Abstract
Rosemary essential oil was evaluated for antifungal potentiality against six major ginseng pathogens: Sclerotinia sclerotiorum, Sclerotinia nivalis, Cylindrocarpon destructans, Alternaria panax, Botrytis cinerea, and Fusarium oxysporum. The in vitro fungicidal effects of two commonly used fungicides, namely mancozeb and fenhexamid, and the volatile organic compounds (VOCs) of Trichoderma koningiopsis T-403 on the mycelial growth were investigated. The results showed that rosemary essential oil is active against all of the pathogenic strains of ginseng root rot, whereas rosemary oil displayed high ability to inhibit the Sclerotinia spp. growth. The highest sensitivity was S. nivalis, with complete inhibition of growth at 0.1% v/v of rosemary oil, followed by Alternaria panax, which exhibited 100% inhibition at 0.3% v/v of the oil. Minimum inhibitory concentrations (MICs) of rosemary oil ranged from 0.1 % to 0.5 % (v/v). Chemical analysis using GC-MS showed the presence of thirty-two constituents within rosemary oil from R. officinals L. Camphore type is the most frequent sesquiterpene in rosemary oil composition. Mancozeb and fenhexamid showed their highest inhibition effect (45% and 30%, respectively) against A. panax. T. koningiopsis T-403 showed its highest inhibition effect (84%) against C. destructans isolate. This study may expedite the application of antifungal natural substances from rosemary and Trichoderma in the prevention and control of phytopathogenic strains in ginseng root infections.
Keywords: Rosemary, essential oil, VOCs, antifungal activity, ginseng root rot
Introduction
Korean ginseng (Panax ginseng L.) is mainly cultivated in South Korea, China, and the US, and currently distributed to many countries around the world. It is a very important perennial herb and has been widely used as a medicine and dietary supplement in Asia for more than a thousand years [1]. Ginseng plants are vulnerable to various soil-borne diseases, primarily several species of fungal pathogens that can cause root rot disease [1,2]. Opportunistic fungal pathogens usually attack the weakened plant parts and ginseng plant seedlings are therefore vulnerable to Alternaria and Fusarium infections [2]. Root rot fungi are the most potentially destructive as they can attack and ruin the roots of older plants. Several species of fungi, such as Alternaria, Botrytis, Cylindrocarpon, Fusarium, and Sclerotinia, have been involved in some forms of ginseng root rot [2]. Therefore, to overcome these problems, most ginseng fields are treated with synthetic agricultural chemicals [3, 4]. However, there is strong disagreement regarding the safety aspects of these synthetic chemicals since they are considered as carcinogenic and responsible for many diseases as well as residual toxicity [5]. Furthermore, the indiscriminate use of these synthetic chemicals has led to the development of multiple resistances. Recently, interest has been growing with regard to phytochemicals as a new source of natural antimicrobial agents.
Essential oils are naturally aromatic and volatile complex mixtures obtained from plant materials [6]. Most all essential oils are classified as Generally Recognized as Safe (GRAS) and possess low risk of resistance evolution in pathogenic microorganisms [7]. Essential oils are known as broad-spectrum antimicrobials, i.e., they are effective against multiple species of pathogenic fungi [8-10]. This is possible because one essential oil can possess multiple active ingredients with antimicrobial properties [11]. In general, essential oils possess greater antifungal activity because of the synergistic effect with their active components; therefore, they are more promising for commercial applications than single compounds [12]. Rosmarinus officinalis L. (family, Lamiaceae), widely known as rosemary, is one of the most popular culinary plants cultivated all over the world [13]. Rosemary is widely recognized as one of the spices having a high number of active ingredients [5]. The principal constituents of rosemary essential oil possess pharmacological value in addition to having antioxidant [13,14] and antimicrobial properties [15-18].
Volatile-mediated interactions between microbes and plants have been gaining more attention in agriculture [19]. For example, Campos et al. [20] suggested the volatiles produced by interacting microorganisms as biological control agents for the control of plant pathogens. Furthermore, it is known that bacterial volatiles promote the growth of the plant and activate induced resistance against phytopathogens [21]. However, the effects of volatile organic compounds emitted by Trichoderma species on plant growth have been recognized only recently [22]. In this study, the in vitro fungicidal effects of rosemary essential oil, the volatile organic compounds (VOCs) of T. koningiopsis T-403, and two commonly used fungicides, namely mancozeb and fenhexamid, on mycelial growth of six soil-borne ginseng pathogenic fungi, were investigated.
Materials and Methods
Sampling and Fungal Cultures
The fungi used in this investigation are pathogenic strains collected from the infected roots of Korean ginseng (Panax ginseng) in a soil biochemistry lab at Kangwon National University, Korea. The ginseng plants were collected from many different Korean ginseng fields. Plants were pulled up, put into zipper bags, and conveyed to the laboratory. Growth of isolates was maintained on potato dextrose agar (PDA), with incubation at 25°C for 7 days. The isolated fungal strains from the infected roots of P. ginseng were identified by direct microscopic examination and the features of the culture according to Domsch et al. [23] and Moubasher [24]. Six phytopathogenic fungi were chosen for this study, i.e., Sclerotinia sclerotiorum, S. nivalis, C. destructans, A. panax, B. cinerea, and F. oxysporum, and the samples were indentified and confirmed as pathogenic strains by the Korean Agricultural Cultural Collection (KACC), Jeonju, Korea. T. koningiopsis T-403 strain, used for its VOC activity, was isolated from ginseng rhizosphere soil and was selected based on its plant growth-promoting traits (unpublished data).
Pathogen Rosemary Oil-Exposure Bioassay
The test was conducted using 100% pure essential oil of rosemary (Rosmarinus officinalis, family: Lamiaceae) purchased from a producer (Sydney Essential Oil Co. Ltd., Australia). For this technique, different dilutions of essential oil were prepared in PDA solution and then a mycelial disc of the pathogen was inoculated on the PDA using a borer with 5 mm diameter, as illustrated by Jiang [25]. Antifungal tests were conducted in triplicate and average values were used. Agar dilution is a suitable method to produce a saturated moisture atmosphere and adjust volatility [26]. PDA plates without essential oil were used as a control. The cultures were kept at 25°C for 10 days. Observations on the antifungal activities of essential oil on the pathogenic fungi were reported after 10 days and the inhibition indices were calculated according to the formula of Messgo-Moumene et al. [27]:
where, C1 is the colony area of an uninhibited phytopathogenic fungus in the control, and C2 is the colony area of a phytopathogenic fungus in the dual culture.
Pathogen-Trichoderma VOC-Exposure Bioassay
The Trichoderma isolate was inoculated in malt extract agar (MEA) medium and incubated at 25°C. One day later, the lid of the Petri dish was exchanged with the bottom of a 3-day-old PDA culture of the phytopathogenic fungi. The halves of the two cultures were sealed together using parafilm tape and incubated for 5 days. The linear growth of the test phytopathogenic fungi was measured. The bioassay was conducted in triplicate. The controls were only inoculated with the phytopathogenic fungi. All of the bioassay procedures were carried out under light- limited conditions to regulate the sporulation of Trichoderma [28]. The inhibition percentage was calculated relative to the control as mentioned above.
Pathogen-Fungicide Assay
The inhibitory effects of mancozeb (75%, Indofil) and fenhexamid (50%, Indofil) on the fungal growth of pathogenic strains were investigated using agar-dilution method. Fungicide solutions were prepared in sterile deionized water and added to cool autoclaved PDA (approximately 50°C) to a final concentration of 20 μg ml-1 for each chemical fungicide. The mixtures were decanted into Petri dishes (100 mm × 15 mm) before hardening. A mycelial agar disc (5 mm in diameter) was cut off from the margin of the freshly growing mycelia of ginseng root- rot fungi and inoculated on the fungicide-amended PDA surface. The pathogen-fungicide assays were carried out in triplicate, and all cultures were incubated at 25°C for 10 days in darkness. Colony diameters were measured and the inhibition indices were calculated in comparison to the control (fungicide-free cultures) [27].
GC-MS Analysis and Conditions
The analysis of rosemary oil was carried out on an Agilent GC-MS (7890A GC and GEOL JP/JMS-Q1050GC MSD) prepared with a splitless injection and a capillary DB-WAX MS column (30 × 0.32mm, 0.5 μm film thickness). The injection port temperature was maintained at 250°C and the column oven temperature of the program was set in a range of 50°C to 250°C (6 /°C min), then raised to 300°C (5°C /min), terminating with a 5 min isothermal at 300°C. Helium (1 ml/min) was the carrier gas and the mass spectrum was detected at 70 eV. The chemical composition was identified by comparison of the mass division patterns of the constituents to those of the WILEY reference standard data and libraries of NIST.
Statistical Analysis
The obtained results were statistically analyzed by using SAS software [29] for all data using version 11.0 of Tukey’s test to compare the averages (p > 0.05).
Results
Antifungal Properties of Rosemary Oil
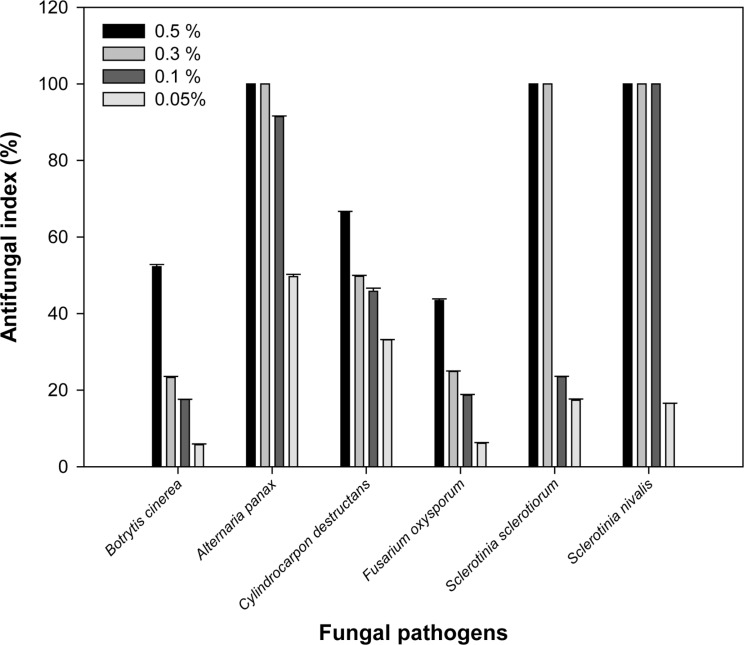
The essential oil exhibited significant antifungal properties against the tested pathogenic fungi. Rosemary oil was effective against S. sclerotiorum, S. nivalis, A. panax, and C. destructans. The highest antimicrobial activity was noticed against S. nivalis strain (100% inhibition) and the lowest antimicrobial effect was detected in F. oxysporum strain (43.4% inhibition) at 0.5% v/v of the oil. Rosemary oil at very low concentration (0.05% (v/v)) could inhibit the mycelial growth of C. destructans by inhibition percentage of (33%) and A. panax by inhibition percentage of 50%. Rosemary oil at 0.1% (v/v) showed complete inhibition only against the phytopathogen S. nivalis, and 91.5% inhibition to A. panax. The oil at 0.3% (v/v) revealed complete inhibition against A. panax, S. sclerotiorum, and S. nivalis, and only 25% inhibition to F. oxysporum, and 23% to B. cinerea. Rosemary oil at 0.3% (v/v) revealed 100% inhibition of mycelium growth to the ginseng pathogenic fungi S. sclerotiorum, and S. nivalis and 50% inhibition to C. destructans. Rosemary oil at 0.5% (v/v) showed 52% inhibition indices to B. cinerea, 66% to C. destructans, and 43% to F. oxysporum, and complete inhibition to A. panax, S. sclerotiorum, and S. nivalis (Fig. 1).
Fig. 1. Antifungal activity of the essential oil of rosemary against ginseng root rot fungi.
B. cinerea; A. panax; C. destructans; F. oxysporum; S. sclerotiorum; S. nivalis. The phytopathogenic fungi (agar dilution tests) were examined after 10 days and the inhibition percentages were calculated.
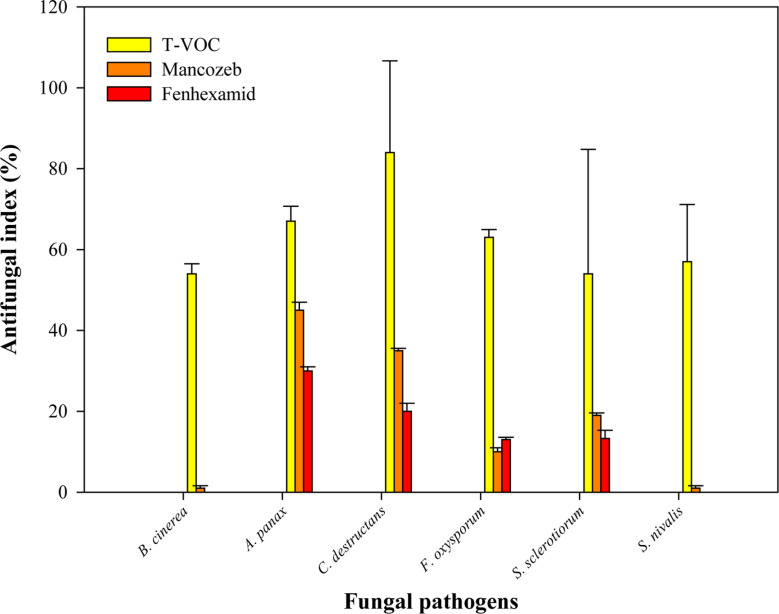
Both fungicides mancozeb and fenhexamid showed their highest inhibition effect against A. panax (45% and 30%, respectively). However, their lowest inhibition effect was against B. cinerea (1% and 0%, respectively). T. koningiopsis T-403 VOCs showed their lowest inhibition effect also against B. cinerea (54.3%). Mancozeb was found to be the most active fungicide, inhibiting the growth of all studied isolates, showing 1% to 45.2% inhibition indices at a concentration of 20 μg ml-1. However, fenhexamid was the inferior fungicide, showing inhibition indices between 0% to 30% at the same concentration (Fig. 2). The chemical fungicides used in this study exhibited inhibition indices of less than 50% in mycelial growth at a concentration of 20 μg ml-1. However, the inhibition index of rosemary oil on the fungal growth was 100% at only 0.1% concentration in S. nivalis, while the T. koningiopsis T-403 VOCs showed inhibition range of 54.7% to 84% (Table 1).
Fig. 2. Antifungal activity of T. koningiopsis T-403 VOCs against ginseng-root rot fungi in comparison to fungicides (20 μg ml-1) on B. cinerea, A. panax, C. destructans, F. oxysporum, S. sclerotiorum, and S. nivalis.
The antifungal indices were detected after 10 days.
Table 1.
Antifungal indices of rosemary essential oil.
| Fungal pathogens | Rosemary oil concentration (%) | T.koningiopsis T-403 | Mancozeb | Fenhexamid | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 0.05 | 0.1 | 0.3 | 0.5 | VOCs | 20 μgml-1 | 20 μgml-1 | |
| Botrytis cinerea | 5.7 ± 0.21e | 17.5 ± 0.06f | 23.3 ± 0.26d | 52.2 ± 0.59c | 54.4 ± 2.48ab | 1.0 ± 0.58e | 0.0 ± 0.00b |
| Alternaria panax | 49.7 ± 0.59a | 91.5 ± 0.15b | 100 ± 0.00a | 100 ± 0.00a | 67.5 ± 3.72a | 45.0 ± 2.00c | 30.0 ± 1.00d |
| Cylindrocarpon destructans | 33.1 ± 0.07b | 45.8 ± 0.80c | 49.7 ± 0.25b | 66.4 ± 0.32b | 84.0 ± 22.63a | 35.0 ± 0.58d | 20.0 ± 2.00d |
| Fusarium oxysporum | 6.1 ± 0.15e | 18.7 ± 0.21e | 24.9 ± 0.15c | 43.4 ± 0.40d | 63.9 ± 1.96b | 10.0 ± 1.00d | 13.0 ± 0.58ab |
| Sclerotinia sclerotiorum | 17.4 ± 0.32c | 23.5 ± 0.10d | 100 ± 0.00a | 100 ± 0.00a | 54.7 ± 30.78ab | 19.0 ± 0.58d | 13.0 ± 2.00d |
| Sclerotinia nivalis | 16.5 ± 0.06d | 100 ± 0.00a | 100 ± 0.00a | 100 ± 0.00a | 57.1 ± 14.14ab | 1.0 ± 0.58c | 0.0 ± 0.00d |
Means with the same letter within a column are not significantly different at p < 0.05.
GC-MS Analysis
The relative components of main compositions in rosemary oil were a-pinene (12.9%), Bicyclo [2.2.1] heptane- 2-one, trimethyl (12.33%), Eucalyptol (11.21%), Camphene (11.04%), b-Pinene (8.09%), D-Limonene (6.16%), a-Phellandrene (1.01%), and β-Caryophyllene (0.38%) (Table 2).
Table 2.
Chemical compositions of rosemary essential oil.
| Peak No. | Compound | RT (min) | Concentration (%) |
|---|---|---|---|
| 1 | Tricyclo[2.2.1.0(2,6)]heptane,1,3,3-trimethyl- | 02:18 | 0.14 |
| 2 | Spiro[4.5]dec-1-ene | 02:46 | 0.1 |
| 3 | Tricyclo[2.2.1.0(2,6)]heptane, 1,7,7-trimethyl- | 03:02 | 5.14 |
| 4 | a-Pinene | 03:14 | 12.8 |
| 5 | Bicyclo[2.2.1]heptane,7,7-dimethyl-2-methylene- | 03:31 | 1.26 |
| 6 | Camphene | 03:53 | 11.04 |
| 7 | b-Pinene | 04:31 | 8.09 |
| 8 | Bicyclo[3.1.0]hexane, 4-methylene-1-(1-methylethyl)- | 04:49 | 2.03 |
| 9 | b-Phellandrene | 04:56 | 0.08 |
| 10 | a-Pinene | 05:23 | 2 |
| 11 | a-Phellandrene | 05:44 | 1.01 |
| 12 | D-Limonene | 06:30 | 6.16 |
| 13 | Bicyclo[3.1.0]hex-2-ene, 4-methyl-1-(1-methylethyl)- | 06:43 | 0.08 |
| 14 | Eucalyptol | 06:59 | 11.21 |
| 15 | 1,4-Cyclohexadiene, 1-methyl-4-(1-methylethyl)- | 07:36 | 0.72 |
| 16 | Benzene, 1-methyl-3-(1-methylethyl)- | 08:15 | 7.48 |
| 17 | Cyclohexene, 4-methyl-3-(1-methylethylidene)- | 08:30 | 0.32 |
| 18 | Cyclohexene, 1-methyl-4-(1-methylethylidene)- | 08:38 | 0.04 |
| 19 | 3-Oxatricyclo[4.1.1.0(2,4)]octane,2,7,7-trimethyl-O | 11:01 | 0.07 |
| 20 | Bicyclo[2.2.1]heptan-2-one, 1,3,3-trimethyl- | 11:40 | 0.08 |
| 21 | Acetaldehyde, (3,3-dimethylcyclohexylidene)-, (E)- | 12:56 | 0.71 |
| 22 | Bicyclo[2.2.1]heptan-2-one, 1,7,7-trimethyl-, (1R)- | 14:48 | 12.33 |
| 23 | 1,6-Octadien-3-ol, 3,7-dimethyl- | 15:24 | 1.1 |
| 24 | 1,6-Octadien-3-ol, 3,7-dimethyl-, 2-aminobenzoate | 15:34 | 0.31 |
| 25 | Aceticacid,1,7,7-trimethyl-bicyclo[2.2.1]hept-2-ylester | 16:16 | 4.14 |
| 26 | 3-Cyclohexen-1-ol, 4-methyl-1-(1-methylethyl)- | 16:46 | 0.12 |
| 27 | Bicyclo[2.2.1]heptan-2-one, 1,7,7-trimethyl-, (1S)- | 17:10 | 0.26 |
| 28 | Cyclohexanol, 1-methyl-4-(1-methylethenyl)- | 17:27 | 0.15 |
| 29 | a-Caryophyllene | 17:58 | 0.38 |
| 30 | Isoborneol | 18:05 | 0.6 |
| 31 | cis-b-Terpineol | 18:43 | 0.12 |
| 32 | 3-Cyclohexene-1-methanol, a,a4-trimethyl- | 19:02 | 9.96 |
Discussion
Ginseng root rot can be caused by several different fungi; some of these fungi are uncommon and can cause more than one disease type and attack the seeds as well. At least twelve pathogenic fungal organisms attack Panax ginseng roots, e.g. Botrytis, Fusarium, Cylindrocarpon, and Sclerotinia [30]. In South Korea, limited studies have been focused on fungicide efficiency against phytopathogenic fungi [31]. The spectrum of the new antifungal agent is generally recognized by specific biological tests on various fungi allowing distinguishing tolerant and sensitive species. The natural polymorphism and accidental mutations may comprise an undeniable level of tolerance to some strains [32]. Mancozeb is a wide-range fungicide for many fungal pathogens, acting on several metabolic pathways in fungal cells [33]. It is a common multi-site dithiocarbamate fungicide, mostly used for preventing spore germination and susceptible to being washed away by rain or irrigation [33]. Fenhexamid is of the hydroxyanilide family [N-(2,3-dichloro-4-hydroxylphenyl)- 1-methylcyclohexanecarboxamide] that prevents RNA and DNA biosynthesis and cell division in fungi [32]. Our results showed that the highest inhibition effect of 20 μg ml-1 mancozeb was 45% against A. panax, while 20 μg ml-1 fenhexamid showed only 30% inhibition activity for the same fungus. Jose et al. [34] investigated the sensitivity of venturia inaequalis isolates to mancozeb in vitro and found that a concentration of only 0.6 μg ml-1 is proposed as a discriminatory dose for determination of sensitivity to mancozeb. Buck [35] used a combination of fungicides with phylloplane yeasts to improve the control of B. cinerea on geranium seedlings, and found that 7.5 μg ml-1 mancozeb did not improve the yeast biocontrol efficacy, and thiophanate-methyl fungicide had an adverse effect on the yeast at the same concentration. Hwang et al. [34] suggested only 10 μg ml-1 for the reduction of mancozeb residue in the postharvest treatments of fresh apples. Shin et al. [31] studied the inhibition indices of six fungicides at different concentrations (0.01, 0.1, 1, and 10 μg ml-1) on the mycelial growth of several C. destructans strains in vitro. Among the fungicides assessed was the dithiocarbamate fungicide mancozveb, which was highly effective on the conidial production and temperately effective on the hyphal growth of C. destructans [31].
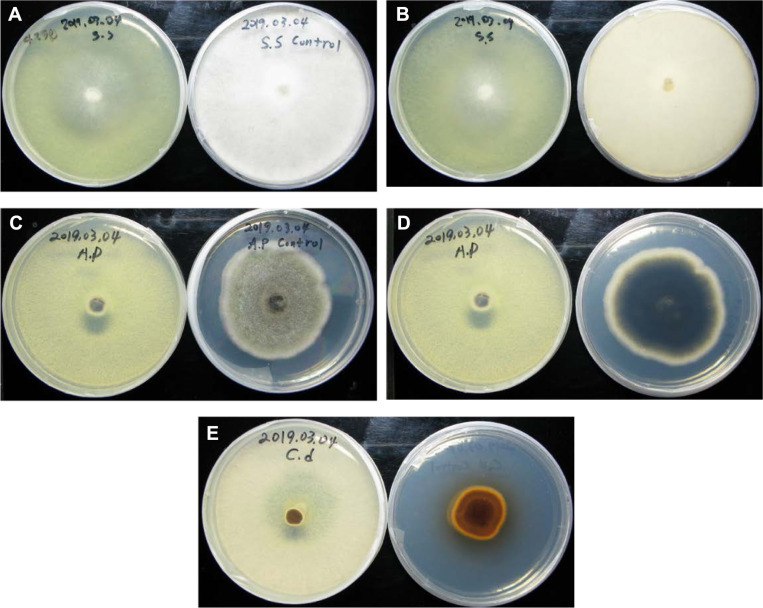
Walker et al. [34] estimated a new indigenous species causing gray mildew in the French vineyards with B. cinerea named B. pseudocinerea, which was originally resistant to fenhexamid. Recently, four different fenhexamid-resistant groups could be detected among the grey mold population [32]. Many Trichoderma species possess the ability to inhibit plant diseases and enhance plant growth and productivity by using an arsenal of mechanisms including myco-parasitism [35], antibiosis [36], induced systemic resistance [37], and supported nutrient efficiency [38]. However, only a few studies have concentrated on Trichoderma volatile compounds and their influence in terms of plant protection. In this study, T. koningiopsis T-403 VOCs showed high inhibition indices (84%) against C. destructans isolate (Fig. 3). T. koningiopsis T-403 was previously demonstrated by our laboratory group to have effective plant growth-promoting activity and inhibitory effect on the plant pathogens after screening many strains. VOCs released by T. pseudokoningii were correlated with the highest Arabidopsis growth promotion [39]. The volatile compounds emitted by Trichoderma can induce plant resistance to pathogens, thus improving plant health [39].
Fig. 3.
Pathogens-T. koningiopsis T-403 VOC-exposure bioassay in 10-days old cultures sealed with parafilm; A) S. sclerotiorum alone (right) and with T. koningiopsis T-403 (left); B) S. Sclerotiorum (control reverse); C) A. panax; D) A. panax (control reverse); E) C. destructans (control reverse).
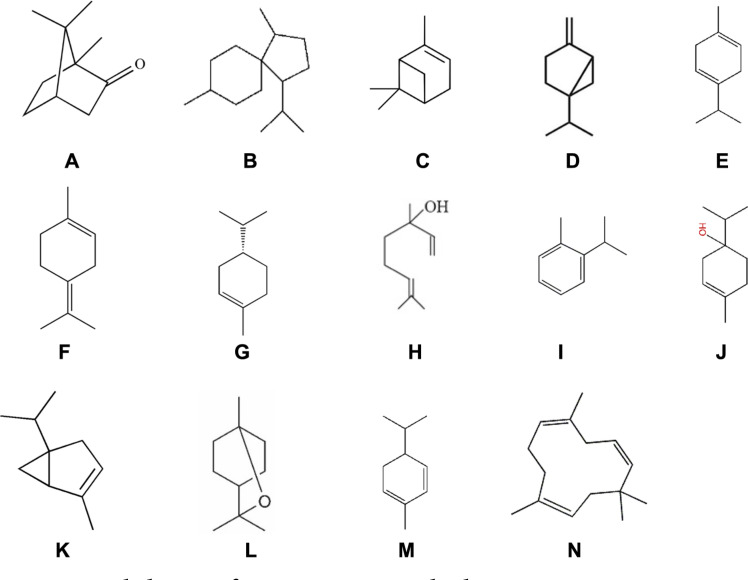
Minimum inhibitory concentrations (MICs) of the essential oil from rosemary (R. officinalis L.) against six ginseng root rot fungi were determined. Rosemary oil possesses significant antifungal effects against all tested pathogenic fungi. Rosemary oil was potent against the fungi A. panax, S. nivalis, S. sclerotiorum, and C. destructans. The best antimicrobial activity was obtained for S. nivalis strain and the lowest effect was found in F. oxysporum strain (Table 1, Fig. 1). The dissimilar result of the essential oil effect against different fungal species may be due to their influence on organelles and biosynthesis rather than only a cell wall. Some previous studies have demonstrated that natural and synthetic antimicrobial agents can cause a significant reduction in the amount of ergosterol, a major sterol component responsible for maintaining cell function and integrity in the fungal cell membrane [40]. Most of the previous studies on rosemary essential oil focused on the antibacterial role. Sirocchi et al. [41] illustrated that incorporation of R. officinalis at only 4% (w/w) to active packaging preserved meats from damage by the cadaverine-producer bacteria Brocothrix thermospacta or any of Enterobacteriaceae members. Sachets that provide a slow discharge of R. officinalis essential oil can also be combined into packaged meat products and used to prevent the meats' putrefaction [42]. The culinary and medicinal uses of rosemary herb are due to its massive arrays of chemical components known as plant metabolites. Among these arrays are low- molecular-weight aromatic chemical compounds called essential oils, and these play a fundamental role in the culinary and antimicrobial properties of the plant [13]. Rosemary essential oil is dominated by α-pinene, 1,8- cineole, α- terpineol, camphene, and borneol as main compounds [43], and these bioactive compounds are responsible for the different medicinal effects of the general antimicrobial [15-17] and antioxidant [14] activities, in addition to other activities, e.g. anticarcinogenic properties [44]. Polyphenolic compounds are another group of components found in rosemary essential oil; however, in recent years the most important of rosemary constituents that have gained significant attention is the unique class of terpenes [45]. Our data revealed that eucalyptol is one of the dominant constituents (11.21%) in rosemary oil (Table 2). It has been well known that terpenes possess antifungal properties. Interestingly, some fungal species were recently discovered to produce eucalyptol in large amounts [46]. In the current detection, thirty-two constituents were identified. Only four constituents, pinene, camphene, limonene, and eucalyptol represent more than 50% of all the chemical composition in rosemary essential oil. Camphore type was the most frequent skeletal sesquiterpene in rosemary oil composition. Fig. 4 displays the main sesquiterpene skeletons of rosemary oil. The chemical analysis of rosemary oil is shown in Table 2. Fu et al. [47] estimated that the main compositions in rosemary oil were cineole (27.2%), pinene (19.4%), camphor (14.3%), camphene (11.5%), Borneol (3.2%), β-Caryophyllene (2.41%), and Bornyl acetate (1.13%). Sienkiewicz et al. [48] reported that the essential oil of rosemary contains thirty-seven constituents with the main ones being cineole (46.5%), camphor (11.5%), pinene (11.1%), β-pinene (9.1%) and camphene (5.3%). The differences in chemical compositions of rosemary oil may be partially due to the variation in the preparation technique, geographical source and maturity stage of R. officinalis plant. Nevertheless, the crude essential oil displayed growth inhibition influence on all pathogenic fungal species examined in the present study (Fig. 5). This study also suggests good examples of antifungal substances from rosemary and Trichoderma which are biodegradable natural products that may be safe for application soon. In conclusion, the fungicidal effects of R. officinalis L. essential oil, the VOCs of T. koningiopsis T-403, and the fungicides mancozeb and fenhexamid, were evaluated to suppress the severe fungal pathogens causing the ginseng root rot. Rosemary oil was effective to prevent the growth of phytopathogenic fungi. S. nivalis exhibited the lowest MIC. GC-MS analysis showed the presence of thirty-two constituents within the essential oil of R. officinals, and some of these are documented to possess antimicrobial properties, e.g. eucalyptol. Our results may assert the importance of essential oils found in common plants in the protection of ginseng crops from mycopathogens. This study will help in the prospective development of biopesticides for the eco-friendly control of plant pathogens and proposes the potential use of the antimicrobial compound from Trichoderma.
Fig. 4. Main sesquiterpenes skeletons of rosemary essential oil.
(A) camphore type, (B) acorane type, (C) pinene type, (D) sabinene type, (E) terpinene type, (F) terpinolene type, (G) limonene type, (H) linalool type, (I) cymene type, (J) terpineol type, (K) thujene type, (L) eucalyptol type, (M) phellandrene type, (N) caryophyllene type .
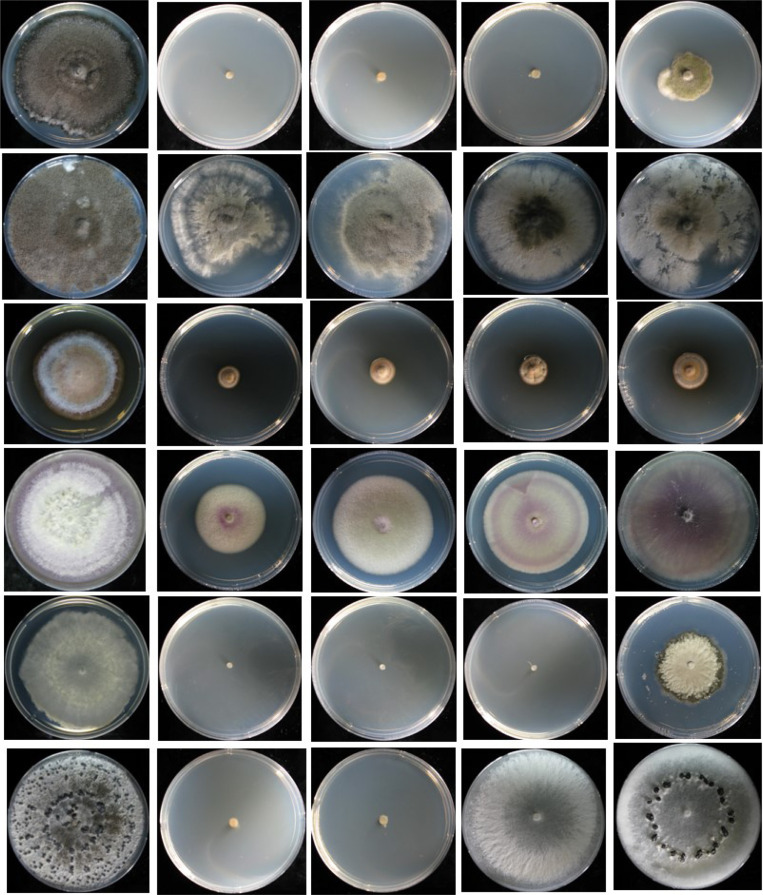
Fig. 5.
Antifungal activity of the rosemarys’ essential oil (left to right) Control, 0.5, 0.3, 0.1, and 0.05 % against ginseng root rot fungi (up to down) B. cinerea; A. panax; C. destructans; F. oxysporum; S. nivalis; S. sclerotiorum, on agar dilution test, phytopathogenic fungi were examined after 10 days and the inhibition percentage was calculated according to the formula of Messgo-Moumene et al. (2015).
Acknowledgment
This study was conducted with the support of a research grant of Kangwon National University in 2020. This work was also supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (Ministry of Science, ICT & Future Planning) (No. 2017R1A2B1009738).
Footnotes
Conflict of Interest
The authors have no financial conflicts of interest to declare.
REFERENCES
- 1.Randall JA, Cook J. American Ginseng in Iowa: Producing IOWA STATE UNIVERSITY F-401 Forestry Extension Ames, Iowa Series on Ginseng. F -400, F401, F402, & F403 2013 [Google Scholar]
- 2.Davis JW, Persons S. Growing and marketing ginseng, goldenseal and other woodland medicinals. Printed in Canada. 2014 First printing June 2014. [Google Scholar]
- 3.Tawaha, K, Alali FQ, Gharaibeh M, Mohammad M, El-Elimat T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem. 2007;104:1372–1378. doi: 10.1016/j.foodchem.2007.01.064. [DOI] [Google Scholar]
- 4.Moreira MR, Ponce AG, dell Valle CE, Roura SI. Inhibitory parameters of essential oils to reduce a foodborne pathogen. LWT. 2005;38:565–570. doi: 10.1016/j.lwt.2004.07.012. [DOI] [Google Scholar]
- 5.Peng Y, Yuan J, Liu F, Ye J. Determination of active components in rosemary by capillary electrophoresis with electrochemical detection. J. Pharm. Biomed. Anal. 2005;39:431–437. doi: 10.1016/j.jpba.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 6.Hyldgaard M, Mygind T, Meyer RL. Essential oils in food preservation: mode of action, synergies and interactions with food matrix components. Front. Microbiol. 2012;3:12. doi: 10.3389/fmicb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardile V, Russo A, Formisano C, Rigano D, Senatore F. Essential oils of Salvia bracteata and Salvia rubifolia from Lebanon: Chemical composition, antimicrobial activity and inhibitory effect on human melanoma cells. J. Ethnopharmacol. 2009;126:265–272. doi: 10.1016/j.jep.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 8.Oussalah M, Caillet S, Saucier L, Lacroix M. Inhibitory effects of selected plant essential oils on the growth of four pathogenic bacteria: E. coli O157:H7, Salmonella typhimurium, Staphylococcus aureus and Listeria monocytogenes . Food Control. 2007;18:414–420. doi: 10.1016/j.foodcont.2005.11.009. [DOI] [Google Scholar]
- 9.Silva F, Ferreira S, Duarte A, Mendonça DI, Domingues FC. Antifungal activity of Coriandrum sativum essential oil, its mode of action against candida species and potential synergism with amphotericin B. Phytomedicine. 2011;19:42–47. doi: 10.1016/j.phymed.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 10.Schnitzler P, Astani A, Reichling J. Screening for antiviral activities of isolated compounds from essential oils. 253643. J. Evid. Based Complement. Alternat. Med. 2011;25:36–43. doi: 10.1093/ecam/nep187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertoli A, Çirak C, Teixeira da Silva JA. Hypericum species as sources of valuable essential oils. Med. Arom. Plant Sci. Biotechnol. 2010;5:29–47. [Google Scholar]
- 12.Tian J, Ban X, Zeng H, He J, Chen Y. The mechanism of antifungal action of essential oil from dill (Anethum graveolens L.) on Aspergillus flavus. PLoS One. 2012;7:e30147. doi: 10.1371/journal.pone.0030147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Habtemariam S. The therapeutic potential of rosemary (Rosmarinus officinalis) diterpenes for Alzheimer's disease. J. Evid. Based Complement. Alternat. Med. 2016 doi: 10.1155/2016/2680409. Article 2016: 2680409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estévez M, Ramírez R, Ventanas S Cava R. Sage and rosemary essential oils versus BHT for the inhibition of lipid oxidative reactions in liver pâté. LWT Food Sci. Technol. 2007;40:58–65. doi: 10.1016/j.lwt.2005.07.010. [DOI] [Google Scholar]
- 15.Valgimigli L. Essential Oils as Natural Food Additives: Composition, Applications, Antioxidant and Antimicrobial Properties. Nova Science Publishers; New York, NY, USA: 2012. [Google Scholar]
- 16.Issabeagloo E, Kermanizadeh P, Taghizadieh M, Forughi R. Antimicrobial effects of rosemary (Rosmarinus officinalis L.) essential oils against Staphylococcus spp. Afr. J. Microbiol. Res. 2012;6:5039–5042. doi: 10.5897/AJMR12.741. [DOI] [Google Scholar]
- 17.Ojeda-Sana AM, van Baren CM, Elechosa M, Ju'arez AMA, Moreno S. New insights into antibacterial and antioxidant activities of rosemary essential oils and their main components. Food Control. 2013;31:189–195. doi: 10.1016/j.foodcont.2012.09.022. [DOI] [Google Scholar]
- 18.Zaouali Y, Bouzaine T, Boussaid M. Essential oils composition in two Rosmarinus officinalis L. varieties and incidence for antimicrobial and antioxidant activities. Food Chem. Toxicol. 2010;48:3144–3152. doi: 10.1016/j.fct.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Piechulla B, Degenhardt J. The emerging importance of microbial volatile organic compounds. Plant Cell Environ. 2014;37:811–812. doi: 10.1111/pce.12254. [DOI] [PubMed] [Google Scholar]
- 20.Campos VP, Pinho RSC, de Freire ES. Volatiles produced by interacting microorganisms potentially useful for the control of plant pathogens. Cienciae Agrotec Lavras. 2010;34:525–535. doi: 10.1590/S1413-70542010000300001. [DOI] [Google Scholar]
- 21.Cortes-Barco AM, Goodwin PH, Hsiang T. Comparison of induced resistance activated by benzothiadiazole, (2R, 3R)- butanediol and an isoparaffin mixture against anthracnose of Nicotiana benthamiana . Plant Pathol. 2010;59:643–653. doi: 10.1111/j.1365-3059.2010.02283.x. [DOI] [Google Scholar]
- 22.Lee S, Hung R, Yap M, Bennett JW. Age matters: the effects of volatile organic compounds emitted by Trichoderma atroviride on plant growth. Arch. Microbiol. 2015;197:723–727. doi: 10.1007/s00203-015-1104-5. [DOI] [PubMed] [Google Scholar]
- 23.Domsch KH, Gams W, Anderson T. Compendium of soil fungi. 1-2. Academic Press; London: 1980. pp. 405–859. [Google Scholar]
- 24.Moubasher AH. Soil fungi in Qatar and other Arab countries. The Centre of Scientific and Applied Research University of Qater; Doha, Qater: 1993. [Google Scholar]
- 25.Jiang L. PhD diss. 2011. Louisiana State University; 2011. Comparison of disk diffusion, agar dilution, and broth microdilution for antimicrobial susceptibility testing of five chitosans. [Google Scholar]
- 26.Ben Kaab S, Rebey IB, Hanafi M, Berhal C, Fauconnier ML, De Clerck C, et al. Rosmarinus officinalis essential oil as an effective antifungal and herbicidal agent. Spanish J. Agr. Res. 2019;17:e1006. doi: 10.5424/sjar/2019172-14043. [DOI] [Google Scholar]
- 27.Messgo-Moumene S, LiY, Bachir K, Houmani Z, Bouznad Z, Chemat F. Antifungal power of citrus essential oils against potato late blight causative agent. J. Essen. Oil Res. 2015;27:169–176. doi: 10.1080/10412905.2014.982877. [DOI] [Google Scholar]
- 28.Dennis C, Webster J. Antagonistic properties of species-groups of Trichoderma: iii. hyphal interaction. Trans. Br. Mycol. Soc. 1971;57:363–369. doi: 10.1016/S0007-1536(71)80050-5. [DOI] [Google Scholar]
- 29.SAS Institute. Statistical Analysis System for Windows [Softaware] SAS; Cary, N.C.: 2011. The SAS 10.2 software. [Google Scholar]
- 30.Punja ZK, Wan A, Goswami RS. Growth, pathogenicity and infection behaviour, and genetic diversity of Rhexocercosporidium panicis isolates from ginseng roots in British Columbia. Canadian J. Plant Pathol. 2008;35:503–513. doi: 10.1080/07060661.2013.843315. [DOI] [Google Scholar]
- 31.Shin J, Fu T, Park K H, Kim KS. The effect of fungicides on mycelial growth and conidial germination of the ginseng root rot fungus, Cylindrocarpon destructans . Mycobiology. 2017;45:220–225. doi: 10.5941/MYCO.2017.45.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Billard A, Fillinger S, Leroux P, Bach J, Lanen C, Lachaise H, Beffa R, Debieu D. Fenhexamid resistance in the Botrytis species complex, responsible for grey mould disease N. Thajuddin (Ed.) Fungicides-Beneficial and Harmful Aspects, InTech Publisher; Rijeka, Croatia: 2011. pp. 61–78. [DOI] [Google Scholar]
- 33.Gullino ML, Tinivella F, Garibaldi A, Kemmitt GM, Bacci L, Sheppard B. Mancozeb: past, present, and future. Plant Dis. 2010;94:1076–1087. doi: 10.1094/PDIS-94-9-1076. [DOI] [PubMed] [Google Scholar]
- 34.Jose LHS, Oliver SV, Paula AC. Sensitivity of Venturia inaequalis chilean isolates to difenoconazole, fenarimol, mancozeb, and pyrimethanil. Chil. J. Agr. Res. 2011;71:39–44. doi: 10.4067/S0718-58392011000100005. [DOI] [Google Scholar]
- 35.Hwang E, Cash J, Zabik M. Postharvest treatments for the reduction of mancozeb in fresh apples. J. Agr. Food Chem. 2001;49:3127–3132. doi: 10.1021/jf010234h. [DOI] [PubMed] [Google Scholar]
- 36.Buck JW. Combinations of fungicides with phylloplane yeasts for improved control of Botrytis cinerea on geranium seedlings. Phytopathology. 2004;94:196–202. doi: 10.1094/PHYTO.2004.94.2.196. [DOI] [PubMed] [Google Scholar]
- 37.Walker AS, Gautier A, Confais C, Martinho D, Viaud M, Le Pêcheur P, et al. Botrytis pseudocinerea, a new cryptic species causing grey mould in French vineyards in sympatry with Botrytis cinerea . Phytopathology. 2011;101:1433–1445. doi: 10.1094/PHYTO-04-11-0104. [DOI] [PubMed] [Google Scholar]
- 38.Szabo M, Csepregi K, Galber M, Fekete C. Control plant-parasitic nematodes with Trichoderma species and nematode- trapping fungi: the role of chi18-5 and chi18-12 genes in nematode egg-parasitism. Biol. Control. 2012;63:121–128. doi: 10.1016/j.biocontrol.2012.06.013. [DOI] [Google Scholar]
- 39.Howell CR. The role of antibiosis in biocontrol. In: Harman GE, Kubicek CP, editors. Trichoderma and Gliocladium London: Taylor and Francis. Vol. 2. 1998. pp. 173–184. [Google Scholar]
- 40.Mathys J, De Cremer K, Timmermans P, Van Kerckhove S, Lievens B, Vanhaecke M, et al. Genome-wide characterization of ISR induced in Arabidopsis thaliana by Trichoderma hamatum T382 against Botrytis cinerea infection. Front. Plant Sci. 2012;3:108. doi: 10.3389/fpls.2012.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Contreras-Cornejo HA, Macías-Rodríguez L, Cortés-Penagos C, López- Bucio J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 2009;149:1579–1592. doi: 10.1104/pp.108.130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee S, Yap M, Behringer G, Hung R, Bennett JW. Volatile organic compounds emitted by Trichoderma species mediate plant growth. Fungal Biol. Biotechnol. 2016;3:7. doi: 10.1186/s40694-016-0025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinto E, Vale-Silva L, Cavaleiro C, Salgueiro L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 2009;58:1454–1462. doi: 10.1099/jmm.0.010538-0. [DOI] [PubMed] [Google Scholar]
- 44.Sirocchi V, Caprioli G, Cecchini C, Coman MM, Cresci A, Maggi F. Biogenic amines as freshness index of meat wrapped in a new active packaging system formulated with essential oils of Rosmarinus officinalis . Inter. J. Food Nutr. Sci. 2013;64:921–928. doi: 10.3109/09637486.2013.809706. [DOI] [PubMed] [Google Scholar]
- 45.Sánchez-González L, Cháfer M, Hernández MA, Chiralt C, González-Martínez Antimicrobial activity of polysaccharide films containing essential oils. Food Control. 2011;22:1302–1310. doi: 10.1016/j.foodcont.2011.02.004. [DOI] [Google Scholar]
- 46.Atti-Santos AC, Rossato M, Pauletti GF, Rota LD, Rech JC, Marcia Regina F, Atti L, Moyna P. Physico-chemical Evaluation of Rosmarinus officinalis L. Essential Oils. 2005;48:1035–1039. doi: 10.1590/S1516-89132005000800020. ISSN 1516-8913 Printed in Brazil. [DOI] [Google Scholar]
- 47.Baser KHC, Buchbauer G. Handbook of Essential Oils: Science, Technology and Applications. CRC Press; Boca Raton, London, New York: 2010. ISBN: 978-1-4200-6315-8. [Google Scholar]
- 48.Bai J, Baldwin EA, Imahori Y, Kostenyuk I, Burns J, Brecht JK. Chilling and heating may regulate C6 volatile aroma production by different mechanisms in tomato (Solanum lycopersicum) fruit. Postharvest Biol. Technol. 2011;60:111–120. doi: 10.1016/j.postharvbio.2010.12.002. [DOI] [Google Scholar]
- 49.Elaissi A, Rouis Z, Mabrouk S, Harzallah-Skhiri F. Correlation between chemical composition and antibacterial activity of essential oils from fifteen Eucalyptus species growing in the Korbous and Jbel Abderrahman Arboreta (North East Tunisia) Molecules. 2012;17:3044–3057. doi: 10.3390/molecules17033044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fu Y, Zu Y, Chen L, Shi X, Wang Z, Sun S, Efferth T. Antimicrobial activity of clove and rosemary essential oils alone and in combination. Phytother. Res. 2007;21:989–994. doi: 10.1002/ptr.2179. [DOI] [PubMed] [Google Scholar]
- 51.Sienkiewicz M, Łysakowska M, Pastuszka M, Bienias W, Kowalczyk E. The potential of use basil and rosemary essential oils as effective antibacterial agents. Molecules. 2013;18:9334–9351. doi: 10.3390/molecules18089334. [DOI] [PMC free article] [PubMed] [Google Scholar]