Abstract
Chelation of iron to iron-binding proteins is a strategy of host defense. Some pathogens counter this via the secretion of low-molecular-weight iron-chelating agents (siderophores). Human phagocytes possess a high-capacity mechanism for iron acquisition from low-molecular-weight iron chelates. Efficient acquisition and sequestration of iron bound to bacterial siderophores by host phagocytes could provide a secondary mechanism to limit microbial access to iron. In the present work we report that human neutrophils, macrophages, and myeloid cell lines can acquire iron from the two Pseudomonas aeruginosa siderophores. Analogous to iron acquisition from other low-molecular-weight chelates, iron acquisition from the siderophores is ATP independent, induced by multivalent cationic metals, and unaffected by inhibitors of endocytosis and pinocytosis. In vivo, this process could serve as an additional mechanism of host defense to limit iron availability to invading siderophore-producing microbes.
Iron is critical to the growth and metabolism of nearly all living organisms, prokaryotic and eukaryotic. Limiting the concentration of free extracellular iron is a strategy of host defense against pathogenic microorganisms that is practiced by many animal species (4, 9, 12, 22). Humans accomplish this by binding extracellular iron to the iron-chelating proteins transferrin and lactoferrin (4, 9, 12, 22). Although there are some exceptions (5), most bacteria are unable to directly utilize iron bound to either of these two human proteins. As a means of acquiring iron under such conditions, many bacterial pathogens secrete highly efficient low-molecular-weight iron chelating agents, termed siderophores (17, 18). These agents compete for and bind available iron. These siderophore-iron complexes are recognized by the bacteria, which then internalize the iron.
The ability to produce siderophores has been linked to the pathogenic potential of many bacterial species including Pseudomonas aeruginosa, Escherichia coli, Vibrio vulnificus, and Vibrio cholerae (11, 13, 14, 23). Siderophore production compromises the effectiveness of the iron limitation approach to host defense. The host could decrease the access of the organism to iron if host inflammatory cells could efficiently acquire and sequester iron chelated to the same siderophores. Once internalized by these cells, such iron would no longer be accessible to the organism.
We have previously demonstrated that human phagocytes and myeloid cell lines possess an inducible high-capacity mechanism for iron acquisition from a variety of low-molecular-weight iron-chelating agents (19, 20). This system has the following features: (i) its activity is induced by a variety of multivalent cationic metals; (ii) it is unaffected when the cells are depleted of ATP or when receptor-mediated endocytosis is inhibited with dihydrocytochalasin B; and (iii) its efficiency is chelate specific (19, 20). Based on these findings, we hypothesized that this phagocyte-associated iron acquisition system could potentially serve as a host defense mechanism against siderophore-producing microbes by allowing these cells to compete with the bacterial pathogen for siderophore-bound iron. To test this hypothesis, we assessed the ability of human phagocytic cells and myeloid cell lines to acquire iron bound to the P. aeruginosa-derived siderophores pyoverdin and pyochelin, as well as the mechanism responsible.
MATERIALS AND METHODS
Purification and iron loading of P. aeruginosa siderophores.
Pyoverdin and pyochelin were purified to uniformity from the growth media of P. aeruginosa strain PA01 (ATCC 15692) using previously described methods (6, 8). For iron acquisition studies, pyochelin and pyoverdin were loaded with 59Fe by incubating each siderophore with 59FeCl3 such that sufficient 59Fe was available to load 40% of the siderophore. This was based on the Fe-siderophore binding stoichiometry of 1:2 and 1:1 for pyochelin and pyoverdin, respectively (6, 8). [14C]pyoverdin and [35S]pyochelin were generated using previously described techniques (2). Briefly, PAO1 bacteria were grown to early stationary phase (absorbance at 600 nm, 0.6) in 1% Casamino Acids (CAA) medium, harvested, washed, and resuspended at 2.4 × 1010 CFU/ml in 20 ml of 5 mM phosphate buffer (pH 7.5) containing 1 mM MgSO4. Concentrated CAA was added to make the resuspension culture 0.25% CAA, and either 0.5 μCi of [14C]ornithine (for pyoverdin) or 0.5 μCi of 35SO42− (for pyochelin) was added to label the siderophores. Reaction cultures were incubated with shaking at 250 rpm at 37°C for 2 h. Bacteria were removed by centrifugation, and the supernatants were subjected to siderophore purification by standard methods (6, 7). The purity of pyochelin (2) and pyoverdin (7) was analyzed by high-performance liquid chromatography. The yield of purified [35S]pyochelin was analyzed by measurement of the absorbance at 350 nm (7), and the yield of purified [14C]pyoverdin was assayed by fluorescence emission at 460 nm when solutions were excited at 400 nm (6). The levels of radioactivity in the two samples were analyzed by liquid scintillation counting. Radiolabeled pyoverdin or pyochelin was then loaded with “cold” iron by the method described above for pyochelin and pyoverdin, except that 57FeCl3 was used.
Human phagocytes.
Human neutrophils and mononuclear phagocytes were separated from other components of the peripheral venous blood of normal human donors by a previously established method (3). Briefly, erythrocytes were removed from heparinized blood by dextran sedimentation. Leukocytes were separated into neutrophils and mononuclear cells by centrifugation through a Ficoll-Hypaque gradient. Neutrophils were washed in Hanks' balanced salt solution (HBSS) and then kept on ice until used on the same day. Monocytes and lymphocytes were maintained in in vitro culture for 5 days in Teflon flasks (RPMI 1680 plus 20% autologous serum) to allow the monocytes to differentiate into monocyte-derived macrophages (MDM) (10). MDM were then separatedfrom lymphocytes based on their adherence to plastic over this period in culture. Prior to their use in iron acquisition assays, MDM were mobilized into solution by being placed on ice (4°C) for 1 h and then gently scraped as previously described (19). This low-temperature scraping leads to negligible cell injury as determined by the measurement of release of intracellular lactate dehydrogenase (19).
HL-60 and U937 cells.
The human promyelocytic HL-60 and promonocytic U937 cell lines were cultivated in RPMI 1640 (University of Iowa Cancer Center, Iowa City, Iowa) plus 10% fetal calf serum and 2 mM glutamine as previously described (19). Prior to use in iron acquisition assays, the cells were washed three times and suspended at the desired concentration in HBSS.
Cellular 59Fe acquisition.
Cellular iron acquisition from pyochelin and pyoverdin was measured by a previously described method (20). Briefly, cells were suspended in HBSS (5 × 106/ml) and placed in 96-well plates (100 μl/well). After equilibration at 37°C under 5% CO2, the desired 59Fe chelate was added at a concentration of 740 nM 59Fe. At defined time points, the cell suspension was centrifuged (500 × g for 5 min at 4°C) and the pellet was washed three times. The amount of 59Fe associated with the cell pellet was then determined with a gamma counter. In each experiment, the 59Fe chelate was added to some wells which contained only HBSS but no cells, to control for possible 59Fe binding to the plate or formation of non-cell-associated iron aggregates which could cosediment with the cell pellet. These values, which generally are <0.05% of the added counts per minute (cpm), were subtracted from those of the corresponding cell-containing samples at each time point to assess cell-specific 59Fe acquisition. In some cases, cells were pretreated with Ga(NO3)3 or other reagents prior to addition of 59Fe, as detailed in Results.
For measurements of the stability of iron association with the cells, cells which had been incubated for defined periods in the presence of the desired 59Fe chelate were washed three times in HBSS and resuspended in HBSS lacking 59Fe. At defined time points, the cells were separated from the HBSS supernatant by centrifugation (500 × g for 5 min at 4°C) and the pellet was washed an additional three times. The amount of 59Fe associated with the cell pellet, as well as the amount in the initial supernatant, was then determined with the gamma counter. In some experiments, iron chelators such as pyochelin, pyoverdin, or nitrilotriacetic acid (NTA) were added to the medium in an attempt to enhance the removal of 59Fe from the cells.
Statistical analyses.
Results obtained under different experimental conditions were compared by Student's paired t test when independent variables were being assessed or by analysis of variance when analyses of trends were being determined. In each case, results were considered significant at P < 0.05.
RESULTS
Acquisition of iron from P. aeruginosa siderophores by phagocytic cells.
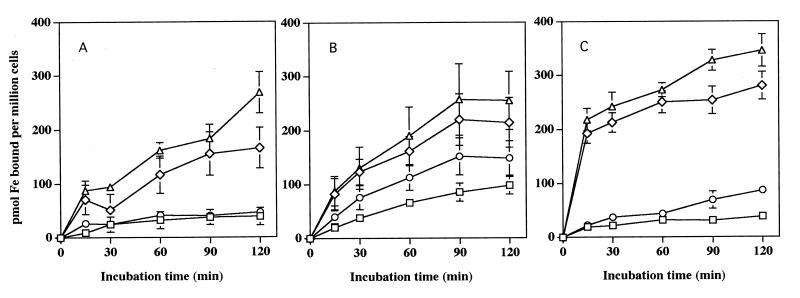
When incubated with [59Fe]pyoverdin or [59Fe]pyochelin, neutrophils, MDM, and HL-60 cells each exhibited a similar capacity to acquire 59Fe from these two P. aeruginosa siderophores over time (Fig. 1). A single experiment in which U937 cells were used yielded similar results (data not shown). Analogous to the iron acquisition we had previously observed from other low-molecular-weight chelates by these cells (19, 20), preincubating the cells with 1 mM Ga(NO3)3 for 30 min resulted in a dramatic increase in the magnitude of 59Fe acquired from either pyochelin or pyoverdin (Fig. 1). Overall iron acquisition from both siderophores occurred more rapidly with the cell lines than with either neutrophils or macrophages when they were pretreated with Ga(NO3)3. Cellular iron acquisition from both pyoverdin and pyochelin appears to be dissociated from acquisition of the siderophores themselves. No cellular uptake of either [35S]ferripyochelin or [14C]ferripyoverdin could be detected under the conditions which led to detectable iron acquisition (data not shown).
FIG. 1.
Acquisition of iron from pyoverdin or pyochelin by phagocytes and other myeloid cells. Shown are the mean and standard error of the mean (n = 3) concentrations of iron acquired from pyoverdin or pyochelin by neutrophils (A), macrophages (B), and HL-60 cells (C) in the absence (□) or presence (◊) of preincubation of the cells with 1 mM Ga(NO3)3 for pyoverdin and in the absence (○) or presence (▵) of preincubation of the cells with 1 mM Ga(NO3)3 for pyochelin. Each cell type examined exhibited the ability to acquire iron from each bacterial siderophore and a marked enhancement of iron acquisition with gallium pretreatment.
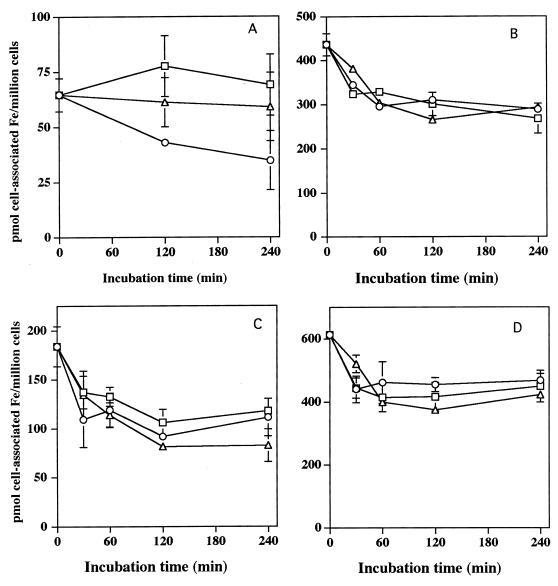
For the above process to be of biological significance, the iron acquired from pyoverdin and pyochelin must be stably associated with the cells. As shown in Fig. 2 and 3, regardless of whether the cells are preincubated with Ga(NO3)3, more than 65% of the iron acquired from pyoverdin or pyochelin by HL-60 cells remained cell associated over a subsequent 4-h incubation in HBSS to which no exogenous iron was added. Addition of either apo-pyochelin or apo-pyoverdin to the cell suspension also failed to remove most of the iron from the cells, regardless of whether it had initially been acquired from pyochelin or pyoverdin (Fig. 2). These data indicate that once cell associated, iron acquired from the siderophore is not readily released into the extracellular space and therefore would be unavailable to the microbe.
FIG. 2.
Stability of cell-associated iron after acquisition from pyoverdin or pyochelin. (A and B) Control (A) and Ga(NO3)3-pretreated (B) HL-60 cells were incubated with [59Fe]pyoverdin for 1 h and then were repetitively washed and resuspended in buffer alone (○) or buffer containing 300 μM apo-pyoverdin (▵) or 100 μM apo-pyochelin (□). Cell-associated 59Fe was then determined over time. (C and D) control (C) and Ga(NO3)3-pretreated (D) HL-60 cells were incubated with [59Fe]pyochelin for 1 h and then repetitively washed and resuspended in buffer alone (○) or buffer containing 300 μM apo-pyoverdin (▵) or 100 μM apo-pyochelin (□). The cell-associated 59Fe concentration was then determined over time. Curves shown are representative of three separate experiments for each condition.
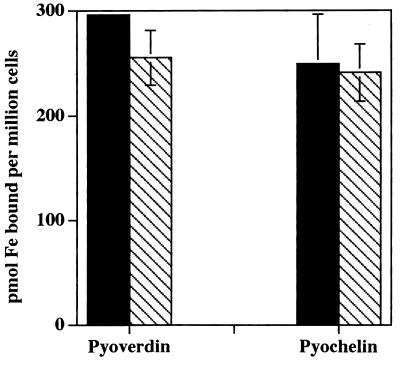
FIG. 3.
Effect of ATP depletion on iron acquisition from pyochelin and pyoverdin by HL-60 cells. Control HL-60 cells (solid bars) or cells which had been pretreated with 1 mM NaCN and 50 mM 2-deoxyglucose (cross-hatched bars) were then incubated with Ga(NO3)3 and suspended in the presence of [59Fe]pyoverdin or [59Fe]pyochelin. After 60 min of incubation, cell-associated 59Fe was determined. No difference (P > 0.05) in cell acquisition of 59Fe was observed from either pyoverdin or pyochelin as a consequence of treatment of the cells with the metabolic inhibitors. Results shown are mean and standard error of the mean (n = 3) concentrations of cell-associated iron from experiments performed in duplicate.
Mechanism of myeloid cell iron acquisition from pyoverdin and pyochelin.
The above findings are consistent with iron acquisition from pyoverdin and pyochelin occurring via the inducible endocytosis-independent mechanism of iron acquisition we have recently described for myeloid cells (19, 20). To provide additional evidence in support of this hypothesis, we used the HL-60 cell line to assess if key features previously identified for this iron acquisition mechanism also apply to iron acquisition from pyoverdin and pyochelin.
As shown in Fig. 3, treatment of HL-60 cells with 1 mM NaCN and 50 mM 2-deoxyglucose, which decreases the ATP levels in HL-60 cells to less than 2% of control levels (20), had no effect on the subsequent ability of the cells to acquire iron from either pyoverdin or pyochelin. The lack of effect of NaCN and 2-deoxyglucose is a feature which distinguishes the iron acquisition mechanism we have described from endocytosis or pinocytosis (15, 16, 21).
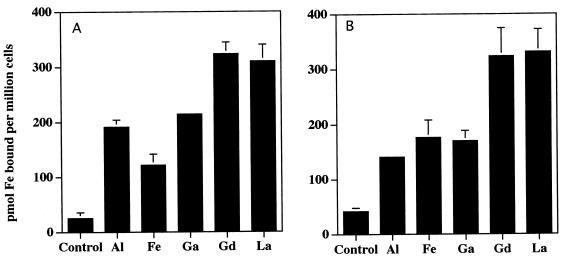
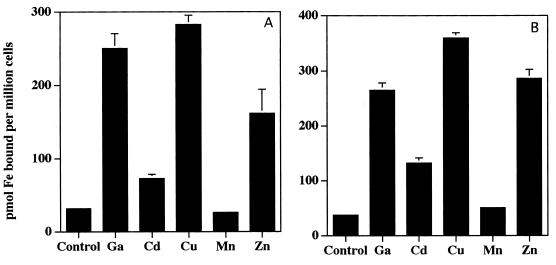
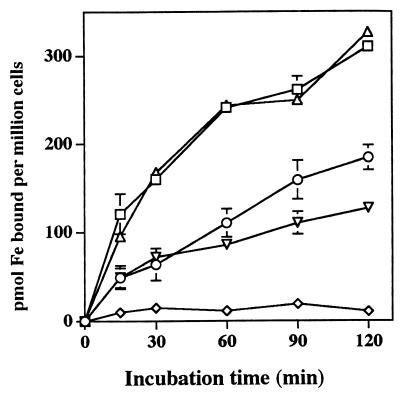
The rate of this form of iron acquisition by human myeloid cells also is induced by a variety of multivalent cationic metals as well as a variety of iron chelates (19, 20). In contrast, divalent metals have been previously shown to have little effect (19, 20). Consistent with these findings, salts of Al3+, La3+, Ga3+, and Gd3+, markedly increase 59Fe acquisition from both pyoverdin and pyochelin by HL-60 cells (Fig. 4). Iron acquisition following cellular exposure to FeCl3 also increased over that of the control but did not quite reach statistical significance (Fig. 4). In contrast to our previous observations with HL-60 acquisition of iron from other low-molecular-weight iron chelates (19, 20), Cu2+, Cd2+, and Zn2+ enhanced the acquisition of iron from both pyochelin and pyoverdin (Fig. 5). Mn2+ had no effect (Fig. 5). Also in contrast to our observations with other iron chelates capable of having their iron acquired by HL-60 cells (20), neither ferripyochelin (up to 100 μM) nor ferripyoverdin (up to 300 μM) induced the rate of HL-60 acquisition of 59Fe complexed to the ferric iron chelator NTA (Fig. 6). In fact, preincubation with ferripyoverdin appears to decrease iron acquisition from NTA by HL-60 cells (Fig. 6). As expected (20), preincubation of the cells with either Ga(NO3)3 or FeNTA enhanced the ability of the cells to acquire Fe from NTA (Fig. 6).
FIG. 4.
Effect of trivalent metals on iron acquisition from pyoverdin (A) and pyochelin (B) by HL-60 cells. Shown is the mean and standard error of the mean (n = 3) concentration of cell-associated 59Fe following a 60-min incubation, in the presence of [59Fe]pyoverdin (A) or [59Fe]pyochelin (B), of control HL-60 cells or cells which had been preincubated with 1 mM trivalent metal (indicated on the x axis). ∗, P < 0.05 relative to control. Specific P values relative to control for are as follows: A1, P < 0.02; Fe, P < 0.07; Ga, P < 0.002; Gd, P < 0.01; and La, P < 0.02 (A) and A1, P < 0.02; Fe, P < 0.06; Ga, P < 0.04; Gd, P < 0.04; and La, P < 0.02 (B).
FIG. 5.
Effect of divalent metals on iron acquisition from pyoverdin (A) and pyochelin (B) by HL-60 cells. Shown is the mean and standard error of the mean (n = 3) concentration of cell-associated 59Fe following a 60-min incubation, in the presence of [59Fe]pyoverdin (A) or [59Fe]pyochelin (B), of control HL-60 cells and cells which had been preincubated with 1 mM divalent metal (indicated on the x axis). Experiments utilizing the trivalent metal Ga were performed as a positive control and are included as a point of reference. ∗, P < 0.05 relative to control. Specific P values relative to control are as follows: Ga, P < 0.05; Cd, P < 0.02; Cu, P < 0.003; Mn, P < 0.2; and Zn, P < 0.05 (A) and Ga, P < 0.0007; Cd, P < 0.003; Cu, P < 0.00005; Mn, P < 0.9; and Zn, P < 0.0007 (B).
FIG. 6.
Effect of ferrisiderophores on of HL-60 iron acquisition from NTA. Shown is the mean and standard error of the mean (n = 4) concentration of cell-associated 59Fe following incubation at the defined time points, in the presence of [59Fe]NTA, of HL-60 cells which had first been preincubated with buffer only (○), 300 μM ferripyoverdin (◊), 100 μM ferripyochelin (▿), 100 μM Ga(NO3)3 (□), or 100 μM FeNTA (▵, single experiment) for 30 min prior to the addition of [59Fe]NTA.
DISCUSSION
Limiting extracellular iron availability has been regarded as an important mechanism of human host defense against microbial pathogens, essentially all of which require iron for optimal growth and metabolism (4, 9, 12, 22). Most investigations have focused on the role of iron chelation by the extracellular host iron-binding proteins transferrin and lactoferrin in achieving this process (4, 9, 12, 22). However, many bacterial pathogens produce siderophores, whose affinity for iron is in the range of lactoferrin and transferrin, effectively negating this iron limitation strategy of the host (17, 18).
In the present work we present data which indicate that human phagocytic cells (neutrophils and macrophages) as well as myeloid cell lines (HL-60 and U937) are capable of acquiring iron from the two siderophores produced by the major human pathogen P. aeruginosa. These data are consistent with the possibility that iron acquisition from bacterial siderophores by phagocytes at sites of infection serves as an additional mechanism for the host to limit iron availability for invading microbes. To our knowledge, this possibility has not been previously proposed.
The mechanism whereby phagocytes and myeloid cells acquire iron from pyoverdin and pyochelin exhibits many of the same characteristics as the mechanism of transferrin-independent iron acquisition from a variety of low-molecular-weight chelating agents we recently detailed in these cell types (19, 20). It is ATP independent, highly inducible by multivalent cationic metals, and unaffected by inhibitors of endocytosis and pinocytosis. In contrast to our earlier experience with this means of cellular iron acquisition, several divalent metals (Cu, Cd, and Zn) also exhibit the ability to enhance the magnitude of iron acquisition from both pyochelin and pyoverdin. Since the mechanism whereby these metals enhance cellular iron acquisition from low-molecular-weight chelating agents is unknown, it is difficult to speculate why divalent metals alter the rate of iron acquisition from the bacterial siderophores but not other low-molecular-weight iron-chelating agents. Nevertheless, since we were unable to detect the uptake of the siderophores themselves, the mechanism probably involves separation of the iron from the siderophore at the cell surface.
The lack of an identifiable buffer or growth medium in which both myeloid cells and P. aeruginosa remain viable and functional precludes the ability to directly test to what extent host phagocytes can disrupt the siderophore-dependent growth of P. aeruginosa. However, in vitro P. aeruginosa requires approximately 7 to 29 μM pyochelin or pyoverdin for rapid growth in the presence of inhibitory levels of transferrin (1). Preliminary data from our laboratory indicate that HL-60 cells have the capacity to acquire at least 50 nmol of iron from pyochelin or pyoverdin per 106 cells over 30 min. Since concentrations of phagocytes can approach 105/μl at sites of infection, the potential for competition between P. aeruginosa bacteria and phagocytic cells for siderophore-bound iron seems quite plausible.
Although one could extrapolate the data reported herein as evidence for a generalized potential of phagocytic cells to disrupt iron metabolism of all siderophore-producing microbes, this would be premature and may be incorrect. We have previously shown that the magnitude of transferrin-independent iron acquisition is chelate specific (19). Interestingly, myeloid cell iron acquisition from deferoxamine, a siderophore produced by Streptomyces pilosus, is quite low (19). Most bacterial siderophores fall into one of two classes, phenol-catechol type or hydroxymate acid type (17, 18). Pyoverdin is a member of the hydroxymate class of siderophores, and thus it might be expected that myeloid cells would be able to acquire iron to a similar extent from other members of this class such as the Escherichia coli siderophore aerobactin. In contrast, pyochelin is a unique compound with no clear relationship to other siderophores (8). Additional work is needed to define the spectrum of microbial siderophores from which human phagocytic cells can remove iron. Unfortunately, most microbial siderophores are not available from commercial sources.
In summary, we have obtained data which demonstrate the ability of human phagocytes and myeloid cell lines to efficiently acquire iron from both of the siderophores produced by P. aeruginosa. These data are consistent with the possibility that such events in vivo serve as an additional mechanism of host defense to limit iron availability to invading siderophore-producing microbes. Further studies to assess the extent to which this observation extends to other microbes and the role of the process in vivo are indicated.
ACKNOWLEDGMENTS
This work was supported in part by the National Institutes of Health (AI28412) and the Department of Veterans Affairs Research Service through a Merit Review Award. It was performed during the tenure of B. E. Britigan as an Established Investigator of the American Heart Association.
REFERENCES
- 1.Ankenbauer R, Sriyosachati S, Cox C D. Effects of siderophores on the growth of Pseudomonas aeruginosa in human serum and transferrin. Infect Immun. 1985;49:132–140. doi: 10.1128/iai.49.1.132-140.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ankenbauer R G, Cox C D. Isolation and characterization of Pseudomonas aeruginosa mutants requiring salicylic acid for pyochelin biosynthesis. J Bacteriol. 1988;170:5364–5367. doi: 10.1128/jb.170.11.5364-5367.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borregaard N, Heiple J M, Simons E R, Clark R A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase. Translocation during activation. J Cell Biol. 1983;97:52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bullen J J, Rogers H J, Griffiths E. Role of iron in bacterial infection. Curr Top Microbiol Immunol. 1978;80:1–35. doi: 10.1007/978-3-642-66956-9_1. [DOI] [PubMed] [Google Scholar]
- 5.Cornelissen C N, Sparling P F. Iron piracy: acquisition of transferrin-bound iron by bacterial pathogens. Mol Microbiol. 1994;14:843–850. doi: 10.1111/j.1365-2958.1994.tb01320.x. [DOI] [PubMed] [Google Scholar]
- 6.Cox C D, Adams P. Siderophore activity of pyoverdin for Pseudomonas aeruginosa. Infect Immun. 1985;48:130–138. doi: 10.1128/iai.48.1.130-138.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox C D, Graham R. Isolation of an iron-binding compound from Pseudomonas aeruginosa. J Bacteriol. 1979;137:357–364. doi: 10.1128/jb.137.1.357-364.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox C D, Rinehart K L, Jr, Moore M L, Cook C J., Jr Pyochelin: novel structure of an iron-chelating growth promoter for Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1981;78:4256–4260. doi: 10.1073/pnas.78.7.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finkelstein R A, Sciortino C V, McIntosh M A. Role of iron in microbe-host interactions. Rev Infect Dis. 1983;5:5759–5777. doi: 10.1093/clinids/5.supplement_4.s759. [DOI] [PubMed] [Google Scholar]
- 10.Gaynor C D, McCormack F X, Voelker D R, McGowan S E, Schlesinger L S. Pulmonary surfactant protein A mediates enhanced phagocytosis of Mycobacterium tuberculosis by a direct interaction with human macrophages. J Immunol. 1995;155:5343–5351. [PubMed] [Google Scholar]
- 11.Henderson D P, Payne S M. Vibrio cholerae iron transport systems: roles of heme and siderophore iron transport in virulence and identification of a gene associated with multiple iron transport systems. Infect Immun. 1994;62:5120–5125. doi: 10.1128/iai.62.11.5120-5125.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kontoghiorghes G J, Weinberg E D. Iron: mammalian defense systems, mechanisms of disease, and chelation therapy approaches. Blood Rev. 1995;9:33–45. doi: 10.1016/0268-960x(95)90038-1. [DOI] [PubMed] [Google Scholar]
- 13.Litwin C M, Rayback T W, Skinner J. Role of catechol siderophore synthesis in Vibrio vulnificus virulence. Infect Immun. 1996;64:2834–2838. doi: 10.1128/iai.64.7.2834-2838.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer J M, Neely A, Stintzi A, Georges C, Holder I A. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect Immun. 1996;64:518–523. doi: 10.1128/iai.64.2.518-523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michl J, Ohlbaum D J, Silverstein S C. 2-Deoxyglucose selectively inhibits Fc and complement receptor-mediated phagocytosis in mouse peritoneal macrophages. I. Description of the inhibitory effect. J Exp Med. 1976;144:1465–1483. doi: 10.1084/jem.144.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michl J, Ohlbaum D J, Silverstein S C. 2-deoxyglucose selectively inhibits Fc and complement receptor-mediated phagocytosis in mouse peritoneal macrophages. II. Dissociation of the inhibitory effects of 2-deoxyglucose on phagocytosis and ATP generation. J Exp Med. 1976;144:1484–1493. doi: 10.1084/jem.144.6.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neilands J B. Siderophores: structure and function of microbial iron transport compounds. J Biol Chem. 1995;270:26723–26726. doi: 10.1074/jbc.270.45.26723. [DOI] [PubMed] [Google Scholar]
- 18.Neilands J B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- 19.Olakanmi O, Stokes J B, Britigan B E. Acquisition of iron bound to low molecular weight chelates by human monocyte-derived macrophages. J Immunol. 1994;153:2691–2703. [PubMed] [Google Scholar]
- 20.Olakanmi O, Stokes J B, Pathan S, Britigan B E. Polyvalent cationic metals induce the rate of transferrin-independent iron acquisition by HL-60 cells. J Biol Chem. 1997;272:2599–2606. doi: 10.1074/jbc.272.5.2599. [DOI] [PubMed] [Google Scholar]
- 21.Ose L, Ose T, Reinersten R, Berg T. Fluid endocytosis in isolated rat parenchymal and non-parenchymal liver cells. Exp Cell Res. 1980;126:109–119. doi: 10.1016/0014-4827(80)90475-9. [DOI] [PubMed] [Google Scholar]
- 22.Weinberg E D, Weinberg G A. The role of iron in infection. Curr Opin Infect Dis. 1995;8:164–169. [Google Scholar]
- 23.Williams P H, Carbonetti N H. Iron, siderophores, and the pursuit of virulence: independence of the aerobactin and enterochelin iron uptake systems in Escherichia coli. Infect Immun. 1986;51:942–947. doi: 10.1128/iai.51.3.942-947.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]