Abstract
Pancreatic solid pseudopapillary neoplasm (SPN) is a rare malignant tumour predominantly affecting young women. The occurrence of peritoneal carcinomatosis (PC) in this setting is an even rarer condition, usually related to perioperative tumour rupture. We present a case of a 43-year-old woman who previously underwent distal splenopancreatectomy after the diagnosis of a pancreatic SPN. Thirteen years later, the patient underwent a radical hysterectomy due to a uterine myoma. Intraoperatively, a peritoneal mass was additionally found and resected. Histological examination revealed an implant with morphology compatible with pancreatic SPN. The patient was then referred to our institution. Staging MRI and CT revealed multiple nodular lesions adjacent to the left colon, suggestive of peritoneal implants. The patient was then submitted to cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) with oxaliplatin and irinotecan. Histological examination confirmed peritoneal involvement by a pancreatic SPN. The postoperative course was unremarkable. Two years after surgery, the patient remains asymptomatic with no evidence of relapse. Despite SPN being cancer with a relatively indolent evolution, one needs to be aware of a possible recurrence several years after the primary resection, mainly in patients with evidence of intraoperative tumour rupture.
Keywords: late recurrence, peritoneal metastasis, hyperthermic intraperitoneal chemotherapy, cytoreduction surgical procedures, pancreatic neoplasms
Introduction
Solid pseudopapillary neoplasm (SPN) of the pancreas is a rare malignant tumour, comprising only 0.13% to 2.7% of all pancreatic neoplasms [1,2]. It predominantly affects young women between 30 to 40 years of age, with a female/male ratio of 8.25-9.78/1 [1,3]. Most patients are diagnosed at an early stage, abdominal pain being the most common complaint [1,3]. Distant metastases occur in 10-15% of patients [2], but peritoneal carcinomatosis (PC) has been reported in only 12 cases [2,4-14]. Although early-stage disease and even locally advanced or metastatic disease are often amenable to complete surgical resection and are associated with long-term survival, the approach to PC is not well established, frequently with disappointing results, even with complete cytoreductive surgery (CCRS) [2]. We present a case of a late recurrence of pancreatic SPN with PC treated with CCRS and hyperthermic intraperitoneal chemotherapy (HIPEC).
This case was previously presented as a meeting abstract/poster at the 40th Congress of the European Society of Surgical Oncology in Lisbon from November 8-10, 2021.
Case presentation
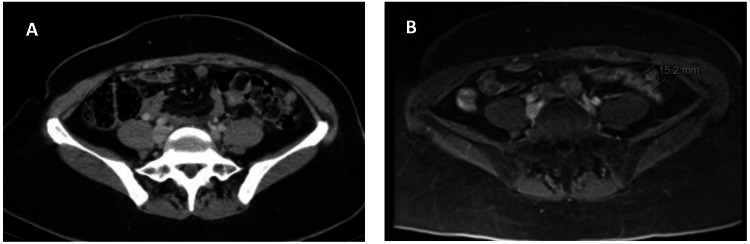
We present a case of a 43-year-old woman previously submitted to distal splenopancreatectomy when she was 30, following the diagnosis of a pancreatic tail SPN in another institution. Thirteen years after the surgery, the patient was submitted to a radical hysterectomy due to a uterine myoma. Intraoperatively, a peritoneal mass of 2.4 x 2.0 cm was incidentally found and resected. Histological examination revealed an implant with morphology compatible with the peritoneal recurrence of a pancreatic SPN. The patient was then referred to our institution. She had no remarkable medical history besides arterial hypertension and type 2 diabetes mellitus. Staging magnetic resonance imaging (MRI) and computed tomography (CT) revealed multiple nodules adjacent to the left colon, suggestive of peritoneal implants (Figure 1)
Figure 1. Preoperative CT (A) and MRI (B) scans revealing metastatic peritoneal nodules adjacent to the left colon.
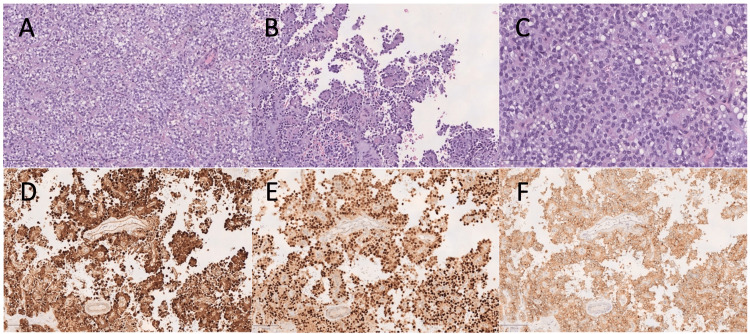
Intraoperatively, the peritoneal carcinomatosis index was 5. The patient was then submitted to CCRS combined with HIPEC with oxaliplatin (360mg/m2) and irinotecan (360mg/m2) at a temperature of 43ºC for 30 min. Histological examination confirmed the involvement of the great omentum, right diaphragmatic peritoneum and multiple peritoneal fragments by a pancreatic SPN (Figure 2).
Figure 2. Histological appearance of one of the neoplastic implants in the great omentum.
Areas of solid (H&E, 100x) (A) and pseudopapillary patterns (H&E, 100x) (B) were seen, with associated fibrovascular stalks containing variable degree of myxoid change and hyalinization. Cells displayed ovoid nuclei with fine chromatin and characteristic longitudinal grooves; the cytoplasm was occasionally vacuolated and exhibited hyaline globules (H&E, 200x) (C). Nuclear and cytoplasmic expression of β-catenin was demonstrated by immunohistochemistry (100x) (D). Additionally, expression of cyclin D1 (100x) (E) and CD10 (100x) (F) was observed. Synaptophysin was expressed focally, whereas chromogranin expression was absent (not shown).
H&E: hematoxylin and eosin stain
The postoperative course was unremarkable, and no adjuvant chemotherapy was administered. The patient remains asymptomatic with no evidence of relapse at two years follow-up.
Discussion
Pancreatic SPN is a rare malignant tumour, and only 12 cases were reported in the literature with progression to PC during the natural history of the disease [2,4-14]. Tumour spillage in the context of intra-operative tumour rupture has been associated with peritoneal recurrence [2]. Data concerning the initial distal splenopancreatectomy in the current case performed in another institution are not available. Peritoneal recurrence after tumour rupture may occur 1 to 19 years after the primary surgery [2], which may raise the issue of a longer follow-up time than other cancer types typically occurring in the gastrointestinal tract, especially in the pancreas.
Since it is a relatively indolent tumour, surgery yields very good long-term results with five-year overall survival (OS) of 93.4-100%, even in the presence of locally advanced or metastatic disease [2,15,16]. Locoregional recurrence occurred in 100% of cases after an R2 resection or tumour rupture [17]. Neoadjuvant treatments have been used to shrink the tumour and avoid intra-operative spillage but their role remains unclear [4]. All these features should prompt an aggressive primary surgical approach to achieve the best oncological results.
Patients with PC from pancreatic SPN treated with surgery alone had disappointing results, with a peritoneal recurrence rate of 58% (7/12). The real recurrence rate might be underestimated owing to the short follow-up time reported in the literature. Nevertheless, none of these patients developed other distant metastasis.
We decided to add HIPEC with oxaliplatin and irinotecan similar to the approach previously described, and after a 24-months follow-up, the patient displays no signs of recurrence [2].
We need to have a longer follow-up to confirm that performing a CCRS-HIPEC is a valid option for patients with pancreatic SPN PC. Nevertheless, the two patients treated with this modality in the literature remain alive, with no signs of recurrence [2].
Conclusions
In spite of the indolent evolution of SPN, there should be a close follow-up, considering a possible recurrence several years later, as it can give rise to extensive peritoneal disease. A well-performed first surgical excision, without tumour spillage, is crucial to avoid a peritoneal recurrence. Nonetheless, the surgery-only approach to patients with PC has yielded disappointing results, with high rates of peritoneal recurrence. Despite the scant data in the literature, an approach with CCRS plus HIPEC might be a more efficient approach to treating patients with PC. Nevertheless, this approach should be further validated by prospective trials in order to properly assess the role of CCRS and HIPEC in this group of patients.
Acknowledgments
Authors Contributions Jorge Nogueiro was responsible for manuscript writing and clinical case orientation, Fábio Gomes for manuscript writing and clinical case orientation, João Pacheco for histological examination, Hugo Santos-Sousa for manuscript writing, Sara Meireles for clinical case orientation, Renato Bessa Melo for clinical case orientation, Marisa Aral for manuscript writing and clinical case orientation, and Barbosa E was responsible for clinical case orientation.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. Papavramidis T, Papavramidis S. https://pubmed.ncbi.nlm.nih.gov/15922212/ J Am Coll Surg. 2005;200:965–972. doi: 10.1016/j.jamcollsurg.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Peritoneal carcinomatosis from solid pseudopapillary neoplasm (Frantz's tumour) of the pancreas treated with HIPEC. Honore C, Goere D, Dartigues P, Burtin P, Dumont F, Elias D. https://ar.iiarjournals.org/content/32/3/1069. Anticancer Res. 2012;32:1069–1073. [PubMed] [Google Scholar]
- 3.Surgical management of solid-pseudopapillary neoplasms of the pancreas (Franz or Hamoudi tumors): a large single-institutional series. Reddy S, Cameron JL, Scudiere J, et al. J Am Coll Surg. 2009;208:950–957. doi: 10.1016/j.jamcollsurg.2009.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malignant potential of solid pseudopapillary neoplasm of the pancreas. Tipton SG, Smyrk TC, Sarr MG, Thompson GB. Br J Surg. 2006;93:733–737. doi: 10.1002/bjs.5334. [DOI] [PubMed] [Google Scholar]
- 5.Adenocarcinoma of the pancreas of childhood: a report of two cases. Benjamin E, Wright DH. Histopathology. 1980;4:87–104. doi: 10.1111/j.1365-2559.1980.tb02901.x. [DOI] [PubMed] [Google Scholar]
- 6.Frantz's tumor: a papillary and cystic tumor of the pancreas in girls. Todani T, Shimada K, Watanabe Y, Toki A, Fujii T, Urushihara N. J Pediatr Surg. 1988;23:116–121. doi: 10.1016/s0022-3468(88)80137-4. [DOI] [PubMed] [Google Scholar]
- 7.Papillary cystic neoplasm of the pancreas. A report of a case presenting with carcinomatosis. Hernandez-Maldonado JJ, Rodriguez-Bigas MA, Gonzalez de Pesante A, Vazquez-Quintana E. https://pubmed.ncbi.nlm.nih.gov/2774363/ Am Surg. 1989;55:552–559. [PubMed] [Google Scholar]
- 8.Malignant papillary cystic tumor of the pancreas. Cappellari JO, Geisinger KR, Albertson DA, Wolfman NT, Kute TE. https://doi.org/10.1002/1097-0142(19900701)66:1<193::AID-CNCR2820660134>3.0.CO;2-6. Cancer. 1990;66:193–198. doi: 10.1002/1097-0142(19900701)66:1<193::aid-cncr2820660134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 9.Papillary-cystic neoplasm of the pancreas. A clinicopathologic study concerning the tumor aging and malignancy of nine cases. Matsunou H, Konishi F. http://10.1002/1097-0142(19900115)65:2<283::aid-cncr2820650217>3.0.co;2-x. Cancer. 1990;65:283–291. doi: 10.1002/1097-0142(19900115)65:2<283::aid-cncr2820650217>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 10.Papillary cystic tumors of the pancreas. Assessment of their malignant potential. Nishihara K, Nagoshi M, Tsuneyoshi M, Yamaguchi K, Hayashi I. Cancer. 1993;71:82–92. doi: 10.1002/1097-0142(19930101)71:1<82::aid-cncr2820710114>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 11.Frantz's tumor (solid and cystic tumor of the pancreas) with liver metastasis: successful treatment and long-term follow-up. Horisawa M, Niinomi N, Sato T, et al. J Pediatr Surg. 1995;30:724–726. doi: 10.1016/0022-3468(95)90701-7. [DOI] [PubMed] [Google Scholar]
- 12.Diffuse peritoneal carcinosis of pseudo-papillary and solid tumor of the pancreas. Role of abdominal injury (Article in French) Lévy P, Bougaran J, Gayet B. https://pubmed.ncbi.nlm.nih.gov/9587521/ Gastroenterol Clin Biol. 1997;21:789–793. [PubMed] [Google Scholar]
- 13.Solid-cystic papillary tumor of the pancreas in children. Zhou H, Cheng W, Lam KY, Chan GC, Khong PL, Tam PK. Pediatr Surg Int. 2001;17:614–620. doi: 10.1007/s003830100005. [DOI] [PubMed] [Google Scholar]
- 14.Peritoneal metastatic disease in a child after excision of a solid pseudopapillary tumour of the pancreas: a unique case. Andronikou S, Moon A, Ussher R. Pediatr Radiol. 2003;33:269–271. doi: 10.1007/s00247-003-0875-z. [DOI] [PubMed] [Google Scholar]
- 15.Solid pseudopapillary neoplasm of the pancreas: a rare entity with unique features. Dinarvand P, Lai J. Arch Pathol Lab Med. 2017;141:990–995. doi: 10.5858/arpa.2016-0322-RS. [DOI] [PubMed] [Google Scholar]
- 16.Solid pseudopapillary neoplasm of the pancreas: management and long-term outcome. Lubezky N, Papoulas M, Lessing Y, et al. Eur J Surg Oncol. 2017;43:1056–1060. doi: 10.1016/j.ejso.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Solid pseudopapillary tumors of the pancreas. Clinical features, surgical outcomes, and long-term survival in 45 consecutive patients from a single center. Butte JM, Brennan MF, Gönen M, et al. J Gastrointest Surg. 2011;15:350–357. doi: 10.1007/s11605-010-1337-1. [DOI] [PubMed] [Google Scholar]