Abstract
Bile acids (BAs), produced in the liver and further transformed in the gut, are cholesterol-derived molecules involved in essential physiological processes. Recent studies suggest that BAs regulate T helper 17 cell function, but the underlying mechanism of this action and their therapeutic value in disease models remains unclear. Using an IL-23 minicircle DNA–based murine model of psoriasiform dermatitis, we showed that oral administration of secondary BAs, including lithocholic acid (LCA), deoxycholic acid, and 3-oxoLCA, significantly improved psoriasiform dermatitis without inducing apparent hepatotoxicity. Of the BAs tested, LCA possessed the greatest potency in treating psoriasiform dermatitis. Intravenous administration of LCA at a much lower dosage (compared with oral treatment) showed a comparable antipsoriatic effect and markedly suppressed the IL-17A response. Ex vivo experiments revealed that LCA reduced IL-17A production in IL-23-stimulated murine T cells in the absence of BA receptors TGR5 or FXR. Strikingly, BAs inhibited CCL20 expression in keratinocytes, which led to reduced migration of CCR6-expressing Jurkat cells cultured in the conditioned medium of stimulated keratinocytes. Thus, BAs improve psoriasiform dermatitis with minimal toxicity via direct inhibition of IL-17A production and blockade of CCL20-mediated trafficking, supporting the potential use of BAs in psoriasis.
INTRODUCTION
Psoriasis is a chronic inflammatory skin disorder that affects up to 3% of the global population (Boehncke and Schön, 2015), with the IL-23/IL-17 immunologic pathway playing a central role in promoting disease onset and perpetuation. Data from in vitro and animal studies indicate that IL-17A, the critical effector cytokine in this pathway, is the principal driver of psoriatic lesion changes (Blauvelt and Chiricozzi, 2018). Overexpression of IL-17A accelerates the proliferation of epidermal keratinocytes (KCs) and stimulates the production of chemokines such as CCL20, which in turn recruits CCR6+ T helper (Th)17 cells and gamma-delta (γδ) T cells into the skin and eventually results in an overly robust inflammatory response.
Bile acids (BAs) are cholesterol-derived natural surfactants that are produced in the liver and secreted into the duodenum (Jia et al., 2018). Although most BAs are transported back into the liver via enterohepatic circulation, a small fraction of this pool escapes reabsorption in the ileum and is subjected to further bacterial transformation in the colon, producing secondary BAs. The principal human primary BAs, chenodeoxycholic acid and cholic acid, are converted to the secondary BAs, lithocholic acid (LCA) and deoxycholic acid (DCA), respectively. Apart from their pivotal role in dietary lipid absorption and cholesterol homeostasis, BAs are increasingly recognized as signaling molecules that regulate immune homeostasis, particularly in the innate immune system (Guo et al., 2016). Nuclear receptors FXR and GPBAR (also known as TGR5) are considered the two most dedicated BA receptors that bind to BAs with high affinity and transduce an array of anti-inflammatory signaling such as suppression of NF-κB pathways (Chen et al., 2019). Recent studies highlight a novel regulatory role of BAs in T-cell homeostasis (Campbell et al., 2020; Song et al., 2020). Supplementation of BAs or engineered bacteria that produce BAs promoted colonic regulatory T cells (Tregs) and ameliorated host susceptibility to inflammatory colitis (Campbell et al., 2020; Song et al., 2020). In addition, an LCA derivative, 3-oxoLCA, has been reported to suppress Th17 differentiation (Hang et al., 2019). These results indicate that BAs could be a potential target in drug development for Th17-mediated inflammatory disease.
In this study, using an IL-23 minicircle DNA (MC)–based murine model of psoriasiform dermatitis (PsD), we showed that several secondary BAs significantly inhibited skin inflammation without causing apparent systemic adverse effects. The antipsoriatic effect of BAs was mediated by direct inhibition of IL-17A production in T cells and blockade of CCR6-CCL20–mediated trafficking.
RESULTS
Oral administration of BAs inhibits PsD
We first asked if BAs can inhibit psoriatic inflammation using an IL-23 overexpressing mouse model induced by hydrodynamic delivery of IL-23 MC initially described by Adamopoulous et al. (2011). The IL-23 MC-induced skin inflammation phenocopies important features of psoriasis, including scaling, erythema, acanthosis, and infiltration of inflammatory cells (Shi et al., 2020). On day 0, a single injection of IL-23 MC was delivered via tail vein to induce inflammation. LCA, DCA, or 3-oxoLCA was then administered via daily oral gavage (Figure 1a). Mice treated with BAs neither exhibited weight loss relative to those fed with vehicle (Figure 1b) nor showed any signs of hepatotoxicity as determined by both histology and serum levels of alanine aminotransferase and aspartate aminotransferase (Supplementary Figure S1a and b). On day 10, serum levels of IL-23 were comparable among all experimental groups (Figure 1c), suggesting that BA treatment did not impair the production of IL-23 by the liver.
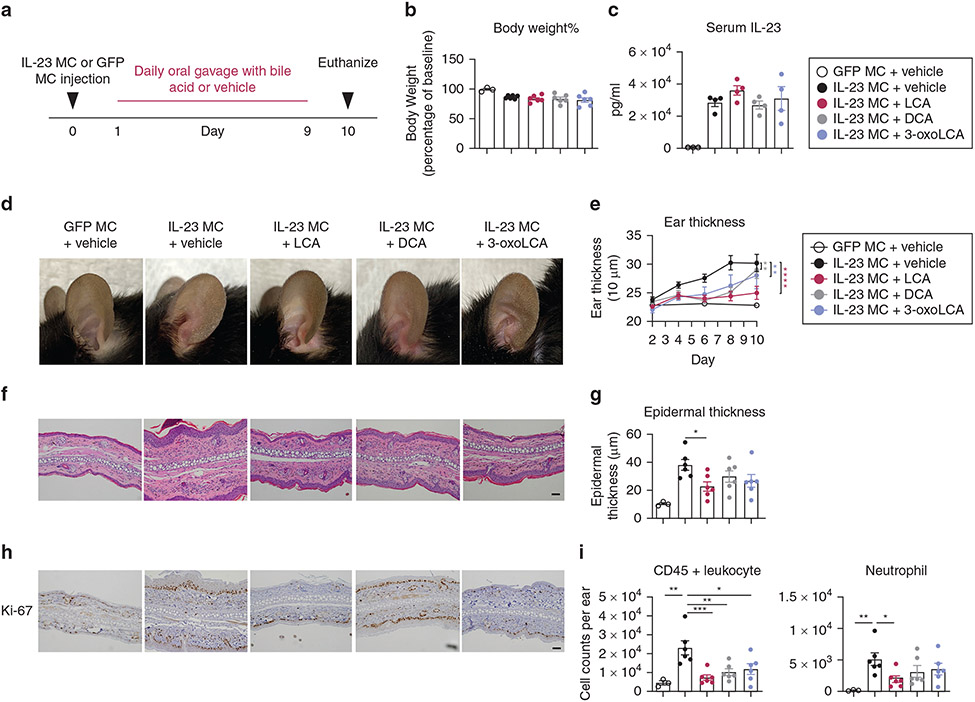
Figure 1. Oral administration of bile acids ameliorates IL-23 MC-induced psoriasiform dermatitis.
(a) Schematic illustration of experimental protocols. Mice were orally administered PBS vehicle or BAs at a dose of 30 mg/kg/per day for 9 consecutive days beginning the day after MC was delivered. (b) percentage of baseline body weight, (c) serum level of IL-23, (d) representative photographs, (e) time course of ear thickness, (f) image of H&E section (Bar = 50 μm), (g) histological analysis of epidermal thickness, (h) representative immunohistochemical images of Ki-67 (Bar = 50 μm) and (i) absolute numbers of CD45+ leukocytes and neutrophils in the ear skin (n = 3–6). Data are presented as mean ± SEM. Data are representative of two independent experiments. * P < 0.05, **P < 0.01. ***P < 0.001, by two-way ANOVA with Bonferroni’s test compared with IL-23 MC + vehicle group in (e) and by one-way ANOVA with Dunnett’s test compared with IL-23 MC + vehicle group in (g, i). BA, bile acid; LCA, lithocholic acid; MC, minicircle DNA.
All BA-treated mice showed markedly less erythema, scaling, and ear thickness (Figure 1d and e). Among the three tested BAs, LCA presented the most potent effect as measured by reductions in ear swelling. In agreement with the clinical phenotype, BAs decreased epidermal thickness although only LCA reached statistical significance (Figure 1f and g). Immunohistochemistry analysis revealed dampened nuclear staining of Ki-67 in the epidermis from the BA-treated group, suggesting a lower proliferation rate of KCs (Figure 1h). Flow cytometry analysis showed less infiltration of CD45+ leukocytes in the ear skin from all groups treated with Bas, whereas only LCA significantly suppressed the infiltration of neutrophils (Figure 1i). We then quantified BA levels in the skin and serum from mice injected with GFP MC or IL-23 MC by liquid chromatography-mass spectrometry. Notably, IL-23 MC-injected mice exhibited lower levels of multiple BAs in the skin and serum than normal control (Supplementary Table S1 and Supplementary Figure S2), suggesting that overexpression of IL-23 might change the metabolism of BAs and supporting our hypothesis that supplementation of BAs could improve skin inflammation.
To further confirm the therapeutic effect of BAs on PsD, we used an alternative model of psoriasis, the imiquimod-induced model. Because LCA exhibited the most potent effect in the IL-23 MC model, we used LCA in subsequent animal experiments. LCA was orally administered daily 2 days before imiquimod application (Supplementary Figure S3a). Similar to what we observed in the IL-23 MC model, mice treated with LCA had less skin inflammation as assessed by clinical, histological, and molecular parameters without loss of body weight (Supplementary Figure S3b-h). Collectively, these data suggest that the secondary BAs, especially LCA, attenuate PsD in mice without obvious hepatotoxicity or other systemic adverse effects.
Intravenous administration of LCA ameliorates PsD
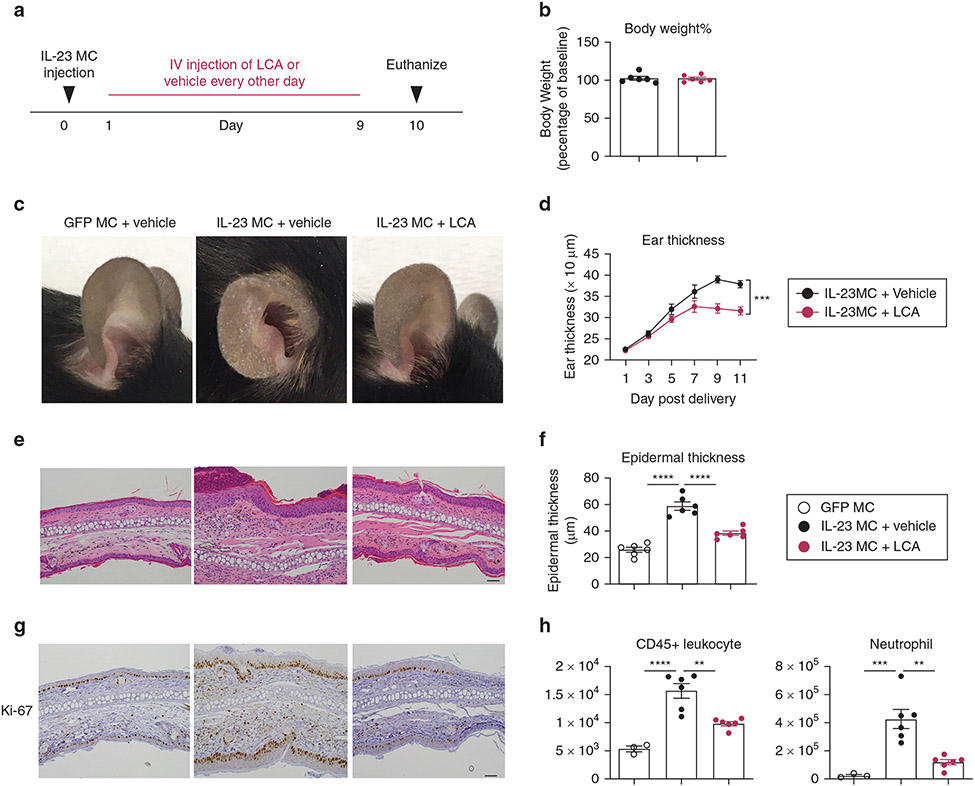
Oral administration of BA supplements has been reported to cause gut reactions such as diarrhea, constipation, stomach pain, and vomiting (Camilleri and Vijayvargiya, 2020; Ely, 2018). To minimize the potential gut irritation, we next explored if intravenous (i.v.) injection of LCA at a lower dosage could achieve similar antipsoriatic effects in mice. IL-23 MC-treated mice were given LCA through tail vein at a much lower dose compared with the protocol for oral treatment (4 mg/kg every other day vs. 30 mg/kg/day) (Figure 2a). No loss of body weight or other adverse effects were observed in the LCA-treated group throughout the experiment (Figure 2b). Similar to oral administration, i.v. injection of LCA markedly improved scaling and ear swelling (Figure 2c and d), attenuated epidermal thickening (Figure 2e and f), decreased the intensity of Ki-67 staining (Figure 2g), and inhibited the infiltration of CD45+ leukocytes and neutrophils (Figure 2h). Together, these results suggest that the i.v. use of LCA at lower dosage was as effective as oral administration in treating PsD.
Figure 2. Intravenous administration of LCA ameliorates IL-23 MC-induced PsD.
(a) Schematic illustration of experimental protocols. Mice were administered i.v. PBS vehicle or LCA at a dose of 4 mg/kg/per day every other day beginning the following day after MC was delivered. (b) Percentage of baseline body weight, (c) representative photographs, (d) time course of ear thickness, (e) image of H&E section (Bar = 50 μm), (f) histological analysis of epidermal thickness, (g) representative images of Ki-67 immunohistochemistry (Bar = 50 μm) and (h) absolute numbers of CD45+ leukocytes and neutrophils in the ear skin. Data are presented as mean ± SEM (n = 3–6). Data are representative of two independent experiments. * P < 0.05, **P < 0.01. ***P < 0.001, by two-way ANOVA with Bonferroni post hoc in (d) and by one-way ANOVA with Dunnett’s test compared with IL-23 MC + vehicle group in (f, h). LCA, lithocholic acid; MC, minicircle DNA.
BAs suppress IL-17A response in vivo
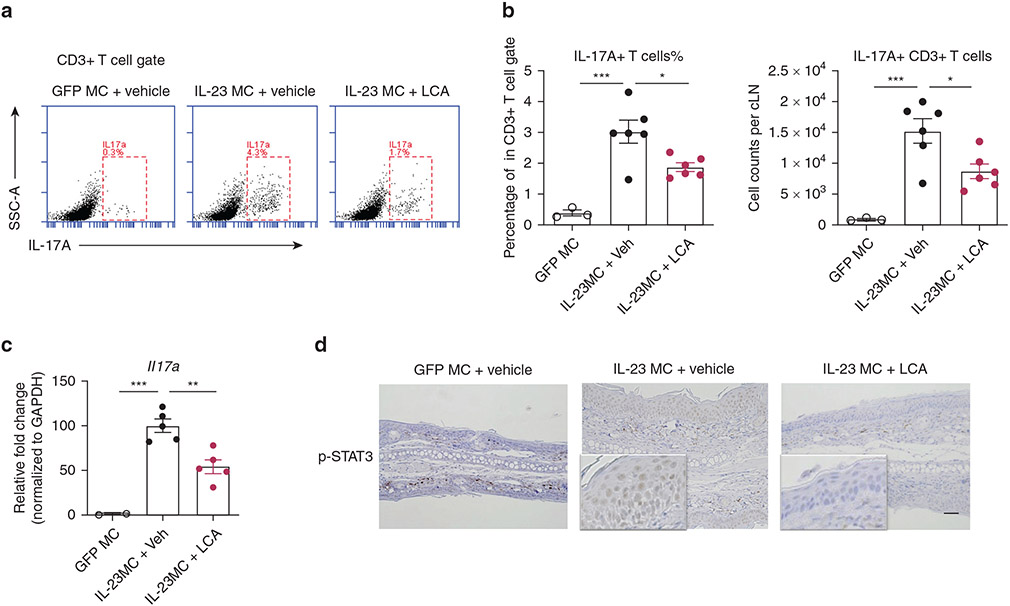
IL-17A has been consistently found to be elevated in psoriatic lesional skin, and therapeutic antibodies to IL-17 have demonstrated efficacy in treating psoriasis (Blauvelt and Chiricozzi, 2018). As expected, the frequency and an absolute number of IL-17A-producing T cells were remarkably increased in the cervical lymph nodes (cLNs) of IL-23 MC-injected mice, and these cells were then substantially decreased in mice with i.v. injections of LCA (Figure 3a and b). RT-qPCR analysis showed fewer transcripts of Il17a in the ear skin of LCA-treated mice (Figure 3c). In addition, the epidermis of LCA-treated mice had reduced phosphorylated signal transducer and activator of transcription (STAT) 3, a transcription factor involved in Th17-mediated inflammation and cell differentiation in comparison with that of untreated mice (Hillmer et al., 2016) (Figure 3d). Similar results showing inhibited phosphorylated levels of STAT3 were obtained in IL-23 MC-injected mice treated with LCA by oral administration (data not shown). Thus, LCA-mediated improvement in skin inflammation was accompanied by a repressed IL-17A response.
Figure 3. LCA suppresses IL-17A response in IL-23 MC-induced PsD.
(a) Representative flow cytometry plots, (b) percentage and absolute numbers of IL-17A–producing T cells in cervical lymph nodes from mice with treatment in Figure 2. (c) Transcripts of Il17a in ear skin and (d) representative immunohistochemical images of p-STAT3 in ear skin (Bar = 50 μm) (n = 3–6). Data are presented as mean ± SEM. Data are representative of two independent experiments. *P < 0.05, **P < 0.01. ***P < 0.001, by one-way ANOVA with Dunnett’s test compared with IL-23 MC + vehicle group in (b, c). LCA, lithocholic acid; LN, lymph node; MC, minicircle DNA.
BAs control not only Th17 but also Treg cell differentiation (Campbell et al., 2020). In patients with psoriasis, the frequency of Tregs is altered in both lesional skin and peripheral blood (Nussbaum et al., 2021). Immunohistochemistry staining, however, revealed comparable infiltration of Foxp3-positive Treg cells in the ear between vehicle-treated or LCA-treated mice (Supplementary Figure S4). Moreover, there was no difference in the ears between the two groups regarding the mRNA levels of Treg-related markers including Foxp3 and Tgfb. In cLNs, LCA failed to affect the accumulation of Tregs as assessed by both flow cytometry and immunohistochemistry. These data suggested that LCA was unlikely to exert significant effects on Tregs in the IL-23 MC-injected mice model.
BAs inhibit IL-17A production in T cells with suppression of Rorc in vitro
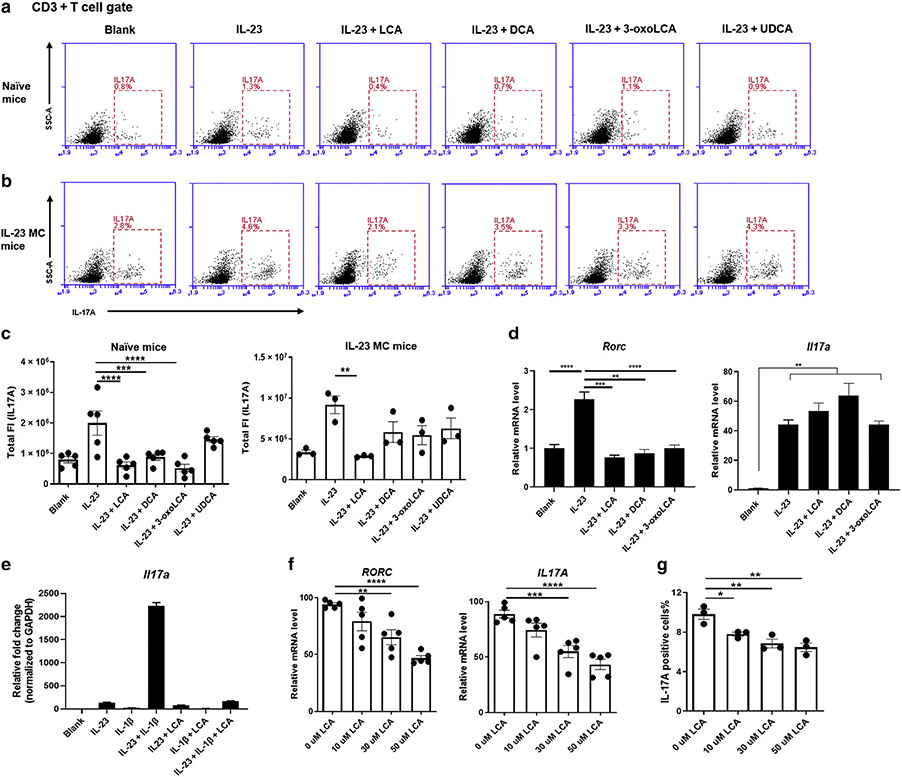
Recent studies have uncovered a novel role for BAs in controlling the immune response by regulating the differentiation of Th17 cells (Hang et al., 2019). We hypothesized that BAs may improve PsD by inhibiting IL-17A production in T cells. Given the fact that γδ T cells rather than CD4+ T cells are the predominant cellular source of IL-17A in murine models of PsD (Mabuchi et al., 2011), we stimulated cLNs cells in vitro with IL-23, a cytokine that drives differentiation of γδ17 T cells (Papotto et al., 2018), instead of using conventional Th17 cell differentiation conditions. Treatment with LCA, DCA, or 3-oxoLCA significantly suppressed the potential to produce IL-17A in CD3+ T cells, whereas ursodeoxycholic acid, also a secondary BA, only showed a marginal suppressive effect (Figure 4a). DCA induced higher rates of apoptotic cLNs cells at 24 hours after stimulation with IL-23, whereas no obvious difference was observed among groups treated with vehicle, LCA, 3-oxoLCA, or ursodeoxycholic acid (Supplementary Figure S5).
Figure 4. LCA inhibits IL-17A production by T cells in vitro.
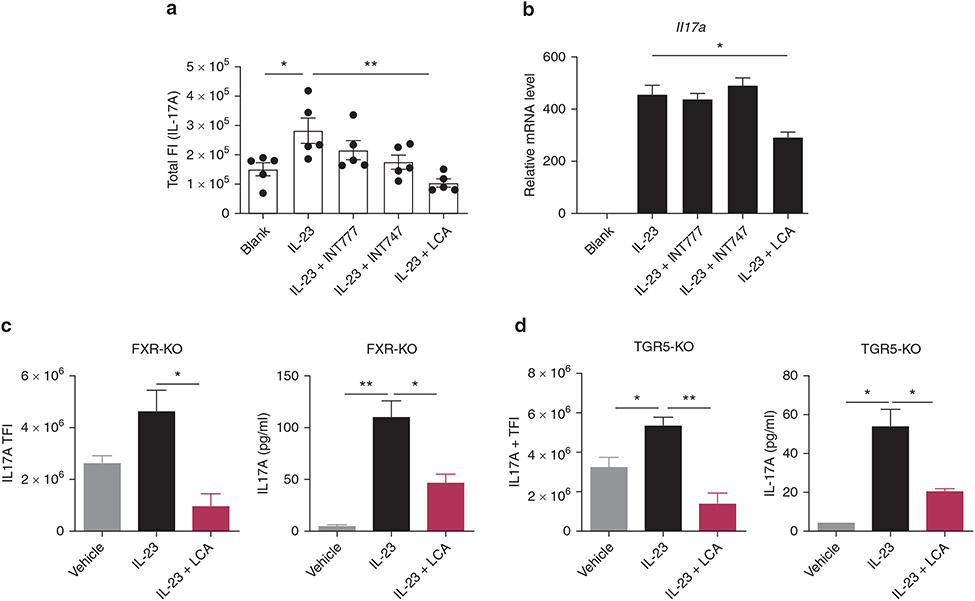
(a, b) Representative flow cytometry plots of CD3+ T cells expressing IL-17A from the cLN of naive mice (a) or IL-23 MC-injected mice (b), after 24 hours incubation in the presence of IL-23 (100 ng/ml) with indicated BAs (50 μM). (c) Total fluorescence intensity of IL-17A in CD3+ T cells with particular treatment indicated. (d) Transcripts of Rorc and Il17a in cLN cells, after 3 hours incubation in the presence of IL-23 (100 ng/ml) with indicated BAs (50 μM) (n = 3). (e) Transcripts of Il17a in cLN cells, after 12 hours incubation in the presence of IL-23 (50 ng/ml) and IL-1β (10 ng/ml) with LCA (50 μM). (f) Transcripts of IL17A and RORC analyzed by qRT-PCR (from five individual donors) and (g) positivity of IL-17A analyzed by flow cytometry in healthy adult human CD4+CCR6+ T cells are inhibited by coculture with LCA overnight in a dose-dependent manner (from three individual donors). Data are presented as mean ± SEM. Data are representative of two independent experiments. *P < 0.05, **P < 0.01. ***P < 0.001. BA, bile acid; cLN, cervical lymph node; DCA, deoxycholic acid; FI, fluorescence intensity; LCA, lithocholic acid; SSC, side scatter; UDCA, ursodeoxycholic acid.
To further examine the effect of BAs on primed T cells, we applied a similar protocol using cLN cells from IL-23 MC-injected mice. In agreement with in vivo studies, only LCA efficiently inhibited IL-17A production (Figure 4b and c). As early as 3 hours after coculture initiation, BAs had already dramatically inhibited the transcription of Rorc, a gene encoding RORγt, which is the pivotal transcription factor in Th17 differentiation (Capone and Volpe, 2020), while the mRNA of Il17a remained unchanged at this time point (Figure 4d). At 24 hours after coculture, the transcripts of both Rorc and Il17a were both profoundly inhibited (Supplementary Figure S6). Previous reports have demonstrated that treatment with both IL-23 and IL-1β induces prominent IL-17A production in γδT cells relative to IL-23 alone (Sutton et al., 2009). As expected, dual treatment with IL-23 and IL-1β led to much higher mRNA levels of Il17a in naive cLN cells, which was profoundly blocked by LCA (Figure 4e). Together, these observations showed the inhibition of Rorc occurred before the suppression of Il17a in cLN cells, indicating that BAs may control IL-17A production via transcriptional regulation of RORγt as previously reported (Hang et al., 2019).
To mimic the crosstalk between immune cells from draining lymph nodes (LNs) and skin, we created an in vitro model consisting of skin fragments and single-cell suspension of cLN cells, followed by IL-23 stimulation overnight. The skin-LN coculture assay expressed much higher levels of Th17-related cytokines than single skin cultures (Supplementary Figure S7a). Remarkably, LCA repressed the transcripts of Th17-related cytokines in cultured tissue as well as the levels of IL-17A proteins in the supernatants (Supplementary Figure S7b and c). γδT cells are the predominant cellular source of IL-17A in murine models of PsD (Qi et al., 2021), whereas in humans, CD4+ Th17 cells are considered the principal source of IL-17A and accumulate in the psoriatic lesions of patients with psoriasis (Blauvelt and Chiricozzi, 2018). To test if our findings from γδ17 T cells are true for human IL-17A producing cells, we purified CD4+CCR6+ T cells (reported to be a marker for Th17 differentiated cells) (Singh et al., 2008) from healthy donors (Supplementary Figure S8) and cultured them with different concentrations of LCA. Exposure to LCA inhibited the transcripts of IL17A and RORC as well as the percentage of IL-17A positivity in CD4+CCR6+x T cells in a dose-dependent manner, without inducing obvious apoptosis (Figure 4f and g and Supplementary Figure S9). Together, these results suggest that BAs inhibit IL-17A production by T cells in both mice and humans, likely through regulation of RORγt.
FXR and TGR5 are not essential for BA-mediated inhibition of IL-17A production
The main receptors that mediate the biological function of BAs, FXR, and TGR5, also act as important modulators of signaling pathways involved in inflammation (Fiorucci et al., 2018). We asked if BAs may inhibit IL-17A production through FXR or TGR5 signaling. For this purpose, we treated IL-23–primed, cLN-derived cells with INT-747, a semi-synthetic FXR agonist, or INT-777, a TGR5 agonist (Schaap et al., 2014). In contrast to LCA, flow analysis showed that neither INT-747 nor INT-777 suppressed the production of IL-17A in murine T cells (Figure 5a). Similarly, INT-747 or INT-777 failed to reduce the expression of IL-17A in skin-LN coculture assay (Figure 5b). We then used FXR-deficient and TGR5-deficient mice to confirm these in vitro results and found that LCA efficiently inhibited IL-17A production in LN cells in the absence of FXR or TGR5 (Figure 5c and d). Taken together, our data suggest that FXR and TGR5 are not required for the inhibitory effect of LCA on IL-17A production.
Figure 5. FXR and TGR5 are not essential for BA-mediated inhibition of IL-17A production.
(a) Total fluorescence intensity of IL-17A on CD3+ T cells from cLNs of wild type mice and (b) transcripts of Il17a in the skin-lymph nodes coculture assay, after 24 hours incubation in the presence of IL-23 (100 ng/ml) with INT-747, INT-777, or LCA (50 μM). (c, d) Total fluorescence intensity of IL-17A on CD3+ T cells and protein levels of IL-17A in the cLN supernatants from (c) FXR-deficient or (d) TGR5-deficient mice after 24 hours incubation in the presence of IL-23 (100 ng/ml), with or without LCA treatment (50 μM). Data are presented as mean ± SEM. Data are representative of two independent experiments. *P < 0.05, **P < 0.01. ***P < 0.001. BA, bile acid; cLN, cervical lymph node; FI, fluorescence intensity; KO, knockout; LCA, lithocholic acid; TFI, total FI.
BAs repress CCL20 production in KCs and inhibit T cell migration
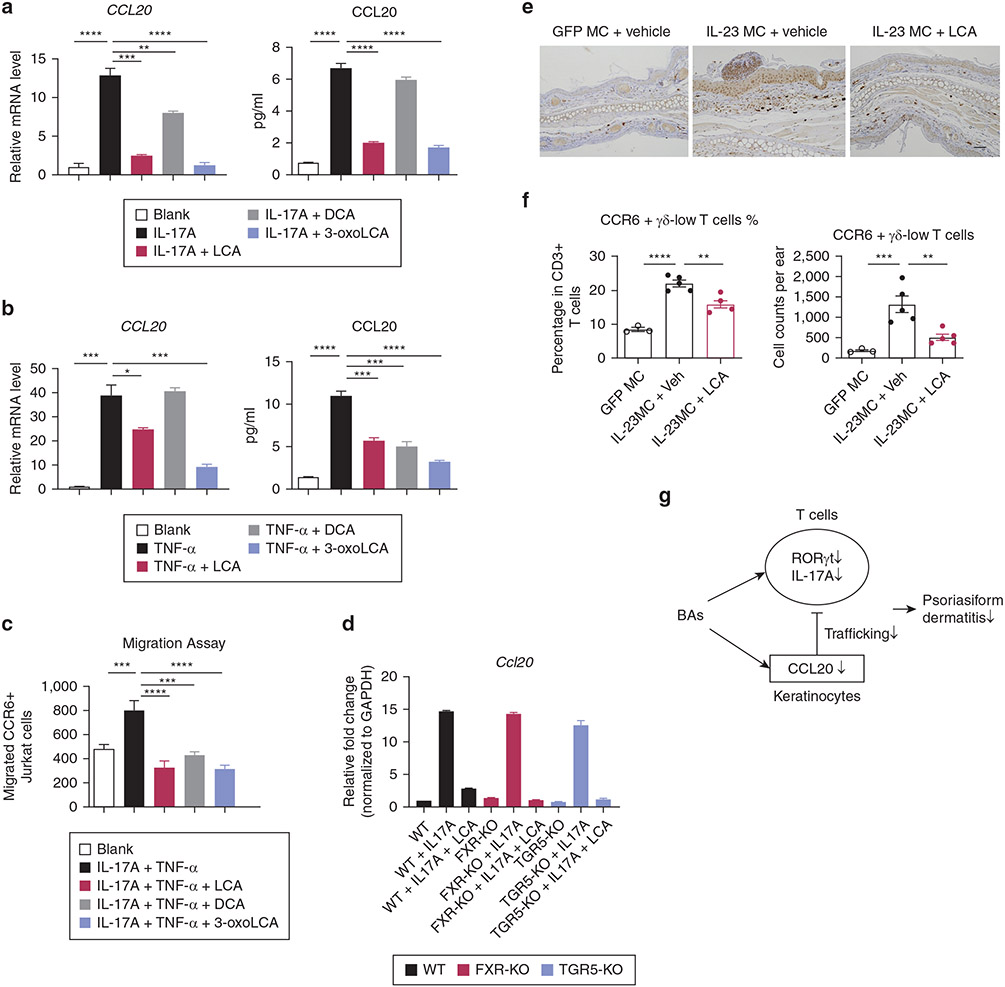
CCL20 is a proinflammatory chemokine that interacts specifically with its sole receptor CCR6 expressed on Th17 cells and γδ17 T cells and mediates the migration of these T cells into the inflammatory site (Furue et al., 2020; Papotto et al., 2018). We then asked whether or not BAs could affect CCL20-CCR6–mediated T cell trafficking. In the IL-23 MC-injected model, KCs rather than endothelial cells or myeloid cells served as the main source of CCL20 in the skin (Supplementary Figure S10). Therefore, we used HaCaT cells, a human epidermal KC line, to study the effect of BAs on CCL20 production. We stimulated KCs with IL-17A or TNF-α, two cytokines that are known to be centrally involved in psoriasis pathophysiology. In line with previous reports, CCL20 was constitutively expressed in cultured KCs, and its production was upregulated by the addition of TNF-α or IL-17A (Harper et al., 2009). Strikingly, LCA or 3-oxoLCA blocked the enhanced expression of CCL20 stimulated by IL-17A and, to a lesser extent, when stimulated by TNF-α (Figure 6a, b).
Figure 6. BAs inhibit CCL20 production by keratinocytes and CCL20-CCR6–mediated T-cell chemotaxis.
(a, b) Transcripts of CCL20 and protein levels of CCL20 in the supernatants from keratinocytes stimulated with IL-17A (a) or TNF-α (b), with the treatment of indicated BAs (50 μM). (c) Migration of CCR6+ Jurkat cells toward medium cultured with stimulated keratinocytes plus BAs. (d) Transcripts of Ccl20 in primary keratinocytes from WT, FXR-KO, and TGR5-KO mice, after stimulation with IL-17A in the presence or absence of LCA (50 μM). (e) Immunohistochemical staining for CCL20 in ear skin from mice treated with GFP MC, IL-23 MC + vehicle, or IL-23 MC + LCA. (f) Percentage and absolute numbers of CCR6+, γδ-low T cells in ear skin. Data are presented as mean ± SEM. Data are representative of two independent experiments. *P < 0.05, **P < 0.01. ***P < 0.001. (g) Schematic of a proposed mechanism for improvement of PsD due to BAs. BA, bile acid; DCA, deoxycholic acid; KO, knockout; LCA, lithocholic acid; MC, minicircle DNA; WT, wild-type.
We then examined if BAs impaired migration of CCR6+ Jurkat cells, a human T cell line, to conditioned HaCaT cell medium by performing a chemotaxis assay. As an experimental control, recombinant CCL20 protein predictably attracted CCR6+ Jurkat cells in a dose-dependent manner but failed to induce normal Jurkat cells’ migration without CCR6 overproduction (Supplementary Figure S11a). Next, we demonstrated the conditioned medium of IL-17A and TNF-α–stimulated KCs attracted more CCR6+ Jurkat cells, which was blocked by treatment with BAs (Figure 6c). Such inhibited chemotaxis was not observed when we used normal CCR6-low Jurkat cells (Supplementary Figure S11b), further supporting that this effect of BAs was dependent on the CCL20-CCR6 axis.
Notably, HaCat cells treated with LCA showed comparable viability versus those cocultured with a vehicle, suggesting LCA at the dosage of 50 μM showed marginal cytotoxicity to KCs (Supplementary Figure S12).
To test the role of FXR or TGR5 signaling in the inhibitory effects of BAs on CCL20 production, we isolated primary KCs from wild-type mice, FXR-knockout mice, and TGR5-knockout mice, and stimulated them with IL-17A overnight in the presence or absence of LCA. IL-17A similarly promoted the expression of CCL20 in wild-type, FXR-knockout, or TGR5-knockout primary KCs, which in each case was substantially blocked by the treatment of LCA (Figure 6d). Together, these findings suggested that neither FXR nor TGR5 is essential for the IL-17A–induced CCL20 production in KCs as well as for the BA-mediated inhibition of CCL20.
In agreement with these in vitro results, we observed decreased expression of CCL20 in epidermis together with reduced infiltration of CCR6+ γδ-low T cells, a cell population accounting for the majority of IL-17A in murine models of PsD (Mabuchi et al., 2011) in the ear skin from mice treated with i.v. injections of LCA (Figure 6e and f). These results suggest that BAs suppressed CCL20-CCR6–mediated trafficking of T cells, which may contribute to their antipsoriatic effect.
DISCUSSION
In this study, we showed that three secondary BAs, including LCA, DCA, and 3-oxoLCA, efficiently inhibit IL-23–mediated PsD without causing apparent hepatotoxicity. The antipsoriatic effect exerted by BAs was associated with the following two mechanisms: (i) inhibiting IL-17A production by T cells, likely via suppression of RORC, and (ii) decreasing CCL20 expression in KCs resulting in impaired trafficking of CCR6+, IL-17A–producing T cells toward the epidermis (Figure 6g).
A dysfunctional BA profile was reported in patients with psoriasis with lower concentrations of the conjugated primary and secondary BAs (Sorokin et al., 2018). In agreement with this concept, some early studies showed that psoriasis can be successfully treated with oral supplementation of dehydrocholic acid or ursodeoxycholic acid, presumably through affecting the gut microflora or suppressing cutaneous phospholipase A2 activity (Gyurcsovics and Bertók, 2003; Itoh et al., 2007). Recent findings revealing a novel role of BAs in controlling Th17 differentiation led us to reconsider the underlying mechanism of BAs in treating psoriasis (Hang et al., 2019). Indeed, our in vitro data demonstrated that BAs directly inhibited the production of IL-17A by T cells, highlighting the IL-17A pathway as the potential mechanistic link between BA and its antipsoriatic effect.
Mounting evidence suggests the anti-inflammatory roles of BAs, particularly in the innate immune system, are mediated by two BA receptors, FXR and TGR5 (Fiorucci et al., 2018). However, considering that BA-treated T cells deficient in FXR or TGR5 had a similarly reduced amount of IL-17A production, these two receptors are unlikely to contribute to the immunoregulatory role of BAs in IL-17A production. Instead, BAs repressed the expression of Rorc at a very early time point before inhibition of IL-17A, suggesting that BAs might suppress IL-17A by downregulating Rorc. Other signaling pathways such as the STAT3 pathway might be also affected by BA treatment, which directly controls IL-17A transcription as the transcripts of STAT3 and the expression of phosphorylated STAT3 were inhibited by LCA (Supplementary Figure S13 and Figure 3d). However, whether these two BA receptors mediate other immune regulatory effects in BA-treated PsD was not addressed in our studies. We observed that IL-23 MC led to decreased transcripts of TGR5 and its downstream targets in mice skin (Nos1, Nos3, Trpv2, and Fgf21) (Supplementary Figure S14) (Jena et al., 2019, 2018), some of which were partially restored by the treatment of LCA. These data suggest that TGR5 might be still involved in antipsoriatic effects of BAs by other mechanisms distinct from IL-17A regulation. Moreover, LCA can also stimulate some nonspecific BA sensors, including vitamin D receptor and pregnane X receptor (Wan and Sheng, 2018), which may account for the superior antipsoriatic effect of LCA compared with the other BAs especially in vitro experiments using cLNs from IL-23 MC-injected mice (Figure 4c). Indeed, we found that the abundance of vitamin D receptors was much higher than that of TGR5 and FXR in cells from cLNs (Supplementary Figure S15). Further investigations are warranted to investigate the molecular mechanism behind BA-improved PsD with special attention to the role of BA receptors.
Chemokines constitute a family of structurally related chemotactic cytokines that regulate the recruitment of leukocytes in physiological and inflammatory conditions (Griffith et al., 2014). Although most chemokines bind to multiple receptors, CCL20 is unique as it attaches to only one known receptor, CCR6. This monogamous pairing plays a crucial role in recruiting IL-17A–producing T cells to local tissues, and both CCL20 and CCR6 are highly expressed in psoriatic skin lesions (Furue et al., 2020). The production of CCL20 in KCs can be rapidly induced by mechanical scratching (Furue et al., 2019) or augmented by cytokines, such as IL-17A and TNF-α (Harper et al., 2009). Herein, we showed that BAs inhibit the expression of CCL20 in KCs both in vivo and in vitro. Although the decreased expression of IL-17A by BA treatment may partially contribute to the suppressed levels of CCL20, our in vitro data demonstrated that BAs can directly inhibit the expression of CCL20. Of great interest, such inhibition was relatively specific, as the expression of other chemokines such as CXCL1 and CXCL2 was not affected (Supplementary Figure S16).
In conclusion, our study showed that BAs ameliorate PsD with minimal toxicity through inhibition of IL-17A production in T cells and blockade of CCL20-mediated trafficking. Administration of BAs or therapeutic strategies that regulate BA metabolism may be beneficial for controlling psoriatic inflammation.
MATERIALS AND METHODS
Mice
Wild-type C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Fxr-deficient mice and Tgr5-deficient mice were maintained in Wan’s Lab. All animal experiments were performed under protocols that were approved by the Institutional Animal Care and Use Committee at the University of California, Davis.
In vivo BA treatment
BAs, INT-747 and INT-777 (MedChemExpress, Monmouth Junction, NJ), were dissolved in DMSO to a stock concentration of 1 mM. For in vivo study, BAs were dissolved by adding each solvent sequentially: 10% DMSO, >90% corn oil for oral administration, and 5% DMSO >95% PBS for i.v. administration. Mice received oral gavage of 0.6-mg BAs once daily (~30 mg/kg) dissolved in a 200-μl vehicle (Hang et al., 2019), or i.v. injections of 80-μg LCA through tail vein every other day (~4 mg/kg) in 100 μl vehicle.
Statistical analysis
Data were analyzed using GraphPad Prism version 6 (GraphPad Software, San Diego, CA). A two-sided unpaired Student’s t-test was used to compare two groups, and a one-way analysis of variance (ANOVA) with a Dunnett post hoc test was used for multiple comparisons unless otherwise indicated. A P-value less than 0.05 was considered statistically significant.
More detailed experimental procedures are described in the Supplementary Materials and Methods.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by a National Psoriasis Foundation Translational Grant to STH and a Guangdong Basic and Applied Basic Research Foundation [grant numbers 2020A1515110320] to ZS. This research was supported in part by the Intramural Research Program of the National Institutes of Health. We thank Dan Hong from Sun Yat-sen Memorial Hospital, Sun Yat-sen University, for her technical support.
Abbreviations:
- BA
bile acid
- cLN
cervical lymph node
- DCA
deoxycholic acid
- i.v.
intravenous
- KC
keratinocyte
- LCA
lithocholic acid
- LN
lymph node
- MC
minicircle DNA
- PsD
psoriasiform dermatitis
- STAT
signal transducer and activator of transcription
- γδ-low
TCR γδ-low expressing
- Th
T helper
- Treg
regulatory T cell
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at www.jidonline.org, and at https://doi.org/10.1016/j.jid.2021.10.027.
Data availability statement
No datasets were generated during the current study, but some or all data are available from the corresponding author by request.
REFERENCES
- Adamopoulos IE, Tessmer M, Chao CC, Adda S, Gorman D, Petro M, et al. IL-23 is critical for induction of arthritis, osteoclast formation, and maintenance of bone mass. J Immunol 2011;187:951–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauvelt A, Chiricozzi A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin Rev Allergy Immunol 2018;55:379–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehncke WH, Schön MP. Psoriasis. Lancet 2015;386:983–94. [DOI] [PubMed] [Google Scholar]
- Camilleri M, Vijayvargiya P. The role of bile acids in chronic diarrhea. Am J Gastroenterol 2020;115:1596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell C, McKenney PT, Konstantinovsky D, Isaeva OI, Schizas M, Verter J, et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 2020;581:475–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone A, Volpe E. Transcriptional regulators of T helper 17 cell differentiation in health and autoimmune diseases. Front Immunol 2020;11:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ML, Takeda K, Sundrud MS. Emerging roles of bile acids in mucosal immunity and inflammation. Mucosal Immunol 2019;12:851–61. [DOI] [PubMed] [Google Scholar]
- Ely PH. Is psoriasis a bowel disease? Successful treatment with bile acids and bioflavonoids suggests it is. Clin Dermatol 2018;36:376–89. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Biagioli M, Zampella A, Distrutti E. Bile acids activated receptors regulate innate immunity. Front Immunol 2018;9:1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furue K, Ito T, Tanaka Y, Yumine A, Hashimoto-Hachiya A, Takemura M, et al. Cyto/chemokine profile of in vitro scratched keratinocyte model: implications of significant upregulation of CCL20, CXCL8 and IL36G in Koebner phenomenon. J Dermatol Sci 2019;94:244–51. [DOI] [PubMed] [Google Scholar]
- Furue K, Ito T, Tsuji G, Nakahara T, Furue M. The CCL20 and CCR6 axis in psoriasis. Scand J Immunol 2020;91:e12846. [DOI] [PubMed] [Google Scholar]
- Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol 2014;32:659–702. [DOI] [PubMed] [Google Scholar]
- Guo C, Xie S, Chi Z, Zhang J, Liu Y, Zhang L, et al. Bile acids control inflammation and metabolic disorder through inhibition of NLRP3 inflammasome [published correction appears in Immunity 2016;45:944]. Immunity 2016;45:802–16. [DOI] [PubMed] [Google Scholar]
- Gyurcsovics K, Bertók L. Pathophysiology of psoriasis: coping endotoxins with bile acid therapy. Pathophysiology 2003;10:57–61. [DOI] [PubMed] [Google Scholar]
- Hang S, Paik D, Yao L, Kim E, Trinath J, Lu J, et al. Bile acid metabolites control TH17 and Treg cell differentiation [published correction appears in Nature 2020;579:E7]. Nature 2019;576:143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper EG, Guo C, Rizzo H, Lillis JV, Kurtz SE, Skorcheva I, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol 2009;129:2175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillmer EJ, Zhang H, Li HS, Watowich SS. STAT3 signaling in immunity. Cytokine Growth Factor Rev 2016;31:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh S, Kono M, Akimoto T. Psoriasis treated with ursodeoxycholic acid: three case reports. Clin Exp Dermatol 2007;32:398–400. [DOI] [PubMed] [Google Scholar]
- Jena PK, Sheng L, Di Lucente J, Jin LW, Maezawa I, Wan YY. Dysregulated bile acid synthesis and dysbiosis are implicated in Western diet-induced systemic inflammation, microglial activation, and reduced neuroplasticity. FASEB J 2018;32:2866–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jena PK, Sheng L, McNeil K, Chau TQ, Yu S, Kiuru M, et al. Long-term Western diet intake leads to dysregulated bile acid signaling and dermatitis with Th2 and Th17 pathway features in mice. J Dermatol Sci 2019;95:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol 2018;15:111–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi T, Takekoshi T, Hwang ST. Epidermal CCR6+ γδ T cells are major producers of IL-22 and IL-17 in a murine model of psoriasiform dermatitis. J Immunol 2011;187:5026–31. [DOI] [PubMed] [Google Scholar]
- Nussbaum L, Chen YL, Ogg GS. Role of regulatory T cells in psoriasis pathogenesis and treatment. Br J Dermatol 2021;184:14–24. [DOI] [PubMed] [Google Scholar]
- Papotto PH, Reinhardt A, Prinz I, Silva-Santos B. Innately versatile: γδ17 T cells in inflammatory and autoimmune diseases. J Autoimmun 2018;87:26–37. [DOI] [PubMed] [Google Scholar]
- Qi C, Wang Y, Li P, Zhao J. Gamma delta T cells and their pathogenic role in psoriasis. Front Immunol 2021;12:627139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaap FG, Trauner M, Jansen PL. Bile acid receptors as targets for drug development. Nat Rev Gastroenterol Hepatol 2014;11:55–67. [DOI] [PubMed] [Google Scholar]
- Shi Z, Garcia-Melchor E, Wu X, Yu S, Nguyen M, Rowland DJ, et al. Differential requirement for CCR6 in IL-23-mediated skin and joint inflammation. J Invest Dermatol 2020;140:2386–97. [DOI] [PubMed] [Google Scholar]
- Singh SP, Zhang HH, Foley JF, Hedrick MN, Farber JM. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J Immunol 2008;180:214–21. [DOI] [PubMed] [Google Scholar]
- Song X, Sun X, Oh SF, Wu M, Zhang Y, Zheng W, et al. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature 2020;577:410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin AV, Domenichiello AF, Dey AK, Yuan ZX, Goyal A, Rose SM, et al. Bioactive lipid mediator profiles in human psoriasis skin and blood. J Invest Dermatol 2018;138:1518–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 2009;31:331–41. [DOI] [PubMed] [Google Scholar]
- Wan YY, Sheng L. Regulation of bile acid receptor activity. Liver Res 2018;2:180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated during the current study, but some or all data are available from the corresponding author by request.