Abstract
G protein-coupled receptors (GPCRs) mediate cellular responses to diverse extracellular stimuli that play vital roles in the regulation of biology, including behavior. Abnormal G protein-coupled receptor kinase (GRK)-mediated regulation of GPCR function is involved in the pathogenesis of hypertension. Among the seven GRK subtypes, GRK4 has attracted attention because of its constitutive activity and tissue-specific expression. Increasing number of studies show that GRK4 affects blood pressure by GPCR-mediated regulation of renal and arterial function. The target receptor of GRK4 is confined not only to GPCRs, but also to other blood pressure-regulating receptors, such as the adiponectin receptor. Genetic studies in humans show that in several ethnic groups, GRK4 gene variants (R65L, A142V, and A486V) are associated with salt-sensitive or salt-resistant essential hypertension and blood pressure responses to antihypertensive medicines. In this article, we present a comprehensive overview of GRK-mediated regulation of blood pressure, focusing on the latest research progress on GRK4 and hypertension and highlighting potential and novel strategies for the prevention and treatment of hypertension.
Keywords: G protein-coupled receptor, G protein-coupled receptor kinase type 4, Dopamine receptor, Angiotensin II type 1 receptor, Hypertension
1. Introduction
Essential hypertension, also known as primary hypertension and idiopathic hypertension, or simply hypertension, is a major contributing risk factor for cardiovascular diseases and other diseases with adverse clinical outcomes, including stroke, heart failure, end-stage-renal disease, and all-cause mortality (Virani et al., 2021). In the United States National Health and Nutrition Examination Survey 2015 to 2018, the prevalence of hypertension was 28.2%, 60.1%, and 77.0% among subjects 20 to 44, 45 to 64, and ≥ 65 years old, respectively (https://www.cdc.gov/nchs/nhanes/). The estimated expenditure for hypertension in the US from 2016 to 2017 was $52.4 billion (Virani et al., 2021). Of 154 health conditions in the US, hypertension ranked 10th in health care costs (Dieleman et al., 2020). Hypertension is a major public health problem worldwide (Fisher & Curfman, 2018; Kostova et al., 2020).
The pathogenesis of hypertension is determined by genetic and environmental factors and their interaction (Harrison, Coffman, & Wilcox, 2021; Wang et al., 2020), in which several organs such as the arteries, brain, endocrine organs, gastrointestinal tract, heart, kidneys, and nerves are involved. Many endocrine factors or neurotransmitters, such as dopamine, angiotensin II (Ang II), endothelin, and epinephrine, regulate sodium homeostasis and vascular reactivity, via their G protein-coupled receptors (GPCRs), to maintain a normal systemic arterial blood pressure (Eroglu, Kocyigit, & Lindholm, 2020; Prieto, Gonzalez, Visniauskas, & Navar, 2021; Rianto, Hoang, Revoori, & Sparks, 2021; Yang, Villar, Jose, & Zeng, 2021). However, in hypertension, GPCR function is aberrant, causing sodium retention and attenuated vasodilation (Prieto et al., 2021; Rianto et al., 2021; Yang et al., 2021). Although the mechanisms leading to aberrant GPCR function are complex, abnormal GPCR phosphorylation plays an important role in the pathogenesis of hypertension.
GPCR kinases (GRKs) comprise seven serine/threonine protein kinases, characterized by their ability to recognize specific GPCRs and phosphorylate their intracellular elements, leading to their uncoupling from G protein subunits, also known as desensitization, promote receptor internalization, and terminate the GPCR-mediated signaling pathway (Benovic, 2021; Gurevich & Gurevich, 2020). Due to the important roles of GPCRs in the development of human diseases, GRKs have attracted considerable attention for their role in the pathogenesis of cardiovascular diseases, including hypertension (de Lucia et al., 2021; Li et al., 2021; Pfleger, Gresham, & Koch, 2019).
Among the GRKs, GRK4 may be the most versatile subtype of GRK in the regulation of blood pressure (Yang, Villar, Jones, Jose, & Zeng, 2015). Several studies have shown that GRK4 plays a vital role in the pathogenesis of hypertension and response to antihypertensive treatment (Jeong et al., 2020; Vandell et al., 2012; Zhang et al., 2020). In this article, we present a comprehensive overview of GRK-mediated regulation of blood pressure, especially focusing on the latest research progress on GRK4 and hypertension, and highlighting potential and novel strategies for the prevention and treatment of hypertension.
2. Classification and characteristics of GRKs
It is well known that there are over 800 genes encoding GPCRs in the human genome. However, of the primary mediators of agonist-dependent phosphorylation of GPCRs, only seven GRKs (GRK1–7) have been identified (Benovic, 2021; Gurevich & Gurevich, 2020; Pfleger et al., 2019). The GRKs comprise a family of seven serine/threonine protein kinases characterized by their ability to recognize and phosphorylate, specifically, agonist-activated GPCRs (Chaudhary & Kim, 2021; Harris, 2012; Santos-Otte et al., 2019). According to their amino acid sequences and ternary structural homologies, the seven mammalian GRKs can be classified into three sub-groups: the GRK1-like subfamily, also called as visual GRK subfamily, which includes rhodopsin kinase GRK1 and visual pigment kinase GRK7; the GRK2-like subfamily, otherwise known as β-adrenergic receptor (β-AR) kinase subfamily, which includes GRK2 (β-ARK1) and GRK3 (β-ARK2); and the GRK4-like subfamily, which includes GRK4, GRK5, and GRK6 (Benovic, 2021; Gurevich & Gurevich, 2020; Pfleger et al., 2019).
The seven GRKs share certain characteristics but are distinct enzymes with specific regulatory properties. They have a similar basic structural architecture, which consists of an amino terminal (N-terminal) domain (~185 amino acids), a central highly conserved catalytic domain (~270 amino acids), and a carboxyl terminal (C- terminal) domain (~105 to 230 amino acids). Each GRK has a well-conserved N-terminus, which is considered to be vital for receptor binding and selective recognition of the activated GPCR. GRKs also contain a regulator of G protein signaling (RGS) homology (RH) domain (~120 amino acids) (Chaudhary & Kim, 2021; Hullmann, Traynham, Coleman, & Koch, 2016; Yu, Sun, Jiao, & Lee, 2018). Differences among GRKs are most notable in the C-terminal domain, which is responsible for their membrane localization. For example, GRK1 and GRK7 are prenylated at the C-terminus; however, GRK2 and GRK3 have a pleckstrin homology domain, which facilitates recruitment to the membrane via interacting with Gβγ subunits. Within the GRK4-subfamily, GRK4 and GRK6 are palmitoylated, whereas GRK5 contains a positively charged lipid-binding amphipathic helix and binds phospholipids via its C-terminus (Homan, Glukhova, & Tesmer, 2013; Ribas et al., 2007). By contrast, the central domain that is involved in its kinase catalytic function is highly conserved among different GRKs. It should be noted that how GRKs recognize GPCRs still remains unclear. A recent study showed direct evidence that upon receptor activation, the N-terminus of GRK1 forms a helix that anchors into the open cytoplasmic cleft of rhodopsin (Chen et al., 2021). The mechanism by which other GRK subtypes recognize GPCRs and vice-versa needs further studies.
The seven GRK subtypes have different tissue distributions. The four GRK subtypes, GRK2, GRK3, GRK5, and GRK6, are widely expressed in mammalian tissues (Benovic, 2021; Gurevich & Gurevich, 2020; Pfleger et al., 2019). By contrast, the other three subtypes, GRK1, GRK4, and GRK7, are found in a limited number of tissues. Among them, GRK1 and GRK7 are found almost exclusively in the retina, whereas GRK4 has very limited distribution in a few organs or tissues (see below) (Benovic, 2021; Gurevich & Gurevich, 2020; Li et al., 2021; Pfleger et al., 2019; Yang et al., 2015; Zhang et al., 2020). The different tissue expressions among GRKs suggest specific properties in the regulation of GPCRs or other targets and different physiological functions in different organs or tissues.
3. Regulation of blood pressure by GRKs
Studies have shown that most GRKs, e.g., GRK2, GRK3, GRK4, GRK5, and GRK6, but not GRK1 and GRK7, are involved in the regulation of blood pressure via different mechanisms (Chaudhary & Kim, 2021; Harris, 2012; Vandell et al., 2012; Yang et al., 2015; Zhang et al., 2020).
Elevated GRK2 expression and activity are associated with increased blood pressure (Cohn et al., 2009; Murga et al., 2019; Napolitano et al., 2012). Hypertensive humans and several animal models of hypertension have elevated expression and activity of GRK2 in lymphocytes, aortas, and vascular smooth muscle cells (VSMCs) (Cohn et al., 2009; Gros et al., 2000; Izzoetal., 2008; Napolitano et al., 2012; Zhao, Vanhoutte, & Leung, 2015). Our previous study also found that maternal diabetes mellitus-programmed hypertension in the offspring is caused by increased GRK2 activity (Luo et al., 2018). Mice with VSM-targeted GRK2 overexpression have impaired (β-AR-mediated vasodilation and increased resting blood pressure (Eckhart, Ozaki, Tevaearai, Rockman, & Koch, 2002). Pharmacological blockade of GRK2 and (β1-AR interaction prevents the development of hypertension in spontaneously hypertensive rats (SHRs), suggesting that inhibition of GRK2 activity could be a strategy for treating hypertension and protecting target organs (Polhemus et al., 2016; Rainbow et al., 2018; Sun et al., 2021). GRK2 downregulation/inhibition also enhances cardiac insulin sensitivity and mild heart hypertrophy with preserved systolic function, that is accompanied with repressed expression of genes related to pathological hypertrophy (Lucas et al., 2014). However, there are a few reports that could be considered as inconsistent with a major role of GRK2 in some hypertensive models (Avendaño et al., 2014; Ciccarelli et al., 2013; Cohn et al., 2008; Oliver et al., 2014; Tutunea-Fatan et al., 2018; Tutunea-Fatan, Caetano, Gros, & Ferguson, 2015). For example, nitric oxide production in adult GRK2 hemizygous mice protects against Ang II-induced hypertension (Avendaño et al., 2014). GRK2 expression is decreased in the aortas of N(G)-nitro-L-arginine methyl ester (L-NAME)-induced hypertensive rats, but unchanged in the mesenteric arteries of SHRs (Oliver et al., 2014). The reasons leading to the differences are still unknown, which need to be elucidated in the future.
Transgenic mice with cardiac-restricted expression of GRK3 have elevated systolic blood pressure and increased cardiac output, associated with cardiac myocyte α1-AR hyper-responsiveness (Vinge et al., 2008). Although α-ARs are important regulators of vascular resistance and GRK3 is expressed in the VSMCs, it is still unclear whether or not GRK3 can regulate blood pressure by affecting vascular resistance. An overall blood pressure lowering effect of GRK3 activation is supported from clinical evidence showing an inverse correlation between GRK3 mRNA expression in lymphocytes and ambulatory systolic and diastolic blood pressure in normotensive and hypertensive humans (Oliver et al., 2010).
VSM-specific overexpression of GRK5 in mice causes hypertension (Keys, Zhou, Harris, Druckman, & Eckhart, 2005). In SHRs, the intramyocardial gene transfer of the amino-terminal region of GRK5 reduces GRK5-mediated exacerbation of cardiac hypertrophy in a blood pressure independent manner (Hullmann et al., 2014; Sorriento et al., 2010; Sorriento et al., 2018). GRK5 and β1-AR expressions are increased in the left ventricles of rats with hypertension induced by L-NAME and in peripheral blood mononuclear cells from humans with heart failure. By contrast, GRK2 expression is increased in all tissues with increased β2-AR expression (Montó et al., 2015). Thus, GRK5 may regulate β1-AR whereas GRK2 may regulate β2-AR. In addition, GRK5 polymorphism (41Q > L) is associated with a decreased risk of cardiovascular outcomes in hypertensive patients treated with atenolol and hydrochlorothiazide (Lobmeyer et al., 2011). This GRK5 polymorphism may be also protective in heart failure by inhibition of β-AR signaling (Liggett et al., 2008).
Renal GRK6 expression is decreased in hypertensive subjects (Xu, Watanabe, Felder, &Jose, 2001). GRK6 deficient mice have enhanced coupling of dopamine D2-like receptors in the striatum, which suggests that GRK6 negatively regulates these receptors (Gainetdinov et al., 2003). In the intestinal cell line IEC-6, GRK6 and GRK4 are responsible for the homologous desensitization of dopamine D1-like receptors (Fraga, Jose, & Soares-da-Silva, 2004). In hypertensive Sprague-Dawley (SD) rats, caused by coarctation of the abdominal aorta, expression of GRK6 in endothelial cells from the common carotid arteries is repressed (Wang et al., 2017). In rats with SHR heart failure, there is a subcellular redistribution of GRK6 from the intercalated discs to the cytoplasm of cardiac myocytes (Yi et al., 2005). In addition, in rats, overexpression of GRK6 in the 6-hydroxydopamine-injured striatum induces the internalization of D1 receptor (D1R) and normalizes the D1R signaling by promoting its desensitization (Ahmed et al., 2010). However, the role of GRK6 in human essential hypertension remains to be determined.
4. Regulation of blood pressure by GRK4
4.1. Features of GRK4
Of all the seven GRKs, GRK4 is the unique subtype with four splice variants (GRK4α, β, γ, and δ) in humans (Fig. 1) (Premont et al., 1996). GRK4α, consisting of 578 amino acids, is the longest and full-length GRK isoform. GRK4β, consisting of 546 amino acids, has no N-terminal exon 2 (32-codon deletion), which contains the phosphatidylinositol bisphosphate (PIP2) binding region. GRK4γ, consisting of 532 amino acids, has no C-terminal exon 15 (46-codon deletion). GRK4δ, consisting of 500 amino acids, the shortest isoform, has no exons 2 and 15 (Jose, Soares-da-Silva, Eisner, & Felder, 2010; Premont et al., 1996). It should be noted that alternative splicing in GRK4β and GRK4δ would lead to the loss of α0 and α1 helices of the RH domain, and GRK4γ and GRK4δ would lose the C-terminal end of α10 and all of the α11 helix, which brings the folding and stability of these three GRK4 isoforms into question (Allen et al., 2015). Unlike humans with four GRK splice variants, rats have five GRK4 splice variants (GRK4A, B, C, D, and E), while mice have only one GRK4. Rat GRK4A has 76% identity with GRK4α, the longest of the human GRK4 splice variants. However, the mouse and rat GRK4 sequences are 90% identical (Premont et al., 1996; Virlon et al., 1998).
Fig. 1.
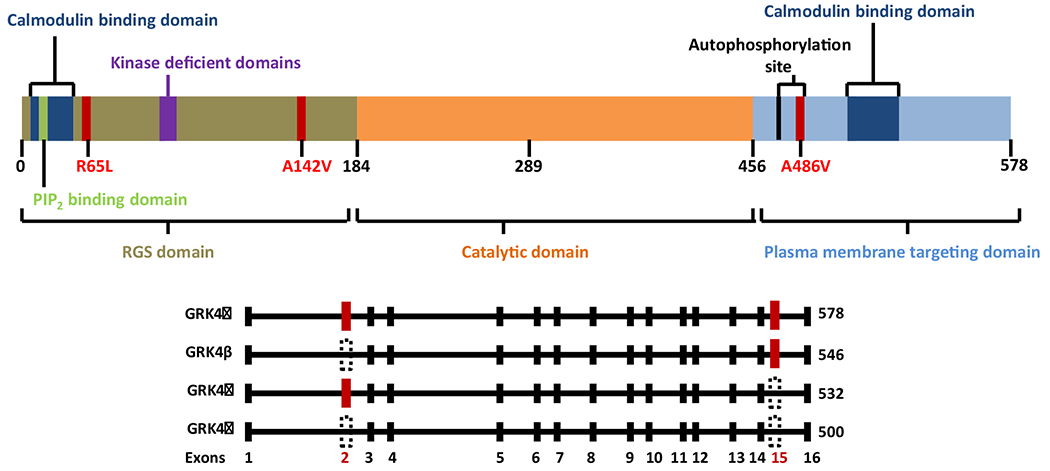
Schematic representation of GRK4 domain architecture.
Top: GRK4 has three primary domains: RGS domain; catalytic domain; and plasma membrane targeting domain. The numbers below the schematic diagram represent relative amino acid residues in GRK4. The positions of the GRK4 gene variants (amino acid substitutions R65L, A142V, and A486V) associated with hypertension are shown in red. The established and purported functional domains of GRK4 are also depicted in different colors.
Bottom: The four GRK4 splice variants (GRK4α, GRK4β, GRK4γ, and GRK4δ) are distinguished by the presence or in-frame deletion of exon 2 (GRK4β), exon 15 (GRK4γ), or both (GRK4δ). The red colored rectangles show the presence of exon 2 and/or exon 15 while the black-dotted triangles show the deletion of exon 2 and/or exon 15.
Abbreviations: GPCR, G protein-coupled receptor; GRK4, G protein-coupled receptor kinase 4; PIP2, phosphatidylinositol bisphosphate; RGS, regulator of G-protein signaling. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Three missense single nucleotide polymorphisms (SNPs) in the coding region of GRK4γ have attracted the most attention in the regulation of blood pressure. The SNPs in nucleotide 448, CGT to CTT (amino acid 65R > L, rs2960306); nucleotide 679, GCC to GTC (amino acid 142A > V, rs1024323); and nucleotide 1711, GCG to GTG (amino acid 486A > V, rs1801058) are associated with hypertension (Jose et al., 2010; Yang et al., 2015), which will be discussed in detail. It should be noted that the GRK4 SNPs (A142V, A486V and R65L) increase its activity, although the mechanisms are not clear at this time.
Unlike the ubiquitously expressed GRK2, GRK3, GRK5, and GRK6, GRK4 is expressed in a limited number of tissues, i.e., arteries, bones, cerebellum, heart, kidneys, small intestines, thyroid, and testes (Jose et al., 2010; Voigt, Holzapfel, Meyer, & Paschke, 2004; Yang et al., 2015). The GRK4/GRK5/GRK6 group has constitutive activity while GRK1, GRK2, GRK3, and GRK7 are activated by binding to ligand-activated GPCRs (Baameur et al., 2010; Fraga et al., 2004; Jose et al., 2010; Li et al., 2015a, b; Yang et al., 2015). The constitutive activity of GRK4 may be due, at least in part, to its ability to bind to inactive Gα and Gβ subunits (Keever, Jones, & Andresen, 2008; Neve, 2006).
4.2. Localization of GRK4
GRK4 has a restricted expression pattern, with highest levels in the testes and myometrium and, to a lesser extent, in a few other organs, including the arteries, brain, kidneys, small intestines, and thyroid, but minimal expression in the normal heart (Jose et al., 2010; Voigt et al., 2004; Yang et al., 2015).
Due to the vital role of the kidney in blood pressure regulation, the distribution of GRK4 in the kidney has been extensively studied. In human, rat, and mouse kidneys, GRK4 is highly expressed in the subapical membranes of renal proximal tubules (S1 and S3 segments), thick ascending limbs of the loop of Henle, distal convoluted tubules, and renal resistance vessels, and much less, in glomeruli (Felder et al., 2002; Sanada et al., 2006a, b; Villar et al., 2009; Yang et al., 2020). GRK4, distributed at the plasma membrane and cytoplasm under basal conditions, becomes internalized at the perinuclear area after activation of GPCRs (Felder et al., 2002; Villar et al., 2009; Yang et al., 2020). Specifically, in the renal plasma membrane microdomains, GRK4 is distributed in non-lipid raft fractions and in lipid raft fractions (Villar et al., 2009). We have also reported that GRK4 is localized in the nuclei of human renal proximal tubule cells and in HEK-293 cells, heterologously expressing hGRK4γ wild type or variants (Wang et al., 2016).
The blood vessels are also critical in the regulation of blood pressure. GRK4 is expressed in the tunica media and adventitia of conductance and resistance arteries from rats and mice (Chen et al., 2014; Zhao et al., 2015). The GRK4 mRNA is well expressed in the aortas of Wistar Kyoto (WKY) rats and SHRs (Zhao etal., 2015). In fact, GRK4 is generally distributed in both large and small arterial vessels, including the thoracic aorta, superior mesenteric arteries, carotid arteries, and renal arteries; there is no difference in GRK4 expression among these vessels. Similar to the GRK4 location in renal proximal tubule cells, GRK4 is also distributed at the membrane and cytoplasm in VSMCs (Chen et al., 2014).
The heart and resistance vessels are the organs involved in the generation of arterial pressure. GRK4 is modestly expressed in the normal heart, but substantially increased after myocardial infarction (Li et al., 2021; Sanada et al., 2006a, b). Our recent study showed that in the heart, GRK4 protein is expressed to a higher extent in cardiomyocytes than in non-cardiomyocytes, such as smooth muscle cells, endothelial cells, and fibroblasts (Li et al., 2021).
4.3. Effect of GRK4 on blood pressure
GRK4 expression and activity in the kidneys and arteries are increased in the hypertensive state, relative to the normotensive state (Table 1). The increase in renal GRK4 levels in hypertension may be organ specific because there is no difference in cardiac GRK4 expression between WKY rats and SHRs (Sanada et al., 2006a, b). Moreover, unlike other GRK subtypes, such as GRK2 and GRK5, which have increased expression secondary to hypertension, GRK4 expression and activity are increased before the onset of hypertension. Therefore, GRK4 may be of importance in the development of hypertension.
Table 1.
Abnormal GRK4 expression and function in hypertensive states.
| Experimental animal models of hypertension or hypertensive humans | Abnormal GRK4 expression and function in the hypertensive state | Blood pressure and targets regulated by GRK4 in the hypertensive state | References |
|---|---|---|---|
| SHRs | Higher mRNA and protein expressions of GRK4 in the renal cortex and aorta but not in the heart of SHRs, compared with WKY rats | Higher systolic blood pressure and renal cortical D1R and ETBR phosphorylation, and increased sodium retention in SHRs compared with WKY rats; impaired ETBR-mediated diuresis and natriuresis in both male and female SHRs; inhibition of contractions to PGE2 induced by previous phenylephrine exposure, and desensitization of TP receptors induced by α1-adrenoceptor activation in the aortae of SHRs, not WKY rats; GRK2 and GRK4 expressions increased in the aortae of SHRs, relative to WKY rats | Sanada et al., 2006; Yang et al., 2020; Zhao et al., 2015 |
| Hypertensive humans | Higher basal (ligand-independent) and D1R-stimulated GRK activities in RPT cells from hypertensive than normotensive subjects | Higher serine phosphorylation of D1R and lower D1R-mediated cAMP accumulation in RPT cells from hypertensive than normotensive subjects | Felder et al., 2002 |
| Long-term exposure to fine particulate matter (PM2.5)-mediated hypertension | Increased mRNA and protein expressions of GRK4 in the kidney of PM2.5-induced hypertension in SD rats and PM2.5-treated RPT cells from WKY rats | Higher systolic and diastolic blood pressures and renal D1R phosphorylation, and decreased D1R expression and receptor-induced natriuresis and diuresis in PM2.5-treated SD rats. Decreased D1R expression, increased D1R phosphorylation and decreased D1R-mediated inhibition of NKA activity in PM2.5-treated RPT cells | Lu et al., 2018 |
| In utero PM2.5 exposure mediated-hypertension in offspring | Increased GRK4 protein expression in renal cortex of offspring of PM2.5-treated dams | Higher systolic blood pressure, lower 24 h urine volume and sodium excretion, decreased renal D1R expression, increased renal D1R phosphorylation, and decreased D1-like receptor-induced natriuresis and diuresis in the offspring of PM2.5-treated dams than the offspring of vehicle-treated dams | Ye et al., 2018 |
| Prenatal LPS exposure-mediated hypertension in offspring | Increased renal GRK4 and GRK2 protein expressions in offspring of LPS-treated dams | Higher systolic blood pressure, lower 24 h urine volume and sodium excretion, decreased renal D1R expression, increased D1R phosphorylation, and decreased D1-like receptor-induced natriuresis and diuresis in the offspring of LPS-treated than vehicle-treated dams | Wang et al., 2014 |
| Prenatal cold stress exposure-mediated hypertension in offspring | Increased arterial GRK4 expression in offspring of prenatal cold stress-treated dams | Higher systolic-, diastolic- and mean blood pressures, impaired D1R-mediated vasodilation, increased D1R phosphorylation and cell internalization in mesenteric artery from offspring of prenatal cold stress-treated than control dams | Sun et al., 2020 |
| Old FBN rats with hypertension | Increased renal GRK4 protein expression | Higher systolic, diastolic, and mean arterial blood pressures, decreased D1R expression, impaired renal D1R-mediated natriuresis, and no change in AT1R expression in RPTs, and increased renal AT1R function and oxidative stress in old rats; GRK4-mediated increase in HEK293 senescence | Chugh et al., 2011; Chugh et al., 2012; Xiao et al., 2017 |
| High salt-induced hypertension | Renal GRK4 expression is lower on normal salt diet but higher on high salt diet in C57BL/6 J than in SJL/J mice | Higher systolic and diastolic arterial pressures, increased renal D5R expression, impaired renal D1R-like receptor-mediated natriuresis, shift to the right of the blood pressure-natriuresis plot in C57BL/6J mice, relative to SJL/J mice fed high salt diet | Escano et al., 2009 |
Abbreviations: AT1R, angiotensin II receptor type 1; cAMP, cyclic adenosine monophosphate; D1R, dopamine D1 receptor; D5R, dopamine D5 receptor; ETBR, endothelin receptor type B; FBN rats, Fischer 344 × Brown Norway rats; GRK4, G protein-coupled receptor kinase 4; LPS, lipopolysaccharides; NKA: Na+/K+-ATPase; PGE2, prostaglandin E2; RPT, renal proximal tubule; SD, Sprague-Dawley; SHRs, spontaneously hypertensive rats; TP, thromboxane prostanoid; WKY, Wistar-Kyoto.
A role for GRK4 in regulating blood pressure is supported by several studies using GRK4 knockout, selective renal reduction of GRK4 expression via the chronic renal cortical interstitial-selective infusion of GRK4 antisense oligodeoxynucleotides or GRK4 small interfering RNA (siRNA) delivered by ultrasound-targeted microbubble destruction, and overexpression of GRK4 variants (Table 2). In contrast to the GRK4 knockdown-mediated amelioration of blood pressure in hypertensive rodents, the expression of human GRK4 gene variants (hGRK4γ 65 L, hGRK4γ 142, or hGRK4γ 486 V) in mice increases blood pressure (Table 2).
Table 2.
In vivo and in vitro studies of GRK4 modification or variants.
| GRK4 modification or variants | Animal or cell | Type of GRK4 modification | Effects of GRK4 modification on blood pressure and related functions | References |
|---|---|---|---|---|
| In vivo studies of GRK4 modification or variants | ||||
| GRK4 knockout | Knockout mice | Global knock mice | Decreases systolic and diastolic blood pressures | Wang et al., 2016 |
| GRK4 siRNA | SHRs | Silencing renal GRK4 expression via UTMD-delivered GRK4 siRNA | Decreases blood pressure; increases sodium excretion and urine volume, reduces D1R phosphorylation and improves D1R-mediated sodium excretion; restores the adiponectin- or ETBR-mediated increase in sodium excretion | Huang et al., 2016; Yang et al., 2020; Zhang et al., 2020 |
| GRK4 As-Odns | SHRs and WKY rats | Renal GRK4 depletion via the chronic renal cortical interstitial infusion of GRK4 As-Odns | Decreases D1R phosphorylation to a greater extent in SHRs than in WKY rats; increases sodium excretion and urine volume, attenuates the increase in arterial blood pressure with age, and decreases protein excretion in SHRs, but not WKY rats | Sanada et al., 2006; Yatabe et al., 2008 |
| GRK4 and AT1R As-Odns | SHRs and WKY rats | Silencing both renal GRK4 and AT1R via intrarenal cortical infusion of both GRK4 and AT1R As-Odns | Decreases blood pressure and increases sodium excretion to a greater extent in SHRs than WKY rats; decreases circulating levels of renin, ang II, and aldosterone, reduces urine protein excretion and improves the GSI in SHRs and WKY rats; greater natriuresis and amelioration of hypertension in SHRs than the rats treated with either GRK4 or AT1R As-Odn | Yatabe et al., 2008 |
| Overexpression of hGRK4γ142V | hGRK4γ WT and hGRK4γ 142 V transgenic mice | Global transgenic mice | Higher blood pressure and renal D1R phosphorylation, impaired D1R-mediated sodium excretion, increased renal AT1R expression and pressor response to Ang II in hGRK4γ 142 V transgenic mice than those in hGRK4γ WT transgenic mice; higher AT1R protein expression in the aorta of GRK4γ 142 V transgenic mice than hGRK4γ WT transgenic mice; hyperphosphorylation of renal ETBR and AdipoR1, impairment of ETBR or AdipoR1 function, less natriuresis and diuresis induced by endothelin or adiponectin in SHRs than WKY rats and GRK4γ 142 V transgenic mice than hGRK4γ WT transgenic mice | Felder et al., 2002; Yang et al., 2020; Wang et al., 2016; Wang et al., 2007; Zhang et al., 2020. |
| Overexpression of hGRK4γ 486 V | hGRK4γ WT and hGRK4γ 486 V transgenic mice | Global transgenic mice | Impairs sodium excretion, increases renal oxidative stress, and increases blood pressure with high salt diet in hGRK4γ 486 V transgenic mice, relative to hGRK4γWT transgenic mice | Diao et al., 2017; Wang et al., 2006 |
| Overexpression of hGRK4γ 65 L | hGRK4γ WT and hGRK4γ 65 L transgenic mice | Global transgenic mice | Increases blood pressure with high salt diet in hGRK4γ 65 L transgenic mice, relative to hGRK4γ WT transgenic mice | Asico and Jose, unpublished data |
| In vitro studies of GRK4 modification or variants | ||||
| Inhibition of GRK4 activity | RPT cells from normotensive humans | Treatment with heparin, an inhibitor of GRK activity | Decreases the expression of GRK2 and GRK4 and attenuates the desensitization of D1R, blunts p44/42 phosphorylation and mitogenesis induced by D3R stimulation in hRPTCs | Watanabe et al., 2002; Villar et al., 2009 |
| Inhibition of GRK4 expression | RPT cells from hypertensive humans | Treatment with antibody recognizing GRK4γ/δ isoforms | Blocks the stimulatory effect of the D1-like receptor agonist, fenoldopam, on GRK activity | Felder et al., 2002 |
| GRK4 As-Odns | RPT cells from hypertensive humans | GRK4 knockdown with its specific As-Odns | Blocks the D1R phosphorylation, blunts fenoldopam-induced D1R desensitization, and restores the D1-like receptor-mediated cAMP production | Felder et al., 2002 |
| GRK4 siRNA | RPT cells from SHRs | GRK4 knockdown with its specific siRNA | Recovers the impaired inhibitory effect of ETBR on Na+/K+-ATPase activity | Yang et al., 2020 |
| GRK4 siRNA | RPT cells from normotensive humans | RPT cells transfected with GRK4 siRNA | Blunts the D3R-mediated mitogenesis | Villar et al., 2009 |
| Inhibition of GRK4 activity | Rat intestinal epithelial cells | Treatment with heparin, an inhibitor of GRK activity | Heparin prevents the loss of inhibition of NHE activity caused by 25 min exposure to SKF-38393, a D1-like receptor agonist | Fraga et al., 2004 |
| Inhibition of GRK4 expression | Rat intestinal epithelial cells | Treatment with the anti-GRK4–6 antibody | Restores the loss of inhibition of NHE activity and stimulation of AC activity caused by 25 min exposure to SKF-38393, a D1-like agonist | Fraga et al., 2004 |
| Overexpression of GRK4 variants | CHO cells | Transfected with GRK4γ with SNPs (A142V, R65L, A486V or combined R65L and A486V) | Increases basal D1R phosphorylation and impairs D1R-mediated cAMP accumulation | Felder et al., 2002 |
| Overexpression of GRK4 splice variants | CHO cells | Transfected with GRK4α, GRK4β, GRK4γ or GRK4δ | GRK4γ and GRK4α increase the phosphorylation of D3R compared with untreated control cells; activation of D3R results in a 3-fold and 2-fold increase in D3R phosphorylation in cells expressing GRK4γ and GRK4α, respectively | Villar et al., 2009 |
| Overexpression of hGRK4γ 142 V | RPT cells from normotensive humans | RPT cells transfected with hGRK4γ 142 V and GRK4γ WT plasmids | Increases activity of Agtr1 and AT1R protein expression | Wang et al., 2016 |
| Overexpression of hGRK4γ 142 V | VSMCs | Transfected with hGRK4γ 142 V and GRK4γ WT plasmids | Higher AT1R protein and mRNA expressions, lower AT1R phosphorylation and protein degradation, higher AT1R-mediated intracellular calcium concentration after stimulation with Ang II in hGRK4γ 142 V than in GRK4γ WT cells | Chen et al., 2014 |
| Overexpression of GRK4 | HEK293 cells | Transfected with full-length GRK4 plasmid | Induces cellular senescence, halts cell proliferation | Xiao et al., 2017; Luo et al., 2019 |
Abbreviations: AC, adenylyl cyclase; AdipoR1, adiponectin receptor 1; Ang II, angiotensin II; As-Odns, antisense oligodeoxynucleotides; AT1R, angiotensin II receptor type 1; cAMP, cyclic adenosine monophosphate; CHO, Chinese hamster ovary; D1R, dopamine D1 receptor; D3R, dopamine D3 receptor; ETBR, endothelin receptor type B; GRK4, G protein-coupled receptor kinase 4; GSI, glomerular sclerosis index; hRPTCs: human renal proximal tubules cells; NHE: sodium-hydrogen exchanger; RPT, renal proximal tubule; SHRs, spontaneously hypertensive rats; siRNA, small interfering RNA; UTMD, ultrasound-targeted microbubble destruction; VSMCs, vascular smooth muscle cells; WKY, Wistar-Kyoto; WT, wild-type.
4.4. Mechanisms involved in the regulation of blood pressure by GRK4
Humans with essential hypertension have aberrant pathophysiological changes, such as increased renal sodium reabsorption and vasoconstriction and impaired arterial vasodilatation, that are not properly regulated by hormones via their receptors (Harrison et al., 2021; Kemp, Howell, Gildea, Keller, & Carey, 2020; Liu et al., 2021; Nizar, Shepard, Vo, & Bhalla, 2018; Olivares-Hernández et al., 2021; Rianto et al., 2021). Accumulating evidence show a role of GRK4 on the dysfunction of GPCRs or non-GPCRs in hypertension.
4.4.1. GRK4 regulates D1R function by affecting its phosphorylation state
The dopaminergic system exerts an autocrine/paracrine regulatory role on renal sodium transport and blood pressure via its five receptor subtypes (Albrecht et al., 1996; Gildea et al., 2010; Jose, Eisner, Drago, Carey, & Felder, 1996; Olivares-Hernández et al., 2021; Yu et al., 2006). Dopamine receptors, belonging to GPCRs, are classified into two families: D1-like receptors (D1R and D5R), which stimulate adenylyl cyclase activity and D2-like receptors (D2R, D3R, and D4R), which inhibit adenylyl cyclase activity. D1-like receptors are the major determinants of dopamine-mediated regulation of sodium transport (Albrecht et al., 1996; Chugh, Lokhandwala, & Asghar, 2011; Harris, 2012; Jose et al., 2010; Villar et al., 2013; Wang et al., 2014; Yang et al., 2021; Ye et al., 2018; Yu et al., 2006). Indeed, during conditions of moderate sodium excess, more than 50% of renal sodium excretion is regulated by D1-like receptors (Yang et al., 2021). However, in hypertensive patients and hypertensive animal models, such as the Dahl salt-sensitive rat and SHR, D1R-mediated natriuresis and diuresis are decreased (Albrecht et al., 1996; Jose et al., 1996).
The impaired function of D1R in hypertension is not due to decreased renal dopamine production, mutation of the D1R gene, or decreased expression of D1R, but is caused by increased D1R phosphorylation and a defect in the coupling of the D1R to its G protein/effector complex in renal proximal tubules (Yu et al., 2006), which is ascribed to increased GRK4 expression or activity (Felder et al., 2002; Jose et al., 2010; Lu et al., 2018; Sun, Chen, Wang, Zhou, & Zeng, 2020) (Fig. 2). SHRs have higher renal GRK4 expression than WKY rats (Sanada et al., 2006a, b). The intrarenal infusion of GRK4 antisense oligodeoxynucleotides or ultrasound-microbubble destruction-targeted GRK4 siRNA delivery to the kidney effectively decreases GRK4 expression, attenuates the augmented D1R phosphorylation, normalizes the sodium balance, and reduces the blood pressure of SHRs (Huang et al., 2016; Sanada et al., 2006a, b). Besides the SHR, GRK4-mediated inhibition of D1R is also reported in other hypertensive animal models (Lu et al., 2018; Sun et al., 2020; Wang et al., 2014; Ye et al., 2018). GRK4γ 142 V transgenic mice have higher blood pressure than GRK4γ wild-type mice, which is related to renal D1R hyperphosphorylation and impaired D1R-mediated natriuresis and dieresis (Felder et al., 2002; Wang et al., 2016; Yang et al., 2020).
Fig. 2.
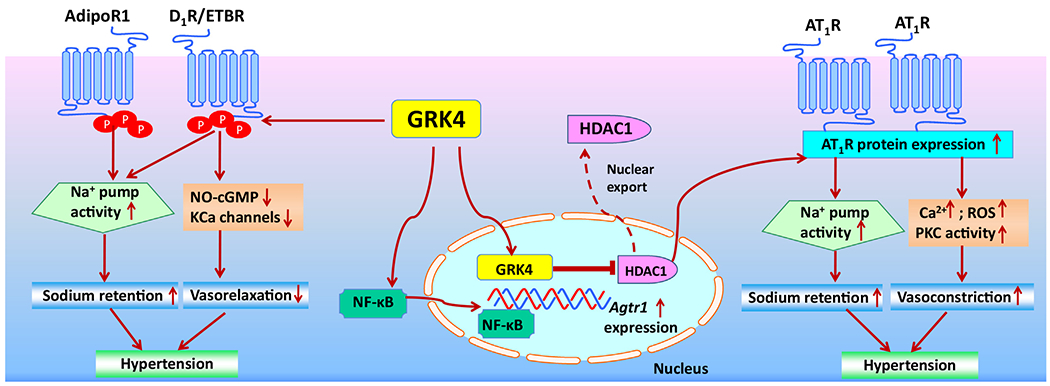
GRK4-mediated regulation of blood pressure via the kidneys and arteries.
GRK4 regulates blood pressure by modulating GPCRs and non-GPCRs in the kidneys and arteries by several mechanisms: 1) renal or arterial GRK4 increases the phosphorylation of D1R, AdipoR1, and ETBR impairs receptor-mediated inhibition of the sodium pump (Na+/K+-ATPase) and attenuates receptor-induced vasorelaxation, subsequently leading to hypertension; 2) renal GRK4 promotes HDAC1 egress from the nucleus into the cytoplasm, which up-regulates Agtr1 expression and the ability of AT1R to increase the activity of the sodium pump (Na+/K+-ATPase), which results in an increase in renal sodium reabsorption and extracellular fluid volume, and consequently hypertension; 3) in the arteries, GRK4 increases NF-κB activity with more NF-κB binding to the AT1R promoter, which increases Agtr1 expression and AT1R protein abundance, intracellular calcium concentration, ROS production, and PKC activity, enhancing vasoconstriction and consequently hypertension.
Abbreviations: AT1R, angiotensin II receptor type 1; AdipoR1, adiponectin receptor 1; cGMP, cyclic guanosine monophosphate; D1R, dopamine D1 receptor; ETBR, endothelin receptor type B; GRK4, G protein-coupled receptor kinase 4; HDAC1, histone deacetylase type 1; KCa: calcium-activated K+ channel; NF-κB, nuclear factor kappa B; NO: nitric oxide; PKC: protein kinase C; ROS, reactive oxygen species.
The regulatory effect of GRK4 on D1R in vivo is confirmed by in vitro studies. GRK4 activity is enhanced in human renal proximal tubule cells from hypertensive subjects, which constitutively phosphorylates D1R even in the absence of D1R agonist (Felder et al., 2002). Inhibition of GRK4 activity with heparin or depletion of GRK4 with antisense oligodeoxynucleotides blunts the D1-like receptor agonist fenoldopam-induced D1R desensitization in human renal proximal tubule cells (Watanabe, Xu, Bengra, Jose, & Felder, 2002). In Chinese hamster ovary cells transfected with GRK4γ variants, including A142V, R65L and A486V, GRK4 activity is also increased that is associated with an increase in basal D1R phosphorylation and impairment of D1R-mediated cAMP production (Felder et al., 2002).
4.4.2. GRK4 regulates AT1R function by affecting its expression and phosphorylation
Ang II, the principal renin-angiotensin system (RAS) effector peptide, exerts its physiological functions via its receptors, type 1 (AT1R) and type 2 (AT2R) (Fatima, Patel, & Hussain, 2021; Ziaja, Urbanek, Kowalska, & Piastowska-Ciesielska, 2021). The vast majority of the actions of Ang II are transduced via AT1R, which increases renal sodium reabsorption and induces vasoconstriction (Paz et al., 2020).
The renal expression of GRK4 and AT1R is higher in kidneys of SHRs than WKY rats (Yatabe et al., 2008). The selective intrarenal cortical infusion of GRK4 antisense oligodeoxynucleotides increases sodium excretion and decreases arterial blood pressure in SHRs (Sanada et al., 2006a, b); these effects are increased with the combined inhibition of renal GRK4 and AT1R (Yatabe et al., 2008). Another study also found that the increased renal GRK4 expression correlates with increased renal AT1R expression and function in old Fischer 344 × Brown Norway rats, suggesting that GRK4-mediated increase in renal AT1R expression may be necessary for the development of age-associated hypertension (Chugh, Lokhandwala, & Asghar, 2012).
AT1R expression and function are regulated by GRK4 variants, as related to regulation of blood pressure (Fig. 2). The hypertension in hGRK4γ 142 V transgenic mice is associated with increased AT1R expression and function in the kidneys and arteries. GRK4γ 142 V transgenic mice that are deficient of AT1R have normal blood pressure (Wang et al., 2016). We have reported that both AT1R expression and AT1R-mediated vasoconstriction are higher in the aorta of hGRK4γ 142 V than hGRK4γ wild type transgenic mice. Moreover, Ang II causes a greater increase in blood pressure while infusion of the AT1R antagonist candesartan causes a greater decrease in blood pressure in hGRK4γ 142 V that hGRK4γ WT transgenic mice (Chen et al., 2014). Agtr1 mRNA and AT1R protein expression and function are greater in VSMCs expressing hGRK4γ 142 V than in cells expressing hGRK4γ WT. In those cells expressing GRK4γ 142 V, the activity of NF-κB, a regulator of AT1R promoter activity, is increased, accompanied by an increase in its binding to the AT1R. The opposite is true for AT1R protein degradation, indicating that the regulation of AT1R expression by hGRK4γ occurs at both transcriptional and post-translational levels (Chen et al., 2014).
Histone deacetylase type 1 (HDAC1) is involved into the regulation of Agtr1 mRNA expression by GRK4. The phosphorylation of HDAC1 by GRK4 promotes the export of HDAC1 from the nucleus to the cytoplasm, which causes an increase in AT1R promoter activity (Wang et al., 2016). The phosphorylation of AT1R is also regulated by GRK4. The phosphorylation of AT1R protein is lower in hGRK4γ 142 V-transduced VSMCs than in hGRK4 WT–transduced cells, which seems to be inconsistent with GRK4 function as a phosphorylating enzyme. However, our further study showed that the interaction between GRK4 and AT1R in VSMCs is reduced in hypertension, as determined by co-immunoprecipitation (Chen et al., 2014). This may be, at least in part, explained by the lower level of AT1R phosphorylation, although the underlying mechanism leading to the reduced GRK4/AT1R vascular interaction is not known. Additional studies are needed to elucidate this GRK4/AT1R vascular interaction.
Similar to hGRK4γ 142 V transgenic mice, hGRK4γ 486 V transgenic mice also have higher blood pressure and greater renal AT1R expression than hGRK4γ WT mice. However, there is a distinct difference that hGRK4γ 142 V transgenic mice are hypertensive on a normal salt diet. By contrast, hGRK4γ 486 V transgenic mice develop hypertension only on a high salt diet (Diao et al., 2017; Wang et al., 2006a, b). The contribution of AT1R in the salt sensitivity of blood pressure in hGRK4γ 486 V transgenic mice remains to be determined.
The studies suggest that GRK4 regulates AT1R expression and its actions in kidneys and arteries. In the kidney, the increased expression of GRK4 or presence of GRK4 variants increases the transcription of AT1R, leading to an increase in AT1R protein expression and renal sodium reabsorption, and subsequently hypertension. In the artery, the increased expression of GRK4 or presence of GRK4 variants increases AT1R protein expression, via both transcriptional and post-translational levels and aggravates AT1R-induced vasoconstriction and subsequently hypertension. However, it should be noted that not only AT1R but also other receptors, such as dopamine receptors, are targets of GRK4. Thus, the effect of GRK4 expression or variants on blood pressure could be ascribed, at least in part, to their regulation of AT1R and other GPCRs.
The activation of AT2R, the other receptor of Ang II, induces natriuresis and lowers blood pressure (Kemp et al., 2020). Our preliminary data indicate that hGRK4γ 142 V transgenic mice have increased phosphorylation of renal AT2R with impaired AT2R-mediated natriuresis, compared with hGRK4γ WT transgenic mice. This is supported by an in vitro study in which overexpression with hGRK4γ 142 V in renal proximal tubule cells impairs AT2R-mediated inhibition of Na+-k+-ATPase activity (Zhang and Yang, unpublished data). Accumulating pieces of evidence indicate that GRK4 regulates blood pressure by affecting several GPCRs, which affect sodium retention, arterial function, consequently leading to elevated blood pressure level.
4.4.3. GRK4 regulation of other GPCRs
More and more GPCRs are reported to be regulated by GRK4. These include endothelin receptor type B (ETBR), thromboxane receptors, and dopamine D3 receptor (D3R).
Endothelin, via its receptor, ETBR, decreases renal tubular sodium transport. However, in hypertensive states, the renal ETBR is hyperphosphorylated and ETBR-mediated sodium excretion is impaired (Dhaun & Webb, 2019). GRK4 is believed to play an important role in this process because down-regulation of renal GRK4 by siRNA normalizes the phosphorylation of ETBR and ameliorates the impaired renal ETBR function in the SHR (Yang et al., 2020). Moreover, hGRK4γ 142 V transgenic mice, which are hypertensive, have increased phosphorylation of renal ETBR and impaired ETBR-mediated natriuresis and diuresis (Yang et al., 2020) (Fig. 2).
Thromboxane receptors may be also regulated by GRK4. GRK4 expression in aortic smooth muscles is higher in SHR than in WKY rats (Zhao et al., 2015). More importantly, in the SHR but not the WKY aorta, α1-AR activation desensitizes thromboxane receptors through activation of PKC-ε (Zhao et al., 2015), which is a positive regulator of GRK4 (Gildea et al., 2013). These findings suggest that GRK4 may be involved in the regulation of aortic thromboxane receptors in hypertension. However, the direct relationships between GRK4 and those receptors have not been fully elucidated.
In addition to D1R, GRK4 also regulates other dopamine receptor subtypes, including D3R. GRK4 is required for D3R-mediated mitogenesis and activation of the mitogen activated protein kinase pathway in human renal proximal tubule cells (Villar et al., 2009). This effect of GRK4 on the regulation of D3R is isoform-specific because even though all four GRK4 isoforms are expressed in human renal proximal tubule cells, only GRK4α and GRK4γ (GRK4γ > GRK4α) isoforms, not GRK4β and GRK4δ, modulate the phosphorylation of D3R (Villar et al., 2009). However, the role of GRK4-mediated D3R regulation in the development of hypertension has not been determined.
4.4.4. GRK4 regulation of non-GPCRs
A few studies also reported that non-GPCRs, such as adiponectin receptor 1 (AdipoR1), are regulated by GRK4. AdipoR1, as with GPCRs, has 7 transmembrane domains. However, the orientation of AdipoR1 is opposite that of GPCRs; its N-terminus is intracellular and its C-terminus is external (Tanabe et al., 2015).
Obesity, characterized by excess body fat, is associated with impaired natriuresis and increased risk of hypertension (Hall et al., 2021; Powell-Wiley et al., 2021). As an endocrine organ, adipose tissue produces and releases adipokines, such as adiponectin (Zhao, Kusminski, & Scherer, 2021). Adiponectin, an adipocytokine produced by adipose tissue can also be produced by renal proximal tubules (Perri et al., 2013). In addition to adiponectin receptor 1 (AdipoR1), there are two other adiponectin receptors, AdipoR2 and T-cadherin; AdipoR1 mRNA is 20 times higher than AdipoR2 in human renal proximal tubule cells (Shen, Hughes, Charlesworth, Kelly, & Peake, 2008). Adiponectin, via AdipoR1, decreases renal sodium transport. However, adiponectin-mediated natriuresis and diuresis are impaired in SHRs. The impaired adiponectin function in hypertension may be due, in part, to the hyperphosphorylation of adiponectin receptor (Zhang et al., 2020). GRK4 is thought to play a role in the impairment of adiponectin receptor function in hypertension (Fig. 2) because hGRK4γ 142 V transgenic mice have increased phosphorylation of renal AdipoR1 and impaired diuretic and natriuretic response to adiponectin, relative to hGRK4γ WT mice. Moreover, renal-selective GRK4 knockdown via renal ultrasound-directed siRNA restores the adiponectin-mediated increase in sodium excretion and reduces blood pressure in SHRs (Zhang et al., 2020). These observations indicate that GRK4 causes the hyperphosphorylation and impaired function of renal AdipoR1 in hypertension. These studies also show that GRK4 can regulate the function of GPCRs and non-GPCRs.
4.4.5. GRK4 regulation of cellular senescence
Cellular senescence, an age-related physiological process, is recognized as a vital contributor to the development of cardiovascular diseases, including hypertension (Gorgoulis et al., 2019). The heterologous expression of GRK4 in HEK293 cells causes cell cycle G1/G0 phase arrest, accompanied by an increase in senescence-associatedβ-galactosidase activity, indicating that GRK4 halts cell proliferation and induces cellular senescence (Luo et al., 2019; Xiao et al., 2017). Therefore, GRK4 may be associated with age-related hypertension, by impairing cellular growth and promoting senescence. However, the causal role of GRK4-mediated regulation of cellular proliferation or senescence on the pathogenesis of hypertension needs to be demonstrated.
5. Regulation of GRK4 expression and activity
GRK4 expression and activity are higher in the hypertensive than normotensive state. It is known that the presence of GRK4 variants leads to increased activity of GRK4, although the mechanisms are still unclear. To our knowledge, until today, there is only one article that reported the possible mechanism on how GRK4 polymorphism affects enzymatic activity (Allen et al., 2015). There are differences in the crystal structure of GRK4α A486V and wild-type GRK4α and other GRKs, e.g., GRK6. Allen et al found that there is a lag in the autophosphorylation of wild-type GRK4α, which is required for full kinase activity. However, this lag is not observed in GRK4α A486V, which has an increased rate of autophosphorylation of a number of residues. These kinetic differences between wild-type GRK4α and GRK4α A486V may be related to their structural differences. The precise effect of GRK4 polymorphisms on its enzymatic activity needs further studies.
In addition to the inherent increase in GRK4 expression in the kidneys and arteries in primary hypertension, environmental factors such as cold stress, fine particulate matter exposure, and infection also increase the expression of GRK4 (Lu et al., 2018; Sun et al., 2020; Wang et al., 2014; Ye et al., 2018). Reactive oxygen species (ROS), may be the vital link between environmental factors and GRK4. For example, long-term exposure of fine particulate matter (PM2.5) in SD rats increases ROS production, renal GRK4 expression, and blood pressure (Lu et al., 2018). The elevated GRK4 expression with long-term exposure to PM2.5 is due to oxidative stress because inhibition of ROS production by tempol, an antioxidant, decreases renal GRK4 expression, alleviates the hyperphosphorylation of renal D1R, increases sodium excretion, and lowers the blood pressure of PM2.5-exposed SD rats (Lu et al., 2018). In utero exposure to PM2.5 and other stressors, such as cold stress and infection, plays an important role in early life-induced hypertension in offspring that may be related to up-regulation of GRK4 expression, at least in arteries and kidneys (Sun et al., 2020; Wang et al., 2014; Ye et al., 2018).
Besides ROS, the increase in GRK4 expression caused by environmental and disease factors may be also a consequence of alterations of some transcription factors and signaling molecules. The GRK4 core promoter resides in the first 1851 bp upstream of its transcription start site (Hasenkamp et al., 2008), suggesting that the DNA-protein and/or protein-protein interaction patterns at this region may affect the transcription and expression of GRK4. The regulatory regions of the GRK4 promoter are independent of cell type and differentiation state (Hasenkamp et al., 2008). The transcription factor c-Myc, by binding to the promoter of GRK4, positively regulates GRK4 protein expression in human renal proximal tubule cells (Gildea et al., 2013). In the PM2.5 regulation of GRK4 expression, c-Myc is the key signal between ROS and increased GRK4 transcription (Lu et al., 2018; Ye et al., 2018). trans-Activator C/EBP family members, including CCAAT/enhancer-binding protein (C/EBP) α, C/EBPβ, or C/EBPδ, also increase GRK4 promoter activity in renal cell lines HEK293T and COS7 cells and osteoblast-like osteosarcoma cell line SaOs-2 (Hasenkamp et al., 2008).
In addition to the regulation of the promoter of GRK4, other regions are also regulated by certain proteins and microRNAs. For example, in the cerebellum, FMRP, an RNA-binding protein, decreases the translation of GRK4 by an interaction between its C-terminal region and the G4RIF domain of GRK4 mRNA (Maurin et al., 2015). A ubiquitous calcium-binding protein calmodulin (CaM) interacts with the GRK4 subfamily, including GRK4, but not the GRK1-like- and the GRK2-like subfamilies, by binding to the C-terminal-or amino-terminal domain (Pronin, Satpaev, Slepak, & Benovic, 1997; Sallese et al., 1997; Sallese et al., 2000). Some miRNAs, such as miR430a and miR218a, have also been implicated in the regulation of GRK4 by targeting the 3′ untranslated region of GRK4 (Guo et al., 2019), suggesting that miRNAs, a class of endogenously-initiated non-coding RNAs, may post-transcriptionally control GRK4 expression via either translational repression or mRNA degradation.
The process of intracellular trafficking may be also involved in the regulation of GRK4. Our previous studies showed that GRK4 is regulated by sorting nexin (SNX), an intracellular transport protein, which is involved in D1R endocytosis and trafficking through the endosomes (Li et al., 2015a, b; Villar et al., 2013). SNX5 directly interacts with GRK4 and prevents GRK4 from targeting the phosphorylation sites of the D1R, which is enhanced after D1R activation (Villar et al., 2013). By contrast, depletion of SNX5 markedly increases the ability of GRK4 to phosphorylate constitutively D1R in human renal proximal tubule cells, consistent with the in vivo studies showing that renal SNX5 depletion increases blood pressure, causes insulin resistance, and decreases D1R-mediated sodium excretion (Li et al., 2015a, b; Li et al., 2018; Villar et al., 2013).
GRKs may be regulated by sex steroids, estrogens, progestins, and androgens. Estrogen, via estrogen receptor α and β, has beneficial effects on the regulation of blood pressure (Colafella & Denton, 2018; Mercuro et al., 2010; Somani, Pawelczyk, De Souza, Kris-Etherton, & Proctor, 2019; Zheng, Ji, Maric, Wu, & Sandberg, 2008). A few studies have found that GRKs, such as GRK2 and GRK6, are regulated by estrogens (Abraham et al., 2018; Ansonoff & Etgen, 2001; Miyoshi, Otsuka, & Shimasaki, 2013) but there is no study on the ability of GRK4 to regulate estrogens or their receptors. In fact, previous studies have shown that there is no difference on GRK4-mediated regulation of blood pressure between male and female hGRK4γ WT mice and hGRK4γ 142 V or hGRK4γ 486 V transgenic mice, or male and female WKY rats and SHRs (Diao et al., 2017; Felder et al., 2002; Wang et al., 2007; Wang et al., 2016).
6. GRK4 and human essential hypertension
6.1. GRK4 polymorphisms and hypertension in humans
Loci in chromosome 4 are associated with hypertension. The GRK4 locus on human chromosome 4p16.3, correlates with essential hypertension and salt sensitivity (Allayee et al., 2001; Casari et al., 1995; Zeng et al., 2008).
Several studies have shown that the three GRK4 gene variants (R65L, A142V, and A486V) are positively associated with essential hypertension in several ethnic groups, including African-Brazilian-, Caucasian and Black American-, Caucasian-Australian-, Chinese-, Italian-, and Japanese-populations (Table 3) (Bengra et al., 2002; Gu et al., 2006; Kimura et al., 2012; Sanada et al., 2016; Speirs et al., 2004; Wang et al., 2006a, b). A meta-analyses confirmed the association between the GRK4 SNPs and hypertension risk among different populations (Liu & Xi, 2012; Zeng et al., 2008; Zhang, Sun, Liu, & Yang, 2015). Not included in the meta-analysis are recent studies of the association of new GRK4 SNPs and hypertension (GRK4 rs1644731) (Jiang et al., 2021), risk of both hypertension and diabetes (GRK4rs1557213) (Du et al., 2021), or cardiovascular disease risk and diabetes (GRK4rs60314379) (Cheng et al., 2021) in Han Chinese population.
Table 3.
Summary of the role of GRK4 variants in hypertension and response to antihypertensive treatment in humans.
| Ethnic Group | Subjects | Single- or multi-locus analyses | Association between GRK4 variants and hypertension | Response to antihypertensive medicines or dietary salt intake | Reference |
|---|---|---|---|---|---|
| Italian | Hypertensive: 60 Normotensive: 60 |
Single-locus | GRK4 486 V correlates with mild hypertension | Bengra et al., 2002 | |
| Ghanaian | Hypertensive: 126 Normotensive: 51 |
Multi-locus | The combination of GRK4 R65L and ACE predicts the hypertensive phenotype with an estimated success of 70.5% | Williams et al., 2004 | |
| Caucasian Australian | Hypertensive: 168 Normotensive: 312 |
Single-locus | GRK4 486 V is associated with hypertension | Speirs et al., 2004 | |
| Chinese | Hypertensive: 503 Normotensive: 490 |
Single-locus | GRK4 A486V is associated with hypertension | Gu et al., 2006 | |
| Chinese | Hypertensive: 503 Normotensive: 490 |
Single-locus | A486 allele is associated with hypertension | Wang et al., 2006 | |
| American | Normotensive adolescents: 664 | Single-locus | GRK4 65 L is associated with stress-induced reduction of urinary sodium excretion in the blacks | Zhu et al., 2006 | |
| Japanese | Newly diagnosed, untreated hypertensives: 184 | Single-locus | GRK4 R65L, A142V, and A486V, are associated with salt-sensitive hypertension; the GRK4 A142V or combination of GRK4 A142V and CYP11B2 is associated with low-renin hypertension; sodium excretion is inversely related to the number of GRK4 variants in hypertension | GRK4 variants (R65L, A142V, and A486V) impair renal dopamine-induced natriuresis, even in the absence of hypertension | Sanada et al., 2006 |
| African Americans | AASK study Hypertensives randomized to metoprolol: 328 | Single-locus | GRK4 A142V is associated with blood pressure response among men. Men with a GRK4 A142 are less responsive to metoprolol if they have the GRK4 L65 variant | Bhatnagar et al., 2009 | |
| African Americans | Hypertensive: 173 Normotensive: 239 |
Single-locus | GRK4 A486V variant is negatively associated with HBP in the nonobese group | Martinez et al., 2010 | |
| European ancestry | Hypertensive: 55; Normotensive: 130 |
Single-locus | GRK4 486 V is associated with salt sensitivity of blood pressure | Carey et al., 2012 | |
| African-derived Brazilian | Participants: 652 | Multi-locus | Combination of NOS3-rs1799983 and GRK A486V is associated with DBP levels | Kimura et al., 2012 | |
| Black subjects | Mild to moderate hypertensive: 40 | Single-locus | GRK4 variants, A142V and R65L, predict blood pressure response to reduction of dietary salt intake | Rayner et al., 2012 | |
| American | Mild-to-moderate hypertensive: 768 | Single-locus | All three GRK4 variants (65 L, 142 V, and 486 V) correlate with increased risk for the primary outcome. | Increasing number of copies of the GRK4 variant 65 L-142 V haplotype is associated with reduced response to the β-blocker monotherapy with atenolol. | Vandell et al., 2012 |
| American | Healthy males: 24 | Multi-locus | Subjects with at least three GRK4 allele variants have impaired natriuretic response to diuretics than those with less than three GRK4 allele variants | Wagner et al., 2012 | |
| Chinese | Participants: 3025 | Single-locus | GRK4 variant rs2488815 correlates with lower values of eGFR | Montasser et al., 2014 | |
| Swiss | Hypertensive: 100 | Single-locus | Subjects with GRK4 65 L and 142 V need more antihypertensive treatment and especially diuretic therapy (particularly for thiazide and thiazide-like diuretics) to reach the same MBP | Muskalla et al., 2014 | |
| Japanese | Hypertensive: 588 Normotensive: 486 |
Single-locus | GRK4 R65L, A142V, or A486V is associated with hypertension | Subjects with GRK4 142 V have a greater decrease in SBP in response to ARBs than non-carrier hypertensive patients | Sanada et al., 2016 |
| Korean | Children: 2163 | Multi-locus | A high sodium intake increases the obesity risk in children with GRK4 A486V; the combination of GRK4 A486V, ACE, and SLC12A3 variants increases the obesity risk in boys, whereas the combination of GRK4 A486V and CYP11B2 variants increases it in girls | Lee et al., 2015 | |
| Black Africans | Hypertensive: 105 | Multi-locus | A high frequency of four NSVs of GRK4 (R65L, A116T, A142V, V486A) in patients with low renin-resistant hypertension | Jones et al., 2017 | |
| American | Patients with breast, lung, ovarian, or other cancers, 38 developed BIH: 114 |
Single-locus | GRK4 variant rs1419044 is associated with BIH | Frey et al., 2017 | |
| Korean | Adults: 15034 | Single-locus | GRK4 65 L TT genotype is inversely associated with hypertension risk | Jeong et al., 2020 | |
| Chinese | cardiovascular disease patients: 326; noncardiovascular disease patients: 1209 |
Multi-locus | GRK4 rs60314379 is associated with cardiovascular disease risk in T2DM | Cheng et al., 2020 | |
| Chinese | Healthy: 1152; T2DM:1152; Hypertensive: 1152 |
Single-locus | GRK4 rs1557213 contributes to the risk of hypertension and T2DM | Du et al., 2021 | |
| Chinese | Hypertensive: 1239 | Single-locus | Patients with GRK4 variants (A142V, A486V or R65L) are more likely to be non-dippers | Patients with GRK4 variants (A142V, A486V or R65L), relative to those without these variants, have a better antihypertensive response to candesartan | Cao et al., 2021 |
| Chinese | Healthy: 65; Hypertensive: 151 |
Multi-locus | GRK4 rs1644731 and RDH8 rs1801058 are associated with hypertension. | Jiang et al., 2021 |
Abbreviations: ACE, angiotensin-converting enzyme; ARBs, angiotensin receptor blockers; BIH, bevacizumab-induced hypertension; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; GRK4, G protein-coupled receptor kinase 4; HBP, high blood pressure; MBP, mean arterial blood pressure; NSVs, nonsynonymous variants; SBP, systolic blood pressure; T2DM, type 2 diabetes mellitus.
About 50% of subjects with hypertension are salt-sensitive (Elijovich et al., 2016). The association between GRK4 variants and salt sensitivity or impaired urinary sodium excretion has been studied in both hypertensive patients and normotensive subjects (Table 3).
The positive association of GRK4 gene variants and hypertension are contrasted by other reports showing no such association (Martinez Cantarin et al., 2010; Rana et al., 2007; Staessen et al., 2008). For example, a study in a family-based random sample of a white population found that GRK4 A142V polymorphism did not contribute to increased blood pressure but the genetic variation in the DRD1 promoter associated with impaired renal sodium handling and blood pressure; no other GRK4 genotypes were studied (Staessen et al., 2008). Another study based on a large, community-based sample in White Americans also showed no association between GRK4 A142V or GRK4 A486V and hypertension (Rana et al., 2007). A study in African Americans aged 18–49 years even showed a negative association between GRK4 variants and hypertension, in which the GRK4 A486V variant was negatively associated with hypertension in the non-obese group (Martinez Cantarin et al., 2010). The reasons for these diverse outcomes are still unknown. Some factors, including ethnicity, age, salt sensitivity, failure to study all the GRK4 variants that are associated with hypertension, and gene-gene interaction should be taken into account.
The role of gene-gene interaction in phenotypes has attracted increasing attention. For example, Pereira et al found that there is an epistatic interaction between GRK4 variants R65L and A142V, and angiotensinogen that affects blood pressure and cardiovascular risk (Pereira Da Silva et al., 2020). In an African population from Ghana, the combination, that is most predictive of hypertension, is GRK4 R65L and angiotensin-converting enzyme (ACE), with an estimated prediction success of 70.5% (Williams et al., 2004). Moreover, the multi-gene interaction is also involved in the salt sensitivity of blood pressure. Sanada et al reported that among Japanese participants, GRK4 R65L, A142V, or A486V impaired a dopaminergic agonist-induced natriuretic effect, and a genetic model of the three GRK4 variants predicted the presence of salt-sensitive hypertension in 94.4% of cases. By contrast, the single-locus model with only GRK4 A142V was 78.4% predictive and a 2-locus model of GRK4 A142V and CYP11B2 C-344 T was 77.8% predictive of low-renin hypertension (Sanada et al., 2006a, b). A study in Korean children aged between 8 and 9 years also showed that the risk of obesity, a well-known pathogenic factor in hypertension, increased with GRK4 A486V, ACE, and SLC12A3 variants in boys, whereas it increased with GRK4 A486V and CYP11B2 variants in girls, as sodium intake increased (Lee et al., 2015).
6.2. Potential role of GRK4 in pharmacogenomics of hypertension
Recently, precision medicine, defined as personalized medicine enhanced by different technologies such as genome-wide association studies, has attracted great attention in the treatment of hypertension (Padmanabhan & Dominiczak, 2021). The presence or absence of GRK4 gene variants has been shown to be valuable in guiding therapeutic antihypertensive strategies. Common variants of GRK4 can predict the blood pressure response to antihypertensive medicines, such as angiotensin receptor blockers (ARBs), β-adrenergic blockers, low salt diet, and diuretics. In addition, studies have also shown the association between GRK4 variants and the blood pressure response to lifestyle modification, such as reduction of dietary salt intake. The role of GRK4 variants in hypertension and response to antihypertensive treatment in humans is summarized in Table 3.
7. Conclusions
In summary, genetic studies have shown a correlation between hypertension and GRK4 variants. Increasing evidence shows that GRK4, via several molecular mechanisms, plays a vital role in regulating the expression and function of blood pressure-related receptors, consequently affecting renal sodium handling, arterial function, and blood pressure (Fig. 2). The downregulation of increased GRK4 activity restores the normal blood pressure-related receptor function. Moreover, pharmacogenomics studies show that GRK4 variants can predict the blood pressure response to antihypertensive medicines. Thus, further studies targeting GRK4 or identifying additional GRK4 variants may provide new therapeutic antihypertensive strategies in the future.
Acknowledgments
This work was supported in part by grants from the National Natural Science Foundation of China (82070442, 31730043), National Key R&D Program of China (2018YFC1312700), Program of Innovative Research Team by National Natural Science Foundation (81721001), and the National Institutes of Health (5R01DK039308, 5R01DK119652, and 5P01HL074940).
Abbreviations:
- Ang II
angiotensin II
- AT1R
angiotensin II receptor type 1
- AT2R
angiotensin II receptor type 2
- AdipoR1
adiponectin receptor 1
- AR
adrenergic receptor
- D1R
dopamine D1 receptor
- D3R
dopamine D3 receptor
- ETBR
endothelin receptor type B
- GPCRs
G protein-coupled receptors
- GRK4
G protein-coupled receptor kinase 4
- hGRK4γ
human GRK4γ
- HDAC1
Histone deacetylase type 1
- L-NAME
L-NG-Nitro arginine methyl ester
- ROS
reactive oxygen species
- SD
Sprague-Dawley
- SHRs
spontaneously hypertensive rats
- SNPs
single nucleotide polymorphisms
- WKY
Wistar Kyoto
- WT
wild-type
- VSMCs
vascular smooth muscle cells
Footnotes
Declaration of Competing Interest
Dr. Jose, who is the Scientific Director of Hypogen, Inc., owns US Patent Number 6660474B1 for GRK4. The other authors report no conflicts.
References
- Abraham AD, Schattauer SS, Reichard KL, Cohen JH, Fontaine HM, Song AJ, et al. (2018). Estrogen regulation of GRK2 inactivates kappa opioid receptor signaling mediating analgesia, but not aversion. The Journal of Neuroscience 38(37), 8031–8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed MR, Berthet A, Bychkov E, Porras G, Li Q, Bioulac BH, et al. (2010). Lentiviral overexpression of GRK6 alleviates L-dopa-induced dyskinesia in experimental Parkinson’s disease. Science Translational Medicine 2(28), 28ra28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht FE, Drago J, Felder RA, Printz MP, Eisner GM, Robillard JE, et al. (1996). Role of the D1A dopamine receptor in the pathogenesis of genetic hypertension. Journal of Clinical Investigation 97(10), 2283–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allayee H, de Bruin TW, Michelle D, Cheng LS, Ipp E, Cantor RM, et al. (2001). Genome scan for blood pressure in Dutch dyslipidemic families reveals linkage to a locus on chromosome 4p. Hypertension 38(4), 773–778. [DOI] [PubMed] [Google Scholar]
- Allen SJ, Parthasarathy G, Darke PL, Diehl RE, Ford RE, Hall DL, et al. (2015). Structure and function of the hypertension variant A486V of G protein-coupled receptor kinase 4. Journal of Biological Chemistry 290(33), 20360–20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansonoff MA, & Etgen AM (2001). Estrogen increases G protein coupled receptor kinase 2 in the cortex of female rats. Brain Research 898(1), 186–189. [DOI] [PubMed] [Google Scholar]
- Avendaño MS, Lucas E, Jurado-Pueyo M, Martínez-Revelles S, Vila-Bedmar R, Mayor F Jr., et al. (2014). Increased nitric oxide bioavailability in adult GRK2 hemizygous mice protects against angiotensin II-induced hypertension. Hypertension 63(2), 369–375. [DOI] [PubMed] [Google Scholar]
- Baameur F, Morgan DH, Yao H, Tran TM, Hammitt RA, Sabui S, et al. (2010). Role for the regulator of G-protein signaling homology domain of G protein-coupled receptor kinases 5 and 6 in beta 2-adrenergic receptor and rhodopsin phosphorylation. Molecular Pharmacology 77(3), 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengra C, Mifflin TE, Khripin Y, Manunta P, Williams SM, Jose PA, et al. (2002). Genotyping of essential hypertension single-nucleotide polymorphisms by a homogeneous PCR method with universal energy transfer primers. Clinical Chemistry 48(12), 2131–2140. [PubMed] [Google Scholar]
- Benovic JL (2021). Historical perspective of the G protein-coupled receptor kinase family. Cells 10(3), 555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar V, O’Connor DT, Brophy VH, Schork NJ, Richard E, Salem RM, et al. (2009). G-protein-coupled receptor kinase 4 polymorphisms and blood pressure response to metoprolol among African Americans: Sex-specificity and interactions. American Journal of Hypertension 22(3), 332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao N, Tang H, Tian M, Gong X, Xu Z, Zhou B, et al. (2021). Genetic variants of GRK4 influence circadian rhythm of blood pressure and response to candesartan in hypertensive patients. Clinical and Experimental Hypertension 43(7), 597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey RM, Schoeffel CD, Gildea JJ, Jones JE, McGrath HE, Gordon LN, et al. (2012). Salt sensitivity of blood pressure is associated with polymorphisms in the sodium-bicarbonate cotransporter. Hypertension 60(5), 1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casari G, Barlassina C, Cusi D, Zagato L, Muirhead R, Righetti M, et al. (1995). Association of the alpha-adducin locus with essential hypertension. Hypertension 25(3), 320–326. [DOI] [PubMed] [Google Scholar]
- Chaudhary PK, & Kim S (2021). The GRKs reactome: Role in cell biology and pathology. International Journal of Molecular Sciences 22(7), 3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Fu C, Chen C, Liu L, Ren H, Han Y, et al. (2014). Role of GRK4 in the regulation of arterial AT1 receptor in hypertension. Hypertension 63(2), 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Plasencia M, Li Z, Mukherjee S, Patra D, Chen CL, et al. (2021). Structures of rhodopsin in complex with G-protein-coupled receptor kinase 1. Nature 595(7868), 600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CF, Lin YJ, Lin MC, Liang WM, Chen CC, & Chen CH (2021). Genetic risk score constructed from common genetic variants is associated with cardiovascular disease risk in type 2 diabetes mellitus. The Journal of Gene Medicine 23(2), Article e3305. [DOI] [PubMed] [Google Scholar]
- Chugh G, Lokhandwala MF, & Asghar M (2011). Oxidative stress alters renal D1 and AT1 receptor functions and increases blood pressure in old rats. American Journal of Physiology. Renal Physiology 300(1), F133–F138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugh G, Lokhandwala MF, & Asghar M (2012). Altered functioning of both renal dopamine D1 and angiotensin II type 1 receptors causes hypertension in old rats. Hypertension 59(5), 1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarelli M, Sorriento D, Franco A, Fusco A, Del Giudice C, Annunziata R, et al. (2013). Endothelial G protein-coupled receptor kinase 2 regulates vascular homeostasis through the control of free radical oxygen species. Arteriosclerosis, Thrombosis, and Vascular Biology 33(10), 2415–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn HI, Harris DM, Pesant S, Pfeiffer M, Zhou RH, Koch WJ, et al. (2008). Inhibition of vascular smooth muscle G protein-coupled receptor kinase 2 enhances alpha1D-adrenergic receptor constriction. American Journal of Physiology. Heart and Circulatory Physiology 295(4), H1695–H1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn HI, Xi Y, Pesant S, Harris DM, Hyslop T, Falkner B, et al. (2009). G protein-coupled receptor kinase 2 expression and activity are associated with blood pressure in black Americans. Hypertension 54(1), 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colafella KMM, & Denton KM (2018). Sex-specific differences in hypertension and associated cardiovascular disease. Nature Reviews Nephrology 14(3), 185–201. [DOI] [PubMed] [Google Scholar]
- De Lucia C, Grisanti LA, Borghetti G, Piedepalumbo M, Ibetti J, Maria LA, et al. (2021). GRK5 contributes to impaired cardiac function and immune cell recruitment in post-ischemic heart failure. Cardiovascular Research. 10.1093/cvr/cvab044 cvab044. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaun N, & Webb DJ (2019). Endothelins in cardiovascular biology and therapeutics. Nature Reviews Cardiology 16(8), 491–502. [DOI] [PubMed] [Google Scholar]
- Diao Z, Asico LD, Villar VAM, Zheng X, Cuevas S, Armando I, et al. (2017). Increased renal oxidative stress in salt-sensitive human GRK4γ486V transgenic mice. Free Radical Biology and Medicine 106, 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieleman JL, Cao J, Chapin A, Chen C, Li Z, Liu A, et al. (2020). US health care spending by payer and health condition, 1996-2016. JAMA 323(9), 863–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du B, Jia X, Tian W, Yan X, Wang N, Cai D, et al. (2021). Associations of SUCNR1, GRK4, CAMK1D gene polymorphisms and the susceptibility of type 2 diabetes mellitus and essential hypertension in a northern Chinese Han population. Journal of Diabetes and its Complications 35(1), Article 107752. [DOI] [PubMed] [Google Scholar]
- Eckhart AD, Ozaki T, Tevaearai H, Rockman HA, & Koch WJ (2002). Vascular-targeted overexpression of G protein-coupled receptor kinase-2 in transgenic mice attenuates beta-adrenergic receptor signaling and increases resting blood pressure. Molecular Pharmacology 61(4), 749–758. [DOI] [PubMed] [Google Scholar]
- Elijovich F, Weinberger MH, Anderson CA, Appel LJ, Bursztyn M, Cook NR, et al. (2016). Salt sensitivity of blood pressure: A scientific statement from the American Heart Association. Hypertension 68(3), e7–e46. [DOI] [PubMed] [Google Scholar]
- Eroglu E, Kocyigit I, & Lindholm B (2020). The endothelin system as target for therapeutic interventions in cardiovascular and renal disease. Clinica Chimica Acta 506, 92–106. [DOI] [PubMed] [Google Scholar]
- Escano CS, Armando I, Wang X, Asico LD, Pascua A, Yang Y, et al. (2009). Renal dopaminergic defect in C57Bl/6J mice. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 297(6), R1660–R1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatima N, Patel SN, & Hussain T (2021). Angiotensin II type 2 receptor: A target for protection against hypertension, metabolic dysfunction, and organ remodeling. Hypertension 77(6), 1845–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder RA, Sanada H, Xu J, Yu PY, Wang Z, Watanabe H, et al. (2002). G protein-coupled receptor kinase 4 gene variants in human essential hypertension. Proceedings of the National Academy of Sciences of the United States of America 99(6), 3872–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher NDL, & Curfman G (2018). Hypertension-a public health challenge of global proportions. JAMA 320(17), 1757–1759. [DOI] [PubMed] [Google Scholar]
- Fraga S, Jose PA, & Soares-da-Silva P (2004). Involvement of G protein-coupled receptor kinase 4 and 6 in rapid desensitization of dopamine D1 receptor in rat IEC-6 intestinal epithelial cells. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 287(4), R772–R779. [DOI] [PubMed] [Google Scholar]
- Frey MK, Dao F, Olvera N, Konner JA, Dickler MN, & Levine DA (2017). Genetic predisposition to bevacizumab-induced hypertension. Gynecologic Oncology 147(3), 621–625. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Bohn LM, Sotnikova TD, Cyr M, Laakso A, Macrae AD, et al. (2003). Dopaminergic supersensitivity in G protein-coupled receptor kinase 6-deficient mice. Neuron 38(2), 291–303. [DOI] [PubMed] [Google Scholar]
- Gildea JJ, Shah I, Weiss R, Casscells ND, McGrath HE, Zhang J, et al. (2010). HK-2 human renal proximal tubule cells as a model for G protein-coupled receptor kinase type 4-mediated dopamine 1 receptor uncoupling. Hypertension 56(3), 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gildea JJ, Tran HT, Van Sciver RE, Bigler W, Carlson JM, & Felder RA (2013). A novel role for c-Myc in G protein-coupled receptor kinase 4 (GRK4) transcriptional regulation in human kidney proximal tubule cells. Hypertension 61(5), 1021–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, et al. (2019). Cellular senescence: Defining a path forward. Cell 179(4), 813–827. [DOI] [PubMed] [Google Scholar]
- Gros R, Chorazyczewski J, Meek MD, Benovic JL, Ferguson SS, & Feldman RD (2000). G-protein-coupled receptor kinase activity in hypertension: Increased vascular and lymphocyte G-protein receptor kinase-2 protein expression. Hypertension 35 (1 Pt 1), 38–42. [DOI] [PubMed] [Google Scholar]
- Gu D, Su S, Ge D, Chen S, Huang J, Li B, et al. (2006). Association study with 33 single-nucleotide polymorphisms in 11 candidate genes for hypertension in Chinese. Hypertension 47(6), 1147–1154. [DOI] [PubMed] [Google Scholar]
- Guo H, Du X, Zhang Y, Wu J, Wang C, Li M, et al. (2019). Specific miRNA-G protein-coupled receptor networks regulate Sox9a/Sox9b activities to promote gonadal rejuvenation in zebrafish. Stem Cells 37(9), 1189–1199. [DOI] [PubMed] [Google Scholar]
- Gurevich EV, & Gurevich VV (2020). GRKs as modulators of neurotransmitter receptors. Cells 10(1), 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JE, Mouton AJ, da Silva AA, Omoto ACM, Wang Z, Li X, et al. (2021). Obesity, kidney dysfunction and inflammation: Interactions in hypertension. Cardiovascular Research 117(8), 1859–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RC (2012). Abnormalities in renal dopamine signaling and hypertension: The role of GRK4. Current Opinion in Nephrology and Hypertension 21(1), 61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DG, Coffman TM, & Wilcox CS (2021). Pathophysiology of hypertension: The mosaic theory and beyond. Circulation Research 128(7), 847–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkamp S, Telgmann R, Staessen JA, Hagedorn C, Dördelmann C, Bek M, et al. (2008). Characterization and functional analyses of the human G protein-coupled receptor kinase 4 gene promoter. Hypertension 52(4), 737–746. [DOI] [PubMed] [Google Scholar]
- Homan KT, Glukhova A, & Tesmer JJ (2013). Regulation of G protein-coupled receptor kinases by phospholipids. Current Medicinal Chemistry 20(1), 39–46. [PubMed] [Google Scholar]
- Huang H, Li X, Zheng S, Chen Y, Chen C, Wang J, et al. (2016). Downregulation of renal G protein-coupled receptor kinase type 4 expression via ultrasound-targeted microbubble destruction lowers blood pressure in spontaneously hypertensive rats. Journal of the American Heart Association 5(10), Article e004028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hullmann JE, Grisanti LA, Makarewich CA, Gao E, Gold JI, Chuprun JK, et al. (2014). GRK5-mediated exacerbation of pathological cardiac hypertrophy involves facilitation of nuclear NFAT activity. Circulation Research 115(12), 976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hullmann J, Traynham CJ, Coleman RC, &Koch WJ (2016). The expanding GRK interactome: Implications in cardiovascular disease and potential for therapeutic development. Pharmacological Research 110, 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo R, Cipolletta E, Ciccarelli M, Campanile A, Santulli G, Palumbo G, et al. (2008). Enhanced GRK2 expression and desensitization of beta AR vasodilatation in hypertensive patients. Clinical and Translational Science 1(3), 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S, Kim JY, Cho Y, Koh SB, Kim N, & Choi JR (2020). Genetically, dietary sodium intake is causally associated with salt-sensitive hypertension risk in a community-based cohort study: A mendelian randomization approach. Current Hypertension Reports 22(7), 45. [DOI] [PubMed] [Google Scholar]
- Jiang W, Wang X, Li R, Wang P, Shan G, Jia X, & Gu M (2021). Targeted capture sequencing identifies genetic variations of GRK4 and RDH8 in Han Chinese with essential hypertension in Xinjiang. PLoS One 16(7), Article e0255311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ES, Spence JD, Mcintyre AD, Nondi J, Gogo K, Akintunde A, et al. (2017). High frequency of variants of candidate genes in black Africans with low renin-resistant hypertension. American Journal of Hypertension 30(5), 478–483. [DOI] [PubMed] [Google Scholar]
- Jose PA, Eisner GM, Drago J, Carey RM, & Felder RA (1996). Dopamine receptor signaling defects in spontaneous hypertension. American Journal of Hypertension 9(4 Pt 1), 400–405. [DOI] [PubMed] [Google Scholar]
- Jose PA, Soares-da-Silva P, Eisner GM, & Felder RA (2010). Dopamine and G protein-coupled receptor kinase 4 in the kidney: Role in blood pressure regulation. Biochimica et Biophysica Acta 1802(12), 1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keever LB, Jones JE, & Andresen BT (2008). G protein-coupled receptor kinase 4gamma interacts with inactive Galpha(s) and Galpha13. Biochemical and Biophysical Research Communications 367(3), 649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp BA, Howell NL, Gildea JJ, Keller SR, & Carey RM (2020). Identification of a primary renal AT2 receptor defect in spontaneously hypertensive rats. Circulation Research 126(5), 644–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keys JR, Zhou RH, Harris DM, Druckman CA, & Eckhart AD (2005). Vascular smooth muscle overexpression of G protein-coupled receptor kinase 5 elevates blood pressure, which segregates with sex and is dependent on Gi-mediated signaling. Circulation 112(8), 1145–1153. [DOI] [PubMed] [Google Scholar]
- Kimura L, Angeli CB, Auricchio MT, Fernandes GR, Pereira AC, Vicente JP, et al. (2012). Multilocus family-based association analysis of seven candidate polymorphisms with essential hypertension in an African-derived semi-isolated Brazilian population. International Journal of Hypertension 2012, Article 859219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostova D, Spencer G, Moran AE, Cobb LK, Husain MJ, Datta BK, et al. (2020). The cost-effectiveness of hypertension management in low-income and middle-income countries: A review. BMJ Global Health 5(9), Article e002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Kim MK, Kim SM, Park H, Park CG, & Park HK (2015). Gender-based differences on the association between salt-sensitive genes and obesity in Korean children aged between 8 and 9 years. PLoS One 10(3), Article e0120111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Homan KT, Vishnivetskiy SA, Manglik A, Tesmer JJ, Gurevich VV, et al. (2015a). G protein-coupled receptor kinases of the GRK4 protein subfamily phosphorylate inactive G protein-coupled receptors (GPCRs). Journal of Biological Chemistry 290(17), 10775–10790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Yang J, Jones JE, Villar VA, Yu P, Armando I, et al. (2015b). Sorting nexin 5 and dopamine D1 receptor regulate the expression of the insulin receptor in human renal proximal tubule cells. Endocrinology 156(6), 2211–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Yang J, Villar VAM, Asico LD, Ma X, Armando I, et al. (2018). Loss of renal SNX5 results in impaired IDE activity and insulin resistance in mice. Diabetologia 61(3), 727–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Fu W, Gong X, Chen Z, Tang L, Yang D, et al. (2021). The role of G protein-coupled receptor kinase 4 in cardiomyocyte injury after myocardial infarction. European Heart Journal 42(14), 1415–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liggett SB, Cresci S, Kelly RJ, Syed FM, Matkovich SJ, Hahn HS, et al. (2008). A GRK5 polymorphism that inhibits beta-adrenergic receptor signaling is protective in heart failure. Nature Medicine 14(5), 510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, & Xi B (2012). Pooled analyses of the associations of polymorphisms in the GRK4 and EMILIN1 genes with hypertension risk. International Journal of Medical Sciences 9(4), 274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Li X, Fu J, Chen K, Liao Q, Wang J, et al. (2021). Increased AT1 receptor expression mediates vasoconstriction leading to hypertension in Snx1−/− mice. Hypertension Research 44(8), 906–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobmeyer MT, Wang L, Zineh I, Turner ST, Gums JG, Chapman AB, et al. (2011). Polymorphisms in genes coding for GRK2 and GRK5 and response differences in antihypertensive-treated patients. Pharmacogenetics and Genomics 21(1), 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Ye Z, Zheng S, Ren H, Zeng J, Wang X, et al. (2018). Long-term exposure of fine particulate matter causes hypertension by impaired renal D1 receptor-mediated sodium excretion via upregulation of G-protein-coupled receptor kinase type 4 expression in Sprague-Dawley rats. Journal of the American Heart Association 7(1), Article e007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas E, Jurado-Pueyo M, Fortuño MA, Fernández-Veledo S, Vila-Bedmar R, Jiménez-Borreguero LJ, et al. (2014). Downregulation of G protein-coupled receptor kinase 2 levels enhances cardiac insulin sensitivity and switches on cardioprotective gene expression patterns. Biochimica et Biophysica Acta 1842, 2448–2456 12 Pt A. [DOI] [PubMed] [Google Scholar]
- Luo H, Chen C, Guo L, Xu Z, Peng X, Wang X, et al. (2018). Exposure to maternal diabetes mellitus causes renal dopamine D1 receptor dysfunction and hypertension in adult rat offspring. Hypertension 72(4), 962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Huang X, Yang J, Huang L, Li R, Wu Q, et al. (2019). Proteomics analysis of G protein-coupled receptor kinase 4-inhibited cellular growth of HEK293 cells. Journal of Proteomics 207, Article 103445. [DOI] [PubMed] [Google Scholar]
- Martinez Cantarin MP, Ertel A, Deloach S, Fortina P, Scott K, Burns TL, et al. (2010). Variants in genes involved in functional pathways associated with hypertension in African Americans. Clinical and Translational Science 3(6), 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurin T, Melko M, Abekhoukh S, Khalfallah O, Davidovic L, Jarjat M, et al. (2015). The FMRP/GRK4 mRNA interaction uncovers a new mode of binding of the fragile X mental retardation protein in cerebellum. Nucleic Acids Research 43(17), 8540–8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercuro G, Dieda M, Piras A, Dessalvi CC, Maffei S, & Roasano GMC (2010). Gender determinants of cardiovascular risk factors and diseases. Journal of Cardiovascular Medicine (Hagerstown). 11(3), 207–220. [DOI] [PubMed] [Google Scholar]
- Miyoshi T, Otsuka F, & Shimasaki S (2013). GRK-6 mediates FSH action synergistically enhanced by estrogen and the oocyte in rat granulosa cells. Biochemical and Biophysical Research Communications 434(2), 401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montasser ME, Shimmin LC, Gu D, Chen J, Gu C, Kelly TN, et al. (2014). Variation in genes that regulate blood pressure are associated with glomerular filtration rate in Chinese. PLoS One 9(3), Article e92468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montó F, Oliver E, Vicente D, Buendía F, Rueda J, Agüero J, et al. (2015). β2- and β1-adrenoceptor expression exhibits a common regulatory pattern with GRK2 and GRK5 in human and animal models of cardiovascular diseases. Journal of Cardiovascular Pharmacology 66(5), 478–486. [DOI] [PubMed] [Google Scholar]
- Murga C, Arcones AC, Cruces-Sande M, Brione SAM, Salaices M, & Mayor F Jr. (2019). G protein-coupled receptor kinase 2 (GRK2) as a potential therapeutic target in cardiovascular and metabolic diseases. Frontiers in Pharmacology 10, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muskalla AM, Suter PM, Saur M, Nowak A, Hersberger M, & Krayenbuehl PA (2014). G-protein receptor kinase 4 polymorphism and response to antihypertensive therapy. Clinical Chemistry 60(12), 1543–1548. [DOI] [PubMed] [Google Scholar]
- Napolitano R, Campanile A, Sarno L, Anastasio A, Maruotti GM, Morlando M, et al. (2012). GRK2 levels in umbilical arteries of pregnancies complicated by gestational hypertension and preeclampsia. American Journal of Hypertension 25(3), 366–371. [DOI] [PubMed] [Google Scholar]
- Neve KA (2006). Novel features of G protein-coupled receptor kinase 4. Molecular Pharmacology 69(3), 673–676. [DOI] [PubMed] [Google Scholar]
- Nizar JM, Shepard BD, Vo VT, & Bhalla V (2018). Renal tubule insulin receptor modestly promotes elevated blood pressure and markedly stimulates glucose reabsorption. JCI Insight 3(16), Article e95107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Hernández A, Figuero-Pérez L, Cruz-Hernandez JJ, González Sarmiento R, Usategui-Martin R, & Miramontes-González JP (2021). Dopamine receptors and the kidney: An overview of health- and pharmacological-targeted implications. Biomolecules 11(2), 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver E, Rovira E, Montó F, Valldecabres C, Julve R, Muedra V, et al. (2010). beta-Adrenoceptor and GRK3 expression in human lymphocytes is related to blood pressure and urinary albumin excretion. Journal of Hypertension 28(6), 1281–1289. [DOI] [PubMed] [Google Scholar]
- Oliver E, Flacco N, Arce C, Ivorra MD, D’Ocon MP, & Noguera MA (2014). Changes in adrenoceptors and G-protein-coupled receptor kinase 2 in L-NAME-induced hypertension compared to spontaneous hypertension in rats. Journal of Vascular Research 51(3), 209–220. [DOI] [PubMed] [Google Scholar]
- Padmanabhan S, & Dominiczak AF (2021). Genomics of hypertension: The road to precision medicine. Nature Reviews Cardiology 18(4), 235–250. [DOI] [PubMed] [Google Scholar]
- Paz O, Riquelme JA, García L, Jalil JE, Chiong M, Santos RAS, et al. (2020). Counter-regulatory renin-angiotensin system in cardiovascular disease. Nature Reviews Cardiology 17(2), 116–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira Da Silva A, Costa MDC, Aguiar L, Matos A, Gil Â, Gorjão-Clara J, et al. (2020). Impact on longevity of genetic cardiovascular risk and lifestyle including red meat consumption. Oxidative Medicine and Cellular Longevity 2020, 1305413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perri A, Vizza D, Lofaro D, Gigliotti P, Leone F, Brunelli E, Malivindi R, De Amicis F, Romeo F, De Stefano R, et al. (2013). Adiponectin is expressed and secreted by renal tubular epithelial cells. Journal of Nephrology 26(6), 1049–1054. [DOI] [PubMed] [Google Scholar]
- Pfleger J, Gresham K, & Koch WJ (2019). G protein-coupled receptor kinases as therapeutic targets in the heart. Nature Reviews. Cardiology 16(10), 612–622. [DOI] [PubMed] [Google Scholar]
- Polhemus DJ, Gao J, Scarborough AL, Trivedi R, McDonough KH, Goodchild TT, et al. (2016). Radiofrequency renal denervation protects the ischemic heart via inhibition of GRK2 and increased nitric oxide signaling. Circulation Research 119(3), 470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell-Wiley TM, Poirier P, Burke LE, Després JP, Gordon-Larsen P, Lavie CJ, et al. (2021). Obesity and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 143(21), e984–e1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premont RT, Macrae AD, Stoffel RH, Chung N, Pitcher JA, Ambrose C, et al. (1996). Characterization of the G protein-coupled receptor kinase GRK4. Identification of four splice variants. Journal of Biological Chemistry 271(11), 6403–6410. [DOI] [PubMed] [Google Scholar]
- Prieto MC, Gonzalez AA, Visniauskas B, & Navar LG (2021). The evolving complexity of the collecting duct renin-angiotensin system in hypertension. Nature Reviews Nephrology 17(7), 481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronin AN, Satpaev DK, Slepak VZ, & Benovic JL (1997). Regulation of G protein-coupled receptor kinases by calmodulin and localization of the calmodulin binding domain. Journal of Biological Chemistry 272(29), 18273–18280. [DOI] [PubMed] [Google Scholar]
- Rainbow RD, Brennan S, Jackson R, Beech AJ, Bengreed A, Waldschmidt HV, et al. (2018). Small-molecule G protein-coupled receptor kinase inhibitors attenuate G protein-coupled receptor kinase 2-mediated desensitization of vasoconstrictor-induced arterial contractions. Molecular Pharmacology 94(3), 1079–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana BK, Insel PA, Payne SH, Abel K, Beutler E, Ziegler MG, et al. (2007). Population-based sample reveals gene-gender interactions in blood pressure in White Americans. Hypertension 49(1), 96–106. [DOI] [PubMed] [Google Scholar]
- Rayner B, Ramesar R, Steyn K, Levitt N, Lombard C, & Charlton K (2012). G-protein-coupled receptor kinase 4 polymorphisms predict blood pressure response to dietary modification in black patients with mild-to-moderate hypertension. Journal of Human Hypertension 26(5), 334–339. [DOI] [PubMed] [Google Scholar]
- Rianto F, Hoang T, Revoori R, & Sparks MA (2021). Angiotensin receptors in the kidney and vasculature in hypertension and kidney disease. Molecular and Cellular Endocrinology 529, Article 111259. [DOI] [PubMed] [Google Scholar]
- Ribas C, Penela P, Murga C, Salcedo A, García-Hoz C, Jurado-Pueyo M, et al. (2007). (2007) the G protein-coupled receptor kinase (GRK) interactome: Role of GRKs in GPCR regulation and signaling. Biochimica et Biophysica Acta 1768(4), 913–922. [DOI] [PubMed] [Google Scholar]
- Sallese M, Mariggiò S, Collodel G, Moretti E, Piomboni P, Baccetti B, et al. (1997). G protein-coupled receptor kinase GRK4. Molecular analysis of the four isoforms and ultrastructural localization in spermatozoa and germinal cells. Journal of Biological Chemistry 272(15), 10188–10195. [DOI] [PubMed] [Google Scholar]
- Sallese M, Iacovelli L, Cumashi A, Capobianco L, Cuomo L, De B, & A. (2000). Regulation of G protein-coupled receptor kinase subtypes by calcium sensor proteins. Biochimica et Biophysica Acta 1498(2–3), 112–121. [DOI] [PubMed] [Google Scholar]
- Sanada H, Yatabe J, Midorikawa S, Hashimoto S, Watanabe T, Moore JH, et al. (2006a). Single-nucleotide polymorphisms for diagnosis of salt-sensitive hypertension. Clinical Chemistry 52(3), 352–360. [DOI] [PubMed] [Google Scholar]
- Sanada H, Yatabe J, Midorikawa S, Katoh T, Hashimoto S, Watanabe T, et al. (2006b). Amelioration of genetic hypertension by suppression of renal G protein-coupled receptor kinase type 4 expression. Hypertension 47(6), 1131–1139. [DOI] [PubMed] [Google Scholar]
- Sanada H, Yoneda M, Yatabe J, Williams SM, Bartlett J, White MJ, et al. (2016). Common variants of the G protein-coupled receptor type 4 are associated with human essential hypertension and predict the blood pressure response to angiotensin receptor blockade. Pharmacogenomics Journal 16(1), 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Otte P, Leysen H, van Gastel J, Hendrickx JO, Martin B, & Maudsley S (2019). G protein-coupled receptor systems and their role in cellular senescence. Computational and Structural Biotechnology Journal 17, 1265–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen YY, Hughes JT, Charlesworth JA, Kelly JJ, & Peake PW (2008). Adiponectin is present in the urine in its native conformation, and specifically reduces the secretion of MCP-1 by proximal tubular cells. Nephrology (Carlton, Vic.) 13(5), 405–410. [DOI] [PubMed] [Google Scholar]
- Somani YB, Pawelczyk JA, De Souza MJ, Kris-Etherton PM, & Proctor DN (2019). Aging women and their endothelium: Probing the relative role of estrogen on vasodilator function. American Journal of Physiology. Heart and Circulatory Physiology 317(2), H395–H404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorriento D, Santulli G, Fusco A, Anastasio A, Trimarco B, & Iaccarino G (2010). Intracardiac injection of AdGRK5-NT reduces left ventricular hypertrophy by inhibiting NF-kappa B-dependent hypertrophic gene expression. Hypertension 56(4), 696–704. [DOI] [PubMed] [Google Scholar]
- Sorriento D, Santulli G, Ciccarelli M, Maione AS, Illario M, Trimarco B, et al. (2018). The amino-terminal domain of GRK5 inhibits cardiac hypertrophy through the regulation of calcium-calmodulin dependent transcription factors. International Journal of Molecular Sciences 19(3), 861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speirs HJ, Katyk K, Kumar NN, Benjafield AV, Wang WY, & Morris BJ (2004). Association of G-protein-coupled receptor kinase 4 haplotypes, but not HSD3B1 or PTP1B polymorphisms, with essential hypertension. Journal of Hypertension 22(5), 931–936. [DOI] [PubMed] [Google Scholar]
- Staessen JA, Kuznetsova T, Zhang H, Maillard M, Bochud M, Hasenkamp S, et al. (2008). Blood pressure and renal sodium handling in relation to genetic variation in the DRD1 promoter and GRK4. Hypertension 51(6), 1643–1650. [DOI] [PubMed] [Google Scholar]
- Sun D, Chen K, Wang J, Zhou L, & Zeng C (2020). In-utero cold stress causes elevation of blood pressure via impaired vascular dopamine D1 receptor in offspring. Clinical and Experimental Hypertension 42(2), 99–104. [DOI] [PubMed] [Google Scholar]
- Sun X, Zhou M, Wen G, Huang Y, Wu J, Peng L, et al. (2021). Paroxetine attenuates cardiac hypertrophy via blocking GRK2 and ADRB1 interaction in hypertension. Journal of the American Heart Association 10(1), Article e016364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe H, Fujii Y, Okada-Iwabu M, Iwabu M, Nakamura Y, Hosaka T, et al. (2015). Crystal structures of the human adiponectin receptors. Nature 520(7547), 312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutunea-Fatan E, Caetano FA, Gros R, & Ferguson SS (2015). GRK2 targeted knockdown results in spontaneous hypertension, and altered vascular GPCR signaling. Journal of Biological Chemistry 290(8), 5141–5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutunea-Fatan E, Abd-Elrahman KS, Thibodeau JF, Holterman CE, Holleran BJ, Leduc R, et al. (2018). GRK2 knockdown in mice exacerbates kidney injury and alters renal mechanisms of blood pressure regulation. Scientific Reports 8(1), 11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandell AG, Lobmeyer MT, Gawronski BE, Langaee TY, Gong Y, Gums JG, et al. (2012). G protein receptor kinase 4 polymorphisms: β-blocker pharmacogenetics and treatment-related outcomes in hypertension. Hypertension 60(4), 957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar VA, Jones JE, Armando I, Palmes-Saloma C, Yu P, Pascua AM, et al. (2009). G protein-coupled receptor kinase 4 (GRK4) regulates the phosphorylation and function of the dopamine D3 receptor. Journal of Biological Chemistry 284(32), 21425–21434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar VA, Armando I, Sanada H, Frazer LC, Russo CM, Notario PM, et al. (2013). Novel role of sorting nexin 5 in renal D1 dopamine receptor trafficking and function: Implications for hypertension. FASEB Journal 27(5), 1808–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinge LE, von Lueder TG, Aasum E, Qvigstad E, Gravning JA, How OJ, et al. (2008). Cardiac-restricted expression of the carboxyl-terminal fragment of GRK3 uncovers distinct functions of GRK3 in regulation of cardiac contractility and growth: GRK3 controls cardiac alpha1-adrenergic receptor responsiveness. Journal of Biological Chemistry 283(16), 10601–10610. [DOI] [PubMed] [Google Scholar]
- Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. (2021). Heart disease and stroke statistics-2021 update: A report from the American Heart Association. Circulation 143(8), e254–e743. [DOI] [PubMed] [Google Scholar]
- Virlon B, Firsov D, Cheval L, Reiter E, Troispoux C, Guillou F, et al. (1998). Rat G protein-coupled receptor kinase GRK4: Identification, functional expression, and differential tissue distribution of two splice variants. Endocrinology 139(6), 2784–2795. [DOI] [PubMed] [Google Scholar]
- Voigt C, Holzapfel HP, Meyer S, & Paschke R (2004). Increased expression of G-protein-coupled receptor kinases 3 and 4 in hyperfunctioning thyroid nodules. Journal of Endocrinology 182(1), 173–182. [DOI] [PubMed] [Google Scholar]
- Wagner F, Malice MP, Wiegert E, McGrath HE, Gildea J, Mitta S, et al. (2012). A comparison of the natriuretic and kaliuretic effects of cicletanine and hydrochlorothiazide in prehypertensive and hypertensive humans. Journal of Hypertension 30(4), 819–827. [DOI] [PubMed] [Google Scholar]
- Wang Z, Asico LD, Escano CS, Felder RA, & Jose PA (2006a). Human G protein coupled receptor kinase type 4γ (hGRK4γ) wild-type prevents salt sensitivity while its variant, hGRK4γ 486V, promotes salt sensitivity in transgenic mice: Role of genetic background [abstract]. Hypertension 48(4), Article e27. [Google Scholar]
- Wang Y, Li B, Zhao W, Liu P, Zhao Q, Chen S, et al. (2006b). Association study of G protein-coupled receptor kinase 4 gene variants with essential hypertension in northern Han Chinese. Annals of Human Genetics 70(Pt 6), 778–783. [DOI] [PubMed] [Google Scholar]
- Wang Z, Armando I, Asico LD, Escano C, Wang X, Lu Q, et al. (2007). The elevated blood pressure of Human GRK4gamma A142V transgenic mice is not associated with increased ROS production. American Journal of Physiology. Heart and Circulatory Physiology 292(5), H2083–H2092. [DOI] [PubMed] [Google Scholar]
- Wang X, Luo H, Chen C, Chen K, Wang J, Cai Y, et al. (2014). Prenatal lipopolysaccharide exposure results in dysfunction of the renal dopamine D1 receptor in offspring. Free Radical Biology and Medicine 76, 242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zeng C, Villar VA, Chen SY, Konkalmatt P, Wang X, et al. (2016). Human GRK4-γ142V variant promotes angiotensin II type I receptor-mediated hypertension via renal histone deacetylase type 1 inhibition. Hypertension 67(2), 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Bao H, Wang KX, Zhang P, Yao QP, Chen XH, et al. (2017). Secreted miR-27a induced by cyclic stretch modulates the proliferation of endothelial cells in hypertension via GRK6. Scientific Reports 7, 41058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Wu T, Neale MC, Verweij R, Liu G, Su S, et al. (2020). Genetic and environmental influences on blood pressure and body mass index in the National Academy of Sciences-National Research Council world war II veteran twin registry. Hypertension 76(5), 1428–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Xu J, Bengra C, Jose PA, & Felder RA (2002). Desensitization of human renal D1 dopamine receptors by G protein-coupled receptor kinase 4. Kidney International 62(3), 790–798. [DOI] [PubMed] [Google Scholar]
- Williams SM, Ritchie MD, Phillips JA 3rd., Dawson E, Prince M, Dzhura E, et al. (2004). Multilocus analysis of hypertension: A hierarchical approach. Human Heredity 57(1), 28–38. [DOI] [PubMed] [Google Scholar]
- Xiao P, Huang X, Huang L, Yang J, Li A, Shen K, et al. (2017). G protein-coupled receptor kinase 4-induced cellular senescence and its senescence-associated gene expression profiling. Experimental Cell Research 360(2), 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Watanabe H, Felder RA, &Jose PA (2001). GRK6 in the kidney in human and rat genetic hypertension [abstract]. FASEB Journal 15, A774. [Google Scholar]
- Yang J, Villar VA, Jones JE, Jose PA, & Zeng C (2015). G protein-coupled receptor kinase 4: Role in hypertension. Hypertension 65(6), 1148–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Li M, Zou X, Chen C, Zheng S, Fu C, et al. (2020). Role of GRK4 in the regulation of the renal ETB receptor in hypertension. FASEB Journal 34(9), 11594–11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Villar VAM, Jose PA, & Zeng C (2021). Renal dopamine receptors and oxidative stress: Role in hypertension. Antioxidants & Redox Signaling 34(9), 716–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatabe J, Sanada H, Midorikawa S, Hashimoto S, Watanabe T, Andrews PM, et al. (2008). Effects of decreased renal cortical expression of G protein-coupled receptor kinase 4 and angiotensin type 1 receptors in rats. Hypertension Research 31(7), 1455–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Lu X, Deng Y, Wang X, Zheng S, Ren H, et al. (2018). In utero exposure to fine particulate matter causes hypertension due to impaired renal dopamine D1 receptor in offspring. Cellular Physiology and Biochemistry 46(1), 148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi XP, Zhou J, Baker J, Wang X, Gerdes AM, & Li F (2005). Myocardial expression and redistribution of GRKs in hypertensive hypertrophy and failure. Anatomical Record Part A-Discoveries in Molecular Cellular and Evolutionary Biology 282(1), 13–23. [DOI] [PubMed] [Google Scholar]
- Yu P, Asico LD, Luo Y, Andrews P, Eisner GM, Hopfer U, Felder RA, et al. (2006). D1 dopamine receptor hyperphosphorylation in renal proximal tubules in hypertension. Kidney International 70(6), 1072–1079. [DOI] [PubMed] [Google Scholar]
- Yu S, Sun L, Jiao Y, & Lee LTO (2018). The role of G protein-coupled receptor kinases in cancer. International Journal of Biological Sciences 14(2), 189–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Villar VA, Eisner GM, Williams SM, Felder RA, & Jose PA (2008). G protein-coupled receptor kinase 4: Role in blood pressure regulation. Hypertension 51(6), 1449–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Sun ZQ, Liu SS, & Yang LN (2015). Association between GRK4 and DRD1 gene polymorphisms and hypertension: A meta-analysis. Clinical Interventions in Aging 11, 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang S, Huang H, Zeng A, Han Y, Zeng C, et al. (2020). GRK4-mediated adiponectin receptor-1 phosphorylative desensitization as a novel mechanism of reduced renal sodium excretion in hypertension. Clinical Science (London) 134(18), 2453–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Vanhoutte PM, & Leung SW (2015). α1-Adrenoceptor activation of PKC-ε causes heterologous desensitization of thromboxane receptors in the aorta of spontaneously hypertensive rats. British Journal of Pharmacology 172(14), 3687–3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Kusminski CM, & Scherer PE (2021). Adiponectin, leptin and cardiovascular disorders. Circulation Research 128(1), 136–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Ji H, Maric C, Wu X, & Sandberg K (2008). Effect of dietary sodium on estrogen regulation of blood pressure in Dahl salt-sensitive rats. American Journal of Physiology. Heart and Circulatory Physiology 294(4), H1508–H1513. [DOI] [PubMed] [Google Scholar]
- Zhu H, Lu Y, Wang X, Snieder H, Treiber FA, Harshfield GA, et al. (2006). The G protein-coupled receptor kinase 4 gene modulates stress-induced sodium excretion in black normotensive adolescents. Pediatric Research 60(4), 440–442. [DOI] [PubMed] [Google Scholar]
- Ziaja M, Urbanek KA, Kowalska K, & Piastowska-Ciesielska AW (2021). Angiotensin II and angiotensin receptors 1 and 2-multifunctional system in cells biology, what do we know? Cells 10(2), 381. [DOI] [PMC free article] [PubMed] [Google Scholar]


