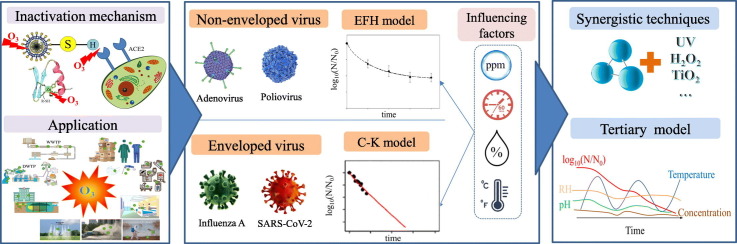
Abstract
The large-scale global COVID-19 has a profound impact on human society. Timely and effectively blocking the virus spread is the key to controlling the pandemic growth. Ozone-based inactivation and disinfection techniques have been shown to effectively kill SARS-CoV-2 in water, aerosols and on solid surface. However, the lack of an unified information and discussion on ozone-based inactivation and disinfection in current and previous pandemics and the absence of consensus on the main mechanisms by which ozone-based inactivation of pandemic causing viruses have hindered the possibility of establishing a common basis for identifying best practices in the utilization of ozone technology. This article reviews the research status of ozone (O3) disinfection on pandemic viruses (especially SARS-CoV-2). Taking sterilization kinetics as the starting point while followed by distinguishing the pandemic viruses by enveloped and non-enveloped viruses, this review focuses on analyzing the scope of application of the sterilization model and the influencing factors from the experimental studies and data induction. It is expected that the review could provide an useful reference for the safe and effective O3 utilization of SARS-CoV-2 inactivation in the post-pandemic era.
Keywords: Ozone, Pandemic, SARS-CoV-2, Inactivation, Kinetic model
Graphical abstract
1. Introduction
The current pandemic caused by novel coronavirus, also known as COVID-19 or severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has not only endangered the lives and health of people in the world, but also severely impacted the global economy. Contagious diseases have historically posed significant risks to public health. Many pathogens have posed serious threats to human health, including SARS (2002–2003), Ebola (2014–2016), H1N1 influenza (2009–2010), Zika virus (2015–2016) and COVID-19 (2019-) (WHO, 2020). Pathogens are expelled from the source of infection by specific means of transmission, eventually invading new susceptible individuals. To cut off the transmission route is to take specific measures to block the transfer of pathogens from the source of infection to the susceptible host, thereby preventing the occurrence of the disease.
Whether it is in the outbreak stage or the post-epidemic era, timely and effectively blocking the transmission route of the virus or other pathogen is a key step in controlling the epidemic spread. An effective means of virus inactivation is indispensable at this time. O3 has strong oxidizing properties and its disinfection is a bacteriolytic-grade while virus-killing method is completely sterilized with no residue. It has a wide range of sterilization and can kill viruses, bacteria, spores, etc. O3 in gaseous form can rapidly spread to the entire disinfection space. Sterilization effect and rate by using O3 is directly dependent on its concentration used. Gaseous O3 disinfection is common in the medical system, and it is considered to be one of the best biocides methods by the WHO (Blanco et al., 2021). The peroxidation effect of O3 damages the structure of bacteria and viruses, thus inhibiting their reproductive cycle and finally eliminating them (Manjunath et al., 2021). By comparing the disinfection effects of UV, O3, and chlorine, Kong et al. (2021) concluded that viruses were more resistant to UV radiation than bacteria, while coronaviruses were more resistant to UV light. Ozonation was an effective method of inactivating SARS-CoV-2.
The utilization rate of conventional O3 inactivation needs to be improved. It is particularly important to predict the mass transfer efficiency during the reaction process in the application system, yet the mechanism of this process is complex and difficult to control. Therefore, analyzing the disinfection process of O3 on viruses from the perspective of inactivation kinetic model is of great significance for the study and popularization of O3 disinfectants. The main purpose of O3 inactivation kinetic study is to determine the reaction rate constant of mass transfer by establishing relevant kinetic models, so as to evaluate the performance of O3 inactivation. However, there is currently a lack of detailed study on the inactivation kinetics of O3 disinfection on pandemic viruses (especially SARS-CoV-2). Meanwhile, the response relationship between the influencing factors of disinfection process and the rate constants of the kinetic model is also lacking in-depth analysis.
Based on the description of the mechanism of O3 inactivation, this review comprehensively summarized the application of O3 in the disinfection of pandemic viruses (especially SARS-CoV-2) from a new perspective on the O3 inactivation process combined with sterilization kinetics. The specific objectives of this review include: (i) discussing the application of O3 inactivation in the past pandemic and the scope of application of the sterilization kinetic model; (ii) clarifying the form and influencing factors of the inactivation kinetic model of O3 inactivation of SARS-CoV-2; and (iii) emphasizing the limitations of O3 disinfection applications and proposing synergistic O3 technologies and a new sterilization kinetic model form that can be applied to the efficient disinfection of SARS-CoV-2. It is expected that this review could provide certain guiding significance for the efficient, safe, and widespread application of O3 based technique for inactivation and disinfection of pathogenic microbes in the future.
2. The characteristics and application of ozone inactivation for harmful microorganisms
O3 has a higher redox potential (2.07 V) than other oxidants such as chlorine and potassium permanganate (Caniani et al., 2021), which is capable to oxidize most of the organic and inorganic matters. O3 is slightly soluble in water with a solubility of pure O3 of 641 mL/L at standard temperature and pressure, which is about 13 times higher than oxygen and 25 times higher than air (Egorova et al., 2015). In water, O3 can be transformed into more reactive oxygen radicals (ROS), such as hydroxyl radicals (OH•), with the effect of indirect oxidation. Its strong oxidizing ability makes it easy to destroy biological structures of bacteria, viruses, and other microorganisms in a very short time (Bayarri et al., 2021).
2.1. Inactivation mechanism of ozone for bacteria
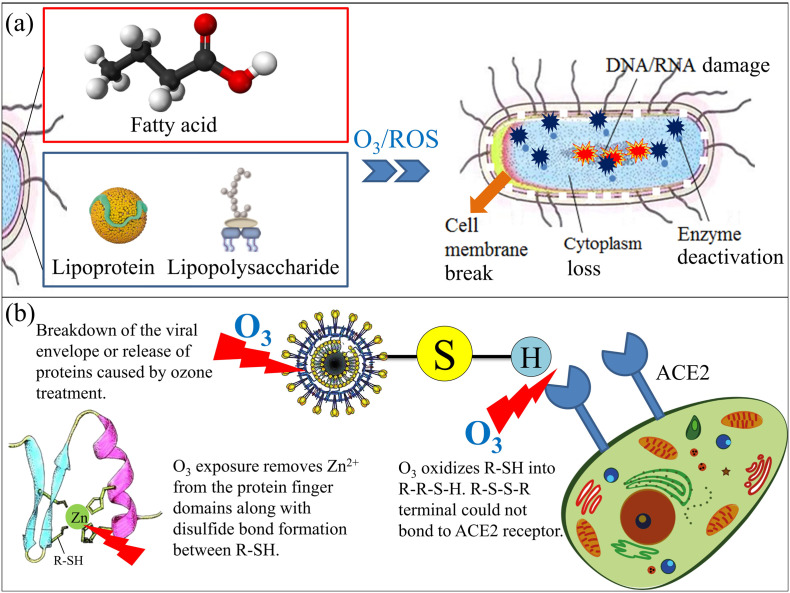
In different environmental media, because of the different active oxides produced by O3 decomposition, the main agents are different. When gaseous O3 is used to react in the gas phase, the direct attack of O3 molecules is the main approach. When ozonized water acts on the surface of the solid phase or in the liquid phase, the O3 molecules and active oxides cooperate to attack pathogens. At present, it is generally considered that the mechanism of O3 disinfection against pathogenic microorganisms is attributed to the diffusion of O3 to the surface of the microbial membrane, through the rapid reaction kinetics of O3 and pathogens. Therefore, O3 reacts with fatty acid in bacterial cell membrane, lipoprotein and lipopolysaccharide in bacterial cell wall, which degrades the membrane and shell structure and changes the permeability of the cell wall, resulting in cytoplasm and shell matrix lost (Sazhina et al., 2018). Furthermore, O3 penetrates membranes, and the reactive oxygen species generated by O3 can further attack genetic material (DNA and RNA) (Cataldo, 2006). These mechanisms can be summarized as follows: i) O3 directly acts on the cell membrane, increasing the permeability of the cell membrane and the reverse osmosis and outflow of the cytoplasm, thereby inactivating the cell; ii) O3 can act on the enzymes of some microorganisms (such as bacteria) to oxidatively decompose the glucose oxidase inside the bacteria, which inactivates the enzyme and hinders the metabolism of bacteria; and iii) O3 can attack genetic material, DNA and RNA, rendering them incapable of genetic transcription. The specific mechanisms are shown in Fig. 1(a).
Fig. 1.
Mechanisms of O3 inactivation.
2.2. Inactivation mechanism of ozone for viruses
Similarly, the mechanism of O3 action on virus is to directly destroy its DNA or RNA, inactivate reverse transcriptase, or disrupt the ability of the virus to bind to target cell receptors (Jiang et al., 2019). During inactivation, O3 destroys proteins and lipids, such as lipoproteins, lipids and glycoproteins, which are involved in redox reactions and are susceptible to oxidation. Meanwhile, O3 also destroys viral envelope glycoproteins and polymers necessary for attachment to host receptors (Manjunath et al., 2021), while the enveloped glycoproteins and the spike of the coronaviruses have the cysteine residues (Tizaoui, 2020). These cysteine residues are composed of sulfhydryl groups called thiol groups (R-SH), which keep a reduced state in the viruses and play an important role in the entry of the virus into the host cells and the fusion of the viral membrane with the host cell membrane. Therefore, these thiol groups will be oxidized into sulphonic acid residues (R-SO3-H) by O3, and the reaction can be enhanced when the temperature is increased (Manjunath et al., 2021). The R-S-S-R terminal (see Fig. 1(b)) could not bond to Angiotensin Converting Enzyme 2 (ACE2) which is a protein receptor located on the membrane surface of the host cell, then the virus could not enter into the host cell. Moreover, studies have shown that with the participation of zinc ions, the cysteine residues could be intact in the virus protein structures, which is significantly important in the viral activity (Lopez et al., 2008). As exposed to oxidative conditions, zinc ion is removed from the protein structure, the disulfide bond is formed between the cysteine residues, thus denaturize the structure of proteins and altering their solubility, consequently destroying the enzyme activity (Ataei-Pirkooh et al., 2021). O3 can also degrade the tryptophan-containing spike protein (Fernandez-Cuadros et al., 2020). O3 significantly alters the structure of viral envelope and genome, resulting in non-pathogenic dysfunction. The specific mechanisms are shown in Fig. 1(b).
3. Use of ozone in controlling the pandemic
3.1. Application of viral disinfection by ozone
A virus is a kind of non-cellular organism with small size and simple structure, containing only one nucleic acid, DNA, or RNA, which must be parasitic in living cells and reproduce by means of replication. Many epidemics caused by viruses have broken out in history, which have seriously endangered human health. During the 1943 epidemic in the U.S., poliovirus was detected in sewage effluent, which was believed to be associated with the New York polio epidemic (Melnick, 1947). Additionally, during the outbreak of H1N1 (swine) flu virus, influenza viruses were also found in wastewater (Sims and Kasprzyk-Hordern, 2020). During the last 20 years, many viruses have severely threatened the whole world, including highly-pathogenic avian influenza H5N1, influenza H1N1, human immunodeficiency virus (HIV), and SARS-Cov-2, which have an extremely high risk of infection (WHO, 2020). As a sterilizing agent, O3 is more effective with a shorter application time and lower regeneration of microorganisms compared to chlorine. Meanwhile, O3 disinfection is not limited by UV light sources, energy consumption, light utilization, operating costs, and maintenance costs for UV disinfection. Table 1 presents the details of O3 inactivation for some of these viruses. In general, O3 had a significant effect on different kinds of viruses. As previously mentioned, the inactivation effect of O3 is influenced by concentration, relative humidity, ambient temperature, characteristics of microorganisms.
Table 1.
Inactivation of epidemic viruses by ozone.
| Virus | Enveloped | O3 concentration | Temperature (°C) | Relative humidity (%) | Time (min) | Inactivation ratio (%) | Reference |
|---|---|---|---|---|---|---|---|
| Human immunodeficiency virus (HIV) | Yes | 4 μg/mL | – | – | 30 | 99.9999 | (Carpendale and Freeberg, 1991) |
| Influenza H1N1 | Yes | 20 ppm | 23–29 | 64–65 | 150 | 99.999 | (Tanaka et al., 2009) |
| 10 ppm | 210 | 99.99 | (Tanaka et al., 2009) | ||||
| Influenza H3N2 | Yes | 20 ppm | Room | 40–95 | 60 | 99.90 | (Hudson et al., 2009) |
| Yellow fever virus | Yes | 20 ppm | Room | 40–95 | 60 | 99.90 | (Hudson et al., 2009) |
| Poliovirus | No | 0–20 ppm | Room | 40–95 | 60 | 99.90 | (Hudson et al., 2009) |
| Adenovirus | No | 20 ppm | 22 | 40–95 | 60 | 99.90 | (Hudson et al., 2009) |
| Highly-pathogenic avian influenza H5N1 | Yes | 0.4–0.5 mg/L | 22 | – | 10 | 99.99 | (Lenes et al., 2010) |
| Hepatitis A HM175/18f | No | 4 ppm | 17 | 52 | 2 | 74.88 | (Brie et al., 2018) |
| SARS-CoV-2 | Yes | 6 ppm | 25 | 60–80 | 55 | 99.99 | (Yano et al., 2020) |
| Respiratory syncytial virus A2 | Yes | 20 ppm | 24 | 80 | 40 | 99.99 | (Blanchard et al., 2020) |
| African swine fever virus | Yes | 5 mg/L | Room | – | 1 | 99.90 | (Zhang et al., 2020) |
Since the early O3 sterilization was mainly applied in water treatment plant (WTP) or waste water treatment plant (WWTP), the familiar microbial medium was mostly water. It is expected to be widely used for disinfection in other media such as solid surfaces and aerosols due to the outbreak of various epidemics. Zhang et al. (2020) indicated that ozonized water could efficiently and rapidly disinfect the African swine fever virus (enveloped) with the concentration of 5–20 mg/L within 1 min. It was suggested that ozonized water of 5 mg/L or above could be extended to the whole disinfection of pig farms or other farms, as well as slaughterhouses and meat processing plants. Similarly, to assess the inactivation methods on a seasonal influenza H1N1 and H5N1 virus (enveloped), Lenes et al. (2010) treated raw water with different concentration of O3 (0.5 mg/L and 1 mg/L) for 10 min. The result was significantly showed that 10 min contact time was enough for O3 to inactivate 99.99% of H5N1 and H1N1 virus.
Recently, a number of studies have focused on how gaseous O3 inactivated viruses on the solid surface media. Blanchard et al. (2020) chose diverse materials as the carriers of influenza A virus A/WSN/33 (enveloped) and human respiratory syncytial virus A2 (enveloped) to demonstrate the high disinfection efficiency of O3. It was conducted that when the relative humidity was greater than or equal to 50 %, the O3 inactivation efficiency would be effectively improved. Moreover, a comparison of the disinfection efficiency of O3 gas between murine norovirus MNV-1 S99 and hepatitis A virus HM175/18f on fresh raspberries was performed (Brie et al., 2018). It was indicated that MNV-1 could be effectively disinfected (>3.3log) by O3 (3 ppm, 1 min), while HAV was useless neither in viral inactivation (<0.6log) nor in genome removal (<0.1log).
3.2. Influencing factors of ozone disinfection
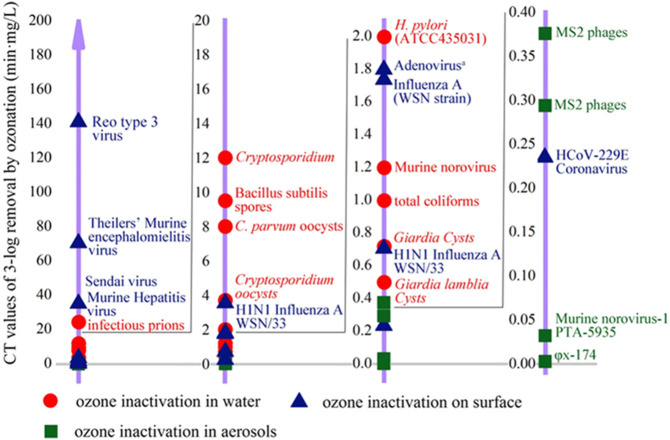
O3 has a broad-spectrum inactivation effect on various pathogenic microbes. However, due to the different biological structures of each microorganism, O3 has diverse inactivation effects on it. Furthermore, different media in which the microorganism is located will also lead to a different inactivation effect. Concentration-Time (CT) value, which is defined as the change in the concentration of disinfectant with the contact time, is a common index to evaluate the strength of O3 disinfection effect (USEPA, 1999). The lower the CT value is, the greater the disinfection ability will be, i.e., the time required to achieving a certain sterilization rate under the same conditions. In order to visually demonstrate the inactivation effect of O3 on microorganisms in different media under similar conditions, Fig. 2 was created by the following rules: i) water or environment temperature of about 20–25 °C; ii) the CT values being stipulated to 3-log inactivation condition, and iii) water pH within 6.5–7.5.
Fig. 2.
The CT values of ozone required for 3-log removal of different microorganisms. When the CT value was 1.8 min·mg/L, the virus with a reduction rate of 3log included Feline calicivirus, Herpes simples-1, influenza H3N2, Murine coronavirus, Poliovirus, Rhinovirus 1A and 14, Vaccinia virus, Vesicular stomatitis virus, and Yellow fever virus in additional to Adenovirus (Blanchard et al., 2020; Bolton et al., 1982; Casasola-Rodriguez et al., 2013; Ding et al., 2014; Dubuis et al., 2020; Finch et al., 1993; Gerrity et al., 2014; Hudson et al., 2009; Kanjo et al., 2000; Kekez and Sattar, 1997; Lee et al., 2021; Lev and Regli, 1992; Lim et al., 2010; Makky et al., 2011; Mik and Groot, 1977; Sato et al., 1990; Schulz et al., 2005).
When inactivation effect in water, aerosols, and on the surface are compared, a significant difference is observed: the CT values achieving 3-log disinfection of surfaces and water seem to be higher than that of in aerosols, the largest gap is even more than four orders of magnitude (Reo type 3 virus VSφx-174). In view of surface disinfection, the disinfection effect of O3 is affected by various factors, including room humidity, response time, type of materials, characteristics of microorganisms, ambient temperature as well as surface properties (Bayarri et al., 2021). Similarly, for water disinfection, response time, water temperature, characteristics of microorganisms, organic matter, pH value, and turbidity are all influence factors (Cristiano, 2020; Kong et al., 2021). Conversely, the main factors of aerosol disinfection are CT values, droplet size, and ambient humidity (Bayarri et al., 2021). The surface area of aerosols contacted with O3 molecules might be much higher. As a result, O3 could effectively attack these microorganisms in the droplet much easier than those in surface and water media, which lead to a lower CT value (Bayarri et al., 2021).
In Fig. 2, H1N1 influenza AWSN/33 exhibited two distinct CT values on surface inactivation. Under the conditions of O3 concentration of 20 ppm with a relative humidity of 80% at an ambient temperature of 24 °C, the CT value of inactivating 99.9% of the viruses on spun high-density polyethylene fabric for 18 min was 0.71 min·mg/L, while the CT value at same conditions on a cloth face mask for 90 min was 3.53 min·mg/L (Blanchard et al., 2020). The difference between surface properties of materials, such as roughness, size, thickness and position, determined the existence of viruses and disinfection efficiency (Bayarri et al., 2021). Likewise, O3 concentration was another key value to inactivation efficiency. In aerosols inactivation, with the same exposed time (0.017 min) and inactivation ratio (3-log) of MS2 phages, the CT values obtained by different O3 concentrations were distinct, that is 9000 ppm O3 led to 0.294 min·mg/L, 11500 ppm O3 led to 0.376 min·mg/L (Kekez and Sattar, 1997).
3.3. Microbial sterilization kinetic models
As one of the key theories in the study of sterilization technology, the kinetic model of microbial sterilization effect can quantitatively evaluate and predict the sterilization effect, which has theoretical guiding significance for the practical application of disinfection. There are various factors that affect O3 sterilization, including CT value, humidity, response time, ambient temperature, characteristics of microorganisms, etc. In order to explore the relationship between these influencing factors, it is necessary to involve the study of the kinetics of microbial sterilization. The first kinetic model was presented in 1908, which was a first-order reaction to model linear disinfection rate (Dalrymple et al., 2010; Rodriguez-Chueca et al., 2015). Generally, the inactivation process can be simplified as a linear kinetic model, and a linear expression relationship between virus inactivation and response time can be constructed on the basis that a population of microorganisms have the same sensitivity to disinfectants (Rodriguez-Chueca et al., 2015). However, the linear model also has lots of limitations that cannot fully express the relationship between various complex factors in the virus inactivation process. Under normal circumstances, the virus inactivation process is nonlinear, presenting different shapes, such as concave (shoulder) and convex (tail). The commonly used sterilization kinetic models are shown in Table 2 , which includes the log-linear model, the Hom model, the Weibull model, the log-logistic model, etc.
Table 2.
The common microbial sterilization kinetic models.
| Model | Expression | Parameters | Applied scope |
|---|---|---|---|
| Chick-Watson model | N0, Nt: the total number of colonies contained in the samples before and after the treatment; C: the concentration of the disinfectant; t: the contact time; k: the first-order disinfection rate |
The Chick-Watson model is used under the assumption that the microorganisms have the same stress resistance, while the logarithm of the decline of microorganisms exhibits a linear change with time. | |
| Hom model | n: the coefficient of dilution; m: an empirical constant; |
This model is suitable for fitting a sterilization curve with an initial lag (m > 1) or a tailing curve (m < 1). | |
| Weibull model | δ: time of first decimal reduction, representing the time required to reduce a logarithmic number of bacteria under certain conditions. p: the shape factor. When p < 1, the curve is concave upward, which means that the sterilization rate increases after the treatment time reaches a certain value; when p > 1, the curve is concave downward, indicating that the sterilization rate decreases after the treatment time reaches a certain value; when p = 1, the curve presents a straight line. |
The Weibull model can be used to describe system behavior with some degree of variability. The model assumes that resistance of microorganisms to bactericidal intensity is different, which is expressed as a nonlinear model of various linear and concave-convex curves. | |
| log-logistic model | A: the difference between the upper and lower asymptotes; σ: the maximum inactivation rate of microorganisms; τ: the time corresponding to the maximum inactivation rate. |
This model is established by considering the different sensitivities of various microorganisms to the sterilization process conditions. |
These models can be used to well validate and predict the sterilization effect of disinfectants. By increasing the parameters of the model, the goodness of the fitting results can be greatly improved. However, a large increase in the number of parameters can sometimes lead to meaningless results prediction.
During microbial inactivation, the appearance of a shoulder or a tail indicates poor fit of the first-order kinetics (Cullen et al., 2009). The shoulder appearing in the curve is often associated with cell clumping, sublethal injury, activation phenomena or multitarget inactivation for spores. Meanwhile, the appearance of the tail is related to the resistance heterogeneity within the microbial population, which is generally intrinsic to the microorganism or acquired during the ozonation (Manas and Pagan, 2005). During the treatment, the shoulder and tail in the curve may also be caused by incomplete initial mixing, rapid reaction between O3 and microorganisms, and environmental factors such as organic matters (Hunt and Marinas, 1997).
3.4. Comparison of kinetics between enveloped and non-enveloped virus
According to the organic structure, viruses can be divided into enveloped and non-enveloped viruses. The viral envelope is an envelope that warps around the viral protein capsid with the main function of helping the virus enter host cells and maintaining the structural integrity of the virion. If this special structure is destroyed, then the virus becomes inactivated (Kong et al., 2021). Viruses with lipid envelopes which are composed of membrane protein and lipid bilayer, such as HIV, Influenza H1N1, SARS-CoV-2, could be effectively inactivated by O3 treatment to impact those components (Bayarri et al., 2021). Studies have indicated that enveloped viruses are more sensitive to sterilizing agents (Lenes et al., 2010). An experiment carried by Murray et al. (2008) on a series of non-enveloped and enveloped viruses, including the PRB strain of influenza A virus, herpes simplex virus type-1, pointed out that virus could be inactivated during O3 exposure by lipid peroxidation and consequent lipid envelop and protein shell destroying, which made enveloped virus extremely sensitive to O3. Recently, Martins et al. (2020) found that SARS-CoV-2 could be disinfected by ozonated water with the destruction of the viral envelope instead of the virus genome. As to the non-enveloped virus, the structure of capsid proteins was firmer than that of the enveloped virus (Kong et al., 2021). Early studies concluded that non-enveloped virus-like Poliovirus 2 was damaged on the viral capsid by application of O3. Relative studies indicated that O3 inactivation of non-enveloped viruses (such as poliovirus) was related to the viral genome damage rather than the capsid protein destruction (Jiang et al., 2019). It was considered that the resistance of viral components to O3 was envelope <nucleic acid< capsid protein (Kong et al., 2021).
The infectivity of both enveloped and non-enveloped viruses depends on functional specific binding proteins on their surfaces. Thus, exposure of these viruses to O3 yields relevant information on the disinfection kinetics of biological structures reacting with O3. Due to structural differences, the O3 consumption of inactivated enveloped and non-enveloped viruses is diverse, while the applicable kinetic models of inactivation are also different. The study of bactericidal kinetic model is of great significance for understanding the inactivation mechanism of viruses. Table 2 showed the inactivation kinetic models of diverse kinds of viruses, including enveloped and non-enveloped virus. In general, enveloped virus (such as influenza A virus, vesicular stomatitis virus and SARS-CoV-2) conformed to the pseudo-first-order kinetic model, while non-enveloped virus (such as murine norovirus, infectious prions, poliovirus) was more suitable for the efficiency factor Hom (EFH) model.
During the outbreak of COVID-19, disinfectants nominated by the U.S. Environmental Protection Agency for inactivating coronaviruses were extensively tested against non-enveloped viruses, and a comprehensive evaluation of enveloped viruses was lacking. Moreover, among these various disinfectants, O3 has been more studied on non-enveloped viruses than that on enveloped viruses (Blanchard et al., 2020).
Five representative animal viruses, including three enveloped viruses (vesicular stomatitis virus, infectious bovine rhinotracheitis virus, influenza A virus) and two non-enveloped viruses (infectious canine hepatitis virus, polio virus type I), were exposed under different concentrations of O3 (0.00, 0.16, 0.64 ppm) (Bolton et al., 1982). For enveloped viruses, the O3 inactivation reaction conformed to a pseudo-first-order kinetic equation, i.e., the decreasing trend of enveloped virus was a first-order reaction under each O3 concentration. The susceptibility of the three enveloped viruses to O3 inactivation was different, depending on their different heat-resistant properties. At 0.64 ppm, the inactivation curves for all three viruses had varying degrees of lag periods (i.e., periods of little or no effect under O3 exposure). Vesicular stomatitis virus had a short and negligible lag phase, influenza A virus has a 6-hour lag phase, and infectious bovine rhinotracheitis virus has a 12 to15 hour lag phase. While, the lag period was longer at 0.16 ppm, which were 6 h, 8 h and 54 h, respectively. By strong contrast, non-enveloped viruses are relatively resistant to O3. Infectious canine hepatitis virus was only slightly inactivated after 42 h of 0.64 ppm O3 exposure, and remained unaffected by 0.16 ppm O3 exposure for 66 h, while polio virus was not affected by 0.61 ppm and 0.64 ppm O3 exposure for 60 h.
In reviewing the inactivation of surface-attached and airborne SARS-CoV-2 by O3, Farooq and Tizaoui improved the Chick-Watson model dominated by liquid-phase disinfection and extended it to gas-phase and solid surface disinfection. The model assumed that the effective concentration of O3 (effectively contacting the surface or virus in the air) was proportional to the O3 gas concentration in the air around the virus cutoff, from which a linear relationship between virus reduction and O3 CT value was derived (Farooq and Tizaoui, 2022). According to the literature review, the inactivation rate constant kv of SARS-CoV-2 is between 6.1 × 10−4 and 38.4 × 10−3 m3/(mg∙min), while the corresponding value range of CTgas was also relatively large. This showed that CTgas could affect the inactivation process of O3, and as mentioned above, related environmental factors (relative humidity, surface properties, etc.) would also have a certain impact.
Torii et al. (2020) reported that the linear relationship between log value of non-enveloped virus (such as coxsackievirus) inactivation and O3 CT value could only be observed at low virus inactivation rates. For most non-enveloped virus, the linear models were not suitable for fitting the kinetics of O3 sterilization, while the nonlinear model could better fit the kinetic change of virus inactivation by O3 treatment. It can be seen from Table 3 that the fitting effect of the Hom model was better than other models for the kinetics of O3 inactivation of non-enveloped viruses. Although the Chick-Watson model is simple and widely used, it cannot reveal the deep connotation of virus inactivation. Nonlinear models have the advantage of illustrating inactivation dynamics including shoulders and tails. When the inactivation trend does not fit the Chick-Watson model, the EFH model can better explain the sterilization kinetics with nonlinear inactivation characteristics especially when shoulder and tail effects are considered. In the determination of poliovirus kinetics, the R2 (the coefficient of determination) of the EFH model and the Chick-Watson model were 0.999 and 0.848 with 0.08 mg/L O3 concentration, 0.996 and 0.477 with 0.25 mg/L O3 concentration, respectively (Sangsanont et al., 2020). The EFH model could better explain the nonlinear disinfection kinetic relationship by changing the power factor of the contact time. The value of n parameter in the model was larger than the value of m parameter, indicating that the O3 concentration had a greater impact on the virus inactivation effect than the contact time. Meanwhile, in the comparison of O3 inactivation of infectious prions both in rendering plant dissolved air flotation treated wastewater, and the municipal final effluent, the EFH model, with a smaller error sum of squares (from 0.001 to 0.121) and a higher coefficient of determination (from 0.958 to 0.998), showed a higher fitness than the Chick-Watson model (Ding et al., 2014).
Table 3.
Kinetic models of O3 inactivation for different viruses.
| Microorganism | Enveloped? | Kinetic model | Notes | Reference |
|---|---|---|---|---|
| Vesicular stomatitis virus | Yes | [Va]t = [Va]0 ⋅ e−kobsd⋅t | The inactivation of enveloped viruses by O3 conformed to a pseudo-first-order kinetic model. The non-enveloped viruses were relatively resistant to O3 inactivation compared to the enveloped viruses. | (Bolton et al., 1982) |
| Influenza A virus | Yes | kobsd ≈ (kO3 ⋅ [03]n) + k37°C | ||
| Infectious bovine rhinotracheitis virus | Yes | |||
| Murine norovirus | No |
|
The efficiency factor Hom model and modified Chick-Watson model fit better than the Chick-Watson model. | Lim et al., 2010 |
| MS2 phage | Yes |
|
The delayed Chick-Watson model was the most suitable model to describe the inactivation process of MS2 phage by O3. | (Cai et al., 2014) |
| Infectious prions | No | C = C0e−k′t |
The efficiency factor Hom model fit better than other models in O3 inactivation of infectious prions both in rendering plant dissolved air flotation treated wastewater, and the municipal final effluent. | Ding et al., 2014 |
| Human adenovirus | No | All second-order inactivation rate constants were similar magnitude. | (Wolf et al., 2018) | |
| Echovirus | No | |||
| Coxsackievirus | No | The expand Chick-Watson model can be used to predict the reduction efficiency of viral heterogeneous consortia. | (Torii et al., 2021) | |
| Poliovirus | No |
|
The efficiency factor Hom model was better than the Chick-Watson model or the modified Chick-Watson model | (Sangsanont et al., 2020) |
| SARS-CoV-2 | Yes | The modified model applied for air and surface inactivation of O3. | Farooq and Tizaoui, 2022 |
4. The role of ozone-based inactivation and disinfection in COVID-19 curbing
Since WHO declared the COVID-19 pandemic as a global health crisis in 2020, to date, the cumulative number of confirmed cases of COVID-19 in the world is 603 million, and the cumulative number of deaths was 6.4 million (WHO, 2022). Severe forms of COVID-19 can evolve into pneumonia, characterized by acute lung injury and acute respiratory failure caused by acute respiratory distress syndrome. COVID-19 is caused by SARS-CoV-2, a new virus composed of protein and RNA. The main transmission routes of SARS-CoV-2 are believed to occur via close contact and respiratory droplet generated through sneezing, coughing, and other sources (Manjunath et al., 2021). Studies indicated that SARS-CoV-2 could even persist on the surface for up to 28 days (Riddell et al., 2020). Thus, effective disinfection methods are imperative and necessary. It is confirmed that the penetration of O3 is more than that of most liquids used as cleaning agents (to prevent infection) (Manjunath et al., 2021).
Moreover, compared with other disinfection technologies, O3 inactivation can not only be utilized in water but also has a wide range of disinfection application prospects in solid and gas media. O3 technology has potential applications for SARS-CoV-2 inactivation in water, gas, and solid phases. O3 disrupts the reproductive cycle of SARS-CoV-2 by per-oxidizing infected cells and destroying the viral capsid. At the same time, cells that have been infected by the virus are eventually exposed to an oxidative environment in the host cell and destroyed in this process (Manjunath et al., 2021).
4.1. Cases studies of ozone based inactivation and disinfection
4.1.1. Ozone disinfection of SARS-CoV-2 in environment
Once excreted by various routes from the body of infected person, SARS-CoV-2 contained in aerosols (droplet, sputum, etc.) remains suspended in the air for some time without any food, which depends on the size of the liquid or solid particles it may be attached to (Eslami and Jalili, 2020). Due to the increasing incidence of virus-containing aerosols, indoor ambient air may contain SARS-CoV-2, so the virus inevitably threatens people in the process of daily activities. In addition, the virus existing on the surface of objects may be disturbed and released into the air again. Therefore, the disinfection of the indoor environment is particularly important to human health. Gaseous O3 penetrates effectively into any part of a room, especially those places hard to reach with ordinary disinfecting liquids or manual cleaning methods, and it decays rapidly into oxygen with a half-life of about 20 min, with no toxic by-products (Alimohammadi and Naderi, 2020). O3 gas is believed to have potential in the viral inactivation of contaminated spaces during the COVID-19 pandemic.
Through a comparative study of existing experiment data, Yao et al. (2020) indicated that as the level of O3 concentration increased from 48.83 to 94.67 μg/m3 accompanied by relative humidity and temperature increase, the transmission of SARS-CoV-2 was reduced. Although the concentration of O3 gas in the atmosphere was low without inactivation effect, it still has a certain inhibitory effect on the air transmission of SARS-CoV-2, so as to confirm the theoretical feasibility of active air disinfection to block the air transmission of SARS-CoV-2.
De Forni et al. (2021) assessed the disinfection effect of O3 generated by a new disinfection device—ICON3 on SARS-CoV-2 in an indoor environment with a low level to prevent the harmful risks to human/ animal health in a high concentration of O3. It was revealed that >99% of SARS-CoV-2 was inactivated with a low concentration of O3 (about 3.18 ppm) in 20 min. Meanwhile, the O3 disinfection effect of ICON3 was validated in rooms of different volumes (15, 30, 60 m3), which showed a linear relationship between the O3 concentration maintenance time and the room volume. Different sizes of the SARS-CoV-2 droplets (10, 3, 0.5 μL) were also exposed to different concentrations of O3 (5.44–1.47 ppm) for 20 min, which presented a significant inactivation result. Studies have shown that severe air pollution accompanied by low wind speed could keep virus particles in the air for a more extended period (Coccia, 2020). Although outdoor disinfection is largely unregulated, its urgency has somewhat hindered risk assessment in public health settings. Outdoor spaces that require extensive spraying must consider the possible secondary adverse effects of spraying. O3 can avoid these problems. Albert et al. (2021) evaluated the application effect of unmanned aerial vehicle spraying of O3 water solution. By optimizing the vehicle characteristics, 97% outdoor surface coverage could be achieved. Atmospheric O3 concentrations were maintained within background levels (<0.004 ppm) during spraying operations with 1 mg/L O3 water solution. Under the O3 concentration of 0.75 mg/L, two strains of SARS-CoV-2 were efficiently inactivated in only 5 min, while the O3 concentration of 0.375 mg/L achieved inactivation of 82–91.5%. These results provided an efficient way to achieve the necessary balance between safety and virus elimination in a public health environment using aqueous O3 solutions in virus-infested areas.
4.1.2. Ozone based inactivation and disinfection of SARS-CoV-2 on solid surface
Walls, furniture, and other surfaces may also be contaminated due to air deposition or direct contact with pollution sources and disease-causing microorganisms so contact with such surfaces will be threatened by pathogenic microorganisms. Therefore, surface disinfection is also particularly important during the fight against COVID-19. When SARS-CoV-2 is deposited on the different types of surfaces, its activity depends on the material it is attached to, and the duration of its infection causing behaviors is still being studied (Eslami and Jalili, 2020).
Franke et al. (2021) tested the disinfection effect of O3 on bacteriophage Φ6 using an automatic room decontamination system, which was used instead of SARS-CoV-2 for safety. Three kinds of different surface materials (ceramic tile, furniture board, and stainless steel) carried of the surrogate virus were placed at two levels. After the disinfection process with the ozone-based technique, the mean reduction factors of ceramic tiles, stainless steel, and furniture board were 6.15 log, 5.31 log, and 4.29 log, respectively. At the same time, Franke et al. (2021) also conducted a control experiment with high relative humidity (90%), and the results showed that without adding O3 as a disinfectant, the virus activity decreased slightly, which indicated that O3 extinguishing virus could only be achieved by combining with humidity.
To elucidate the inactivation effect of O3 gas on SARS-CoV-2, Percivalle et al. (2021) chose eight different surfaces, including stainless steel, painted and not painted aluminum, Plexiglas, glass, plastic, FFP2 mask, and surgical gown, spotted droplets containing SARS-CoV-2 copies, placed these samples in an environmental controlled box, exposed the samples with different concentration of O3 gas (0.5, 1, 2 ppm) for 40 min and 60 min. Interestingly, the disinfection efficiency of gaseous O3 did not proportionate with its concentration and did not depend on the type of surface. Three concentrations of O3 gas effectively inactivated SARS-CoV-2 on the eight different characters within 40 min. Additionally, control experiments on dry surfaces have shown that a specific ratio of humidity and hydration within droplets ensured the survival of SARS-CoV-2 on solid surfaces.
Personal protective equipment is a significant barrier to ensure the safety and health of medical staff. In the case of a shortage of medical resources, the reuse of personal protective equipment is a major test for inactivating SARS-CoV-2. Clavo et al. (2020) indicated that the utilization of O3 treatment had an excellent inactivated effect on personal protective equipment contaminated with SARS-CoV-2. According to his study, the virus disinfected effects of several O3 treatments of SARS-CoV-2-contaminated protective clothing and FFP2 masks (filtering face masks with a minimum efficiency of 92%) were evaluated, including changes in exposure time and O3 concentration. Meanwhile, it was also explored whether the inactivation effect of O3 on SARS-CoV-2 was related to relative humidity. When the O3 concentration was higher than 2000 ppm, SARS-CoV-2 on the surface of contaminated personal protective equipment could be disinfected in <10 min. When the O3 concentration reached 10,000 ppm, it could disinfect SARS-CoV-2 after only 30 s. At lower O3 concentrations (4–12 ppm), these effects depended on relative humidity conditions.
4.1.3. Ozone based inactivation and disinfection of SARS-CoV-2 in water
According to previous studies, SARS-CoV-2 RNA has been found in the influent of WWTP in many regions of the world. There was a positive correlation between the number of infected patients and the virus concentration (Ahmed et al., 2020; Hata et al., 2021; Nemudryi et al., 2020). Similarly, as the front line of the epidemic, the wastewater from hospitals contains many pathogenic microorganisms. The public would be at risk of infection if discharged the wastewater without proper treatment. Especially against the backdrop of the COVID-19 pandemic, reducing health risks to the public and the environment is of great importance. Through a qualitative and quantitative assessment from 422 relative articles, Verinda et al. (2021) concluded that SARS-CoV-2 was still detected from several treated wastewater. Therefore, it is necessary for domestic and hospital wastewater to be adequately disinfected and treated before being transported or discharged. Zhang et al. (2021) concluded that coronaviruses were slightly resistant to UV disinfection but were quickly inactivated by O3 and chlorine.
Tran et al. (2021) indicated that SARS-CoV-2 in aquatic media was more sensitive to water temperature, pH value, and the presence of disinfectant in the water. According to studies of the Water Environment Federation and the United States Environmental Protection Agency, SARS-CoV-2 has not been detected after the disinfection process both in drinking water and wastewater (USEPA, 2020; WEF, 2020). Foladori et al. (2022) similarly stated that through primary, secondary and tertiary treatment, the concentration of SARS-CoV-2 and other viruses in the influent of WWTP could be effectively decreased, from 20 to 3.0E+06 GU/L (Genomic Units/L) in the influent to 2.50E+05 GU/L after secondary biological treatment to negative concentration in the effluent, some of which was the attribution of disinfection. According to Morrison et al. (2020), O3 may be a highly effective disinfectant to inactivate SARS-CoV-2 in water. When the concentration of O3 was 0.2–0.8 ppm in the water, SARS-CoV-2 in virus stock was significantly reduced within 1 min with 2 log reduction (Martins et al., 2020). The SARS-CoV-2 RNA quantification in supernatants treated by ozonated water showed a remarkable reduction in genome copies of progeny SARS-CoV-2 per copy of housekeeping gene RNase-P, which suggested that ozonated water inactivated the structure of SARS-CoV-2 rather than its genome at a low concentration. Volkoff et al. (2021) tested the inactivation effect of O3 on SARS-CoV-2 sourced from municipal wastewater in USA. The results showed that the number of SARS-CoV-2 RNA gene copy reduced 2% and 11% at an average O3 concentration of 4.5 and 9 ppm for 60 and 90 min, respectively, which pointed out a considerable reduction of SARS-CoV-2 RNA with the treatment by O3. These existing studies demonstrated a significant effect of O3 treatment on the decrease of SARS-CoV-2 RNA in the aquatic environment. A study on a WWTP using the activated sludge process showed that the conventional process was less effective for SARS-CoV-2 removal, while O3 oxidation could reduce the number of SARS-CoV-2 in the effluent (Westhaus et al., 2021). This indicated that O3 can be either used as an effective strategy for wastewater disinfection during COVID-19 pandemic, or combined employed with other processed (Advance Oxidation Processes, AOPs). However, the feasibility of this technology involves safety requirements, sewage volume, availability of disinfectants, investment and service costs, post-maintenance, etc. (Wang et al., 2020). Therefore, the prospect of O3 application needs to be further explored.
4.2. The limitation of ozone application
4.2.1. The side-effect on the environment
Although O3 inactivation presents excellent safety and effectiveness, there are still concerns about the human health consequences of continued exposure to O3, with the limited and immature technological means to actually guarantee the O3 levels below the toxicity threshold as well as the best inactivation efficiency. O3 is a toxic gas in which toxicity is mainly manifested in the damage of the gaseous O3 molecules in the air to the human's respiratory tract, making it one of the main components of air pollution. For instance, when the O3 concentration in the air is 0.1 ppm, it will stimulate the upper respiratory tract and urinary tract of the human body. When it was 1.0–2.0 ppm, it could cause rhinitis, cough, nausea, retching, and asthma. When the concentration was 2–5 ppm, inhalation for 10–20 min will cause breathing difficulties, bronchospasm, and retrosternal pain. Furthermore, inhalation for 4 h at 10 ppm O3 could cause death, while inhalation at 50 ppm could cause death within minutes (Zanardi et al., 2015). Moreover, several authorities such as the U.S. Environmental Protection Agency, the Occupational Safety and Health Administration, and the Food and Drug Administration, have specified the O3 limitation of human exposure of 0.08 ppm for 8 h, 0.10 ppm for 8 h, 0.05 ppm for 8 h, respectively (Farooq and Tizaoui, 2022; Quevedo-león et al., 2020). Such low O3 levels in a closed laboratory seem feasible. Yet, in any living or working place, it seems to be a challenge to maintain a constant flow of O3 for an extended period of time while air exchange continuing, like hospitals, hotels, station waiting halls, etc., where people are mixed and relatively concentrated. Although O3 has great application potential in the air inactivation on SARS-CoV-2, its characteristic is not suitable to use in the crowd during normal business hours. The air inactivation could be adopted by O3 combined with other disinfection methods when the business is closed.
4.2.2. The detrimental effects on the surfaces
Although O3 inactivation has its own advantage over other disinfection methods in surface application as a strong oxidizing agent, the damage of O3 to surface materials is beyond doubt. Unsaturated organic compounds adsorbed on the surface of solid materials or constituted by themselves can react with O3 to generate oxidation byproducts, which have adverse effects on human health and air quality (Shen and Gao, 2018). Under O3 exposure, the molecular chain network structure and cross-linking points of natural rubber products will be destroyed, and the physical properties such as tensile strength and elongation at break of the materials will be reduced accordingly. Thus the surface morphology, molecular structure and mechanical properties of the materials will be significantly changed, resulting in the aging and deformation of natural rubber products (Zheng et al., 2021). O3 is also capable to have irreversible chemical reactions with the unsaturated bonds and reducing groups in the chemical structure of plastic materials, resulting in the oxidative degradation of plastic materials and therefore value loss (Singh and Sharma, 2008). Moreover, plastic materials exposed to O3 would release microplastic particles after changes in surface morphology, causing secondary pollution. As the exposure time increasing, the release abundance of microplastic gradually raised, while the surface crack extension and fragmentation caused by O3 exposure were the main factors of microplastic generation (Zhang et al., 2022). High concentrations of O3 could react with unsaturated organic matters of indoor materials, causing material fading, generation and release of ketones, aldehydes and other substances (Poppendieck et al., 2007).
4.2.3. Restriction in aqueous environment
When using O3 for water disinfection, it is also important to consider the impact of the resultant disinfection byproducts on the environment, human health, etc. O3 can oxidize and decompose organic matters in water bodies, generating organic byproducts including aldehydes, ketones, carboxylic acids, lipids and others, most of which can be biodegraded and removed (Greene et al., 2012). Most organic byproducts can be removed by biodegradation, among which acids are harmless to human, but aldehydes have been proven to be carcinogenic (Feron et al., 1991), and the drinking water quality index specifies formaldehyde as 0.9 mg/L by WHO (Slompo and Silva, 2019). When bromide is contained in water, O3 oxidation produces bromate and a variety of brominated organic byproducts (Joshi et al., 2022). Bromate has also been proven to be carcinogenic (Moore and TaoChen, 2006) and existence in drinking water with limits of 10 μg/L by European Union (Gunten, 2003). The amount of O3 should be controlled within a reasonable range. Since excessive O3 residue after disinfection is not only toxic to fish and other aquatic animals, but also damages fish scales, gills and other body structures, destroys the immune defense barrier composed of mucus and microbial communities on the fish body surface, resulting in a risk of fish diseases (Jhunkeaw et al., 2021; Paller and Heidinger, 1980).
4.3. Kinetic models for SARS-CoV-2
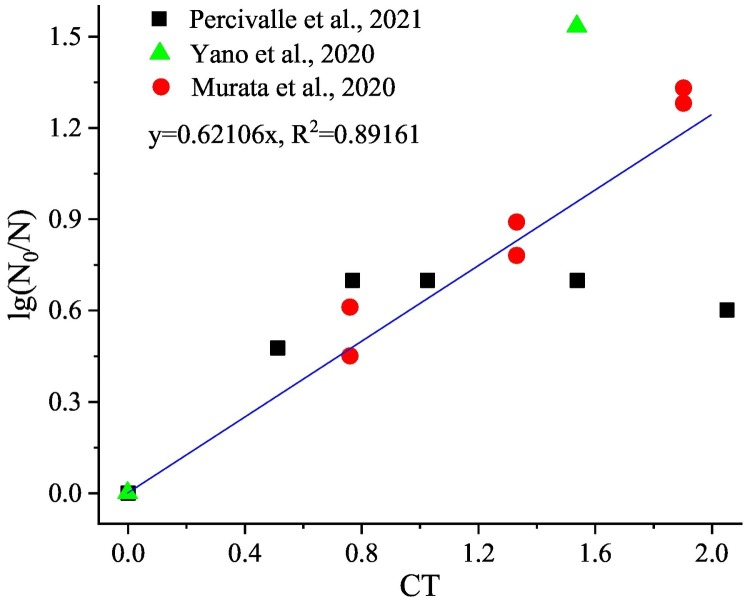
Since most of the current studies are focused on O3 inactivation of SARS-CoV-2 on solid surfaces, the relevant parameters selected here are derived from O3 inactivation experiments on solid surfaces. Studies have verified that SARS-CoV-2 is an enveloped, positive single-stranded RNA coronavirus (Xia et al., 2021). The inactivation kinetics of O3 on enveloped viruses conformed to the first-order kinetic model. Therefore, under the exclusion of material properties (selecting stainless steel as the contact material), the correlation between different concentrations, relative humidity (RH), contact time and log-reduction is analyzed with data derived from the relevant studies of Percivalle et al. (2021), Yano et al. (2020), Murata et al. (2021). It is shown that the log10-reduction value is significantly positively correlated with the CT value, and the Pearson correlation coefficient is 0.800 (p < 0.01). The log10-reduction value is not correlated with RH, and the Pearson correlation coefficient is 0.046. Furthermore, through linear regression analysis, the following relationship could be obtained:
| (1) |
where, N0 and N were the number of SARS-CoV-2 units at certain time and initial time, with a good correlation (R2 = 0.89161). The specific distribution of the values is shown in Fig. 3 .
Fig. 3.
Relationship between lg(N0/N) and CT value for O3 inactivation of SARS-CoV-2 (Murata et al., 2021; Percivalle et al., 2021; Yano et al., 2020).
The above resultant is the same as the study of Farooq and Tizaoui (2022) which gave a similar linear formula, and the Eq. (1) of this study is similar to the form of the Chick-Watson model in the kinetic of inactivation reaction. From the concept of CT value, it could be recognized that the influence of the combined effect of O3 concentration and contact time on the virus inactivation rate was greater than the effect of concentration or time alone. There was a pseudo rate constant k in the Chick-Watson model and Eq. (1). This parameter was affected by inactivation conditions including RH, surface material properties, temperature, etc., which would alter the mass transfer efficiency of O3, and finally react to the O3 concentration, then influence the sterilization effect.
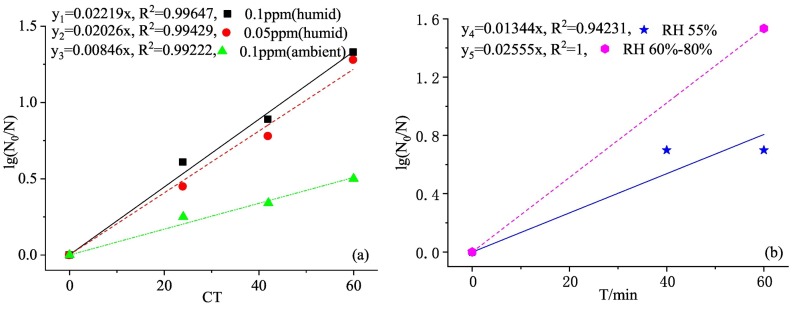
Fig. 4 shows the log-linear model and its fit degree of O3 inactivation of SARS-CoV-2 at different O3 concentrations and RH. It could be seen from Fig. 4(a) that under the same RH (80%), the k value of the fitting curve with high O3 concentration (y1) is greater than that with lower concentration (y2), and its R2 is larger with better fitting effect. Similarly, it could be known from Fig. 4(b) that under the same O3 concentration, the k value of the fitting curve with high RH (y4) is greater than that with low RH (y5), and the comparison of y1 and y3 in Fig. 4(a) could also obtain the same conclusion. The response fitting effect with high RH is better with greater R2.
Fig. 4.
(a)The log-linear model and fit degree of O3 inactivation of SARS-CoV-2 at different concentrations (Murata et al., 2021). (b) The log-linear model and fit degree of O3 inactivation of SARS-CoV-2 at different relative humidity (RH) (Percivalle et al., 2021; Yano et al., 2020).
The O3 concentration was proportional to the inactivation reaction rate. High concentration of O3 could provide more O3 molecules and OH• to react with the viral lipid envelop, increasing the reaction rate. Zucker et al. (2021) used pseudoviruses to replace SARS-CoV-2 to test the inactivation effect of O3 on solid surface. Under the O3 exposure of 1000 ppm for 20 min, 100 ppm for 30 min and 30 ppm for 40 min, the virus inactivation rate could reach 95%. The inactivation rates of three concentrations of O3 (30 ppm, 100 ppm, 1000 ppm) within a 30-min contact time could reach 90%, 94% and 99%, respectively. Therefore, increasing the O3 concentration can effectively reduce the virus infectivity. Similarly, Inagaki et al. (2020) found that in the process of studying the rapid inactivation effect of O3 water against SARS-CoV-2, when the concentration of O3 water was 1, 4, 7 and 10 mg/L, the virus inactivation rates for 5-second contact time were 81.4%, 93.2%, 96.6% and 96.6%, respectively, whereas the virus inactivation rates for 10-second contact time were 75.4%, 93.2%, 96.6% and 97.5%, respectively. High concentration O3 water was very effective for virus inactivation with a dose-dependent relationship. Although the effect of O3 water on microorganisms may depend on the external conditions, high concentrations of O3 water (≥10 mg/L) would produce sufficient inactivation even in the presence of inhibitors (such as proteins).
Additionally, the increase in air humidity promotes the probability of the reaction between water molecules and O3 and the generation of OH• with stronger oxidizing ability, which further improves the disinfection efficiency. Moreover, during gas phase disinfection, O3 needed to be transferred from the gas phase to the liquid phase before it could attack the virus. The strong reactivity of O3 made the reaction completely take place in the mass transfer liquid membrane during the entire mass transfer process. Studies have shown that the combination of low concentrations of O3 and high RH could form a powerful disinfectant with an inactivation rate as high as 99% (Grignani et al., 2020). Clavo et al. (2020) discussed the effect of RH on O3 treatment of personal protective equipment contaminated with SARS-CoV-2. At RH of 63% and O3 concentration of 8–12 ppm, virus amplification was detected in both protective clothing and masks after exposure for 30 and 50 min, while increasing RH to 99%, reducing O3 concentration to 4–6.5 ppm. The viruses in the protective clothing were completely eliminated after the exposure of 30 and 50 min. Tizaoui et al. (2022) evaluated the effect of CT and RH in synergistic inactivation of SARS-CoV-2 and concluded that the diffusion rate of O3 on the liquid surface was 100 times than that of the dry surface, i.e., in order to achieve the same sterilization effect, the amount of O3 gas used to inactivate the virus was 100 times than that of the liquid virus. When verifying the inactivated effect of different RH (17%–70%) on drying SARS-CoV-2, the author found that with the increase of RH, the virus inactivation rate also increased accordingly. The RH threshold was 13%–70%, and O3 could not effectively kill the virus both below or above this range. Mazur-Panasiuk et al. (2021) also verified that the O3 disinfection effect of SARS-CoV-2 substitutes on solid surface was different under different RH, i.e., the virus inactivation rate fluctuated between 0 and 0.9 log under low RH, while it could rise to 0.6–2.1 log under high RH.
4.4. Influencing factors of disinfection effects
For different medium, O3 concentration and contact time are the key factors affecting the effectiveness of O3 disinfection. Generally, in a certain contact time, the higher the O3 concentration is, the better the disinfection effect will be (Cordoba-Lanus et al., 2022), while in a certain O3 concentration, the longer the contact time is, the better the disinfection effect will be (Volkoff et al., 2021). However, there is a threshold of O3 concentration and contact time for the effect of inactivation on pathogenic microorganisms. Too low O3 concentration might have no inactivation effect (Pelleu et al., 1974), while too high concentration will result in an insignificant increase in reaction efficiency and waste (Kowalski et al., 2003). Similarly, too short contact time will make the reaction inadequate. While for too long contact time, the inactivation curve will produce a tailing, i.e., the change in O3 inactivation rate will slow down or even remain unchanged with increasing time, while the O3 inactivation efficiency will increase rapidly with time only during the inactivation reaction period (Huang et al., 2012). Criscuolo et al. (2021) proposed an optimal O3 concentration (4 ppm) and disinfection time (30 min) combination to reach an effective inactivation (>90%) of SARS-CoV-2 on almost all tested materials, while the low concentration (0.2 ppm) that was non-toxic to humans required 4 times as much time to achieve the same reduction rate.
RH is not only the important factor affecting the survival of SARS-CoV-2 (Noorimotlagh et al., 2021b), but also a critical factor to affect the O3 inactivation. In the study of Tizaoui et al. (2022) when the RH was 99%, the inactivation rate of protective clothing with 30 and 50 min of O3 exposure was 100%, but SARS-CoV-2 on the mask was not effectively disinfected. The likely reason was that the higher humidity created a higher level of condensation on the surface of the mask, hindering the contact of O3 with the virus. In daily disinfection occasions (such as hospitals, stations, campuses, etc.), extremely high humidity (99%) is neither common nor feasible. It will both cause surface condensation affecting the inactivation effect, and may cause rust or even damage to the equipment. Other studies have also pointed out that extremely high RH may reduce or increase O3 inactivation (Volkoff et al., 2021). Bayarri et al. (2021) reported that it was best to control RH at 70–90% when using O3 to inactivate viruses. Tizaoui et al. (2022) pointed out that 13–70% RH was the best threshold for O3 inactivation of SARS-CoV-2. Thus, combined with the characteristics of the sterilization kinetic models, the inactivation rate constant k with different RH can be given. The change rule and internal mechanism of the k parameter influenced by RH can be clarified. Then the effective RH threshold of virus inactivation can be finally determined. Therefore, the relevant environmental factors should be effectively integrated to optimize the optimal dosage of O3. The synergistic effect of CT and RH is the key to O3 inactivation of viruses, so it is possible to explore the possible changes of CT values at lower levels of humidity, and to formulate feasible O3 inactivation conditions through the reasonable distribution of (CT, RH).
In addition to the above main influencing factors, the O3 inactivation efficiency is also influenced by temperature, environmental media, etc. Temperature is the key factor of O3 stability in air (Epelle et al., 2022), as well as the decisive factor to the solubility of O3 in water (Ziyaina and Rasco, 2021), which can indirectly affect the inactivation efficiency of O3. Studies have found that SARS-CoV-2 exists in different environmental media (water, ambient air, solid surfaces) and thus the O3 inactivation differs considerably in different media.
5. What can we learn so far from COVID-19 pandemics regarding the role of ozone application?
5.1. Strategies for ozone application in environment
It has been demonstrated that SARS-CoV-2 could be used in various solid surfaces, air and water environment with a certain O3 concentration and in short time to achieve ideal effects. During the COVID-19 pandemic, the use of this tolerated, sustained O3 exposure to protect individuals from infection is justified.
In the laboratory environment, O3 does have a strong positive effect on the inactivation of SARS-CoV-2, yet in practical application, the efficiency of O3 cannot be quantitatively inferred and absolutely effectively defined. The essential difference between the laboratory scene and the practical environment lies in the degree of control of variables, such as relative humidity, temperature, environmental airtightness, etc. Therefore, in specific life scenarios close to the laboratory environment, the inactivation effectiveness of O3 on SARS-CoV-2 is still positive. At present, O3 disinfection is mainly based on static disinfection. High concentrations of O3 not only endanger human health corrode building materials but also have an impact on the environment. The longer disinfection time and the unattended disinfection method limit the application scope of O3 disinfection. In addition, the static disinfection method cannot keep the microbial concentration in the indoor air at a very low level for a long time. At the same time, the space is inconvenient to use, which also limits the application scope of O3 disinfection. O3 is a gas with inferior stability, so if the low O3 concentration in the space cannot be maintained accurately in real-time, the so-called inactivation becomes meaningless.
Blanco et al. (2021) gave some suggestions on the application of O3 to inactivate SARS-CoV-2 indoors. Using O3 at a concentration of 10–20 mg/m3 for 10–50 min can disinfect items in small chambers, while for large rooms, O3 at a concentration of 30–50 mg/m3 can be used for 20–30 min. O3 of high doses is suited in time-critical situations. If time permits, low doses of 5–10 mg/m3 can be uses to disinfect for 4 h. Since the damage caused by O3 to human respiratory system, lower-dose O3 of <0.1 mg/m3 is useful to stop the spread of SARS-CoV-2 in places where people are present. For high-risk areas like hospital, it is recommended to disinfect multiple times a day, and additional cleaning of high-risk environmental surface in hospitals should also be considered, along with enhanced disinfection operations for work equipment such as keyboard, phone, scanner and other object surface (Seif et al., 2021).
It has been shown that O3 can be efficiently decomposed using metals and their oxides (e.g. TiO2, SiO2, Al2O3) with catalyst carriers (e.g. activated carbon) (Li et al., 2020). Huang et al. (2012) have used MnO2/Al2O3 and MnO2/AC as catalysts to test their ability on treating O3 tail gas, and the results were <10 ppb, which was lower than the O3 limitation concentration recommended by the relevant standards. Based on this, a degradation device containing catalysts can be used to decompose residual O3 in the enclosed room after disinfection until it is not measurable. Meanwhile, opening windows for ventilation is also a good choice, from which excess O3 gas indoors will be discharged outdoors with the airflow. In addition, studies have shown that SARS-CoV-2 can be transmitted through indoor air, and improving indoor ventilation systems in high-traffic places such as hospitals can effectively reduce the airborne transmission of the virus (Noorimotlagh et al., 2021a).
5.2. The wiser use of ozone in the COVID-19 pandemic
The COVID-19 epidemic has had a profound impact on human society. The experience in controlling and blocking the spread of COVID-19 has confirmed that the existence of environmental vector pathogens has a significant risk of secondary transmission. Ongoing studies have shown that O3 can be effectively applied to the disinfection of pathogens on water, air, and object surfaces and has many advantages. Therefore, in the post-epidemic era, achieving efficient and wiser use of O3 for pathogens such as viruses, including SARS-CoV-2 control, is still the focus of attention.
5.2.1. Synergistic techniques of ozone disinfection
In practical applications, although high concentrations of O3 have a good inactivation effect on microorganisms, O3 concentrations exceeding the threshold will endanger human health and corrode materials. Moreover, the inactivation efficiency of O3 is relatively expensive. Therefore, a reasonable ozone-assisted collaborative disinfection system has been gradually paid more attention. To improve the disinfection effect with relatively low O3 concentration and shorten the disinfection time, the synergistic inactivation technology combing O3 disinfection with other physical (such as ultraviolet) or chemical disinfection methods (such as hydrogen peroxide) should be paid more attention. The specific inactivation effect is shown in Table 4 .
Table 4.
Inactivation effect of ozone synergistic technologies.
| Methods | Pathogens | Inactivated effect | Experiment condition | Reference |
|---|---|---|---|---|
| O3 + H2O2 | Salmonella enterica serovar Typhimurium | 5.2 log CFU/fruit on smooth surface; 4.2 log CFU/fruit on the stem scar | gaseous O3 with 800 and 1600 ppm; aerosolized H2O2 generated from 2.5%, 5% and 10% H2O2; 30 min reaction | (Fan et al., 2020) |
| O3 + UV | Escherichia coli | 100 % killing rates in <5 min | contaminated water; UV types of 15 W LP and 150 W MP; O3 levels of 1 and 2 ppm | (Tawabini et al., 2013) |
| H2O2 + UVC + O3 | Aspergillus spores | 1.23 log reduction on lemon surface | H2O2 3.5%; UVC 2.1 mW/cm2; O3 2g/h | (Hasani et al., 2019) |
| Ultrasound + O3 | Escherichia coli | 99 % inactivation after 4 min | Ultrasound 100 W; O3 1 mg/L, 160 W, 10% gas flow | (Al-Hashimi et al., 2015) |
| O3 + TiO2 | Escherichia coli | 6.22 log reduction after 10 min | freshwater; TiO2 1 g/L; O3 102 mg/L | (Rodriguez-Chueca et al., 2015) |
| UV + Ag-TiO2 + O3 | Escherichia coli | 2.41 log reduction with 0.5 s | UV 6.5 mW/cm2; O3 9.84 mg/L; 20 °C; pH 8.01; water flow rate 500 L/h; | (Wu et al., 2011) |
In the comparative experiment of the AOPs for the inactivation of Salmonella typhimurium on tomatoes, Fan et al. (2020) found that both O3 alone reduction (<0.6 log CFU/fruit on smooth surface and the stem scar) and aerosolized hydrogen peroxide alone reduction (2.1 log CFU/fruit on the smooth surface and 0.8 log CFU/fruit on stem scar) were lower than that of the combination treatments reduction (5.2 log CFU/fruit on the smooth surface and 4.2 log CFU/fruit on stem scar). A large number of studies demonstrated that the inactivation effect of O3 synergistic disinfection technology on pathogens is obviously better than that of the pure O3 method in general (Azuma et al., 2022).
The synergy between hydrogen peroxide and O3 is mainly because of the hydroxyl radicals generated by the direct reaction of hydrogen peroxide and O3 molecules. The mechanism of its action is shown as follows (Merenyi et al., 2010):
The mechanism of action of UV synergistic O3 technology is shown as follows (Izadifard et al., 2017):
The mechanism of the synergistic technology between ultrasound and O3is shown as follows (Kang, 1998). In the formula, “(((” represents the ultrasonic identifier, while “O(3P)” represents the reactive oxygen species generated after the O3 molecule is decomposed by ultrasonic excitation.
It can be concluded that these AOPs help to generate more hydroxyl radicals in the reaction system, thereby significantly improving the inactivation effect. A variety of studies on the utilization of pure O3 technology and O3 synergistic technology to disinfect microorganisms have been reported. However, there is still a lack of studies of these AOPs on the inactivation of SARS-CoV-2. In the post-epidemic era, in view of the high transmission ability of SARS-CoV-2 and the differences in its survival characteristics in different environmental media, the study on the inactivation of SARS-CoV-2 by O3 synergistic technology in other places and media can be carried out. The focus of studies is to determine the optimal combination mode of O3 synergistic technology and its applicable conditions and concentration range. Meanwhile, the mechanism of action between O3 synergistic technology and SARS-CoV-2 in the inactivation process can also be studied to further clarify the disinfection mechanism and ensure the efficiency of disinfection on SARS-CoV-2.
5.2.2. Establishment of multivariate models
The kinetic equations of O3 inactivation currently studied are based on traditional inactivation kinetic models, such as Chick-Watson model and EFH model, while the model parameters are estimated based on the change of microbial concentration over time in the experiment, which consider relatively simple factors. However, the model parameters will change in different experimental environments, and the inactivation effect is also different due to different microorganisms. Because the microbial population in the laboratory is different from the population structure in the environment, the virus adapts to disinfection stress through genetic selection or mutation, resulting in the emergence of a population resistant to disinfectants (Dolan et al., 2018), so the conclusions drawn in the laboratory (such as concentration, temperature, RH, etc.) are not necessarily suitable for practical applications. Therefore, viral heterogeneity and technology-specific factors can be incorporated into kinetic parameters. Meanwhile, the uncertainties in viral tolerance stemming from the genetic properties of virus populations should also be taken into account in the kinetic model of viral inactivation, which is beneficial to adopt effective disinfection strategies for different viruses.
After considering a number of issues including the genetic characteristics of the virus, the influence of disinfection factors, the unpredictable virus resistance to disinfectants and other factors on the disinfection kinetic model, such as analyzing the change law of the inactivation rate, a tertiary model may be established by integrating multiple models. The multivariate model can integrate target microbial inactivation kinetic factors and disinfection technology-dependent effects, and comprehensively describe the influencing factors of microbial population changes and their changing laws over time. This will provide a more scientific basis for O3 inactivation applications.
6. Conclusions
O3 based inactivation and disinfection is an efficient, broad-spectrum and green method, owning to its characteristics of thorough sterilization and no dead ends in disinfection with significant effects on water and air safety. O3 has shown strong inactivation effects on pandemic viruses, including swine flu virus, H1N1, influenza A virus, HIV, and SARS-CoV-2. The inactivation mechanism is to diffuse O3 to the surface of microbial membranes or capsids protein, thereby oxidatively degrading membrane and shell structure, resulting in the loss of cytoplasm and shell matrix and leading to genetic material destruction and co-enzyme deactivation.
The bactericidal kinetic model can quantitatively evaluate the bactericidal effect of O3. The non-enveloped virus represented by poliovirus conformed to the EFH model, while the enveloped virus represented by SARS-CoV-2 conformed to the pseudo-first-order kinetic model. The pseudo rate constant in the pseudo-first-order kinetic model was affected by RH, temperature, etc. Both RH and O3 concentrations need to be set to a reasonable threshold for application in a safe environment. On the basis of the existing inactivation kinetic model, the definite and uncertain factors can be integrated to establish a tertiary model, which can take effective disinfection strategies for different conditions. In the view of the high transmission ability of SARS-CoV-2 and its robust survival characteristic in different environmental media, other physical and chemical techniques can be used to synergistically inactivate the virus in future studies.
CRediT authorship contribution statement
Yamei Cai: Literature review, manuscript drafting.
Yaqian Zhao: Supervision, discussion, manuscript correction and finalizing.
Asheesh Kumar Yadav: Supervision, manuscript reviewing.
Bin Ji: Discussion, figure plotting.
Peiying Kang: Discussion.
Ting Wei: Discussion.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Editor: Pavlos Kassomenos
Data availability
Data will be made available on request.
References
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O'Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert S., Amarilla A.A., Trollope B., Sng J.D.J., Setoh Y.X., Deering N., Modhiran N., Weng S.H., Melo M.C., Hutley N., Nandy A., Furlong M.J., Young P.R., Watterson D., Grinham A.R., Khromykh A.A. Assessing the potential of unmanned aerial vehicle spraying of aqueous ozone as an outdoor disinfectant for SARS-CoV-2. Environ. Res. 2021;196 doi: 10.1016/j.envres.2021.110944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hashimi A.M., Mason T.J., Joyce E.M. Combined effect of ultrasound and ozone on bacteria in water. Environ. Sci. Technol. 2015;49:11697–11702. doi: 10.1021/es5045437. [DOI] [PubMed] [Google Scholar]
- Alimohammadi M., Naderi M. Effectiveness of ozone gas on airborne virus inactivation in enclosed spaces: a review study. Ozone: Sci. Eng. 2020;43:21–31. doi: 10.1080/01919512.2020.1822149. [DOI] [Google Scholar]
- Ataei-Pirkooh A., Alavi A., Kianirad M., Bagherzadeh K., Ghasempour A., Pourdakan O., Adl R., Kiani S.J., Mirzaei M., Mehravi B. Destruction mechanisms of ozone over SARS-CoV-2. Sci. Rep. 2021;11:18851. doi: 10.1038/s41598-021-97860-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma T., Usui M., Hayashi T. Inactivation of antibiotic-resistant bacteria in wastewater by ozone-based advanced water treatment processes. Antibiotics. 2022;11:210. doi: 10.3390/antibiotics11020210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayarri B., Cruz-Alcalde A., Lopez-Vinent N., Mico M.M., Sans C. Can ozone inactivate SARS-CoV-2? A review of mechanisms and performance on viruses. J. Hazard. Mater. 2021;415 doi: 10.1016/j.jhazmat.2021.125658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard E.L., Lawrence J.D., Noble J.A., Xu M., Joo T., Ng N.L., Schmidt B.E., Santangelo P.J., Finn M.G. Enveloped Virus Inactivation on Personal Protective Equipment by Exposure to Ozone. 2020. medRxiv. [DOI] [Google Scholar]
- Blanco A., Ojembarrena F.B., Clavo B., Negro C. Ozone potential to fight against SAR-COV-2 pandemic: facts and research needs. Environ. Sci. Pollut. Res. Int. 2021;28:16517–16531. doi: 10.1007/s11356-020-12036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton D.C., Zee Y.C., Osebold J.W. The biological effects of ozone on representative members of five groups of animal viruses. Environ. Res. 1982;27:476–484. doi: 10.1016/0013-9351(82)90102-5. [DOI] [PubMed] [Google Scholar]
- Brie A., Boudaud N., Mssihid A., Loutreul J., Bertrand I., Gantzer C. Inactivation of murine norovirus and hepatitis a virus on fresh raspberries by gaseous ozone treatment. Food Microbiol. 2018;70:1–6. doi: 10.1016/j.fm.2017.08.010. [DOI] [PubMed] [Google Scholar]
- Cai X., Han J.C., Li R., Zhang Y., Liu Y., Liu X., Dai R. Phage MS2 inactivation in pure and filtered water: effect of pseudo-kinetics and other factors. Ozone: Sci. Eng. 2014;36(1):86–93. doi: 10.1080/01919512.2013.836953. [DOI] [Google Scholar]
- Caniani D., Caivano M., Mazzone G., Masi S., Mancini I.M. Effect of site-specific conditions and operating parameters on the removal efficiency of petroleum-originating pollutants by using ozonation. Sci. Total Environ. 2021;800 doi: 10.1016/j.scitotenv.2021.149393. [DOI] [PubMed] [Google Scholar]
- Carpendale M.T.F., Freeberg J.K. Ozone inactivates HIV at noncytotoxic concentrations. Antivir. Res. 1991;16:281–292. doi: 10.1016/0166-3542(91)90007-E. [DOI] [PubMed] [Google Scholar]
- Casasola-Rodriguez B., Orta de Velasquez M.T., Luqueno-Martinez V.G., Monje-Ramirez I. Quantification of helicobacter pylori in the viable but nonculturable state by quantitative PCR in water disinfected with ozone. Water Sci. Technol. 2013;68:2468–2472. doi: 10.2166/wst.2013.512. [DOI] [PubMed] [Google Scholar]
- Cataldo F. Ozone degradation of biological macromolecules: proteins, hemoglobin, RNA, and DNA. Ozone: Sci. Eng. 2006;28:317–328. doi: 10.1080/01919510600900290. [DOI] [Google Scholar]
- Clavo B., Cordoba-Lanus E., Rodriguez-Esparragon F., Cazorla-Rivero S.E., Garcia-Perez O., Pinero J.E., Villar J., Blanco A., Torres-Ascension C., Martin-Barrasa J.L., Gonzalez-Martin J.M., Serrano-Aguilar P., Lorenzo-Morales J. Effects of ozone treatment on personal protective equipment contaminated with SARS-CoV-2. Antioxidants (Basel) 2020;9 doi: 10.3390/antiox9121222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. An index to quantify environmental risk of exposure to future epidemics of the COVID-19 and similar viral agents: theory and practice. Environ. Res. 2020;191 doi: 10.1016/j.envres.2020.110155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordoba-Lanus E., Garcı´a-Pe´rez O., Esparrago´n F.R.G., Bethencourt-Estrella C.J., Torres-Mata L.B., Blanco A., Villar J.S., Sanz O., Dı´az J.J., Barrasa J.L.M.N., Serrano-Aguilar P., Piñero J.-E., Clavo B., Lorenzo-Morales J. Ozone treatment effectively eliminates SARS-CoV-2 from infected face masks. PLoS ONE. 2022;17 doi: 10.1371/journal.pone.0271826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscuolo E., Diotti R.A., Ferrarese R., Alippi C., Viscardi G., Signorelli C., Mancini N., Clementi M., Clementi N. Fast inactivation of SARS-CoV-2 by UV-C and ozone exposure on different materials. Emerg. Microbes Infect. 2021;10:206–209. doi: 10.1080/22221751.2021.1872354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristiano L. Could ozone be an effective disinfection measure against the novel Coronavirus (SARS-CoV-2)? J. Prev. Med. Hyg. 2020;61:E301–E303. doi: 10.15167/2421-4248/jpmh2020.61.3.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen P.J., Tiwari B.K., O’Donnell C.P., Muthukumarappan K. Modelling approaches to ozone processing of liquid foods. Trends Food Sci. Technol. 2009;20:125–136. doi: 10.1016/j.tifs.2009.01.049. [DOI] [Google Scholar]
- Dalrymple O.K., Stefanakos E., Trotz M.A., Goswami D.Y. A review of the mechanisms and modeling of photocatalytic disinfection. Appl. Catal. B Environ. 2010;98:27–38. doi: 10.1016/j.apcatb.2010.05.001. [DOI] [Google Scholar]
- De Forni D., Poddesu B., Cugia G., Gallizia G., La Licata M., Lisziewicz J., Chafouleas J., Lori F. BioRxiv; 2021. Low Ozone Concentration and Negative Ions for Rapid SARS-CoV-2 Inactivation. [DOI] [Google Scholar]
- Ding N., Neumann N.F., Price L.M., Braithwaite S.L., Balachandran A., Belosevic M., Gamal El-Din M. Ozone inactivation of infectious prions in rendering plant and municipal wastewaters. Sci. Total Environ. 2014;470–471:717–725. doi: 10.1016/j.scitotenv.2013.09.099. [DOI] [PubMed] [Google Scholar]
- Dolan P.T., Whitfield Z.J., Andino R. Mechanisms and concepts in RNA virus population dynamics and evolution. Ann. Rev. Virol. 2018;5:69–92. doi: 10.1146/annurev-virology-101416-041718. [DOI] [PubMed] [Google Scholar]
- Dubuis M.E., Dumont-Leblond N., Laliberté C., Veillette M., Duchaine C.J.P.O. Ozone efficacy for the control of airborne viruses: bacteriophage and norovirus models. PLoS ONE. 2020;15 doi: 10.1371/journal.pone.0231164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorova G.V., Voblikova V.A., Sabitova L.V., Tkachenko I.S., Tkachenko S.N., Lunin V.V. Ozone solubility in water. Mosc. Univ. Chem. Bull. 2015;70:207–210. doi: 10.3103/s0027131415050053. [DOI] [Google Scholar]
- Epelle E.I., Emmerson A., Nekrasova M., Macfarlane A., Cusack M., Burns A., Mackay W., Yaseen M. Microbial inactivation: gaseous or aqueous ozonation? Ind. Eng. Chem. Res. 2022;61:9600–9610. doi: 10.1021/acs.iecr.2c01551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslami H., Jalili M. The role of environmental factors to transmission of SARS-CoV-2 (COVID-19) AMB Express. 2020;10:92. doi: 10.1186/s13568-020-01028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X., Sokorai K.J.B., Gurtler J.B. Advanced oxidation process for the inactivation of salmonella typhimurium on tomatoes by combination of gaseous ozone and aerosolized hydrogen peroxide. Int. J. Food Microbiol. 2020;312 doi: 10.1016/j.ijfoodmicro.2019.108387. [DOI] [PubMed] [Google Scholar]
- Farooq S., Tizaoui C. A critical review on the inactivation of surface and airborne SARS-CoV-2 virus by ozone gas. Crit. Rev. Environ. Sci. Technol. 2022 doi: 10.1080/10643389.2022.2043094. [DOI] [Google Scholar]
- Fernandez-Cuadros M.E., Albaladejo-Florin M.J., Pena-Lora D., Alava-Rabasa S., Perez-Moro O.S. Ozone (O3) and SARS-CoV-2: physiological bases and their therapeutic possibilities according to COVID-19 evolutionary stage. SN Compr. Clin. Med. 2020;1–9 doi: 10.1007/s42399-020-00328-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feron V.J., Til H.P., Vrijer F., Woutersen R.A., Cassee F.R., Bladeren P.J.V. Aldehydes: occurrence, carcinogenic potential, mechanism of action and risk assessment. Mutat. Res. Genet. Toxicol. 1991;259:363–385. doi: 10.1016/0165-1218(91)90128-9. [DOI] [PubMed] [Google Scholar]
- Finch G.R., Black E.K., Gyurek L., Belosevic M. Ozone inactivation of cryptospondium parvum in DemandFree phosphate buffer determined by in vitro excystation. Appl. Environ. Microbiol. 1993;59:4203–4210. doi: 10.1128/aem.59.12.4203-4210.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foladori P., Cutrupi F., Cadonna M., Manara S. Coronaviruses and SARS-CoV-2 in sewerage and their removal: step by step in wastewater treatment plants. Environ. Res. 2022;207 doi: 10.1016/j.envres.2021.112204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke G., Knobling B., Brill F.H., Becker B., Klupp E.M., Belmar Campos C., Pfefferle S., Lutgehetmann M., Knobloch J.K. An automated room disinfection system using ozone is highly active against surrogates for SARS-CoV-2. J. Hosp. Infect. 2021;112:108–113. doi: 10.1016/j.jhin.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrity D., Owens-Bennett E., Venezia T., Stanford B.D., Plumlee M.H., Debroux J., Trussell R.S. Applicability of ozone and biological activated carbon for potable reuse. Ozone: Sci. Eng. 2014;36:123–137. doi: 10.1080/01919512.2013.866886. [DOI] [Google Scholar]
- Greene A.K., Güzel-Seydim Z.B., Seydim A.C. In: Ozone in Food Processing. O’Donnell C., Tiwari B.K., Cullen P.J., Rice R.G., editors. Blackwell Publishing Ltd.; UK: 2012. Chemical and physical properties of ozone; pp. 19–32. [Google Scholar]
- Grignani E., Mansi A., Cabella R., Castellano P., Tirabasso A., Sisto R., Spagnoli M., Fabrizi G., Frigerio F., Tranfo G. Safe and effective use of ozone as air and surface disinfectant in the conjuncture of Covid-19. Gases. 2020;1:19–32. doi: 10.3390/gases1010002. [DOI] [Google Scholar]
- Gunten U.V. Ozonation of drinkingwater: Part II. Disinfection and by-product formation in presence of bromide, iodide or chlorine. Water Res. 2003;37:1469–1487. doi: 10.1016/s0043-1354(02)00458-x. [DOI] [PubMed] [Google Scholar]
- Hasani M., Chudyk J., Murray K., Lim L.-T., Lubitz D., Warriner K. Inactivation of salmonella, listeria monocytogenes, aspergillus and penicillium on lemons using advanced oxidation process optimized through response surface methodology. Innovative Food Sci. Emerg. Technol. 2019;54:182–191. doi: 10.1016/j.ifset.2019.04.010. [DOI] [Google Scholar]
- Hata A., Hara-Yamamura H., Meuchi Y., Imai S., Honda R. Detection of SARS-CoV-2 in wastewater in Japan during a COVID-19 outbreak. Sci. Total Environ. 2021;785(1):143578. doi: 10.1016/j.scitotenv.2020.143578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.-L., Lee M.-G., Tai J.-H. Controlling indoor bioaerosols using a hybrid system of ozone and catalysts. Aerosol Air Qual. Res. 2012;12:73–82. doi: 10.4209/aaqr.2011.07.0098. [DOI] [Google Scholar]
- Hudson J.B., Sharma M., Vimalanathan S. Development of a practical method for using ozone gas as a virus decontaminating agent. Ozone: Sci. Eng. 2009;31:216–223. doi: 10.1080/01919510902747969. [DOI] [Google Scholar]
- Hunt N.K., Marinas B.J. Kinetics of escherichia coli inactivation with ozone. Water Res. 1997;31:1355–1362. doi: 10.1016/S0043-1354(96)00394-6. [DOI] [Google Scholar]
- Inagaki H., Saito A., Sudaryatma P.E., Sugiyama H., Okabayashi T., Fujimoto S. Rapid inactivation of SARS-CoV-2 with ozoned water. Ozone Sci. Eng. 2020;43:1–5. doi: 10.1080/01919512.2021.1889318. [DOI] [Google Scholar]
- Izadifard M., Achari G., Langford C.H. Degradation of sulfolane using activated persulfate with UV and UV-ozone. Water Res. 2017;125:325–331. doi: 10.1016/j.watres.2017.07.042. [DOI] [PubMed] [Google Scholar]
- Jhunkeaw C., Khongcharoen N., Rungrueng N., Sangpo P., Panphut W., Thapinta A., Senapin S., St-Hilaire S., Dong H.T. Ozone nanobubble treatment in freshwater effectively reduced pathogenic fish bacteria and is safe for Nile tilapia ( Oreochromis niloticus ) Aquaculture. 2021;534 doi: 10.1016/j.aquaculture.2020.736286. [DOI] [Google Scholar]
- Jiang H.J., Chen N., Shen Z.Q., Yin J., Qiu Z.G., Miao J., Yang Z.W., Shi D.Y., Wang H.R., Wang X.W., Li J.W., Yang D., Jin M. Inactivation of poliovirus by ozone and the impact of ozone on the viral genome. Biomed. Environ. Sci. 2019;32:324–333. doi: 10.3967/bes2019.044. [DOI] [PubMed] [Google Scholar]
- Joshi R., Ratpukdi T., Knutson K., Bhatnagar A., Khan E. Bromate formation control by enhanced ozonation: a critical review. Crit. Rev. Environ. Sci. Technol. 2022:1154–1198. doi: 10.1080/10643389.2020.1850169. [DOI] [Google Scholar]
- Kang J.-W. Kinetics and mechanism of the sonolytic destruction of methyl tert-butyl ether by ultrasonic irradiation in the presence of ozone. Environ. Sci. Technol. 1998;32:3194–3199. doi: 10.1021/es970874u. [DOI] [Google Scholar]
- Kanjo Y., Kimata I., Isek M., Miyanaga S., Okada H., Banno C., Matsumoto M., Shimada Y. Inactivation of cryptosporidium spp. Oocysts with ozone. Water Sci. Technol. 2000;41:119–125. doi: 10.1029/1999WR900289. [DOI] [Google Scholar]
- Kekez M.M., Sattar S.A. A new ozone-based method for virus inactivation: preliminary study. Phys. Med. Biol. 1997;42:2027–2039. doi: 10.1088/0031-9155/42/11/002. [DOI] [PubMed] [Google Scholar]
- Kong J., Lu Y., Ren Y., Chen Z., Chen M. The virus removal in UV irradiation, ozonation and chlorination. Water Cycle. 2021;2:23–31. doi: 10.1016/j.watcyc.2021.05.001. [DOI] [Google Scholar]
- Kowalski W.J., Bahnfleth W.P., Striebig B.A., Whittam T.S. Demonstration of a hermetic airborne ozone disinfection system: studies on E. Coli. AIHA J. 2003;64:222–227. doi: 10.1080/15428110308984811. [DOI] [PubMed] [Google Scholar]
- Lee J., Bong C., Pan K.B., Abafog A.T., Park S. Fast and easy disinfection of coronavirus-contaminated face masks using ozone gas produced by a dielectric barrier discharge plasma generator. Environ. Sci. Technol. Lett. 2021;8:339–344. doi: 10.1021/acs.estlett.1c00089. [DOI] [PubMed] [Google Scholar]
- Lenes D., Deboosere N., Menard-Szczebara F., Jossent J., Alexandre V., Machinal C., Vialette M. Assessment of the removal and inactivation of influenza viruses H5N1 and H1N1 by drinking water treatment. Water Res. 2010;44:2473–2486. doi: 10.1016/j.watres.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Lev O., Regli S. Evaluation of ozone disinfection systems: characteristic time T. J. Environ. Eng. 1992;118:268–285. doi: 10.1061/(ASCE)0733-9372(1992)118:4(477). [DOI] [Google Scholar]
- Li X.T., Ma J., He H. Recent advances in catalytic decomposition of ozone. J. Environ. Sci. 2020;94:14–31. doi: 10.1016/j.jes.2020.03.058. [DOI] [PubMed] [Google Scholar]
- Lim M.Y., Kim J.M., Lee J.E., Ko G. Characterization of ozone disinfection of murine norovirus. Appl. Environ. Microbiol. 2010;76:1120–1124. doi: 10.1128/AEM.01955-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez L.A., Riffle A.J., Pike S.L., Gardner D., Hogue B.G. Importance of conserved cysteine residues in the coronavirus envelope protein. J. Virol. 2008;82:3000–3010. doi: 10.1128/JVI.01914-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makky E.A., Park G.S., Choi I.W., Cho S.I., Kim H. Comparison of Fe(VI) (FeO4(2-)) and ozone in inactivating Bacillus subtilis spores. Chemosphere. 2011;83:1228–1233. doi: 10.1016/j.chemosphere.2011.03.030. [DOI] [PubMed] [Google Scholar]
- Manas P., Pagan R. Microbial inactivation by new technologies of food preservation. J. Appl. Microbiol. 2005;98:1387–1399. doi: 10.1111/j.1365-2672.2005.02561. [DOI] [PubMed] [Google Scholar]
- Manjunath S.N., Sakar M., Katapadi M., Geetha Balakrishna R. Recent case studies on the use of ozone to combat coronavirus: problems and perspectives. Environ. Technol. Innov. 2021;21 doi: 10.1016/j.eti.2020.101313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins R.B., Castro I.A., Pontelli M., Souza J.P., Lima T.M., Melo S.R., Siqueira J.P.Z., Caetano M.H., Arruda E., de Almeida M.T.G. SARS-CoV-2 inactivation by ozonated water: a preliminary alternative for environmental disinfection. Ozone: Sci. Eng. 2020;43:108–111. doi: 10.1080/01919512.2020.1842998. [DOI] [Google Scholar]
- Mazur-Panasiuk N., Botwina P., Kutaj A., Woszczyna D., Pyrc K. Ozone treatment is insufficient to inactivate SARS-CoV-2 surrogate under field conditions. Antioxidants. 2021;10:1480. doi: 10.3390/antiox10091480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick J.L. Poliomyelitis virus in urban sewage in epidemic and in nonepidemic times 1. Am. J. Epidemiol. 1947;45:240–253. doi: 10.1093/oxfordjournals.aje.a119132. [DOI] [PubMed] [Google Scholar]
- Merenyi G., Lind J., Naumov S., Sonntag C.V. Reaction of ozone with hydrogen peroxide (peroxone process) a revision of current mechanistic concepts based on thermokinetic and quantum-chemical considerations. Environ. Sci. Technol. 2010;44:3505. doi: 10.1021/es100277d. [DOI] [PubMed] [Google Scholar]
- Mik G.D., Groot I.D. Mechanisms of inactivation of bacteriophage φX174 and its DNA in aerosols by ozone and ozonized cyclohexene. J. Hyg. 1977;78:199. doi: 10.1017/s0022172400056096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M.M., TaoChen Mutagenicity of bromate: implications for cancer risk assessment. Toxicology. 2006;221:190–196. doi: 10.1016/j.tox.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Morrison C., Atkinson A., Zamyadi A., Kibuye F., McKie M., Hogard S., Mollica P., Jasim S., Wert E.C. Critical review and research needs of ozone applications related to virus inactivation: potential implications for SARS-CoV-2. Ozone: Sci. Eng. 2020;43:2–20. doi: 10.1080/01919512.2020.1839739. [DOI] [Google Scholar]
- Murata T., Komoto S., Iwahori S., Sasaki J., Nishitsuji H., Hasebe T., Hoshinaga K., Yuzawa Y. Reduction of severe acute respiratory syndrome Coronavirus-2 infectivity by admissible concentration of ozone gas and water. Microbiol. Immunol. 2021;65:10–16. doi: 10.1111/1348-0421.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B.K., Ohmine S., Tomer D.P., Jensen K.J., Johnson F.B., Kirsi J.J., Robison R.A., O'Neill K.L. Virion disruption by ozone-mediated reactive oxygen species. J. Virol. Methods. 2008;153:74–77. doi: 10.1016/j.jviromet.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Nemudryi A., Nemudraia A., Wiegand T., Surya K., Buyukyoruk M., Cicha C., Vanderwood K.K., Wilkinson R., Wiedenheft B. Temporal detection and phylogenetic assessment of SARS-CoV-2 in municipal wastewater. Cell. Rep. Med. 2020;1 doi: 10.1016/j.xcrm.2020.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorimotlagh Z., Jaafarzadeh N., Martínez S.S., Mirzaee S.A. A systematic review of possible airborne transmission of the COVID-19 virus (SARS-CoV-2) in the indoor air environment. Environ. Res. 2021;193 doi: 10.1016/j.envres.2020.110612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorimotlagh Z., Mirzaee S.A., Jaafarzadeh N., Maleki M., Kalvandi G., Karami C. A systematic review of emerging human coronavirus (SARS-CoV-2) outbreak: focus on disinfection methods, environmental survival, and control and prevention strategies. Environ. Sci. Pollut. Res. 2021;28:1–15. doi: 10.1007/s11356-020-11060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller M.H., Heidinger R.C. Mechanisms of delayed ozone toxicity to bluegill Lepomis machrochirus rafinesque. Environ. Pollut. Ser. A Ecol. Biol. 1980;22:229–239. doi: 10.1016/0143-1471(80)90017-3. [DOI] [Google Scholar]
- Pelleu G.B., Jr., Holleman N.G., R.F.B Ozone and glycol vapor decontamination of air in a closed room. J. Dent. Res. 1974;53:1132–1137. doi: 10.1177/00220345740530051101. [DOI] [PubMed] [Google Scholar]
- Percivalle E., Clerici M., Cassaniti I., Nepita E.V., Marchese P., Olivati D., Catelli C., Berri A., Baldanti F., Marone P., Bruno R., Triarico A., Lago P. SARS-CoV-2 viability on different surfaces after gaseous ozone treatment: a preliminary evaluation. J. Hosp. Infect. 2021;110:33–36. doi: 10.1016/j.jhin.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppendieck D., Hubbard H., Ward M., Weschler C., Corsi R.L. Ozone reactions with indoor materials during building disinfection. Atmos. Environ. 2007;41:3166–3176. doi: 10.1016/j.atmosenv.2006.06.060. [DOI] [PubMed] [Google Scholar]
- Quevedo-león R., Bastías-Montes J.M., Espinoza-Tellez T., Ronceros B., Balic I., Muñoz O. Inactivation of coronaviruses in food industry: the use of inorganic and organic disinfectants, ozone, and UV radiation. Sci. Agrop. 2020;11:257–266. doi: 10.17268/sci.agropecu.2020.02.14. [DOI] [Google Scholar]
- Riddell S., Goldie S., Hill A., Eagles D., Drew T.W. The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virol. J. 2020;17:145. doi: 10.1186/s12985-020-01418-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Chueca J., Ormad Melero M.P., Mosteo Abad R., Esteban Finol J., Ovelleiro Narvion J.L. Inactivation of Escherichia coli in fresh water with advanced oxidation processes based on the combination of O3, H2O2, and TiO2. Kinetic modeling. Environ. Sci. Pollut. Res. Int. 2015;22:10280–10290. doi: 10.1007/s11356-015-4222-3. [DOI] [PubMed] [Google Scholar]
- Sangsanont J., Kurisu F., Furumai H., Katayama H. Ozone disinfection kinetics of poliovirus 1 determined by cell culture assay, RT-qPCR, and ethidium monoazide qPCR reduction in a continuous quench-flow reactor. J. Appl. Microbiol. 2020;129:1530–1540. doi: 10.1111/jam.14787. [DOI] [PubMed] [Google Scholar]
- Sato H., Wananabe Y., Miyata H. Virucidal effect of ozone treatment of laboratory animal viruses. Jikken Dobutsu Exp. Anim. 1990;39:223–229. doi: 10.1538/expanim1978.39.2_223. [DOI] [PubMed] [Google Scholar]
- Sazhina N.N., Evteeva N.M., Titov V.N. Experimental evaluation of velocity constants for ozone interaction with individual fatty acids. Bull. Exp. Biol. Med. 2018;165:356–359. doi: 10.1007/s10517-018-4169-8. [DOI] [PubMed] [Google Scholar]
- Schulz C.R., Clarkson Davis C., Bonacquisti T., Navratil R. The impacts of new CT requirements for designing ozone systems forCryptosporidiumInactivation. Ozone: Sci. Eng. 2005;27:129–138. doi: 10.1080/01919510590925248. [DOI] [Google Scholar]
- Seif F., Noorimotlagh Z., Mirzaee S.A., Kalantar M., Barati B., Fard M.E., Fard N.K. The SARS-CoV-2 (COVID-19) pandemic in hospital: an insight into environmental surfaces contamination, disinfectants’ efficiency, and estimation of plastic waste production. Environ. Res. 2021;202 doi: 10.1016/j.envres.2021.111809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J., Gao Z. Ozone removal on building material surface: a literature review. Build. Environ. 2018;134:205–217. doi: 10.1016/j.buildenv.2018.02.046. [DOI] [Google Scholar]
- Sims N., Kasprzyk-Hordern B. Future perspectives of wastewater-based epidemiology: monitoring infectious disease spread and resistance to the community level. Environ. Int. 2020;139 doi: 10.1016/j.envint.2020.105689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B., Sharma N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 2008;93:561–584. [Google Scholar]
- Slompo N.D.M., Silva G.H.R.D. Disinfection of anaerobic/aerobic sanitary effluent using ozone: formaldehyde formation. Water Environ. Fed. 2019;91:898–905. doi: 10.1002/wer.1128. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Sakurai M., Ishii K. Inactivationof influenza virus by ozone gas. IHI Eng. Rev. 2009;42:108–111. [Google Scholar]
- Tawabini B., Khalil A., Abussaud B. Reduction of Escherichia coli bacteria from contaminated water by combining hydrogen peroxide, ozone and ultraviolet light. Water Supply. 2013;13:782–789. doi: 10.2166/ws.2013.064. [DOI] [Google Scholar]
- Tizaoui C. Ozone: a potential oxidant for COVID-19 virus (SARS-CoV-2) Ozone: Sci. Eng. 2020;42:378–385. doi: 10.1080/01919512.2020.1795614. [DOI] [Google Scholar]
- Tizaoui C., Stanton R., Statkute E., Rubina A., Lester-Card E., Lewis A., Holliman P., Worsley D. Ozone for SARS-CoV-2 inactivation on surfaces and in liquid cell culture media. J. Hazard. Mater. 2022;428 doi: 10.1016/j.jhazmat.2022.128251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii S., Itamochi M., Katayama H. Inactivation kinetics of waterborne virus by ozone determined by a continuous quench flow system. Water Res. 2020;186 doi: 10.1016/j.watres.2020.116291. [DOI] [PubMed] [Google Scholar]
- Torii S., Miura F., Itamochi M., Haga K., Katayama K., Katayama H. Impact of the heterogeneity in free chlorine, UV254, and ozone susceptibilities among coxsackievirus B5 on the prediction of the overall inactivation efficiency. Environ. Sci. Technol. 2021;55:3156–3164. doi: 10.1021/acs.est.0c07796. [DOI] [PubMed] [Google Scholar]
- Tran H.N., Le G.T., Nguyen D.T., Juang R.-S., Rinklebe J., Bhatnagar A., Lima E.C., Iqbal H.M.N., Sarmah A.K., Chao H.-P. SARS-CoV-2 coronavirus in water and wastewater: a critical review about presence and concern. Environ. Res. 2021;193 doi: 10.1016/j.envres.2020.110265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA . Disinfection Profiling and Benchmarking Guidance Manual. Environmental Protection Agency; 1999. https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=20002249.txt [Google Scholar]
- USEPA . Coronavirus and Drinking Water and Wastewater. 2020. https://www.epa.gov/coronavirus/coronavirus-and-drinking-water-and-wastewater [Google Scholar]
- Verinda S.B., Yulianto E., Gunawan G., Nur M. Ozonated nanobubbles - a potential hospital wastewater treatment during the COVID-19 outbreak in Indonesia to eradicate the persistent SARS-CoV-2 in HWWs? Ann. Trop. Med. Public Health. 2021;24 doi: 10.36295/asro.2021.24197. [DOI] [Google Scholar]
- Volkoff S.J., Carlson T.J., Leik K., Smith J.J., Graves D., Dennis P., Aris T., Cuthbertson D., Holmes A., Craig K., Marvin B., Nesbit E. Demonstrated SARS-CoV-2 surface disinfection using ozone. Ozone: Sci. Eng. 2021;43:296–305. doi: 10.1080/01919512.2020.1863770. [DOI] [Google Scholar]
- Wang J., Shen J., Ye D., Yan X., Zhang Y., Yang W., Li X., Wang J., Zhang L., Pan L. Disinfection technology of hospital wastes and wastewater: suggestions for disinfection strategy during coronavirus disease 2019 (COVID-19) pandemic in China. Environ. Pollut. 2020;262 doi: 10.1016/j.envpol.2020.114665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEF . The Water Professional’s Guide to COVID-19. 2020. https://wef.org/news-hub/wef-news/the-water-professionals-guide-to-the-2019-novel-coronavirus/ [Google Scholar]
- Westhaus S., Weber F.-A., Schiwy S., Linnemann V., Brinkmann M., Widera M., Greve C., Janke A., Hollert H., Wintgens T., Ciesek S. Detection of SARS-CoV-2 in raw and treated wastewater in Germany –Suitability for COVID-19 surveillance and potential transmission risks. Sci. Total Environ. 2021;751 doi: 10.1016/j.scitotenv.2020.141750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . Disease Outbreaks by Year. World Health Organization; 2020. http://www.who.int/csr/don/archive/year/en/ [Google Scholar]
- WHO . WHO Coronavirus (Covid-19) Overview. World Health Organization; 2022. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 [Google Scholar]
- Wolf C., Gunten U.V., Kohn T. Kinetics of inactivation of waterborne enteric viruses by ozone. Environ. Sci. Technol. 2018;52(4):2170–2177. doi: 10.1021/acs.est.7b05111. [DOI] [PubMed] [Google Scholar]
- Wu D., You H., Zhang R., Chen C., Du J. Inactivation of Escherichia coli using UV/AgTiO2/O3-mediated advanced oxidation: application to ballast water disinfection. J. Chem. Technol. Biotechnol. 2011;86:1521–1526. doi: 10.1002/jctb.2667. [DOI] [Google Scholar]
- Xia B., Shen X., He Y., Pan X., Liu F.-L., Wang Y., Yang F., Fang S., Wu Y., Duan Z., Zuo X., Xie Z., Jiang X., Xu L., Chi H., Li S., Meng Q., Zhou H., Zhou Y., Cheng X., Xin X., Jin L., Zhang H.-L., Yu D.-D., Li M.-H., Feng X.-L., Chen J., Jiang H., Xiao G., Zheng Y.-T., Zhang L.-K., Shen J., Li J., Gao Z. SARS-CoV-2 envelope protein causes acute respiratory distress syndrome (ARDS)-like pathological damages and constitutes an antiviral target. Cell Res. 2021;31:847–860. doi: 10.1038/s41422-021-00519-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano H., Nakano R., Suzuki Y., Nakano A., Kasahara K., Hosoi H. Inactivation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by gaseous ozone treatment. J. Hosp. Infect. 2020;106:837–838. doi: 10.1016/j.jhin.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M., Zhang L., Ma J., Zhou L. On airborne transmission and control of SARS-cov-2. Sci. Total Environ. 2020;731 doi: 10.1016/j.scitotenv.2020.139178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanardi I., Borrelli E., Valacchi G., Travagli V., Bocci V.J.C.M.C. Ozone: a multifaceted molecule with unexpected therapeutic activity. Curr. Med. Chem. 2015;23:304–314. doi: 10.2174/0929867323666151221150420. [DOI] [PubMed] [Google Scholar]
- Zhang L., Luo Y., Wang W., Sun Y., Zhang J., Fatima M., Jia X., Qiu H.J. Efficient inactivation of african swine fever virus by ozonized water. Vet. Microbiol. 2020;247 doi: 10.1016/j.vetmic.2020.108796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Xu Q., Shi Y.L., Chen Z., Lu Y., Yang H.W., Xie Y.F., Hou L. Study on the influence of operational and management processes of a water reclamation plant since COVID-19 situation. Environ. Pollut. 2021;285 doi: 10.1016/j.envpol.2021.117257. [DOI] [PubMed] [Google Scholar]
- Zhang X., Lin T., Wang X. Investigation of microplastics release behavior from ozone-exposed plastic pipe materials. Environ. Pollut. 2022;296 doi: 10.1016/j.envpol.2021.118758. [DOI] [PubMed] [Google Scholar]
- Zheng T., Zheng X., Zhan S., Zhou J., Liao S. Study on the ozone aging mechanism of natural rubber. Polym. Degrad. Stab. 2021;186 doi: 10.1016/j.polymdegradstab.2021.109514. [DOI] [Google Scholar]
- Ziyaina M., Rasco B. Inactivation of microbes by ozone in the food industry: a review. Afr. J. Food Sci. 2021;15:113–120. doi: 10.5897/AJFS2020.2074. [DOI] [Google Scholar]
- Zucker I., Lester Y., Alter J., Werbner M., Yecheskel Y., GalTanamy M., Dessau M. Pseudoviruses for the assessment of coronavirus disinfection by ozone. Environ. Chem. Lett. 2021;19:1779–1785. doi: 10.1007/s10311-020-01160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.