Abstract
Background
We conducted a systematic review examining the cost effectiveness of a 3-month course of isoniazid and rifapentine, known as 3HP, given by directly observed treatment, compared to 9 months of isoniazid that is directly observed or self-administered, for latent tuberculosis infection. 3HP has shown to be effective in reducing progression to active tuberculosis and like other short-course regimens, has higher treatment completion rates compared to standard regimens such as 9 months of isoniazid. Decision makers would benefit from knowing if the higher up-front costs of rifapentine and of the human resources needed for directly observed treatment are worth the investment for improved outcomes.
Methods
We searched PubMed, Embase, CINAHL, LILACS, and Web of Science up to February 2022 with search concepts combining latent tuberculosis infection, directly observed treatment, and cost or cost-effectiveness. Studies included were in English or French, on human subjects, with latent tuberculosis infection, provided information on specified anti-tubercular therapy regimens, had a directly observed treatment arm, and described outcomes with some cost or economic data. We excluded posters and abstracts, treatment for multiple drug resistant tuberculosis, and combined testing and treatment strategies. We then restricted our findings to studies examining directly-observed 3HP for comparison. The primary outcome was the cost and cost-effectiveness of directly-observed 3HP.
Results
We identified 3 costing studies and 7 cost-effectiveness studies. The 3 costing studies compared directly-observed 3HP to directly-observed 9 months of isoniazid. Of the 7 cost-effectiveness studies, 4 were modelling studies based in high-income countries; one study was modelled on a high tuberculosis incidence population in the Canadian Arctic, using empiric costing data from that setting; and 2 studies were conducted in a low-income, high HIV-coinfection rate population. In five studies, directly-observed 3HP compared to self-administered isoniazid for 9 months in high-income countries, has incremental cost-effectiveness ratios that range from cost-saving to $5418 USD/QALY gained. While limited, existing evidence suggests 3HP may not be cost-effective in low-income, high HIV-coinfection settings.
Conclusion
Cost-effectiveness should continue to be assessed for programmatic planning and scale-up, and may vary depending on existing systems and local context, including prevalence rates and patient expectations and preferences.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-022-14766-6.
Keywords: Tuberculosis, Preventive treatment, Rifapentine, Isoniazid, Systematic review, Cost-effectiveness
Introduction
One-quarter of the world has latent tuberculosis infection (LTBI); an estimated 10% will eventually develop active tuberculosis (TB) [1]. This LTBI population functions as the reservoir driving ongoing incidence of active TB, even in the absence of continued transmission. Finding and treating LTBI is important to control TB and for the ultimate goal of eliminating TB [1–3].
For many years, LTBI has been treated with isoniazid administered daily for 9 months (9H), which reduces reactivation by 90% but incurs risk of hepatoxicity [4, 5]. Isoniazid is widely-available, and costs very little.
More recently, shorter options for LTBI treatment have been adopted [6], including a three-month regimen of once-weekly isoniazid and rifapentine (3HP) [7]; 4 months of daily rifampicin (4R) [8]; or 3 months of daily isoniazid and rifampicin [6]. Shorter courses of treatment are similarly effective and easier for patients to complete [9]. 4R is associated with a theoretical risk of rifampicin resistance, though not empirically demonstrated [10]. 3HP is effective [7] but is usually given via directly-observed treatment (DOT), which requires more visits and human resources, and has corresponding budgetary implications. Self-administration of 3HP met the threshold for non-inferiority to DOT in [11], and is an approved strategy for, the US [12]. However, rifapentine is significantly more expensive than isoniazid.
Directly-observed preventative therapy (DOPT) for treatment of LTBI is a commonly used programmatic strategy, particularly in populations at high-risk of developing active TB [13–17]. This treatment support might mitigate the risk related to congregate or crowded living [14, 15], cultural and/or linguistic barriers [13, 14]. Barriers such as lack of trust in authorities, and histories of racism and colonialism may pose challenges for DOPT [18, 19]. While DOPT can be used with any LTBI regimen, it is recommended with 3HP because of the weekly dosing interval and significant pharmacokinetic impact of a missed dose.
A 2011 systematic review evaluated cost effectiveness evidence for LTBI treatment overall [20]. It excluded high-risk populations including HIV-coinfected patients, but did not restrict drug regimens, including self-administered regimens and DOT. Isoniazid was cost-effective for the general population in high-resource settings compared to no treatment, but there was insufficient evidence for low-resource settings. The systematic review included only one study directly comparing two drug regimens.
Since 2011, several more cost-effectiveness studies have been published. With 3HP, the requirement for direct observation coupled with expensive medication incurs more up-front costs and could be cause for policymakers to hesitate. Economic evidence to inform these decisions is critical.
We performed a systematic review on the cost-effectiveness of 3HP, recognizing potential variation across settings, geographic regions, and specific populations.
Methods
A systematic review was performed to determine the cost and cost-effectiveness of 3HP DOT. We searched PubMed, Embase, CINAHL, LILACS, and Web of Science up to February 28, 2022, with search concepts combining latent tuberculosis, drug therapy or directly observed preventive therapy, and cost, economic, or cost effectiveness (see Additional file 1 for full details on the search strategy).
Studies included were in English or French, on human subjects, with LTBI, provided information on specified anti-tubercular regimens, had a directly-observed arm, and described outcomes with some cost or economic data. We excluded posters and abstracts, studies on drug-resistant TB, and studies that combine testing and treating as a single intervention. Two reviewers (KB, WAL) screened all records independently; disagreements were resolved by consensus and if necessary by a third reviewer (AAZ). We included only studies examining 3HP DOT cost or cost-effectiveness. Data extraction was based on modified criteria from CHEERS checklist [21]. The eligible studies were extracted independently by two reviewers (KB, WAL).
Where there was no description of whether treatment was self-administered therapy (SAT) or administered by DOT, we assumed the usual strategy of administration based on drug regimen (e.g. 3HP was originally studied as DOT [7], 9H is usually self-administered) or based on national standards (e.g. LTBI treatment in Taiwan is routinely given by DOT) or by inference from information given regarding the frequency of visits or dosing.
We included studies that report data on cost with no information or follow-up with regard to effectiveness. We report on cost studies and cost-effectiveness studies separately, with outcomes on cost per patient and incremental cost effectiveness ratios (ICERs), respectively. For cases when authors provided itemized costs, such as for medications, medical professionals’ time, and laboratory and radiology investigations, we calculated estimated total costs per regimen for comparison. Quality of the included studies for the cost and cost-effectiveness review was assessed independently by two reviewers (KB, WAL) based on a modified Drummond checklist [22]. We did not plan for a meta-analysis given the range of geographical and contextual factors, including baseline adherence rate, LTBI prevalence, co-morbidity prevalence particularly HIV co-infection, usual care for LTBI treatment, and patient expectations.
For cost-effectiveness studies, we present results on 3HP DOT and 9H SAT. For studies in which there was no 9H SAT arm, we compared 3HP DOT to the comparator most similar to 9H SAT for which data was available (e.g. 9H DOT). ICERs were re-calculated using 9H SAT as baseline where possible. Costs have been converted to 2020USD based on Consumer Price Index [23].
Cost and cost-effectiveness analysis studies on self-administered 3HP (3HP SAT) alone, with no DOT arm, were excluded from the formal systematic review. Our primary outcome is cost and cost-effectiveness for 3HP.
Results
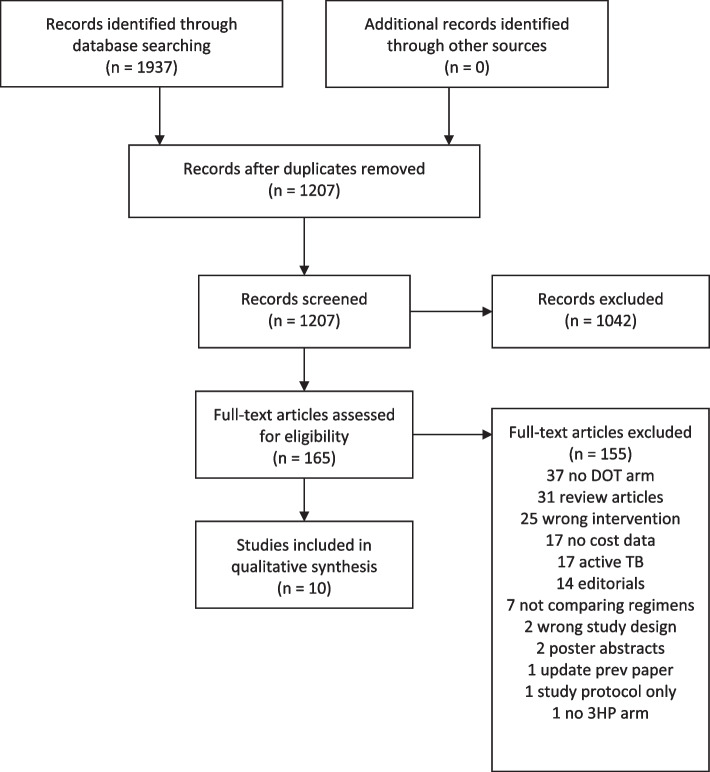
We identified 1937 records from database searches, 730 were duplicates, leaving 1207 for screening. Of these, 165 underwent full text review. Ten papers were included in this systematic review (see Fig. 1). We identified three costing-only studies and seven cost-effectiveness studies. Key study parameters are shown in Table 1. (See Additional file 2 for full details.)
Fig. 1.
PRISMA 2009 Flow Diagram. Directly-observed 3HP cost and cost-effectiveness systematic review [24]
Table 1.
Study characteristics
| Author, year | Population, context, setting | study perspective | reference case | comparators | time horizon | Primary outcome | cost vs CEAc | Secondary/ health outcomes | WTP threshold | source of cost data | source of effectiveness data | currency, price date | choice of model | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Costing studies | Huang 2016 [25] | general populationa, Taiwan, hospital | health system | 9H DOT | 3HP DOT | 5 years | cost/ patient | cost | treatment completion, active TB, cost/case avoided | NA | empiric | published data | USDd2014 | NA |
| Chen 2018 [26] | rheumatoid arthritis patients, Taiwan, clinic | health system | 9H DOT | 3HP DOT | 2 years | cost/ patient | cost | treatment completion, active TB, cost/case avoided | NA | empiric | NA | USDd2014 | NA | |
| Wheeler 2019 [27] | Inmates, USA, prison clinic | health system | 9H DOT | 3HP DOT | no long-term follow-up | cost/ patient | cost | treatment completion | NA | empiric | NA | USD 2012 | NA | |
| Cost effectiveness studies (HIC context) | Holland 2009 [28] | general population, USA, NSb | societal | no treatment | 9H SAT, 9H DOT, 3HP DOT, 4R | lifetime | ICER | CEA | active TB | $50,000 | mixed | mostly published | USD 2008 | Markov |
| Holland 2011 [29] | general population, USA, NSb | societal | 9H SAT | 3HP SAT, 3HP DOT, 1HP SAT | lifetime | ICER | CEA | active TB | 0 | non-empiric | published | USD 2011 | Markov | |
| Shepardson 2013 [30] | general population, USA, clinic | health system and societal | 9H SAT | 3HP DOT | 20 years | ICER | CEA | active TB | NSb | mixed | published data | USD 2010 | individual-based stochastic | |
| Doan 2019 [31] | general population, USA, NSb | health system | serial radiographic surveillance | 3HP DOT, 3HP SAT, 3RH, 4R, 6H, 9H | 20 years or death | ICER | CEA | active TB | $50,000 | non-empiric | published data | USD 2018 | Markov | |
| Pease, 2021 [32] | general population, Iqaluit, Canada | health system | 9H DOT (twice weekly) | 3HP DOT | 30 years | ICER | CEA | Cases and deaths averted | NSb | mostly empiric | programmatic | CAD 2019 | Markov | |
| Cost effectiveness studies (LMIC context) | Johnson 2018 [33] | people living with HIV, Uganda, HIV clinic | health system | 9H SAT | 3HP-DOT | 20 years | ICER | CEA | active TB | sensitivity-tested $1000, $3000, $5000, $7000, $9000 | non-empiric | published data | USD 2017 | Markov |
| Ferguson, 2020 [34] | people living with HIV, Uganda, HIV clinic | health system | 3HP DOT | 1HP SAT | 20 years | ICER | CEA | Active TB, TB deaths | NSb | Non-empiric | Published data | USD 2019 | Markov |
abut no women of child-bearing age, no children < 12 years old
bNS not stated
cCEA = cost-effectiveness analysis
ddenotes currency year not explicitly stated, so year inferred for subsequent CPI calculation
Costing-only studies
The three costing-only studies compare treatments that are directly observed; none has a SAT group. Two are from hospitals in Taiwan [25, 26], where DOPT is the standard of care. The other pertains to Californian prisoners [27]. All 3 costing studies reported completion rates, and consistently reported improved completion rates [25–27] for 3HP compared to 9H (See Table 2).
Table 2.
Cost/patient, as found in costing studies and compared to input costs/patient in cost-effectiveness studies, adjusted to 2020 USD, and completion rates
| Study type | Cost/ patient 9H DOT | Cost/ patient 9H SAT | Cost/ patient 3HP DOT | Treatment completion 9H DOT | Treatment completion 9H SAT | Treatment completion 3HP DOT | ||
|---|---|---|---|---|---|---|---|---|
| Costing studies | Huang [25] | Costing | $784 | $286 | 0.873 | 0.97 | ||
| Chen [26] | Costing | $784 | $286 | 0.783 | 0.905 | |||
| Wheeler [27] | Costing | $636 | $676 | 0.42 | 0.9 | |||
| Cost effectiveness studies (HIC) | Holland 2009 [28] | CE | $285 | $605 | 0.53 | 0.94 | ||
| Holland 2011 [29] | CE | $287 | $609 | 0.53 | 0.9 | |||
| Shepardson [30] | CE | $479 | $691 | 0.68 | 0.84 | |||
| Doan [31] | CE | $496 | $619 | 0.52 | 0.85 | |||
| Pease [32] | CE | $801 | $381 | 0.75 | 0.82 | |||
| Cost effectiveness studies (LMIC context) | Johnson [33] | CE | $17 | $94 | 0.47 | 0.74 | ||
| Ferguson [34] | CE | $23 | 0.74 |
Costs are rounded to nearest whole dollar and adjusted to 2020 USD using Consumer Price Index [23]
All three costing studies are in contexts that have systems in place for DOT, so no new investments had to be made. With shorter duration and longer dosing interval, 3HP is favoured because of fewer total visits.
Treatment completion rates vary widely between studies, particularly for 9H (see Table 2). This heterogeneity may reflect regional or cultural differences in treatment acceptance and adherence.
Costing studies did not include any downstream costs of LTBI treatment, notably for serious adverse events (SAEs) and for active tuberculosis. Therefore, they had fewer component costs compared to the cost-analysis studies (see Table 3).
Table 3.
Items included in cost inputs for cost-effectiveness and cost studies
| Author | TB meds | physician time | nurse time | other worker time | “clinic visit” | travel | radiology | lab tests | patient time (lost wages) | treatment SAEs | hospitalization SAE | treatment active TB | hospitalization active TB | contact tracing | HIV meds | secondary transmission | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Costing studies | Huang [25] | Y | Y | ||||||||||||||
| Chen [26] | Y | Y | |||||||||||||||
| Wheeler [27] | Y | Y | Y | Y | Y | Y | |||||||||||
| Cost effectiveness studies | Holland 2009 [28] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |||
| Holland 2011 [29] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | ||||
| Shepardson 2013 [30] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | ||||
| Doan [31] | Y | Y | Y | Y | Y | Y | Y | Y | Y | ||||||||
| Pease [32] | Y | Y | Y | Y | Y | Y | Y | Y | Y | ||||||||
| LMIC context | Johnson [33] | Y | Y | Y | Y | ||||||||||||
| Ferguson [34] | Y | Y | Y | Y |
Cost-effectiveness studies
Of seven identified cost-effectiveness studies, four are modelled on US data, a high-resource setting [28–31]. Pease [32] focuses on the Canadian Arctic that is high income, high TB prevalence, geographically remote, and a majority-Indigenous population. Johnson [33] and Ferguson [34] examine cost-effectiveness in a low-resource, high HIV prevalence setting, using a Ugandan HIV clinic as their model, with different reactivation rate, mortality rate, treatment standards, and willingness-to-pay (WTP) (Table 4 and Additional file 3) compared with North America.
Table 4.
Selected epidemiologic and cost input parameters in 2020 USD used across included studies
| Author | Holland 2009 [28] | Holland 2011 [29] | Shepardson [30] | Doan [31] | Pease [32] | Johnson [33] | Ferguson [34] |
|---|---|---|---|---|---|---|---|
| Selected epidemiologic parameters | |||||||
| Risk reduction 9H SAT | 0.93 | 0.93 | 0.92 | 0.96 | 0.93b | 0.58 | na |
| Risk reduction 3HP DOT | 0.93 | 0.93 | 0.95 | 0.975 | 0.93 | 0.63 | 0.90 |
| Treatment completion 9H SAT | 0.53 | 0.53 | 0.68 | 0.52 | 0.75b | 0.47 | na |
| Treatment completion 3HP DOT | 0.94 | 0.9 | 0.84 | 0.85 | 0.82 | 0.74 | 0.74 |
| Adverse events 9H SAT | 0.014 | 0.014 | 0.055 | 0.023 | 0.065b | 0.034 | na |
| Adverse events 3HP DOT | 0.014 | 0.05 | 0.082 | 0.016 | 0.057 | 0.034 | 0.034 |
| Selected cost parametersa | |||||||
| Treatment cost per regimen 9H SAT | 285 | 287 | 479 | 496 | 801b | 18 | na |
| Treatment cost per regimen 3HP DOT | 605 | 609 | 691 | 619 | 381 | 94 | 23 |
| Treatment cost of SAEs | 190 | 190 | 201 | Included in cost of hospitalization | Included in cost of hospitalization | Not included | Not included |
| Cost of hospitalization SAEs | 6396 | 6372 | 6738 | 6670 | 2616 | Not included | Not included |
| Cost active TB meds | 2460 | 2426 | 3542 | 3812 | 1517 | 230 | 230 |
| Cost active TB hospitalization | 12,016 | 11,969 | 30,260 | 29,463 | 66,495 | Not included | Not included |
na not applicable. “Not included” denotes not included in the model. SAEs = serious adverse events
aCosts in 2020 USD after adjustment using Consumer Price Index [23]
bregimen was 9H DOT
The five cost-effectiveness studies in high-income settings vary on whether 3HP or 9H is better tolerated (Table 4). Pease [32] is unique with less difference in treatment completion between 3HP and 9H DOT, with higher 9H treatment cost (since it was DOT rather than SAT); and lower 3HP DOT treatment cost. Additionally, the cost of treating active TB is higher, likely due to geographical remoteness.
Five cost-effectiveness studies in high-income settings demonstrate widely-variable ICERs ranging from 3HP being cost-saving compared to 9H, to an ICER of $5418 per QALY gained, though all found that 3HP DOT is cost-effective with ICER values well under a WTP of USD $50,000 per QALY gained. Methods for the five were comparable, with a few inclusion differences such as contact tracing, secondary transmission, travel times, patient costs, and radiology (Table 3). All seven cost-effectiveness studies list one or more clinical outcomes; active TB cases per 1000 patients is common to all, ranging from 9.1 to 37 per 1000 patients treated with 9H, and 3.9 to 38 per 1000 patients treated with 3HP (Table 5).
Table 5.
Outcomes, adjusted to 2020 USD
| Cost/ patient 9H SAT | Cost/ patient 3HP DOT | Effectiveness (QALY gained/patient 9H SAT) | Effectiveness (QALY gained/patient 3HP DOT) | ICER 3HP DOT compared to 9H SAT (2020USD/QALY gained) | Total TB cases/ 1000 patients on 9H SAT | Total TB cases/ 1000 patients on 3HP DOT | |
|---|---|---|---|---|---|---|---|
| Holland 2009 [28] | $817 | $933 | 22.64505 | 22.67083 | a4511 | 20.3 | 8.7 |
| Holland 2011 [29] | $833 | $868 | 22.64937 | 22.66836 | a1818 | 22 | 13 |
| Shepardson 2013—health system [30] | $606 | $739 | 0.044d | 0.019d | 5418 | 9.1 | 3.9 |
| Shepardson 2013—societal [30] | $837 | $864 | 0.044d | 0.019d | 1081 | 9.1 | 3.9 |
| Shepardson 2014—health system [35] | Not stated | Not stated | Not stated | Not stated | 2054 | 9.1 | 3.9 |
| Shepardson 2014—societal [35] | Not stated | Not stated | Not stated | Not stated | 3HP dominates | 9.1 | 3.9 |
| Doan [31] | $1095 | $900 | 15.6161 | 15.6539 | a3HP dominates | 23.2 | 10.6 |
| Pease [32] | $920c | $626 | 20.13c | 20.14 | 3HP dominates | 30.16 | 27.79 |
| Johnson [33] | $2576 | $2640 | 10.843 | 10.837 | b9927 DALY averted | 37 | 28 |
| Ferguson [34] | Not studied | $1541 | Not studied | 7.3697 | Not applicable | Not studied | 21.3 |
Costs in 2020 USD after adjustment using Consumer Price Index [23]
aICERs recalculated using 9H SAT as reference
b2020USD/ DALY averted in a low-resource setting
c9H DOT was reference
dShepardson reports effectiveness as mean QALY loss
All authors report from a health system perspective, except for Shepardson who provides two analyses as denoted
Pease [32] compares 9H DOT against 3HP DOT in a high-incidence, high-income, remote Arctic setting and finds that 3HP DOT is cost-saving in almost all scenarios. Shepardson in a US setting makes analyses from health system and societal perspectives [30], demonstrating 3HP DOT’s ICER of USD $1081 per QALY gained from a societal perspective (Table 5). After a rifapentine cost-reduction, Shepardson recalculated the ICER and found 3HP DOT to be cost-saving compared to 9H SAT from a societal perspective [35]. Note that Shepardson reports effectiveness as mean QALY loss, rather than QALY gained, though ICERs are calculated as incremental cost per QALY gained (see Table 5).
Holland 2009 also directly compares 9H DOT to 9H SAT and found it not to be cost-effective, with an ICER well over USD $50,000 per QALY gained [28].
The study by Johnson, in a low-resource, high-prevalence setting with high HIV co-morbidity omits many costs, such as for treating adverse events or for hospitalization, while HIV treatment costs are included. This leads to higher overall cost per DALY averted [33]. Completion rates for both regimens are lower than other studies, particularly for 3HP DOT (see Table 4). Risk reductions on both regimens are lower, contributing to a higher ICER. Risk of adverse events, including drug-drug interactions, were assumed to be equivalent. Johnson concludes that in a low-resource setting, ICER is above a WTP of USD$1000/DALY averted (Table 5).
Based on a similar model, Ferguson [34] performed a cost-effectiveness analysis for 1HP compared to 3HP. 1HP is administered daily by self-administration, in contrast to 3HP which is administered weekly by DOT. There is no isoniazid arm and regimens are presumed equally effective [36], so this is not comparable to other studies and there is no inherent ICER. Compared to the Johnson study [33], input costs and overall costs are lower for 3HP DOT.
For high-resource settings, the ICER is expressed as dollars per Quality-Adjusted Life Year (QALY) gained but for a low-resource setting, the ICER is expressed as dollars per Disability-Adjusted Life Year (DALY) averted as per convention, and so these are not comparable.
The quality of the included studies, assessed on a modified Drummond checklist [22], is provided in Additional file 4. Of note, the costing studies [25–27] do not include all the costs incurred, particularly long-term, downstream costs/savings. They also do not include sensitivity analyses. Modelling studies in HICs by Doan [31], Holland [28, 29], and Shepardson [30] have no discussion related to ethical and distribution issues, though Pease [32] includes this. The modelling studies set in LMICs [33, 34] do not include the cost of adverse events. Chen [26] and Huang [25], who study Taiwanese patients, do not address generalizability of their findings.
Discussion and conclusion
This is the first systematic review on the cost-effectiveness of 3HP compared to 9H for LTBI. All studies in highly-resourced contexts found that 3HP DOT is cost-effective at a WTP of $50,000 per QALY gained, compared to 9H SAT or 9H DOT, despite the higher cost of rifapentine and the costs of DOT. Doan and Pease found it to be cost-saving [31, 32]. Shepardson’s 2014 update found 3HP to be cost-saving from a societal perspective [35]. However, in LMICs, 3HP DOT may not be cost effective. Johnson [33] found 3HP to have an ICER above a WTP of USD$1000/DALY averted. Ferguson did not compare 3HP to 9H [34].
Self-administered treatment studies were specifically excluded from this systematic review. Because missing a dose in a weekly regimen can lead to subtherapeutic drug levels, treatment failure, and development of drug resistance, we focus on 3HP DOT as it is habitually administered and studied. In a non-inferiority trial, 3HP SAT was found to be non-inferior to 3HP DOT in the US [11]. Scant literature examines 3HP SAT cost and cost-effectiveness. Denholm [37] demonstrated in a single Australian centre that 3HP SAT costs less than 9H SAT, at $375 compared to $441 USD per person treated, driven by more outpatient visits in the 9H arm. Yuen [38] examined costs of 3HP SAT compared to 6H SAT in Pakistan, demonstrating that in this LMIC, 3HP is also less costly, particularly after a rifapentine price reduction which resulted in 3HP SAT costing $294 USD compared to $399 for 6H SAT. Holland 2011 and Doan included a 3HP SAT regimen in their models: both studies found it more cost-effective than 3HP DOT and 9H SAT. [29, 31]
The heterogeneity found in the costing and cost-effectiveness studies might be in part because of qualitative aspects to how DOPT is operationalized. The literature supports measures that improve the ease of LTBI treatment, such as shorter courses of better-tolerated medication, such as 3HP and 4R in contrast to 9H [39, 40]. Providing treatment in locations and via structures that are convenient to patients also reduces barriers, for example in schools [41, 42], in residences [14, 15, 43], or with methadone treatment [16, 44–46]. The convenience of treatment is difficult to separate as a driver of adherence, from the effect of a DOPT strategy. DOT might also be a proxy for frequency of treatment support, as an opportunity for patients to ask questions, report side-effects, and generally engage with providers [47].
In contrast and depending on context and other program characteristics, DOT can also be perceived as punitive and paternalistic and therefore reduce trust and engagement with providers. Patients in high-risk populations find DOT to be humiliating and discriminating [18, 19]. Patients and providers alike acknowledge that the interaction to persuade compliance with DOT is based on authority and subtle threats [18]. Patients with positive experiences have opportunity to negotiate flexibility and had continuity of providers [18].
Our review has several limitations. The wide range of ICERs and of incident cases of active TB indicate uncertain findings. This is reinforced by variation regarding key sensitivity variables, such as expected increase in adherence rates. Several variables likely have local, regional, and cultural differences, for example the already-high baseline adherence rate in Taiwan. Cost of living and costs of medications and materials are higher in remote locations such the Canadian Arctic. Reactivation rates are highly correlated with HIV co-infectivity rates.
There are limitations in using conventional WTP measures. The ICER for 3HP DOT in a low-resource context [33] is above the conventional WTP—three times national GDP—but remains well below the cost of HIV treatment in that context, which is a baseline assumption of that model and an accepted, funded, real-world practice. This contradiction illustrates the utility of reporting relative cost-effectiveness in relation to other, accepted interventions [48]. Further, cost-effectiveness should be accompanied by other considerations in each context such as budget impact, feasibility, transparency, equity, and consistency [49].
From our systematic review, we conclude from the literature that 3HP DOT is cost-effective over the 9H SAT standard at WTP of less than $50,000 per QALY gained in high-income countries.
Supplementary Information
Additional file 1. Search strategy for 3HP cost effectiveness.
Additional file 2: S1 Table. Study characteristics.
Additional file 3: S2 Table. Key input parameters for modelling studies.
Additional file 4: S3 Table. Quality scores based on Drummond checklist.
Acknowledgements
The authors wish to thank Edwina Veerasingam for database assistance.
Abbreviations
- LTBI
Latent tuberculosis infection
- TB
Tuberculosis
- 9H
9 months isoniazid
- 3HP
3 months weekly isoniazid and rifapentine
- 4R
4 months daily rifampicin
- DOT
Directly observed treatment
- DOPT
Directly observed preventative treatment
- CHEERS
Consolidated Health Economic Evaluation Reporting Standards
- SAT
Self-administered treatment
- ICER
Incremental cost-effectiveness ratio
- 1HP
1 month daily isoniazid and rifapentine
- USD
United States dollars
- CEA
Cost-effectiveness analysis
- SAE
Serious adverse effects
- WTP
Willingness to pay
Authors’ contributions
WAL and AAZ designed the study; WAL created the search strategy with the assistance of OD; searches were undertaken and updated by WAL and OD; WAL and KB screened articles for eligibility and extracted the data; WAL analyzed and synthesized data and wrote the initial draft of the manuscript. REC and AAZ provided critical review, guidance, and advice. All authors reviewed, edited, and approved the final manuscript.
Funding
This research received no financial support.
Availability of data and materials
All data generated or analysed during this study are included in this published article and in supplementary information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
WAL, KB, and OD have no competing interests to declare. REC has previously received consulting fees from Sanofi. Sanofi has previously donated study drugs to Johns Hopkins University. AAZ is an associate editor for BMC Infectious Diseases.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Global tuberculosis report 2020. Geneva: World Health Organization; 2020. Licence: CC BY-NC-SA 3.0 IGO.
- 2.Rangaka MX, Cavalcante SC, Marais BJ, Thim S, Martinson NA, Swaminathan S, et al. Controlling the seedbeds of tuberculosis: diagnosis and treatment of tuberculosis infection. Lancet. 2015;386(10010):2344–2353. doi: 10.1016/S0140-6736(15)00323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lönnroth K, Migliori GB, Abubakar I, D’Ambrosio L, de Vries G, Diel R, et al. Towards tuberculosis elimination: an action framework for low-incidence countries. Eur Respir J. 2015;45(4):928–952. doi: 10.1183/09031936.00214014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Comstock GW, Ferebee SH, Hammes LM. A controlled trial of community-wide isoniazid prophylaxis in Alaska. Am Rev Respir Dis. 1967;95(6):935–943. doi: 10.1164/arrd.1967.95.6.935. [DOI] [PubMed] [Google Scholar]
- 5.Kopanoff DE, Snider DE, Jr, Caras GJ. Isoniazid-related hepatitis: a U.S. Public Health Service cooperative surveillance study. Am Rev Respir Dis. 1978;117:991–1001. doi: 10.1164/arrd.1978.117.6.991. [DOI] [PubMed] [Google Scholar]
- 6.Sterling TR, Njie G, Zenner D, et al. Guidelines for the Treatment of Latent Tuberculosis Infection: Recommendations from the National Tuberculosis Controllers Association and CDC, 2020. MMWR Recomm Rep. 2020;69(1):1–11. doi: 10.15585/mmwr.rr6901a1externalicon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sterling TR, Vilarino ME, Borisov AS, Shang N, Gordin F, Bliven-Sizemore E, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365:2155–2166. doi: 10.1056/NEJMoa1104875. [DOI] [PubMed] [Google Scholar]
- 8.Menzies D, Adjobimey M, Ruslami R, Trajman A, Sow O, Kim H, et al. Four months of rifampin or nine months of isoniazid for latent tuberculosis in adults. N Engl J Med. 2018;379(5):440–453. doi: 10.1056/NEJMoa1714283. [DOI] [PubMed] [Google Scholar]
- 9.Pease C, Hutton B, Yazdi F, Wolfe D, Hamel C, Quach P, et al. Efficacy and completion rates of rifapentine and isoniazid (3HP) compared to other treatment regiments for latent tuberculosis infection: a systematic review with network meta-analysis. BMC Infect Dis. 2017;17:265–276. doi: 10.1186/s12879-017-2377-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.den Boon S, Matteelli A, Getahun H. Rifampicin resistance after treatment for latent tuberculous infection: a systematic review and meta-analysis. Int J Tuberc Lung Dis. 2016;20(8):1065–1071. doi: 10.5588/ijtld.15.0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belknap R, Holland D, Feng PJ, Millet JP, Cayla JA, et al. Self-administered versus directly observed once-weekly isoniazid and rifapentine treatment of latent tuberculosis infection: a randomized trial. Ann Intern Med. 2017;167:689–697. doi: 10.7326/M17-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borisov AS, Bamrah Morris S, Njie GJ, et al. Update of recommendations for use of once-weekly isoniazid-Rifapentine regimen to treat latent mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2018;67:723–726. doi: 10.15585/mmwr.mm6725a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNab BD, Marciniuk DD, Alvi RA, Tan L, Hoeppner VH. Twice weekly isoniazid and rifampin treatment of latent tuberculosis infection in Canadian Plains Aborigines. Am J Respir Crit Care Med. 2000;162(3Pt1):989–993. doi: 10.1164/ajrccm.162.3.9804117. [DOI] [PubMed] [Google Scholar]
- 14.Bishara H, Ore L, Vinitsky O, Bshara H, Armaly N, Weiler-Ravell D. Cost of nurse-managed latent tuberculous infection treatment among hard-to-reach immigrants in Israel. Int J Tuberc Lung Dis. 2015;19(7):799–804. doi: 10.5588/ijtld.14.0674. [DOI] [PubMed] [Google Scholar]
- 15.Chaisson RE, Armstrong J, Stafford J, Golub J, Bur S. Safety and tolerability of intermittent rifampin/pyrazinamide for the treatment of latent tuberculosis infection in prisoners. JAMA. 2002;288:165–166. doi: 10.1001/jama.288.2.165. [DOI] [PubMed] [Google Scholar]
- 16.Chaisson RE, Barnes GL, Hackman J, Watkinson L, Kimbrough L, Metha S, et al. A randomized, controlled trial of interventions to improve adherence to isoniazid therapy to prevent tuberculosis in injection drug users. Am J Med. 2001;110:610–615. doi: 10.1016/S0002-9343(01)00695-7. [DOI] [PubMed] [Google Scholar]
- 17.Tulsky JP, Pilote L, Hahn JA, Zolopa AJ, Burke M, Chesney M, et al. Adherence to isoniazid prophylaxis in the homeless: a randomized controlled trial. Arch Intern Med. 2000;160:697–702. doi: 10.1001/archinte.160.5.697. [DOI] [PubMed] [Google Scholar]
- 18.Sakbakken M, Bjune GA, Frich JC. Humiliation or care? A qualitative study of patients’ and health professionals’ experiences with tuberculosis treatment in Norway. Scand J Caring Sci. 2012;26:313–323. doi: 10.1111/j.1471-6712.2011.00935.x. [DOI] [PubMed] [Google Scholar]
- 19.De Vries SG, et al. Barriers and facilitators to the uptake of tuberculosis diagnostic and treatment services by hard-to-reach population in countries of low and medium tuberculosis incidence: a systematic review of qualitative literature. Lancet Infect Dis. 2017;17:e128–e143. doi: 10.1016/S1473-3099(16)30531-X. [DOI] [PubMed] [Google Scholar]
- 20.Chavan S, Newlands D, Smith C. A systematic review of economic evaluations of chemoprophylaxis for tuberculosis. J Trop Med. 2011:130976. 10.1155/2011/130976. [DOI] [PMC free article] [PubMed]
- 21.Husereau D, Drummond M, Petrou S, Greenberg D, Augustovski F, Briggs AH, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. BMJ. 2013;346:f1049. doi: 10.1136/bmj.f1049. [DOI] [PubMed] [Google Scholar]
- 22.Drummond M, et al. Methods for the economic evaluation of health care programmes. 2. Oxford: Oxford University Press; 1997. [Google Scholar]
- 23.World Bank. Consumer Price Index https://data.worldbank.org/indicator/FP.CPI.TOTL Accessed 24 Feb 2020.
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6(7):e10000097. doi: 10.1371/journal.pmed10000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang YW, Yang SF, Yeh YP, Tsao TCY, Tsao SM. Impacts of 12-dose regimen for latent tuberculosis infection: treatment completion rate and cost-effectiveness in Taiwan. Medicine. 2016;95(34):1–5. doi: 10.1097/MD.0000000000004126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen YM, Liao TL, Chen HH, Chen DY. Three months of once-weekly isoniazid plus rifapentine (3HP) in treating latent tuberculosis infection is feasible in patients with rheumatoid arthritis. Ann Rheum Dis. 2018;77(11):1688–1689. doi: 10.1136/annrheumdis-2018-213097. [DOI] [PubMed] [Google Scholar]
- 27.Wheeler C, Mohle-Boetani J. Completion rates, adverse effects, and costs of a 3-month and 9-month treatment regimen for latent tuberculosis infection in California inmates, 2011-2014. Public Health Rep. 2019;134(1_suppl):71S–79S. doi: 10.1177/0033354919826557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holland DP, Sanders GD, Hamilton CD, Stout JE. Costs and cost-effectiveness o four treatment regimens for latent tuberculosis infection. Am J Respir Crit Care Med. 2009;179(11):1055–1060. doi: 10.1164/rccm.200901-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holland DP, Sanders GD, Hamilton CD, Stout JE. Potential economic viability of two proposed Rifapentine-based regimens for treatment of latent tuberculosis infection. PLoS One. 2011;6(7):e22276. doi: 10.1371/journal.pone.0022276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shepardson D, Marks SM, Chesson H, Kerrigan A, Holland DP, Scott N, et al. Cost-effectiveness of a 12-dose regimen for treating latent tuberculous infection in the United States. Int J Tuberc Dis. 2013;17(12):1531–1537. doi: 10.5588/ijtld.13.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doan TN, Fox GJ, Meehan MT, Scott N, Ragonnet R, Viney K, et al. Cost-effectiveness of 3 months of weekly rifapentine and isoniazid compared with other standard treatment regimens for latent tuberculosis infection: a decision analysis study. J Antimicrob Chemother. 2019;74(1):218–227. doi: 10.1093/jac/dky403. [DOI] [PubMed] [Google Scholar]
- 32.Pease C, Alvarez G, Mallick R, Patterson M, Finn S, Habis Y, Schwartzman K, Kilabuk E, Mulpuru S, Zwerling A. Cost-effectiveness analysis of 3 months of weekly rifapentine and isoniazid compared to isoniazid monotherapy in a Canadian arctic setting. BMJ Open. 2021;11:e047514. doi: 10.1136/bmjopen-2020-047514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson KT, Churchyard GJ, Sohn H, Dowdy DW. Cost-effectiveness of preventive therapy for tuberculosis with isoniazid and rifapentine versus isoniazid alone in high-burden settings. Clin Infect Dis. 2018;67(7):1072–1078. doi: 10.1093/cid/ciy230. [DOI] [PubMed] [Google Scholar]
- 34.Ferguson O, Jo Y, Pennington J, Johnson K, Chaisson RE, Churchyard G, Dowdy D. Cost-effectiveness of one month of daily isoniazid and rifapentine versus three months of weekly isoniazid and rifapentine for prevention of tuberculosis among people receiving antiretroviral therapy in Uganda. J Int AIDS Soc. 2020;23:e25623. doi: 10.1002/jia2.25623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shepardson D, MacKenzie WR. Update on cost-effectiveness of a 12-dose regimen for latent tuberculous infection at new rifapentine prices. Int J Tuberc Lung Dis. 2014;18(6):751. doi: 10.5588/ijtld.14.0052. [DOI] [PubMed] [Google Scholar]
- 36.Swindells S, Ramchandani R, Gupta A, Benson CA, Leon-Cruz J, Mwelase N, Jean Juste MA, Lama RJ, Valencia J, et al. One month of Rifapentine plus isoniazid to prevent HIV-related tuberculosis. N Engl J Med. 2019;380:1001–1011. doi: 10.1056/NEJMoa1806808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denholm JT, McBryde ES, Eisen D, Street A, Matchett E, Chen C, Shultz T, Biggs B, Leder K. SIRCLE: a randomised controlled cost comparison of self-administered short-course isoniazid and rifapentine for cost-effective latent tuberculosis eradication. Intern Med J. 2017;47:1433–1436. doi: 10.1111/imj.13601. [DOI] [PubMed] [Google Scholar]
- 38.Yuen CM, Majidulla A, Jaswal M, Safdar N, Malid AA, Khan AJ, Becerra MC, Keshavjee S, Lu C, Hussain H. Cost of delivering 12-dose isoniazid and rifapentine versus 6 months of isoniazid for tuberculosis infection in a high-burden setting. Clin Infect Dis. 2021;73(5):e1135–e1141. doi: 10.1093/cid/ciaa1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Macaraig MM, Jalees M, Lam C, Burzynski J. Improved treatment completion with shorter treatment regimens for latent tuberculosis infection. Int J Tuberc Lung Dis. 2018;22(11):1344–1349. doi: 10.5588/ijtld.18.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McClintock AH, Eastment M, McKinney CM, et al. Treatment completion for latent tuberculosis infection: a retrospective cohort study comparing 9 months of isoniazid, 4 months of rifampin and 3 months of isoniazid and rifapentine. BMC Infect Dis. 2017;17(1):146. doi: 10.1186/s12879-017-2245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holzschuh EL, Province S, Johnson K, Walls C, Shemwell C, Martin G, et al. Use of video directly observed therapy for treatment of latent tuberculosis infection—Johnson County, Kansas, 2015. MMWR. 2017;66(14):387–389. doi: 10.15585/mmwr.mm6614a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips L, Carlile J, Smith D. Epidemiology of a tuberculosis outbreak in a rural Missouri high school. Pediatrics. 2004;113(6):514. doi: 10.1542/peds.113.6.e514. [DOI] [PubMed] [Google Scholar]
- 43.Chan PC, Yang CH, Chang LY, Wang KF, Lu BY, Lu CY. Et a. latent tuberculosis infection treatment for prison inmates: a randomised controlled trial. Int J Tuberc Lung Dis. 2012;16(5):633–638. doi: 10.5588/ijtld.11.0504. [DOI] [PubMed] [Google Scholar]
- 44.Batki SL, Gruber VA, Bradley JM, Bradley M, Delucchi K. A controlled trial of methadone treatment combined with directly observed isoniazid for tuberculosis prevention in injection drug users. Drug Alcohol Depend. 2002;66:283–293. doi: 10.1016/S0376-8716(01)00208-3. [DOI] [PubMed] [Google Scholar]
- 45.O’Connor PG, Shi JM, Henry S, Durante AJ, Friedman L, Selwyn PA. Tuberculosis chemoprophylaxis using a liquid isoniazid-methadone admixture for drug users in methadone maintenance. Addiction. 1999;94:1071–1075. doi: 10.1046/j.1360-0443.1999.947107112.x. [DOI] [PubMed] [Google Scholar]
- 46.Scholten JN, Driver CR, Munsiff SS, et al. Effectiveness of isoniazid treatment for latent tuberculosis infection among human immunodeficiency virus (HIV)-infected and HIV-uninfected injection drug users in methadone programs. Clin Infect Dis. 2003;37:1686–1692. doi: 10.1086/379513. [DOI] [PubMed] [Google Scholar]
- 47.Karumbi J, Garner P. Directly observed therapy for treating tuberculosis. Cochrane Database Syst Rev. 2015;5:CD003343. doi: 10.1002/14651858.CD003343.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S. Thresholds for the cost–effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93:118–124. doi: 10.2471/BLT.14.138206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bertram MY, Lauer JA, De Joncheere K, Edejer T, Hutubessy R, Kieny M-P, Hill SR. Cost–effectiveness thresholds: pros and cons. Bull World Health Organ. 2016;94:925–930. doi: 10.2471/BLT.15.164418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Search strategy for 3HP cost effectiveness.
Additional file 2: S1 Table. Study characteristics.
Additional file 3: S2 Table. Key input parameters for modelling studies.
Additional file 4: S3 Table. Quality scores based on Drummond checklist.
Data Availability Statement
All data generated or analysed during this study are included in this published article and in supplementary information files.