Abstract
The reported numbers of Covid‐19 cases and deaths were compared for 18 countries (14 in Western Europe, plus Australia, Brazil, Israel and the USA) to assess the effect of historic and current national BCG immunizations. In view of the high death rate for Covid‐19 patients over 70 years of age, and given the fact that BCG vaccination is typically given early in life, we compared countries that had introduced BCG in the 1950s with those that had not. No effect on Covid‐19 case fatality rate (CFR) or number of deaths per population could be demonstrated. Since some countries test for Covid‐19 more than others, the effect of tests performed per million population on reported deaths per million was also assessed, but again did not demonstrate an effect of BCG vaccination in the 1950s. Whether countries had never used the vaccine, had historically used it but since ceased to do so, or were presently vaccinating with BCG did not correlate with national total number of deaths or CFR. We conclude that there is currently no evidence for a beneficial effect of BCG vaccination on Covid‐19 reported cases or fatalities.
Keywords: BCG, Covid‐19, Mycobacterium tuberculosis vaccine, Sars‐CoV‐2, TBC, trained immunity, tuberculosis
Introduction
Bacillus Calmette‐Guérin (BCG) is a vaccine containing an attenuated strain of Mycobacterium bovis that has been in use for nearly a century, with some genetic variations accruing in the strains being used (Crispen 1989; Luca and Mihaescu 2013). BCG was designed to protect against tuberculosis (Mycobacterium tuberculosis), but the vaccine can result in immune protection well beyond its target organism (Netea and Crevel 2014). This can be explained by a third leg of the immune system that is activated upon microbial exposure besides the classical native and adapted immunity and for which the name ‘trained immunity’ was coined. Trained immunity does not involve permanent genetic changes but depends on epigenetic and transcriptional changes that are generally relatively short‐lived ((Netea et al. 2016, Netea et al. 2020). Neonatal vaccination with BCG can reduce infant mortality rates from other bacterial infections, as was demonstrated in Guinea‐Bissau (Kristensen et al. 2000). More importantly, the BCG anti‐bacterial vaccine was shown to reduce viremic loads of attenuated yellow fever virus in experimentally infected individuals, presumably due to epigenetic reprogramming of parts of the immune system (Arts et al. 2018). This broad immunity enhancement has recently led researchers to propose that BCG vaccination might be beneficial for combating Covid‐19 (Curtis et al. 2020).
The mounting evidence for the relationship between BCG vaccination and Covid‐19 is based on an inferred association among two somewhat ambiguous data sets; reports of national BCG vaccine coverage and current Covid‐19 reporting (Ozdemir et al. 2020; Gursel and Gursel 2020), although none of the submitted papers to date have acknowledged the numerous confounders that make these data so ambiguous. These variables, such as the differences in testing strategies, reporting biases, a nation's ability to respond to the pandemic, prevalence of co‐morbidities and different stages of the pandemic across various countries, will all have significant impacts on suspected correlations between BCG vaccination and Covid‐19 severity, and thus all must be looked at critically to avoid confirmational bias in interpretations. Demography is a further factor affecting the Covid‐19 case fatality rate (CFR), especially when comparing countries with an ageing population versus countries with a lower average age. Lastly, in some countries the epidemic is further advanced than in others, which further affects the CFR: early in the epidemic the deaths, which can take weeks to occur, lag behind reported infections and are typically low, then CFR increases as patients begin succumbing. Therefore, at this still early stage of the pandemic, the association between Covid‐19 manifestations and BCG vaccination should be considered as a hypothesis only, and should be tested through appropriate carefully designed studies (Kumar and Meena 2020).
Covid‐19 is currently manifested in the human population in four distinct ways. Approximately 15–20% (this number is in flux) of the population that becomes infected will be asymptomatic or paucisymptomatic (Keeley et al. 2020). Upon admission to hospital, 92% of patients have symptoms, and 8% are asymptomatic (Mei et al. 2020). Another 60% (range 40–80%) will have a range of mild symptoms (Chen et al. 2020a), but even so might be incurring serious organ damage that will haunt them later in life (Mitrani et al. 2020). Another roughtly 10‐25% of cases will have severe to critical symptoms resulting in pneumonia leading to acute respiratory distress (ARDS), coagulopathies, and a form of hyperactive immune response termed a cytokine storm (Ragab et al. 2020; Mangalmurti and Hunter 2020; Guan et al. 2020; Guo et al. 2020). Finally, roughly 3–5% of confirmed symptomatic cases of Covid‐19 will die from multiple organ failures or other sequelae. The CFR for confirmed Covid‐19 cases reported to WHO stands at 4·33% (13 405 694 confirmed cases and 580 552 deaths) (15 July 2020; https://coronavirus.jhu.edu/map.html). A report from the United States Centers for Disease Control and Prevention COVID‐19 Response Team showed that 80% of deaths associated with COVID‐19 were among adults aged ≥65 years; a major European report found the number to be even higher, 91%; and if the 8% of deaths among 45–64 is included, 99% of all Covid‐19 deaths are accounted for, and the majority of had specific comorbidities that further predisposed them to the cytokine storm (Vestergaard et al. 2020).
Preventing, slowing, stopping and reversing the Covid‐19 cytokine storm is now the number one goal for scientists and physicians around the world, and there have been dozens, if not hundreds, of suggestions as how to do it. One of these has been the suggestion to use the BCG vaccine to prophylactically limit the infection and prevent the storm. Clinical trials have now been initiated to assess if BCG vaccination can achieve those goals. Until more data are available, the WHO currently recommends using BCG vaccination only in carefully monitored randomized clinical trials (WHO 2020).
If the hypothesis that BCG vaccination affects severity and outcome of Covid‐19 is correct, then this should be best reflected by lower infection fatality rates reported by various countries that included this vaccine in their national vaccination programs, currently or in the past. One publication described such an association (Ozdemir et al. 2020), where countries that currently include BCG in their national vaccination programs were compared to countries that excluded it. However, that comparison was skewed, as most of the included countries currently practicing BCG vaccination are in Africa where the epidemic arrived later and cases so far have more often remained unreported, while all the countries included in the analysis without current nation‐wide BCG vaccination programs were in Europe, where the epidemic is significantly more advanced and reporting should have been more inclusive (though by no means complete). A similar approach was followed by Gursel and Gursel (2020), who compared (mostly European) countries that have never routinely used BCG, or had ceased to do so, with countries that continued its use in immunization programs. They compared the number of cases and deaths per million, which was lower for countries with current BCG vaccination (P < 0·0001). Countries that had ceased using the vaccine in the last two decades had significantly (P = 0·109) fewer Covid‐19 confirmed deaths per million cases compared to countries that stopped three to four decades ago (Gursel and Gursel 2020).
It is now well‐established that Covid‐19 is the most serious for individuals who are at the higher age spectrum, have underlying conditions, such as cardiovascular disease, obesity, diabetes or a combination of these, are male, and are regular smokers (Chen et al. 2020). This does not mean that young, healthy individuals cannot be infected, only they do not appear to suffer from severe symptoms as frequently as the aforementioned patient groups, and age cohorts younger than 60 show much lower fatality rates (The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team 2019; CDC COVID‐19 Response Team 2020, Aggarwal et al. 2020). For these reasons, it is not informative to compare fatality rates between countries that currently use BCG vaccination on a national scale with countries that do not, as vaccines are typically administered at a young age, while the Covid‐19 fatalities are restricted mostly to elderly patients. For a correlation to be assessed between the severity of Covid‐19 and the vaccination state, we suggest that the analysis should concentrate on people who are at the higher end of the age spectrum, who would or would not have been vaccinated in their youth.
With this in mind, and being aware of all the caveats listed above, we compared some epidemiological parameters of Covid‐19 between countries and took the historic BCG vaccination data into account. We assessed whether nationally reported Covid‐19 cases and fatality rates correlated with BCG vaccination for individuals who were born from 1950 and onwards and who are therefore younger than 70. Before that year, few countries employed the vaccine, and Covid‐19 patients born prior to 1945 will have a substantial risk of dying, regardless of any protective effect of previous vaccinations. Hence, we compared the reported incidence and outcome of Covid‐19 infections for a number of countries that are well into the developing pandemic and compared the BCG vaccination programs in place since the 1950s to assess if a protective effect of BCG vaccination could be demonstrated.
Results and discussion
The 18 countries we compared included those reporting >100 000 total cases of Covid‐19 on 7 May 2020 (USA, Spain, Italy, UK, Russia, France, Germany, Brazil, Turkey and Iran). Of these, the UK and France had BCG vaccination programs in place from the 1950s onwards. In order to include countries that had relatively low reported numbers of cases while the epidemic was nationally far enough advanced in time, Austria, Ireland and Israel were included, all three of which had BCG vaccination programs introduced in the 1950s. To include countries not using BCG in the 1950s, which could be compared to these in terms of population size, Portugal, the Netherlands, Belgium and Switzerland were added. Furthermore, Australia was included because they had a vaccination regime in place since the 1950s that was similar to that of the UK. The dates countries introduced BCG vaccination (if at all) is summarized in Table 1.
Table 1.
Data used in this study
| Covid‐19 cases* | Covid‐19 deaths* | Deaths/mil pop* | Nationwide BCG vaccination† | Strain† | Current vaccination | |
|---|---|---|---|---|---|---|
| USA | 1 292 623 | 76 928 | 232 | Never | Not applicable | No |
| Spain | 256 855 | 26 070 | 558 | From 1965, at birth | Danish | No |
| Italy | 215 858 | 29 958 | 495 | Never | Not applicable | No |
| UK | 206 715 | 30 615 | 451 | From 1953, at age 12–14 years | Evans Medical/Medeva | No |
| Russia | 177 160 | 1625 | 11 | Start date unknown, at birth | Not specified | Yes |
| France | 174 791 | 25 987 | 398 | From 1950, at birth | Danish SSI 1331 | No |
| Germany | 169 430 | 7392 | 88 | From 1960, at birth | Danish SSI 1331 | No |
| Brazil | 135 693 | 9188 | 43 | Start date unknown, at birth to 1 year | BCG Moreau Rio | Yes |
| Turkey | 133 721 | 3641 | 43 | From 1952, at birth to 1 year | Serum Inst. of India | Yes |
| Iran | 103 135 | 6486 | 77 | From 1984, at birth to 1 year | Pasteur Inst. 1173‐P2 | Yes |
| Belgium | 51 420 | 8415 | 726 | Never | Not applicable | No |
| Netherlands | 41 774 | 5288 | 309 | Never | Not applicable | No |
| Switzerland | 30 126 | 1810 | 209 | From 1960, at birth to 1 year | Merieux | No |
| Portugal | 26 715 | 1,105 | 108 | From 1964, at birth to 1 year | Not specified | Yes |
| Ireland | 22 385 | 1,403 | 284 | From 1950, at birth | Danish SSI 1331 | Yes |
| Israel | 16 381 | 240 | 28 | From 1955, at birth | Not specified | No |
| Austria | 15 752 | 609 | 68 | From 1952, at birth to 1 year | Not specified | No |
| Australia | 6896 | 97 | 4 | From 1950, at age 12–14 years | Not specified | No |
mil pop, million population.
* As of 7 May 2020. Source: Johns Hopkins University. See Table S1 for data extracted on 18 July 2020.
† Source: http://www.bcgatlas.org (Zwerling et al. 2011).
Figure 1a shows the correlation between the number of reported cases and the number of reported deaths. The arithmetic average CFR of 5·2% for these 18 countries is indicated as a dotted line. Two of the six countries that employed BCG in the 1950s have CFRs that lay well below this average (Australia and Israel), while France is above it. To date, Russia has reported relatively low numbers of deaths compared to its number of cases, which could be due to the fact that at the time of analysis the epidemic may be at an earlier stage of progression compared to most other countries included here. The true number of cases is obviously much higher than what each of these countries has reported, with a factor that will vary per country. Fatality rates are also not equally assessed, as some countries do not count deaths occurring outside of the hospital, be it in elderly care facilities or in private homes, while countries also use different definitions of Covid‐19‐related deaths (positive by tests or positive based on symptoms).
Figure 1.
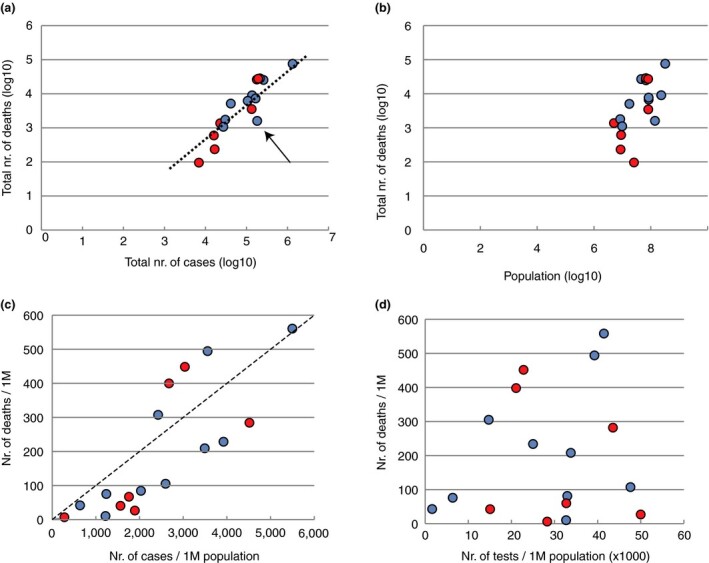
Analysis of 18 countries: Australia, Austria, Belgium, Sweden, Switzerland, Canada, Brazil, Turkey, Germany, France, Russia, United Kingdom, Italy, Spain and USA (ordered for increasing number of total reported cases on the day of data download, 7 May 2020). Panel a: Reported total number of deaths per reported total number of cases. The dotted line shows the average case fatality rates of these countries. The arrow indicates Russia. Panel b: Reported total number of deaths per national population size. Panel c: Number of deaths and number of cases, both expressed per million population. The hatched line represents 100 deaths per million for 1000 cases per million. Panel d: Number of deaths per number of tests performed, both per million population. In all panels, blue indicates countries not employing BCG in the 1950s, regardless of whether they started to use this vaccine later. See Fig. S1 for data extracted on 18 July 2020 ( BCG in 1950s;
BCG in 1950s;  no BCG in 1950s). [Colour figure can be viewed at wileyonlinelibrary.com]
no BCG in 1950s). [Colour figure can be viewed at wileyonlinelibrary.com]
Population size was also taken into account. There was only a weak correlation between the reported number of deaths and the population size of the analysed countries (Fig. 1b). Four of the countries that used BCG in the 1950s have relatively small population sizes, while Turkey and the UK have larger populations. Expressing the number of cases per million population provides a rather poor correction for the vast differences in local population densities, as the epidemic is highly unevenly distributed within countries. For instance, one‐third of all cases in Spain occurred in the Madrid area that harbours only 14% of the total national population. Fatality rates are also deeply affected by the state of local healthcare services. The quality of healthcare not only varies per country, but even countries with excellent healthcare may not be able to locally offer this while facilities are being overloaded with cases at the peak of the outbreak, as was evident in Lombardy (Italy), Madrid (Spain) or New York City (NY, USA). When the number of cases and the number of deaths were both expressed per million population (Fig. 1c), four countries were found above the line of 100 deaths/M for 1000 cases/M: the Netherlands, France, UK and Italy. Two of these had vaccinated in the 1950s and two had not.
The degree of testing varies per country, for instance due to limited testing capacity, which affects the total number of detected cases. If fewer tests are performed, total cases can be artificially low, pushing the CFR up. Testing may be mostly restricted to symptomatic, hospitalized individuals in some countries, or be more widespread to include asymptomatic communities in others. This will result in a difference of detected cases, skewing the CFR towards low or high rates, respectively. We could find no clear correlation between the number of tests performed per million population and the number of reported deaths per million (Fig. 1d). Countries with BCG vaccination in the 1950s reported either low (Australia, Israel, Austria, Turkey) or high (Ireland, UK, France) numbers of deaths per million population.
When a country introduced BCG vaccination in the 1950s, it may have taken years to reach nation‐wide coverage, if that level was reached at all. When BCG coverage was compared between EU countries in 2000–2004, four countries still vaccinated all children, with reported coverages of >90% in Ireland and France, 83% in Portugal and 75% in UK (Infuso et al. 2006). Of the countries that did not include BCG in their vaccination program in the 1950s, most introduced it later, but very few have continued the practice to the present (Table 1). We used these data to determine if differences could be seen when comparing figures from countries that had never applied any vaccination with BCG on a national scale. These included Italy, the Netherlands, the USA and Belgium (although in these countries small specific target groups may have been recommended for vaccination). Ireland, Portugal Turkey, Russia, Brazil and Iran currently vaccinate, in contrast with the other countries. No trend could be detected between countries that had never vaccinated and those that currently vaccinate, in terms of total number of cases per population (Fig. 2a). The total number of deaths was generally higher in countries that had never vaccinated, and the three countries currently using BCG vaccination had medium (Portugal, Ireland) to high (Brazil) death rates (Fig. 2b).
Figure 2.
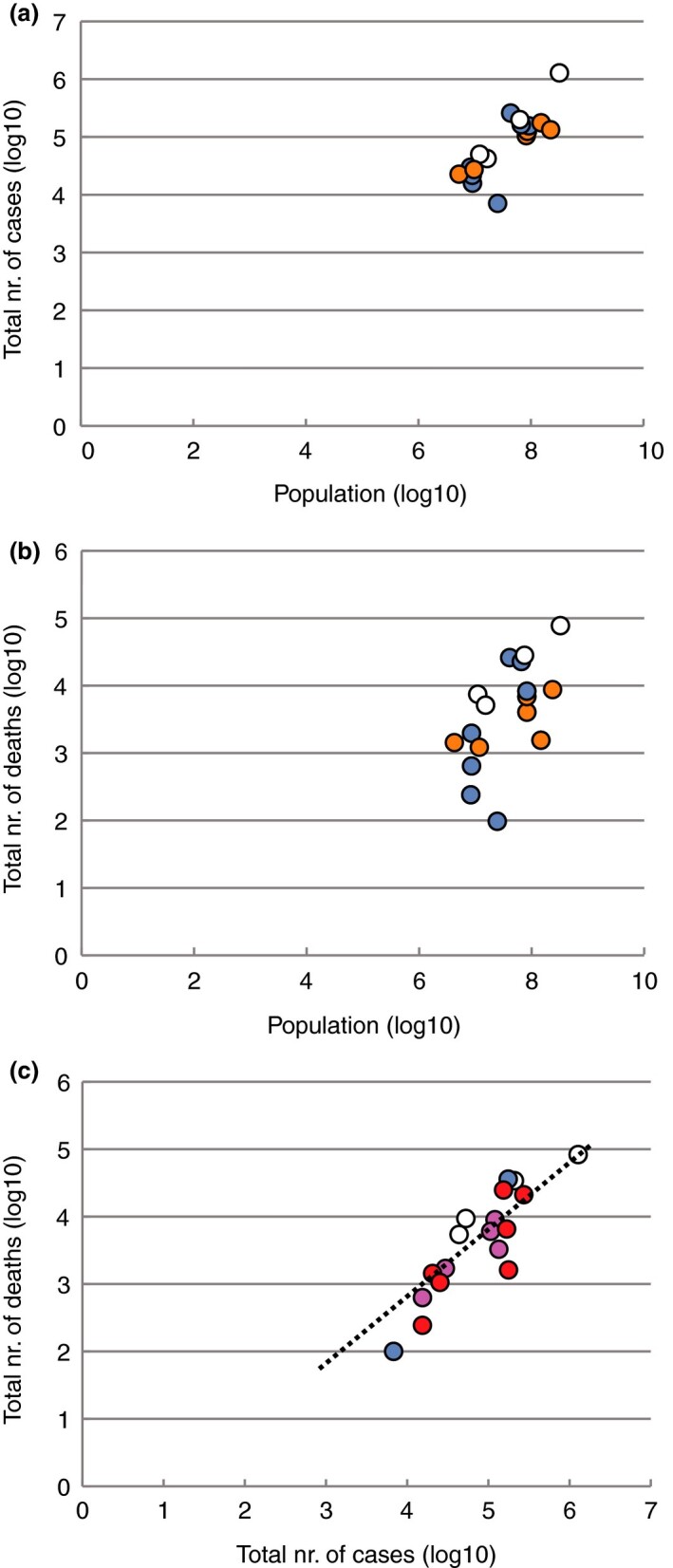
Effect of current BCG vaccination and age at which the vaccination was administered. Panel a: Reported total number of cases, and panel b, total number of deaths, plotted against the population sizes (both on a logarithmic scale). Countries are coloured for those currently using BCG vaccination on a national scale, those who have ceased to do so, and those that never used large‐scale BCG vaccination. Panel c: Total number of deaths against the total number of cases (as in Fig. 1a), now coloured for the time the vaccine was administered. See Fig. S2 for data extracted on 18 July 2020 (a:  BCG at present;
BCG at present;  BCG (historic);
BCG (historic);  no BCG; b:
no BCG; b:  BCG at present;
BCG at present;  BCG (historic);
BCG (historic);  no BCG; c:
no BCG; c:  BCG at birth;
BCG at birth;  BCG at birth–1y;
BCG at birth–1y;  BCG >10j;
BCG >10j;  no BCG). [Colour figure can be viewed at wileyonlinelibrary.com]
no BCG). [Colour figure can be viewed at wileyonlinelibrary.com]
If the effect of BCG on ‘immune training’ truly affects Covid‐19 outcome, the strongest effect should be expected when the vaccine was administered soon after birth, when epigenetically controlled patterns of gene expression are still most malleable. The age at which BCG was administrated varied between countries. Only four countries started vaccination early (from or prior to 1960) and vaccinated at birth: France (CFR: 14·8%), Germany (CFR: 4·3%), Ireland (CFR: 6·2%) and Israel (CFR: 0·6%); Spain introduced vaccination at birth in 1965 (CFR: 10·1%) (Table 1). In other countries, the vaccine was or is being delivered during a time span from birth up to one year of age. Both Australia and the UK typically vaccinated during childhood, but their Covid‐19 CFR rates differ by a factor of 10 (1·4 and 14·8%, respectively). Irrespective of whether the vaccine was used in the past or present, Fig. 2c shows that there is no grouping of countries with a particular age at which the vaccination was administered, with respect to reported CFR.
At revision stage, on July18, we downloaded the numbers for the selected countries again (Table S1) and the figures were reproduced with these updated numbers. This did not change the overall conclusions (Figs S1 and S2).
A number of publications were published on the subject of BCG vaccination and Covid‐19 as this manuscript was under review. These either supported the view that BCG could be protective (Ebina‐Shibuya et al. 2020; Escobar et al. 2020; Hauer et al. 2020; Madan et al. 2020; Macedo and Febra 2020; Sharma et al. 2020), or reported the lack of such an effect (Meena et al. 2020; Hamiel et al. 2020). However, all studies used datasets from spring. Sharma et al. used data from 29 May, Madan and colleagues downloaded data from April 1, Macedo and Febra assessed the situation on 4 April 2020, Shibuya and coworkers downloaded their data on April 10 and Escobar and colleagues assessed the BCG index (taking into account the age of vaccinated individuals and the number of years vaccination programmes were in place), using data from 21 April 2020. However, it took time for the pandemic to reach different continents: in April it had hardly touched South America or Africa. Now it is clear that countries such Brazil, India and South Africa, which all have current BCG vaccination programs, suffer from high attack rates, thus countering a presumed protective effect against infection (death rates are lagging behind even further and may not always be accurately assessed or reported in these countries). When data from 13 May 2020 were analysed, a protective effect of BCG on Covid‐19 was not detected (Meena et al. 2020). Methodological flaws include ignoring confounding factors that are likely to affect the outcome of Covid‐19 (which we did not correct for, either) as has been pointed out (Riccò et al. 2020), and the proposed duration of immunological protection lasting at most 15 years (Kantor 2020) also weakens conclusions (including those based on our Fig. 1). Nevertheless, the latest developments of the pandemic in a number of countries suggest that protection by BCG against Covid‐19 does not occur.
From our analyses, and subject to the caveats expressed as to the data sources, at this moment in time the involvement of BCG vaccination as having an ameliorative effecter as prophylactic treatment against Covid‐19 is not proven.
Materials and methods
Data on Covid‐19 cases were extracted from the Johns Hopkins University database (https://www.worldometers.info/coronavirus) on 7 May 2020. Historic BCG vaccination programs for individual countries were extracted from http://www.bcgatlas.org (Zwerling et al. 2011) (last accessed on 7 May 2020). The data we used are summarized in Table 1. The type of strain used for vaccination is added for completeness, but given the variation in strain usage, the effect of strain was not analysed. Figures were produced with Excel.
During revision of the manuscript, the data were again extracted for the same selection of countries on 18 July 2020. These data are summarized in the Supplementary file.
Conflict of interest
The authors have no conflict of interest to declare.
Supplementary Material
Figure S1. Analysis of the same 18 countries used in Fig. 1 of original submission, now with data downloaded on 18 July 2020.
Figure S2. Effect of current BCG vaccination and age at which the vaccination was administered, as Fig. 2, now with data downloaded on 18 July 2020.
Table S1. Data extracted at revision stage, as of 18 July, 2020.
Contributor Information
T.M. Wassenaar, Molecular Microbiology and Genomics Consultants Zotzenheim Germany; European Molecular Biology Laboratory (EMBL).
G.S. Buzard, Independent scolar Middletown MD USA
D.J. Newman, Newman Consulting LLC Wayne PA USA
References
- Aggarwal,S., Garcia‐Telles,N., Aggarwal,G., Lavie,C., Lippi,G. and Henry,B.M. (2020) Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID‐19): early report from the United States. Diagnosis (Berl) 7, 91–96. [DOI] [PubMed] [Google Scholar]
- Arts,R.J.W., Moorlag,S.J.C.F.M., Novakovic,B., Li,Y., Wang,S.Y., Oosting,M., Kumar,V., Xavier,R.J. et al. (2018) BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe 23, 89–100.e5. [DOI] [PubMed] [Google Scholar]
- CDC COVID‐19 Response Team (2020) Severe outcomes among patients with coronavirus disease 2019 (COVID‐19) — United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep 69, 343–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen,J., Lu,H., Melino,G., Boccia,S., Piacentini,M., Ricciardi,W., Wang,Y., Shi,Y. et al. (2020a) COVID‐19 infection: the China and Italy perspectives. Cell Death Dis 11, 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen,N., Zhou,M., Dong,X., Qu,J., Gong,F., Han,Y., Qiu,Y., Wang,J. et al. (2020b) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395, 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispen,R. (1989) History of BCG and its substrains. Prog Clin Biol Res 310, 35–50. [PubMed] [Google Scholar]
- Kristensen,I., Aaby,P. and Jensen,H. (2000) Routine vaccinations and child survival: follow up study in Guinea‐Bissau, West Africa. BMJ 321, 1435–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis,N., Sparrow,A., Ghebreyesus,T.A. and Netea,M.G. (2020) Considering BCG vaccination to reduce the impact of COVID‐19. Lancet 395, 1545–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina‐Shibuya,R., Horita,N., Namkoong,H. and Kaneko,T. (2020) National policies for paediatric universal BCG vaccination were associated with decreased mortality due to COVID‐19. Respirology, 10.1111/resp.13885. Advance online publication [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar,L.E., Molina‐Cruz,A. and Barillas‐Mury,C. (2020) BCG vaccine protection from severe coronavirus disease 2019 (COVID‐19). Proc Natl Acad Sci 117, 17720–17726. 10.1073/pnas.2008410117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan,W.‐J., Ni,Z.‐Y., Hu,Y., Liang,W.‐H., Ou,C.‐Q., He,J.‐X., Liu,L., Shan,H. et al. (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382, 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo,G., Ye,L., Pan,K., Chen,Y., Xing,D., Yan,K., Chen,Z., Ding,N. et al. (2020) New insights of emerging SARS‐CoV‐2: epidemiology, etiology, clinical features, clinical treatment, and prevention. Front Cell Dev Biol 8, 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gursel,M. and Gursel,I. (2020) Is global BCG vaccination‐induced trained immunity relevant to the progression of SARS‐CoV‐2 pandemic? Allergy 75, 1815–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamiel,U., Kozer,E. & Youngster,I. (2020) SARS‐CoV‐2 rates in BCG‐vaccinated and unvaccinated young adults. JAMA, 3232340–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauer,J., Fischer,U., Auer,F. and Borkhardt,A. (2020) Regional BCG vaccination policy in former East‐ and West Germany may impact on both severity of SARS‐CoV‐2 and incidence of childhood leukemia. Leukemia 34, 2217–2219. 10.1038/s41375-020-0871-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor,I.N. (2020) BCG versus COVID‐19? Medicina (B Aires) 80, 292–294. [PubMed] [Google Scholar]
- Keeley,A.J., Evans,C.M. and de Silva,T.I. (2020) Asymptomatic SARS‐CoV‐2 infection: the tip or the iceberg? Thorax 75, 621–622. [DOI] [PubMed] [Google Scholar]
- Kumar,J. and Meena,J. (2020) Demystifying BCG vaccine and COVID‐19 relationship. Indian Pediatr 57, 588–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infuso,A., Falzon,D. and Network,Euro T.B. (2006) European survey of BCG vaccination policies and surveillance in children, 2005. Euro Surveill 11, 6–11. [DOI] [PubMed] [Google Scholar]
- Luca,S. and Mihaescu,T. (2013) History of BCG vaccine. Maedica (Buchar) 8, 53–58. [PMC free article] [PubMed] [Google Scholar]
- Macedo,A. and Febra,C. (2020) Relation between BCG coverage rate and COVID‐19 infection worldwide. Med Hypotheses 142, 109816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan,M., Pahuja,S., Mohan,A., Pandey,R.M., Madan,K., Hadda,V., Tiwari,P., Guleria,R. et al. (2020) TB infection and BCG vaccination: are we protected from COVID‐19? Public Health 185, 91–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangalmurti,N. and Hunter,C.A. (2020) Cytokine storms: understanding COVID‐19. Immunity. 53, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meena,J., Yadav,A. and Kumar,J. (2020) BCG vaccination policy and protection against COVID‐19. Indian J Pediatr 10.1007/s12098-020-03371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei,X., Zhang,Y., Zhu,H., Ling,Y., Zou,Y., Zhang,Z., Guo,H., Liu,Y. et al. (2020) Observations about symptomatic and asymptomatic infections of 494 patients with COVID‐19 in Shanghai, China. Am J Infect Control 10.1016/j.ajic.2020.06.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrani,R.D., Dabas,N. and Goldberger,J.J. (2020) COVID‐19 cardiac injury: implications for long‐term surveillance and outcomes in survivors. Heart Rhythm S1547‐5271(20)30625‐1. 10.1016/j.hrthm.2020.06.026. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea,M.G. and van Crevel,R. (2014) BCG‐induced protection: effects on innate immune memory. Semin Immunol 26, 512–517. [DOI] [PubMed] [Google Scholar]
- Netea,M.G., Joosten,L.A., Latz,E., Mills,K.H., Natoli,G., Stunnenberg,H.G., O'Neill,L.A. and Xavier,R.J. (2016) Trained immunity: a program of innate immune memory in health and disease. Science 22, 352(6284):aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea,M.G., Domínguez‐Andrés,J., Barreiro,L.B., Chavakis,T., Divangahi,M., Fuchs,E., Joosten,L.A.B., van der Meer,J.W.M. et al. (2020) Defining trained immunity and its role in health and disease. Nat Rev Immunol 20, 375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdemir,C., Kucuksezer,U.C. and Tamay,Z.U. (2020) Is BCG vaccination effecting the spread and severity of COVID‐19? Allergy 75, 1824–1827. [DOI] [PubMed] [Google Scholar]
- Ragab,D., Salah Eldin,H., Taeimah,M., Khattab,R. and Salem,R. (2020) The COVID‐19 cytokine storm; what we know so far. Front Immunol 11, 1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccò,M., Gualerzi,G., Ranzieri,S. and Bragazzi,N.L. (2020) Stop playing with data: there is no sound evidence that Bacille Calmette‐Guérin may avoid SARS‐CoV‐2 infection (for now). Acta bio‐medica: Atenei Parmensis 91, 207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma,A., Kumar Sharma,S., Shi,Y., Bucci,E., Carafoli,E., Melino,G., Bhattacherjee,A. and Das,G. (2020) BCG vaccination policy and preventive chloroquine usage: do they have an impact on COVID‐19 pandemic? Cell Death Dis 11(7), 516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team (2020) Vital surveillances: The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID‐19) — China. China CDC Weekly, 2, 113–122. [PMC free article] [PubMed] [Google Scholar]
- Vestergaard,L.S., Nielsen,J., Richter,L., Schmid,D., Bustos,N., Braeye,T., Denissov,G., Veideman,T.. et al. (2020) Excess all‐cause mortality during the COVID‐19 pandemic in Europe ‐ preliminary pooled estimates from the EuroMOMO network, March to April 2020. Euro Surveill 25, 10.2807/1560-7917.ES.2020.25.26.2001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2020) Bacille Calmette‐Guérin (BCG) vaccination and COVID‐19. https://www.who.int/news‐room/commentaries/detail/bacille‐calmette‐gu%C3%A9rin‐(bcg)‐vaccination‐and‐covid‐19 (last accessed July 15, 2020).
- Zwerling,A., Behr,M.A., Verma,A., Brewer,T.F., Menzies,D. and Pai,M. (2011) The BCG world atlas: a database of global BCG vaccination policies and practices. PLoS Med 8, e1001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Analysis of the same 18 countries used in Fig. 1 of original submission, now with data downloaded on 18 July 2020.
Figure S2. Effect of current BCG vaccination and age at which the vaccination was administered, as Fig. 2, now with data downloaded on 18 July 2020.
Table S1. Data extracted at revision stage, as of 18 July, 2020.


