Abstract
Objective:
Mood instability is associated with the onset of bipolar disorder (BD) in youth with a family history of the illness. In a clinical trial with youth at high risk for BD, we examined the association between mood instability and symptomatic, psychosocial, and familial functioning over an average of 2 years.
Method:
Youth (aged 9–17 years) with major depressive disorder or other specified BD, current mood symptoms, and a family history of BD were rated by parents on a mood instability scale. Participants were randomly assigned to 4 months of family-focused therapy or enhanced care psychoeducation, both with medication management as needed. Independent evaluators rated youth every 4 to 6 months for up to 4 years on symptom severity and psychosocial functioning, whereas parents rated mood instability of the youth and levels of family conflict.
Results:
High-risk youth (N = 114; mean age 13.3 ± 2.6 years; 72 female) were followed for an average of 104.3 ± 65.8 weeks (range, 0–255 weeks) after randomization. Youth with other specified BD (vs major depressive disorder), younger age, earlier symptom onset, more severe mood symptoms, lower psychosocial functioning, and more familial conflict over time had higher mood instability ratings throughout the study period. Mood instability mediated the association between baseline diagnosis and mother/offspring conflict at follow-up (Z = 2.88, p = .004, αβ = 0.19, 95% CI = 0.06–0.32). Psychosocial interventions did not moderate these associations.
Conclusion:
A questionnaire measure of mood instability tracked closely with symptomatic, psychosocial, and family functioning in youth at high risk for BD. Interventions that are successful in reducing mood instability may enhance long-term outcomes among high-risk youth.
Clinical trial registration information:
Early Intervention for Youth at Risk for Bipolar Disorder; https://clinicaltrials.gov/; NCT01483391
Keywords: emotion regulation, affective reactivity, mania, depression, family processes, family therapy
Mood instability—also called mood or affective lability—is a transdiagnostic feature of children with mood, behavioral, and emotional disturbances.1–3 The term refers to frequent, sudden, and unpredictable shifts in emotional states. Youth with mood instability change rapidly from states such as irritability or rage to sadness or anxiety; euphoria or expansiveness to withdrawal or disinterest; or giddiness or hilarity to crying inconsolably. The construct is similar to affective reactivity, which refers to high-intensity reactions to interpersonal events or internal stimuli, followed by a slow return to one’s emotional baseline.2,4,5
In patients with bipolar disorder (BD), subthreshold fluctuations in mood states can be observed between manic and depressive episodes, even when patients are in remission.6 Inter-episode mood instability appears to be more pronounced in younger than in older patients with BD.7,8 Several dimensions of mood instability, as rated by parents, have been found to distinguish offspring of parents with BD from offspring of parents with other psychiatric disorders: elevation/activation (eg, bursts of silliness or excessive familiarity with strangers), irritability (eg, unpredictable loss of temper), and anxiety–depression (eg, sudden periods of sadness or tearfulness).5
In adults with BD I or II, mood instability is associated with impaired psychosocial functioning, increased stress, and decreased quality of life.1,9,10 In younger patients, lability of moods is associated with impairments in family functioning, particularly when mood shifts involve activation and aggression. In adolescents with BD I and II, impulsive expressions of irritability, hostility, and aggression are more closely associated with family distress and conflict than depression or withdrawal.11 Among children and teens with bipolar spectrum disorders, mania symptoms are more strongly associated with parenting stress than are depressive symptoms.12
Importantly, mood instability is a risk factor for the onset of BD I or II in high-risk individuals. In a 15-year study of community volunteers, Angst et al.13 found that “ups and downs” were the strongest predictor of which persons developed BD over time, independent of a family history of mania. In studies of youth with other specified (ie, subthreshold) BD14 and offspring of parents with BD,15 high levels of parent-reported mood instability were associated with an increased likelihood of conversion to BD I or II over 5 to 8 years, above and beyond baseline levels of depression or anxiety.
This study was conducted to evaluate the presence, functional impact, and malleability of mood instability in youth at high risk for BD who participated in a randomized trial of psychosocial intervention. High risk was defined as having the following: (1) a lifetime history of major depressive disorder (MDD) or other specified bipolar disorder (OSBD), with recurrent and brief periods of elevation and activation; (2) mood symptoms in the 1 to 2 weeks before study entry; and (3) a family history of BD I or II. The inclusion of youth with MDD with a family history of BD reflected 3 considerations: (1) about 50% of adults with BD I or II report that their first episode was a major depressive episode16; (2) youth with a family history of BD are at high risk for conversion to BD I/II in the 4 to 5 years after onset of a major depressive episode, with conversion estimates ranging from 15% to 40% in 2 years17–22; and (3) depressed youth with unstable moods are at particularly high risk for conversion.23,24
High-risk participants were randomly assigned to 4 months of family-focused therapy (FFT) or enhanced usual care (brief family psychoeducation and individual support), both with pharmacotherapy as needed. The primary findings were that, over an average of 2 years of follow-up, youth assigned to FFT had longer intervals before new mood episodes and lower levels of suicidal ideation and behavior than youth assigned to enhanced usual care.25,26 The design of the trial enabled us to examine mood instability as a correlate of changes in symptom status and psychosocial functioning among high-risk youth, as well as its malleability by targeted psychosocial interventions.
Our first study objective was to determine whether age, mood diagnoses, and levels of symptom severity in high-risk youth were cross-sectionally or longitudinally associated with levels of parent-reported mood instability. We expected the following: (1) that mood instability scores would be higher in younger (ie, school-aged or early adolescent) high-risk children than in late adolescent youth; (2) that high-risk youth with OSBD would have higher levels of mood instability (and particularly, elevation/activation) over time than high-risk youth with MDD; and (c) that clinician-rated mood symptom severity would track closely with parent ratings of mood instability in youth over time.
The second objective was to examine whether levels of mood instability are correlated with levels of psychosocial and family functioning in high-risk youth, especially those with subthreshold mania symptoms. We hypothesized the following: (1) that youth with higher levels of mood instability would have lower social functioning and higher levels of conflict with parents at follow-up than would youth with more stable moods; and (2) that the relation between baseline mood diagnosis (ie, OSBD vs MDD) and levels of family conflict at follow-up would be mediated by levels of mood instability.
The third objective was to explore whether mood instability could be modified by psychosocial interventions. Because of its focus on enhancing communication and reducing family conflict, FFT may have the effect of decreasing environmental triggers for mood instability in high-risk youth. Thus, we hypothesized that FFT would be associated with greater decreases over time in youths’ mood instability compared to enhanced usual care. Verifying these hypotheses, we reasoned, would clarify the nature of targets for early intervention in children vulnerable to BD.
METHOD
Participants
Recruitment for the trial ran from October 6, 2011, to September 15, 2016, in outpatient clinics at the UCLA School of Medicine, Los Angeles, CA; University of Colorado, Boulder and Aurora, CO; and Stanford University School of Medicine, Stanford, CA. Parents of potential research participants responded to online, radio, television, or print advertisements or were referred by pediatricians. At the initial visit, youth and their parent(s) received an explanation of study procedures and signed institutional review board–approved assent or consent forms. More detail on study procedures is given in the first report from this trial.25
Youth met the following eligibility criteria: (1) age between 9 years 0 months and 17 years 11 months; (2) lifetime DSM-IV-TR27 and, after 2013, DSM-528 criteria for lifetime MDD or OSBD (formerly BD, not otherwise specified); (3) current mood symptoms, with Young Mania Rating Scale29 scores ≥11 or Children’s Depression Rating Scale, Revised30 scores ≥29 in the prior 1 to 2 weeks (eMethods in Supplement 1, available online); (4) has at least one parent or grandparent willing to participate; and (5) has one or more first- or second-degree relatives with a lifetime history of BD I or II. Criteria for OSBD were adapted from the Course and Outcome of Bipolar Youth study8,31: recurrent and distinct 1- to 3-day periods with abnormally elevated, expansive, or irritable mood, plus 2 (3, if irritable mood only) symptoms of mania that reflected a change from baseline mood and totaled at least 10 days in the child’s lifetime. We excluded youth who met DSM-5 criteria for pervasive developmental disorders or current substance/alcohol use disorders.
Diagnostic Assessment
Diagnoses of research participants were based on the Kiddie Schedule for Affective Disorders and Schizophrenia, Present and Lifetime Version (KSADS-PL) for DSM-IV32 and later for DSM-5,33 administered separately to the youth and at least one parent about the youth. Participants were only admitted to the study once there was diagnostic agreement between the KSADS-PL interviewer and a child psychiatrist based on a separate evaluation. Interrater reliability (intraclass rs) averaged 0.74 to 0.84 across diagnosticians for depression and hypo/mania KSADS items (eMethods in Supplement 1, available online). Parents were queried about their own psychiatric history using the MINI International Neuropsychiatric Interview.34 Diagnoses of first- and second-degree relatives who could not be directly interviewed were based on reports from parents using the Family History Screen instrument.35
Outcome Assessments
Mood instability was based on parent ratings of the offspring using the Children’s Affective Lability Scale (CALS),36 completed at each study assessment (baseline, every 4 months for the first year, and then every 6 months for up to 4 years). The 20-item CALS covers specific child behaviors in the past 3 months, each rated on 1 (never or rarely occurs) to 5 (1 or more times a day) scales. Internal reliability for the CALS was 0.92 (Cronbach’s α). A total score was the primary variable of interest, with secondary analyses concerning factor scores derived by Birmaher et al.5 in offspring of parents with BD: elevation/activation (eg, “Has bursts of silliness for little or no apparent reason”), irritability (eg, “Suddenly loses his/her temper when you would not expect it”), and anxiety–depression (“Has bursts of being nervous or fidgety”) (Table S1, Supplement 1, available online).
To examine the severity of mood symptoms of the youth at each interval, independent evaluators who were unaware of participants’ treatment assignments administered the Adolescent Longitudinal Interval Follow-up Evaluation37 to the youth and one parent and rated each week of the prior 4- or 6-month interval on Psychiatric Status Ratings (PSRs) of depression, hypomania, and mania. The depression PSR was scored on a 1 (symptoms absent) to 6 (severe symptoms) scale, whereas the mania and hypomania PSRs were combined into a single 8-point hypo/mania scale ranging from 1 (no symptoms) to 6 (syndromal hypomania), with scores of 7 to 8 indicating severe or extremely severe mania (eMethods, Supplement 1, available online). Interrater reliabilities (intraclass rs) for weekly PSRs for depression and hypo/mania were 0.88 to 0.99, calculated across raters at 3 sites.
At each assessment, independent evaluators made 1 to 100 (low to high) ratings of the child’s psychosocial functioning on the Children’s Global Assessment Scale38 covering the prior 2 weeks. The evaluator rated the scale from all sources of information obtained from the child and parent at the time of interview. Family functioning was measured with the parent-rated Conflict Behavior Questionnaire (CBQ),39 which contains 20 true/false items assessing the level of argumentativeness, frustration in communication, and relational distress in parent–child dyads over the previous 3 months (example items in eMethods in Supplement 1, available online). To standardize scores across different family constellations, and because mothers were the primary caregivers in over 80% of the families, we examined only mothers’ CBQ ratings of dyadic conflict. Cronbach’s α for the CBQ was 0.87.
Treatment Protocols
After the baseline assessment, eligible participants were randomly allocated to FFT or enhanced usual care, both lasting 4 months. Allocation was based on a dynamic allocation procedure40 that balanced groups within sites on mood diagnosis, age, and medications at time of study referral (mood stabilizers/antipsychotics vs neither). Study psychiatrists conducted a baseline evaluation with the child and, if both the child and parent(s) agreed, offered biweekly and then monthly medication management sessions using study-based pharmacotherapy guidelines.41
In FFT, the child participant, parents, and (when possible) siblings attended 12 sessions lasting 60 minutes each (8 weekly, 4 biweekly) in the 4 months after randomization. Sessions focused on psychoeducation about mood disorders, communication enhancement training, and problem-solving skills training. Youth and family members in the 4-month enhanced usual care condition received 3 weekly 60-minute sessions of family psychoeducation followed by 3 monthly sessions of individual support focused on implementing a mood management plan. Clinicians’ manuals for both interventions are available at https://www.semel.ucla.edu/champ/downloads-clinicians (details in eMethods in Supplement 1, available online).
Statistical Analyses
For the first study objective, we examined whether the demographic characteristics (age, sex, race, or ethnicity), diagnoses (OSBD vs MDD), comorbid disorders (presence/absence of internalizing [eg, anxiety] and/or externalizing [eg, attention-deficit/hyperactivity disorder {ADHD}, disruptive behavior] disorders), and symptom severity ratings (mean weekly depression and hypo/mania PSRs) of the youth were related to CALS scores at baseline, using analyses of variance and Pearson correlations. Secondary analyses examined whether mothers with BD I or II were more likely to rate their offspring as having high mood instability than were mothers who did not have BD.
Next, in mixed-effect regression models (PROC MIXED in SAS42), repeated CALS total scores collected in 4- to 6-month follow-up intervals were regressed on the child’s baseline mood diagnosis and mean weekly PSR depression and hypo/mania scores from the same assessment intervals. In each model, age, sex, comorbid disorders, and treatment condition were entered as additional independent variables. CALS and CBQ scores were square root transformed to improve the normality of the residuals. Data analyses used all available follow-up points. Post hoc sensitivity analyses were conducted to determine whether the reduced sample of participants with CALS data after 30 months was unduly influencing the group contrasts from the full longitudinal models.
The second objective concerned the association between individual and family functioning and mood instability scores. We conducted separate repeated-measure mixed-effect regression models to evaluate whether Children’s Global Assessment Scale ratings and mother-rated CBQ (family conflict) ratings were associated with mean CALS total scores in the same assessment interval. Next, we examined the temporal relationship between baseline mood diagnosis and mother/offspring CBQ scores across intervals, with “lagged” CALS total scores (ie, ratings from the prior assessment interval) as the mediator. We fitted a structural equation model (Mplus Version 843) testing the significance of indirect effects and estimating 95% confidence intervals via Monte Carlo integration. All submodels in the structural model adjusted for values of the dependent variable from the previous assessment period. Continuous variables in the model were standardized (eMethods, Supplement 1, available online).
The third set of mixed-effect regression models assessed whether youth who were randomly assigned to FFT had decreasing levels of mood instability at follow-up compared to youth assigned to enhanced usual care. We examined both linear and quadratic trajectories of symptoms. Finally, in exploratory analyses of variance conducted at baseline and 12 months, we examined whether youth with more intensive medication regimens had higher CALS scores than youth with less intensive regimens (eMethods in Supplement 1, available online).
Power analyses were undertaken to examine whether the 2-group design could identify significant changes in symptom outcomes over 2 years, assuming a sample size of 150 and 20% attrition. For measures obtained every 4 to 6 months, we estimated 80% power to detect a change from no difference between treatment conditions at baseline to a difference of d = 0.57 SDs at end of treatment (α = 0.01). For effects of treatment group on PSRs averaged across follow-up intervals, we estimated 90% power to detect a constant difference of d = 0.50 (α = 0.01).
RESULTS
Sample Composition
Of 127 youth who entered the trial, baseline parent-rated CALS scores (covering 3 months before intake) were available for 114 (89.8%). The 114 youth were on average 13.3 ± 2.6 years of age (range, 9.0–17.8 years); 73 were girls (64.04%), 21 (18.4%) were persons of color, and 22 (19.3%) were of Hispanic ethnicity (Table 1). Baseline CALS questionnaires were completed by 93 mothers, 15 fathers, and 6 other relatives. The 114 youth with parent-rated CALS scores did not differ from the 13 without CALS scores on demographic or symptom variables. Of the 114 participants, 65 (57.0%) met DSM-IV-TR and DSM-5 criteria for MDD, whereas 49 (43.0%) met DSM-5 and Course and Outcome of Bipolar Youth criteria for OSBD. Youth with OSBD were no more likely to have comorbid internalizing or externalizing disorders than were youth with MDD (Table 1).
TABLE 1.
Demographic and Clinical Variables for Youth at High Risk for Bipolar Disorder (N = 114)
| Characteristic | Mean | SD |
|---|---|---|
| Age, y | 13.3 | 2.6 |
| Age at first mood symptoms, y | 11.5 | 2.8 |
| Socioeconomic status, class 1 to 5a | 3.9 | 0.9 |
| Young Mania Rating Scale, baseline (last 1 wk) | 12.5 | 7.0 |
| Children’s Depression Rating Scale, Revised, baseline (last 2 wk) | 46.6 | 14.2 |
| Children’s Global Assessment Scale, baseline (last 2 wk) | 54.4 | 10.1 |
| Children’s Global Assessment Scale, most severe past episode | 44.1 | 8.1 |
| A-LIFE Psychiatric Status Rating of Depression (scale of 1–6), baseline mean for 18 wk before intake | 3.7 | 1.0 |
| A-LIFE Psychiatric Status Rating of Hypo/mania (scale of 1–8), baseline mean for 18 wk before intake | 1.6 | 0.7 |
| n | % | |
| Sex, female Race | 73 | 64.0 |
| Race | ||
| African American | 8 | 7.0 |
| Asian | 9 | 7.9 |
| American Indian or Alaska Native | 1 | 0.9 |
| Native Hawaiian or Pacific Islander | 3 | 2.6 |
| White | 92 | 80.7 |
| Unknown | 1 | 0.9 |
| Hispanic ethnicity | 22 | 19.3 |
| Primary diagnosis | ||
| Major depressive disorder | 65 | 57.0 |
| Bipolar disorder, not otherwise specified | 49 | 43.0 |
| Comorbid disordersb | ||
| None | 17 | 14.9 |
| Internalizing disorders only | 40 | 35.1 |
| Externalizing disorders only | 25 | 21.9 |
| Internalizing and externalizing disorders | 32 | 28.1 |
| Baseline medicationsc | ||
| None | 49 | 43.0 |
| Antipsychotic | 28 | 24.6 |
| Anticonvulsant | 17 | 14.9 |
| Antidepressant | 43 | 37.7 |
| Anxiolytic | 3 | 2.6 |
| Psychostimulant/other ADHD agent | 24 | 21.1 |
| Family history of bipolar disorder | ||
| First-degree relatives only | 72 | 63.2 |
| Second-degree relatives only | 17 | 14.9 |
| First- and second-degree relatives | 25 | 21.9 |
Note: A-LIFE = Adolescent Longitudinal Interval Follow-Up Evaluation; ADHD = attention-deficit/hyperactivity disorder.
Higher values for socioeconomic status (Hollingshead—Redlich scale) indicate higher educational level and occupation.
Youth with internalizing disorders all had anxiety disorders; 3 youth also had eating disorders. Youth with externalizing disorders had ADHD, conduct disorder, or oppositional defiant disorder, alone or in combination. One child met the DSM-5 criteria for both major depressive disorder and disruptive mood dysregulation disorder.
Numbers do not sum to the sample size because participants could be taking more than 1 class of medication.
Of 114 participants, 54 were randomly assigned to FFT and 60 to enhanced usual care. At intake, 66 youth (57.9%) opted to take medications under supervision of a study psychiatrist; 48 (42.1%) did not opt for pharmacotherapy. Of the 114 participants with baseline CALS ratings, 105 (51 in FFT, 54 in EC) had at least one CALS rating at follow-up and were included in the longitudinal models (Consolidated Standards of Reporting Trials [CONSORT] diagram, Figure 1). Although we attempted to follow participants for 4 years, the average length of follow-up was 104.3 ± 65.8 weeks (range, 0–255 weeks).
FIGURE 1. Consolidated Standards of Reporting Trials (CONSORT) Diagram.
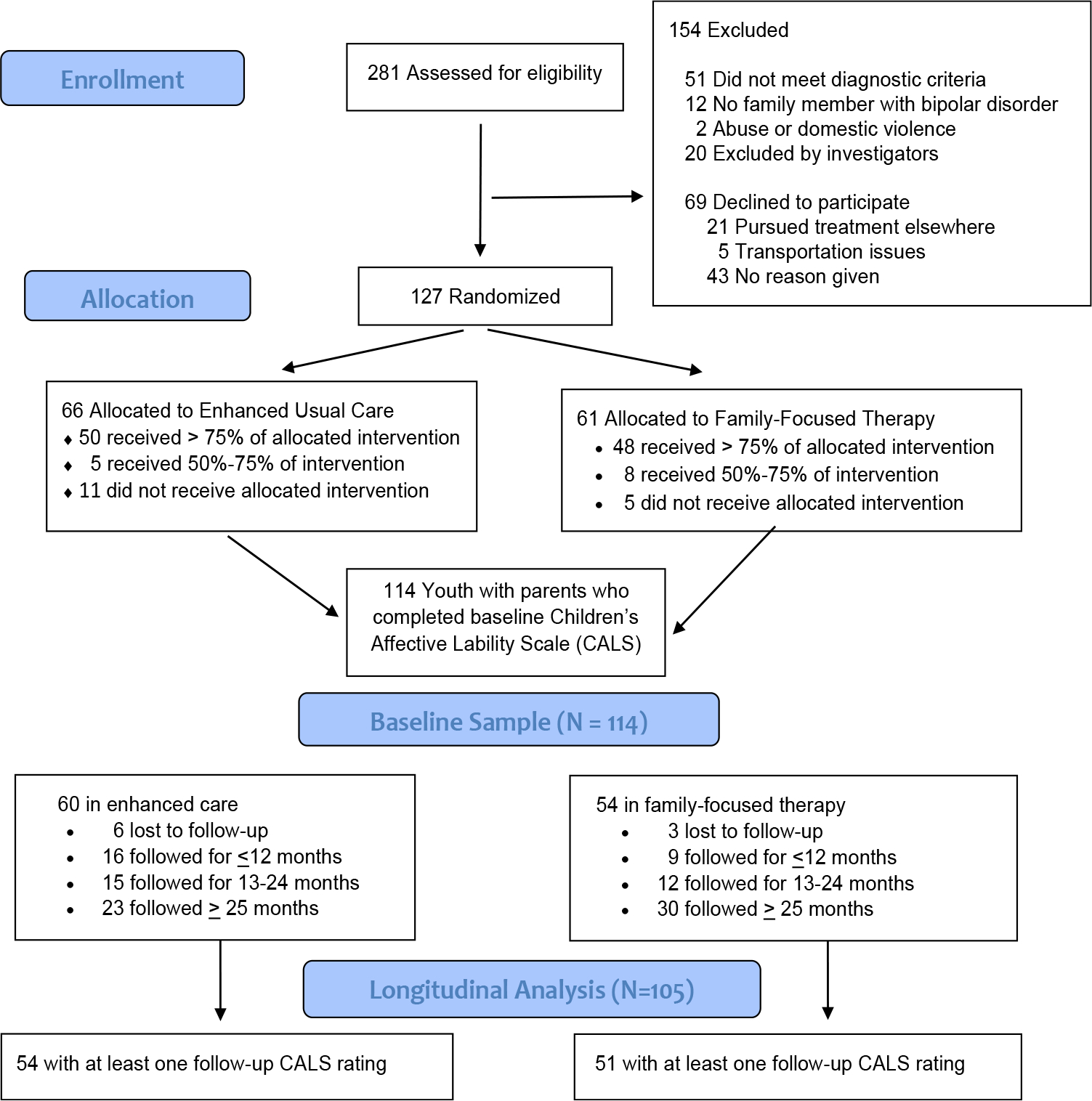
Note: Of 127 participants allocated to interventions, 56 were enrolled at UCLA, 44 at University of Colorado, and 27 at Stanford University Schools of Medicine.
Mood Instability, Demographic Variables, and Symptom Severity at Baseline
Baseline parent-rated CALS total scores did not covary with sex, race, or ethnicity of the youth. As expected, younger children had higher baseline CALS total scores than older children (r114 = −0.41, p < .0001). Among the CALS subscores, age was negatively correlated with CALS elevation/activation (r114 = −0.35, p < .0001) and irritability (r114 = −0.45, p < .0001) but not with anxiety–depression. Mean age of mood symptom onset was also negatively correlated with CALS total scores (r = −0.48, p < .0001) and subscores (all p < .0001).
Youth with OSBD had higher parent-rated CALS total scores at intake (F1,112 = 12.16, p < .001; partial ή2 = 0.10, 95% CI = 0.02–0.21), as well as higher CALS elevation/activation, irritability, and anxiety–depression subscores compared to youth with MDD (Table 2). There were no independent effects of comorbid disorders or interactions between mood diagnoses and comorbid disorders on CALS total scores or subscores. Mothers with diagnoses of BD I or II did not rate their offspring higher in CALS total scores or subscores than mothers without BD.
TABLE 2.
Relationship of Baseline Demographic and Clinical Variables to Children’s Affective Lability Scale Scores in Youth at High Risk for Bipolar Disorder (N = 114)
| Children’s Affective Lability Scale |
|||||
|---|---|---|---|---|---|
| Baseline mean (SD) | |||||
| Baseline variable | n | Total score | Elevation/activation | Irritability | Anxiety—depression |
| Male | 41 | 48.83 (13.60) | 14.29 (4.95) | 20.78 (7.34) | 9.73 (3.35) |
| Female | 73 | 48.86 (16.36) | 14.70 (5.89) | 19.60 (7.70) | 10.18 (3.29) |
| Major depressive disorder | 65 | 44.58 (13.01) | 12.94 (4.49) | 18.48 (7.12) | 9.40 (3.10) |
| Other specified bipolar disorder | 49 | 54.51 (16.51) | 16.69 (6.13) | 22.08 (7.71) | 10.84 (3.42) |
| Comorbid disorders | |||||
| None | 17 | 44.59 (14.76) | 13.82 (5.58) | 18.12 (7.90) | 8.88 (3.24) |
| Internalizing only | 40 | 46.83 (17.25) | 13.20 (5.20) | 18.60 (7.97) | 10.73 (3.79) |
| Externalizing | 25 | 50.72 (12.50) | 16.44 (5.53) | 20.64 (6.76) | 9.24 (2.62) |
| Internalizing and externalizing | 32 | 52.19 (14.94) | 15.17 (5.94) | 22.34 (7.09) | 10.34 (3.01) |
| Baseline Correlations | |||||
| Age | 114 | −0.42*** | −0.35*** | −0.45*** | −0.09 |
| Age at symptom onset | 88 | −0.48*** | −0.47*** | −0.42*** | −0.20 |
| PSR depression (18 wk before intake) | 111 | −0.08 | −0.12 | −0.11 | 0.11 |
| PSR (Hypo)mania (18 wk before intake) | 111 | 0.31** | 0.35** | 0.25* | 0.15 |
| Children’s Global Assessment Scale (prior 2 wk) | 87 | −0.24* | −0.16 | −0.20 | −0.28** |
| Mother-rated conflict behavior Questionnaire (prior mo) | 85 | 0.42*** | 0.32** | 0.54*** | 0.13 |
Note: The Children’s Affective Lability Scale (CALS) is rated by parents based on the 3 months before study intake. PSR = Psychiatric Status Rating. In univariate analyses of variance comparing group means (upper half of table), groups labeled with superscript letters “a” and “b” differ at p < .005. In univariate analyses of variance comparing group means (upper half of table), groups labeled with superscript letters “c” and “d” differ at p < .05.
p < .001
p < .01, and
p < .05 for baseline correlations.
Mood Instability Scores Over Time
Parent-rated CALS scores decreased between baseline and 30 months and then increased thereafter (quadratic effect: F1,298 = 19.80, p < .0001; linear effect, F1,112 = 48.79, p < .0001). The increases after 30 months are based on the scores of 22 participants (17.3% of sample) who were still being followed at that point.
As indicated in Figure 2, CALS total scores at each 4- to 6-month follow-up interval tracked closely with mean weekly PSRs for depression (F1,293 = 54.63, p < .0001) and hypo/mania (F1,293 = 15.47, p < .0001) calculated for the same intervals. In the same model, independent effects of mood diagnosis (ie, higher scores among youth with OSBD) (F1,293 = 5.67, p = .018), comorbid disorders (F3,293 = 3.05, p = .029), and age (F1,293 = 17.21, p < .0001) were observed on CALS total scores at follow-up. Post hoc tests indicated that youth who had both internalizing and externalizing comorbid disorders had higher average CALS total scores over time than youth who had no comorbid disorders (Tukey–Kramer, p < .05) (Table S2, Supplement 1, available online). There were no sex differences on the trajectory of CALS scores.
FIGURE 2. Parent-Rated Mood Instability and EvaluatorRated Psychiatric Status Ratings Among Youth at High Risk for Bipolar Disorder.
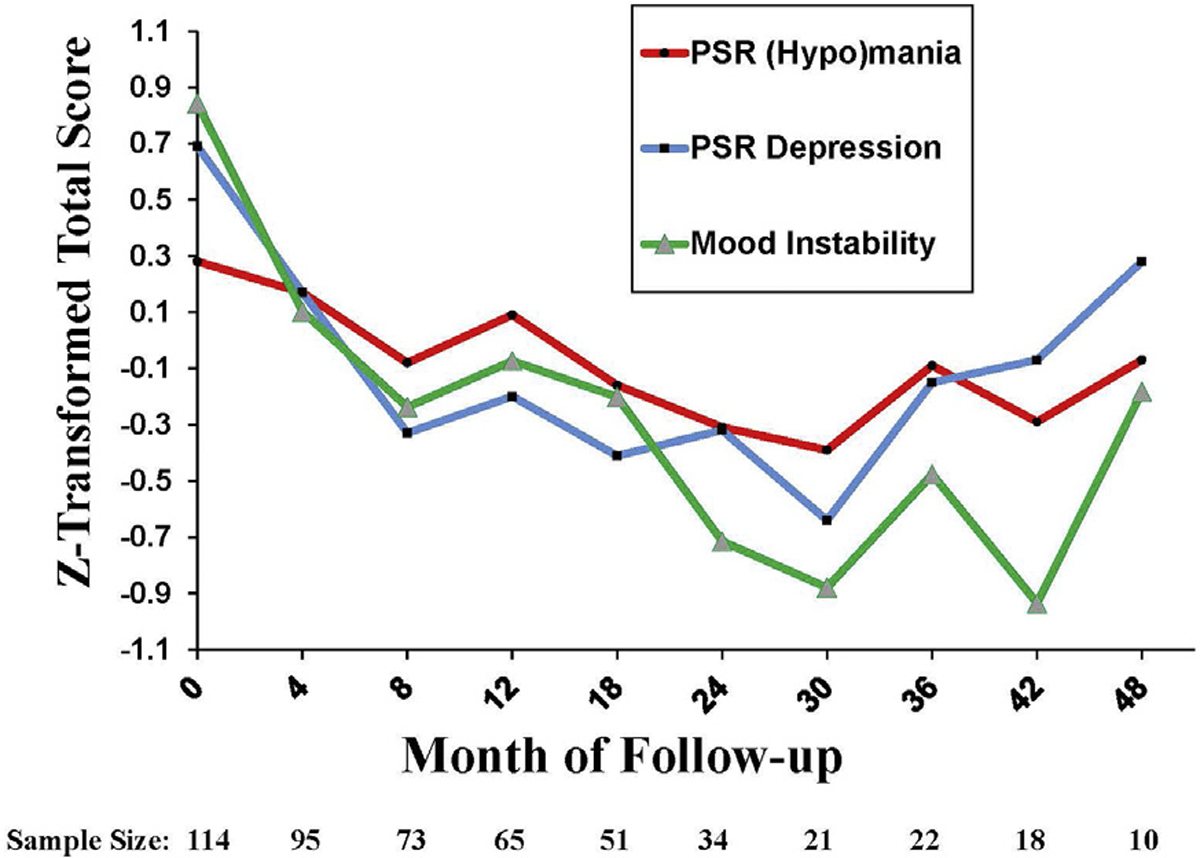
Note: A total of 114 participants had baseline mood instability (Children’s Affective Lability Scale [CALS]) scores. Of these, 105 participants had at least one follow-up period with both mood instability and PSR ratings and were included in longitudinal analyses. All scores were z transformed to allow comparability over time. PSR = Psychiatric Status Ratings from the Adolescent Longitudinal Interval Follow-up Evaluation.
Youth with OSBD were distinguished by higher CALS elevation/activation subscores (F1,296 = 13.02, p = .0004) and anxiety–depression subscores (F1,410 = 4.19, p = .04) at repeated follow-up intervals compared to youth with MDD, but the 2 diagnostic groups did not differ on CALS irritability subscores at follow-up. Youth with internalizing and externalizing comorbid disorders had higher irritability subscores than youth with no comorbid disorders (F3,410 = 4.06, p = .007; Tukey–Kramer comparison, p < .01). Sensitivity analyses comparing diagnostic groups and excluding data points collected beyond 30 months indicated that the sporadic increases in CALS scores after 30 months did not exert substantial influence on effects reported for the full longitudinal models (eResults in Supplement 1 and Figure S1, available online).
Psychosocial Treatments and Pharmacotherapy
In a mixed effect regression model, random assignment to FFT or enhanced usual care was not related to CALS total scores or subscores of the youth at follow-up, and there were no significant linear or quadratic interactions of treatment group with study visit. These results were not affected by including as covariates age, mood diagnosis, comorbid disorders, or PSR scores. Furthermore, the 2 groups did not differ in sensitivity analyses that considered only the first 30 months of follow-up (Figure S1, Supplement 1, available online).
At baseline, youth who were prescribed antipsychotics, mood stabilizers, anticonvulsants, antidepressants, anxiolytics, or anti-ADHD agents (n = 65) did not differ from youth who were not prescribed these agents (n = 49) in regard to CALS total scores. In the reduced sample available at 12 months, youth who were taking at least one psychiatric medication (n = 47) had higher CALS total scores than youth who were not taking any psychiatric medications (n = 18; F1,63 = 8.21, p = .006). Mood instability scores were also related to the number of medications prescribed at 12 months (mean = 1.48 ± 1.26; r65 = 0.31, p = .01). In addition, 12-month CALS scores were higher among 16 youths who were prescribed antipsychotics (F1,63 = 8.37, p = .005), and among 22 youth who were taking stimulant or nonstimulant ADHD agents (F1,63 = 6.38, p = .014) compared to youth who were not prescribed these medications.
Mood Instability as a Mediator of Psychosocial Functioning
In a repeated-measure mixed-effects model, the trajectory of Children’s Global Assessment of Functioning scores was closely associated with the trajectory of CALS total scores over time (F1,253 = 83.79, p < .0001). Lower global functioning scores were related to higher CALS elevation/activation, irritability, and anxiety–depression subscores over time (for all comparisons, p < .0001). In the domain of family conflict, mother-rated CBQ scores in the follow-up intervals were strongly related to CALS scores during the same intervals (F1,188 = 81.07, p < .0001), as well as mean weekly PSR depression scores (F1,188 = 12.61, p = .0005) and younger age (F1,188 = 8.37, p = .004). In addition, CBQ scores were higher in youth with OSBD than in youth with MDD throughout follow-up (F1,223 = 5.93, p = .016).
Next, we considered the temporal relationships among these variables. After controlling for lagged CALS scores and study visit, there was a significant effect of mood diagnosis on the hypothesized mediator, namely, CALS total scores, indicating that youth with OSBD had higher mood instability scores over time than youth with MDD (Z = 3.10, p = .002) (Figure 3). There was no interaction between diagnosis and baseline CALS scores on follow-up CALS scores. Furthermore, there were significant effects of lagged CALS scores (the mediator) on mother-rated CBQ scores such that youth with more mood instability in one assessment interval had higher mother/offspring conflict scores in the next interval (Z = 6.70, p < .001) (Figure 3). The indirect effect of primary mood diagnosis on CBQ scores at follow-up via the mediator (lagged CALS scores) was significant (Z = 2.88, p = .004, αβ = 0.19, 95% CI = 0.06–0.32).
FIGURE 3. Time-Lagged Model of Association Between Baseline Diagnosis, Children’s Affective Lability Scale (CALS) Scores, and Mother-Rated Conflict Behavior Questionnaire (CBQ) Scores in Youth at High Risk for Bipolar Disorder.
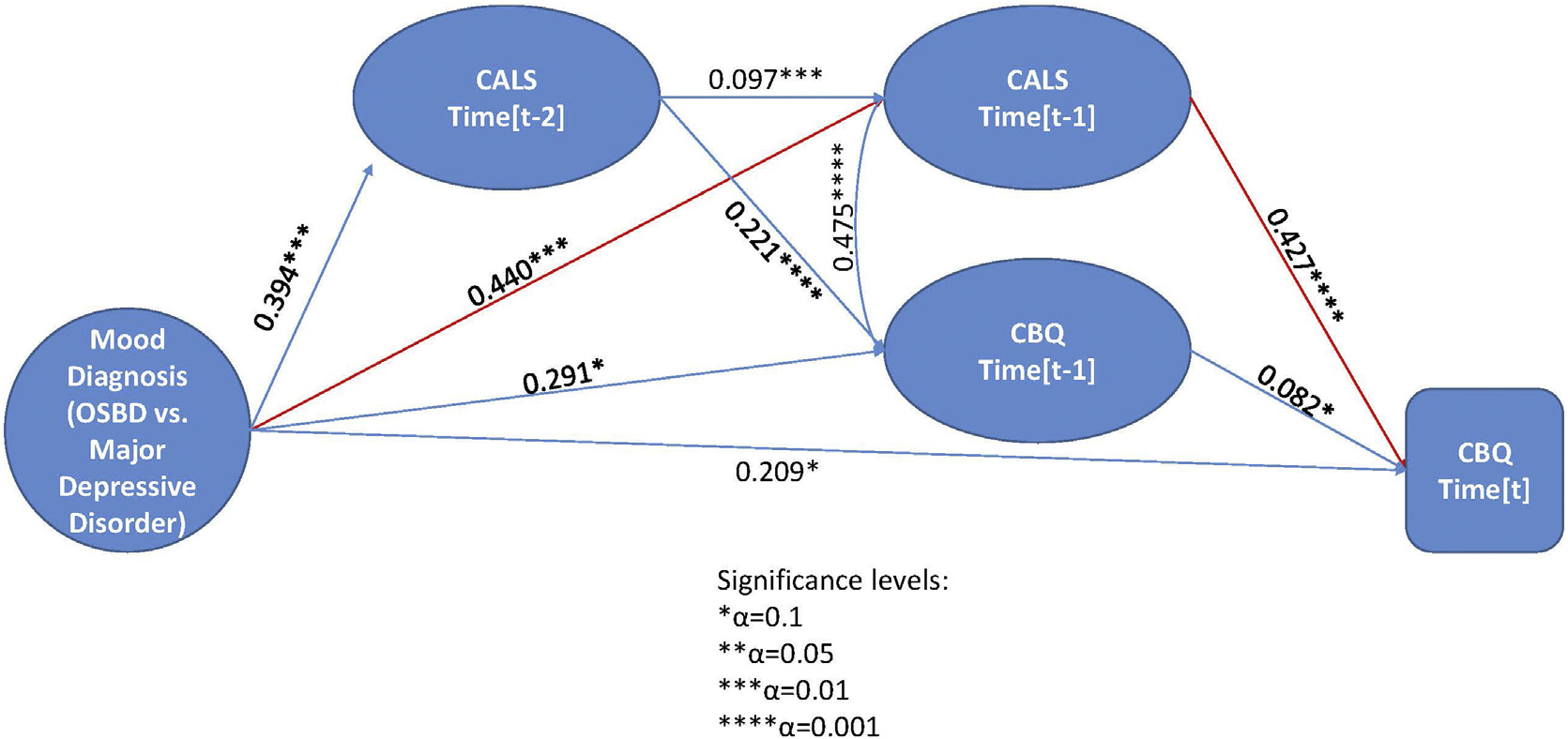
Note: CALS scores reflect parents’ ratings of the child’s mood instability in the prior 3 months, whereas CBQ scores reflect mothers’ perceptions of dyadic conflict with the offspring. Instruments were rated at baseline, every 4 months in year 1, and every 6 months thereafter, up to a possible 48 months of follow-up. Time = t refers to a current 4- to 6-month follow-up interval, whereas t-1 indicates the prior 4- to 6-month interval and t-2 refers to the interval before that one. MDD = major depressive disorder; OSBD = other specified bipolar disorder.
When covarying for lagged PSR depression and hypo/mania scores, CALS scores still significantly mediated the effect of baseline mood diagnosis on follow-up CBQ scores (indirect effect: Z = 2.41, p = .016, αβ = 0.20, 95% CI = 0.04–0.35). The direct effect of mood diagnosis on CBQ scores was nonsignificant after accounting for lagged CBQ scores (Z = 1.57, p = .10) and study visit, as well as the mediation effect of lagged CALS scores.
DISCUSSION
In this study of youth at high risk for BD, younger age, earlier symptom onset, a diagnosis of OSBD (vs MDD), complex comorbidities, and higher levels of depressive and hypo/manic symptoms were associated with higher parent-rated mood instability scores over time. When considering different types of mood instability, sudden onsets of elevated mood and activation (eg, bursts of silliness or giddiness, excessive familiarity with others) and anxiety–depression behaviors were more frequent among youth with OSBD than among those with MDD. However, fluctuating expressions of irritability were equally common over time in these 2 high risk presentations. Parent-reported irritability distinguished high-risk youth with combinations of externalizing and internalizing comorbid disorders from youth without complex comorbidities.
A secondary observation was the association of mood instability with individual child functioning and mother–offspring relationship conflict. We observed that youth with more mood instability in one assessment interval had higher mother–offspring conflict in the next interval. Furthermore, the association between a baseline diagnosis of OSBD (vs MDD) and higher mother–offspring conflict in a follow-up assessment interval was mediated by the child’s level of mood instability in the prior assessment interval. It is not surprising that youth who have sudden emotional outbursts or rapidly lose interest in activities have trouble maintaining friendships or interacting in a positive way with parents. In a similar vein, in adolescents with BD I or II, intermittent aggression was associated with poorer social functioning in teens, higher conflict in families, and lower quality of family relationships.11
In high-risk youth whose parents have mood disorders, dyadic child–parent conflict may reflect mood reactivity of the parent as well as the child. We did not observe associations between maternal lifetime diagnoses of BD I or II and their ratings of mood instability in the offspring. However, we did not measure mood instability among parents, or whether those parents with histories of BD I or II were in mood episodes at the time that they completed the CALS. Emotionally dysregulated youth with longer durations of exposure to parents with BD are at an increased risk for onset of mood disorders in adulthood.44
As previously reported in this trial, FFT with pharmacotherapy was associated with longer time until the emergence of new mood episodes and larger reductions in suicidal ideation and behavior among high-risk youth at follow-up, compared to enhanced usual care with pharmacotherapy.25,26 In the present study, we found that mood instability scores decreased longitudinally over the study period and did not differentially improve with FFT compared to enhanced usual care. Reductions in mood instability over time may reflect reductions in emotional reactivity as children age, which is consistent with findings from prior studies of mood instability,7,8 as well as the finding in this study that younger children had higher CALS scores at baseline.
Interestingly, CALS scores increased after 30 months in participants who remained in the study. Because this subgroup represented only 17.3% of the intent-to-treat sample, we could not determine whether increases in mood instability characterized youth with identifiable risk factors or whether these participants stayed in the study longer because their moods had not stabilized. Future studies may be able to determine whether youth whose mood instability scores increase over time are at especially high risk for onset of BD I or II compared to youth who show age- or treatment-related decreases.
When youth opted for pharmacotherapy as well as psychosocial interventions (57% of participants), study psychiatrists followed a standardized set of treatment guidelines.41 Medication regimens were not associated with mood instability scores at study entry. However, after 12 months of treatment, youth with higher mood instability scores were receiving higher-intensity regimens. Complex polypharmacy involving antipsychotic agents is often used to treat mood lability, regardless of the disorder.3,45 In high-risk youth, persistent mood lability associated with psychosocial impairment and family conflict may lead to more requests from parents for modifications of drug regimens. Because medication choices or doses were changed when clinically indicated, we could not evaluate whether specific regimens were more or less effective in treating mood instability.
The parent-rated CALS scale is not a diagnostic instrument, but is an efficient way of tracking symptom trajectories and psychosocial impairment in high-risk youth. Because it takes only 5 minutes for parents to complete and is easily hand scored, it will be considerably easier for clinicians to administer than Adolescent Longitudinal Interval Follow-up Evaluations or Young Mania Rating Scale interviews, which are lengthier and require extensive training. Additional clinical information may be gleaned from children’s self-reports of mood lability, which were not obtained in this study. Often, youth have insight into the environmental precipitants of their mood changes, which may include family conflicts, peer or school stressors, changes in sleep patterns, or interruption of daily routines. The clinical utility of questionnaire-based measures of mood instability compared to ecological momentary assessments, in which youth rate moods daily or weekly using smartphones, deserves exploration.
The present study has implications for treatment planning for youth at high risk for BD. A feasibility study found that adults with BD who had frequent inter-episode mood swings could be retained in a dialectical behavior therapy–informed group treatment, with reductions in affective lability over 9 months compared to usual care.46 In an open trial, youth with mood lability and a family history of BD who received an 8-week mindfulness intervention reported reductions in mood lability and less suppression of negative emotions over 3 months.47 Thus, psychological interventions that emphasize mindful meditation and distress tolerance may hold promise for high-risk children with mood instability. Future clinical trials should examine whether intervening specifically on mood instability in high-risk youth helps delay or prevent the onset of syndromal BD and enhances psychosocial functioning in adulthood.
Supplementary Material
Acknowledgments
Financial support for this study was provided by the National Institute of Mental Health (NIMH) grants R01 MH093676, R01MH093666, R34MH117200, and R01MH123575. The funding sources had no role in the design or conduct of the study; collection, management, analysis and interpretation of data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
The authors thank the following individuals for providing administrative support and study diagnostic or follow-up evaluations: Casey Armstrong, MA, Samantha Frey, BA, Brittany Matkevich, BA, and Margaret Van de Loo, BA (UCLA School of Medicine, Los Angeles, CA); Jennifer Pearlstein, MA and Aimee-Noelle Swanson, PhD (Stanford University, Stanford, CA); and Addie Bortz, MA, Anna Frye, BA, Zachary Millman, PhD, Rochelle Rofrano, BA, Aimee Sullivan, PhD, and Meagan Whitney, BA (University of Colorado, Boulder CO). The authors thank the following individuals for providing care for study patients: Danielle Denenny, PhD, Alissa Ellis, PhD, Marcy Forgey Borlik, MD, Danielle Keenan-Miller, PhD, Eunice Kim, PhD, Sarah Marvin, PhD, Lisa O’Donnell, PhD, and Robert Suddath, MD (UCLA School of Medicine); Victoria Cosgrove, PhD, Meghan Howe, LCSW, MSW, and Donna Roybal, MD (Stanford University); and Melissa Batt, MD, Emily Carol, PhD, Christopher Hawkey, PhD, Susan Lurie, MD, Dan Nguyen, MD, and Dawn Taylor, PhD (University of Colorado, Boulder). Independent fidelity ratings of therapy sessions were provided by Eunice Kim, PhD, UCLA School of Medicine. The following individuals provided consultation on study procedures, pharmacotherapy protocols, and statistical analyses: David Axelson, MD (Ohio State University Medical Center), Amy Garrett, PhD (University of Texas Health Science Center San Antonio); Antonio Hardan, MD (Stanford University); Michael Gitlin, MD (UCLA School of Medicine); Judy Garber, PhD (Vanderbilt University); and Gerhard Hellemann, PhD (University of Alabama at Birmingham).
The authors thank the members of the independent data safety monitoring board including Howard Markman, PhD (University of Denver, Denver, CO), Frederick Wamboldt, MD (University of Colorado Anschutz Medical Campus, Aurora, CO), and Charles Judd, PhD (University of Colorado, Boulder, CO). This study was reviewed and continuously approved by the medical institutional review boards of the UCLA Semel Institute, David Geffen School of Medicine at UCLA, Los Angeles, CA; the University of Colorado, Boulder and Anschutz Medical Campus, Aurora, CO; and Stanford University School of Medicine, Stanford, CA.
Footnotes
Disclosure: Dr. Miklowitz has received research support from NIMH, the Danny Alberts Foundation, the Attias Family Foundation, the Carl and Roberta Deutsch Foundation, the Kayne Family Foundation, AIM Youth Mental Health, and the Max Gray Fund; and book royalties from Guilford Press and John Wiley and Sons. Dr. Weintraub has received research support from NIMH and AIM Youth Mental Health. Dr. Singh has received research support from Stanford’s Maternal Child Health Research Institute and Stanford’s Department of Psychiatry and Behavioral Sciences, NIMH, the National Institute on Aging, the Patient Centered Outcomes Research Institute, Johnson and Johnson, and the Brain and Behavior Research Foundation. She has reported serving on the advisory board for Sunovion and Skyland Trail, as a consultant for Johnson and Johnson, and has previously consulted for X, moonshot factory, Alphabet Inc., and Limbix Health. She has received royalties from American Psychiatric Association Publishing and Thrive Global. Dr. Walshaw has received research support from Blue-bird Biotech and Second Sight. Dr. Birmaher has received research support from NIMH and royalties from Random House, UpToDate, and Lippincott, Williams and Wilkins. Dr. Chang has served as a consultant on the speaker’s bureau for Sunovion. Dr. Schneck has received research support from NIMH and the Ryan White Foundation. Mr. Merranko has reported no biomedical financial interests or potential conflicts of interest.
This article was reviewed under and accepted by Deputy Editor Elizabeth A. McCauley, PhD.
There are no linked research data sets for this article. Data will be made available through correspondence with the author within 12 months after publication of this article.
Contributor Information
David J. Miklowitz, Semel Institute for Neuroscience and Human Behavior, University of California, Los Angeles.
Marc J. Weintraub, Semel Institute for Neuroscience and Human Behavior, University of California, Los Angeles.
Manpreet K. Singh, Stanford University School of Medicine, California.
Patricia D. Walshaw, Semel Institute for Neuroscience and Human Behavior, University of California, Los Angeles.
John A. Merranko, University of Pittsburgh Medical Center, Pennsylvania.
Boris Birmaher, University of Pittsburgh Medical Center, Pennsylvania.
Kiki D. Chang, Private practice, Palo Alto. California.
Christopher D. Schneck, School of Medicine, University of Colorado Anschutz Medical Campus, Aurora.
REFERENCES
- 1.Stanislaus S, Faurholt-Jepsen M, Vinberg M, et al. Mood instability in patients with newly diagnosed bipolar disorder, unaffected relatives, and healthy control individuals measured daily using smartphones. J Affect Disord. 2020;271:336–344. 10.1016/j.jad.2020.03.049 [DOI] [PubMed] [Google Scholar]
- 2.Marwaha S, He Z, Broome M, et al. How is affective instability defined and measured? A systematic review. Psychol Med. 2014;44(9):1793–1808. 10.1017/S0033291713002407 [DOI] [PubMed] [Google Scholar]
- 3.Patel R, Lloyd T, Jackson R, et al. Mood instability is a common feature of mental health disorders and is associated with poor clinical outcomes. BMJ Open. 2015;5(5):e007504. 10.1136/bmjopen-2014-007504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koenigsberg HW. Affective instability: toward an integration of neuroscience and psychological perspectives. J Personal Disord. 2010;24:60–82. 10.1521/pedi.2010.24.1.60 [DOI] [PubMed] [Google Scholar]
- 5.Birmaher B, Goldstein BI, Axelson DA, et al. Mood lability among offspring of parents with bipolar disorder and community controls. Bipolar Disord. 2013;15(3):253–263. 10.1111/bdi.12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonsall MB, Wallace-Hadrill SM, Geddes JR, Goodwin GM, Holmes EA. Nonlinear time-series approaches in characterizing mood stability and mood instability in bipolar disorder. Proc Biol Sci. 2012;279(1730):916–924. 10.1098/rspb.2011.1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKnight R, Bilderbeck A, Miklowitz DJ, Hinds C, Goodwin GM, Geddes JR. Longitudinal mood monitoring in bipolar disorder: course of illness as revealed through a short messaging service. J Affect Disord. 2017;223:139–145. 10.1016/j.jad.2017.07.029 [DOI] [PubMed] [Google Scholar]
- 8.Birmaher B, Axelson D, Strober M, et al. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63(2):175–183. 10.1001/archpsyc.63.2.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faurholt-Jepsen M, Frost M, Busk J, et al. Is smartphone-based mood instability associated with stress, quality of life, and functioning in bipolar disorder? Bipolar Disord. 2019;21(7):611–620. 10.1111/bdi.12796 [DOI] [PubMed] [Google Scholar]
- 10.O’Donnell LA, Ellis AJ, Van de Loo MM, et al. Mood instability as a predictor of clinical and functional outcomes in adolescents with bipolar I and bipolar II disorder. J Affect Disord. 2018;236:199–206. 10.1016/j.jad.2018.04.0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keenan-Miller D, Peris T, Axelson D, Kowatch RA, Miklowitz DJ. Family functioning, social impairment, and symptoms among adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(10):1085–1094. 10.1016/j.jaac.2012.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez Algorta G, MacPherson HA, Youngstrom EA, et al. Parenting stress among caregivers of children with bipolar spectrum disorders. J Clin Child Adolesc Psychol. 2018; 47(suppl 1):S306–S320. 10.1080/15374416.2017.1280805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angst J, Gamma A, Endrass J. Risk factors for the bipolar and depression spectra. Acta Psychiatr Scand Suppl 2003;(418):15–19. 10.1034/j.1600-0447.108.s418.4.x [DOI] [PubMed] [Google Scholar]
- 14.Birmaher B, Merranko JA, Goldstein TR, et al. A risk calculator to predict the individual risk of conversion from subthreshold bipolar symptoms to bipolar disorder I or II in youth. J Am Acad Child Adolesc Psychiatry. 2018;57(10):755–763. 10.1016/j.jaac.2018.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hafeman DM, Merranko J, Goldstein TR, et al. Assessment of a person-level risk calculator to predict new-onset bipolar spectrum disorder in youth at familial risk. JAMA Psychiatry. 2017;74(8):841–847. 10.1001/jamapsychiatry.2017.1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lish JD, Dime-Meenan S, Whybrow PC, Price RA, Hirschfeld RM. The National Depressive and Manic-Depressive Association (NDMDA) survey of bipolar members. J Affect Disord. 1994;31(4):281–294. 10.1016/0165-0327(94)90104-x [DOI] [PubMed] [Google Scholar]
- 17.Angst J, Sellaro R, Stassen HH, Gamma A. Diagnostic conversion from depression to bipolar disorders: results of a long-term prospective study of hospital admissions. J Affect Disord. 2005;84(2–3):149–157. 10.1016/S0165-0327(03)00195-2 [DOI] [PubMed] [Google Scholar]
- 18.DelBello MP, Carlson GA, Tohen M, Bromet EJ, Schwiers M, Strakowski SM. Rates and predictors of developing a manic or hypomanic episode 1 to 2 years after a first hospitalization for major depression with psychotic features. J Child Adolesc Psychopharmacol. 2003;13(2):173–185. 10.1089/104454603322163899 [DOI] [PubMed] [Google Scholar]
- 19.Duffy A, Alda M, Crawford L, Milin R, Grof P. The early manifestations of bipolar disorder: a longitudinal prospective study of the offspring of bipolar parents. Bipol Disord. 2007;9(8):828–838. 10.1111/j.1399-5618.2007.00421.x [DOI] [PubMed] [Google Scholar]
- 20.Fiedorowicz JG, Endicott J, Leon AC, Solomon DA, Keller MB, Coryell WH. Subthreshold hypomanic symptoms in progression from unipolar major depression to bipolar disorder. Am J Psychiatry. 2011;168(1):40–48. 10.1176/appi.ajp.2010.10030328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geller B, Fox LW, Clark KA. Rate and predictors of prepubertal bipolarity during follow-up of 6- to 12-year-old depressed children. J Am Acad Child Adolesc Psychiatry. 1994; 33(4):461–468. 10.1097/00004583-199405000-00003 [DOI] [PubMed] [Google Scholar]
- 22.Geller B, Zimerman B, Williams M, Bolhofner K, Craney LL. Bipolar disorder at prospective follow-up of adults who had prepubertal major depressive disorder. Am J Psychiatry. 2001;158(1):125–127. 10.1176/appi.ajp.158.1.125 [DOI] [PubMed] [Google Scholar]
- 23.Kochman FJ, Hantouche EG, Ferrari P, Lancrenon S, Bayart D, Akiskal HS. Cyclothymic temperament as a prospective predictor of bipolarity and suicidality in children and adolescents with major depressive disorder. J Affect Disord. 2005;85(1–2):181–189. 10.1016/j.jad.2003.09.009 [DOI] [PubMed] [Google Scholar]
- 24.Nadkarni RB, Fristad MA. Clinical course of children with a depressive spectrum disorder and transient manic symptoms. Bipolar Disord. 2010;12(5):494–503. 10.1111/j.1399-5618.2010.00847.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miklowitz DJ, Schneck CD, Walshaw PD, et al. Effects of family-focused therapy vs enhanced usual care for symptomatic youths at high risk for bipolar disorder: a randomized clinical trial. JAMA Psychiatry. 2020;77(5):455–463. 10.1001/jamapsychiatry.2019.4520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miklowitz DJ, Merranko JA, Weintraub MJ, et al. Effects of family-focused therapy on suicidal ideation and behavior in youth at high risk for bipolar disorder. J Affect Disord. 2020;275:14–22. 10.1016/j.jad.2020.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (Text Revision) (DSM-IV-TR). American Psychiatric Press; 2000. [Google Scholar]
- 28.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (5th ed., DSM-5). American Psychiatric Press; 2013. [Google Scholar]
- 29.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity, and sensitivity. Br J Psychiatry. 1978;133:429–435. 10.1192/bjp.133.5.429 [DOI] [PubMed] [Google Scholar]
- 30.Poznanski EO, Mokros HB. Children’s Depression Rating Scale, Revised (CDRS-R) Manual. Western Psychological Services; 1995. [Google Scholar]
- 31.Axelson DA, Birmaher B, Strober M, et al. Phenomenology of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63(10):1139–1148. 10.1001/archpsyc.63.10.1139 [DOI] [PubMed] [Google Scholar]
- 32.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- 33.Kaufman J, Birmaher B, Axelson D, Perepletchikova F, Brent D, Ryan N. Kiddie Schedule for Affective Disorders and Schizophrenia, Present and Lifetime Version, 2013 (K-SADS-PL 2013). Western Psychiatric Institute and Clinic; 2013. [Google Scholar]
- 34.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33; https://www.ncbi.nlm.nih.gov/pubmed/9881538 [PubMed] [Google Scholar]
- 35.Weissman MM, Wickramaratne P, Adams P, Wolk S, Verdeli H, Olfson M. Brief screening for family psychiatric history: the Family History Screen. Arch Gen Psychiatry. 2000;57(7):675–682. 10.1001/archpsyc.57.7.675 [DOI] [PubMed] [Google Scholar]
- 36.Gerson AC, Gerring JP, Freund L, et al. The Children’s Affective Lability Scale: a psychometric evaluation of reliability. Psychiatr Res 1996;65(3):189–198. 10.1016/s0165-1781(96)02851-x [DOI] [PubMed] [Google Scholar]
- 37.Keller MB, Lavori PW, Friedman B, et al. The longitudinal interval follow-up evaluation: a comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44:540–548. 10.1001/archpsyc.1987.01800180050009 [DOI] [PubMed] [Google Scholar]
- 38.Shaffer D, Gould MS, Brasic J, et al. A Children’s Global Assessment Scale (CGAS). Arch Gen Psychiatry. 1983;40:1228–1231. 10.1001/archpsyc.1983.01790100074010 [DOI] [PubMed] [Google Scholar]
- 39.Prinz RJ, Foster SL, Kent RN, O’Leary KD. Multivariate assessment of conflict in distressed and nondistressed mother-adolescent dyads. J Appl Behav Anal. 1979;12: 691–700. 10.1901/jaba.1979.12-691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Begg CB, Iglewicz B. A treatment allocation procedure for sequential clinical trials. Biometrics. 1980;36(1):81–90; http://www.ncbi.nlm.nih.gov/pubmed/7370375 [PubMed] [Google Scholar]
- 41.Schneck CD, Chang KD, Singh MK, DelBello MP, Miklowitz DJ. A pharmacologic algorithm for youth who are at high risk for bipolar disorder. J Child Adolesc Psychopharmacol. 2017;27(9):796–805. 10.1089/cap.2017.0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ger D, Everitt BS. Handbook of Statistical Analyses Using SAS. second ed. CRC Press; 2001. [Google Scholar]
- 43.Muthén LK, Muthén BO. Mplus Statistical Analysis with Latent Variables: User’s Guide (Version 8). Muthén & Muthén; 2017. [Google Scholar]
- 44.Doucette S, Levy A, Flowerdew G, et al. Early parent-child relationships and risk of mood disorder in a Canadian sample of offspring of a parent with bipolar disorder: findings from a 16-year prospective cohort study. Early Interv Psychiatry. 2016;10(5):381–389. 10.1111/eip.12195 [DOI] [PubMed] [Google Scholar]
- 45.Goldberg JF, Brooks JO 3rd, Kurita K, et al. Depressive illness burden associated with complex polypharmacy in patients with bipolar disorder: findings from the STEP-BD. J Clin Psychiatry. 2009;70(2):155–162. 10.4088/jcp.08m04301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wright K, Dodd AL, Warren FC, et al. Psychological therapy for mood instability within bipolar spectrum disorder: a randomised, controlled feasibility trial of a dialectical behaviour therapy-informed approach (the ThrIVe-B programme). Int J Bipolar Disord. 2021;9(1):20. 10.1186/s40345-021-00226-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hafeman DM, Ostroff AN, Feldman J, et al. Mindfulness-based intervention to decrease mood lability in at-risk youth: preliminary evidence for changes in resting state functional connectivity. J Affect Disord. 2020;276:23–29. 10.1016/j.jad.2020.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


