Abstract
Fever, a nonspecific acute-phase response, has been associated with improved survival and shortened disease duration in infections, but the mechanisms of these beneficial responses are poorly understood. We previously reported that increasing core temperature of bacterial endotoxin (LPS)-challenged mice to the normal febrile range modified expression of tumor necrosis factor alpha (TNF-α), interleukin 1β (IL-1β), and IL-6, three cytokines critical to mounting an initial defense against microbial pathogens, but survival was not improved in the warmer animals. We speculated that our inability to show a survival benefit of optimized cytokine expression in the warmer animals reflected our use of LPS, a nonreplicating agonist, rather than an infection with viable pathogens. The objective of this study was to determine if increasing murine core temperature altered cytokine expression and improved survival in an experimental bacterial peritonitis model. We showed that housing mice at 35.5°C rather than 23°C increased core temperature from 36.5 to 37.5°C to 39.2 to 39.7°C, suppressed plasma TNF-α expression for the initial 48 h, delayed gamma interferon expression, improved survival, and reduced the bacterial load in mice infected with Klebsiella pneumoniae peritonitis. We showed that the reduced bacterial load was not caused by a direct effect on bacterial proliferation and probably reflected enhanced host defense. These data suggest that the increase in core temperature that occurs during bacterial infections is essential for optimal antimicrobial host defense.
Septic shock is a severe, often life-threatening consequence of gram-negative bacterial infection, which occurs in 500,000 to 750,000 patients per year in the United States (1). Fever, a nonspecific acute-phase response, has been associated with improved survival (5, 22) and shortened disease duration (10, 19, 22) in infections, but the mechanisms of these beneficial responses are poorly understood. The proinflammatory cytokines interleukin-1β (IL-1β) and tumor necrosis factor alpha (TNF-α) are essential for survival in host infections (6, 8). However, dysregulated expression of these cytokines also causes multiorgan injury, shock, and death (21). Mice lacking a functional TNF-α gene not only show the expected susceptibility to lethal infections with intracellular pathogens but surprisingly mount a dysregulated, progressive, lethal inflammatory response to stimuli that are not normally lethal (20). The role of IL-6 is complex and not completely understood. It limits the early inflammatory response by inhibiting expression of TNF-α and IL-1, but IL-6 also induces expression of acute-phase reactants and generation of T- and B-cell-dependent immune responses (13, 16). Thus, it may be an early cytokine mediator of the transition from innate to antigen-specific immune responses. IL-6 knockout mice mount an exaggerated inflammatory response to bacterial endotoxin (lipopolysaccharide [LPS]) (24), supporting the essential role for early IL-6 generation in limiting inflammation. Gamma interferon (IFN-γ) increases macrophage capacity for killing intracellular pathogens, enhances TNF-α and IL-1β expression, and enhances generation of T-cell-dependent responses. However, IFN-γ also enhances the lethal potential of TNF-α when they are systemically coexpressed (9).
The increased mortality caused by therapeutic interventions with cytokine-modifying agents or broadly acting anti-inflammatory medications underscores how inadvertently perturbing the essential orchestration of host defense mechanisms can reduce survival during infections. We previously reported that increasing core temperature from basal levels to the normal febrile range enhanced an early, self-limited pulse of TNF-α generation, increased IL-6 expression, and delayed IL-1β expression in a murine LPS-challenge model of sepsis, leading us to speculate that fever may play an essential role in orchestrating host defenses (17, 18). However, raising core temperature in the LPS-challenged mice tended to reduce rather than improve survival. We speculated that our inability to show a survival benefit of enhanced host defense in the warmer animals in this study reflected our use of LPS, a nonreplicating agonist, rather than an infection with viable pathogens. The objective of the present study was to determine if increasing murine core temperature altered cytokine expression and improved survival in an experimental bacterial peritonitis model.
MATERIALS AND METHODS
Bacterial culture.
Klebsiella pneumoniae 1:K2 strain B5055 and the Caroli substrain of B5055 were obtained from Ida and Frits Orskov (State Serum Institute, Copenhagen, Denmark) and stored as frozen glycerol stocks. The bacteria were cultured in tryptic soy broth (Sigma) at 37°C, and growth was monitored by measuring optical density at 650 nm (OD650). Bacteria were harvested during logarithmic growth, washed three times with sterile phosphate-buffered saline, pH 7.2 (PBS), adjusted to 0.3 OD units (approximately 108 bacteria per ml), and used to inoculate mice. Inoculum size was confirmed by enumerating colony growth on MacConkey agar.
Experimental peritonitis.
Eight- to ten-week-old male CD-1 mice weighing 25 to 30 g were purchased from Harlan-Sprague Co. (Indianapolis, Ind.), housed in the University of Maryland at Baltimore (UMAB) animal facility under the supervision of a full-time veterinarian, and used within 4 weeks of delivery. Mice were adapted to standard plastic cages for at least 4 days prior to study. Groups of four to six mice were inoculated with a single intraperitoneal (i.p.) injection of washed bacteria or PBS alone and placed in 23 or 35.5°C cages in the same room. Core temperature was monitored in a subset of the mice, using a rectal thermistor probe (Cole-Palmer) inserted to 2.5 cm. All animal protocols were approved by the Institutional Animal Care and Use Committee of UMAB. To avoid the influence of diurnal cycling, all experiments were started at approximately the same time each day (between 8:00 and 10:00 a.m.). In some experiments, groups of mice were sacrificed at the indicated time after inoculation by cervical dislocation after anesthesia with methoxyflurane. Heparinized blood was collected via cardiac puncture. Peritoneal lavage was performed with 5 ml of Hanks basal salt solution (Bethesda Research Laboratories, Gaithersburg, Md.), and lung, spleen, kidney, and liver were collected using sterile technique as we previously described (17).
Measurement of cytokine concentration.
Mouse TNF-α, IFN-γ, IL-6, and IL-1β were measured in the UMAB Cytokine Core Laboratory using standard two-antibody enzyme-linked immunosorbent assay with commercial antibody pairs and recombinant standards (TNF-α and IL-6 from Endogen, Boston, Mass.; IFN-γ from R&D, Minneapolis, Minn.; and IL-1β from Genzyme, Cambridge, Mass.). Polystyrene plates (Maxisorb; Nunc) were coated with capture antibody in PBS overnight at 25°C. The plates were washed four times with 50 mM Tris–0.2% Tween 20 (pH 7.2) and then blocked for 90 min at 25°C with assay buffer (PBS containing 4% bovine serum albumin and 0.01% thimerosal [pH 7.2]). The plates were washed, 50 μl of assay buffer was added to each along with 50 μl of sample or standard prepared in assay buffer, and the plates were incubated at 37°C for 2 h. After washing, strepavidin-peroxidase polymer in casein buffer (Research Diagnostics, Mount Pleasant, N.J.) was added and incubated at 25°C for 30 min. The plate was washed, 100 μl of substrate (tetramethylbenzidene; Dako, Carpenteria, Calif.) was added, and the plate was incubated for 20 to 30 min. The reaction was stopped with 100 μl of 2 N HCl, and the OD450 (minus OD650) was read on a microplate reader (Molecular Devices, Sunnyvale, Calif.). The data were analyzed using a computer program (SoftPro; Molecular Devices). The TNF-α, IL-6, IFN-γ, and IL-1β assays had lower detection limits of 8, 3, 3.9, and 1.5 pg/ml, respectively.
Measurement of bacterial load.
Organs were weighed and homogenized in 1 ml of 0.9% NaCl between sterile frosted glass slides. The solid tissue was allowed to sediment for 10 min at room temperature. Homogenate supernatants and blood were serially diluted, and 10-μl aliquots of each were plated on MacConkey agar and incubated at 37°C overnight; the number of colonies was enumerated and expressed per milliliter of blood or peritoneal fluid or gram (wet weight) of tissue.
In vitro bacterial proliferation assay.
K. pneumoniae cultures in 250 ml of tryptic soy broth (Difco) were inoculated with an overnight liquid inoculum. Parallel cultures were incubated in 37 and 39.5°C shaking incubators, and OD650 was monitored using a spectrophotometer (Beckman, Fullerton, Calif.). To study the effect of longer exposure to elevated temperature on bacterial proliferation, K. pneumoniae culture plates were cultured at 37 or 39.5°C for 2 days and used to directly inoculate the tryptic soy broth.
Statistical analysis.
All data are presented as mean ± standard error (SE). Differences between two groups were tested using an unpaired Student t test. Differences among more than two groups were tested by a Fisher protected least-squares difference test applied to a one-way analysis of variance. Survival was analyzed using a log-rank test.
RESULTS
Effect of experimental peritonitis and ambient temperature on core temperature and survival.
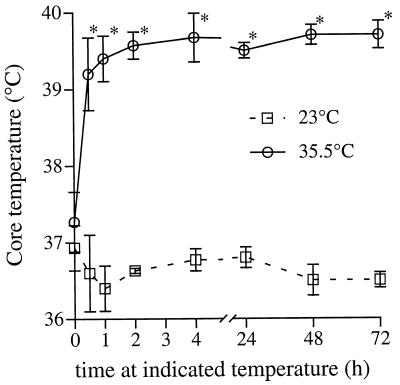
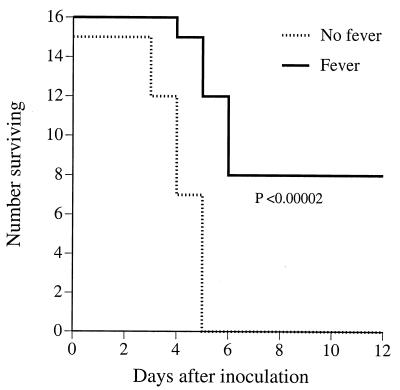
Basal core temperature of mice housed at 23°C ranged between 36.5 and 37.8°C (Fig. 1). Following inoculation with 100 CFU of K. pneumoniae strain 5055 and transfer to the 35.5°C ambient temperature, core temperature rapidly increased from 37.2 ± 0.39°C to 39.2 ± 0.47°C within 0.5 h and remained at that level for the following 72 h of observation (Fig. 1). By comparison, the infected mice that remained at 23°C failed to generate a fever over this period of time. For the remainder of this report, we will refer to the mice housed at 23°C as afebrile and those housed at 35.5°C as febrile. As previously reported (15), inoculation with 100 CFU of the Caroli variant of K. pneumoniae strain 5055 was uniformly lethal in afebrile mice housed at usual laboratory temperature (23°C) (Fig. 2). Inoculation with only 25 CFU of K. pneumoniae was also uniformly lethal (data not shown). In striking contrast, survival in the febrile mice inoculated with 100 CFU of K. pneumoniae was 50% (Fig. 2).
FIG. 1.
Effect of ambient temperature on core temperature in mice after inoculation with K. pneumoniae. Mice were inoculated i.p. with 100 CFU of K. pneumoniae strain 5055 and then placed in 23 or 35.5°C ambient temperature; core temperature was sequentially measured using a rectal thermistor probe. Mean ± SE; n = 4; ∗, P < 0.05 compared with mice at 23°C ambient temperature.
FIG. 2.
Influence of core temperature on survival after inoculation with K. pneumoniae. Mice were inoculated i.p. with 100 CFU of the Caroli strain of K. pneumoniae and then placed in 23°C (No fever) or 35.5°C (Fever) ambient temperature; survival was followed over 12 days.
Bacterial load after peritonitis in febrile and afebrile mice.
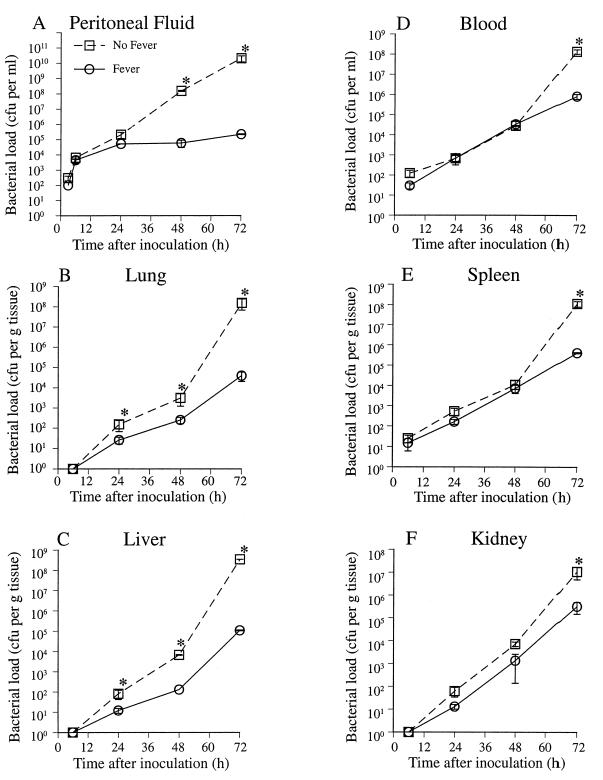
To determine the influence of core temperature on bacterial proliferation and dissemination in vivo, we quantitated the bacterial load in the peritoneal cavity, blood, and homogenates of distal organs following i.p. inoculation with 100 CFU of K. pneumoniae strain 5055 in afebrile and febrile mice (Fig. 3). In the afebrile mice, the bacterial load recoverable in peritoneal lavage increased logarithmically over 72 h, reaching 2.1 × 1010 CFU per ml. In contrast, in the febrile animals, the peritoneal bacterial load plateaued after 24 h, reaching only 2.2 × 105 CFU per ml by 72 h after inoculation. Bacteria were detected in blood and spleen as early as 6 h after inoculation in both groups of mice. The blood and splenic bacterial load increased in both groups at similar rates over the first 48 h after inoculation, but bacterial proliferation subsequently accelerated in the afebrile mice. By 72 h after inoculation, blood and splenic CFU were >2 orders of magnitude higher in the afebrile mice ([1.3 ± 0.33] × 108 versus [8.1 ± 2.2] × 105 CFU per ml of blood; [1.09 ± 0.33] × 108 versus [4.0 ± 0.27] × 105 CFU per g of splenic tissue). Bacterial CFU were not detected in lung, liver, and kidney until 24 h after inoculation and subsequently increased more rapidly in the afebrile mice. By 72 h after inoculation, the bacterial load in the lung and liver was >3 orders of magnitude higher in the afebrile animals ([1.53 ± 0.86] × 108 versus [3.92 ± 1.89] × 104 CFU per g of lung tissue; [3.5 ± 0.02] × 108 versus [1.08 ± 0.06] × 105 CFU per g of liver tissue), and the bacterial load in kidney was 33-fold higher in the afebrile mice ([1.08 ± 0.59] × 107 versus [3.22 ± 1.73] × 105 CFU per g of kidney tissue). Bacterial load was also analyzed upon death in nonsurvivors (Table 1). At death, febrile mice had bacterial loads in liver, lung, and spleen that were 6- to 40-fold lower than in afebrile mice.
FIG. 3.
Influence of core temperature on bacterial clearance after inoculation with K. pneumoniae. Mice were inoculated i.p. with 100 CFU of K. pneumoniae strain 5055 and then placed in 23°C (No Fever) or 35.5°C (Fever) ambient temperature. Six mice in each group were sacrificed at the indicated times, and the bacterial CFU in blood, peritoneal lavage fluid, and organ homogenates was determined by plating on MacConkey agar. Mean ± SE; n = 6; ∗, P < 0.05 compared with the “no fever” mice.
TABLE 1.
Bacterial load at time of death in afebrile and febrile micea
| Group | Mean CFU/g of tissue (107) ± SE
|
|||
|---|---|---|---|---|
| Lung | Liver | Spleen | Kidney | |
| Afebrile | 22.0 ± 9.01 | 15.3 ± 6.8 | 85.3 ± 58.5 | 5.7 ± 2.2 |
| Febrile | 3.7 ± 1.7b | 1.0 ± 0.4b | 2.1 ± 0.9b | 3.6 ± 2.9 |
Dilutions of homogenized organs were cultured on MacConkey agar, and colonies were enumerated.
Difference from value for afebrile mice with P < 0.05.
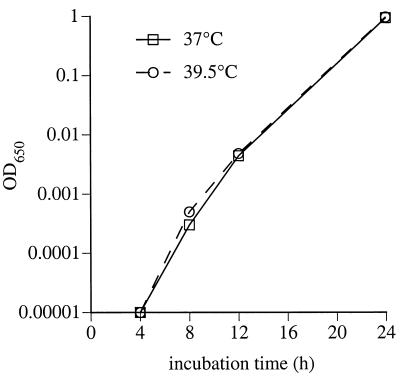
To determine if exposure to the higher temperatures directly inhibited bacterial proliferation, we cultured K. pneumoniae strain 5055 in tryptic soy broth in vitro at 37 or 39.5°C while monitoring OD650. During a 20-h incubation, the rate and duration of logarithmic growth were similar at the two temperatures (Fig. 4). Liquid cultures inoculated from culture plate incubated for 48 h at 37 or 39.5°C also had nearly identical proliferation rates (data not shown), indicating that >2-day exposure to 39.5°C did not reduce bacterial proliferation rate.
FIG. 4.
Direct influence of temperature on bacterial proliferation in vitro. One hundred milliliters of LB medium was inoculated with 10 CFU of K. pneumoniae strain 5055 and cultured at 37 or 39.5°C in a shaking incubator; the OD650 was sequentially measured as a measure of bacterial proliferation.
Influence of core temperature on cytokine levels in bacterial peritonitis.
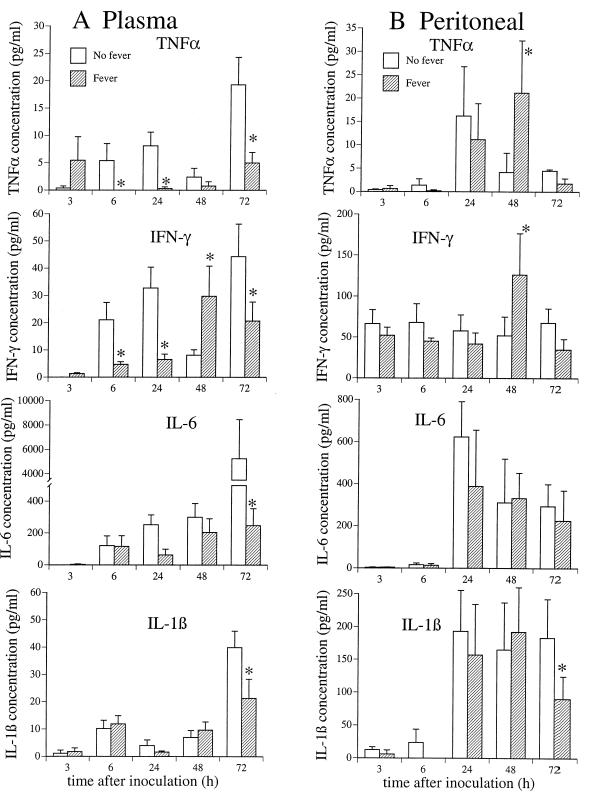
We previously showed that exposing macrophages to febrile temperature in vitro suppressed TNF-α expression (11, 12, 18). We also reported that increasing core temperature in LPS-challenged mice accelerated and enhanced an early, self-limited pulse of TNF-α in plasma but subsequently suppressed persistent TNF-α expression and enhanced the expression of IL-6 (17, 18). To determine if core temperature had a similar influence on cytokine expression during infections, we serially measured plasma cytokine levels in the afebrile and febrile mice following inoculation with 100 CFU of K. pneumoniae strain 5055 (Fig. 5). TNF-α was not detected until 6 h after inoculation in the afebrile mice. Plasma TNF-α levels plateaued at 5 to 8 pg/ml between 6 and 24 h after inoculation, reached a nadir of 2.4 pg/ml by 48 h, and increased again to 19 pg/ml by 72 h coincident with a marked increase in bacterial load and obvious signs of distress. In contrast, in the febrile mice, plasma TNF-α was detectable in two of eight mice 3 h after inoculation but was not detectable between 6 and 48 h after inoculation. After remaining undetectable for 48 h, TNF-α appeared in plasma, but at levels only one-fourth as high as in the afebrile mice (7 pg/ml). Plasma IL-6 levels were similar in the two groups for the first 2 days after inoculation but subsequently increased in the afebrile mice, reaching levels at 72 h that were 21-fold higher than in the febrile mice (5,253 ± 3,196 versus 224 ± 104 pg/ml). Plasma IFN-γ was detected 6 h after inoculation, peaked at 24 h, decreased to a nadir at 48 h, and increased to a second peak at 72 h in the afebrile animals. In contrast, plasma IFN-γ levels did not peak until 48 to 72 h after inoculation in the febrile mice. Plasma IL-1β levels were similar in the two groups of mice. Cytokine levels in peritoneal fluid were similar in the afebrile and febrile mice, except for a coincident increase in TNF-α and IFN-γ in the febrile mice 48 h after LPS, coinciding with the peak in plasma IFN-γ levels.
FIG. 5.
Influence of core temperature on cytokine expression after inoculation with K. pneumoniae. Mice were inoculated i.p. with 100 CFU of K. pneumoniae strain 5055 and then placed in 23°C (No fever) or 35.5°C (Fever) ambient temperature. Six mice in each group were sacrificed at the indicated times. Heparinized blood was collected from the left ventricle, and the peritoneum was lavaged with 5 ml of Hanks basal salt solution. The concentrations of the indicated cytokines in plasma (A) and peritoneal lavage fluid (B) were measured by enzyme-linked immunosorbent assay and quantified using a recombinant standard curve. Cytokine concentrations are expressed per milliliter of plasma and peritoneal fluid. Mean ± SE; n = 6; ∗, P < 0.05 compared with the “no fever” mice.
DISCUSSION
We previously reported that briefly increasing core temperature in anesthetized LPS-challenged mice altered the acute-phase cytokine response by enhancing an early peak in TNF-α expression but failed to reduce LPS-induced mortality (18). We extended these studies by comparing survival, cytokine expression, and bacterial clearance in mice maintained with 36.5 and 39.5°C core temperature for several days during infection with a clinically relevant and lethal pathogen, K. pneumoniae.
K. pneumoniae strain 5055 Caroli is reported to be uniformly lethal in mice (15), but the mice in these previous studies were maintained at normal laboratory temperatures. Our data confirm previous reports that young mice fail to generate a fever in response to infection or LPS challenge when they are denied access to external sources of heat (14). In this study, reconstituting fever by providing an increased ambient temperature increased survival from 0 to 50% in animals injected i.p. with this uniformly lethal strain of K. pneumoniae. The protective effects of reconstituting febrile core temperature in mice infected with K. pneumoniae were similar to those reported in mice infected with lethal herpes simplex (2) and rabies (3) viruses and in lizards infected with the gram-negative bacterium Aeromonas hydrophila (4).
The results of the present study show that the improved survival correlated with a profound reduction in bacterial proliferation in the febrile animals. Bacterial proliferation in vitro was similar at basal (37°C) and febrile-range (39.5°C) temperatures for up to 20 h. Logarithmic growth rates of bacteria could not be maintained beyond this time interval in liquid culture. However, exposing K. pneumoniae grown on agar plates to 39.5 rather than 37°C for 2 days did not affect subsequent growth rate after inoculating liquid culture medium. These results indicate that the reduced bacterial proliferation in the febrile animals resulted from alterations in host-pathogen interaction rather than a direct toxic effect of the warmer temperature on the bacteria. The early temperature-related divergence of bacterial load in the peritoneal fluid suggests that host defenses at the primary site of infection were enhanced in the warmer animals. Bacterial load in liver and lung diverged 24 h after inoculation, at which time levels of bacteremia remained similar in the two groups, suggesting that local antimicrobial host defenses may also have been enhanced in these organs in the febrile mice. Furthermore, the lower pathogen load at time of death in the febrile animals suggests that different pathogenic processes may cause death in the febrile and afebrile animals. Specifically, we speculate that collateral damage from host defenses may play a greater role in causing death in the febrile mice, while overwhelming bacterial infection may be the major cause of death in the afebrile mice.
We previously reported that increasing core temperature in anesthetized mice challenged with LPS enhanced the early peak in plasma TNF-α while suppressing persistent expression (17, 18). In the present study, plasma TNF-α expression was suppressed over the first 2 days of infection, but only two of eight febrile mice showed enhanced early TNF-α expression as previously reported for LPS-challenged mice (17, 18). In the previous studies, the magnitude of early TNF-α enhancement was directly proportional to the dose of LPS administered. In mice challenged with ≤1 μg of LPS, no enhancement in early TNF-α expression was evident. In the present study, mice were challenged with 100 CFU of Klebsiella, an amount which contains only approximately 0.1 pg of LPS (7). These data suggest that febrile core temperature is predominantly suppressive for TNF-α expression during bacterial infections. This finding in the febrile animals is consistent with the early deactivation of TNF-α gene expression in macrophages in vitro previously reported by our laboratory (11, 12, 18), an effect caused by destabilization of TNF-α mRNA (11), and an early cessation of transcription (21a). The latter effect is mediated in part by partial activation of heat shock factor 1. We previously reported that submaximal heat shock protein gene activation occurs in animals exposed to febrile temperature (18), providing further correlative evidence for a possible link between heat shock and cytokine regulation during fever.
In contrast with TNF-α, expression of IFN-γ in plasma was delayed rather than suppressed. The combined effect of fever on plasma IFN-γ and TNF-α prevented simultaneous exposure to both cytokines, thus avoiding their potential synergistic harmful effects (9). On the other hand, in the febrile mice, a coincident peak in IFN-γ and TNF-α occurred in the peritoneal compartment, the primary site of infection, which may enhance local antimicrobial defenses at the primary site of infection (9). Taken together, these data show that the effects of fever on cytokine expression are complex and are specific for each cytokine and body compartment.
In summary, we have shown that generating an increase in core temperature during bacterial infection modifies cytokine expression, reduces bacterial growth and dissemination by enhancing host defenses, and improves host survival but may increase the risk of death from other processes. The molecular mechanisms of these effects are presently under investigation in our laboratory.
ACKNOWLEDGMENTS
This work was supported by PHS grant R01AI42117.
We thank Monica DeBoer and Katie Buffum for technical assistance.
REFERENCES
- 1.Angus D D, Linde-Zwirble W T, Clermont G, Newbold R C, Pinsky M R. How much sepsis is there in the US? Am J Respir Crit Care Med. 1996;153:A837. [Google Scholar]
- 2.Armstrong C. Some recent research in the field of neurotropic viruses with especial reference to lymphocytic choriomeningitis and herpes simplex. Mil Surg. 1942;91:129–145. [Google Scholar]
- 3.Bell J F, Moore G F. Effects of high ambient temperature on various stages of rabies virus infection in mice. Infect Immun. 1974;10:510–515. doi: 10.1128/iai.10.3.510-515.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernheim H A, Bodel P T, Askenase P W, Atkins E. Effects of fever on host defense mechanisms after infection in the lizard Dipsosaurus dorsalis. Br J Exp Pathol. 1978;59:76–84. [PMC free article] [PubMed] [Google Scholar]
- 5.Bryant R E, Hood A F, Hood C E, Koenig M G. Factors affecting mortality of gram-negative rod bacteremia. Arch Intern Med. 1971;127:120–128. [PubMed] [Google Scholar]
- 6.Cross A, Asher L, Seguin M, Yuan L, Kelly N, Hammack C, Sadoff J. The importance of a lipopolysaccharide-initiated, cytokine-mediated host defense mechanism in mice against extraintestinally invasive Escherichia coli. J Clin Investig. 1995;96:676–686. doi: 10.1172/JCI118110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cross A S, Opal S M, Sadoff J C, Gemski P. The choice of bacteria in animal models of sepsis. Infect Immun. 1993;61:2741–2747. doi: 10.1128/iai.61.7.2741-2747.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cross A S, Sadoff J C, Kelly N, Bernton E, Gemski P. Pretreatment with recombinant murine tumor necrosis factor a/cachectin and murine interleukin 1a protects mice from lethal bacterial infection. J Exp Med. 1989;169:2021–2027. doi: 10.1084/jem.169.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doherty G M, Lange J R, Langstein H N, Alexander H R, Buresh C M, Norton J A. Evidence for IFN-γ as a mediator of the lethality of endotoxin and tumor necrosis factor-α. J Immunol. 1992;149:1666–1670. [PubMed] [Google Scholar]
- 10.Dorn T F, DeAngelis C, Baumgardner R A, Mellits E D. Acetaminophen: more harm than good for chicken pox? J Pediatr. 1989;114:1045–1048. doi: 10.1016/s0022-3476(89)80461-5. [DOI] [PubMed] [Google Scholar]
- 11.Ensor J E, Crawford E K, Hasday J D. Warming macrophages to febrile range destabilizes tumor necrosis factor-α mRNA without inducing heat shock. Am J Physiol. 1995;269:C1140–C1146. doi: 10.1152/ajpcell.1995.269.5.C1140. [DOI] [PubMed] [Google Scholar]
- 12.Ensor J E, Wiener S M, McCrea K A, Viscardi R M, Crawford E K, Hasday J D. Differential effects of hyperthermia on macrophage interleukin-6 and tumor necrosis factor-α expression. Am J Physiol. 1994;266:C967–C974. doi: 10.1152/ajpcell.1994.266.4.C967. [DOI] [PubMed] [Google Scholar]
- 13.Gauldie J, Richards C, Harnish D, Lansdorp P, Baumann H. Interferon β2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci USA. 1987;84:7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habicht G S. Body temperature in normal and endotoxin-treated mice of different ages. Mech Aging Dev. 1981;16:97–104. doi: 10.1016/0047-6374(81)90037-3. [DOI] [PubMed] [Google Scholar]
- 15.Held T, Mielke M, Chedid M, Unger M, Trautmann M, Huhn D, Cross A. Granulocyte colony-stimulating factor worsens the outcome of experimental Klebsiella pneumoniae pneumonia through direct interaction with the bacteria. Blood. 1998;91:2525–2535. [PubMed] [Google Scholar]
- 16.Hirano T. Interleukin-6 and its relation to inflammation and disease. Clin Immunol Immunopathol. 1992;62:S60–S65. doi: 10.1016/0090-1229(92)90042-m. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Q, DeTolla L, Kalvakolanu I, Fitzgerald B, Hasday J D. Fever upregulates expression of pyrogenic cytokines in endotoxin-challenged mice. Am J Physiol. 1999;276:R1653–R1660. doi: 10.1152/ajpregu.1999.276.6.R1653. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Q, DeTolla L, Kalvakolanu I, Van Roojien N, Singh I S, Fitzgerald B, Cross A S, Hasday J D. Febrile range temperature modifies early systemic tumor necrosis factor alpha expression in mice challenged with bacterial endotoxin. Infect Immun. 1999;67:1539–1546. doi: 10.1128/iai.67.4.1539-1546.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackowiak P A, Wasserman S S, Levine M M. An analysis of the quantitative relationship between oral temperature and severity of illness in experimental shigellosis. J Infect Dis. 1992;166:1181–1184. doi: 10.1093/infdis/166.5.1181. [DOI] [PubMed] [Google Scholar]
- 20.Marino M W, Dunn A, Grail D, Inglese M, Noguchi Y, Richards E, Jungbluth A, Wada H, Moore M, Williamson B, Basu S, Old L. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci USA. 1997;94:8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reinhat K, Eyrich K, Sprung C, editors. Sepsis: current perspectives in pathophysiology and therapy. New York, N.Y: Springer-Verlag; 1994. [Google Scholar]
- 21a.Singh, I. S., S. Calderwood, I. Kalvakolanu, R. Viscardi, and J. D. Hasday. Inhibition of tumor necrosis factor-α transcription in macrophages exposed to febrile range temperature: a possible role for heat shock factor-1. J. Biol. Chem., in press. [DOI] [PubMed]
- 22.Stanley E D, Jackson G G, Panusarn C, Rubenis M, Dirda V. Increased viral shedding with aspirin treatment of rhinovirus infection. JAMA. 1975;231:1248–1251. [PubMed] [Google Scholar]
- 23.Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei X, Achong M. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Investig. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]