (CCl3 and CCl3O2) by a mixed-function cytochrome oxidase complex (NADPH)- cytochrome P450 electron transport chain in the hepatic smooth endoplasmic reticulum. These substances make hepatotoxic CCl4 and initiate lipid peroxidation in the membranes. CCl4 enters the body in various ways, such as air, water, food, and even skin. It goes through the whole body but has more concentration in the liver, kidneys, muscles, adipose tissue, and blood. The results showed the levels of fatty acids increase in the brain and kidney tissues after CCl4 administration. In the groups given Walnut extract against CCl4, it was found that the GSH level increased and the MDA level reduced in all tissues. Given lipophilic vitamin levels, it was found that α-tocopherol levels increased in the brain and liver tissues in the group receiving additional Walnut in comparison with the controls, and cholesterol levels increased in the tissues, except the kidney, in all groups in comparison with the control group. Phenolic compounds, vitamins, and carotenoids existing in fruits such as Walnut possess antioxidant activity and are effective compounds to prevent diseases relating to oxidative stress.
4.9. Alzheimer’s disease
AD is the common reason for dementia - a general term for memory loss and other cognitive abilities just as essential to alter with everyday life. Dementia should not be considered a normal part of growing up. Many people’s memory fades a bit as they grow up, so the difference between usual age-related memory problems and early signs of AD is not that significant (Mucke, 2009). Aβ has 40–42 amino acids in length and is made by proteolytic cleavage of the more significant amyloid precursor protein, which is a transmembrane protein. Hyperphosphorylated tau, a protein in microtubule assembly and stabilization. Hyperphosphorylation of tau is very typical to find in diseases with tau filaments and might as well be required for toxicity (Goedert and Spillantini, 2006). Oxidative stress and neuroinflammation induced by Aβ in the brain are thought to be most likely the pathogenesis of AD (Zou et al., 2016). According to Zou et al. (2016) study, a highly concentrated Walnut peptides diet ameliorated the cognitive and memory disorders of mice. Their study also showed positive effects of restoration of levels of antioxidant enzymes and inflammatory mediators with consumption of Walnut peptides. The goal of the Morris Water Maze test was to check the results of Walnut peptides on the impairment of spatial learning and memory in Aβ25–35-induced AD mice. The time that the mice needed to find the platform was lowered progressively during the test. The test showed considerable differences in the time of the animal between different test days and different dietary situations. The model group mice took longer to find the platform on the test days than those in the sham group, meaning that the hippocampus injection of Aβ25–35 could induce many neurologic and cognitive disorders. The test also showed a big difference in spatial ability to learn, between the model group and the Walnut peptide treatment groups. However, the difference was not significant between the model group and the low-dose Walnut peptides group. The test also showed that injection of Aβ25–35 efficiently impaired spatial learning and memory, which could be easily stopped via a Walnut peptides diet. In comparison to the sham animals, the step-down avoidance test showed a significant lowering of avoidance time and a highering of the error count in the Aβ25–35-injected group, SOD and GSH activities were hugely lowered in the hippocampus of Aβ25–35-treated mice, treatment could quickly force an increase of the AChE level in hippocampus and depressed the enhancement of AChE. Moreover, NO level in the hippocampus was more in the Aβ25–35 group; just the treatment with Aβ25–35 hugely increased the expressions of iNOS and activated NF-κB p65.
Wang et al. (2019) examined the effect of Walnut on Aβ reduction. walnuts contain an active peptide called PW5(Pro-Pro-Lys-Asn-Trp), which has anti-Aβ42 accumulation activity. The results of research on mice showed that PW5, with its anti-ab activities, could be effective in improving the patient's cognition. Serum levels of norepinephrine also increased significantly in response to PW5. As a result of experiments, it can be said that PW5 in Walnut protein can have a good effect on brain function and the prevention of AD. It is stated in another article written by Muthaiyah. et al., Aß produces free radicals that eventually lead to oxidative stress and cell death.
Walnuts contain antioxidant compounds that could affect preventing oxidative stress. In a study conducted by wang et al. it was shown that walnut protein hydrolysates had antioxidant activity. Three out of 48 peptides identified, can act as an antioxidant inside and outside the cell. These peptides include QGRPWG, PSRADIY, and AYNIPVNIAR (Wang et al., 2022). The main focus of Wang et al. study (Wang et al., 2019) was whether walnuts can prevent cytotoxicity and oxidative damage caused by Aß. One result of the article was that Walnut extract could reduce Aß-induced cell death, DNA damage, and ROS production. As a result, eating a diet containing Walnut extract can effectively prevente memory loss and AD. In this article, Chauhan et al (Fotuhi and Do, 2010). Examined the role of Walnuts in improving learning ability in a transgenic mouse model of AD. There have been reports of the role of Aβ and oxidative stress in developing AD. Walnuts are rich in compounds that contain melatonin and vitamin E, which are antioxidant compounds. Mice were grouped into two groups, the control, and experimental groups, and the difference between these two groups was in the Walnut diet. Decreased learning ability and anxiety-related behaviors were observed in control mice that did not have Walnuts in their diet. A significant improvement in memory and learning ability was observed in mice with diet Walnuts. Finally, the results showed that a Walnut diet could quickly stop and delay AD.
In conclusion, Aβ is a massive cause of amyloid plaques in AD patients. Aβ is involved in producing free radicals that ultimately lead to oxidative stress. As a result, researchers have recently been looking for antioxidant compounds to prevent oxidative stress, inflammation, and cell death. Walnut is one of these anti-antioxidant and anti-inflammatory compounds. The positive effects of Walnuts on preventing AD are as follows: Walnuts can play a role in preventing beta-amyloid-dependent cytotoxicity by reducing the production of free radicals and reducing damage to cell membranes and DNA. As a result, a diet rich in Walnuts can prevent AD-related dementia by reducing Aβ fibrillation and oxidative stress (Chauhan and Chauhan, 2012).
Some laboratory research has indicated that Walnut extract can inhibit Aβ fibrillization and solubilize its fibrils and has a protective effect against AD-induced oxidative stress and cellular death, which leads to the inhibition of AD (Muthaiyah et al., 2014). According to Muthaiyah et al. (2014), the mice which consumed Walnut showed better improvements in their memory stage, the number of errors in the EPM assay (a test measuring anxiety in laboratory animals) in these mice was lower, and also their duration of staying information in mind was longer than controls (AD- Tg mice which no consuming Walnut). Their findings showed that long-term activity of a diet with 6% or 9% Walnuts in AD -Tg mice significantly lowered or even stopped deficits in memory and learning compared to AD-Tg mice on a diet that does not include Walnuts, and there was not a big difference in the activity of these mice (compared to wild-type control mice). For individuals with AD, diets with 6% or 9% Walnuts (equivalent to 1 oz. 1.5 oz. Walnuts per day in humans) ameliorated memory loss, improved learning, and motor skills, reduced anxiety-related behavior, and slowed the progression of AD.
In another study, Joseph et al. (2009) showed that Walnut can affect aging and health span; the consumption of Walnut can slow the process of aging and, thus, the process of brain degeneration. Oxidative stress results from the shift toward ROS production in the equilibrium between ROS generation and the antioxidant defense system. In the brain, this is essential since, during periods of oxidative stress, Bcl-2 (a protein that participates in apoptosis) increases and accelerates cellular apoptosis and aging. In the process of aging, the expression of cyclooxygenase 2 appears to be related to α-β deposition in the hippocampus and inflammatory prostaglandins increase in the hippocampus and in other areas during aging. Since the prostaglandin synthesis pathway seems to be a significant cause of ROS in the brain and other organ systems. A high Walnut diet in the old rats had very positive results in cognitive function as those seen with berry fruit or Concord grape supplementation. Preliminary analyses of the stress signals that may be affected with Walnut supplementation suggest that the (n-3) PUFA, a-linolenic acid, was very effective in lowering stress signals such as cytokines and NFκB.
Based on the fact that eating Walnuts can improve brain function, Sala-Vila et al. (2020b) conducted experiments. The tests were performed on a group of middle-aged people at risk for AD. In these experiments, the relationship between Walnut consumption and MRI-assessed brain phenotypes was investigated. One hundred eighty-seven people who consumed Walnuts more than once a week in this experiment showed lower WMH volume (WMH is a marker for diseases related to small cerebral arteries involved in AD). They also showed higher GMV levels (gray matter volume), indicating a healthy aging process. As a result of these experiments, it can be said that Walnut consumption has beneficial effects on brain phenotypes and regional GMV.
Honglin et al (Lv et al., 2021). examined the effect of Walnuts on memory improvement in mice with AD. Since the Walnut kernel is a traditional Chinese medicine for improving brain function, experiments were performed to investigate its effect on brain function. The results are as follows: The first result was that eating Walnuts improves learning and memory. The second one was that eating Walnuts increases acetylcholine and reduces oxidative stress. The third result was that the consumption of Walnuts enhances the function of the nervous system and has a positive effect on neural stem cells. The results also showed that Walnut meal protein treatment could regulate genes. These results above show us that eating Walnuts can be helpful in improving memory and thus preventing AD.
Researchers found that Walnut extract could reduce oxidative stress and cytotoxicity caused by Ab in PC12 cell lines. The beneficial activity is achieved by reducing the formation of free radicals and impairing membrane and DNA damage. This beneficial impact might be attributed to the antioxidant polyphenols found in Walnut. A Walnut-rich diet may be a promising method for preventing and delaying the onset of AD (Essa et al., 2012).
In an article written by Orhan et al. (2011), the main focus was on evaluating the neuroprotective effects of the leaf and fruit extracts. Based on the results obtained in experiments, it can be said that anticholinesterase activity is in contrast to AChE and butyrylcholinesterase, which are essential enzymes in AD. The effect of antioxidants was also investigated in these experiments. Extracts were able to inhibit DPPH radicals, unlike H2O2. The sections were not able to inhibit butyrylcholinesterase and AChE. As a result, we can say that the common belief among locals about the positive effects of Walnuts consumption on brain health can be explained by its antioxidant and rich content of total phenol and flavonoids. Rajaram et al (Rajaram et al., 2017). designed experiments to investigate the effect of Walnuts on healthy aging. These experiments showed that Walnuts could have many benefits in delaying the onset of age-related diseases, including retinal pathology and cognitive impairment. These benefits of Walnuts are due to their effect on vascular health and heart protection, primarily because of the bioactive compounds (such as polyphenols and n-3 fatty acids). Mirazkar et al (Pandareesh et al., 2018). found that Walnuts can play an essential role in learning and memory. To better understand the function of Walnuts in reducing oxidative stress, they performed experiments on mice of different ages. The final findings showed that Walnuts could play a role in reducing oxidative stress and oxidative damage to proteins and lipids in AD by inhibiting free radicals and protecting the antioxidant status. As a result, a diet rich in Walnuts can help reduce the risk of AD and delay its onset.
Nowadays, some kinds of neurotoxicant materials, such as organophosphate, have gotten completely widespread in our environment. These chemicals used to be poisonous in warfare agents to threaten the military and civilian populations. But now, not only used on the battlefields and in the city regions. Bio terrorists are now using this chemical to inflict mass casualties. It can rapidly deactivate Acetylcholinesterase enzymes in synaptic areas and confront the body with cholinergic crisis, as acetylcholine plays a vital role at these points. To prevent these kinds of problems, some types of nutraceuticals, such as α-Linolenic acid, are being used. These materials are abundant in vegetable products like Walnut and flex. These products can be used to treat neurodegenerative diseases such as organophosphate and other ones. α-Linolenic acid has a vast safety margin which leads us to use it in widespread ways for strengthening the transcription and transplantation programs in our brains. Also, α-Linolenic acid has a very prominent ability to activate some vital and mechanical pathways, which can be helpful to restore brain function and treat neurodegenerative diseases.
Table 2 summarizes the studies on the effect of walnut and its active constituents on ANDs.
Table 2.
A summary of the studies on the effect of walnut and its active constituents.
| First author | Year | Type of study | Neurological disease | Name of walnut species | Type of extract or phytochemical | Dose | duration | Mechanistic pathway |
|---|---|---|---|---|---|---|---|---|
| Jifeng Wu (Wu et al., 2017) | 2017 | In vivo | Glioblastoma | – | Juglone | 10, 20, and 40 μM | 48 h |
|
| Valeria Pavan (Pavan et al., 2017) | 2016 | In vivo | Glioblastoma | Juglans nigra | Juglone | – | – |
|
| Jiang Wang (Wang et al., 2017) | 2017 | In vitro | Glioblastoma | Juglans mandshurica | Juglone ( 5-hydroxy-1,4-napthoquinone) | 5, 10 and 20 µM | 24 h |
|
| Carlo Genovese (Genovese et al., 2020) | 2020 | In vitro | Glioblastoma | Juglans regia L. | Juglone (from walnut septum in Trecastagni) | 17,18–275 µg/ml | 24 h and 48 h |
|
| Raheleh Jahanbani (Jahanbani et al., 2021) | 2021 | In vivo | Epilepsy | – | Walnut kernel proteins | 5, 10, 20, 50, and 100 mg/Kg | 48 h |
|
| Majid Asadi-Shekaari (Asadi-Shekaari et al., 2014) | 2014 | In vivo | Epilepsy | Juglans regia | Walnut kernel extracts | 50, 100, 200 and 500 mg/kg | – |
|
| Majid Asadi-Shekaari (Asadi-Shekaari et al., 2012b) | 2012 | In vivo | Epilepsy | Juglans regia | Walnut kernel extracts | – | 2 month( pre-treatment) |
|
| Musthafa Mohamed Essa (Essa et al., 2019) | 2019 | In vivo | Parkinson’s disease | Juglans regia | Walnut kernel extracts lipids | 20 mg/kg body weight day | 28 days |
|
| Jin Gyu Choi (Choi et al., 2016) | 2016 | Both | Parkinson’s disease | Juglans regia L. | Juglandis Semen(the polyphenol-rich aqueous walnut extract) | 15–100 µM | 24 h |
|
| Seon Young Choi (Choi et al., 2012) | 2012 | In vivo | Parkinson’s disease | – | Naphthazarin | 0.1 or 1 mg/kg | 5 days |
|
| Anamitra Ghosh (Ghosh et al., 2013) | 2013 | In vivo | Parkinson’s disease | Black walnut | Napthoquinone in leaves, roots, and bark of black walnut plants | (3 mg/kg) 24 h before the first MPTP injection | 6 days |
|
| Sameri, Maryam Jafar (Sameri et al., 2011) | 2011 | In vivo | Parkinson's Disease | – | Gallic acid | 8 micro gram | 10 days |
|
| Ali Ganji Ganji et al., (2019) |
2019 | In vivo | multiple sclerosis | Juglans regia | Walnut oil | 5 ml/kg/day | 21 days |
|
| Mohammad Shabani Shabani et al., (2012) |
2012 | In vivo | Neurotoxicity | Juglans regia | – | 6% | 5 weeks |
|
| Claudia Ceci Ceci et al., (2020) |
2020 | In vivo RCT |
Neurotoxicity |
Juglans ailanthifolia & Juglans regia |
Ellagic acid (EA) | Rats: 50 mg/kg/day Humans: 45 g/day |
Humans: 7 days |
|
| Zahra Batool Batool et al., (2019) |
2019 | In vivo | Neurotoxicity | – | – | 400 mg/kg/day | 28 days |
|
| Feng et al (Feng et al., 2018). | 2018 | In vivo | Neurotoxicity | – | Defatted walnut meal protein hydrolysates (DWMPH) | 1 g/kg/day | 90 days |
|
| Fisher et al (Fisher et al., 2017). | 2017 | In vitro | Neurotoxicity | Juglans regia | Ground with skin | 0% (control), 6%, and 9% | 15 weeks |
|
| Chunlei Liu (Liu et al., 2019a) |
2019 | In vitro | Neurotoxicity | Juglans mandshurica Maxim. | EVSGPGLSPN | 12.5 μM, 25 μM, 50 μM, and 100 μM | 24 h |
|
| Jialin Xu Xu et al., (2018) |
2018 | In vivo | Neuroinflammation | – | Urolithin A and Urolithin B | 3, 10, 30 μM | 24 h |
|
| Willis et al (Willis et al., 2010). | 2010 | In vitro | anti-inflammatory effects of walnuts in microglia |
Juglans regia | methanolic extract |
ranged from 0.00001 to 10 mg of walnuts per milliliter of solution |
overnight |
|
| Poulose et al (Poulose et al., 2013). | 2013 | In vivo | maintain protein homeostasis | Juglans regia | ground walnuts | 6% containing 60 g walnuts/kg and 9% containing 90 g walnuts/kg |
15 weeks |
|
| Fisher et al (Fisher et al., 2021). | 2021 | In vitro | Neuroinflammation | Juglans regia | Walnut oil | 100 ng/ml | 48 h, 1, 2 or 4 weeks |
|
| Fanrui Zhao et al (Zhao et al., 2021). | 2021 | In vivo | Memory | Juglans mandshurica Maxim. | walnut-derived 99 peptide Tyr-Val-Leu-Leu-Pro-Ser-Pro-Lys (YVLLPSPK) | 60 mg/kg/day | 6 weeks |
|
| Jianqiao Liao et al (Liao et al., 2020). | 2020 | In vivo | Memory | – | Walnut oil | 10 ml/kg walnut oil daily | 8 weeks |
|
| Saida Haider et al (Haider et al., 2018). | 2018 | In vivo | Memory | Juglans regia | walnut suspension | 400 mg/kg/day | 4 weeks |
|
| Jong Min Kim Kim et al., (2020) |
2020 | In vivo | Memory | Juglans regia L. | Leaf extract | 20 mg/kg of body weight oral administration or 10 mg/kg by injection |
Group A 41 days and group B 31 days |
|
| Zahra Batool Batool et al., (2017) |
2017 | In vivo | Memory | – | – | 400 mg/kg/day | 28 days |
|
| Ansari, Javad Mohajer (Ansari et al., 2016) | 2016 | In vivo | Traumatic Brain Injury | Juglans regia | 6% of daily food | Walnut kernel (WK) | 60 days |
|
| Papoutsi, Z (Papoutsi et al., 2008) | 2008 | In vitro | Traumatic Brain Injury | Juglans regia L. | 10–200 mg/ml | Walnut methanolic extract | 24days |
|
| Aydın, Sevinç (Aydın et al., 2015) | 2015 | In vivo | Traumatic Brain Injury | Juglans regia L. | The fruit was frozen (50 g) and then prepared with a special method | Walnut fruits extract | 45 days |
|
| Li et al (Li et al., 2017). | 2017 | In vivo | Alzheimer’s disease | Juglans regia L | walnut protein hydrolysate | Low dose (0.20 g/kg) medium dose (0.33 g/kg) high dose (0.66 g/kg) |
5 days |
|
| Balu Muthaiyah (Muthaiyah et al., 2014) | 2014 | In vivo | Alzheimer’s Disease | Juglans regia L. | English walnut kernels | 6% or 9% walnut-enriched diet | 9 – 10 months |
|
| Ilkay Eedoghan Orhan (Orhan et al., 2011) | 2011 | In vitro | Alzheimer’s Disease | Juglans regia L. | leaves and fruits of J. regia(Dichloromethane- Acetone- Ethyl acetate-Methanol) | 50, 100 and 200 mg /ml | – |
|
| Balu Muthaiyah (Muthaiyah et al., 2011) | 2011 | In vitro | Alzheimer’s Disease | Juglans regia L. | Walnut extracts such as Phenolics, α-linolenic acid- flavonoids and gamma tocopherol | 2 and 4 μg GAE | 12 and 24 h |
|
| Sujatha Rajaram (Rajaram et al., 2017) | 2017 | In vitro (single blind randomized clinical trial) | Alzheimer’s Disease | Juglans regia L. | – | 15% of daily energy intake as walnuts | 2 years |
|
| Neha Chauhan (Chauhan et al., 2004) | 2004 | In vitro | Alzheimer’s Disease | Juglans regia L. | (Methanolic Extract of Walnut) MEOW | (2.5, 5.0, 10.0 ml) of MEOW | 1–2–3 days |
|
| Mirazkar D. Pandareesh (Pandareesh et al., 2018) | 2018 | In vivo | Alzheimer’s Disease | Juglans regia L. | – | 6%and 9% walnuts enriched diet | 5, 10, or 15 months |
|
| Lv et al (Lv et al., 2021). | 2021 | Animal study | Alzheimer's disease | Walnut | 800 and 1600 mg/kg/d | 6 weeks |
|
|
| Chauhan et al. (2010) | 2010 | Animal study | Alzheimer's disease | Walnut | custom-mix diets (pellets) containing 6% walnuts or 9% walnuts | 4 months |
|
|
| Sala-Vila et al (Sala-Vila et al., 2020b). | 2020 | Cohort Study | Alzheimer's disease | Walnut | ≥ 1 serving/week |
|
||
| Min Wang Wang et al., (2019) |
2019 | In vitro In vivo |
Alzheimer | Juglans regia | PW5 WPH |
80/ 400 pw5 mg/kg/day | 12 weeks |
|
| Juan Zou Zou et al., (2016) |
2016 | In vivo | Alzheimer | Juglans Sigilata Dode | walnut peptides | 200/ 400/ 800 mg/kg/ day | 5 weeks |
|
| Bishop, Nicholas J (Bishop and Zuniga, 2021) | 2021 | Human study | Investigation of cognitive function in older adults | – | walnut | (none, low intake (0·01–0·08 1 oz. servings per day) and moderate intake (>0·08 1 oz. servings per day) | – |
|
| Ji Liu Liu et al., (2019a) |
2019 | In vivo | Oxidative stress | – | Walnut seed coat Walnut seed |
100 (WSC) mg/kg/day 600 (WS) mg/kg/day |
8 weeks |
|
| Shuguang Wang (Wang et al., 2021) |
2021 | In vivo | Oxidative stress | Juglans regia | WPH | 333, 666 mg/ kg/ day |
21 days |
|
| Shuguang Wang Wang et al., (2020) |
2020 | In vivo | Oxidative stress | Juglans regia | WPH | 666 mg/kg and 400 mg/kg |
21 days |
|
| Dayong Ren Ren et al., (2018) |
2018 | In vivo | Oxidative stress | Juglans mandshurica Maxim. | MWHP | 200, 400, 800 mg/kg/day | 30 days |
|
| Jahanbani, Raheleh (Jahanbani et al., 2021) | 2021 | In vivo | Anti-seizure effects | Juglans regia L. | 5, 10, 20, 50, 100 mg/kg | walnut peptide | A month |
|
5. Conclusion
Walnut is a beneficial compound for health, especially the nervous system. In this study effects of Walnut on ANDs such as AD, PD, GBM, epileptic seizures, MS, neurotoxicity, neuroinflammation, and traumatic brain injuries as well as their potential effects on memory and cognition were reviewed. Evidence suggests that Walnut can decrease the risk of oxidative stress and neuroinflammation caused by Aβ protein deposition in the human brain, preventing AD. Walnut and its compounds (such as vitamin E, melatonin, ellagitannins, and urolithins) prevent the formation of fibrillar Aβ -protein and defibrillate them. It contains antioxidant compounds that prevent oxidative stress and damage to lipids and proteins caused by Aβ fibrils and the MAO-B enzyme, which is involved in PD (Djedjibegovic et al., 2020, Pandareesh et al., 2018, Muthaiyah et al., 2011). As a result, it can play an important role in avoiding Aβ-dependent DNA damage, ROS production by MAO-B, and neuronal cell death (Tatton et al., 2003, Chauhan and Chauhan, 2012). In PD, dopaminergic neurons undergo cell death.
In neurodegenerative diseases, the activity of the MAO-B enzyme increases, causing oxidative stress and cell death. Compounds in Walnut work as antioxidant properties and MAO inhibitor factors. These products with anti-inflammatory compounds are effective in treating neuroinflammatory disorders like MS (Ceci et al., 2020). More importantly, Walnut extracts have potential antiproliferative effects on the most aggressive primary cerebral neoplasm, GBM. The potential therapeutic agent can trigger apoptosis in glioma tumor cells and limit tumor migration by restricting angiogenesis and improving the quality of life and life expectancy of patients with GBM. Oxidative stress is associated with the appearance of epilepsy and seizure development. Walnuts can protect neurons by increasing the seizure-induction threshold and reducing the severity of seizures to an acceptable amount.
Walnuts contain alpha-linolenic acid and omega-3, which can protect cells against cisplatin-induced neurotoxicity. Also, micronutrients can reduce the toxicity of metals like cadmium. The results show that Walnut can improve the speed of learning and, with the effect of its proteins, increase the synthesis of acetylcholine and decrease its destruction.
In this review, it has been shown that Walnut has beneficial effects on ANDs. Also, it can act as a preventive compound for these diseases with no significant side effects. We suggest that further in vivo and in vitro studies and clinical trials, should be carried out to consider the underlying mechanisms of Walnut in these disorders and gain a better understanding of this product’s effects on human health.
Funding
None.
Conflict of Interest
None.
Contributor Information
Amirmohammad Khalaji, Email: amirm.khalaji@gmail.com.
Niloofar Deravi, Email: niloofarderavi@sbmu.ac.ir.
References
- Anon, 2022; 10.1016@S1474–44221930411-9.pdf.
- Ansari J.M., Eftekhar-Vaghefi S.H., Shahrokhi N., Basiri M., Pour F.M., Asadi-Shekaari M. Pre-treatment effects of walnut kernel (Juglans regia) on brain edema, neuronal death and neurological scores in male rat after traumatic brain injury. J. Appl. Pharm. Sci. 2016;6(10):102–106. [Google Scholar]
- Asadi-Shekaari M., Kalantaripour T.P., Nejad F.A., Namazian E., Eslami A. The anticonvulsant and neuroprotective effects of walnuts on the neurons of rat brain cortex. Avicenna J. Med. Biotechnol. 2012;4(3):155. [PMC free article] [PubMed] [Google Scholar]
- Asadi-Shekaari M., Eslami A., Kalantaripour T., Joukar S.J.M.P., 2014, Practice. Potential mechanisms involved in the anticonvulsant effect of walnut extract on pentylenetetrazole-induced seizure, 23(6):538–542. [DOI] [PMC free article] [PubMed]
- Asadi-Shekaari M., Kalantaripour T.P., Nejad F.A., Namazian E., Eslami AJAJoMB, 2012b, The anticonvulsant and neuroprotective effects of walnuts on the neurons of rat brain cortex, 4(3):155. [PMC free article] [PubMed]
- Atkinson G.P., Nozell S.E., Harrison D.K., Stonecypher M.S., Chen D., Benveniste E.N.J.O., 2009, The prolyl isomerase Pin1 regulates the NF-κB signaling pathway and interleukin-8 expression in glioblastoma, 28(42):3735–3745. [DOI] [PMC free article] [PubMed]
- Aydın S., Gökçe Z., Yılmaz Ö.J.T.J.O.B., 2015, The effects of Juglans regia L.(walnut) extract on certain biochemical paramaters and in the prevention of tissue damage in brain, kidney, and liver in CCl4 applied Wistar rats, 40(3):241–250.
- Bahramnjead E., Kazemi Roodsari S., Rahimi N., Etemadi P., Aghaei I., Dehpour ARJNr Effects of modafinil on clonic seizure threshold induced by pentylenetetrazole in mice: involvement of glutamate, nitric oxide. GABA, serotonin Pathw. 2018;43(11):2025–2037. doi: 10.1007/s11064-018-2623-7. [DOI] [PubMed] [Google Scholar]
- Bahremand A., Nasrabady S.E., Ziai P., Rahimian R., Hedayat T., Payandemehr B., et al., 2010, Involvement of nitric oxide-cGMP pathway in the anticonvulsant effects of lithium chloride on PTZ-induced seizure in mice, 89(2–3):295–302. [DOI] [PubMed]
- Bashkatova V., Narkevich V., Vitskova G., Vanin AJPiN-P, 2003, Psychiatry B. The influence of anticonvulsant and antioxidant drugs on nitric oxide level and lipid peroxidation in the rat brain during penthylenetetrazole-induced epileptiform model seizures, 27(3):487–492. [DOI] [PubMed]
- Bashkatova V., Vitskova G., Narkevich V., Vanin A., Mikoyan V., Rayevsky K.J.J.O.M.N., 2000, Nitric oxide content measured by ESR-spectroscopy in the rat brain is increased during pentylenetetrazole-induced seizures, 14(3):183–190. [DOI] [PubMed]
- Batool Z., Agha F., Ahmad S., Liaquat L., Tabassum S., Khaliq S., et al. Attenuation of cadmium-induced decline in spatial, habituation and recognition memory by long-term administration of almond and walnut supplementation: Role of cholinergic function. Pak. J. Pharm. Sci. 2017;30(1 Suppl):273–279. [PubMed] [Google Scholar]
- Batool Z., Agha F., Tabassum S., Batool T.S., Siddiqui R.A., Haider S. Prevention of cadmium-induced neurotoxicity in rats by essential nutrients present in nuts. Acta Neurobiol. Exp. 2019;79(2):169–183. [PubMed] [Google Scholar]
- Becker E.B., Bonni A. Pin1 mediates neural-specific activation of the mitochondrial apoptotic machinery. Neuron. 2006;49(5):655–662. doi: 10.1016/j.neuron.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Berg D.A., Belnoue L., Song H., Simon A. Neurotransmitter-mediated control of neurogenesis in the adult vertebrate brain. Development. 2013;140(12):2548–2561. doi: 10.1242/dev.088005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhullar K.S., Rupasinghe H. Polyphenols: multipotent therapeutic agents in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2013;2013 doi: 10.1155/2013/891748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop N.J., Zuniga K.E. Investigating walnut consumption and cognitive trajectories in a representative sample of older US adults. Public Health Nutr. 2021;24(7):1741–1752. doi: 10.1017/S1368980020001287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmachari G. CRC Press,; Boca Raton: 2013. Chemistry and Pharmacology of Naturally Occurring Bioactive Compounds. [Google Scholar]
- Browne T., Holmes G.J.N.E.J.O.M. Prim. care: Epilepsy. 2001;344(15):1145–1151. doi: 10.1056/NEJM200104123441507. [DOI] [PubMed] [Google Scholar]
- Ceci C., Graziani G., Faraoni I., Cacciotti I. Strategies to improve ellagic acid bioavailability: from natural or semisynthetic derivatives to nanotechnological approaches based on innovative carriers. Nanotechnology. 2020;31(38) doi: 10.1088/1361-6528/ab912c. [DOI] [PubMed] [Google Scholar]
- Chatrabnous N., Yazdani N., Tavallali V., Vahdati K.J.L. Preserv. Qual. fresh Walnuts Using Plant Extr. 2018;91:1–7. [Google Scholar]
- Chatrabnous N., Yazdani N., Vahdati K.J.J.O.N. Determ. Nutr. Value Oxid. Stab. fresh Walnut. 2018;9(1):11–20. [Google Scholar]
- Chauhan N., Wang K.C., Wegiel J., Malik M.N. Walnut extract inhibits the fibrillization of amyloid beta-protein, and also defibrillizes its preformed fibrils. Curr. Alzheimer Res. 2004;1(3):183–188. doi: 10.2174/1567205043332144. PMID: 15975066. [DOI] [PubMed] [Google Scholar]
- Chauhan A., Chauhan V. Brain Aging and Therapeutic Interventions. Springer,; 2012. Potential beneficial effects of a diet with walnuts in aging and Alzheimer’s disease; pp. 239–252. [Google Scholar]
- Chen R., Cohen A.L., Colman H.J.Ctoio. Target. Ther. Patients High. -grade gliomas: , Present, Future. 2016;17(8):1–11. doi: 10.1007/s11864-016-0418-0. [DOI] [PubMed] [Google Scholar]
- Choi J.G., Park G., Kim H.G., Oh D.-S., Kim H., Oh M.S. In vitro and in vivo neuroprotective effects of walnut (Juglandis semen) in models of Parkinson’s disease. Int. J. Mol. Sci. 2016;17(1):108. doi: 10.3390/ijms17010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S.Y., Son T.G., Park H.R., Jang Y.J., Oh S.B., Jin B., et al. Naphthazarin has a protective effect on the 1–methyl‐4–phenyl‐1, 2, 3, 4–tetrahydropyridine‐induced Parkinson's disease model. J. Neurosci. Res. 2012;90(9):1842–1849. doi: 10.1002/jnr.23061. [DOI] [PubMed] [Google Scholar]
- Damier P., Kastner A., Agid Y., Hirsch E.C. Does monoamine oxidase type B play a role in dopaminergic nerve cell death in Parkinson's disease. Neurology. 1996;46(5):1262. doi: 10.1212/wnl.46.5.1262. [DOI] [PubMed] [Google Scholar]
- De Matteis V., Rizzello L., Ingrosso C., Liatsi-Douvitsa E., De Giorgi M.L., De, Matteis G., et al. Cultiv. -Depend. Anticancer Antibact. Prop. Silver Nanopart. Synth. Using Leaves Differ. Olea Eur. trees. 2019;9(11):1544. doi: 10.3390/nano9111544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delanty N., MAJAon Dichter. Antioxidant therapy in neurologic disease. 2000;57(9):1265–1270. doi: 10.1001/archneur.57.9.1265. [DOI] [PubMed] [Google Scholar]
- Devi P.U., Manocha A., Vohora D.J., 2008, Eoop. Seizures, antiepileptics, antioxidants and oxidative stress: an insight for researchers, 9(18):3169–3177. [DOI] [PubMed]
- Djedjibegovic J., Marjanovic A., Panieri E., Saso L. Ellagic acid-derived urolithins as modulators of oxidative stress. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/5194508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essa M.M., Vijayan R.K., Castellano-Gonzalez G., Memon M.A., Braidy N., Guillemin G.J. Neuroprotective effect of natural products against Alzheimer’s disease. Neurochem. Res. 2012;37(9):1829–1842. doi: 10.1007/s11064-012-0799-9. [DOI] [PubMed] [Google Scholar]
- Essa M.M., Subash S., Dhanalakshmi C., Manivasagam T., Al-Adawi S., Guillemin G.J., et al. Dietary supplementation of walnut partially reverses 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine induced neurodegeneration in a mouse model of Parkinson’s disease. Neurochem. Res. 2015;40(6):1283–1293. doi: 10.1007/s11064-015-1593-2. [DOI] [PubMed] [Google Scholar]
- Essa M.M., Subash S., Dhanalakshmi C., et al. Correction to: Dietary Supplementation of Walnut Partially Reverses 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine Induced Neurodegeneration in a Mouse Model of Parkinson’s Disease. Neurochem. Res. 2019;44(2684) doi: 10.1007/s11064-019-02887-1. [DOI] [PubMed] [Google Scholar]
- Feigin V.L., Vos T., Nichols E., Owolabi M.O., Carroll W.M., Dichgans M., et al. The global burden of neurological disorders: translating evidence into policy. Lancet Neurol. 2020;19(3):255–265. doi: 10.1016/S1474-4422(19)30411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L., Wang X., Peng F., Liao J., Nai Y., Lei H., et al. Walnut Protein Hydrolysates Play a Protective Role on Neurotoxicity Induced by d-Galactose and Aluminum Chloride in Mice. Molecules. 2018;23:9. doi: 10.3390/molecules23092308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D.R., Poulose S.M., Bielinski D.F., Shukitt-Hale B. Serum metabolites from walnut-fed aged rats attenuate stress-induced neurotoxicity in BV-2 microglial cells. Nutr. Neurosci. 2017;20(2):103–109. doi: 10.1179/1476830514Y.0000000150. [DOI] [PubMed] [Google Scholar]
- Fisher D., Cahoon D., Shukitt-Hale B.J.C.D.I.N., 2021, Walnut Oil and Blueberry Treatments Have Beneficial Individual but Not Synergistic Effects on Neuroinflammation In Vitro, 5(Supplement_2):904–.
- Fotuhi M., Do D. Factors determining the size of the hippocampus. Alzheimer'S. Dement. 2010;4(6):S69. [Google Scholar]
- Ganji A., Farahani I., Palizvan M.R., Ghazavi A., Ejtehadifar M., Ebrahimimonfared M., et al. Therapeutic effects of walnut oil on the animal model of multiple sclerosis. Nutr. Neurosci. 2019;22(3):215–222. doi: 10.1080/1028415X.2017.1371389. [DOI] [PubMed] [Google Scholar]
- Genovese C., Cambria M.T., D'angeli F., Addamo A.P., Malfa G.A., Siracusa L., et al. Double Eff. Walnut septum Extr. (Juglans regia L. ) Count. A172 glioblastoma Cell Surviv. Bact. Growth. 2020;57(5):1129–1144. doi: 10.3892/ijo.2020.5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Saminathan H., Kanthasamy A., Anantharam V., Jin H., Sondarva G., et al. The peptidyl-prolyl isomerase Pin1 up-regulation and proapoptotic function in dopaminergic neurons: relevance to the pathogenesis of Parkinson disease. J. Biol. Chem. 2013;288(30):21955–21971. doi: 10.1074/jbc.M112.444224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Spillantini M.G. A century of Alzheimer's disease. Science. 2006;314(5800):777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- González-Sarrías A., Núñez-Sánchez M.A.N., Tomás-Barberán F.A., Espín J.C. Neuroprotective effects of bioavailable polyphenol-derived metabolites against oxidative stress-induced cytotoxicity in human neuroblastoma SH-SY5Y cells. J. Agric. Food Chem. 2017;65(4):752–758. doi: 10.1021/acs.jafc.6b04538. [DOI] [PubMed] [Google Scholar]
- Habibie A., Yazdani N., Saba M.K., Vahdati K.J.S.H. Ascorbic Acid. Inc. Walnut Green. husk Extr. Preserv. Postharvest Qual. cold Storage fresh Walnut Kernels. 2019;245:193–199. [Google Scholar]
- Haider S., Batool Z., Ahmad S., Siddiqui R.A., Haleem D.J. Walnut supplementation reverses the scopolamine-induced memory impairment by restoration of cholinergic function via mitigating oxidative stress in rats: a potential therapeutic intervention for age related neurodegenerative disorders. Metab. Brain Dis. 2018;33(1):39–51. doi: 10.1007/s11011-017-0120-3. [DOI] [PubMed] [Google Scholar]
- Hanif F., Muzaffar K., Perveen K., Malhi S.M., Simjee S.U.J.A.P.J.O.C.P.A. Glioblastoma multiforme: a Rev. its Epidemiol. Pathog. Clin. Present. Treat. 2017;18(1):3. doi: 10.22034/APJCP.2017.18.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harandi S., Abbasnejad M., Mirnajafi-Zadeh J., Esmaeili-Mahani S., Esmaeilpour K., Asadi-Shekaari M., et al., 2013, Inhibitory effects of walnut consumption on amygdala kindling model of epilepsy in male Wistar rats, 17(7):360–369.
- Harandi S., Abbasnejad M., Mirnajafi-Zadeh J., Esmaeilpour K., Masoumi Y., Asadi-Shekaari M., et al., 2013b, Effect of walnut (Juglans regia L) consumption on anticonvulsant low-frequency stimulation in rats, 17(4):177–187.
- Hsieh C.-L., Tang N.-Y., Chiang S.-Y., Hsieh C.-T., Lin J.-G.J.L.S. Anticonvulsive and free radical scavenging actions of two herbs, Uncaria rhynchophylla (MIQ) Jack and Gastrodia elata Bl., in kainic acid-treated rats. 1999;65(20):2071–2082. doi: 10.1016/s0024-3205(99)00473-7. [DOI] [PubMed] [Google Scholar]
- Hu M., Liu Z., Lv P., Wang H., Zhu Y., Qi Q., et al. Autophagy and Akt/CREB signalling play an important role in the neuroprotective effect of nimodipine in a rat model of vascular dementia. Behav. Brain Res. 2017;325:79–86. doi: 10.1016/j.bbr.2016.11.053. [DOI] [PubMed] [Google Scholar]
- Ishimoto H., Shibata M., Myojin Y., Ito H., Sugimoto Y., Tai A., et al. In vivo anti-inflammatory and antioxidant properties of ellagitannin metabolite urolithin A. Bioorg. Med. Chem. Lett. 2011;21(19):5901–5904. doi: 10.1016/j.bmcl.2011.07.086. [DOI] [PubMed] [Google Scholar]
- Jahanban-Esfahlan A., Ostadrahimi A., Tabibiazar M., Amarowicz R.J.Ijoms. A comprehensive review on the chemical constituents and functional uses of walnut (Juglans spp.) husk, 2019; 20(16):3920. [DOI] [PMC free article] [PubMed]
- Jahanbani R., Ghaffari S.M., Salami M., Vahdati K., Sepehri H., Sarvestani N.N., et al. Antioxid. Anticancer Act. Walnut (Juglans regia L. ) Protein hydrolysates Using Differ. Proteases. 2016;71(4):402–409. doi: 10.1007/s11130-016-0576-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanbani R., Ghaffari M., Vahdati K., Salami M., Khalesi M., Sheibani N., et al. Kinet. Study Protein Hydrolys. Inhib. angiotensin Convert. Enzym. Pept. hydrolysate Extr. Walnut. 2018;24(1):77–85. [Google Scholar]
- Jahanbani R., Bahramnejad E., Rahimi N., Shafaroodi H., Sheibani N., Moosavi-Movahedi A.A., et al. Anti-seizure effects of walnut peptides in mouse models of induced seizure: The involvement of GABA and nitric oxide pathways. Epilepsy Res. 2021;176 doi: 10.1016/j.eplepsyres.2021.106727. [DOI] [PubMed] [Google Scholar]
- Joseph J.A., Shukitt-Hale B., Willis L.M. Grape juice, berries, and walnuts affect brain aging and behavior. J. Nutr. 2009;139(9):1813S–1817SS. doi: 10.3945/jn.109.108266. [DOI] [PubMed] [Google Scholar]
- Joseph J.D., Yeh E.S., Swenson K.I., Means A.R. The peptidyl-prolyl isomerase Pin1. Prog. Cell Cycle Res. 2003;5:477–487. [PubMed] [Google Scholar]
- Kim K.B., Lee S., Kim J.H. Neuroprotective effects of urolithin A on H2O2-induced oxidative stress-mediated apoptosis in SK-N-MC cells. Nutr. Res. Pract. 2020;14(1):3–11. doi: 10.4162/nrp.2020.14.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Ladomersky E., Mozny A., Kocherginsky M., O’Shea K., Reinstein Z.Z., et al. Glioblastoma age-Relat. Neurol. Disord. adults. 2021;3(1):vdab125. doi: 10.1093/noajnl/vdab125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalska M., Owecki M., Prendecki M., Wize K., Nowakowska J., Kozubski W., et al. Aging and neurological diseases. Senescence-Physiol. Or. Pathol. 2017:63–94. [Google Scholar]
- Leray E., Moreau T., Fromont A., Edan G. Epidemiology of multiple sclerosis. Rev. Neurol. 2016;172(1):3–13. doi: 10.1016/j.neurol.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Li W., Zhao T., Zhang J., Xu J., Sun-Waterhouse D., Zhao M., et al. Effect of walnut protein hydrolysate on scopolamine-induced learning and memory deficits in mice. J. Food Sci. Technol. 2017;54(10):3102–3110. doi: 10.1007/s13197-017-2746-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian X.Y., Zhang Z.Z., Stringer J.L.J.E. Anticonvulsant activity of ginseng on seizures induced by chemical convulsants. 2005;46(1):15–22. doi: 10.1111/j.0013-9580.2005.40904.x. [DOI] [PubMed] [Google Scholar]
- Liao J., Nai Y., Feng L., Chen Y., Li M., Xu H. Walnut oil prevents scopolamine-induced memory dysfunction in a mouse model. Molecules. 2020;25:7. doi: 10.3390/molecules25071630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Guo Y., Zhao F., Qin H., Lu H., Fang L., et al. Potential mechanisms mediating the protective effects of a peptide from walnut (Juglans mandshurica Maxim.) against hydrogen peroxide induced neurotoxicity in PC12 cells. Food Funct. 2019;10(6):3491–3501. doi: 10.1039/c8fo02557f. [DOI] [PubMed] [Google Scholar]
- Liu J., Chen D., Wang Z., Chen C., Ning D., Zhao S. Protective effect of walnut on d‐galactose‐induced aging mouse model. Food Sci. Nutr. 2019;7(3):969–976. doi: 10.1002/fsn3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löscher W.J.S. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. 2011;20(5):359–368. doi: 10.1016/j.seizure.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Luan F., Wang Z., Yang Y., Ji Y., Lv H., Han K., et al. Juglans mandshurica Maxim.: A Rev. its Tradit. Usages, Phytochem. Const., Pharmacol. Prop. 2021;11 [Google Scholar]
- Lv H., Chen D., Xu C., Ma Y., Yang J., Chen C., et al., 2021, Positive Effects of Walnut Meal Extracts on Memory Impairment of Alzheimer's Disease Mice Induced by D-Galactose.
- Macleod S., Appleton R.E.J.A.O.D.I.C.-E. Pract. N. antiepileptic Drugs. 2007;92(6):182–188. doi: 10.1136/adc.2007.106880. [DOI] [PubMed] [Google Scholar]
- Mallajosyula J.K., Kaur D., Chinta S.J., Rajagopalan S., Rane A., Nicholls D.G., et al. MAO-B elevation in mouse brain astrocytes results in Parkinson's pathology. PloS One. 2008;3(2) doi: 10.1371/journal.pone.0001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B.R.J.C.L.L.I.N. Memory dysfunction. 2015;21(3 Behavioral Neurology and Neuropsychiatry):613. [DOI] [PMC free article] [PubMed]
- Mohammed Junaidh K., Muhammed Sajid E., Mohammed Ashif A.J.E. A Rev. Walnut.: Its Pharmacol. Prop. role Hum. life. 2022;2809:3. [Google Scholar]
- Mucke L. Alzheimer's disease. Nature. 2009;461(7266):895–897. doi: 10.1038/461895a. [DOI] [PubMed] [Google Scholar]
- Muthaiyah B., Essa M.M., Chauhan V., Chauhan A. Protective effects of walnut extract against amyloid beta peptide-induced cell death and oxidative stress in PC12 cells. Neurochem. Res. 2011;36(11):2096–2103. doi: 10.1007/s11064-011-0533-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthaiyah B., Essa M.M., Lee M., Chauhan V., Kaur K., Chauhan A. Dietary supplementation of walnuts improves memory deficits and learning skills in transgenic mouse model of Alzheimer's disease. J. Alzheimer'S. Dis. 2014;42(4):1397–1405. doi: 10.3233/JAD-140675. [DOI] [PubMed] [Google Scholar]
- Nagatsu T., Sawada M. Molecular mechanism of the relation of monoamine oxidase B and its inhibitors to Parkinson’s disease: possible implications of glial cells. Oxid. Stress Neuroprotection. 2006:53–65. doi: 10.1007/978-3-211-33328-0_7. [DOI] [PubMed] [Google Scholar]
- Naletova I., Cucci L.M., D’Angeli F., Anfuso C.D., Magrì A., La Mendola D., et al. A tunable nanoplatform nanogold Funct. angiogenin Pept. anti-angiogenic Ther. brain Tumours. 2019;11(9):1322. doi: 10.3390/cancers11091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z.-J., Zhang Y.-G., Chen S.-X., Thakur K., Wang S., Zhang J.-G., et al. Exploration of walnut components and their association with health effects. Crit. Rev. Food Sci. Nutr. 2021;62:1–17. doi: 10.1080/10408398.2021.1881439. [DOI] [PubMed] [Google Scholar]
- Orhan I.E., Suntar I.P., Akkol E.K. In vitro neuroprotective effects of the leaf and fruit extracts of Juglans regia L.(walnut) through enzymes linked to Alzheimer's disease and antioxidant activity. Int. J. Food Sci. Nutr. 2011;62(8):781–786. doi: 10.3109/09637486.2011.585964. [DOI] [PubMed] [Google Scholar]
- Osonoe K., Mori N., Suzuki K., Osonoe MJBr, 1994, Antiepileptic effects of inhibitors of nitric oxide synthase examined in pentylenetetrazol-induced seizures in rats, 663(2):338–340. [DOI] [PubMed]
- Ostrom Q.T., Patil N., Cioffi G., Waite K., Kruchko C., Barnholtz-Sloan J.S.J.N.-O. CBTRUS Stat. Rep.: Prim. brain Other Cent. Nerv. Syst. Tumors Diagn. U. S. 2013–2017. 2020;22(Supplement_1):iv1–iv96. [Google Scholar]
- Oyrer J., Maljevic S., Scheffer I.E., Berkovic S.F., Petrou S., Reid C.A.J.P.R. Ion.-. Channels Genet. Epilepsy.: Genes Mech. Dis. -Target. Ther. 2018;70(1):142–173. doi: 10.1124/pr.117.014456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakrah S., Rahemi M., Nabipour A., Zahedzadeh F., Kakavand F., Vahdati K.J.J.O.F.P., et al. Sens. Nutr. Attrib. Persian Walnut Kernel Influ. Matur. Stage, Dry. Method, Cultiv. 2021;45(6) [Google Scholar]
- Pakrah S., Rahemi M., Haghjooyan R., Nabipour A., Kakavand F., Zahedzadeh F., et al. Comp. Phys. Biochem. Prop. Dried Fresh Kernels Persian Walnut. 2022:1–8. [Google Scholar]
- Pandareesh M.D., Chauhan V., Chauhan A. Walnut supplementation in the diet reduces oxidative damage and improves antioxidant status in transgenic mouse model of Alzheimer’s disease. J. Alzheimer's Dis. 2018;64(4):1295–1305. doi: 10.3233/JAD-180361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoutsi Z., Kassi E., Chinou I., Halabalaki M., Skaltsounis L., Moutsatsou P. Walnut extract (Juglans regia L.) and its component ellagic acid exhibit anti-inflammatory activity in human aorta endothelial cells and osteoblastic activity in the cell line KS483. Br. J. Nutr. 2008;99(4):715–722. doi: 10.1017/S0007114507837421. [DOI] [PubMed] [Google Scholar]
- Pavan V., Ribaudo G., Zorzan M., Redaelli M., Pezzani R., Mucignat-Caretta C., et al. Antiproliferative activity of Juglone derivatives on rat glioma. 2017;31(6):632–638. doi: 10.1080/14786419.2016.1214830. [DOI] [PubMed] [Google Scholar]
- Petrosillo G., Matera M., Casanova G., Ruggiero F., Paradies G. Mitochondrial dysfunction in rat brain with aging: involvement of complex I, reactive oxygen species and cardiolipin. Neurochem. Int. 2008;53(5):126–131. doi: 10.1016/j.neuint.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Polman C.H., Reingold S.C., Banwell B., Clanet M., Cohen J.A., Filippi M., et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulose S.M., Bielinski D.F., Shukitt-Hale B. Walnut diet reduces accumulation of polyubiquitinated proteins and inflammation in the brain of aged rats. J. Nutr. Biochem. 2013;24(5):912–919. doi: 10.1016/j.jnutbio.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Poulose S.M., Miller M.G., Shukitt-Hale B. Role of walnuts in maintaining brain health with age. J. Nutr. 2014;144(4):561S–566SS. doi: 10.3945/jn.113.184838. [DOI] [PubMed] [Google Scholar]
- Qiang L., Yang Y., Ma Y.-J., Chen F.-H., Zhang L.-B., Liu W., et al. Isol. Charact. Cancer stem Cells Hum. glioblastoma Cell lines. 2009;279(1):13–21. doi: 10.1016/j.canlet.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Rajaram S., Valls-Pedret C., Cofán M., Sabaté J., Serra-Mir M., Pérez-Heras A.M., et al. The Walnuts and Healthy Aging Study (WAHA): protocol for a nutritional intervention trial with walnuts on brain aging. Front. Aging Neurosci. 2017;8:333. doi: 10.3389/fnagi.2016.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recknagel R.O., Glende E.A., Jr, Dolak J.A., Waller R.L.J.P. therapeutics. Mech. Carbon tetrachloride Toxic. 1989;43(1):139–154. doi: 10.1016/0163-7258(89)90050-8. [DOI] [PubMed] [Google Scholar]
- Ren D., Zhao F., Liu C., Wang J., Guo Y., Liu J., et al. Antioxidant hydrolyzed peptides from Manchurian walnut (Juglans mandshurica Maxim.) attenuate scopolamine-induced memory impairment in mice. J. Sci. Food Agric. 2018;98(13):5142–5152. doi: 10.1002/jsfa.9060. [DOI] [PubMed] [Google Scholar]
- Ritesh K., Suganya A., Dileepkumar H., Rajashekar Y., Shivanandappa T.J.T.R., 2015, A single acute hepatotoxic dose of CCl4 causes oxidative stress in the rat brain, 2:891–895. [DOI] [PMC free article] [PubMed]
- Rock K., McArdle O., Forde P., Dunne M., Fitzpatrick D., O'Neill B., et al. A Clin. Rev. Treat. Outcomes glioblastoma multiforme— Valid. A Non-Trial Popul. Results A Random Phase III Clin. Trial: Has. A more Radic. Approach Improv. Surviv. ? 2012;85(1017):e729–e733. doi: 10.1259/bjr/83796755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryo A., Togo T., Nakai T., Hirai A., Nishi M., Yamaguchi A., et al. Prolyl-isomerase Pin1 accumulates in Lewy bodies of Parkinson disease and facilitates formation of α-synuclein inclusions. J. Biol. Chem. 2006;281(7):4117–4125. doi: 10.1074/jbc.M507026200. [DOI] [PubMed] [Google Scholar]
- Sala-Vila A., Valls-Pedret C., Rajaram S., Coll-Padrós N., Cofán M., Serra-Mir M., et al. Effect of a 2-year diet intervention with walnuts on cognitive decline. The Walnuts And Healthy Aging (WAHA) study: a randomized controlled trial. Am. J. Clin. Nutr. 2020;111(3):590–600. doi: 10.1093/ajcn/nqz328. [DOI] [PubMed] [Google Scholar]
- Sala-Vila A., Crous-Bou M., Sánchez-Benavides G., de Arenaza-Urquijo E.M., Suárez-Calvet M., Milà-Alomà M., et al. Eating a Weekly Serving of Walnuts Relates to Beneficial Brain Imaging Phenotypes in a Cohort at Increased Risk of Alzheimer's Disease. Curr. Dev. Nutr. 2020;4(Supplement_2):1234. [Google Scholar]
- Sameri M.J., Sarkaki A., Farbood Y., Mansouri S. Motor disorders and impaired electrical power of pallidal EEG improved by gallic acid in animal model of Parkinson's disease. Pak. J. Biol. Sci.: PJBS. 2011;14(24):1109–1116. doi: 10.3923/pjbs.2011.1109.1116. [DOI] [PubMed] [Google Scholar]
- Schapira A., Mann V., Cooper J., Dexter D., Daniel S., Jenner P., et al. Anatomic and disease specificity of NADH CoQ1 reductase (complex I) deficiency in Parkinson's disease. J. Neurochem. 1990;55(6):2142–2145. doi: 10.1111/j.1471-4159.1990.tb05809.x. [DOI] [PubMed] [Google Scholar]
- Schapira A.H. Monoamine oxidase B inhibitors for the treatment of Parkinson’s disease. CNS Drugs. 2011;25(12):1061–1071. doi: 10.2165/11596310-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Shabab T., Khanabdali R., Moghadamtousi S.Z., Kadir H.A., Mohan G. Neuroinflammation pathways: a general review. Int. J. Neurosci. 2017;127(7):624–633. doi: 10.1080/00207454.2016.1212854. [DOI] [PubMed] [Google Scholar]
- Shabani M., Nazeri M., Parsania S., Razavinasab M., Zangiabadi N., Esmaeilpour K., et al. Walnut consumption protects rats against cisplatin-induced neurotoxicity. Neurotoxicology. 2012;33(5):1314–1321. doi: 10.1016/j.neuro.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Shekh-Ahmad T., Lieb A., Kovac S., Gola L., Wigley W.C., Abramov A.Y., et al. Combination antioxidant therapy prevents epileptogenesis and modifies chronic epilepsy. 2019;26 doi: 10.1016/j.redox.2019.101278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichiri M. The role of lipid peroxidation in neurological disorders. J. Clin. Biochem. Nutr. 2014 doi: 10.3164/jcbn.14-10. 14-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorvon S.D.J.E. Drug Treat. Epilepsy Century ILAE: Second 50 years, 1959–2009. 2009;50:93–130. doi: 10.1111/j.1528-1167.2009.02042.x. [DOI] [PubMed] [Google Scholar]
- Sian J., Dexter D.T., Lees A.J., Daniel S., Agid Y., Javoy‐Agid F., et al. Alterations in glutathione levels in Parkinson's disease and other neurodegenerative disorders affecting basal ganglia. Annals of Neurology: Official. J. Am. Neurol. Assoc. Child Neurol. Soc. 1994;36(3):348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- Spencer P.S., Lein P. Neurotoxicity. Encycl. Toxicol. 2014 [Google Scholar]
- Tatton W., Chalmers-Redman R., Tatton N. Neuroprotection by deprenyl and other propargylamines: glyceraldehyde-3-phosphate dehydrogenase rather than monoamine oxidase B. J. Neural Transm. 2003;110(5):509–515. doi: 10.1007/s00702-002-0827-z. [DOI] [PubMed] [Google Scholar]
- Thurman D.J., Begley C.E., Carpio A., Helmers S., Hesdorffer D.C., Mu J., et al. The primary prevention of epilepsy: A report of the Prevention Task Force of the International League Against Epilepsy. 2018;59(5):905–914. doi: 10.1111/epi.14068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullman M.J. Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. Am. J. Manag Care. 2013;19(2 Suppl):S15–S20. [PubMed] [Google Scholar]
- Vu D.C., Nguyen T.H., Ho T.L.J.R.A. Overv. Phytochem. Potential Health-Promot. Prop. Black Walnut. 2020;10(55):33378–33388. [Google Scholar]
- Wang J., Liu K., Wang X.-F., Sun D.-J.J.O.R. Juglone reduces Growth Migr. U251 glioblastoma Cells disrupts angiogenesis. 2017;38(4):1959–1966. doi: 10.3892/or.2017.5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Liu J., John A., Jiang Y., Zhu H., Yang B., et al. Structure identification of walnut peptides and evaluation of cellular antioxidant activity. Food Chem. 2022;388 doi: 10.1016/j.foodchem.2022.132943. [DOI] [PubMed] [Google Scholar]
- Wang M., Amakye W.K., Guo L., Gong C., Zhao Y., Yao M., et al. Walnut‐derived peptide PW5 ameliorates cognitive impairments and alters gut microbiota in APP/PS1 transgenic mice. Mol. Nutr. Food Res. 2019;63(18):1900326. doi: 10.1002/mnfr.201900326. [DOI] [PubMed] [Google Scholar]
- Wang S., Zheng L., Zhao T., Zhang Q., Liu Y., Sun B., et al. Inhibitory Effects of Walnut (Juglans regia) Peptides on Neuroinflammation and Oxidative Stress in Lipopolysaccharide-Induced Cognitive Impairment Mice. J. Agric. Food Chem. 2020;68(8):2381–2392. doi: 10.1021/acs.jafc.9b07670. [DOI] [PubMed] [Google Scholar]
- Wang S., Su G., Zhang X., Song G., Zhang L., Zheng L., Zhao M. Characterization and Exploration of Potential Neuroprotective Peptides in Walnut (Juglans regia) Protein Hydrolysate against Cholinergic System Damage and Oxidative Stress in Scopolamine-Induced Cognitive and Memory Impairment Mice and Zebrafish. J. Agric. Food Chem. 2021;69(9):2773–2783. doi: 10.1021/acs.jafc.0c07798. Epub 2021 Mar 1. PMID: 33645974. [DOI] [PubMed] [Google Scholar]
- Weber L.W., Boll M., Stampfl AJCrit, 2003, Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model, 33(2):105–136. [DOI] [PubMed]
- Weinreb O., Amit T., Bar-Am O., Youdim M.B. Rasagiline: a novel anti-Parkinsonian monoamine oxidase-B inhibitor with neuroprotective activity. Prog. Neurobiol. 2010;92(3):330–344. doi: 10.1016/j.pneurobio.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Wiesinger H.J.P.I.N. Arginine Metab. Synth. nitric oxide Nerv. Syst. 2001;64(4):365–391. doi: 10.1016/s0301-0082(00)00056-3. [DOI] [PubMed] [Google Scholar]
- Willis L.M., Shukitt-Hale B., Cheng V., Joseph J.A. Dose-dependent effects of walnuts on motor and cognitive function in aged rats. Br. J. Nutr. 2008;101(8):1140–1144. doi: 10.1017/S0007114508059369. [DOI] [PubMed] [Google Scholar]
- Willis L.M., Shukitt-Hale B., Joseph J.A. Modulation of cognition and behavior in aged animals: role for antioxidant-and essential fatty acid–rich plant foods. Am. J. Clin. Nutr. 2009;89(5):1602S–1606SS. doi: 10.3945/ajcn.2009.26736J. [DOI] [PubMed] [Google Scholar]
- Willis L.M., Bielinski D.F., Fisher D.R., Matthan N.R., Joseph J.A. Walnut extract inhibits LPS-induced activation of BV-2 microglia via internalization of TLR4: possible involvement of phospholipase D2. Inflammation. 2010;33(5):325–333. doi: 10.1007/s10753-010-9189-0. [DOI] [PubMed] [Google Scholar]
- Wu J., Zhang H., Xu Y., Zhang J., Zhu W., Zhang Y., et al. Juglone induces apoptosis Tumor stem- Cells ROS-p38 Pathw. glioblastoma. 2017;17(1):1–7. doi: 10.1186/s12883-017-0843-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Yuan C., Wang G., Luo J., Ma H., Xu L., et al. Urolithins Attenuate LPS-Induced Neuroinflammation in BV2Microglia via MAPK, Akt, and NF-κB Signaling Pathways. J. Agric. Food Chem. 2018;66(3):571–580. doi: 10.1021/acs.jafc.7b03285. [DOI] [PubMed] [Google Scholar]
- Yang Y., Niu C.-S., Cheng C.-D.J.O.R. Pin1-Nanog Expr. Hum. glioma Is. Correl. Adv. Tumor Progress. 2013;30(2):560–566. doi: 10.3892/or.2013.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaro A., Aliyu M., Garba K., Hassan S.J.T.Jo.N.P.R. Anti-Seizure Act. Extr. Jatropha gossypiifolia Linn. (Euphorbiac-.-. ) 2018;2(2):99–102. [Google Scholar]
- Zhao F., Liu C., Fang L., Lu H., Wang J., Gao Y., et al. Walnut-Derived Peptide Activates PINK1 via the NRF2/KEAP1/HO-1 Pathway, Promotes Mitophagy, and Alleviates Learning and Memory Impairments in a Mice Model. J. Agric. Food Chem. 2021;69(9):2758–2772. doi: 10.1021/acs.jafc.0c07546. [DOI] [PubMed] [Google Scholar]
- Zou J., Cai P.-S., Xiong C.-M., Ruan J.-L. Neuroprotective effect of peptides extracted from walnut (Juglans Sigilata Dode) proteins on Aβ25-35-induced memory impairment in mice. J. Huazhong Univ. Sci. Technol. [Med. Sci. ] 2016;36(1):21–30. doi: 10.1007/s11596-016-1536-4. [DOI] [PubMed] [Google Scholar]