Abstract
Objective:
Multiple myeloma (MM) and monoclonal gammopathy of undetermined significance (MGUS), the precursor of MM, are plasma cell neoplasms. The evolution of the treatment of MM in recent years has dramatically improved the outcome for these patients. Currently, multidisciplinary studies are being conducted to elucidate the etiopathogenesis of the disease and develop specific treatment agents and prognostic markers. The present study investigates the relationships between immunoexpression of CD138, Pan-Ras, CCL-3, DKK-1, and MUM-1 and disease progression in cases of MM and MGUS.
Materials and Methods:
Immunohistochemical staining for CD138, Pan-Ras, CCL-3, DKK-1, and MUM-1 were performed on bone marrow biopsy samples from 94 MM and 20 MGUS patients diagnosed between 2011 and 2018. Immunohistochemical results were examined semiquantitatively, and the associations between the immunohistochemical, clinical, and biochemical markers utilized for MM and MGUS patient staging were analyzed.
Results:
Pan-Ras, DKK-1, and MUM-1 staining results were significantly higher in MM compared to MGUS (p=0.005, 0.001, and 0.001, respectively). The mean CCL-3 expression in patients with MGUS was 23.15%, while it was 18.68% in cases of MM (p=0.413). CCL-3 expression was significantly higher in high-risk MGUS cases compared to other risk groups according to the Mayo Clinic Risk Stratification for MGUS. According to the International Staging System and the Revised International Staging System, CD138 expression was higher among stage II and stage III patients than stage I patients.
Conclusion:
Differences in Pan-Ras, MUM-1, DKK-1, and CCL-3 expressions between MM and MGUS suggest that these molecules may play a role in the progression of MGUS to MM. CCL-3, an immunohistochemical marker, may be predictive of MGUS progression, while CD138 is associated with more advanced stages of MM.
Keywords: Multiple myeloma, Monoclonal gammopathy of undetermined significance, DKK-1, Pan-Ras, CCL-3, MUM-1
Abstract
Amaç:
Multipl myelom (MM) ve MM’nin prekürsörü olan önemi belirsiz monoklonal gamopati (MGUS), plazma hücreli neoplazilerdir. Güncel çalışmalarda bu hastalıkların etiyopatogenezini aydınlatmak, spesifik tedavi ajanları ve prognostik belirteçler geliştirmek için multidisipliner çalışmalar yürütülmektedir. Çalışmamızda MM ve MGUS’lerde CD138, Pan-Ras, CCL-3, DKK-1 ve MUM1 immünoekspresyonun hastalık evreleriyle olan ilişkisini araştırmayı hedefledik.
Gereç ve Yöntemler:
2011-2018 yılları arasında tanı alan 94 MM ve 20 MGUS olgusuna ait kemik iliği biyopsilerine, CD138, Pan-Ras, CCL-3, DKK-1, MUM-1 immünohistokimyasal (İHK) boyaması yapıldı. İHK sonuçları semi kantitatif olarak değerlendirildi ve MM ve MGUS olgularının hastalık evreleriyle olan ilişkileri değerlendirildi.
Bulgular:
Pan-Ras, DKK-1 ve MUM-1 immünoekspresyonu, MM’li olgularda MGUS’li olgulara göre anlamlı olarak daha yüksek saptandı (p=0,005, 0,001, ve 0,001, sırasıyla). MGUS olgularında ortalama CCL-3 immünoekspresyonu %23,15 iken, MM olgularında %18,68 idi (p=0,413). MGUS olgularında Mayo Klinik risk sınıflandırması (MCRS) modeline göre, yüksek riskli MGUS olgularında diğer risk gruplarına kıyasla CCL-3 ekspresyonu önemli ölçüde artmış olarak saptandı. CD138 ekspresyonu, ISS ve R-ISS sınıflama sistemlerine göre, evre 2 ve evre 3 hastalarda evre 1 hastalara göre artmış olarak tespit edildi.
Sonuç:
MM ve MGUS olgularında Pan-Ras, MUM-1, DKK-1 ve CCL-3 ekspresyonlarındaki farklılıklar, bu belirteçlerin MGUS-MM progresyonunda önemli roller üstlendiğini göstermektedir. Kemik iliği biyopsilerinde, kolay ve pratik bir şekilde, CCL-3 immün belirteci MGUS progresyonunda prediktif; CD138 ise MM olgularında ileri evre tayininde kullanılabilir.
Introduction
Multiple myeloma (MM) is a plasma cell neoplasm defined by the proliferation of clonal plasma cells in the bone marrow and the release of a monoclonal protein in serum and/or urine in the majority of cases. MM accounts for 10%-15% of hematological malignancies and 1% of all malignancies, with a median age at diagnosis of 69 years [1,2]. In recent years, the advancement of MM treatment has greatly improved the outcomes for these patients [3,4,5]. Monoclonal gammopathy of unknown significance (MGUS) is an asymptomatic precursor plasma cell neoplasm that progresses to MM in roughly 1% of cases per year. The etiopathogenesis of progression from MGUS to MM has not been elucidated yet [2,6,7,8].
Many preclinical and clinical investigations have been conducted to investigate the cytogenetic pathogenesis of MGUS and MM. The hematopoietic niche is critical in the genesis and progression of MGUS and MM. Tremendous effort has recently been made to produce niche-oriented therapeutic agents [3,4]. Treatment regimens for lytic lesions in the bone, which are regarded as one of the most serious disease consequences, have gained ground among researchers [5].
To estimate the prognosis of MM, many risk scoring systems have been designed. The International Staging System (ISS) is the most widely accepted and frequently used of them. Serum β2-microglobulin and serum albumin levels are used in this staging system [9].
With a focus on molecular pathogenesis and the prognostic utility of molecular changes, the ISS was recently revised with the inclusion of cytogenetic high-risk-related mutations and serum lactate dehydrogenase levels, and the Revised ISS (R-ISS) was created [10,11,12]. The use of these staging systems, however, varies by institution.
There are efforts to stratify MGUS cases according to the risk of progression. The Mayo Clinic Risk Stratification (MCRS) for MGUS is the most recognized of these efforts, and it is also referenced in the 2010 manual of the International Myeloma Working Group. Based on 20 years of follow-up, the Mayo Clinic model advises that cases be classified as being of low risk, low-medium risk, medium-high risk, or high risk [1,2,6,7].
In MM and MGUS, myeloma cells and atypical plasma cells express syndecan-1, also known as CD138, an integral membrane protein that allows cells to communicate with the extracellular matrix [13]. CD138 has been linked to myeloma cell adhesion and communication [13,14,15,16].
Chemokine ligand 3 (CCL-3)/macrophage inflammatory protein-1 (MIP-1) is a chemokine produced by myeloma cells that are involved in the niche stage of MM and MGUS tumor pathogenesis. CCL-3 receptors were found on bone marrow-derived stem cells (BMSCs), osteoclasts, and osteoblasts. CCL-3 has also been linked to disease-related bone damage [17,18]. Another function of CCL-3, which is significant for the pathogenesis of MM, is its role in myeloma cell survival in the bone marrow niche [3,19,20].
MUM-1/IRF-4, or the MM oncogene, is a gene that belongs to the interferon regulator family and is associated with different stages of plasma cell development. It is involved in the development of MM and its precursor forms, as well as the differentiation of T-helper (Th-17) cells, which are crucial in the tumor microenvironment, and cytokine release. Several studies [21,22,23] have found that immunoexpression of MUM-1 is increased in the advanced stages of MM.
Dickkopf-1 (DKK-1) is a cytokine that plays a key role in the relationship between tumors and the microenvironment. DKK-1 inhibits osteoblastogenesis via antagonizing the wingless integrated cell signaling pathway. In MM, a similar mechanism stops BMSCs from becoming mature osteoblasts. The increasing number of bone complications caused by DKK-1-mediated events in advanced stages of MM suggests that the DKK-1 immunohistochemical marker could be used to determine prognosis [18,24,25,26].
Although K-RAS, N-RAS, and H-RAS mutations are mostly associated with epithelial neoplasms, they are also detected at frequencies of 9% to 30% in MM. Although they have also been detected in MGUS and other MM precursor diseases, it is noteworthy that the frequency of mutations is higher in MM compared to MGUS. Patients with MM have a poorer prognosis if there is a mutation in the RAS genes. Several studies have reported mutations of RAS subtypes at varying frequencies in the pathogenesis of MM [27,28,29,30].
The aim of this study was to evaluate the impact of a set of immunohistochemical markers in bone marrow biopsies on the disease progression of patients with MM and MGUS.
Materials and Methods
Patients
This study was designed in the Marmara University Faculty of Medicine’s Department of Pathology. At the beginning of the study, bone marrow biopsies were used to make the initial diagnosis, and the Department of Pathology at the Marmara University Faculty of Medicine Pendik Research Hospital investigated the data of 443 patients diagnosed with MM or MGUS between January 2011 and March 2018. Biopsies for the evaluation of remission and/or recurrence were not considered. The study included bone marrow specimens from 20 patients with MGUS and 94 patients with MM who had records of disease stages and cytogenetic analysis results. Clinical data were gathered from patient records in the same center’s Department of Hematology. A hematologist from that department informed us about the ISS, R-ISS, and MCRS data of the patients, reflecting the results of the applied disease staging systems. The amounts of serum β2-microglobulin and albumin were used to assess the ISS stage. Serum lactate dehydrogenase levels and cytogenetic data were added to the ISS data to assess the R-ISS stage. Non-immunoglobulin (Ig)G isotype (IgA and IgM), M protein levels, and serum-free light chain ratio were determined for MGUS patients for MCRS staging. Patients who did not have sufficient clinical data or tissue samples for immunohistochemistry analyses were excluded. The Ethics Committee of Marmara University approved the study (09.2018.277).
Immunohistochemical Analysis
Bone marrow biopsy materials were preserved in formalin-fixed paraffin-embedded blocks after 3 hours of decalcification in EDTA at a concentration of 10%. Sections of 4 µm in thickness were mounted on positively charged slides. Staining of CCL-3 (PA1-38160, polyclonal, rabbit, Thermo Fischer, 1:50), MUM-1 (D0-7, monoclonal, mouse, Dako, ready-to-use), DKK-1 (SAB1404944, monoclonal, mouse, Sigma-Aldrich, 1:100), and Pan-Ras (Ras10, monoclonal, mouse, Thermo Fischer, 1:100) markers were performed using an automated immunohistochemistry device (BenchMark ULTRA XT automated stainer, Ventana Medical System, Inc., Tucson, AZ, USA). Immunohistochemical analyses of CD138 (MI15, monoclonal, mouse, Cellmark, 1:100), kappa light chain (L1C1, monoclonal, mouse, Thermo Scientific-Lab Vision, 1:200), and lambda light chain (HP6054, monoclonal, mouse, Genemed, 1:50) were performed by re-evaluating the first diagnosis slides of the patients using the same techniques.
Membranous staining with CD138, nuclear staining with MUM-1, and cytoplasmic staining with Pan-Ras, CCL-3, and DKK-1 were considered positive. Results were noted by two blinded researchers who specialized in pathology.
While calculating the staining percentages of Pan-Ras, CCL-3, DKK-1, and MUM-1 markers, all slides were scanned and then CD138 positive neoplastic cells were taken into account; the total number of hematopoietic cells was not counted. A ratio of any given marker represents the percentage of positively stained cells among the total number of neoplastic plasma cells.
Statistical Analysis
The NCSS 2007 program (NCSS Statistical Software, Kaysville, UT, USA) was used for statistical analysis. Descriptive statistical methods (mean, standard deviation, median, frequency, percentage, minimum, maximum) were used to evaluate the data. Shapiro-Wilk tests and graphical analyses were used to determine whether quantitative data were normally distributed. Student’s t-test was used to compare normally distributed quantitative variables and the Mann-Whitney U test was used to compare non-normally distributed quantitative variables between two groups. The Kruskal-Wallis test was used to compare quantitative variables that were not normally distributed between three or more groups, and the Bonferroni-Dunn test was used for pairwise comparisons. Pearson chi-square and Fisher exact tests were used to compare qualitative data. Statistical significance was accepted as p<0.05.
Results
Demographic Characteristics
The mean age of all patients was 65 (range: 27-92) years and the ratio of men to women was 1.08.
Staging and Results of Clinical Risk Scores
Patients with MM were classified at the time of diagnosis according to the ISS, and 44 patients (46.8%) were stage I, 20 (21.2%) were stage II, and 30 (32%) were stage III. The patients were also classified at diagnosis according to the R-ISS, and 17 patients (18%) were stage I, 56 (59.5%) were stage II, and 21 (22.5%) were stage III (Table 1). Patients with MGUS were classified according to the MCRS. Four patients (20%) were in the low-risk group, 7 (35%) were in the low-to-intermediate-risk group, 5 (25%) were in the intermediate-to-high-risk group, and 4 (20%) were in the high-risk group (Table 2).
Table 1. ISS and R-ISS distributions of multiple myeloma cases.

Table 2. Mayo Clinic Risk Stratification distribution of monoclonal gammopathy of undetermined significance cases.

Immunohistochemical Findings
The results of the immunohistochemical examinations are shown in Table 3. According to these data, Pan-Ras staining rates were higher among patients with MM than those with MGUS (p=0.005). The Pan-Ras staining patterns are shown in Figure 1.
Table 3. Comparison of the immunohistochemical results of multiple myeloma and monoclonal gammopathy of undetermined significance cases.

Figure 1.
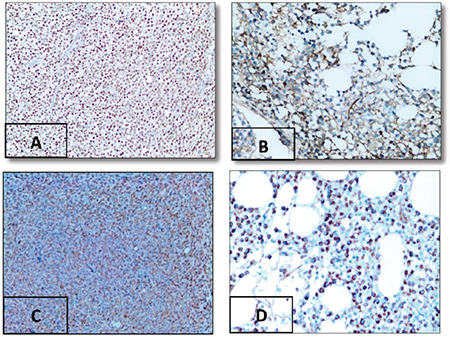
Expression of immunohistochemical markers in multiple myeloma: (A) MUM-1 expression in atypical plasma cells, 90%, 400x. (B) Pan-Ras expression in atypical plasma cells, 30%, 400x. (C) DKK-1 expression in atypical plasma cells, 95%, 200x. (D) CCL-3 expression in atypical plasma cells, 80%, 400x.
There was no statistically significant difference between patients with MM and MGUS in terms of CCL-3 staining rates (p>0.05).
DKK-1 and MUM-1 expression levels of patients with MM were significantly higher than those of patients with MGUS (p=0.001 for both). The MUM-1 staining patterns are shown in Figures 1 and 2.
Figure 2.
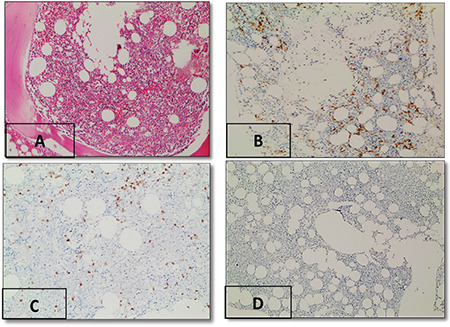
Expression of immunohistochemical markers in monoclonal gammopathy of undetermined significance. (A) Hematoxylin and eosin staining, 40x. (B) CD138 expression in atypical plasma cells, 6%, 40x. (C) MUM-1 expression in atypical plasma cells, 80%, 40x. (D) Pan-Ras expression in atypical plasma cells, 0%, 40x.
Analysis of the Relationship Between Immunohistochemical Findings and ISS and R-ISS in MM
CD138 staining rates were higher among patients with advanced ISS stages (p=0.002) (Table 4). This suggests that there are more atypical plasma cells in the advanced ISS stages. After pairwise comparisons were made to determine the group causing the significant difference, the staining rate of stage III cases was found to be higher than that of stage I (p=0.003). When the R-ISS classification was taken into account, the advanced stages once again showed higher rates of CD138 staining (p=0.043) (Table 5). Pairwise comparisons revealed that the staining rates of stage III cases were higher than those of stages I and II (p=0.037). According to R-ISS stages, Pan-Ras, CCL-3, and MUM-1 showed higher expression rates at advanced stages, but these differences were not statistically significant.
Table 4. Comparison of ISS stages and immunohistochemical results.

Table 5. Comparison of immunohistochemical findings according to R-ISS stages.

Comparisons of the immunohistochemical findings according to ISS (I-II-III) and R-ISS (I-II-III) stages are shown in Tables 4 and 5.
Analysis of the Relationship Between Immunohistochemical Findings and MCRS Groups in MGUS
The correlations between the results of immunohistochemical staining and the risk groups are shown in Table 6. The “no risk,” “low risk,” and “medium risk” groups were evaluated together and the “high risk” group was evaluated separately.
Table 6. Comparison of immunohistochemical findings of patients in high-risk group with those of patients in other groups according to the Mayo Clinic Risk Stratification for MGUS.

CCL-3 staining rates of cases classified as “high risk” according to MCRS criteria were found to be significantly higher than those of other groups (p=0.018). The immunoexpression of all markers was increased in the “high risk” group compared to the “no risk/low risk/medium risk” group, although this finding was not statistically significant. There were no correlations between the expression rates of CCL-3 and other markers.
Discussion
MM and MGUS are plasma cell neoplasms and the etiopathogenesis of these diseases has not been fully clarified. They particularly affect people over 65 years of age, and despite great advances in treatment, MM remains a largely incurable disease. The mechanisms of disease progression have also not yet been elucidated [1,2,6,7,30].
Several studies have reported different rates of distribution according to ISS and R-ISS stages at the time of diagnosis in cases of MM [10,11]. Palumbo et al. [10] reported that 38% of their participants were ISS stage I, 38% were ISS stage II, and 24% were ISS stage III. Jimenez-Zepeda et al. [11] reported that 30.3% of their patients were R-ISS stage I, 46.5% were R-ISS stage II, and 23.2% were R-ISS stage III. In our study, 46.8% of the patients were ISS stage I, 21.3% were ISS stage II, and 31.9% were ISS stage III, while 18.1% were R-ISS stage I, 59.6% were R-ISS stage II, and 22.3% were R-ISS stage III.
When we categorized MGUS cases using the MCRS criteria, we found that 4 (20%) were in the “no risk” group, 7 (35%) were in the “low-to-moderate risk” group, 5 (25%) were in the “medium-to-high risk” group, and 4 (20%) were in the “high risk” group. Rajkumar et al. [6] reported that the majority of cases in a large series of patients were classed as “medium risk” at the time of diagnosis, which is similar to our findings [8].
Numerous studies in the literature have focused on the immunoexpression rate and staining pattern of CD138, as well as its association with disease prognosis in MM and its precursors [14,16,31]. Kim et al. [32] studied CD138 levels using serum immunoelectrophoresis and suggested a possible link between CD138 levels and disease stage. In our study, we found a statistically significant difference between CD138 staining rates and ISS and R-ISS stages among MM patients, which was consistent with previous research [14,15,16]. Kawano et al. [31] and Foster et al. [33] found increases in CD138 expression using flow cytometry and immunohistochemistry, and they attributed those increases to poor disease prognosis. When we evaluated CD138 staining in cases of MGUS using the MCRS criteria, we found no significant differences. This could be due to the narrow distribution range of plasma cell ratios (3%-9%) in MGUS, as well as the small number of cases included in our investigation.
Many similar mutations have been found in cytogenetic anomalies in MM and MGUS. One of these mutations is in the RAS gene, which has been linked to the development of MM in patients with MGUS [28,30]. RAS has been associated with the development of various cancers, including hematological malignancies [28,29,30]. Zangari et al. [34] reported that inhibition of Pan-Ras prevented the development and progression of MM in rats. Our study has shown that the rate of Pan-Ras immunoexpression in plasmacytic cells was higher in MM patients than in MGUS patients (p=0.005). This finding supports the significance of Pan-Ras in disease progression, and this observation is further supported by previous studies on the role of the RAS gene in the development of MM from MGUS [7,27]. All of these findings suggest that the Pan-Ras immune marker can be utilized to evaluate patients with MGUS and early-stage MM, and that therapeutic agents can be developed accordingly. Another possible supporting evidence of the role of Pan-Ras in plasma cell neoplasm progression is its higher expression levels in advanced risk groups. When our cohort’s MGUS patients were classified using the MCRS system, the Pan-Ras immunoexpression rate was higher in the high-risk group, but this difference was not statistically significant (Table 6). We found no correlation between Pan-Ras expression and ISS stages. However, when the results were evaluated using R-ISS criteria, which involve cytogenetic analysis in addition to the ISS criteria, it was clear that the expression of Pan-Ras was increased among the higher R-ISS stages (mean values of 36.8%, 37.15%, and 46.50% in R-ISS stages I, II, and III, respectively). When all of these data were evaluated together, the presence of a statistically significant difference between cases of MGUS and MM and a difference between stages in the R-ISS system in terms of increased expression indicated consistency with the pathogenesis of RAS mutations in the progression of MGUS to MM [7,27]. These findings suggest that the Pan-Ras immune marker could be utilized to follow the progression of MGUS to MM and predict progression to MM.
MIP-1a (MIP-1 alpha/CCL-3) is a chemotactic cytokine released by macrophages. CCL-3 also stimulates the synthesis of molecules that promote the proliferation of MM cells in the bone marrow niche, such as the receptor activator of nuclear factor-kappa-B ligand (RANKL), interleukin-6 (IL-6), vascular endothelial growth factor, and tumor necrosis factor (TNF). CCL-3 also plays active roles in the early stages of neoplasms, such as in the adhesion of neoplastic cells in the microenvironment [26]. This is supported by a higher rate of immunoexpression in the high-risk group compared to other MCRS groups in the present study. CCL-3 may also have a role in the development of lytic bone lesions in the pathogenesis of MM by inhibiting osteoblastic cells and activating osteoclastic cells [25,35]. Politou et al. [20] explained the mechanism of action of CCL-3 with the change in the osteoblastic/osteoclastic activity balance in the bone, primarily and more predominantly by reducing osteoblastic activity. Palma et al. [25] found that elevated levels of CCL-3 and DKK-1 were associated with lytic bone lesions in cases of MM, MGUS, and smoldering MM (SMM). Ng et al. [24] found unexpectedly high levels of CCL-3 in the serum of MGUS patients in their study. Although lytic bone lesions are not expected in MGUS, they did discover impaired bone formation in MGUS patients. They explained this discovery with the advanced radiological examination method they used in their study. As a result, they were able to identify clinically unexpected lytic bone lesions at the microstructural level in cases of MGUS [24]. Taken together, these findings may suggest that CCL-3 has different roles in different stages of the pathogenesis of plasmacytic neoplasms. In addition to predicting an advanced stage of MGUS, we can also suggest that high CCL-3 levels may portend the possible progression of bone lesions in plasma cell neoplasms; therefore, treatment regimens can be developed accordingly.
DKK-1 is a cytokine expressed by myeloma cells and it is also involved in the development of bone lesions in MM. Because there is no definitive treatment for bone complications in MM, research on this marker has grown in popularity [5,18,26]. In our study, we found that DKK-1 expression was higher among MM patients than MGUS patients. Palma et al. [25] evaluated DKK-1 levels in bone marrow aspiration samples from patients with SMM and found that levels were higher among patients with progressive SMM. They hypothesized that DKK-1 could be a valuable marker for disease progression [25]. Although there was no statistically significant difference between the high-risk and other risk groups of the MCRS system in our study, increased DKK-1 expression in the high-risk group suggests that it may be a useful marker in assessing the progression risk of MGUS cases. Ng et al. [24] found similar results for DKK-1 and CCL-3. They explained that situation with bone lesions that were detectable at the microstructural level [24]. However, when we reviewed the current literature, we did not find any studies that compared DKK-1 levels in the progression of MGUS to SMM and MM.
MUM-1 plays critical roles in myeloma pathogenesis, being involved in cell cycle control, energy metabolism, and cell death [21,22]. Shaffer et al. [23] found that when MUM-1 expression is inhibited, myeloma cells die suddenly. In our study, we discovered that MUM-1 immunoexpression was substantially higher in cases of MM than MGUS. Heintel et al. [21] were the first to show that increased MUM-1 expression, as evaluated by polymerase chain reaction (PCR), was related to a worse prognosis in patients with MM. According to the same study, the levels of MUM-1 expression detected by immunohistochemistry and PCR were not correlated, and more research on this topic is needed [21]. Although MUM-1 immunoexpression was not significantly correlated with ISS and R-ISS stages in our study, we observed that the expression rate increased in parallel with ISS and R-ISS staging (staining rates for ISS I, II, and III were 36.5%, 41.2%, and 47.13%, respectively, and staining rates for R-ISS I, II, and III were 32.24%, 39.61%, and 51.43%, respectively). Although not statistically significant, we found that MUM-1 expression was higher in the high-risk group (42%) compared to other groups (8%) when cases of MGUS were evaluated according to MCRS risk groups. Based on these previous findings and our current findings, we believe that MUM-1 can serve as a reliable marker for assessing the progression of MGUS to MM and possibly predicting aggressive behavior in cases of MM.
Based on the findings of this study, we propose that the immunohistochemical evaluation of plasmacytic neoplasms can facilitate predictions regarding the risk of the development of MGUS to MM as well as the biological behavior of cases of MM. Immunohistochemical analysis has advantages over molecular approaches in that it is a low-cost, quick method that may be performed on bone marrow biopsies at the time of diagnosis. In general, MGUS-SMM-MM progression and the prediction of the aggressive course of MM are complicated, multistep, and multifactorial questions. Although CD138, Pan-Ras, DKK-1, MUM-1, and CCL-3 staining results seem to provide essential information in predicting such progression, we believe that larger studies with additional various markers should be performed on this subject.
Study Limitations
This study has some limitations. The number of MGUS patients in the study was relatively small. Another limitation is that only the patients with cytogenetic analysis results were included in the study. The statistically insignificant results that we found while evaluating the effectiveness of some markers may be due to the limited number of cases.
Conclusion
In our examination of MM and MGUS patients, we discovered that Pan-Ras, DKK-1, and MUM-1 were expressed at higher rates by neoplastic cells in MM than in MGUS. This finding shows that the aforementioned molecules may play essential roles in the course of progression from MGUS to MM. Immunotherapeutic agents for these markers may be considered for treatment options. Because the CD138 expression rate represents the number of plasma cells in bone marrow, it is much higher in advanced ISS and R-ISS stages. Thus, the percentage of CD138 expression could be associated with a more advanced stage by showing the neoplastic plasma cell burden in the bone marrow and it can be used as a predictive marker for aggressive behavior in MM patients, even though it is not used in the ISS and R-ISS systems.
The increased expression of CCL-3 in MGUS cases compared to MM, as well as the higher expression of CCL-3 in the high-risk group of MGUS compared to the other groups according to the MCRS criteria, could be crucial in early MM oncogenesis.
Our research has focused on immunohistochemistry markers that are significant in the pathogenesis of plasma cell neoplasms, as well as the predictive utility of those markers in plasma cell neoplasm progression. Further studies are warranted to better assess the role of immunohistochemical biomarkers, such as markers indicating tumor proliferative capacity, in MGUS progression and MM prognosis.
Footnotes
Ethics
Ethics Committee Approval: The Ethics Committee of Marmara University approved the study (09.2018.277).
Authorship Contributions
Concept: İ.Ş.İ., T.T., H.K.T.; Design: İ.Ş.İ, T.T., H.K.T.; Data Collection or Processing: İ.Ş.İ, T.T., H.K.T.; Analysis or Interpretation: İ.Ş.İ, T.T., H.K.T.; Literature Search: İ.Ş.İ, T.T., H.K.T.; Writing: İ.Ş.İ., T.T., H.K.T.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: This study was funded by the Scientific Research and Projects Board of Marmara University, İstanbul, Turkey (grant number: SAG-C-TUP-090518-0227).
References
- 1.Landgren O, Weiss BM. Patterns of monoclonal gammopathy of undetermined significance and multiple myeloma in various ethnic/racial groups: support for genetic factors in pathogenesis. Leukemia. 2009;23:1691–1697. doi: 10.1038/leu.2009.134. [DOI] [PubMed] [Google Scholar]
- 2.Landgren O, Graubard BI, Kumar S, Kyle RA, Katzmann JA, Murata K, Costello R, Dispenzieri A, Caporaso N, Mailankody S, Korde N, Hultcrantz M, Therneau TM, Larson DR, Cerhan JR, Rajkumar SV. Prevalence of myeloma precursor state monoclonal gammopathy of undetermined significance in 12372 individuals 10-49 years old: a population-based study from the National Health and Nutrition Examination Survey. Blood Cancer J. 2017;7:618. doi: 10.1038/bcj.2017.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghobrial IM, Detappe A, Anderson KC, Steensma DP. The bone-marrow niche in MDS and MGUS: implications for AML and MM. Nat Rev Clin Oncol. 2018;15:219–233. doi: 10.1038/nrclinonc.2017.197. [DOI] [PubMed] [Google Scholar]
- 4.Xu S, De Veirman K, De Becker A, Vanderkerken K, Van Riet I. Mesenchymal stem cells in multiple myeloma: a therapeutical tool or target? Leukemia. 2018;32:1500–1514. doi: 10.1038/s41375-018-0061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vallet S, Filzmoser JM, Pecherstorfer M, Podar K. Myeloma bone disease: update on pathogenesis and novel treatment strategies. Pharmaceutics. 2018;10:202. doi: 10.3390/pharmaceutics10040202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajkumar SV, Kyle RA, Buadi FK. Advances in the diagnosis, classification, risk stratification, and management of monoclonal gammopathy of undetermined significance: implications for recategorizing disease entities in the presence of evolving scientific evidence. Mayo Clin Proc. 2010;85:945–948. doi: 10.4065/mcp.2010.0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss B, Kuehl WM. Advances in understanding monoclonal gammopathy of undetermined significance as a precursor of multiple myeloma. Expert Rev Hematol. 2010;3:165–174. doi: 10.1586/ehm.10.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyle RA, San-Miguel JF, Mateos MV, Rajkumar SV. Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. Hematol Oncol Clin North Am. 2014;28:775–790. doi: 10.1016/j.hoc.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Rajkumar SV. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:719–734. doi: 10.1002/ajh.24402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, Richardson P, Caltagirone S, Lahuerta JJ, Facon T, Bringhen S, Gay F, Attal M, Passera R, Spencer A, Offidani M, Kumar S, Musto P, Lonial S, Petrucci MT, Orlowski RZ, Zamagni E, Morgan G, Dimopoulos MA, Durie BG, Anderson KC, Sonneveld P, San Miguel J, Cavo M, Rajkumar SV, Moreau P. Revised International Staging System for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol. 2015;33:2863–2869. doi: 10.1200/JCO.2015.61.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jimenez-Zepeda VH, Duggan P, Neri P, Rashid-Kolvear F, Tay J, Bahlis NJ. Revised International Staging System applied to real world multiple myeloma patients. Clin Lymphoma Myeloma Leuk. 2016;16:511–518. doi: 10.1016/j.clml.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Kastritis E, Terpos E, Roussou M, Gavriatopoulou M, Migkou M, Eleutherakis- Papaiakovou E, Fotiou D, Ziogas D, Panagiotidis I, Kafantari E, Giannouli S, Zomas A, Konstantopoulos K, Dimopoulos MA. Evaluation of the Revised International Staging System in an independent cohort of unselected patients with multiple myeloma. Haematologica. 2017;102:593–599. doi: 10.3324/haematol.2016.145078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayer-Garner IB, Sanderson RD, Dhodapkar MV, Owens RB, Wilson CS. Syndecan-1 (CD138) immunoreactivity in bone marrow biopsies of multiple myeloma: shed syndecan-1 accumulates in fibrotic regions. Mod Pathol. 2001;14:1052–1058. doi: 10.1038/modpathol.3880435. [DOI] [PubMed] [Google Scholar]
- 14.Aref S, Goda T, El-Sherbiny M. Syndecan-1 in multiple myeloma: relationship to conventional prognostic factors. Hematology. 2003;8:221–228. doi: 10.1080/1024533031000153630. [DOI] [PubMed] [Google Scholar]
- 15.Jiang H, Zhang W, Shang P, Zhang H, Fu W, Ye F, Zeng T, Huang H, Zhang X, Sun W, Man-Yuen Sze D, Yi Q, Hou J. Transfection of chimeric anti-CD138 gene enhances natural killer cell activation and killing of multiple myeloma cells. Mol Oncol. 2014;8:297–310. doi: 10.1016/j.molonc.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Quran SZ, Yang L, Magill JM, Braylan RC, Douglas-Nikitin VK. Assessment of bone marrow plasma cell infiltrates in multiple myeloma: the added value of CD138 immunohistochemistry. Hum Pathol. 2007;38:1779–1787. doi: 10.1016/j.humpath.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terpos E, Politou M, Szydlo R, Goldman JM, Apperley JF, Rahemtulla A. Serum levels of macrophage inflammatory protein-1 alpha (MIP-1α) correlate with the extent of bone disease and survival in patients with multiple myeloma. Br J Haematol. 2003;123:106–109. doi: 10.1046/j.1365-2141.2003.04561.x. [DOI] [PubMed] [Google Scholar]
- 18.Terpos E, Ntanasis-Stathopoulos I, Gavriatopoulou M, Dimopoulos MA. Pathogenesis of bone disease in multiple myeloma: from bench to bedside. Blood Cancer J. 2018;8:7. doi: 10.1038/s41408-017-0037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fairfield H, Falank C, Avery L, Reagan MR. Multiple myeloma in the marrow: pathogenesis and treatments. Ann N Y Acad Sci. 2016;1364:32–51. doi: 10.1111/nyas.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Politou M, Terpos E, Anagnostopoulos A, Szydlo R, Laffan M, Layton M, Apperley JF, Dimopoulos MA, Rahemtulla A. Role of receptor activator of nuclear factor-kappa B ligand (RANKL), osteoprotegerin and macrophage protein 1-alpha (MIP-1a) in monoclonal gammopathy of undetermined significance (MGUS) Br J Haematol. 2004;126:686–689. doi: 10.1111/j.1365-2141.2004.05092.x. [DOI] [PubMed] [Google Scholar]
- 21.Heintel D, Zojer N, Schreder M, Strasser-Weippl K, Kainz B, Vesely M, Gisslinger H, Drach J, Gaiger A, Jäger U, Ludwig H. Expression of MUM1/IRF4 mRNA as a prognostic marker in patients with multiple myeloma. Leukemia. 2008;22:441–445. doi: 10.1038/sj.leu.2404895. [DOI] [PubMed] [Google Scholar]
- 22.Bai H, Wu S, Wang R, Xu J, Chen L. Bone marrow IRF4 level in multiple myeloma: an indicator of peripheral blood Th17 and disease. Oncotarget. 2017;8:85392–85400. doi: 10.18632/oncotarget.19907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaffer AL, Emre NC, Lamy L, Ngo VN, Wright G, Xiao W, Powell J, Dave S, Yu X, Zhao H, Zeng Y, Chen B, Epstein J, Staudt LM. IRF4 addiction in multiple myeloma. Nature. 2008;454:226–231. doi: 10.1038/nature07064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng AC, Khosla S, Charatcharoenwitthaya N, Kumar SK, Achenbach SJ, Holets MF, McCready LK, Melton LJ 3rd, Kyle RA, Rajkumar SV, Drake MT. Bone microstructural changes revealed by high-resolution peripheral quantitative computed tomography imaging and elevated DKK1 and MIP-1α levels in patients with MGUS. Blood. 2011;118:6529–6534. doi: 10.1182/blood-2011-04-351437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palma BD, Guasco D, Pedrazzoni M, Bolzoni M, Accardi F, Costa F, Sammarelli G, Craviotto L, De Filippo M, Ruffini L, Omedè P, Ria R, Aversa F, Giuliani N. Osteolytic lesions, cytogenetic features and bone marrow levels of cytokines and chemokines in multiple myeloma patients: role of chemokine (C-C motif) ligand 20. Leukemia. 2016;30:409–416. doi: 10.1038/leu.2015.259. [DOI] [PubMed] [Google Scholar]
- 26.Roodman GD. Pathogenesis of myeloma bone disease. Leukemia. 2009;23:435–441. doi: 10.1038/leu.2008.336. [DOI] [PubMed] [Google Scholar]
- 27.Rasmussen T, Kuehl M, Lodahl M, Johnsen HE, Dahl IM. Possible roles for activating RAS mutations in the MGUS to MM transition and in the intramedullary to extramedullary transition in some plasma cell tumors. Blood. 2005;105:317–323. doi: 10.1182/blood-2004-03-0833. [DOI] [PubMed] [Google Scholar]
- 28.Chng WJ, Gonzalez-Paz N, Price-Troska T, Jacobus S, Rajkumar SV, Oken MM, Kyle RA, Henderson KJ, Van Wier S, Greipp P, Van Ness B, Fonseca R. Clinical and biological significance of RAS mutations in multiple myeloma. Leukemia. 2008;22:2280–2284. doi: 10.1038/leu.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Billadeau D, Jelinek DF, Shah N, LeBien TW, Van Ness B. Introduction of an activated N-ras oncogene alters the growth characteristics of the interleukin 6-dependent myeloma cell line ANBL6. Cancer Res. 1995;55:3640–3646. [PubMed] [Google Scholar]
- 30.Bergsagel PL, Kuehl WM. Molecular pathogenesis and a consequent classification of multiple myeloma. J Clin Oncol. 2005;23:6333–6338. doi: 10.1200/JCO.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 31.Kawano Y, Fujiwara S, Wada N, Izaki M, Yuki H, Okuno Y, Iyama K, Yamasaki H, Sakai A, Mitsuya H, Hata H. Multiple myeloma cells expressing low levels of CD138 have an immature phenotype and reduced sensitivity to lenalidomide. Int J Oncol. 2012;41:876–884. doi: 10.3892/ijo.2012.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JM, Lee JA, Cho IS, Ihm CH. Soluble syndecan-1 at diagnosis and during follow up of multiple myeloma: a single institution study. Korean J Hematol. 2010;45:115–119. doi: 10.5045/kjh.2010.45.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foster MC, Christensen R, Egan J, Whitley J, Chiu WK, Serody J, Gabriel D, Ivanova A, Dunphy CH, Shea TC. Prognostic impact of bone marrow CD138 immunohistochemistry (IHC) prior to autologous stem cell. J Clin Oncol. 2009;27:8600a (abstract). [Google Scholar]
- 34.Zangari M, Shin I, Branca A, Yoo H, Fallo S, Davies FE, Hoffman B, Fruchtman SM, Yoon D. Rigosertib, a pan RAS inhibitor, decreases mouse and human myeloma cell growth in preclinical models. Blood. 2016;128:5664. [Google Scholar]
- 35.Vallet S, Pozzi S, Patel K, Vaghela N, Fulciniti MT, Veiby P, Hideshima T, Santo L, Cirstea D, Scadden DT, Anderson KC, Raje N. A novel role for CCL3 (MIP-1α) in myeloma-induced bone disease via osteocalcin downregulation and inhibition of osteoblast function. Leukemia. 2011;25:1174–1181. doi: 10.1038/leu.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]


