Abstract

The chemical industry aims to reduce its greenhouse gas emissions (GHGs) by adopting biomass as a renewable carbon feedstock. However, biomass is a limited resource. Thus, biomass should preferentially be used in processes that most reduce GHG emissions. However, a lack of harmonization in current life cycle assessment (LCA) literature makes the identification of efficient processes difficult. In this study, 46 fermentation processes from literature are harmonized and analyzed on the basis of their GHG reduction compared with fossil benchmarks. The GHG reduction per amount of sugar used is defined as Sugar-to-X efficiency and used as a performance metric in the following. The analyzed processes span a wide range of Sugar-to-X efficiencies from −3.3 to 6.7 kg of CO2 equiv per kg of sugar input. Diverting sugar from bioethanol production for fuels to the fermentation and bioconversion processes with the highest Sugar-to-X efficiency could reduce the chemical industry’s GHG emissions by an additional 130 MT of CO2 equiv without requiring any more biobased feedstocks.
Keywords: biobased chemicals, biobased feedstock, life cycle assessment, climate change, bioconversion, chemical processes
Short abstract
This study presents a harmonized life cycle assessment of 46 biobased processes to determine their resource efficiency in reducing climate impacts.
Introduction
The production of chemicals and plastics is predicted to account for approximately 50% of growth of global oil consumption by 2050, with direct CO2 emissions from the chemical industry increasing by 30% if no disruptive measures are taken.1 Renewable carbon sources are required to reduce these climate impacts. One promising renewable carbon feedstock for chemical production is biomass.2,3 The adoption of biomass is, therefore, facilitated by multiple national4,5 and transnational6 bioeconomy action plans.
This development is supported by the recent reviews on life cycle assessments (LCA) for biobased chemicals by Montazeri et al.7 and for biobased polymers by Spierling et al.8 that suggest biobased chemicals and polymers can reduce GHG emissions for most products.
Nevertheless, the proposed large-scale allocation of biomass to chemical production has not remained without criticism9 of environmental burden-shifting, indirect land-use change (iLUC), and competition between food and material production.10 Taking these concerns into account, it is unclear if sufficient sustainable biomass will be available to switch from a fossil-based chemical industry to one based on biomass. Estimates of the worldwide potential of sustainable biomass differ widely between 45 and 450 EJ.11−16 Given this uncertainty, sustainable biomass should be considered a scarce resource, and thus, the development of biobased processes should prioritize technologies that make efficient use of limited biomass resources. In this work, we focus on the efficient abatement of climate impacts, specifically, as concerns about global warming are the leading driver in the push to transform the chemical industry.
However, the identification of resource-efficient technologies requires sound quantification and comparison of climate impacts. Unfortunately, LCA studies of biobased processes in literature are usually not directly comparable.7,8 This lack of comparability can mainly be attributed to the following reasons.
First, assumptions often differ for the environmental impacts of processes in the background system, e.g., processes that are not directly modeled but adapted from LCA databases. Background processes are often used for energy supply, the fossil reference system, and biomass feedstock. The impact of biomass feedstock, especially, can vary widely. For instance, Renouf et al. studied the impact of fermentable sugar production, an essential input of many biobased chemicals.17 They determined that sugar’s climate impact varies by a factor of 5 depending on whether sugar cane or corn is used. The impact of biomass cultivation also varies strongly with local conditions: Pelton found that corn production’s climate impacts lie between 4.9 kg of CO2 equiv/bushel and 17.7 kg of CO2 equiv/bushel for the 10th and 90th percentile of counties in the U.S.18 Even for a fixed feedstock and production process, impacts can vary considerably because of methodological choices. Trisopolos et al. studied the effect of allocation and substitution approaches on the LCA of glucose production via corn wet-milling and arrived at impacts of 0.7–1.1 kg of CO2 equiv/kg.19 Differences in the environmental impact of the fossil-based reference chemicals can further hinder interpretation. For instance, when studying the replacement of fossil-based adipic acid, Cok et al. assume the global warming impact of fossil-based adipic acid as 8.8 kg of CO2 equiv/kg,20 Corona et al. assume between 12.9 and 22.6,21 and Aryapratama et al. assume 25.6 kg of CO2 equiv/kg,22 mainly because of different assumptions about N2O abatement. For the study of biobased ethylene, several authors used nearly identical inventories for biobased ethylene production.23−25 However, they arrive at GHG reduction between −0.4 and 2.4 kg of CO2 equiv/kg for biobased ethylene because of differences in the background system. These variations in the background system make it challenging to identify the most efficient biobased process by comparing individual studies’ results without an in-depth analysis of the background system. Thus, even if studies considered resource efficiencies, comparison of the efficiencies between studies is challenging. The required harmonization is tedious and a major barrier for comparing individual processes.
In addition to the large uncertainties introduced by the feedstock and the background system, studies differ in selecting the functional unit. While most studies use 1 kg of chemical/polymer as a functional unit, other functional units are also used, such as clamshell containers,26 packing films,27 or plastic bottles.28 Conversion from a product to a mass basis is generally possible but introduces another layer of complexity.
Lastly, the difference in resource efficiency between biobased processes is often not a key concern for LCA studies of biobased materials. However, for bioenergy, approaches considering the efficient use of resources were already proposed more than ten years ago,29 and multiple studies look into the efficient use of biomass for energy services. For instance, Steubing et al.30 consider the optimal use of waste biomass in Europe; Codina Gironès et al.31 assess the CO2 mitigation potential of biomass in Switzerland; Calvo-Serrano et al.32 optimize the allocation of biomass for fuel, electricity, and ethylene production; and Daioglou et al. developed future scenarios for biobased energy use on the basis of the TIMER model.33
However, for biobased chemicals, such comprehensive assessments of efficiency are lacking. While some studies consider multiple biomass processes, they still only consider a fraction of the published data. Adom et al. assess the production of seven biobased chemicals for integration in the GREET model.34 However, no efficiencies are assessed. Gerssen-Gondolach et al. study the land-use efficiency for the chemicals ethylene, PLA, PHA, PTT, and butanol in different production scenarios and predict that PLA will have the highest land-use efficiency in the future.35 However, because of the early stage of their assessment, uncertainties are high, and since their publication, a large number of additional pathways to biobased production have been published. We, thus, conclude that a study assessing the resource efficiency of current biobased processes is still missing from the literature.
This study identifies the most climate-efficient biomass utilization pathways by applying a consistent LCA methodology and background system to assess 46 bioconversion processes based primarily on simple sugars and their value chains to 16 biobased chemicals. We calculate the greenhouse gas reduction for all biobased processes and value chains using consistent harmonized background, reference, and feedstock data and are thereby able to rank the biobased bioconversion processes by their climate impact reduction efficiency.
Methodology
Goal and Scope
Our study aims to identify processes that utilize biobased feedstocks most efficiently to reduce climate impacts. However, as discussed above, the nature of biobased feedstocks varies widely in the environmental impact of production, local availability, and suitability for different processes. Thus, defining a single efficiency for all feedstocks requires some equivalence between the different feedstock types. This need does not arise if the selection of processes is limited to bioconversion processes since they can use the same resource: fermentable sugar. While focusing on bioconversion processes removes any pyrolysis-based processes and the direct catalytical conversion of biomass from the scope of this study, bioconversion processes already cover many critical biobased processes. For example, 7 of 12 processes with near potential identified by National Renewable Energy Laboratory (NREL) are bioconversion processes.36 Furthermore, bioconversion yields the biobased chemical with by far the largest production volume worldwide, ethanol.
Functional Unit and System Boundaries
For each biobased process evaluated in this study, our system boundaries include the biobased process and its upstream activities. Furthermore, it is assumed that all carbon taken from the atmosphere is released at the end of life. Additionally, the system boundaries include the fossil-based production chain to produce an equivalent output to the biobased process’s output (Figure 1). Since the product of the biobased and fossil-based process are assumed to be functionally equivalent, it is also assumed that the use phase is equivalent and can, thus, be neglected,
Figure 1.
System boundaries and functional unit used for the LCA. The functional unit is 1 kg of sugar used by the biobased process. The output of the fossil system is set to be equivalent to the output of the biobased system. If the biobased process produces more than one output, system expansion is used. The system boundaries are cradle to grave, assuming eventual release of all carbon as CO2 at end-of-life and equal impacts for equivalent bio- and fossil-based products during the use phase.
The resource efficiency of a process is calculated by defining the functional unit as 1 kg of sugar consumed by the biobased production pathway. The difference in global warming impact between bio- and fossil-based production is then defined as the Sugar-to-X efficiency (S2X).
| 1 |
As the Sugar-to-X (S2X) efficiency is expressed relative to the sugar input, the contribution of sugar production to the S2X efficiency is the same for all processes. Thus, changes in the sugar production’s environmental impact result in identical changes in the S2X efficiency for all biobased processes, which eliminates one of the largest uncertainties between LCA studies of biobased processes. This feature allows us to rank the biobased processes according to their potential to reduce climate impacts without the uncertainties of biobased feedstock production.
We calculate the S2X efficiency by considering four production scenarios for the biobased feedstock: sugar-based on corn stover, sugar cane, and corn with either no or high iLUC. The production of the sugar considers all life cycle stages from the cultivation of the feedstock to transportation and the final processing to sugar. However, the different scenarios only affect the absolute value of the S2X efficiency, not the ranking of the processes relative to each other. As a base case, we assume that the sugar is produced from corn in the U.S. and disregard contributions from iLUC, with a cradle-to-gate climate impact of sugar production of 0.65 kg of CO2 equiv per kg of sugar.37 For corn stover, sugar cane, and corn with the inclusion of indirect land-use change, the global warming impact of 1 kg of sugar is 0.0, 0.26, and 1.06 kg of CO2 equiv, respectively.38 The scale of land-use change is still a topic under active discussion. Scully et al. found that land-use change for fuel ethanol production in the U.S. varies between −2 to 30 g of CO2 equiv/MJ with a single outlier reported at 104 g of CO2 equiv/MJ.39 Here, we assume land-use change emissions of 0.41 kg of CO2 equiv/kg of sugar from corn, which is equivalent to 30 g of CO2 equiv/MJ for fuel ethanol production providing a pessimistic bound. For sugar derived from corn stover, we assume a pretreatment process with diluted acid based on the biochemical sugar model by NREL.40 Furthermore, C6 and C5 sugars are both assumed to achieve the same yield in the fermentation. Since it is very optimistic for any given process to achieve the same yield without any further process changes, this assumption provides an upper limit to the S2X efficiency. The biobased production begins with bioconversion but can include further conversion steps. For instance, biobased ethylene production includes the fermentative conversion of sugar to ethanol and the gas-phase dehydration of ethanol to ethylene,41 while succinic acid can be produced directly from sugar in a single bioconversion step.42 Furthermore, different separation sections are considered for the same product (Table 1).
Table 1. List of Processes Reviewed and Harmonized for This Studya.
The table lists the bioconversion/reaction type and the major separation units used. Processes are labeled following the scheme: product–prefix–first author–year. The prefix is added if one author proposes multiple processes for the same product and is being kept close to the naming conventions of the original publication. Most of the time, this refers to differences in downstream processing, though in some instances the bioconversion, itself, changes. If the difference between processes is not in bioconversion/reaction or separation, a comment on the difference between processes is provided. A more detailed breakdown of the processes is found in the Supporting Information.
Biobased processes can have one or more outputs, e.g. the production of butadiene also yields 2-butanol and butene. In such a case, the additional outputs will also be produced by the reference production system. Ideally, the reference system produces identical products. However, an identical product is not always available. In this case, a functional substitute is used. A functional substitute does not have the same chemical structure but is assumed to fulfill the same function; e.g., biobased succinic acid could substitute fossil-based adipic acid in nylon due to its diacid functionality, or bioethanol can substitute gasoline.20 For functional substitution, we assume equivalence on an mass basis for chemicals and an energy basis for fuels. The following functional substitutes are considered: succinic acid substituting adipic acid, PLA substituting PET, itaconic acid substituting acrylic acid, and ethanol substituting gasoline. For succinic acid, PLA, and itaconic acid, we assume that 1 kg of biobased chemical can replace 1 kg of fossil-based chemical. For ethanol, we assume that 1 kg of ethanol substitutes 0.587 kg of gasoline due to ethanol’s lower heating value.
As the end-of-life stage, the release of all stored carbon as CO2 without any energy recovery is assumed. This assumption is conservative for the life cycle emissions and allows us to capture differences in the carbon content of functional substitutes. Apart from carbon released during the end of life, it is assumed that use phase and end of life are the same for functional substitutes and, thus, cancel in the S2X efficiency.
Inventory Data for Bio- and Fossil-Based Processes
In the previous section, we established the S2X efficiency as the metric for biobased processes. However, for a comprehensive assessment, this metric should be applied to as many biobased processes as possible. We identify these biobased processes by screening the LCA and process design literature to extract inventories of biobased processes for our study. Apart from publications mentioned in current reviews of the LCA literature, we searched SCOPUS with the search term ″(“life cycle assessment” OR “lca” OR “techno economic” OR “process design”) AND (“biobased”) to find additional publications. This search led to 610 results that were first screened by title and abstract to identify relevant publications, with that number further reduced to 94 because most of the found studies were not LCA studies and were, thus, disregarded. Since conducting an LCA study requires complete inventories, we only considered studies that contained either complete inventory data or sufficient data to reconstruct the inventory. Our literature screening yielded 26 studies that contain sufficient inventory data for 46 processes producing 16 different chemicals (Table 1).
Not all reviewed studies contained complete inventories. In this case, we tried to reconstruct the inventory using the original publication, additional sources, or further assumptions. Any steps undertaken to reconstruct inventory data are discussed in detail for each process in the Supporting Information. Several seemingly promising studies had to be disregarded because no inventory data could be extracted. A more transparent deposition of inventory data for LCA studies would be helpful for any similar future studies and should be encouraged throughout the LCA community.43
Some bioconversion processes discussed in the literature use a feedstock different than sugar, e.g., glycerol, and would, thus, not fit our methodology. However, these processes are included if similar yields are reported for the bioconversion from sugar as from the original feedstock. It is assumed that the downstream processing is not affected by switching feedstock as long as similar titer and yields are reached. All processes that were originally considered a different bioconversion medium are discussed in more detail in the Supporting Information.
While the biobased inventory data is based on literature, the fossil-based inventory data is based on industrial data from IHS Markit, which has been used in other studies to model large fossil-based production networks.44,45 A list of all fossil-based value chains and the name of each selected individual process is available in the Supporting Information. The background system is modeled with ecoinvent (see Supporting Information).
Results
Merit Order Curve for Biobased Processes
The S2X efficiency is used to rank all 46 biobased processes identified in our literature screening in a merit order curve (Figure 2). As discussed previously, the order of the processes in this merit order curve does not depend on the feedstock since the input is the same for all processes. However, the absolute Sugar-to-X efficiency does depend on the feedstock.
Figure 2.
Merit order curve for the S2X efficiency of biobased processes and value chains. The legend shows the fossil-based chemicals substituted by the biobased chemicals. The fossil-based chemical is not always substituted by a chemically identical biobased chemical. The dashed lines show the position of the zero lines if the feedstocks corn stover, sugar cane, or corn + iLUC would be considered instead of corn. Since all processes are normalized to 1 kg of sugar input, a change in feedstock would result in a constant offset for all products. Processes are labeled following the scheme: product, prefix, first author, year. The prefix is added if one author proposes multiple processes for the same product and is being kept close to the naming conventions of the original publication (Table 1). Tabulated results are available in the Supporting Information.
Using corn as feedstock, 34 of the 46 processes have a positive S2X efficiency, while 11 processes have a negative S2X efficiency. However, the S2X efficiency has a large range from 6.1 kg of CO2 equiv/kg of sugar for the replacement of adipic acid with succinic acid produced by the direct process proposed by Cok et al.,20 to −3.0 kg of CO2 equiv/kg of sugar for the replacement of PET with PLA produced via the fungi-based process proposed by Manandhar et al.58 Furthermore, the S2X efficiency varies considerably between process variants producing the same product.
Three alternatives to corn as sugar source are considered in this study: corn stover, sugar cane, and corn + iLUC (dashed lines Figure 2). Changing the feedstock results in the same change in S2X efficiency for all processes. When corn stover is used as feedstock, the S2X efficiency is 0.7 kg of CO2 equiv/kg of sugar higher than for the base case of corn, 0.4 kg of CO2 equiv/kg of sugar higher for sugar cane, and 0.4 kg of CO2 equiv/kg of sugar lower for corn + iLUC. Thus, the type of biomass feedstock is primarily relevant for processes with an S2X efficiency within this range of variation. In these cases, the feedstock type decides whether this process reduces climate impacts compared with fossil-based production. This affects primarily biobased ethanol or products derived from biobased ethanol. While biobased ethanol derived from corn still has a positive S2X efficiency of 0.2 kg of CO2/kg of sugar, this S2X efficiency becomes negative once iLUC is considered and would increase impacts compared with gasoline. The same is true for most of the processes to produce ethylene and butadiene.
In the following, individual products are discussed in more detail, and differences in the processes that lead to different S2X efficiencies are highlighted in order of decreasing S2X efficiency.
Succinic and Adipic Acid
The processes with the highest S2X efficiency (6.1–3.5 kg of CO2 equiv/kg of sugar) all substitute adipic acid, either directly with biobased adipic acid or functionally with succinic acid. Their considerable reduction potential is based on avoding the large climate impacts of adipic acid’s fossil-based production. This fossil-based adipic acid production emits 11.2 kg of CO2 equiv from cradle to grave. Our calculation for the climate impacts of fossil-based adipic acid is even conservative, as 100% N2O abatement is assumed. Other studies arrive at climate impacts of fossil-based adipic acid exceeding 20 kg of CO2 equiv/kg (cradle to grave).21
The S2X efficiency of Cok et al.’s20 succinic acid production processes is generally higher than that of the processes by Adome et al.34 because of the significantly lower yield assumed by Adome et al. (0.5 kg of succinic acid/kg of sugar) compared with Cok et al. (0.67 kg of succinic acid/kg of sugar). In contrast, the impacts due to energy and auxiliary demand are very similar. Both studies propose processes with separations on the basis of crystallization, which is either direct (DC-Cok) or first increases the concentration of succinic acid by electrodialysis (EDI-Cok, EDI-Adom) or liquid–liquid extraction (LLE-Adome). The DC-Cok process has a S2X efficiency of 6.1 kg of CO2 equiv/kg of sugar versus 4.6 kg of CO2 equiv/kg for LLE-Adome and 4.9 kg of CO2 equiv/kg of sugar (Cok) versus 3.5 kg of CO2 equiv/kg of sugar (Adome) for the EDI process. Cok et al. propose a third process where ammonium sulfate is produced as a byproduct (AS-Cok), and separation occurs via ultra- and nanofiltration with S2X efficiency of 5.4 kg of CO2 equiv/kg of sugar.
Succinic acid production based on purification via adsorption on ZSM-5 zeolite and desorption with hot water followed by evaporative crystallization are proposed by Efe et al.65 and Klein et al.64 The desorption process’s large energy demand leads to a much lower S2X efficiency than the process by Cok et al.20 and Adome et al.34 discussed above. Efe et al.65 analyze the effect of different titers on the process. For the base case titer of 13.7 g/L (low), the process has an S2X efficiency of 1.4 CO2 equiv/kg of sugar. When the titer is doubled to 27.2 g/L (high), the S2X efficiency increases to 4.0 CO2 equiv/kg of sugar. However, the use of Actinobacillus succinogenes in the processes by Adome et al.34 suggests a substantially higher titer of 45.5–53.2 g/L, which might explain the better performance of their processes.66 The process proposed by Klein et al.64 has a heat requirement about twice as high as low-Efe, which leads to an overall negative S2X efficiency of −2.2 CO2 equiv/kg of sugar. Nevertheless, the assumed titer of the bioconversion is 21.8 g/L is 50% higher than the titer of low-Efe. Both Efe et al. and Klein et al. conduct a techno-economic assessment instead of an LCA for their processes, and thus, higher heat requirements might be an economically worthwhile trade-off.
For adipic acid production, two different processes have been proposed. Aryapratama et al.22 consider the direct bioconversion of C6 sugars to adipic acid with a yield and titer of approximately 1.49 kg of sugar/kg of adipic acid and 1.4 g/L, respectively. The adipic acid is purified via evaporative crystallization. The process proposed by Corona et al.21 considers the bioconversion of depolymerized lignin aromatics to muconic acid, followed by hydrogenation to adipic acid. For the bioconversion, a yield close to 100% and a titer of 80 g/L is assumed, which are projections onto possible targets. Overall, both processes result in similar and high S2X efficiencies of 5.0 and 4.4 CO2 equiv/kg of sugar for Aryapratama et al. and Corona et al., respectively. The higher S2X efficiency of succinic acid production compared with adipic acid production makes a strong case for functional substitution where technically possible.
1,3-Propanediol and 1,4-Butanediol
1,3-Propanediol and 1,4-butanediol show the next highest S2X efficiency. Again, high S2X efficiencies result from the high climate impacts of the fossil pathways with 11.0 and 5.8 kg of CO2 equiv/kg for 1,3-propanediol and 1,4-butanediol, respectively.
For 1,3-propanediol, Adome et al.34 propose an extraction process (LLE-Adome) using an ethanol/K2HPO4 mixture that lowers the energy requirements of the process compared with the vacuum distillation proposed by Anex et al.,46 which leads to a better S2X efficiency of 4.1 kg of CO2 equiv/kg of sugar for LLE-Adome compared with 3.9 kg of CO2 equiv/kg of sugar for Anex et al. because both processes have a similar yield of around 2 kg of sugar/kg of 1,3-propanediol. It should be noted that the consumption of K2HPO4 is substantial, with 0.63 kg/kg of 1,3-propanediol, and adds significantly to the climate impact. The second process proposed by Adome et al. (3HPA-Adome) first ferments glucose to 3-hydroxypropionic acid (3HPA) and then converts it to 1,3-propanediol. However, the 3HPA-Adome process has an overall lower yield of 2.8 kg of sugar/kg of 1,3-propanediol that results in the lowest S2X efficiency of all 1,3-propanediol processes, with 1.6 kg of CO2 equiv/kg of sugar, though it should be noted that the bioconversion to 3HPA originally utilized glycerol.
For the production of 1,4-butanediol, two processes are proposed. The process by Satam et al.47 directly ferments sugar to 1,4-butanediol and separates it by vapor scrubbing and distillation, while Adome et al.34 first ferment sugar to succinic acid, which is then hydrogenated to 1,4-butanediol. Both processes have a similar yield of 0.5 kg of 1,4-butanediol/kg of sugar. However, the two-step process of Adome et al. requires about twice the energy, thus leading to a lower S2X efficiency of 1.1 kg of CO2 equiv/kg of sugar versus 1.9 kg of CO2 equiv/kg of sugar for Satam et al.
Lactic Acid and Polylactic Acid
Nine studies for the production of lactic either or polylactic acid (PLA) are identified. Processes that only produce lactic acid (Dunn et al.,57 Manandhar et al.,58 Ögmundarson et al.60) are extended by a process from IHS markit to convert the lactic acid to PLA. This process contributes about 0.64 kg of CO2 equiv/kg of PLA to the climate impacts in addition to the lactic acid production. We assume that this PLA would avoid the fossil production of PET.
The production of PLA shows a large spread in S2X efficiency, varying between +2.1 and −3.0 CO2 equiv/kg of sugar. Two processes, Papong et al.63 and Lokesh et al.,62 have a positive S2X efficiency of 1.9 and 2.1, respectively. However, the process by Lokesh et al. has no heating requirement, which seems unlikely, and thus, this process should be treated with caution. The gate-to-gate impact (2.1 kg of CO2 equiv/kg of PLA) of Papong et al. agrees reasonably well with the LCAs published by commercial PLA producers (1.9 kg of CO2 equiv/kg of PLA).67
However, the remaining processes for PLA production all have negative or close to zero S2X efficiencies. The S2X efficiency is close to zero for Ögmundarson et al.60 (0.3) and Dacosta et al.56 (0.1). The S2X efficiencies for Dunn et al.57 (−0.8), Morales et al.59 (−1.1), and Manandhar et al.58 (−1.5 to −3.0) are significantly lower and lead to no positive S2X, regardless of feedstock. This large discrepancy in S2X efficiency is not due to any differences in yield but is primarily related to drastically different heat requirements. Additionally, the process of Dunn et al. requires large amounts of yeast, and the process by Ögmundarson et al. requires large amounts of triethanolamine and lime, which also adds significant environmental impact to the process. This large and consistent difference in energy requirement between industrial and simulated results points to some fundamental differences in lactic acid and PLA production modeling and begs further investigation.
2-Butanol, Aniline, Acrylic Acid, Isoprene, and Itaconic Acid
For the production of 2-butanol, aniline, acrylic acid, isoprene, and itaconic acid, only one process, or in the case of 2-butanol three variants of the same process, are available.
2-Butanol has a S2X efficiency of 1.2–1.1 kg of CO2 equiv/kg of sugar. 2-Butanol is followed by biobased aniline and isoprene with S2X efficiencies of 1.1 and 0.5 kg of CO2 equiv/kg of sugar, respectively. More research might be warranted into 2-butanol, isoprene, and aniline because of their high S2X efficiency but few available studies. Especially, a more direct route toward isoprene might be interesting, as the process by Lundberg et al.54 ferments sugar to mesaconic acid, followed by multiple reactions, while more direct metabolic pathways are available.68
Biobased acrylic acid via 3HPA, as proposed by Adome et al.,34 has a barely positive S2X efficiency of 0.03 kg of CO2 equiv/kg of sugar. This low S2X efficiency is primarily due to the low impact of fossil-based acrylic acid production (2.8 kg of CO2 equiv/kg of acrylic acid), not by gate-to-gate impacts of biobased acrylic acid production (0.9 kg of CO2 equiv/kg of acrylic acid).
Apart from the production of acrylic acid via 3HPA, we consider itaconic acid as a potential replacement for acrylic acid. Two scenarios (A1 and A2) for itaconic acid are proposed by Nieder-Heitmann et al.55 The scenarios differ in the titer of the bioconversion with 148 g/L and 88.4 g/L for A1 and A2, respectively. Scenario A1 has a slightly positive S2X efficiency of 0.2 kg of CO2 equiv/kg of sugar, while A2 has an S2X efficiency of −1.7 kg of CO2 equiv/kg of sugar. The S2X is low for both processes because of their high heating demands and the low global warming impact of fossil-based acrylic acid production. However, with further process optimization or other applications, itaconic acid could have climate benefits in the future.
Ethanol, Ethylene, Butadiene, Isobutanol
The last large block of processes produces the chemicals ethanol, ethylene, and butadiene, with all processes having an S2X efficiency in the range of 0.35 to −0.3 kg of CO2 equiv/kg of sugar. For ethanol, the low S2X efficiency is based on the low impact of the fossil-based reference (0.587 kg gasoline) with 2.3 kg of CO2 equiv/kg of ethanol. For the case of ethylene and butadiene, low yields lead to a low S2X efficiency with 3.8–4.0 kg of sugar/kg of ethylene and 5.0–8.6 kg of sugar/kg of butadiene. However, in the case of butadiene, significant amounts of coproducts are additionally generated.
All ethylene processes identified in the literature rely on the same technology: the gas-phase dehydration of ethanol to ethylene. Many of the processes reference the design by Knochar et al.41 and achieve similar high yields between 98% and 100%. The difference in their environmental impact thus mainly results in different energy requirements. However, the top-performing ethylene process by Chen et al.28 has no heating input, which seems unrealistic, given that the dehydration of ethanol to ethylene is endothermic, and the process should thus be treated with caution.
The production of butadiene in a gas-phase reaction from ethanol produces many side products, such as ethylene, butanol, acetaldehyde, and others. The yield of butadiene and the side products drive the S2X efficiency of the four butadiene processes considered here, which lies between 0.25 and −0.27 kg of CO2 equiv/kg of sugar.
Ethanol is the biobased chemical with the currently largest production volume worldwide. However, we only identified one inventory to produce ethanol from sugar directly, as most studies integrate feedstock processing with ethanol production. Ethanol achieves an S2X efficiency of 0.2 kg of CO2 equiv/kg of sugar, which is within the range of ethylene. The high production volume of ethanol, coupled with its low S2X efficiency, provides the opportunity to optimize the biobased industry by shifting production from biobased ethanol to chemicals with higher S2X efficiency—an opportunity that we are exploring in the next section.
pHBA
The production of pHBA has a negative S2X efficiency of −2.6 kg of CO2 equiv/kg of sugar. However, the process described by Krömer et al.61 is an example for a conceptual framework to assess the possible costs and life cycle impacts of biobased aromatics, not an optimized process design. Furthermore, the comparison with benzene might not be 100% fair as pHBA offers more functionality than benzene, and thus the low S2X efficiency, in this case, should not discourage any further research into biobased aromatics.
Relation of S2X Efficiency to Oxygen Content
The maximum S2X efficiency increases with the oxygen content of the substituted product (Figure 3). Two factors are likely the main contributors to this effect. First, products with a high oxygen content generally substitute products with similarly high oxygen content, with ethanol substituting gasoline being the exception. As less energy is needed to reduce the glucose for such products, less CO2 is created during bioconversion, and thus, higher yields are achieved. Second, fossil-based feedstocks such as natural gas and oil have a very low oxygen content. Thus, multiple reaction steps are often required to reach a target molecule with higher oxygen content. These multiple reaction steps lead to higher impacts for fossil-based oxygenated products. It therefore seems preferable to focus research and development efforts on substituting oxygen-containing molecules, since large S2X efficiencies can be expected here. Still, this heuristic can be misleading because, for example, acrylic acid has a high oxygen content in the substituted product but a low S2X efficiency due to the relative ease of fossil-based acrylic acid production. While oxygen content might give some indication, a more in-depth analysis of fossil-based production is still required.
Figure 3.

Relation between oxygen content of the substituted product and the S2X efficiency. The dashed line indicates the Pareto front of S2X efficiency and oxygen content from this study. However, the line does not reflect a fundamental law, and points above the line could exist even if they were not identified in this study. The oxygen content of petrol is assumed to be 2%.
Changes of the Background and Reference System
The calculation of the S2X efficiency normalizes the systems for the input of sugar to eliminate the effect of uncertainties in the sugar production in direct comparisons. Still, the S2X efficiency is sensitive toward the background system and the fossil-based reference processes because each process consumes different amounts of utilities and substitutes different products. Thus, the given merit order curve represents a current state of the chemical industry and might change with changes in the background system and reference processes.
Concerning the background system, the S2X efficiency would be most sensitive toward changes in heat production because heat demands contribute most to the global warming impacts of the processes. A switch from the assumed heating with natural gas to heating with clean electricity or biomass would strongly increase the S2X efficiency of all processes. Furthermore, some processes have substantial electricity demands. As a result, a lowering of the electric grid intensity would favor succinic acid production by EDI-Cok and EDI-Adom and 1,4-butanediol by Adom because of the relatively large electricity consumption. In the same way, a lowering of global warming impacts in lime production would most favor lactic acid production processes.
Moreover, the reference processes will also change in the future. Currently, the chemical industry is based on fossil resources for feedstock and utilities. However, the overall global warming emissions of the chemical industry are expected to decrease as new feedstock is adapted and electrification is increased. An overall lower global warming impact of the reference chemical industry would lower the S2X efficiency and might also change the merit order of the processes discussed in this study. However, it should be noted that a future lower S2X efficiency resulting from a lower climate impact of the chemical industry is still desirable because this would indicate change toward a more sustainable future.
Avoided Emissions by Optimized Feedstock Allocation
The limited availability of biomass is a crucial consideration for the large-scale adoption of biobased processes. First-generation feedstocks such as corn and wheat have drawn considerable criticism because of concerns about the disruption of food supply and environmental impacts caused by indirect land-use change.10 Because of these concerns, the E.U. plans to phase out first-generation biobased ethanol by 2030.69
Energy crops or waste biomass might have less undesired side effects but are still only available in limited quantities. However, even today, it is possible to increase biobased products’ contribution toward lower carbon emissions without requiring any more feedstock by reallocating feedstock currently used in processes with low a S2X efficiency, such as ethanol, to more efficient processes. In a future, where ethanol might get phased out as combustion fuel, the S2X efficiency could serve as an indicator of what bioprocess to prioritize.
The biobased chemical with the largest production volume is ethanol at around 100 Mt/year in 2019, with the production mainly centered around the U.S. and Brazil.70 In comparison, the production volume of all bioplastics is only around 2 Mt/year in 2019.71 However, while ethanol is by far the most produced biobased chemical, it has a low S2X efficiency compared with other chemicals such as succinic acid or 1,3-propanediol. Thus, the overall global warming impact reduction of the biobased industry could be significantly increased by allocating biomass currently used in ethanol production, around 220 Mt of sugar per year, to other biobased chemicals with a higher S2X efficiency, even if the reduction in fuel ethanol production results in a higher consumption of gasoline.
We calculate these additional climate impact reductions by first calculating the difference in S2X efficiency between biobased ethanol production and all other processes and then allocating sugar to the process with the highest S2X efficiency until the market demand for this process is reached (see Table S5). We then continue with the next highest S2X efficiency process until the feedstock limit of 220 Mt of sugar/year is reached. Since the additional reductions only depend on the difference in S2X efficiency between ethanol and the target chemical, they are independent of the feedstock, and it therefore should not matter whether Brazilian sugar cane or U.S. corn is used as the feedstock for ethanol.
Because of the concerns about the missing energy inputs, PLA production by Lokesh et al.62 and ethylene production by Chen et al.28 are excluded from this assessment.
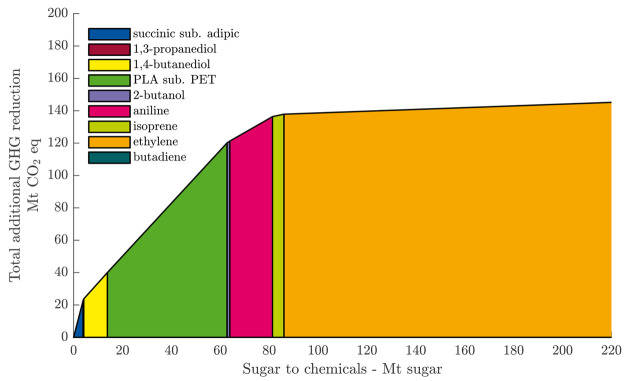
Following this approach, the current biobased industry could reduce an additional 134 Mt of CO2 equiv of emission compared with the base case of ethanol production (Figure 4). All sugar currently used for ethanol production could be used for a process with a higher S2X efficiency than ethanol.
Figure 4.
Additional reduction in GHG emissions due to substituting bioethanol fuel production with biobased chemical production. Products with high S2X efficiency are assumed to substitute ethanol first until their current production volume is reached.
As in the merit order curve, adipic acid substitution by succinic acid has the highest S2X efficiency. However, the absolute reduction potential is limited by the relatively low production volumes of adipic acid. Adipic acid is followed by 1,3-propanediol and 1,4-butanediol, which also have high S2X efficiencies but relatively small production volumes. Nevertheless, the production of adipic acid, 1,3-propanediol, and 1,4-butanediol instead of ethanol could reduce climate impacts by an additional 40 Mt of CO2 (30% of all additional reductions) while only consuming 6.3% of the biomass feedstock. The three top chemicals are followed by PLA substituting PET, which offers the largest additional reduction of 63 Mt of CO2 because of its combination of high S2X efficiency and the high production volume of PET. However, PLA will likely not substitute PET in all applications, thereby reducing the real-world additional reductions.
2-Butanol and aniline still offer substantial improvements in S2X efficiency compared with ethanol and have, in the case of aniline, decent market sizes combined, which allows for an additional 20 Mt of CO2 reductions. Isoprene only brings minor contributions because of its low S2X efficiency and small market size.
Finally, ethylene offers only slight improvements over ethanol regarding S2X efficiency, and these improvements are likely within the uncertainty of this assessment. Thus, it is debatable whether the processing of ethanol to biobased ethylene archives any additional reduction.
If we suppose the production of biobased ethylene is not regarded as desirable, then in that case, 130 Mt of sugar are left for ethanol production, for which a better use might be found in new, yet to be developed, and matured biobased processes.
Conclusions
Biobased chemicals are gaining renewed interest in reducing the climate intensity of the chemical industry. However, the availability of sustainable biobased feedstock remains an open question. This question is exasperated by the current state of LCA for biobased chemicals, which makes it challenging to identify biobased processes that efficiently use limited biomass resources to avoid climate change.
In this work, we screened and harmonized the current literature on bioconversion processes to determine their resource efficiency in reducing climate impacts to identify promising biobased processes by defining the Sugar-to-X efficiency as a measure of resource efficiency. Our analysis shows substantial differences in efficiency between both biobased products and process designs. High S2X efficiencies are achieved by diacids and diols, such as succinic acid, adipic acid, 1,4-butanediol, and 1,3-propanediol. Generally, as oxidation content in the target molecule increases, so does their S2X efficiency. S2X efficiency likely increases because of higher yields for more oxidized molecules and the higher climate impact of fossil-based production for highly oxidized molecules. However, many highly oxidized chemicals have only a limited market capacity. Thus, we found that even with their high reduction potential, their absolute reduction in climate impacts is small.
While outside of the scope of this study, economic effects will be crucial for the contribution of bioconversion processes toward climate change mitigation. The most environmentally beneficial processes might not be the most economical processes and, thus, reshuffle the abatement curve and reduce overall reduction. A shift in production from ethanol to other biochemicals might increase profit margin but also supply, which affects market prices. With carbon pricing being adopted by more and more regulatory bodies, a high S2X efficiency indicates a large economic lever to exploit higher carbon prices.
Nevertheless, replacing the current ethanol production for fuel use with biobased chemicals could reduce 130 Mt of CO2 emissions per year. Notably, the replacement of only 6.3% of the current ethanol production with high reduction biobased chemicals could already achieve 30% of this potential, which makes a strong case to favor high-value biobased chemicals over biofuel production. The presented Sugar-to-X (S2X) efficiency allows identifying biobased chemicals promising to reduce climate impacts in a resource-efficient way.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acssuschemeng.2c03275.
B.W. and A.B. acknowledge funding from the European Union’s Horizon 2020 research and innovation program under grant agreement no. 720695 (GreenSolRes).
The authors declare no competing financial interest.
Supplementary Material
References
- International Energy Agency . The Future of Petrochemicals: Towards More Sustainable Plastics and Fertilisers. https://www.oecd.org/publications/the-future-of-petrochemicals-9789264307414-en.htm (accessed 01.06.2022).
- OECD . The Application of Biotechnology to Industrial Sustainability:A Primer. http://www.oecd.org/science/emerging-tech/1947629.pdf (accessed 01.06.2022). [Google Scholar]
- Bozell J. J. Feedstocks for the Future - Biorefinery Production of Chemicals from Renewable Carbon. Clean Soil Air Water 2008, 36 (8), 641–647. 10.1002/clen.200800100. [DOI] [Google Scholar]
- Bundesministeriumfür Bildung und Forschung ; Bundesministerium für Ernährung und Landwirtschaft (Nationale Bioökonomiestrategie. https://www.bmel.de/SharedDocs/Downloads/DE/Broschueren/Nationale-Biooekonomiestrategie-Zusammenfassung.pdf?__blob=publicationFile&v=5 (accessed 01.06.2022).
- House T. W. National Bioeconomy Blueprint, April 2012. Industrial Biotechnology 2012, 8 (3), 97–102. 10.1089/ind.2012.1524. [DOI] [Google Scholar]
- Europäische Kommission . A sustainable bioeconomy for Europe:Strengthening the connection between economy, society and the environment: updated bioeconomy strategy; Publications Office of the European Union, 2018, https://op.europa.eu/en/publication-detail/-/publication/edace3e3-e189-11e8-b690-01aa75ed71a1/ (accessed 01.06.2022). [Google Scholar]
- Montazeri M.; Zaimes G. G.; Khanna V.; Eckelman M. J. Meta-Analysis of Life Cycle Energy and Greenhouse Gas Emissions for Priority Biobased Chemicals. ACS Sustainable Chem. Eng. 2016, 4 (12), 6443–6454. 10.1021/acssuschemeng.6b01217. [DOI] [Google Scholar]
- Spierling S.; Knüpffer E.; Behnsen H.; Mudersbach M.; Krieg H.; Springer S.; Albrecht S.; Herrmann C.; Endres H.-J. Bio-based plastics - A review of environmental, social and economic impact assessments. Journal of Cleaner Production 2018, 185, 476–491. 10.1016/j.jclepro.2018.03.014. [DOI] [Google Scholar]
- Searchinger T. D.; Wirsenius S.; Beringer T.; Dumas P. Assessing the efficiency of changes in land use for mitigating climate change. Nature 2018, 564 (7735), 249–253. 10.1038/s41586-018-0757-z. [DOI] [PubMed] [Google Scholar]
- Searchinger T.; Heimlich R.; Houghton R. A.; Dong F.; Elobeid A.; Fabiosa J.; Tokgoz S.; Hayes D.; Yu T.-H. Use of U.S. croplands for biofuels increases greenhouse gases through emissions from land-use change. Science (New York, N.Y.) 2008, 319 (5867), 1238–1240. 10.1126/science.1151861. [DOI] [PubMed] [Google Scholar]
- Searle S.; Malins C. A reassessment of global bioenergy potential in 2050. GCB Bioenergy 2015, 7 (2), 328–336. 10.1111/gcbb.12141. [DOI] [Google Scholar]
- BERINGER T. I.; LUCHT W.; SCHAPHOFF S. Bioenergy production potential of global biomass plantations under environmental and agricultural constraints. GCB Bioenergy 2011, 3 (4), 299–312. 10.1111/j.1757-1707.2010.01088.x. [DOI] [Google Scholar]
- Field C. B.; Campbell J. E.; Lobell D. B. Biomass energy:The scale of the potential resource. Trends in ecology & evolution 2008, 23 (2), 65–72. 10.1016/j.tree.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Fischer G.; Schrattenholzer L. Global bioenergy potentials through 2050. Biomass and Bioenergy 2001, 20 (3), 151–159. 10.1016/S0961-9534(00)00074-X. [DOI] [Google Scholar]
- Long H.; Li X.; Wang H.; Jia J. Biomass resources and their bioenergy potential estimation:A review. Renewable and Sustainable Energy Reviews 2013, 26, 344–352. 10.1016/j.rser.2013.05.035. [DOI] [Google Scholar]
- de Vries B. J.; van Vuuren D. P.; Hoogwijk M. M. Renewable energy sources:Their global potential for the first-half of the 21st century at a global level: An integrated approach. Energy Policy 2007, 35 (4), 2590–2610. 10.1016/j.enpol.2006.09.002. [DOI] [Google Scholar]
- Renouf M. A.; Wegener M. K.; Nielsen L. K. An environmental life cycle assessment comparing Australian sugarcane with US corn and UK sugar beet as producers of sugars for fermentation. Biomass and Bioenergy 2008, 32 (12), 1144–1155. 10.1016/j.biombioe.2008.02.012. [DOI] [Google Scholar]
- Pelton R. Spatial greenhouse gas emissions from US county corn production. Int. J. Life Cycle Assess 2019, 24 (1), 12–25. 10.1007/s11367-018-1506-0. [DOI] [Google Scholar]
- Tsiropoulos I.; Cok B.; Patel M. K. Energy and greenhouse gas assessment of European glucose production from corn – a multiple allocation approach for a key ingredient of the bio-based economy. Journal of Cleaner Production 2013, 43, 182–190. 10.1016/j.jclepro.2012.12.035. [DOI] [Google Scholar]
- Cok B.; Tsiropoulos I.; Roes A. L.; Patel M. K. Succinic acid production derived from carbohydrates:An energy and greenhouse gas assessment of a platform chemical toward a bio-based economy. Biofuels, Bioprod. Bioref. 2014, 8 (1), 16–29. 10.1002/bbb.1427. [DOI] [Google Scholar]
- Corona A.; Biddy M. J.; Vardon D. R.; Birkved M.; Hauschild M. Z.; Beckham G. T. Life cycle assessment of adipic acid production from lignin. Green Chem. 2018, 20 (16), 3857–3866. 10.1039/C8GC00868J. [DOI] [Google Scholar]
- Aryapratama R.; Janssen M. Prospective life cycle assessment of bio-based adipic acid production from forest residues. Journal of Cleaner Production 2017, 164, 434–443. 10.1016/j.jclepro.2017.06.222. [DOI] [Google Scholar]
- Alvarenga R. A. F.; Dewulf J.; De Meester S.; Wathelet A.; Villers J.; Thommeret R.; Hruska Z. Life cycle assessment of bioethanol-based PVC. Biofuels, Bioprod. Bioref. 2013, 7 (4), 386–395. 10.1002/bbb.1405. [DOI] [Google Scholar]
- Belboom S.; Léonard A. Does biobased polymer achieve better environmental impacts than fossil polymer?:Comparison of fossil HDPE and biobased HDPE produced from sugar beet and wheat. Biomass and Bioenergy 2016, 85, 159–167. 10.1016/j.biombioe.2015.12.014. [DOI] [Google Scholar]
- Liptow C.; Tillman A.-M. A Comparative Life Cycle Assessment Study of Polyethylene Based on Sugarcane and Crude Oil. Journal of Industrial Ecology 2012, 16 (3), 420–435. 10.1111/j.1530-9290.2011.00405.x. [DOI] [Google Scholar]
- Madival S.; Auras R.; Singh S. P.; Narayan R. Assessment of the environmental profile of PLA, PET and PS clamshell containers using LCA methodology. Journal of Cleaner Production 2009, 17 (13), 1183–1194. 10.1016/j.jclepro.2009.03.015. [DOI] [Google Scholar]
- Lokesh K.; Matharu A. S.; Kookos I. K.; Ladakis D.; Koutinas A.; Morone P.; Clark J. Hybridised sustainability metrics for use in life cycle assessment of bio-based products: resource efficiency and circularity. Green Chem. 2020, 22 (3), 803–813. 10.1039/C9GC02992C. [DOI] [Google Scholar]
- Chen G.-Q.; Patel M. K. Plastics derived from biological sources:Present and future: a technical and environmental review. Chem. Rev. 2012, 112 (4), 2082–2099. 10.1021/cr200162d. [DOI] [PubMed] [Google Scholar]
- Cherubini F.; Strømman A. H. Life cycle assessment of bioenergy systems: state of the art and future challenges. Bioresource technology 2011, 102 (2), 437–451. 10.1016/j.biortech.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Steubing B.; Zah R.; Ludwig C. Heat, electricity, or transportation? The optimal use of residual and waste biomass in Europe from an environmental perspective. Environ. Sci. Technol. 2012, 46 (1), 164–171. 10.1021/es202154k. [DOI] [PubMed] [Google Scholar]
- Codina Gironès V.; Peduzzi E.; Vuille F.; Maréchal F. On the Assessment of the CO2Mitigation Potential of Woody Biomass. Front. Energy Res. 2018, 5, 151. 10.3389/fenrg.2017.00037. [DOI] [Google Scholar]
- Calvo-Serrano R.; Guo M.; Pozo C.; Galán-Martín Á.; Guillén-Gosálbez G. Biomass Conversion into Fuels, Chemicals, or Electricity? A Network-Based Life Cycle Optimization Approach Applied to the European Union. ACS Sustainable Chem. Eng. 2019, 7 (12), 10570–10582. 10.1021/acssuschemeng.9b01115. [DOI] [Google Scholar]
- Daioglou V.; Wicke B.; Faaij A. P. C.; van Vuuren D. P. Competing uses of biomass for energy and chemicals:Implications for long-term global CO 2 mitigation potential. GCB Bioenergy 2015, 7 (6), 1321–1334. 10.1111/gcbb.12228. [DOI] [Google Scholar]
- Adom F.; Dunn J. B.; Han J.; Sather N. Life-cycle fossil energy consumption and greenhouse gas emissions of bioderived chemicals and their conventional counterparts. Environ. Sci. Technol. 2014, 48 (24), 14624–14631. 10.1021/es503766e. [DOI] [PubMed] [Google Scholar]
- Gerssen-Gondelach S. J.; Saygin D.; Wicke B.; Patel M. K.; Faaij A. Competing uses of biomass:Assessment and comparison of the performance of bio-based heat, power, fuels and materials. Renewable and Sustainable Energy Reviews 2014, 40, 964–998. 10.1016/j.rser.2014.07.197. [DOI] [Google Scholar]
- Biddy M. J.; Scarlata C.; Kinchin C.. Chemicals from Biomass: A Market Assessment of Bioproducts with Near-Term Potential; National Renewable Energy Laboratory, U.S. Department of Energy: Golden, CO, 2016, 10.2172/1244312. [DOI]
- Winter B.; Meys R.; Bardow A. Towards aromatics from biomass: Prospective Life Cycle Assessment of bio-based aniline. Journal of Cleaner Production 2021, 290, 125818. 10.1016/j.jclepro.2021.125818. [DOI] [Google Scholar]
- IHS Markit . Aniline: Chemical Economics Handbook. https://ihsmarkit.com/index.html (accessed 01.12.2017).
- Scully M. J.; Norris G. A.; Alarcon Falconi T. M.; MacIntosh D. L. Carbon intensity of corn ethanol in the United States: state of the science. Environ. Res. Lett. 2021, 16 (4), 43001. 10.1088/1748-9326/abde08. [DOI] [Google Scholar]
- Tao L.; Davis R.. NREL 2017 Biochemical Sugar Model. https://www.nrel.gov/extranet/biorefinery/aspen-models/ (accessed 01.06.2022).
- Knochar N. K.; Merims R.; Padia A. S. Ethylene from Ethanol. Chemical Engineering Progress 1981, 77 (6), 66–70. [Google Scholar]
- Bechthold I.; Bretz K.; Kabasci S.; Kopitzky R.; Springer A. Succinic Acid: A New Platform Chemical for Biobased Polymers from Renewable Resources. Chem. Eng. Technol. 2008, 31 (5), 647–654. 10.1002/ceat.200800063. [DOI] [Google Scholar]
- Pauliuk S.; Majeau-Bettez G.; Mutel C. L.; Steubing B.; Stadler K. Lifting Industrial Ecology Modeling to a New Level of Quality and Transparency: A Call for More Transparent Publications and a Collaborative Open Source Software Framework. Journal of Industrial Ecology 2015, 19 (6), 937–949. 10.1111/jiec.12316. [DOI] [Google Scholar]
- Kätelhön A.; Meys R.; Deutz S.; Suh S.; Bardow A. Climate change mitigation potential of carbon capture and utilization in the chemical industry. Proc. Natl. Acad. Sci. U.S.A. 2019, 116 (23), 11187–11194. 10.1073/pnas.1821029116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanes R. J.; Carpenter A. Evaluating opportunities to improve material and energy impacts in commodity supply chains. Environ. Syst. Decis 2017, 37 (1), 6–12. 10.1007/s10669-016-9622-5. [DOI] [Google Scholar]
- Anex R. P.; Ogletree A. L.. Life-Cycle Assessment of Energy-Based Impacts of a Biobased Process for Producing 1,3-Propanediol. In Feedstocks for the future: Renewables for the production of chemicals and materials; Bozell J. J., Ed.; ACS Symposium Series, Vol. 921; American Chemical Society, 2006; pp 222–238. 10.1021/bk-2006-0921.ch017. [DOI] [Google Scholar]
- Satam C. C.; Daub M.; Realff M. J. Techno-economic analysis of 1,4-butanediol production by a single-step bioconversion process. Biofuels, Bioprod. Bioref. 2019, 13 (5), 1261–1273. 10.1002/bbb.2016. [DOI] [Google Scholar]
- Pereira J. P. C.; van der Wielen L. A. M.; Straathof A. J. J. Perspectives for the microbial production of methyl propionate integrated with product recovery. Bioresource technology 2018, 256, 187–194. 10.1016/j.biortech.2018.01.118. [DOI] [PubMed] [Google Scholar]
- Cespi D.; Passarini F.; Vassura I.; Cavani F. Butadiene from biomass, a life cycle perspective to address sustainability in the chemical industry. Green Chem. 2016, 18 (6), 1625–1638. 10.1039/C5GC02148K. [DOI] [Google Scholar]
- Moncada J.; Gursel I. V.; Worrell E.; Ramírez A. Production of 1,3-butadiene and ε-caprolactam from C6 sugars:Techno-economic analysis. Biofuels, Bioprod. Bioref. 2018, 12 (4), 600–623. 10.1002/bbb.1876. [DOI] [Google Scholar]
- Chen L.; Pelton R. E.; Smith T. M. Comparative life cycle assessment of fossil and bio-based polyethylene terephthalate (PET) bottles. Journal of Cleaner Production 2016, 137, 667–676. 10.1016/j.jclepro.2016.07.094. [DOI] [Google Scholar]
- Hong J.; Zhang Y.; Xu X.; Li X. Life cycle assessment of corn- and cassava-based ethylene production. Biomass and Bioenergy 2014, 67, 304–311. 10.1016/j.biombioe.2014.05.014. [DOI] [Google Scholar]
- Posen I. D.; Griffin W. M.; Matthews H. S.; Azevedo I. L. Changing the renewable fuel standard to a renewable material standard:Bioethylene case study. Environ. Sci. Technol. 2015, 49 (1), 93–102. 10.1021/es503521r. [DOI] [PubMed] [Google Scholar]
- Lundberg D. J.; Lundberg D. J.; Zhang K.; Dauenhauer P. J. Process Design and Economic Analysis of Renewable Isoprene from Biomass via Mesaconic Acid. ACS Sustainable Chem. Eng. 2019, 7 (5), 5576–5586. 10.1021/acssuschemeng.9b00362. [DOI] [Google Scholar]
- Nieder-Heitmann M.; Haigh K. F.; Görgens J. F. Process design and economic analysis of a biorefinery co-producing itaconic acid and electricity from sugarcane bagasse and trash lignocelluloses. Bioresource technology 2018, 262, 159–168. 10.1016/j.biortech.2018.04.075. [DOI] [PubMed] [Google Scholar]
- Fernandez-Dacosta C.; Wassenaar P. N.; Dencic I.; Zijp M. C.; Morao A.; Heugens E. H.; Shen L. Can we assess innovative bio-based chemicals in their early development stage?:A comparison between early-stage and life cycle assessments. Journal of Cleaner Production 2019, 230, 137–149. 10.1016/j.jclepro.2019.05.115. [DOI] [Google Scholar]
- Dunn J. B.; Adom F.; Sather N.; Han J.; Snyder S.; He C.; Gong J.; Yue D.; You F.. Life-cycle Analysis of Bioproducts and Their Conventional Counterparts in GREET; U.S. Department of Energy, Argonne National Laboratory, Argonne, IL; distributed by Office of Scientific and Technical Information, U.S. Department of Energy: Oak Ridge, TN, 2015. 10.2172/1250468. [DOI]
- Manandhar A.; Shah A. Techno-Economic Analysis of Bio-Based Lactic Acid Production Utilizing Corn Grain as Feedstock. Processes 2020, 8 (2), 199. 10.3390/pr8020199. [DOI] [Google Scholar]
- Morales M.; Dapsens P. Y.; Giovinazzo I.; Witte J.; Mondelli C.; Papadokonstantakis S.; Hungerbühler K.; Pérez-Ramírez J. Environmental and economic assessment of lactic acid production from glycerol using cascade bio- and chemocatalysis. Energy Environ. Sci. 2015, 8 (2), 558–567. 10.1039/C4EE03352C. [DOI] [Google Scholar]
- Ögmundarson Ó.; Sukumara S.; Laurent A.; Fantke P. Environmental hotspots of lactic acid production systems. GCB Bioenergy 2020, 12 (1), 19–38. 10.1111/gcbb.12652. [DOI] [Google Scholar]
- Krömer J. O.; Ferreira R. G.; Petrides D.; Kohlheb N. Economic Process Evaluation and Environmental Life-Cycle Assessment of Bio-Aromatics Production. Frontiers in bioengineering and biotechnology 2020, 8, 403. 10.3389/fbioe.2020.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokesh K.; Matharu A. S.; Kookos I. K.; Ladakis D.; Koutinas A.; Morone P.; Clark J. Hybridised sustainability metrics for use in life cycle assessment of bio-based products: resource efficiency and circularity. Green Chem. 2020, 22 (3), 803–813. 10.1039/C9GC02992C. [DOI] [Google Scholar]
- Papong S.; Malakul P.; Trungkavashirakun R.; Wenunun P.; Chom-in T.; Nithitanakul M.; Sarobol E. Comparative assessment of the environmental profile of PLA and PET drinking water bottles from a life cycle perspective. Journal of Cleaner Production 2014, 65, 539–550. 10.1016/j.jclepro.2013.09.030. [DOI] [Google Scholar]
- Klein B. C.; Silva J. F.; Junqueira T. L.; Rabelo S. C.; Arruda P. V.; Ienczak J. L.; Mantelatto P. E.; Pradella J. G.; Junior S. V.; Bonomi A. Process development and techno-economic analysis of bio-based succinic acid derived from pentoses integrated to a sugarcane biorefinery. Biofuels, Bioprod. Bioref. 2017, 11 (6), 1051–1064. 10.1002/bbb.1813. [DOI] [Google Scholar]
- Efe Ç.; van der Wielen L. A.; Straathof A. J. Techno-economic analysis of succinic acid production using adsorption from fermentation medium. Biomass and Bioenergy 2013, 56, 479–492. 10.1016/j.biombioe.2013.06.002. [DOI] [Google Scholar]
- Zheng P.; Dong J.-J.; Sun Z.-H.; Ni Y.; Fang L. Fermentative production of succinic acid from straw hydrolysate by Actinobacillus succinogenes. Bioresource technology 2009, 100 (8), 2425–2429. 10.1016/j.biortech.2008.11.043. [DOI] [PubMed] [Google Scholar]
- Vink E. T.; Davies S. Life Cycle Inventory and Impact Assessment Data for 2014 Ingeo Polylactide Production. Industrial Biotechnology 2015, 11 (3), 167–180. 10.1089/ind.2015.0003. [DOI] [Google Scholar]
- Tiso T.; Winter B.; Wei R.; Hee J.; de Witt J.; Wierckx N.; Quicker P.; Bornscheuer U. T.; Bardow A.; Nogales J.; Blank L. M. The metabolic potential of plastics as biotechnological carbon sources - Review and targets for the future. Metabolic engineering 2022, 71, 77–98. 10.1016/j.ymben.2021.12.006. [DOI] [PubMed] [Google Scholar]
- European Union DIRECTIVE (EU) 2018/2001 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 11 December 2018 on the promotion of the use of energy from renewable sources (recast). Official Journal of the European Union; 2018, https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018L2001 (accessed 01.06.2022).
- Renewable Fuels Association . Ethanol Industry Outlook; 2019, https://d35t1syewk4d42.cloudfront.net/file/1031/RFA2019Outlook.pdf (accessed 01.06.2022).
- European Bioplastics . Bioplastics: facts and figures.https://docs.european-bioplastics.org/publications/EUBP_Facts_and_figures.pdf (accessed 01.06.2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.