Abstract
How myofilaments operate at short mammalian skeletal muscle lengths is unknown. A common assumption is that thick (myosin-containing) filaments get compressed at the Z-disc. We provide ultrastructural evidence of sarcomeres contracting down to 0.44 µm—approximately a quarter of thick filament resting length—in long-lasting contractions while apparently keeping a regular, parallel thick filament arrangement. Sarcomeres produced force at such extremely short lengths. Furthermore, sarcomeres adopted a bimodal length distribution with both modes below lengths where sarcomeres are expected to generate force in classic force–length measurements. Mammalian fibres did not restore resting length but remained short after deactivation, as previously reported for amphibian fibres, and showed increased forces during passive re-elongation. These findings are incompatible with viscoelastic thick filament compression but agree with predictions of a model incorporating thick filament sliding through the Z-disc. This more coherent picture of mechanical mammalian skeletal fibre functioning opens new perspectives on muscle physiology.
Keywords: skeletal muscle fibre, myosin, actin, titin, delta-state, muscle physiology, structural biology
1. Introduction
Muscles are the motors of versatile movements of and throughout the body. Striated muscles actuate the passive skeletal system, and their active mechanical properties stem from highly organized, serially and parallelly arranged functional units, the sarcomeres. The sarcomeres contain two sets of filaments, the thin (actin-containing) and thick (myosin-containing) filaments. When the muscle is activated, small motors distributed along the thick filaments build temporary cross-bridges with overlapping thin filaments driving muscle contraction [1]. Muscle length changes result from a sliding motion of the filaments relative to each other [2,3]. Thus, the sarcomere's ability to produce isometric muscle force depends on its length, resulting in the fundamental force–length relationship (FLR). The classic fibre FLR consists of piecewise linear segments [4]. Isometric fibre force increases first steeply, then shallower with length (ascending limb) before reaching a plateau region and, subsequently, decreases with length (descending limb). The mechanistic and ultrastructural basis of the ascending limb of this relationship, and particularly its steep range, is not fully understood today.
The sliding filament [2,3] and cross-bridge theories [1] straightforwardly explain the FLR's plateau region and its descending limb based on thin and thick filament overlap. The loss of isometric force capacity in the ascending limb's shallow region has been associated with increased lattice spacing [5] and thin filaments entering the adjacent half-sarcomere through the M-line, forming inefficient cross-bridges [6]. The slope change on the FLR's ascending limb corresponds to the length where the thick filaments' tips reach the Z-disc, a thin meshed filament structure [7] defining the sarcomere boundary. The steep slope of the FLR is typically explained by thick filament folding or compression (figure 1, left illustration) and a possibly decreasing number of cross-bridges [4,5,11], though there is neither experimental evidence nor a structurally convincing model that supports this hypothesis.
Figure 1.

Active force–length relationship, FLR. Active isometric forces (normalized to maximum isometric force, Fim) of the first experimental block (triangles, over sarcomere length, SL) approximate the classic FLR (thick grey line) above SLs of approximately 1.4 µm. At SL less than approximately 1.4 µm, fibres remained taut after cessation of activation and fibre forces deviate from the classic FLR, showing a hump at 1–1.4 µm. Active isometric forces (including the hump) are approximated by the Rode et al. [8] model (dashed magenta line; model parameters: thick filament length 1.65 µm [9], effective bare region length 0.125 µm [4], thin filament length 1.13 µm [10], swivelled cross-bridge force [8] 0 pN). The model predicts a negative slope at SL of approximately 1.2 µm. Note that optically measured SL using light microscopy at lengths below 1.4 µm rather overestimates true mean SL (cf. Figure 5). Assuming a systematic overestimation of SLs observed, a left shift in the relation along the abscissa is expected. When sarcomeres shorten to lengths below 1.65 µm, thick filaments might compress or buckle at the Z-disc (left illustration, classic assumption) or slide through the Z-disc (right illustration). Right inset: α-actinin molecules (black and grey) cross-link the tetragonal thin filament (helices) grids of opposite half-sarcomeres (blue and green, respectively) to form the Z-disc structure. Thin filament grids of opposite polarity are assumed to align as a consequence of the thick filament (thick rods) sliding through the Z-disc. Thick filaments fill the previously empty spots within the tetragonal thin filament grid of the adjacent half-sarcomere.
By contrast, [12] provided early but largely neglected evidence against thick filament folding or compression. First, amphibian skeletal muscle fibre forces increased slowly upon activation to considerable values (25% of maximum isometric force) at short lengths where the classical FLR [4] is zero. Second, fibres did not restore their resting lengths but remained short after cessation of activation. If thick filaments were compressed or folded during the contraction, they should then push the fibre toward resting length. Third, activating fibres at optimal length after contractions with prolonged activation at very short lengths yielded much lower (approx. half) isometric forces. These findings led Ramsey and Street to postulate a new, irreversible state that the fibre had entered, the delta-state [12]. Other studies also reported delayed and slow force generation at extremely short muscle lengths [13–16], but an interpretation of the delta-state remained highly controversial.
Competing with the postulation of a new, irreversible fibre state, a recent model [8] suggests mechanical explanations for the above-mentioned phenomena. Combining classical theories of contraction with a hypothetical thick filament sliding through the Z-disc (figure 1, right illustration), the model predicts the entire FLR including a hump at very short lengths ([8], their fig. 5). Interestingly, the hump contains a range of negative slope. In general, isometric end-held contractions in FLR regions of negative slope between regions of positive slope can theoretically result in bimodal sarcomere length distributions [17]. Here, the equilibration of sarcomeres takes time, especially when considering the decreased maximum contraction speed in the range of the FLR's steep ascending limb [18]. Hence, fibre dynamics might explain the delayed and slow force rise in end-held fibre contractions at very short lengths. Further, sarcomeres might get stuck due to friction and/or titin–actin binding [19–22] explaining the absent recovery of fibre resting length. Finally, short, still-stuck sarcomeres and long sarcomeres taking up fibre elongation when restoring optimal fibre length could result in impaired force-producing capability. These plausible mechanical explanations suggest that fibres may not switch irreversibly to a new state when contracting to very short lengths but are temporarily mechanically impaired.
In this paper, we investigate skinned mammalian fibre behaviour in end-held isometric contractions. First, we explore whether the delta-state occurs in mammalian muscle fibres by measuring the FLR in the ascending limb segment and below with long-lasting activation, observing fibre behaviour after deactivation, and measuring active isometric force after re-elongating fibres. Second, we visually test the hypotheses of a regular appearance of thick filaments and the occurrence of bimodal sarcomere length distributions by observing the ultrastructure of longitudinal sections of extremely shortened fibres. Third, we test whether muscle fibres are only temporarily mechanically impaired and do not switch irreversibly to a delta-state when contracting to extremely short lengths. For that, we examine if passive forces are increased when re-elongating fibres with possibly stuck sarcomeres. Moreover, we compare their active isometric force after a simple passive stretch with that after a modified passive lengthening protocol aiming at reducing sarcomere length inhomogeneity. We hypothesize that isometric forces will be closer to reference forces in the latter case. The results of the study contribute to a more coherent understanding of skeletal muscle fibre behaviour and a structural understanding of its force-producing mechanisms.
2. Material and methods
2.1. Fibre preparation and measurement
Muscle preparation, storage and activation techniques for permeabilized single muscle fibres were followed [23,24]. Muscles were taken from nine Wistar rats immediately after death (6 ± 3 months, 491 ± 93 g, cage sedentary, 12 h : 12 h light : dark cycle, housing temperature: 22°C). The experiments have been approved by the German animal protection law (Tierschutzgesetz, §4(3); permit no.: T170/18ST). All experiments were performed on glycerinated skinned single fibre segments from extensor digitorum longus muscles (EDL). After permeabilization in a skinning solution, the bundles were stored at −20°C in a storage solution (cf. table 1 in [23]). On the day of the experiments, small segments of the skinned fibre bundles were dissected, and single muscle fibres were isolated. Then, fibre bundles were treated with a relaxing solution (cf. table 1 in [23]) containing Triton X-100 (1% v/v) for 2–3 min at 4°C to ensure the removal of internal membranes without affecting the contractile apparatus [25,26]. Afterwards, the fibre extremities were clamped by aluminium foil ‘T-clips’ (Institute of Applied Physics, Ultrafast Optics, Jena, Germany). Kinetic experiments with single skeletal muscle fibres were performed with a standard fibre test system (Aurora Scientific 1400A). Fibre-clip units were mounted between attachment hooks connected to a lever arm of a high-speed length controller (322 C-I, Aurora Scientific, Canada) and a force transducer (403a, Aurora Scientific, Canada). The two ends of the fibre were fixed with glutaraldehyde in rigour solution and glued to the clips with fingernail polish diluted with acetone [27] which minimizes sarcomere length inhomogeneity by reducing fibre end compliance [28]. During kinetic experiments, sarcomere length (SL) was measured using a high-speed camera system (901B, Aurora Scientific, Canada) in combination with a 20× ELWD dry-objective (NA 0.40, Nikon) and an accessory lens (2.5×, Nikon) within a region of interest of the muscle fibre. The autocorrelation function/Sine-fit (ACF/Sine-fit) of the Aurora software is used to calculate the SL as the mean length of 10–20 sarcomeres in series. The sarcomere length was set to 2.5 ± 0.05 µm. The corresponding fibre length was defined as the individual optimal fibre length (L0). The width w and height h of the fibre was measured at three different positions in the central segment of the relaxed fibre with a 10× dry-objective (NA 0.30, Nikon) and a 10× eyepiece and then averaged. The fibre cross-sectional area (CSA) was determined assuming an elliptical cross-section of single muscle fibres [29] (πhw/4). The two possible viewing angles of the built-in prism were assumed to coincide with the ellipse's main axes. The CSA was 4330 ± 1700 µm2 with a fibre length L of 0.94 ± 0.24 mm (39 fibres from nine rats). All preparations were performed on a cooling unit at 4–6°C.
2.2. Experimental protocol
All experiments were conducted at a constant temperature of 12°C ± 0.1°C. Active isometric end-held contractions were performed by immersing a fibre first for 60 s in a pre-activating solution for equilibration, then for an experiment-dependent time (duration of activation 13 s for reference contractions at L0) in an activating solution (pCa = 4.5), and finally for 420 s in a relaxing solution (pCa = 9.0). Brenner's [30] cycling protocol was applied before and after typical reference contractions (at L0) to stabilize the sarcomere pattern and maintain the mechanical fibre integrity for an extended period. Two isometric reference contractions at L0 were performed at the beginning of each experimental block. Passive ramps were performed at a low speed to preserve the fibres [23,31]. Fibres were each used only for one block of experiments.
The first experimental block (CSA = 3570 ± 1170 µm2; 21 fibres from six rats) investigated the mammalian FLR for comparison with amphibian FLR [12]. The second (CSA = 6050 ± 1200 µm2, seven fibres from one rat) and third experimental blocks (CSA = 4690 ± 2000 µm2, 11 fibres from four rats) investigated the effect of passive fibre stretches on passive and reference forces of impaired fibres (that remained taut after contracting to very short lengths). In the second block, passive movements of impaired fibres were kept to a minimum by applying standard passive stretches (similar to [12]). In the third block, impaired fibres were passively overextended to 1.26 L0 superimposing a cycling protocol [30] to the passive stretch before re-measuring their reference force. Fibres were considered undamaged and were included in the analyses if (i) no ruptures or lesions were visible; (ii) force increased/decreased less than 1% during the last second of activation. The fibres (n = 39) developed 83.0 ± 18.3 kPa maximum isometric tension (first block: 78.5 ± 19.9 kPa, second block: 81.8 ± 14.6 kPa, third block: 92.4 ± 14.8 kPa) indicating consistent and repeatable preparation routines and fibre functionality.
2.2.1. First block—force–length relationship
After reference contractions at L0, the distance between the transducers was set to 0.58 L0. At this length, muscle fibres were initially slack. Then, fibres were activated for 38 s up to three times. If fibres remained taut throughout the relaxation period, fibre length was further reduced in 0.05 L0 steps down to a minimal 0.23 L0 and activated once for 38 s at each length. Otherwise, after a new reference contraction at L0, the fibre length was set to 0.05 L0 shorter length than before, i.e. 0.53 L0 and fibres were again activated up to three times. Then, the protocol was continued as described before. To represent the whole FLR over SL, occasional measurements were performed in the range of the classical FLR [4] with 13 s activation time.
2.2.2. Second block—active and passive forces, standard stretch
After the second reference contraction at L0 (exp I) and a passive stretch (from 0.23 L0 to 1.26 L0), fibres were activated at 0.23 L0 for 48 s (exp II). Subsequently, impaired, taut fibres were passively stretched with a standard ramp [12] to L0 at 0.0025 L0 s−1 before a further reference contraction (13 s activation time) was performed (exp III), omitting Brenner's cycling protocol. Finally, a further passive stretch was measured.
2.2.3. Third block—active and passive forces, cycled overextension
Experiments repeated the protocols of the second block; however, the passive stretches performed were aimed at gently pulling the hypothetically stuck sarcomeres apart with superimposed cyclic changes in length. Fibres were pulled from 0.23 L0 to 1.26 L0 at 0.0075 L0 s−1, every 2.5 s interrupted by an adapted cycling protocol [30] (three quick ramps followed by 1 s rest, for L < 0.58 L0: 0.05 L0 initial shortening, followed by 0.055 L0 lengthening, and finally 0.005 L0 shortening; for L > 0.58 L0: 0.2 L0 initial shortening, followed by 0.25 L0 lengthening, and finally 0.05 L0 shortening). Cycled overextensions took the same time as the standard passive stretches of the second block.
2.3. Transmission electron microscopy
Transmission electron microscopy (TEM) images were obtained for ultrastructural observation of single skeletal muscle fibre segments in a longitudinal direction. The permeabilized fibres were fixed with a protocol adapted from [32]. Briefly, after finishing the mechanical experiments, muscle fibres were fixed at the predefined muscle length and contraction state in a mixture of 2% paraformaldehyde, and 2.5% glutaraldehyde in 0.2 M phosphate buffer (pH 7.4) for 2 h at room temperature. Afterwards, the prefixed muscle fibre was removed from the rig and placed in the fixative solution overnight at 4°C. The next day, the fibres were washed threefold with 0.2 M phosphate buffer (pH 7.4) for 30 min each, incubated with post-fix solution (1% OsO4, 1.5% K3Fe(III)(CN)6 in 0.1 M phosphate buffer) for 2 h at 4°C. The fibres were transferred to glass vials for dehydration by using increasing acetone concentrations (50% 30 min, 70% 30 min followed by staining with 70% acetone + 0.5% uranyl acetate overnight at 4°C, 90% 30 min, 96% 30 min, 100% 30 min, 100% + 0.5% DMP-30 (2,4,6-Tris-(dimethylaminomethyl)-phenol) 30 min). After dehydration, the samples were embedded in epoxy resin which was polymerized for 48 h at 63°C in a Memmert UN30 incubator (Memmert GmbH, Schwabach, Germany). From these blocks, ultrathin sections (40–70 nm) were cut (Leica EM UC7, Leica, Wien, Austria) and collected on Formvar and carbon-coated copper grids, post-stained with uranyl acetate aqueous solution for 20 min and lead citrate for 2 min. TEM imaging was performed using an FEI Tecnai Spirit transmission electron microscope (ThermoFisher Scientific) equipped with a Tietz TemCam F416 CMOS camera (TVIPS, Gauting, Germany). SL was obtained across a region in the fibres' centre.
2.4. Statistical methods
Data were recorded at 1 kHz. Real-time software (600A, Aurora Scientific, Canada) was used for data acquisition. Matlab (MathWorks, Natick, MA, United States) was used for data analysis. All data were presented as mean value ± standard deviation (mean ± s.d.) unless otherwise stated. All statistical analyses were calculated with SPSS (IBM Corp., Armonk, NY, USA). To show possible variations between passive forces within the second and third blocks, repeated measures variance analyses (rmANOVA) were conducted. Significance levels were set to p < 0.05. To reveal differences between normalized active isometric forces at L0 and passive forces at L0 and 1.26 L0 in the second versus the third experimental block after the normal versus cycled stretch, t-tests for independent samples were performed.
All data were tested for normal distribution using z-kurtosis (kurtosis/standard error kurtosis) and z-skewness (skewness/standard error skewness) as well as the Shapiro-Wilk test. If not pointed out separately in the results section, normal distribution can be assumed for all statistical calculations (smallest p-value of all Shapiro-Wilk tests: p = 0.096). Significant main effects or interactions of the rmANOVA were followed up using Bonferroni post hoc tests. The effect size f was calculated as (: partial eta-square) and classified as strong [33] if f exceeded 0.4. For t-tests, the Pearson correlation coefficient was calculated as (t: statistical t-value; df: degree of freedom) and classified as strong [33] if r exceeded 0.5.
3. Results
3.1. Ultrastructural measurement of sarcomere length distribution
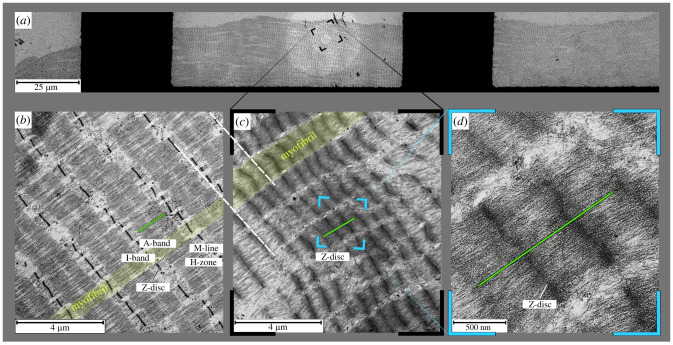
Sarcomeres along the entire fibre contracted to short SLs (figure 2). The sarcomere structure was preserved when the fibre contracted to very short lengths (SL of control fibre: approximately 2.3 µm, figure 2b, versus SL of short fibre: approximately 0.8 µm, figure 2c,d). Z-discs are still clearly visible at SLs down to 0.5 µm (figure 2d). Moreover, filaments still appear in parallel arrangements perpendicular to the Z-discs (figure 2c,d). Light stripes from the bottom left to upper right confine myofibrils (figure 2b,c). The regular thick filament (green line) length (1.65 µm [34]) is approximately threefold longer than the observed SL in figure 2d.
Figure 2.
Electron microscopic (EM) images of EDL fibre segments. (a) Overview of a whole fibre segment at 0.28 L0 contracting at very short SL. The fibre view is partially blocked (black areas) by the copper grid used in the EM observation. (b) Control with sarcomeres at 2.3 µm. (c) The magnification of a representative region is indicated in (a). (d) The magnification of a representative region is indicated in (c). The green rod represents a thick filament at a regular length of 1.65 µm [34] that coincides with the anisotropic band length visible in (b). (d) shows three sarcomeres with dense zones that appear dark on each side of the Z-disc. Scale bars indicate length. The myofibrils of our skinned fibres (one highlighted yellow in (b) and one in (c)) increased in width with shortening.
SLs exhibited bimodal distributions (figure 3a,d) after fibres were exposed to long-lasting activations at very short end-held fibre lengths (0.28 L0, n = 1, 0.38 L0, n = 1). The fibre at 0.38 L0 exhibited SL peaks at 0.6 and 1.3 µm (figure 3d) with a mean SL of 1.07 µm. The fibre at 0.28 L0 exhibited fewer distinct peaks, one at a similar short SL of 0.65 µm while the second peak, compared with the fibre at 0.38 L0, occurred at a shorter SL of 0.95 µm (figure 3a, mean SL 0.87 µm). EM images show that SL is not randomly distributed but show regions with longer SL and with shorter SL (figure 3b,e). Regions with longer SL appear brighter. A clear parallel filament structure is visible at both fibre lengths (figure 3c,f).
Figure 3.
Sarcomere length (SL) distributions in delta-state fibre segments. Electron microscopic images at two short fibre lengths. SLs were measured at 510 spots for 0.28 L0 and 450 spots for 0.38 L0 within depicted overlayed grids (a,d, insets). Fibres exhibited (a,d) bimodal SL distributions in the fibre ultrastructure after contraction to (b,c) 0.28 L0 and (e,f) 0.38 L0. For comparison, the regular thick filament length (1.65 µm) is indicated with green rods. Scale bars indicate length. Fibre lengths are expressed relative to the optimum fibre length L0. Sarcomere lengths are reported in absolute values (µm). In (c) there is a dense zone on each side of the Z-disc. In (f), such a zone is barely visible.
3.2. Active isometric force–length relationship
The isometric active forces of our mammalian fibres (first experimental block) show a similar hump at short lengths (figure 1, symbols) to amphibian fibres measured by [12].
The measured forces roughly coincide with a hump in active force that we predicted using a model considering thick filament sliding through the Z-disc [8] (figure 1, dashed magenta line). Notably, the forces of swivelled cross-bridges needed to be tuned to 0 pN in the model to approximate the experimental data. Further, fibres remained taut after cessation of activation at sarcomere lengths smaller than approximately 1.4 µm corresponding to 0.56 L0.
3.3. Active fibre forces in isometric end-held contractions
The second and third experimental blocks investigated the active forces of fibres in a sequence of isometric end-held contractions, first at L0 (exp I), then at 0.23 L0 (exp II), and again at L0 (exp III, cf. figure 4). Between exp II and exp III, fibres were stretched to L0 in the second block but overstretched to 1.26 L0 in the third block. Active isometric force Fisom in exp II was 0.26 ± 0.05 Fim. In exp III, Fisom was 0.64 ± 0.02 Fim for the second block (figure 4, grey) and 0.77 ± 0.03 Fim for the third block (figure 4, green). Forces developed much slower at very short (figure 4, purple) than at optimal length (figure 4, blue, green, grey). Furthermore, there was almost no force development in the first three seconds of activation in exp II.
Figure 4.

Active force–time traces in a sequence of end-held isometric contractions. Forces (mean ± s.d., normalized to maximum isometric force Fim at optimal length L0) were obtained in the second and third experimental blocks in the sequence exp I, exp II and exp III. Fibres were fully activated at 0 s. Blue dot: isometric forces (Fisom) at L0 (n = 18); purple dot: Fisom at short length 0.23 L0 (n = 18). Fisom at L0 (green dot, n = 11) after passive overextension (cycled stretch to 1.26 L0) exceeds (**: t = 9.507, p < 0.001, r = 0.92, a large effect) that after the standard stretch to L0 (grey dot, n = 7) by 20%. Fisom at L0 (blue dot) as well as at short length 0.23 L0 (purple dot) do not differ significantly between experimental blocks (t-test, p = 0.14; variances are homogeneous, Levene's test F = 1.024, p = 0.33, n = 18; normal distribution of pooled data assumed: Shapiro-Wilk test, p = 0.633) and were therefore pooled (n = 18).
Figure 5 illustrates the typical appearance of fibres before and after contractions at L0 and short lengths. Fibres were slack at short lengths before activation (figure 5b, top), became taut during contraction (figure 5a, bottom middle), and remained taut afterwards (figure 5b, bottom). During activation at a short length, fibres became less translucent and showed contraction bands [12] and an inhomogeneous sarcomere pattern, making the SL measurement difficult (figure 5a, bottom middle). SL was measured in regions showing a clear striation pattern (figure 5a, left circle). The contraction bands largely disappeared during and after cycled overextension.
Figure 5.
Fibre appearance before and after activation. (a) Activated fibre at optimal (top, SL = 2.5 µm) and short length (bottom, SL = 1.1 µm). Details of the fibre are 5× magnified (circles). Contraction at short length induced contraction bands [12], i.e. dark and bright regions of the muscle fibre (bottom middle) that probably coincide with regions of shorter and longer sarcomeres in electron micrographic images (figure 3b,d). SLs were measured in brighter contraction bands where the sarcomere pattern was visible (left circle) with light microscopy. (b) Fibre at a short length through the built-in prism before (sagged, top) and after (taut, bottom) activation.
3.4. Passive fibre forces during slow stretches
Passive ramp experiments ensued end-held isometric contractions in the second and third experimental blocks. Statistics are summarized in table 1. In the second block (figure 6, left), a one-way rmANOVA showed a significant strong main effect (effect size: f = 3.22) of experiment type (exp I, exp II and exp III) on passive forces at L0. As the sphericity of the data could not be assumed (Mauchly-W: p < 0.05), a Greenhouse–Geisser adjustment was made [35] with ε < 0.75. The Bonferroni corrected post hoc analysis showed strong differences between all three forces (highest p-value: p = 0.005, smallest effect size: r = 0.91). A t-test for dependent samples showed significant (p < 0.001) and strong differences (r = 0.98) between passive forces (blue and green) at 1.26 L0. In the third block (figure 6, right), a two-way rmANOVA showed significant differences in passive forces depending on the factors experiment (exp I, exp II and exp III) and length (L0 and 1.26 L0) (figure 6, right); experiment and length revealed strong interaction (effect size: f = 3.22). Bonferroni-corrected pairwise comparisons showed significant differences for all pairs (highest p-value: p = 0.033) except for passive force after exp I and exp III at L0. The effects were strong (smallest effect size: r = 0.79). The data and residuals were not normally distributed in one case (passive force after exp I at L0). However, the rmANOVA is robust to violation of the normality assumption even for small sample sizes [36].
Table 1.
Passive forces F were normalized to maximum isometric force Fim and fibre lengths L to optimal fibre length L0. All subgroups were significantly and strongly different except for passive forces during stretch after exp I and exp III in the third block.
| fibre length (L/L0) | stretch after exp I (F/Fim) | stretch after exp II (F/Fim) | stretch after exp III (F/Fim) | statistical test | p | |
|---|---|---|---|---|---|---|
| second block (n = 7) | 1.0 | 0.007 ± 0.004 | 0.058 ± 0.016 | 0.023 ± 0.003 | 1-way rmANOVA | <0.001 |
| 1.26 | 0.050 ± 0.008 | — | 0.112 ± 0.012 | t-Test | <0.001 | |
| third block (n = 8) | 1.0 | 0.006 ± 0.008 | 0.039 ± 0.016 | 0.009 ± 0.005 | 2-way rmANOVA | <0.005 |
| 1.26 | 0.053 ± 0.041 | 0.084 ± 0.041 | 0.071 ± 0.036 |
Figure 6.
Passive force–length traces in between a sequence of active end-held isometric contractions. Passive force (mean ± s.d., normalized to maximum isometric force, Fim, at optimal length, L0) during slow stretch after consecutive end-held isometric contractions (exp I) at L0 (blue), exp II at 0.23 L0 (purple) and exp III again at L0 (green). Cycled overextension curves (third block) result from force values taken every 2.5 s (each in the middle of a 1 s constant length phase after a cycle). Passive forces during stretch after exp II start at very short lengths and strongly exceed those after exp I and exp III. Further, passive forces after exp III strongly exceed those after exp I in the second block, but less so in the third block. Comparing the second and third blocks, passive forces after exp II (purple) and after exp III (green) are higher in the second block. Dots indicate forces that were statistically compared. For statistics see text and table 1.
T-tests for independent samples showed significant differences in passive forces between experimental blocks after exp II (figure 6, purple, p = 0.041) at L0 and after exp III (figure 6, green, p = 0.013) at 1.26 L0. In both cases, variance homogeneity can be assumed (Levene-test, p > 0.05). Passive forces after exp II (r = 0.53, strong effect) and exp III (r = 0.62, strong effect) were higher in the second block.
4. Discussion
Providing ultrastructural, kinetic and kinematic evidence, this study shows that the delta-state occurs in mammalian skeletal muscle fibres, extends our knowledge about the delta-state, and confirms several hypotheses derived from the assumption that thick filaments slide through the Z-disc [8]. Consistent with a predicted negative slope of the force–length relationship at short SL (figure 1, the dashed magenta line at approximately 1.2 µm [8]) SLs developed a bimodal distribution at short lengths (figure 3) that manifested as contraction bands in fibres contracting at very short SL (figure 5). Moreover, and consistent with our hypothesis of stuck sarcomeres, passive forces were elevated in stretches following prolonged activation at short lengths (figure 6). Finally, active force production at optimal length recovered partially (figure 4) when impaired fibres were exposed to passive overextension and cycling. These findings suggest that the delta-state has a mechanical cause, is at least partially reversible, and is compatible with thick filament sliding through the Z-disc when muscle fibres contract to short lengths.
4.1. Ultrastructural evidence of short sarcomeres
We report the first electron micrographic measurements of skeletal muscle sarcomere lengths (SLs) of nearly a quarter of thick filament length (figure 3). Images were obtained after long-lasting contractions with substantial force development of mammalian skeletal muscle fibres at very short lengths (cf. figure 2). For example, a fibre at 0.28 L0 exhibited a mean SL of 0.87 µm, with the shortest individual SL of 0.44 µm and the longest individual SL of 1.36 µm (figure 3a), which is below the length where sarcomeres are assumed to produce force [4]. Longitudinal fibre sections suggest a parallel arrangement of thick filaments perpendicular to the Z-disc (figures 2 and 3), indicating an orderly process of sarcomere shortening. This finding conflicts with the assumption that thick filaments buckle or get compressed when fibres shorten to SL below 1.65 µm (figure 1, left illustration). Thick filament buckling would probably be associated with an observable irregular, non-parallel myofilament arrangement. Elastic compression of thick filaments from 1.65 µm down to lengths of 0.44 µm would require not only the compression of the thinner tips of the thick filaments but its backbone as well. Required forces would exceed the maximum isometric force Fim by two to three orders of magnitude [8,37]. Thick filament compression over time due to possible viscous properties is also very unlikely, as discussed later (see §4.4).
Based on a model [8] incorporating thick filament sliding through the Z-disc (figure 1, right illustration) that predicts a region of negative slope in the FLRs hump region (figure 1, dashed magenta line), we hypothesized a bimodal SL distribution in fibres contracting to very short lengths. Our results confirm this hypothesis (figures 2 and 3). In general, the force developed by sarcomeres increases when shortening within an FLR region of negative slope, tending to elongate initially slightly longer sarcomeres [17]. This process continues until both the longer and the shorter sarcomeres reach a range of positive slope and isometric force equilibrium, resulting in a bimodal SL distribution. For shorter fibre lengths, the peaks of the bimodal distribution were closer together while the peak of shorter SLs remained at a similar length (figure 3a,d), suggesting a lower limit of sarcomere shortening.
It is not straightforward to imagine myofilament sliding down to SLs of 0.44 µm. One of the challenges here is the cross-linking of filaments at the Z-disc and the M-line. The tetragonal arrangement of thin filaments at the Z-disc [7] might be helpful to enable thick filaments to slide through the Z-disc at approximately 1.65 µm SL [8], the resting length of the thick filament [38]. Moreover, lateral spacing seems not an issue at this length [8]. Recent evidence suggests that the thick filaments slide through the Z-disc at such SL [39]. Next, thick filaments would encounter the M-line of the adjacent sarcomere at approximately 0.8 µm SL. It has never been shown that thick filaments can slide through the M-line. If they do, they finally encounter the Z-disc of the adjacent sarcomere at approximately 0.5 µm SL (cf. figure 2d, the thick filament model spans three sarcomeres).
It is known that the thin filaments' flexural stiffness is much lower than that of the thick filaments [37]. Still, thin filaments are capable to pass the sarcomere's M-line, and—after unphysiological detachment from the Z-disc—they can move orderly to the opposite Z-disc [6]. If swivelled cross-bridges [40] do not occur—and this might vary from species to species—thin filaments' low flexural stiffness might lead to their deformation. In this case, thin filaments of 1 µm length might not reach the M-line of the adjacent sarcomere at approximately 0.66 µm SL. However, if they do, they might slide through the M-line and would reach the Z-disc of the adjacent sarcomere at 0.5 µm SL, like the thick filaments, and this might contribute to the explanation of why the sarcomeres do not contract much further.
4.2. Occurrence of the delta-state in mammalian fibres
The delta-state of fibres is associated with several phenomena and was demonstrated in amphibian muscle fibres [12] and single-skinned cardiac cells of rat ventricles [16]. The delta-state occurs when fibres are allowed to contract to very short SLs during long-lasting activation. The first phenomenon is that isometric forces occur in a range below the mean SL of 1.4 µm [12–14], where the classic FLR (figure 1, thick grey line) is zero. We reproduced this finding for mammalian skeletal muscle (figure 1, symbols).
As in experiments by [12], fibres became less translucent, showed contraction bands when activated at very short lengths (figure 5a), remained short after cessation of activation at short SL (figure 5b, bottom), and force developed slowly in end-held contractions requiring long-lasting (48 s) activation to reach a near-plateau (figure 4, purple line). Further, subsequent reference contractions at optimal length L0 reached only 60% of regular Fim (figure 4, grey dot). Similarly, [12] reported approximately 50% Fim of impaired fibres at L0 and found delayed and slow force generation at very short lengths. They assumed that the resistance of the sarcolemma was responsible for this delay. Our results indicate that this is not the case. We found similar delays of force production (approx. 2 to 3 s, figure 4, purple line) in our experiments with single muscle fibres with a partially removed sarcolemma. Fibres need to take up the slack (figure 5b, top) before force production and the maximal contraction velocity decreases with decreasing SL [18]. Together, this might explain the late onset of force production in end-held contractions at short SLs.
4.3. Is the delta-state reversible?
From the assumption that the delta-state could be caused by thick filaments sliding through the Z-disc until sarcomeres get stuck, we developed the hypothesis that fibres are only reversibly mechanically impaired and do not switch to a new, irreversible state when contracting to extremely short lengths as proposed by [12]. Compatible with our hypothesis, our results show increased passive forces when stretching a fibre that previously contracted to a very short length (figure 6, purple lines versus blue lines). We aimed at restoring fibre structure through passive overextension with a superimposed cycling protocol to restore the normal functioning of the fibre. From our results, we can conclude that this was partially successful. First, passive forces during the cycled stretch were lower than those obtained in the standard stretch (figure 6, purple lines). Second, passive forces after the following reference contraction at optimal length (figure 6, green line) were lower in the experiments with cycled overextension, with no difference in passive force at L0 compared with reference passive forces before contraction to very short lengths (figure 6, right, blue line). However, passive force at 1.26 L0 was still increased compared with reference passive force (figure 6, right, green versus blue line), indicating incomplete recovery of fibre function. Third, cycled overextension increased Fim by 20% compared with the impaired Fim obtained after minimal mechanical movements (standard stretch to L0, figure 4, green versus grey line). As the stretching protocol was only minimally tuned, it is conceivable that optimized passive fibre movements can lead to even better fibre recovery. Finally, fibres showed a much more homogeneous sarcomere pattern after cycled overextension than after the standard stretch, and, after stretching, fibres were slack again at a short length. In sum, it seems possible that fibres can return from the delta-state to their normal functioning.
4.4. Explanations of fibre delta-state behaviour
The observed passive forces in stretches following contractions to very short lengths (figure 6, purple data) speak against spring-like compression or folding of thick filaments [5,11,41–43] (figure 1, left illustration) in the steep part of the FLR's ascending limb. Spring-like compressed thick filaments would push the fibre back to its resting length and would not lead to increased passive forces when re-elongating the contracted fibre to L0. However, an at-first-glance appealing assumption of viscous behaviour of thick filaments (hypothesized by [44]) potentially explains observed delta-state behaviour. If the thick filaments get compressed over time, sarcomeres can get shorter than the regular thick filament length of 1.65 µm, thus enabling active force production at very short lengths. In subsequent stretches, titin, which connects the thick filament tips to the Z-disc, might be stretched, producing passive forces at short SL because compressed viscous thick filaments resist stretching. However, our ultrastructural and mechanical findings make this idea implausible. First, SL was very inhomogeneous in contractions to very short lengths. More specifically, SLs were bimodally distributed (figure 3), and this cannot easily be explained with a viscous thick filament compression. Second, it is hard to imagine how the meshed Z-disc should stop the stiff thick filaments. For example, the much softer thin filaments [37] slide easily through the meshed M-line at optimal muscle length. Third, if a viscous effect is present, thick filaments, and thus sarcomeres, should re-elongate to resting length in the absence of a compressing force. This is not the case. Fibres remain persistently short after cessation of activation (figure 5b, bottom), and passive forces are produced during very slow elongation of the fibres (taking overall 400 s). Fourth, thick filaments would have to be very easily compressible in the range from 1.65 to 1.4 µm (steep part of classic FLR) and then suddenly become nearly incompressible (reflecting the required long-lasting activation).
Another possibility potentially explaining the increased passive forces after contractions at short SL is related to the idea that thick filaments slide through the Z-disc [8]. If this is the case, then titin filaments, which connect thick filament tips to the Z-disc, get pulled through the Z-disc as well. It is widely thought that titin can attach to the actin filament [19,21,45,46]. Titin–actin attachments and pulling titin through the Z-disc would tend to hamper the shortening motion and might contribute to stuck sarcomeres. A further candidate that could stop thick filament sliding through the Z-disc and lead to persistently stuck sarcomeres might be myosin-binding protein C. This protein probably links thin and thick filaments [47] and, assuming thick filament sliding through the Z-disc, would reach the Z-disc at SL around classic zero force (figure 1). Like titin, it could get pulled through the Z-disc, potentially leading to stuck sarcomeres. Moreover, titin's role in decreasing the maximal sarcomere contraction velocity in the range of the steep part of the ascending limb [18] might be more important than assumed previously. We had to tune the parameter accounting for swivelled cross-bridge force (that has been assumed to dampen muscle contraction [8]) to zero in the model [8] reconstructing the FLR (figure 1, dashed magenta line) to achieve the experimentally determined hump size at short SL (figure 1, symbols). This points to a critical titin contribution to muscle damping at short fibre lengths.
The forces in the hump region represent a cloud of data points (figure 1, symbols) instead of following the model-predicted line (figure 1, dashed magenta line). This might be mainly related to the occurrence of a region of negative slope in the FLR hump. The distribution of SLs depends on the dynamics of the contraction and the initial conditions. The still-increasing force (figure 4, purple trace) shows that a perfect equilibrium is not yet achieved. Hence, different forces can be obtained at the same end-held length, contributing to the scattered appearance of data.
4.5. Physiological feasibility and meaning of thick filament sliding through the Z-disc
So far, apart from rare evidence for chick fibres [48], the sliding of thick filaments through the Z-disc has been thought to occur exclusively in insect muscles and the chameleon tongue muscle [49]. These muscles possess a special Z-disc. Recently, [39] suggested thick filament sliding through the Z-disc in homozygous TiZ-BioIDp/p mice. Their data not only shows contact between Z-discs and myosin filaments but rather indicates that ‘both myosin heavy and light chain proteins […] pass the Z-disc boundary under physiological conditions in heart and skeletal muscle’ [39]. We propose that the thick filaments slide through the Z-disc in all movements where muscles work in the steep part of the FLR's ascending limb. Hampered sliding of thick filaments caused e.g. by lesions in the Z-disc, might contribute to muscle weakness in Duchenne muscular dystrophy, Sjögren's syndrome, or after eccentric exercise. Moreover, cramps bear some similarities with our experiments at very short lengths in that they often occur in lasting contractions at short muscle lengths and can be relieved by externally supplied stretching [50].
Vertebrate skeletal muscle is known to differ significantly in its ultrastructural characteristics (e.g. variations in filament mechanical properties (for reviews see [37]), Z-disc meshwork, M-line structure (for reviews see [51,52])) between fibre types (slow- and fast-twitch muscle fibres). However, the Z-disc is in general characterized by the symmetry of thin filaments arranged in parallel and antiparallel in a tetragonal lattice and the flexible binding sites at each end of the α-actinins connecting the thin filaments [52]. It provides the appropriate number of channels with an appropriate cross-sectional area to allow the thick filaments to slide through it [8]. Further, the Z-disc increases in area by 10% [53] and changes its cross-sectional small-square form to a basket weave pattern in activated muscle [52,54,55], possibly facilitating thick filament sliding through it. In turn, the decrease in channel area and the conformational change upon deactivation might contribute to thick filaments getting stuck at very short lengths, potentially explaining observed pulling forces when re-elongating the deactivated fibres. The structural and geometrical framework of the Z-disc bears major similarities across fibre types and might make thick filament sliding through the Z-disc a general possibility of vertebrate muscle contraction when operating at SL below 1.65 µm (approx. 0.72 L0).
On average, muscles span a working range from approximately 0.8 L0 to approximately 120% L0 [38]. The authors of the meta-analysis [38] divided the muscles into two clusters, ‘short’ and ‘long’. They suggested, that ‘short’ muscles operate more on the ascending limb. Brown et al. [56] reported minimum lengths less than 0.7 L0 for cat leg muscles (semitendinosus, sartorius) which suggests that their sarcomeres work in the steep part of the ascending limb of the FLR. For the human soleus muscle, Kawakami & Fukunaga [57] reported in vivo sarcomere lengths from 1.5 to 2.2 µm (0.65 to 0.91 L0). Thus, the soleus's sarcomeres work in the steep and shallow parts of the FLR's ascending limb. Hence, there is some evidence that the in vivo sarcomere working range can comprise the steep part of the FLR's ascending limb, a range in which our results suggest that thick filaments slide through the Z-disc.
Before the existence of exo- and endo-skeletal structures, muscles probably adhered to gelatinous material such as in jellyfish and sponges [58]. Therefore, these primordial muscles had to be equipped with a protective mechanism preventing extreme or uncontrolled muscle shortening and muscular dysfunctions in the first living beings with muscles, which had no skeleton yet. Sliding of thick filaments through the Z-disc might have provided a protective mechanism, damping muscle contraction (through titin and/or swivelled cross-bridges) while maintaining the structural integrity of the muscle.
5. Conclusion
The delta-state is not an amphibian fibre artefact but is present in mammalian skeletal muscle fibres. The partial recovery of active force production at optimal length through a passive stretching protocol points to a mechanical nature of the delta-state. Our structural evidence suggests that the thick filaments maintain a regular parallel arrangement when sarcomeres contract down to a small fraction of thick filament resting length, challenging the textbook assumption that thick filaments get compressed at the Z-disc. Subsequently, re-elongating the short, deactivated fibres requires pulling forces. This is consistent with thick filaments sliding through the Z-disc and Ca2+-dependent Z-disc behaviour that might lead to stuck thick filaments upon deactivation. Moreover, we found a bimodal sarcomere length distribution in fibres that contracted to very short lengths which is in accordance with a region of negative slope of the force–length relationship at very short lengths predicted with a model assuming thick filament sliding through the Z-disc. In sum, our results yield essential clues to the relation between fibre structure, fibre force generation and muscle function with possible implications for evolution and (patho-)physiological muscle behaviour. However, a structural proof of thick filament sliding through the Z-disc or alternative explanations are still missing.
Acknowledgements
The authors are grateful to Prof. Dr Stefan Raunser and Dr Oliver Hofnagel for EM support. We thank Dr Tino Stöckel and PD Dr Norman Stutzig for checking the statistics.
Ethics
The study was conducted according to the guidelines of the Declaration of Helsinki and approved according to the German animal protection law (Tierschutzgesetz, §4 (3); permit no.: T170/18ST).
Data accessibility
The statistical data underlying figures 1–4, 6 and table 1 are provided as data Excel files. At the request of the authors, readers are herein alerted to the fact that additional materials related to this manuscript may be found on Figshare: https://doi.org/10.6084/m9.figshare.21621132 [59].
Authors' contributions
A.T.: conceptualization, data curation, funding acquisition, investigation, methodology, resources, validation, writing—original draft, writing—review and editing; M.H.: conceptualization, formal analysis, investigation, validation, visualization, writing—original draft; A.K.: investigation, writing—review and editing; C.R.: conceptualization, funding acquisition, investigation, project administration, supervision, validation, writing—original draft, writing—review and editing; T.S.: conceptualization, funding acquisition, investigation, resources, supervision, validation, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) – project number 405834662 – under grants SI841/17-1, RO 5811/1-1, as well as partially funded by the DFG as part of the German Excellence Strategy – EXC 2075–390 740 016.
References
- 1.Huxley AF. 1957. Muscle structure and theories of contraction. Prog. Biophys. Biophys. Chem. 7, 255-318. [PubMed] [Google Scholar]
- 2.Huxley HE, Hanson J. 1954. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature 173, 973-976. [DOI] [PubMed] [Google Scholar]
- 3.Huxley AF, Niedergerke R. 1954. Structural changes in muscle during contraction; interference microscopy of living muscle fibres. Nature 173, 971-973. ( 10.1038/173971a0) [DOI] [PubMed] [Google Scholar]
- 4.Gordon AM, Huxley AF, Julian FJ. 1966. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J. Physiol. 184, 170-192. ( 10.1113/jphysiol.1966.sp007909). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams CD, Salcedo MK, Irving TC, Regnier M, Daniel TL. 2013. The length–tension curve in muscle depends on lattice spacing. Proc. R. Soc. B 280, 20130697. ( 10.1098/rspb.2013.0697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trombitás K, Tigyi-Sebes A. 1984. Cross-bridge interaction, with oppositely polarized actin filaments in double-overlap zones of insect flight muscle. Nature 309, 168-170. ( 10.1038/309168a0) [DOI] [PubMed] [Google Scholar]
- 7.Knappeis GG, Carlsen F. 1962. The ultrastructure of the Z disc in skeletal muscle. J. Cell Biol. 13, 323-335. ( 10.1083/jcb.13.2.323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rode C, Siebert T, Tomalka A, Blickhan R. 2016. Myosin filament sliding through the Z-disc relates striated muscle fibre structure to function. Proc. R. Soc. B 283, 20153030. ( 10.1098/rspb.2015.3030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zatsiorsky VM, Prilutsky BI. 2012. Biomechanics of skeletal muscles. Champaign, IL: Human Kinetics. ( 10.5040/9781492595298) [DOI] [Google Scholar]
- 10.ter Keurs HE, Luff AR, Luff SE. 1984. Force — sarcomere-length relation and filament length in rat extensor digitorum muscle. Adv. Exp. Med. Biol. 170, 511-525. ( 10.1007/978-1-4684-4703-3_44) [DOI] [PubMed] [Google Scholar]
- 11.MacIntosh BR, Gardiner PF, McComas AJ. 2006. Skeletal muscle: form and function, 2nd edn. Champaign, IL: Human Kinetics. [Google Scholar]
- 12.Ramsey RW, Street SF. 1940. The isometric length-tension diagram of isolated skeletal muscle fibers of the frog. J. Cell. Physiol. 15, 11-34. [Google Scholar]
- 13.Moss RL. 1979. Sarcomere length-tension relations of frog skinned muscle fibres during calcium activation at short lengths. J. Physiol. 292, 177-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rüdel R, Taylor SR. 1971. Striated muscle fibers: facilitation of contraction at short lengths by caffeine. Science 172, 387-388. [DOI] [PubMed] [Google Scholar]
- 15.Schoenberg M, Podolsky RJ. 1972. Length-force relation of calcium activated muscle fibers. Science 176, 52-54. ( 10.1126/science.176.4030.52) [DOI] [PubMed] [Google Scholar]
- 16.Fabiato A, Fabiato F. 1976. Dependence of calcium release, tension generation and restoring forces on sarcomere length in skinned cardiac cells. Eur. J. Cardiol. 4(Suppl), 13-27. [PubMed] [Google Scholar]
- 17.Heidlauf T, Klotz T, Rode C, Siebert T, Röhrle O. 2017. A continuum-mechanical skeletal muscle model including actin-titin interaction predicts stable contractions on the descending limb of the force-length relation. PLoS Comput. Biol. 13, 1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edman KAP. 1979. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J. Physiol. 291, 143-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dutta S, Tsiros C, Sundar SL, Athar H, Moore J, Nelson B, Gage MJ, Nishikawa K. 2018. Calcium increases titin N2A binding to F-actin and regulated thin filaments. Sci. Rep. 8, 1-11. ( 10.1038/s41598-018-32952-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagy A. 2004. Differential actin binding along the PEVK domain of skeletal muscle titin. J. Cell Sci. 117, 5781-5789. ( 10.1242/jcs.01501) [DOI] [PubMed] [Google Scholar]
- 21.Bianco P, Nagy A, Kengyel A, Szatmári D, Mártonfalvi Z, Huber T, Kellermayer MSZ. 2007. Interaction forces between F-actin and titin PEVK domain measured with optical tweezers. Biophys. J. 93, 2102-2109. ( 10.1529/biophysj.107.106153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Hessel AL, Unger A, Ing D, Recker J, Koser F, Freundt JK, Linke WA. 2020. Graded titin cleavage progressively reduces tension and uncovers the source of A-band stability in contracting muscle. Elife 9, 1-23. ( 10.7554/eLife.64107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomalka A, Rode C, Schumacher J, Siebert T. 2017. The active force–length relationship is invisible during extensive eccentric contractions in skinned skeletal muscle fibres. Proc. R. Soc. B 284, 20162497. ( 10.1098/rspb.2016.2497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomalka A, Weidner S, Hahn D, Seiberl W, Siebert T. 2021. Power amplification increases with contraction velocity during stretch-shortening cycles of skinned muscle fibers. Front. Physiol. 12, 1-16. ( 10.3389/fphys.2021.644981) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fryer MW, Owen VJ, Lamb GD, Stephenson DG. 1995. Effects of creatine phosphate and P(i) on Ca2+ movements and tension development in rat skinned skeletal muscle fibres. J. Physiol. 482, 123-140. ( 10.1113/jphysiol.1995.sp020504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linari M, Caremani M, Piperio C, Brandt P, Lombardi V. 2007. Stiffness and fraction of myosin motors responsible for active force in permeabilized muscle fibers from rabbit psoas. Biophys. J. 92, 2476-2490. ( 10.1529/biophysj.106.099549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burmeister Getz E, Cooke R, Lehman SL. 1998. Phase transition in force during ramp stretches of skeletal muscle. Biophys. J. 75, 2971-2983. ( 10.1016/S0006-3495(98)77738-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chase PB, Kushmerick MJ. 1988. Effects of pH on contraction of rabbit fast and slow skeletal muscle fibers. Biophys. J. 53, 935-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song SK, Shimada N, Anderson PJ. 1963. Orthogonal diameters in the analysis of muscle fibre size and form. Nature 200, 1220-1221. ( 10.1038/2001220a0) [DOI] [PubMed] [Google Scholar]
- 30.Brenner B. 1983. Technique for stabilizing the striation pattern in maximally calcium-activated skinned rabbit psoas fibers. Biophys. J. 41, 99-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Degens H, Yu F, Li X, Larsson L. 1998. Effects of age and gender on shortening velocity and myosin isoforms in single rat muscle fibres. Acta Physiol. Scand. 163, 33-40. [DOI] [PubMed] [Google Scholar]
- 32.Eastwood AB, Wood DS, Bock KL, Sorenson MM. 1979. Chemically skinned mammalian skeletal muscle. I. The structure of skinned rabbit psoas. Tissue Cell 11, 553-566. ( 10.1016/0040-8166(79)90062-4) [DOI] [PubMed] [Google Scholar]
- 33.Cohen J. 1988. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- 34.Stephenson DG. 2003. Relationship between isometric force and myofibrillar MgATPase at short sarcomere length in skeletal and cardiac muscle and its relevance to the concept of activation heat. Clin. Exp. Pharmacol. Physiol. 30, 570-575. [DOI] [PubMed] [Google Scholar]
- 35.Girden ER. 1992. ANOVA: repeated measures. Sage university papers. Quantitative applications in the social sciences. no. 07-084. Newbury Park, CA: Sage. [Google Scholar]
- 36.Norman G. 2010. Likert scales, levels of measurement and the ‘laws’ of statistics. Adv. Heal. Sci. Educ. 15, 625-632. ( 10.1007/s10459-010-9222-y) [DOI] [PubMed] [Google Scholar]
- 37.Miller MS, Tanner BCW, Nyland LR, Vigoreaux JO. 2010. Comparative biomechanics of thick filaments and thin filaments with functional consequences for muscle contraction. J. Biomed. Biotechnol. 2010, 473423. ( 10.1155/2010/473423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burkholder TJ, Lieber RL. 2001. Review sarcomere length operating range of vertebrate muscles during movement. J. Exp. Biol. 204, 1529-1536. [DOI] [PubMed] [Google Scholar]
- 39.Rudolph F, et al. 2020. Deconstructing sarcomeric structure–function relations in titin-BioID knock-in mice. Nat. Commun. 11, 1-10. ( 10.1038/s41467-020-16929-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reedy MC, Beall C, Fyrberg E. 1989. Formation of reverse rigor chevrons by myosin heads. Nature 339, 481-483. ( 10.1038/340301a0) [DOI] [PubMed] [Google Scholar]
- 41.Zoladz JA. 2018. Muscle and exercise physiology. Amsterdam, The Netherlands: Elsevier, Academic press. ( 10.1016/C2017-0-01877-3) [DOI] [Google Scholar]
- 42.Brown IE, Scott SH, Loeb GE. 1996. Mechanics of feline soleus: II design and validation of a mathematical model. J. Muscle Res. Cell Motil. 17, 221-233. ( 10.1007/BF00124244) [DOI] [PubMed] [Google Scholar]
- 43.Nordin M, Frankel VH. 2010. Basic biomechanics of the musculoskeletal system, 5th edn. Philadelphia, PA: Lippincott, Williams & Wilkins. [Google Scholar]
- 44.Till O, Siebert T, Blickhan R. 2010. A mechanism accounting for independence on starting length of tension increase in ramp stretches of active skeletal muscle at short half-sarcomere lengths. J. Theor. Biol. 266, 117-123. ( 10.1016/j.jtbi.2010.06.021) [DOI] [PubMed] [Google Scholar]
- 45.Rode C, Siebert T, Blickhan R. 2009. Titin-induced force enhancement and force depression: a ‘sticky-spring’ mechanism in muscle contractions? J. Theor. Biol. 259, 350-360. ( 10.1016/j.jtbi.2009.03.015) [DOI] [PubMed] [Google Scholar]
- 46.Nishikawa KC, Monroy JA, Uyeno TE, Yeo SH, Pai DK, Lindstedt SL. 2012. Is titin a ‘winding filament’? A new twist on muscle contraction. Proc. R. Soc. B 279, 981-990. ( 10.1098/rspb.2011.1304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luther PK, Winkler H, Taylor K, Zoghbi ME, Craig R, Padron R, Squire JM, Liu J. 2011. Direct visualization of myosin-binding protein C bridging myosin and actin filaments in intact muscle. Proc. Natl Acad. Sci. USA 108, 11 423-11 428. ( 10.1073/pnas.1103216108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hagopian M. 1970. Contraction bands at short sarcomere length in chick muscle. J. Cell Biol. 47, 790-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herrel A, Meyers JJ, Timmermans J-P, Nishikawa KC. 2002. Supercontracting muscle: producing tension over extreme muscle lengths. J. Exp. Biol. 205, 2167-2173. ( 10.1242/jeb.205.15.2167) [DOI] [PubMed] [Google Scholar]
- 50.Swash M, Czesnik D, de Carvalho M. 2019. Muscular cramp: causes and management. Eur. J. Neurol. 26, 214-221. ( 10.1111/ene.13799) [DOI] [PubMed] [Google Scholar]
- 51.Millman BM. 1998. The filament lattice of striated muscle. Physiol. Rev. 78, 359-391. [DOI] [PubMed] [Google Scholar]
- 52.Luther PK. 2009. The vertebrate muscle Z-disc: sarcomere anchor for structure and signalling. J. Muscle Res. Cell Motil. 30, 171-185. ( 10.1007/s10974-009-9189-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burgoyne T, Morris EP, Luther PK. 2015. Three-dimensional structure of vertebrate muscle Z-band: the small-square lattice Z-band in rat cardiac muscle. J. Mol. Biol. 427, 3527-3537. ( 10.1016/j.jmb.2015.08.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goldstein MA, Michael LH, Schroeter JP, Sass RL. 1988. Structural states in the Z band of skeletal muscle correlate with states of active and passive tension. J. Gen. Physiol. 92, 113-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goldstein MA, Michael LH, Schroeter JP, Sass RL. 1989. Two structural states of Z-bands in cardiac muscle. Am. J. Physiol. – Hear. Circ. Physiol. 256, H552-H559. ( 10.1152/ajpheart.1989.256.2.h552) [DOI] [PubMed] [Google Scholar]
- 56.Brown IE, Liinamaa TL, Loeb GE. 1996. Relationships between range of motion, Lo, and passive force in five strap-like muscles of the feline hind limb. J. Morphol. 230, 69-77. () [DOI] [PubMed] [Google Scholar]
- 57.Kawakami Y, Fukunaga T. 2006. New insights into in vivo human skeletal muscle function. Exerc. Sport Sci. Rev. 34, 16-21. [DOI] [PubMed] [Google Scholar]
- 58.Seipel K, Schmid V. 2005. Evolution of striated muscle: jellyfish and the origin of triploblasty. Dev. Biol. 282, 14-26. ( 10.1016/j.ydbio.2005.03.032) [DOI] [PubMed] [Google Scholar]
- 59.Tomalka A, Heim M, Klotz A, Rode C, Siebert T. 2022. Data from: Ultrastructural and kinetic evidence support that thick filaments slide through the Z-disc. Figshare. ( 10.6084/m9.figshare.21621132) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The statistical data underlying figures 1–4, 6 and table 1 are provided as data Excel files. At the request of the authors, readers are herein alerted to the fact that additional materials related to this manuscript may be found on Figshare: https://doi.org/10.6084/m9.figshare.21621132 [59].