Abstract
The role of intestinal lymphocytes and gamma interferon (IFN-γ) production in protective immunity to Eimeria tenella infection was evaluated in two inbred strains of chickens (SC and TK) that display different patterns of susceptibility to coccidiosis. Oral inoculation of either strain with E. tenella led to parasite invasion of the intestinal cecum and cecal tonsils. Greater fecal oocyst shedding was seen in TK chickens. Flow cytometric analyses of cecal tonsil lymphocytes demonstrated greater numbers of CD4+ and T-cell receptor γδ-positive (TCR1+) cells in SC chickens and elevated numbers of CD8+ and TCR2+ cells in TK chickens following primary infection. IFN-γ mRNA expression was significantly increased in cecal tonsil and intraepithelial lymphocytes at days 6 and 8, respectively, after primary infection in SC compared to TK chickens. While no differences were noted between cecal tonsil lymphocytes of the two strains following secondary infection, TK chickens showed elevated IFN-γ transcript levels in intestinal intraepithelial lymphocytes at this time. Selective depletion of CD4+, but not CD8+, cecal tonsil lymphocytes in SC chickens resulted in a reduced IFN-γ mRNA expression, indicating that CD4+ cells are the primary source of this cytokine. Collectively, these results indicate that local lymphocyte responses and production of IFN-γ are influenced by host genetic factors.
Apicomplexan protozoa of the genus Eimeria are a common cause of coccidiosis. Following ingestion of infective oocysts, coccidial parasites undergo a complex life cycle ultimately impairing the gastrointestinal tract and resulting in nutrient malabsorption, body weight loss, and in severe cases, death (13). Cell-mediated immunity (CMI) plays a major role in host protection against coccidiosis in chickens (3, 27, 32) and mice (36, 49). Alterations in lymphocyte subpopulations and cytokine production during Eimeria infections in both animals have been investigated to clarify the nature of protective immunity (2, 12, 25). These studies have shown that gamma interferon (IFN-γ) is an important component of the host protective CMI (7, 26). Chicken IFN-γ has been cloned (9, 19, 41, 48), and monoclonal antibodies (MAbs) against the recombinant protein have been used to further characterize CMI during coccidiosis (50).
Different species of Eimeria are known to display tissue tropism within the avian intestinal tract. For example, E. tenella primarily infects the cecum and cecal tonsils located at the ileocecal junction, which contain the major source of lymphocytes in the cecum. It was therefore of interest to examine the roles of lymphocyte subpopulations and IFN-γ production in the cecal tonsils of chickens infected with E. tenella. To perform these studies, we took advantage of the fact that genetically divergent, inbred strains of chickens, SC (B2B2) and TK (B15B21), display different degrees of susceptibility to E. tenella infection. SC chickens consistently produce fewer fecal oocysts than TK chickens following E. tenella infection. These two strains were therefore examined with regard to the changes in intestinal lymphocyte subpopulations and cytokine production following E. tenella infection to ascertain the relative importance of these two parameters in acquired immunity to coccidiosis.
MATERIALS AND METHODS
Animals, parasites, and experimental infections.
Fertilized chicken eggs of SC and TK chickens were obtained from Hyline International Production Center (Dallas Center, Iowa) and hatched at Livestock and Poultry Sciences Institute facilities, Agricultural Research Service, U. S. Department of Agriculture (Beltsville, Md.). The strain of E. tenella used was developed from a single oocyst isolation, originally from Wisconsin, and maintained at the Immunology and Disease Resistance Laboratory (Beltsville, Md.). Chickens were kept in brooder batteries in clean buildings until 3 weeks of age and then transferred to separate housing for experimental infection. Unless otherwise noted, chickens were inoculated esophageally with 104 sporulated oocysts on day 0 and subsequently given a secondary infection with 105 oocysts on day 21 post-primary infection (ppi). Chickens were given feed and water ad libitum, and constant light was provided throughout the study.
Oocyst counting.
Fecal oocysts shedding was monitored in individual birds between days 5 and 9 following primary and secondary infections. Fecal samples were homogenized in a blade grinder, and two 35-ml samples were collected from each suspension. The oocysts were diluted in 0.2 M sucrose to 1:10 to 1:10,000 and counted microscopically in a McMaster chamber. Total oocyst number was calculated as oocyst count × dilution factor × (fecal sample volume/counting chamber volume).
Preparation and staining of tissue sections.
Cecal tonsils and the middle section of the cecum were removed, embedded in Tissue-Tek freezing compound, and quickly frozen on dry ice. Tissue blocks were sectioned at 5 μm, placed onto poly-l-lysine-coated slides, immediately air dried, fixed in acetone at 4°C for 10 min, and stored at −70°C until use. Prior to staining, the slides were allowed to equilibrate to room temperature and nonspecific binding was blocked by 10-min incubation with normal goat serum at room temperature. Slides were incubated at 37°C in a humidity chamber with a MAb (HB-8335; American Type Culture Collection, Manassas, Va.) which detects E. tenella sporozoites and merozoites and washed three times with phosphate-buffered saline; bound MAb was detected with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G (IgG; Sigma, St. Louis, Mo.), and the slides were washed with phosphate-buffered saline. Slides were examined and photographed using Vectashield mounting medium (Vector Laboratories, Burlingame, Calif.) with a Zeiss photomicroscope (Carl Zeiss, Hanover, Md.).
Lymphocyte preparation.
Intestinal intraepithelial lymphocytes (IELs) were prepared as described elsewhere (24). The region of the intestine approximately 2 cm below the duodenal loop and 2 cm above the ileocecal junction was removed, opened longitudinally, and rinsed with Ca+2- and Mg+2-free Hanks' balanced salt solution (CMF-HBSS; Sigma) containing 10 mM dithothreitol (Sigma). The intestine was cut into 3-cm fragments and incubated with swirling in 150 ml of CMF-HBSS for 10 min at 38°C, and the supernatant was discarded. Intestinal sections were resuspended in CMF-HBSS containing 10−4 M EDTA and incubated with constant swirling at 38°C for 20 min; lymphocytes were isolated from the supernatant by discontinuous 30% Percoll density gradient centrifugation at 600 × g for 25 min at 24°C. To prepare lymphocytes from the spleen and cecal tonsils, single cell suspensions were produced by gentle pressing through a stainless steel mesh, and red blood cells and debris were removed by centrifugation through Histopaque-1077 (Sigma). Cells at the interface were collected, washed twice with HBSS, and adjusted to the desired concentration. The viability of all cell preparations was consistently greater than 95%.
FACS.
Lymphocytes from spleen and cecal tonsils and IELs were adjusted to 107 cells/ml in fluorescence-activated cell sorting (FACS) buffer (HBSS, 3% bovine serum albumin, 0.05% NaN3), and 100 μl of cells was incubated with 100 μl of previously optimized dilutions of MAbs to chicken CD4 (28), CD8 (28), T-cell receptor αβ (TCR2), T-cell receptor γδ (TCR1), or HB2 (negative control, anti-human T cells; American Type Culture Collection) at 4°C for 45 min with occasional shaking and washed three times in FACS buffer by centrifugation. Cells were incubated with FITC-conjugated goat anti-mouse IgG (1:400) at 4°C for 30 min with occasional shaking, washed and resuspended in 500 μl of FACS buffer, and analyzed with an EPICS-XL-MCL flow cytometer (Coulter, Hialeah, Fla.). A minimum of 104 viable cells from each experiment was analyzed. Data are presented as the ratio of the number of cells stained with specific MAb from E. tenella-infected chickens compared to cells stained with MAb from noninfected control chickens following subtraction from both groups of background staining by an irrelevant MAb (HB2).
Depletion of T lymphocyte subpopulations.
Cecal tonsil lymphocytes (2 × 107) were sequentially incubated with 2 ml of MAb (culture supernatant) against chicken CD8 or CD4 (28) antigens for 30 min at 4°C and rabbit complement (1:100; Cedarlane Laboratories, Hornby, Ontario, Canada) for 1 h at 41°C. MAb HB2 was used as a negative control. Viable cells were isolated by centrifugation through Histopaque-1077, washed twice, resuspended in HBSS, and analyzed by FACS. Cell depletion was routinely greater than 99.5%.
RT-PCR.
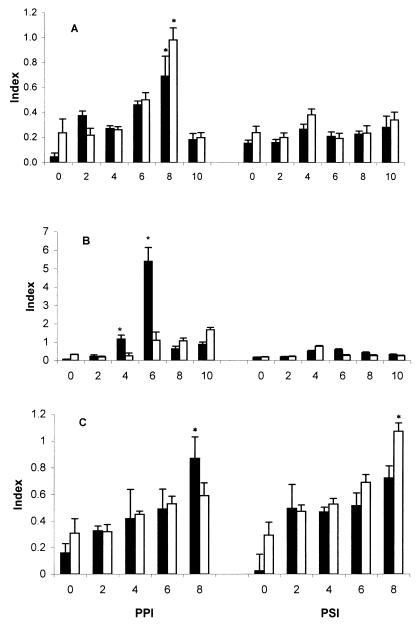
IFN-γ mRNAs were quantified by reverse transcriptase-mediated PCR (RT-PCR) as described elsewhere (7). Briefly, total RNA was isolated from 107 IELs, cecal tonsil lymphocytes or splenic lymphocytes by using TRIzol (Life Technologies, Gaithersburg, Md.), and cDNA was synthesized from 1.5 μg using oligo(dT) and Moloney murine leukemia virus reverse transcriptase (SuperScript II; Life Technologies). IFN-γ competitor cDNA (366 bp) was generated from the cDNA by PCR with forward primer cIFNf1 (5′-ACAGATCTGAGGAGCTCTATACTCTG-3′) and reverse primer cIFNr1 (5′-AAAGATCTACAATAATAGGTCCACCGTCAGC-3′). Primer sequences and predicted sizes of the IFN-γ target and β-actin (control) amplification products are listed in Table 1. Primer sequences were chosen to permit amplification to span one genomic intron, thereby eliminating genomic DNA contamination. Total and IFN-γ competitor cDNAs were mixed and coamplified in a 20-μl reaction mixture containing 0.15 mM primers and 0.1 U of Taq DNA polymerase (Life Technologies). The reactions were performed for 32 cycles in a PTC-100 programmable thermal cycler (MJ Research, Watertown, Mass.) under the following conditions: 95°C for 30 s (denaturation), 57°C for 20 s (polymerization), and 72°C for 40 s (annealing). The concentrations of total and IFN-γ competitor cDNAs were predetermined and fell within the linear region of the dose-response amplification curve under the conditions used. PCR products were separated on a 1.6% Metaphor–0.4% GTG agarose gel and stained with ethidium bromide. The intensities of IFN-γ target and competitor bands were quantified using Sigma Gel software (Jandel, San Rafael, Calif.), and the relative staining density (d) of the IFN-γ target band was calculated with the formula d = a/(a + b), where a represents the intensity of the IFN-γ target band and b represents the intensity of the IFN-γ competitor band. A relative value for each sample (index in Fig. 4) was obtained by normalizing the value of d to the intensity of the β-actin band.
TABLE 1.
Primer sequences and predicted sizes of IFN-γ target, IFN-γ competitor, and β-actin PCR products
| Gene | Full size (bp) | Competitor cDNA (bp) | Primer sequencea |
|---|---|---|---|
| IFN-γ | 480 | 366 | ACTTACAACTTGTTTGTTCTGTCTGTC (f) |
| GCAATTGCATCTCCTCTGAGACTGG (r) | |||
| β-Actin | 582 | TCTGGTGGTACCACAATGTACCCT (f) | |
| CCAGTAATTGGTACCGGCTCCTC (r) |
(f), forward; (r), reverse.
FIG. 4.
E. tenella-induced IFN-γ mRNA expression in spleen, cecal tonsil, and intraepithelium. SC (closed bar) and TK (open bar) chickens (six per group) were given a primary infection with 104 oocysts of E. tenella at day 0 and a secondary infection with 105 oocysts at day 21. Single cells from spleen (A), cecal tonsils (B), and IELs (C) were isolated at the indicated days ppi or psi for mRNA preparation. The amount of IFN-γ mRNA in 107 cells as determined in RT-PCR was quantified using the formula d = a/(a + b), where a represents the staining intensity of the target IFN-γ band and b represents the intensity of the IFN-γ competitor band. The y axis shows the index, a relative value of d normalized against an internal control, β-actin; representative data from two independent studies are shown. ∗ indicates significant difference at P < 0.05.
Statistical analysis.
Results were compared by Student's t test using the SAS package (SAS Institute Inc., Cary, N.C.). A P value of less than 0.05 was considered statistically significant.
RESULTS
Fecal oocyst shedding in SC and TK chickens.
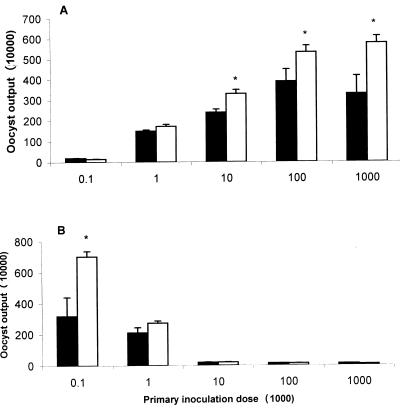
As shown in Fig. 1A, both SC and TK strain chickens shed progressively more oocysts with increasing oocyst dose following primary infection, while SC chickens excreted significantly fewer oocysts than TK chickens at 104, 105, and 106 oocysts (P < 0.05); no differences were noted at doses of 102 and 103 oocysts. Following secondary infection (Fig. 1B), oocyst excretion decreased with increasing oocyst doses, and TK chickens again shed significantly more oocysts compared to SC chickens (P < 0.05). In general, higher primary oocysts induced greater immune protection than lower doses following secondary infection. A primary inoculation dose of 104 oocysts was chosen for further study based on the relatively high oocyst output with minimal clinical damage to epithelial cells, villi, and the mucosal surface in the cecum and cecal tonsils (data not shown).
FIG. 1.
Fecal oocyst shedding in chickens infected with E. tenella. SC and TK chickens were inoculated with 102, 103, 104, 105, or 106 sporulated oocysts of E. tenella at day 0 (A; primary infection) and 105 oocysts at day 21 (B; secondary infection). Fecal samples were collected from individual chickens from days 5 to 9 ppi (A) and psi (B). Each data point shows the average oocyst count from three chickens; representative data from two independent studies are shown. ∗ indicates significant difference at P < 0.05.
Intracellular development of coccidia in the cecum and cecal tonsils.
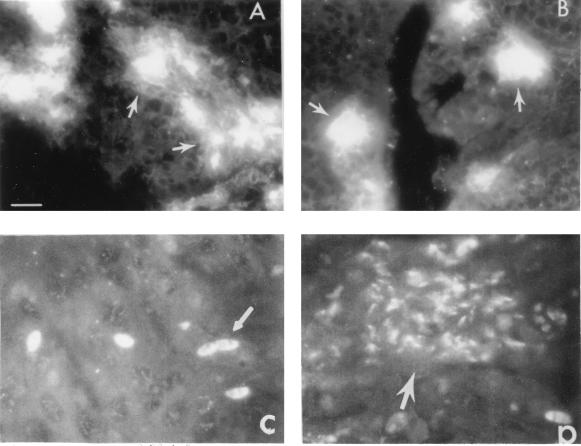
Figures 2A and B illustrate parasite localization within the cecum and cecal tonsil of SC chickens at day 3 ppi. The staining pattern in both tissues was virtually indistinguishable using an E. tenella MAb that recognizes both sporozoite- and merozoite-associated antigens. Similar results were seen using tissue sections from TK chickens (data not shown). Invading sporozoites which were seen inside cecal tonsil cells at 24 h postinfection (Fig. 2C) developed into meronts at 48 h postinfection (Fig. 2D). These results clearly demonstrated that E. tenella undergoes intracellular development in the cecum as well as cecal tonsils.
FIG. 2.
Indirect immunofluorescence staining of tissue sections from cecum and cecal tonsils from chickens infected with E. tenella. Developing E. tenella are shown in cecum (A) and cecal tonsil (B) at 72 h postinfection; sporozoites (C) and meronts (D) are shown in cecal tonsil at 24 (C) and 48 (D) h after infection with 105 sporulated oocysts of E. tenella. Each slide contained tissues from three chickens, and three slides were evaluated for the presence of developing parasites. Sporozoites and developing meronts were identified with anti-E. tenella MAb and FITC-conjugated goat anti-mouse IgG and microscopically examined in a Zeiss microscope (bar = 250 μm). The arrows indicate areas with high numbers of intracellular parasites undergoing asexual development (A and B), sporozoites (C), and merozoites (D).
Changes in T-lymphocyte subpopulations in cecal tonsils during primary and secondary E. tenella infections.
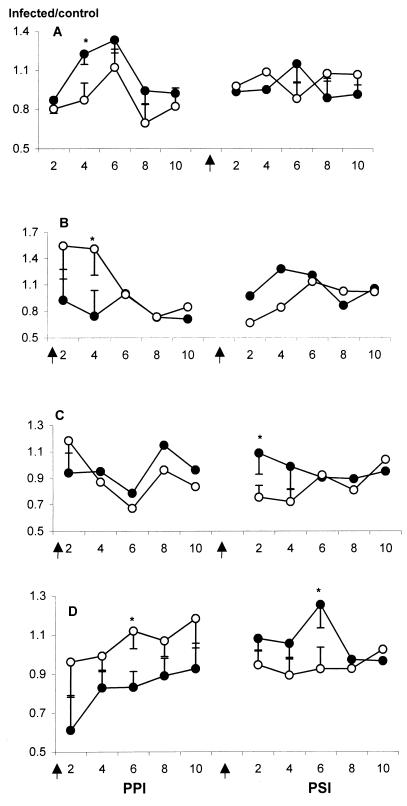
The ability of E. tenella parasites to infect intestinal cecal tonsils prompted us to examine changes in lymphocyte subpopulations expressing the CD4, CD8, TCRγδ, and TCRαβ antigens. As previously described (25), the number of CD4+, CD8+, TCRγδ+, and TCRαβ+ cells in intestinal tissues in uninfected SC and TK chickens varied (data not shown). As shown in Fig. 3A, the number of CD4+ cells in SC chickens increased at days 4 ppi, whereas these cells were consistently lower in TK chickens. In contrast, TK CD4+ cells were generally more numerous than SC CD4+ cells following secondary infection, with the exception of day 6 post-secondary infection (psi). CD8+ cells were noticeably higher in TK chickens at day 4 ppi but lower at days 2 and 4 psi (Fig. 3B), whereas following secondary infection, CD8+ cells were increased in SC compared to TK chickens at days 2 and 4 psi. Cells expressing the TCRγδ antigen were generally higher in SC chickens following both primary and secondary inoculations (Fig. 3C). In contrast, TCRαβ+ cells in TK chickens exceeded those in SC chickens after primary infection but were reduced compared to the SC strain following secondary infection (Fig. 3D). These alterations in TCRγδ+- and TCRαβ+-expressing cells were not seen with lymphocytes isolated from the peripheral blood or spleen (data not shown).
FIG. 3.
Kinetics of T-lymphocyte subpopulation changes in the cecal tonsils following E. tenella infection. SC (closed) and TK (opened) chickens were given a primary infection with 104 oocysts of E. tenella at day 0 and a secondary infection with 105 oocysts at day 21 ppi (arrow). Cecal tonsil lymphocytes (six chickens per group) were prepared at the indicated days ppi or psi, stained with MAbs detecting CD4 (A), CD8 (B), TCRγδ (C), or TCRαβ (D), and analyzed by a flow cytometry. The y axis indicates the ratios of T cells from infected to uninfected chickens; representative data from two independent studies are shown. ∗ indicates significant difference at P < 0.05.
IFN-γ mRNA expression following the primary and the secondary E. tenella infections.
To investigate the involvement of IFN-γ in CMI during coccidial infection, RT-PCR was used to quantify IFN-γ mRNA expression in the spleen, cecal tonsils, and IELs. As shown in Fig. 4A, IFN-γ mRNA expression in splenic lymphocytes progressively increased over time until day 8 ppi in both SC and TK chickens, with the latter displaying significantly higher levels at this time (P < 0.05). In contrast, following secondary infection, no significant differences in IFN-γ mRNA expression were seen in either strain. Highest levels of IFN-γ transcripts were detected in cecal tonsil lymphocytes, particularly in SC chickens, at days 4 and 6 ppi (Fig. 4B; P < 0.05). As in the spleen, no differences were noted in these cells following secondary infection. A notable exception was IELs, where gradually increasing levels of IFN-γ mRNA were observed until day 8; levels were significantly higher in SC chickens than in TK chickens at day 8 ppi, while TK chickens showing higher level of IFN-γ mRNA than SC chickens at day 8 psi (Fig. 4C) (P < 0.05).
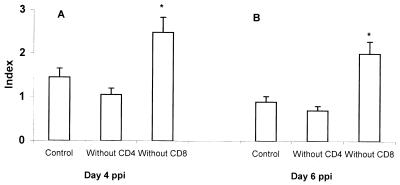
To investigate the cell type(s) producing IFN-γ in cecal tonsil lymphocytes, we infected SC chickens with 105 oocysts of E. tenella, isolated cecal tonsil lymphocytes at days 4 and 6 ppi by density gradient centrifugation, depleted CD4+ or CD8+ cells by treatment with MAb plus complement, and quantified IFN-γ mRNA in the remaining cells by RT-PCR. As shown in Fig. 5, depletion of CD4+ cells from cecal tonsil lymphocytes taken at days 4 or 6 ppi resulted in reduced IFN-γ transcript expression, whereas depletion of CD8+ cells increased mRNA expression more than twofold compared to control treatment (P < 0.05). The latter observation may be the result of a possible regulatory effect on IFN-γ mRNA expression by CD8+ cells. Furthermore, since IFN-γ mRNA expression was not completely abolished by depletion of either cell type, it is likely that lymphocytes other than CD4+ cells are involved in local IFN-γ production.
FIG. 5.
IFN-γ mRNA expression in cecal tonsil lymphocytes depleted of CD4+ or CD8+ cells. SC chickens were infected with 105 sporulated oocysts of E. tenella, and cecal tonsil lymphocytes were isolated by density gradient centrifugation at days 4 (A) and 6 (B) ppi. Cecal tonsil lymphocytes (12 chickens per group) were treated with CD4 or CD8 MAb plus complement to deplete the respective cell types. The amount of IFN-γ mRNA in 107 cells as determined in RT-PCR was quantified using the formula d = a/(a + b), where a represents the staining intensity of the target IFN-γ band and b represents the intensity of the IFN-γ competitor band. The y axis shows the index, a relative value of d normalized against an internal control, β-actin. ∗ indicates significant difference at P < 0.05.
DISCUSSION
The major findings of this study are as follows: (i) oral inoculation of chickens with E. tenella led to invasion of both the cecum and cecal tonsils, with higher fecal oocyst shedding in SC than TK chickens, (ii) greater numbers of CD4+ cells were seen in the cecal tonsils in SC compared to TK chickens following primary infection, whereas the predominance of CD8+ cells was seen in TK compared to SC chickens following secondary infection with E. tenella, (iii) SC chickens showed higher IFN-γ mRNA expression in the cecal tonsils compared to TK chickens after primary infection, and (iv) CD4+ but not CD8+ cells are primarily involved in IFN-γ production in the cecal tonsils following E. tenella infection.
The availability of inbred strains of chickens with different degrees of susceptibility to coccidiosis provides an opportunity to examine the effects of various parameters on protective immunity to coccidiosis. Major histocompatibility complex (MHC) and non-MHC genes have been shown to influence oocyst production and host immune responses to primary and secondary infections with Eimeria (6, 29). In coccidiosis, some chicken strains which are relatively susceptible to primary infection show a high degree of resistance to challenge infection, whereas others are susceptible to both primary and secondary infections (5, 29). SC and TK chickens used in this study possess different MHC genes and show different levels of susceptibility to E. acervulina (24, 25, 31, 32). The present study demonstrated that SC chickens, which are more resistant to E. acervulina, are also less susceptible to E. tenella infection compared to TK chickens. The higher resistance of SC chickens may be related to their ability to produce higher levels of antigen-specific antibodies early in infection, their enhanced T-cell response to Eimeria antigens, and/or their greater innate coccidial inhibitory activity (29, 31). Similarly, differences in resistance to Eimeria were also found in mice; resistant mice (BALB/c) produced fewer oocysts than those with a susceptible background (C57BL/6) (17, 39).
We found, rather unexpectedly, that E. tenella infects not only the cecum but also the cecal tonsils. Developing E. tenella schizonts were also seen in the bursa of Fabricius (unpublished observation). The cecal tonsils, located at the ileocecal junction, contain dense accumulations of lymphocytes and other cell types involved in antigen stimulation. Lymphocytes in cecal tonsils consist of 45 to 55% B cells and 35% T cells and are involved both in antibody production and CMI (1). Their immunological maturation and overall size are dependent on the degree of antigenic stimulation in the intestine (8). Several investigations have indicated that cecal tonsil lymphocytes may be involved in the intestinal immune response to Eimeria. For example, during E. tenella infection, an increased number of leukocytes (43) and lymphoid nodules were found in the base of cecal tonsils, accumulating as dense aggregates of lymphocytes containing irregularly scattered lymphoid tissues and germinal centers (8). Cecal tonsil lymphocytes exhibit considerable heterogeneity in surface phenotype and presumably in function (22). The presence of developing parasites in the cecal tonsils and bursa of Fabricius supports the notion that IELs are involved in the transport of coccidia to other tissues (27).
In the intestine, IELs play a critical role in a complex intercellular network during local infection processes. Cellular communication networks within the intestinal mucosa are bidirectional, with mucosal immune cells transmitting and receiving regulatory signals to and from other residents of the mucosa (46, 47). In chickens, a variety of specialized lymphoid organs (e.g., cecal tonsils and bursa of Fabricius) and cell types (epithelial, lymphoid, antigen-presenting, and natural killer cells) have evolved in the gut tissues to defend against harmful intestinal pathogens such as coccidia (27). In the study reported here, the number of CD4+ cecal tonsil lymphocytes in SC chickens increased at days 4 and 6 ppi but remained consistently low during the same time period in TK chickens. CD8+ cells, on the other hand, were noticeably more numerous in TK than in SC chickens following primary infection but significantly more numerous in SC chickens psi. It is tempting to speculate that the CD4+/CD8+ cell ratio in these two chicken strains is related to their observed differences in resistance to coccidiosis. Although the nature of effector mechanism controlling disease resistance to E. tenella remains to be clarified, it is probable that both CD4+ and CD8+ cells are involved at different phases of host protective immunity. We cannot exclude another possibility, that a small subset of CD4+ or CD8+ cells may be responsible for resistance or susceptibility to coccidiosis as shown in Cryptosporidium muris (33).
In addition, TCRγδ+ and TCRαβ+ cells also may contribute differently to the host immune response to coccidia. One of the most profound features of mucosal immunity, compared to that of other tissues, is the presence of a relatively high percentage of TCRγδ+ cells (25, 27). Intestinal TCRγδ+ cells influence the growth and differentiation of epithelial cells, as evidenced by the fact that mice lacking TCRγδ+ cells showed severely impaired development of the intestinal epithelia (4, 18). Because TCRγδ+ cells recognize ligands that do not stimulate TCRαβ+ cells, activation of TCRγδ+ cells may be inherently unique and separable from that of TCRαβ+ cells (42). In this regard, TCRγδ+ cells mediate specific cellular immune functions without the requirement for antigen processing and directly recognize invading pathogens or damaged cells (39, 45). We observed that the number of TCRγδ+ cecal tonsil lymphocytes in SC chickens was generally higher than that in TK chickens following primary and secondary infections. In contrast, TCRγδ+ cell numbers were lower in SC than TK chickens during primary infection but higher following secondary infection.
Other investigations have also characterized the dynamics of intestinal lymphocyte subpopulations as a consequence of primary or secondary infection with Eimeria (25). In E. acervulina infection, the proportions of CD4+, CD8+, and TCRγδ+ cells in duodenal IELs were significantly increased in chickens inoculated with E. acervulina (2). After primary E. tenella infection, TCRαβ+ IELs appeared to be the main responding cell type in the cecum (44), whereas both TCRαβ+ and TCRγδ+ cells were found to cluster around sporozoites after secondary infection. However, the relative importance of these cells to resistance or susceptibility to coccidiosis was not clear. In murine E. vermiformis infection, TCRαβ+ knockout mice displayed defects in protective immunity whereas TCRγδ knockout mice showed exaggerated intestinal damage, apparently due to a failure to regulate the consequences of the T-cell response. However, Rose et al. (38) concluded that TCRγδ+ lymphocytes are not crucial to the establishment or control of primary infection with E. vermiformis.
With regard to the activities of cytokines during avian coccidiosis, IFN-γ appears to play the predominant role (7, 26). Chicken IFN-γ, like its mammalian homologue, regulates acquired immunity to Eimeria by activating lymphocytes and enhancing expression of MHC class II genes (21, 41). IFN-γ production in mice (35) and chickens (31, 50) has been used as a measure of T-cell responses to Eimeria antigens. Successful cloning of chicken IFN-γ and expression of a functional recombinant IFN-γ protein (9, 19, 20, 41) will undoubtedly lead to further understanding of its physiologic and immunologic roles in coccidiosis (26, 30). In a recent study, recombinant chicken IFN-γ protected chick fibroblasts from virus-mediated lysis, induced nitrite secretion from macrophages in vitro, and enhanced MHC class II antigen expression on macrophages (21, 32, 41).
In a previous study, we demonstrated that IFN-γ production in the intestine was higher in the intestinal tissues where coccidia develop, IFN-γ mRNA expression was significantly elevated in infected chickens compared to uninfected controls, IFN-γ levels were increased in SC compared to TK chickens, and production of this cytokine was seen in the intestine prior to the circulation (50). Correlation of disease resistance with early local production of IFN-γ indicates an important role of this cytokine in protective immunity. In the present study, we extended this concept of site-specific immunity to coccidia by quantifying the expression of IFN-γ mRNA in various lymphoid tissues where E. tenella undergoes intracellular development. Using a sensitive RT-PCR, IFN-γ transcript levels were shown to be higher in the cecal tonsils than in the spleen or IELs during the course of infection, particularly in SC compared to TK chickens.
The ability of SC chickens to express greater levels of IFN-γ transcripts in cecal tonsil lymphocytes may be related to their enhanced disease resistance. IFNs have been reported to be inimical to parasites, probably because of their ability to inhibit parasite development (11, 26), promote production of free radicals (10, 34), activate antibody-dependent cell-mediated cytotoxicity (14), and/or promote the release of cytoplasmic granules containing perforin and proteases (15, 16). In any event, the data presented here support the hypothesis that local IFN-γ production at the sites of parasite infestation is an important component of the host immune response to coccidia. Future studies to identify the nature of cell(s) involved in IFN-γ production (23, 24, 40) and their interaction in eliciting local protective immunity to coccidia will lead to logical control strategies against this disease.
ACKNOWLEDGMENTS
We thank Erik P. Lillehoj for critical review of the manuscript.
This work was supported by CSRS USDA NRI grant 98-35204-6471.
REFERENCES
- 1.Befus A D, Johnston N, Leslie G A, Bienenstock J. Gut-associated lymphoid tissue in the chicken. I. Morphology, ontogeny, and some functional characteristics of Peyer's patches. J Immunol. 1980;125:2626–2632. [PubMed] [Google Scholar]
- 2.Bessay M, Le Vern Y, Kerboeuf D, Yvore P, Quere P. Changes in intestinal intra-epithelial and systemic T-cell subpopulations after an Eimeria infection in chickens: comparative study between E. acervulina and E. tenella. Vet Res. 1996;27:503–514. [PubMed] [Google Scholar]
- 3.Bhogal B S, Jacobson E B, Tse H Y, Schmatz D M, Ravino O J. Parasite exposure elicits a preferential T-cell response involved in protective immunity against Eimeria species in chickens primed by an internal-image anti-idiotypic antibody. Infect Immun. 1989;57:2804–2810. doi: 10.1128/iai.57.9.2804-2810.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boismenu R, Havran W L. Modulation of epithelial cell growth by intraepithelial T cells. Science. 1994;266:1253–1255. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- 5.Bumstead N, Millard B J. Variation in susceptibility of inbred lines of chickens to seven species of Eimeria. Parasitology. 1992;104:407–413. doi: 10.1017/s0031182000063654. [DOI] [PubMed] [Google Scholar]
- 6.Bumstead N, Millard B. Genetics of resistance to coccidiosis: response of inbred chicken lines to infection by Eimeria tenella and Eimeria maxima. Br Poult Sci. 1987;28:705–715. doi: 10.1080/00071668708417006. [DOI] [PubMed] [Google Scholar]
- 7.Choi K D, Lillehoj H S, Zalenga D S. Changes in local IFN-γ and TGF-β4 mRNA expression and intraepithelial lymphocytes following Eimeria acervulina infection. Vet Immunol Immunopathol. 1999;71:263–275. doi: 10.1016/s0165-2427(99)00103-8. [DOI] [PubMed] [Google Scholar]
- 8.del Cacho E, Gallego M, Sanz A, Zapata A. Characterization of distal lymphoid nodules in the chicken caecum. Anat Rec. 1993;237:512–517. doi: 10.1002/ar.1092370411. [DOI] [PubMed] [Google Scholar]
- 9.Digby M R, Lowenthal J W. Cloning and expression of the chicken interferon-gamma gene. J Interferon Cytokine Res. 1995;15:939–945. doi: 10.1089/jir.1995.15.939. [DOI] [PubMed] [Google Scholar]
- 10.Dimier I H, Bout D T. Inhibition of Toxoplasma gondii replication in IFN-activated human intestinal epithelial cells. Immunol Cell Biol. 1997;75:511–514. doi: 10.1038/icb.1997.80. [DOI] [PubMed] [Google Scholar]
- 11.Fayer R. Quinine inhibition of host cell penetration by Eimerian sporozoites in vitro. J Parasitol. 1971;57:901–905. [PubMed] [Google Scholar]
- 12.Findly R C, Roberts S J, Hayday A C. Dynamic response of murine gut intraepithelial T cells after infection by the coccidian parasite Eimeria. Eur J Immunol. 1993;23:2557–2564. doi: 10.1002/eji.1830231027. [DOI] [PubMed] [Google Scholar]
- 13.Fitzgerald P R. The economic impact of coccidiosis in domestic animals. Adv Vet Sci Comp Med. 1980;24:121–143. [PubMed] [Google Scholar]
- 14.Fleischer B. Effector cells in avian spontaneous and antibody-dependent cell-mediated cytotoxicity. J Immunol. 1980;125:1161–1166. [PubMed] [Google Scholar]
- 15.Gabay J E, Almeida R P. Antibiotic peptides and serine protease homologs in human polymorphonuclear leukocytes: defensins and azurocidin. Curr Opin Immunol. 1993;5:97–102. doi: 10.1016/0952-7915(93)90087-9. [DOI] [PubMed] [Google Scholar]
- 16.Hudig D, Ewoldt G R, Woodard S L. Proteases and lymphocyte cytotoxic killing mechanisms. Curr Opin Immunol. 1993;5:90–96. doi: 10.1016/0952-7915(93)90086-8. [DOI] [PubMed] [Google Scholar]
- 17.Joysey H S, Wakelin D, Rose M E. Resistance to infection with Eimeria vermiformis in mouse radiation chimeras is determined by donor bone-marrow cells. Infect Immun. 1988;56:1399–1401. doi: 10.1128/iai.56.5.1399-1401.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kagnoff M F. Current concepts in mucosal immunity. III. Ontogeny and function of T cells in the intestine. Am J Physiol. 1998;274:G455–G458. doi: 10.1152/ajpgi.1998.274.3.G455. [DOI] [PubMed] [Google Scholar]
- 19.Kaiser P, Sonnemans D, Smith L M. Avian IFN-γ genes: sequence analysis suggests probable cross-species reactivity among galliforms. J Interferon Cytokine Res. 1998;18:711–719. doi: 10.1089/jir.1998.18.711. [DOI] [PubMed] [Google Scholar]
- 20.Kaiser P, Wain H M, Rothwell L. Structure of the chicken interferon-gamma gene, and comparison to mammalian homologues. Gene. 1998;207:25–32. doi: 10.1016/s0378-1119(97)00600-8. [DOI] [PubMed] [Google Scholar]
- 21.Kaspers B, Lillehoj H S, Jenkins M C, Pharr G T. Chicken interferon-mediated induction of major histocompatibility complex class II antigens on peripheral blood monocytes. Vet Immunol Immunopathol. 1994;44:71–84. doi: 10.1016/0165-2427(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 22.Klein J R. Whence the intestinal intraepithelial lymphocyte? J Exp Med. 1996;184:1203–1206. doi: 10.1084/jem.184.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar P A, Das S K, Rao J R. Effect of immunostimulation on cytotoxic activity of intestinal intraepithelial lymphocytes of chickens in infectious bursal disease and Eimeria tenella infections. Acta Vet Hung. 1998;46:1–11. [PubMed] [Google Scholar]
- 24.Lillehoj H S. Intestinal intraepithelial and splenic natural killer cell responses to eimerian infections in inbred chickens. Infect Immun. 1989;57:1879–1884. doi: 10.1128/iai.57.7.1879-1884.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lillehoj H S. Analysis of Eimeria acervulina-induced changes in the intestinal T lymphocyte subpopulations in two chicken strains showing different levels of susceptibility to coccidiosis. Res Vet Sci. 1994;56:1–7. doi: 10.1016/0034-5288(94)90188-0. [DOI] [PubMed] [Google Scholar]
- 26.Lillehoj H S, Choi K D. Recombinant chicken interferon-gamma-mediated inhibition of Eimeria tenella development in vitro and reduction of oocyst production and body weight loss following Eimeria acervulina challenge infection. Avian Dis. 1998;42:307–314. [PubMed] [Google Scholar]
- 27.Lillehoj H S, Trout J M. Avian gut-associated lymphoid tissues and intestinal immune responses to Eimeria parasites. Clin Microbiol Rev. 1996;9:349–360. doi: 10.1128/cmr.9.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lillehoj H S, Lillehoj E P, Weinstock D, Schat K A. Functional and biochemical characterizations of avian T lymphocyte antigens identified by monoclonal antibodies. Eur J Immunol. 1988;18:2059–2065. doi: 10.1002/eji.1830181228. [DOI] [PubMed] [Google Scholar]
- 29.Lillehoj H S, Ruff M D, Bacon L D, Lamont S J, Jeffers T K. Genetic control of immunity to Eimeria tenella. Interaction of MHC genes and non-MHC linked genes influences levels of disease susceptibility in chickens. Vet Immunol Immunopathol. 1989;20:135–148. doi: 10.1016/0165-2427(89)90094-9. [DOI] [PubMed] [Google Scholar]
- 30.Lowenthal J W, York J J, O'Neil T E, Rhodes S, Prowse S J, Strom D G, Digby M R. In vivo effects of chicken interferon-gamma during infection with Eimeria. J Interferon Cytokine Res. 1997;17:551–558. doi: 10.1089/jir.1997.17.551. [DOI] [PubMed] [Google Scholar]
- 31.Martin A, Lillehoj H S, Kaspers B, Bacon L D. Mitogen-induced lymphocyte proliferation and interferon production following coccidia infection. Avian Dis. 1994;38:262–268. [PubMed] [Google Scholar]
- 32.Martin A, Awadalla S, Lillehoj H S. Characterization of cell-mediated responses to Eimeria acervulina antigens. Avian Dis. 1995;39:538–547. [PubMed] [Google Scholar]
- 33.McDonald V, Robinson H A, Kelly J P, Bancroft G J. Cryptosporidium muris in adult mice: adoptive transfer of immunity and protective roles of CD4 versus CD8 cells. Infect Immun. 1994;62:2289–2294. doi: 10.1128/iai.62.6.2289-2294.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ovington K S, Smith N C. Cytokines, free radicals and resistance to Eimeria. Parasitol Today. 1992;8:422–426. doi: 10.1016/0169-4758(92)90196-9. [DOI] [PubMed] [Google Scholar]
- 35.Rose M E, Smith A L, Wakelin D. Gamma interferon-mediated inhibition of Eimeria vermiformis growth in cultured fibroblasts and epithelial cells. Infect Immun. 1991;59:580–586. doi: 10.1128/iai.59.2.580-586.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rose M E, Wakelin D, Hesketh P. Eimeria vermiformis: differences in the course of primary infection can be correlated with lymphocyte responsiveness in the BALB/c and C57BL/6 mouse, Mus musculus. Exp Parasitol. 1990;71:276–283. doi: 10.1016/0014-4894(90)90032-8. [DOI] [PubMed] [Google Scholar]
- 37.Rose M E, Hesketh P, Wakelin D. Immunization against experimental coccidiosis produces contrasting results in inbred mice of differing susceptibility to infection. Infect Immun. 1994;62:733–737. doi: 10.1128/iai.62.2.733-737.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rose M E, Hesketh P, Rothwell L, Gramzinski R A. T-cell receptor lymphocytes and Eimeria vermiformis infection. Infect Immun. 1996;64:4854–4858. doi: 10.1128/iai.64.11.4854-4858.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schild H, Mavaddat N, Litzenberger C, Ehrich E W, Davis M M, Bluestone J A, Matis L, Draper R K, Chien Y H. The nature of major histocompatibility complex recognition by T cells. Cell. 1994;76:29–37. doi: 10.1016/0092-8674(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 40.Smith A L, Rose M E, Wakelin D. The role of natural killer cells in resistance to coccidiosis: investigations in a murine model. Clin Exp Immunol. 1994;97:273–279. doi: 10.1111/j.1365-2249.1994.tb06080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song K D, Lillehoj H S, Choi K D, Zarlenga D, Han J Y. Expression and functional characterization of recombinant chicken interferon-gamma. Vet Immunol Immunopathol. 1997;58:321–333. doi: 10.1016/s0165-2427(97)00034-2. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka Y, Morita C T, Tanaka Y, Nieves E, Brenner M B, Bloom B R. Natural and synthetic non-peptide antigens recognized by human T cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 43.Vervelde L, Vermeulen A N, Jeurissen S H. In situ characterization of leucocyte subpopulations after infection with Eimeria tenella in chickens. Parasite Immunol. 1996;18:247–256. doi: 10.1046/j.1365-3024.1996.d01-94.x. [DOI] [PubMed] [Google Scholar]
- 44.Vervelde L, Jeurissen S H. The role of intra-epithelial and lamina propria leucocytes during infection with Eimeria tenella. Adv Exp Med Biol. 1995;371B:953–958. [PubMed] [Google Scholar]
- 45.Wallach M, Smith N C, Petracca M, Miller C M, Eckert J, Braun R. Eimeria maxima gametocyte antigens: potential use in a subunit maternal vaccine against coccidiosis in chickens. Vaccine. 1995;13:347–354. doi: 10.1016/0264-410x(95)98255-9. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Klein J R. Thymus-neuroendocrine interactions in extrathymic T cell development. Science. 1994;265:1860–1862. doi: 10.1126/science.8091211. [DOI] [PubMed] [Google Scholar]
- 47.Wang J, Whetsell M, Klein J R. Local hormone networks and intestinal T cell homeostasis. Science. 1997;275:1937–1939. doi: 10.1126/science.275.5308.1937. [DOI] [PubMed] [Google Scholar]
- 48.Weining K C, Schultz U, Munster U, Kaspers B, Staeheli P. Biological properties of recombinant chicken interferon-gamma. Eur J Immunol. 1996;26:2440–2447. doi: 10.1002/eji.1830261026. [DOI] [PubMed] [Google Scholar]
- 49.Yun C H, Estrada A, Van Kessel A, Gajadhar A, Redmond M, Laarveld B. Immunomodulatory effects of oat beta-glucan administered intragastrically or parenterally on mice infected with Eimeria vermiformis. Microbiol Immunol. 1998;42:457–465. doi: 10.1111/j.1348-0421.1998.tb02309.x. [DOI] [PubMed] [Google Scholar]
- 50.Yun, C. H., H. S. Lillehoj, and K. D. Choi. Chicken IFN-γ monoclonal antibodies and their application in enzyme-linked immunosorbent assay. Vet. Immunol. Immunopathol., in press. [DOI] [PubMed]