Abstract
Licorice (Glycyrrhiza uralensis) contains several compounds that have been reported to alleviate menopausal symptoms via interacting with estrogen receptors (ERs). The compounds exist mainly in the form of glycosides, which exhibit low bioavailability and function. To bioconvert liquiritin and isoliquiritin, the major estrogenic compounds, to the corresponding deglycosylated liquiritigenin and isoliquiritigenin, respectively, licorice was fermented with Monascus, which has been demonstrated to deglycosylate other substances. The contents of liquiritigenin and isoliquiritigenin in Monascus-fermented licorice increased by 10.46-fold (from 38.03 μM to 379.75 μM) and 12.50-fold (from 5.53 μM to 69.14 μM), respectively, compared with their contents in non-fermented licorice. Monascus-fermented licorice exhibited 82.5% of the ERβ binding activity of that observed in the positive control (17 β-estradiol), whereas the non-fermented licorice exhibited 54.1% of the binding activity in an in vivo ER binding assay. The increase in the ERβ binding activity was associated with increases in liquiritigenin and isoliquiritigenin contents. Liquiritigenin acts as a selective ligand for ERβ, which alleviates menopausal symptoms with fewer side effects, such as heart disease and hypertension, compared with a ligand for ERα. In addition, Monascus-fermented licorice contained 731 mg/kg of monacolin K, one of the metabolites produced by Monascus that reduces serum cholesterol. Therefore, Monascus-fermented licorice is a promising material for the prevention and treatment of menopausal syndrome with fewer side effects.
Keywords: Monascus albidulus, licorice, menopausal symptoms, liquiritigenin, estrogen receptor β, monacolin K
Introduction
Decreased levels of estrogens cause menopause syndrome in women after middle age. Menopausal symptoms, such as hot flushes, night sweats, depression, sleep disturbances, vaginal dryness, bone loss, and changes in cardiovascular and metabolic function, considerably affect the quality of life for middle-aged women [3, 33]. Hormone replacement therapy (HRT) that uses pharmaceutical estrogens has been adopted to treat menopausal symptoms [10]. However, according to the Women's Health Initiative (WHI), HRT potentially has side effects that could lead to breast cancer and heart attacks, although it can effectively alleviate menopausal symptoms [17]. Therefore, HRT is limited to treatment of menopausal symptoms with minimal doses of replaced hormones [7]. Currently, many women prefer natural substitutes, such as herbal preparations or nutraceuticals, which are considered generally safer compared with HRT. Phytoestrogens are plant compounds that are considered to act similar to estrogens based on their analogous chemical structures [7]. Isoflavones, lignans, and coumestans are the major phytoestrogens [29]. Particularly, isoflavones, such as genistein and S-equol from soy, have been reported to be effective in alleviating menopausal symptoms [1, 15]. However, the effectiveness of other phytoestrogens in the treatment of menopausal symptoms is yet to be evaluated. In addition, some reports have raised concerns over potential long-term side effects, such as stroke, heart disease, and breast cancer [3, 17, 33]. Therefore, currently, phytoestrogen has not been considered as a therapeutic agent.
Replaced hormones and phytoestrogens alleviate menopausal symptoms by binding to estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ) [34, 36]. ERα and ERβ are very similar structurally; however, their ligand-binding domains are very different with regard to their selectivity to ligands [27]. ERα and ERβ exhibit entirely different functions [32]. Generally, when estrogen-like compounds bind selectively to ERβ, they can function as tumor suppressors; therefore, estrogen-like compounds that bind to ERβ can alleviate menopausal symptoms without the side effects associated with menopausal therapies, such as breast cancer [32].
Licorice represents one of the most important traditional herbal medicines in Korea, China, Japan, and East Asia in general [44]. Some studies have suggested beneficial health effects of licorice and its bioactive constituents [18]. Licorice root extracts are often consumed as dietary supplements by women at menopause [5, 18]. According to Boonmuen et al.(2016), licorice root extracts harbor diverse compounds, including ER agonists and antagonists [5, 18]. Particularly, liquiritigenin, isoliquiritigenin, calycosin, methoxychalcone, vestitol, and glycycoumarin are estrogen agonists. Among them, liquiritigenin, an aglycone form of liquiritin, is a well-known selective agonist for the tumor-suppressive ERβ [30]. Liquiritigenin is notably less stimulatory of genes promoting proliferation and motility when compared with 17 β-estradiol (E2) [12]. Licorice is derived from three medicinal plants, Glycyrrhiza uralensis, Glycyrrhiza glabra, and Glycyrrhiza inflata. Each species has different compound profiles, and some compounds are specific markers for each species [40]. The contents of ER agonists, such as liquiritin, isoliquiritin, and liquiritigenin, are significantly higher in G. uralensis than in G. glabra or G. inflata. However, the mean content of liquiritigenin, an ERβ marker compound, in G. uralensis, is very low [21, 39]. Hwang et al. (2015) reported the bioconversion of liquiritin into liquiritigenin using Laetiporus sulphureus to increase liquiritigenin content in licorice; however, their study focused on liquiritin and liquiritigenin contents, but not on the bioconversion of associated compounds, such as liquiritin apioside and isoliquiritin [16].
Monascus spp. have been applied in the bioconversion of glycosides into their corresponding aglycones to enhance their functionality and bioavailability [14, 24]. In addition, Monascus spp. produce several bioactive compounds, such as monacolin K and γ-aminobutyric acid (GABA), during fermentation [9, 23, 26], which could provide additional functionality to bioconversion effects. Particularly, monacolin K (also known as mevinolin or lovastatin) lowers blood cholesterol levels by inhibiting the activity of HMG-CoA reductase, a key enzyme in the cholesterol biosynthesis pathway [8]. Therefore, Monascus-fermented licorice could benefit women at menopause by enhancing cardiovascular functions.
In the present study, licorice was fermented with Monascus to enrich the preferred estrogen-like compounds that bind selectively to ERβ. Several estrogenic compounds, including liquiritin and liquiritin apioside, were bioconverted into their corresponding aglycones, which could exhibit relatively higher functional activity and bioavailability. Monascus-fermented licorice exhibited the high ERβ binding activity and contained monacolin K. Therefore, Monascus-fermented licorice is a promising and alternative functional material for the management of menopausal symptoms without the side effects associated with other common therapies.
Materials and Methods
Chemicals and Media
Monacolin K, citrinin, liquiritin, liquiritigenin, trifluoroacetic acid, acetic acid, and p-nitrophenyl-β-D-glucopyranoside were purchased from Sigma-Aldrich Co. (USA), isoliquiritin and isoliquiritigenin from ChemFaces (China), and liquiritin apioside and isoliquiritin apioside from Chengdu Biopurify Phytochemicals Ltd. (China). All HPLC-grade solvents were purchased from Fisher Scientific Korea Ltd. (Korea). Extraction solution and methanol were purchased from Samchun Pure Chemical (Korea). Potato dextrose agar (PDA) was purchased from Acumedia Inc.(USA), and glucose from Duksan Pure Chemicals (Korea). Yeast extract-peptone-dextrose and yeast nitrogen base media were purchased from Sigma-Aldrich Co. G. uralensis base media were composed of 2.0 5.0% G. uralensis extracts and 2% glucose. Licorice (G. uralensis) was purchased from a regional market in Chungcheongbuk-do, Korea. Licorice extracts were prepared by adding 5 L of distilled water to 1.2 kg of licorice, followed by boiling at 95oC for 5 h. The extraction supernatant recovered by filtration with a Whatman filter paper No. 1 (UK) was concentrated to a solid content of 15% using a vacuum evaporator.
Strains and Growth Conditions
Thirteen Monascus strains that had been isolated from Monascus fermentation products were used [19, 20]. In addition, four type strains, Monascus pilosus KCCM 60084, Monascus purpureus KCCM 60016, Monascus ruber KCTC 6122, and Monascus kaoliang KCCM 60154, obtained from the Korea Culture Center of Microorganisms (KCCM, Korea) or the Korean Collection for Type Cultures (KCTC, Korea), were used in the initial screening (Table 1).
Table 1.
Contents of liquiritigenin and isoliquiritigenin, and monacolin K production in licorice fermented with Monascus strains.
| Strain | Origin | Fermented solid contents (%) | Monacolin K (mg/kg) | Liquiritigenin (mg/g) | Isoliquiritigenin (mg/g) | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Acid form | Lactone form | Total | |||||
| M. pilosus A | Red mold rice, Korea | 48.90 ± 2.37 | 834.06 ± 176.20 | 2,198.29 ± 188.64 | 3,032.35 ± 247.99 | 0.74 ± 0.16 | 0.39 ± 0.07 |
| M. purpureus B | Red mold rice, Korea | 56.26 ± 4.12 | 136.86 ± 27.48 | 389.72 ± 44.26 | 526.58 ± 57.89 | 1.73 ± 0.22 | 0.22 ± 0.06 |
| M. pilosus KMU106 | Red mold rice, Korea | 54.59 ± 2.37 | 142.40 ± 37.41 | NDc | 142.4 ± 37.41 | 2.66 ± 0.31 | 0.42 ± 0.08 |
| M. pilosus D | Red mold rice, Korea | 52.48 ± 2.16 | 142.79 ± 11.16 | ND | 142.79 ± 11.16 | 0.74 ± 0.11 | 0.31 ± 0.12 |
| M. pilosus F | Red mold rice, Korea | 50.13 ± 5.37 | 139.28 ± 18.14 | ND | 139.28 ± 18.14 | 0.61 ± 0.14 | 0.13 ± 0.04 |
| M. pilosus KMU108 | Red mold rice, Korea | 53.31 ± 4.18 | 147.45 ± 25.18 | ND | 147.45 ± 25.18 | 3.83 ± 0.71 | 0.60 ± 0.04 |
| M. pilosus I | Red mold rice, Korea | 52.43 ± 3.76 | 146.46 ± 31.43 | ND | 146.46 ± 31.43 | 3.60 ± 0.34 | 0.63 ± 0.05 |
| M. pilosus K | Red mold rice powder, China | 45.96 ± 3.72 | 807.37 ± 90.11 | 2,426.45 ± 213.86 | 3,233.82 ± 300.41 | 1.87 ± 0.18 | 0.34 ± 0.05 |
| M. pilosus M | Red mold rice powder, China | 52.19 ± 6.54 | 151.09 ± 21.87 | ND | 151.09 ± 21.87 | 3.18 ± 0.26 | 0.53 ± 0.12 |
| M. albidulus N | Red mold rice powder, China | 44.82 ± 3.11 | 874.01 ± 48.64 | 2,870.10 ± 128.19 | 3,744.11 ± 166.87 | 3.22 ± 0.31 | 0.40 ± 0.08 |
| M. pilosus O | Red mold rice powder, China | 52.67 ± 1.48 | 130.90 ± 12.89 | ND | 130.9 ± 12.89 | 3.14 ± 0.48 | 0.64 ± 0.08 |
| M. pilosus T | Red mold rice powder, China | 54.22 ± 2.86 | 130.90 ± 6.48 | ND | 130.9 ± 6.48 | 2.24 ± 0.29 | 0.67 ± 0.11 |
| M. pilosus U | Red mold rice powder, China | 51.68 ± 5.12 | 140.58 ± 17.78 | ND | 140.58 ± 17.78 | 3.94 ± 0.34 | 0.68 ± 0.16 |
| M. pilosus KCCM60084 | KCCMa | 53.33 ± 3.42 | 142.64 ± 22.68 | ND | 142.64 ± 22.68 | 3.44 ± 0.16 | 0.63 ± 0.07 |
| M. purpureus KCCM60016 | KCCM | 55.30 ± 3.76 | 144.77 ± 17.64 | 39.18 ± 2.84 | 183.95 ± 20.68 | 2.19 ± 0.15 | 0.38 ± 0.08 |
| M. ruber KCTC6122 | KCTCb | 57.38 ± 4.29 | 145.13 ± 7.66 | 225.99 ± 29.87 | 371.12 ± 38.04 | 0.37 ± 0.08 | 0.16 ± 0.08 |
| M. kaoliang KCCM60154 | KCCM | 53.13 ± 4.86 | 152.62 ± 10.04 | 92.59 ± 11.64 | 245.21 ± 20.80 | 3.18 ± 0.24 | 0.52 ± 0.12 |
aKCCM, Korea Culture Center of Microorganisms.
bKCTC, Korean Collection for Type Cultures.
cND, not detected.
Fermentation was carried out at 30°C with shaking at 200 rpm for 150 h in a 2% licorice medium.
Spores were harvested using a 0.85% saline buffer after culturing each of the strains on PDA plates for 5 days at 30°C. Spores were inoculated into 50 ml of G. uralensis base media at 1.0×106 spore/ml. A 2% or 5% licorice medium was used for the initial screening or licorice fermentation. All broth cultures were incubated for 3 days (initial screening) or 10 days (fermentation) at 30°C with constant shaking at 200 rpm. The culture broth was freeze-dried using a freeze-dryer (FDU-1200, EYELA, Japan) for the analysis of fermentation products.
For an in vivo ER binding assay, the yeasts harboring pRR-ERβ-5Z (plasmid #23062) or pRR-ERα-5Z (plasmid #23061) were constructed by transforming each plasmid into Saccharomyces cerevisiae W300a obtained from Prof. S. J. Park (Yonsei University, Seoul, Korea) [11, 31]. Both plasmids were purchased from Addgene (USA). Yeast transformation was carried out using an MP BIO Alkali-Cation Yeast Transformation Kit (MP Biomedicals, USA) according to the kit protocol. Yeast was routinely cultured and maintained on a minimal media [11].
Quantitative Analysis of Monacolin K, Citrinin, Liquiritin, Liquiritin Apioside, Liquiritigenin, Isoliquiritin, Isoliquiritin Apioside, and Isoliquiritigenin
Quantitative analyses of monacolin K and citrinin were carried out using HPLC as described previously for the analysis of Monascus-fermented red ginseng [14]. Liquiritin, isoliquiritin, liquiritigenin, isoliquiritigenin, liquiritin apioside and isoliquiritin apioside were also analyzed quantitatively [21] using an Agilent HPLC equipped with a HiQ sil C18W column (250 × 4.6 mm, particle size 5 μm, pore size 120 Å; Kya Tech Corporation, Japan). For each chemical analysis, 50 mg of a sample was extracted using 500 μl of methyl alcohol for 40 min with gentle shaking. All extracted samples were filtered using a 0.45-μl syringe filter (Life Sciences, USA). For the liquiritin, liquiritin apioside, isoliquiritin and isoliquiritn apioside HPLC analyses, samples (20 μl) were separated using an acetonitrile gradient mobile phase (20%acetonitrile for 10 min for the initial run, followed by 20–40%gradient for 2 min, 40% for 13 min, 40 20% gradient for 2 min, and 20% for 23 min) at a flow rate of 0.5 ml/min. Signals were detected using a UV detector (Agilent 1260 Infinity LC, Agilent Technologies, USA) at a wavelength of 254 and 370 nm for liquiritin and liquiritin apioside and for isoliquiritin and isoliquiritin apioside, respectively. For the liquiritigenin and isoliquiritigenin HPLC analyses, samples (20 μl) were separated using an acetonitrile gradient mobile phase (10–30% acetonitrile gradient for 10 min for the initial run, followed by 30–50%gradient for 35 min, 50–70% gradient for 10 min, 70–75% gradient for 10 min, 75–10% for 10 min, and 10% for 10 min) at a flow rate of 1 ml/min. Signals were detected at a wavelength of 274-nm and 370-nm wavelengths for liquiritigenin and for isoliquiritigenin, respectively.
β-Glucosidase Assay
β-Glucosidase in the culture broth was measured as described previously with minor modifications [14]. Substrate solution was prepared by dissolving p-nitrophenyl-β-D-glucopyranoside in a 50 mM potassium phosphate buffer. Five hundred microliters of the aliquot was stored at –20°C until use. Two hundred microliters of supernatant from the culture broths was centrifuged at 13,750 ×g for 10 min, mixed with 500 μl of preheated substrate solution, and then incubated at 30°C for 30 min. Released p-nitrophenol was measured using a spectrophotometer at 405 nm. One unit of β-glucosidase activity was defined as the release of 1 μmole of p-nitrophenol per min.
Yeast Estrogen Binding Assay
The ERα and β binding assays were performed using S. cerevisiae harboring pRR-ERα-5Z or pRR-ER β -5Z [31] as described by Fox (2008) [11]. Yeast was pre-cultured in a minimal medium for approximately 24 h until the optical density (OD) at 600 nm exceeded 1. Subsequently, culture was diluted to OD = 0.4 with a fresh medium. Diluted yeast culture (200 μl) and 5 μl of Monascus-fermented licorice extract were transferred to a 96-well plate. The plate was incubated for 18 h at 30°C. Culture (50 μl) was mixed with a 200 μl of Lac-Z buffer in a new 96-well plate, and then incubated at 30°C for 15 min. Color development was monitored at 405 nm. The ER binding activity was expressed as % relative absorbance to the 17 β-estradiol treatment whose maximum absorbance was set to 100% [11].
Results and Discussion
Screening for Monascus Strains Suitable for the Bioconversion of Liquiritin and Isoliquiritin in Licorice (G. uralensis)
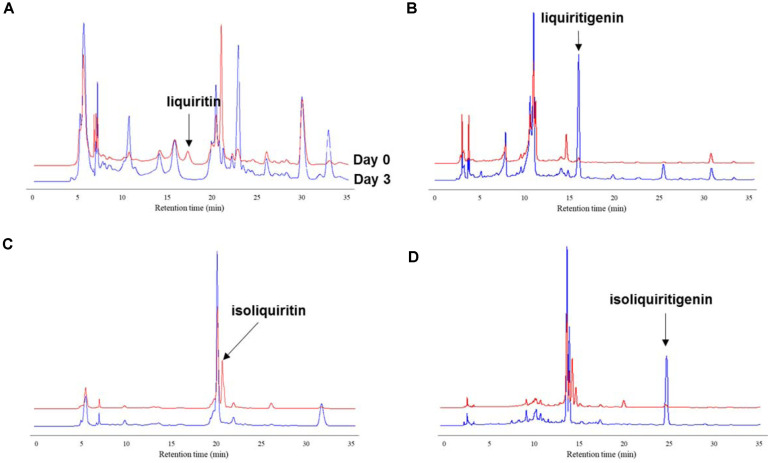
We have reported previously that Monascus spp. can convert glycosides into their corresponding aglycones [14, 24]. In the present study, we attempted to bioconvert the glycosidic components in licorice, such as liquiritin and liquiritin apioside, into liquiritigenin, a well-known selective agonist for ERβ. First, we tested and screened for Monascus strains suitable for the deglycosylation of liquiritin and isoliquiritin in licorice. We have previously isolated 17 Monascus strains from Monascus-fermented products [19, 20]. Each strain exhibited the strain-specific characteristics with regard to enzyme and secondary metabolite production, such as monacolin K, pigments, and citrinin, under different fermentation substrates [14, 24]. Therefore, the strains were evaluated for the deglycosylation of liquiritin and isoliquiritin and for the production of monacolin K in licorice media. Liquiritin and isoliquiritin in licorice were all converted into the corresponding deglycosylated compounds by fermentation with Monascus spp. The liquiritin peak (retention time 17.2 min) disappeared (Fig. 1A), and the liquiritigenin peak (retention time 15.8 min) emerged (Fig. 1B) after 3 days of fermentation. Similarly, the isoliquiritin peak (retention time 20.9 min) disappeared (Fig. 1C) and the isoliquiritigenin peak (retention time 24.8 min) was observed (Fig. 1D) after 3 days of fermentation.
Fig. 1.
HPLC chromatograms of liquiritin (A), liquiritigenin (B), isoliquiritin (C), and isoliquiritigenin (D).
The liquiritigenin and isoliquiritigenin contents in the freeze-dried Monascus-fermented licorice ranged from 0.37 to 3.94 mg/g and from 0.13 to 0.68 mg/g, respectively (Table 1). M. pilosus U exhibited the highest bioconversion activities for liquiritigenin and isoliquiritigenin, which were observed at 3.94 and 0.68 mg/g, respectively, and was followed by M. pilosus KMU108, M. pilosus I, and M. albidulus N (Table 1). On the other hand, most strains produced monacolin K at various levels, ranging from 130.9 to 3,744.1 mg/g, and M. pilosus A, M. purpureus B, M. pilosus K, and M. albidulus N produced over 500 mg/g of monacolin K, which is the minimum level to be claimed as a functional food in the case of Monascus-fermented red yeast rice (2014 Health Functional Food Code, Ministry of Food and Drug Safety (MFDS), Korea). In addition, most of the strains produced citrinin at very low concentrations, and citrinin was not detected in M. albidulus N-fermented licorice (data not shown). Since citrinin is a nephrotoxic and hepatotoxic fungal toxin [4, 22], the selection of a strain that produces little or no citrinin is very critical for functional food materials. The level of citrinin production in Monascus spp. is known to be substrate dependent [35]. Therefore, M. albidulus N was selected as the strain suitable for the bioconversion of liquiritin and isoliquiritin in licorice. It has been identified previously as M. albidulus [19, 20] and was designated as M. albidulus KMU112.
Bioconversion of Liquiritin and Isoliquiritin in Licorice during Fermentation with M. albidulus KMU112
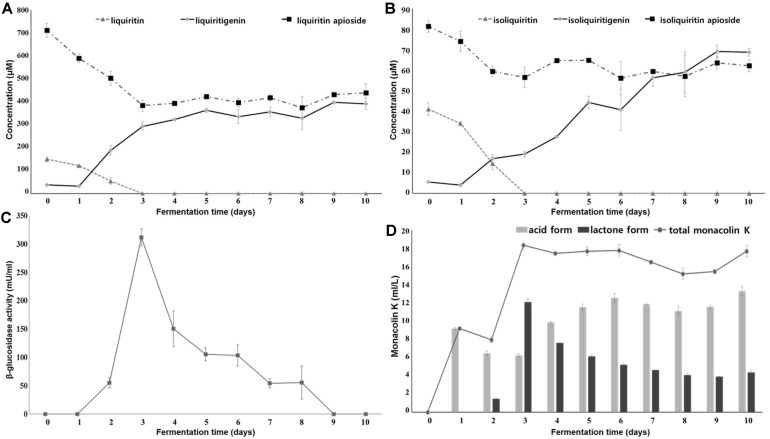
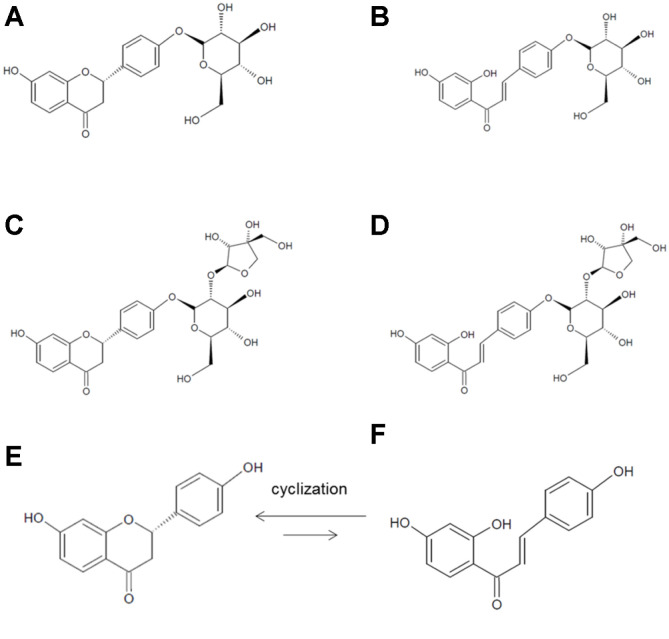
The liquiritin concentration was 149.64 μM, whereas that of liquiritigenin was very low at 38.03 μM in a 5% preculture licorice medium (Fig. 2A), suggesting that most of liquiritin exists in a glycosylated form in licorice. After 3 days of fermentation, liquiritin was no longer detected, and liquiritigenin concentrations increased to 322.63 μM on day 4 of fermentation and reached a maximum of 397.75 μM on day 9 of fermentation (Fig. 2A). Such an unexpected increase in the concentration of liquiritigenin suggested the existence of liquiritin analogs in licorice and their bioconversion into liquiritigenin during Monascus fermentation. Liquiritin apioside is another liquiritin glycoside (Fig. 3), observed at 1.27% on average in G. uralensis and more than 1% in various licorice species [21]. Consequently, some of liquiritigenin could have been derived from liquiritin apioside, and the concentrations were 712.05 μM before fermentation and 383.82 μM on day 3 of fermentation, respectively (Fig. 2A).
Fig. 2.
Bioconversion of liquiritin/liquiritin apioside and isoliquiritin/isoliquiritin apioside, β-glucosidase activity, and monacolin K production during licorice fermentation. (A) Bioconversion of liquiritin and liquiritin apioside to liquiritigenin; (B) bioconversion of isoliquiritin and isoliquiritin apioside to isoliquiritigenin; (C) β-glucosidase activity; (D) monacolin K production.
Fig. 3.
Chemical structures of liquiritin (A), isoliquiritin (B), liquiritin apioside (C), isoliquiritin apioside (D), liquiritigenin (E), and isoliquiritigenin (F).
The concentrations of isoliquiritin and isoliquiritigenin were 40.99 μM and 5.53 μM, respectively, in the licorice medium, suggesting that a glycolylated form has higher concentration than an aglycoylated form, such as liquiritin/liquiritgenin. Isoliquiritin was also not detected after 3 days of fermentation, and the concentrations of isoliquiritigenin increased steadily during the fermentation period and reached a maximum of 69.14 μM on day 9. Some of the isoliquiritigenin could also be derived from isoliquiritin apioside, which is an isoliquiritin glycoside (Fig. 3). Such bioconversion was supported by the 30.63% reduction in isoliquiritin apioside from 81.32 μM to 56.41 μM on the day 3 culture (Fig. 2B).
Liquiritin and isoliquiritin are bioconverted into liquiritigenin and isoliquiritigenin by β-glucosidase [2, 45]. The β-glucosidase activity was detected in fermented licorice and reached a maximum of 312.02 mU/ml after 3 days of fermentation, suggesting that liquiritin and isoliquiritin of licorice were bioconverted by β-glucosidase in M. albidulus KMU112 (Fig. 2C). Conversely, the concentrations of liquiritin apioside and isoliquiritin apioside decreased until day 3 and then maintained a constant concentration, and the decreases represented 46.10% and 30.63% reductions from the initial concentrations, respectively. The reason for the partial bioconversion of liquiritin/isoliquiritin apiosides remains unclear; however, apiosides seem to be converted in different ways compared with liquiritin/isoliquiritin. According to Sato (2007) [37], liquiritin/liquiritin apioside and isoliquiritin/isoliquiritin apioside were bioconverted completely by naringinase, which has both α-L-rhamnosidase and β-glucosidase functions. Therefore, liquiritin apioside and isoliquiritin apioside could be bioconverted by enzymes other than β-glucosidase [37].
Since liquiritigenin and isoliquiritigenin can be interconverted naturally [38], the final liquiritigenin and isoliquiritigenin concentrations may not be proportional to each bioconversion. In summary, glycosylated forms of liquiritin/isoliquiritin and liquiritin apioside/isoliquiritin apioside were successfully biotransformed into their corresponding deglycosylated forms by fermentation.
Monacolin K Production during Licorice Fermentation by M. albidulus KMU112
Total monacolin K was detected at 18.35 mg/L on day 3 of culture, and the concentration was maintained until day 10 of fermentation (Fig. 2D). The active monacolin K (acid form) was produced mainly during the early fermentation period (9.21 mg/l on day 1), and then the concentration increased gradually up to the maximum level on day 6 of fermentation (12.58 mg/l). Conversely, the inactive monacolin K (lactone form) was detected after day 2, and reached a maximum (12.11 mg/l) on day 3, followed by a gradual decrease. The pH of the culture broth decreased until day 3 of fermentation and then increased after, suggesting that an increase in the active acid form after day 3 was in part derived from the inactive lactone form due to change in pH [28]. The active and inactive monacolin K concentrations in the freeze-dried powder of the 10-day culture were 551.03 mg/kg and 180.55 mg/kg, respectively. Monacolin K production has been demonstrated to be affected by substrates. The monacolin K concentrations in Monascus-fermented soybean, fortified mugwort, or ginseng were 1,366, 2,219, and 3,089 mg/kg [14, 24, 25]. In addition, the concentration of a substrate influences monacolin K production so that monacolin K yield was generally higher under lower substrate concentrations [41]. In the present study, 5% licorice medium instead of 2% licorice medium was used for strain screening to increase liquiritigenin and isoliquiritingenin concentrations. The amounts of active monacolin K in fermented licorice exceed the monacolin K standard concentrations in the Monascus-fermented red yeast rice functional food (2014 Health Functional Food Code, MFDS, Korea). Therefore, the fermented licorice could prevent the cardiovascular diseases associated with the adverse side effects of the female climacteric syndrome, which is an additional benefit of fermented licorice.
In Vivo ER Binding Assay of Monascus-Fermented Licorice Extracts
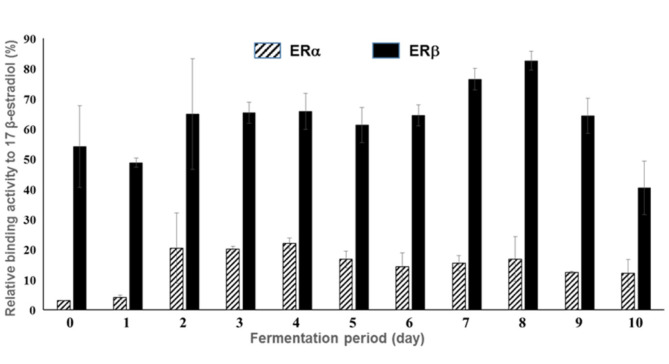
The ERα and ERβ binding activities of the Monascus-fermented licorice extracts were evaluated using recombinant yeasts that contained the ERα or ERβ gene fused to a β-galactosidase reporter gene. The ERα and ERβ binding activities of the extracts of pre-fermented licorice media were 3.04% and 54.06% that of the positive control, 17 β-estradiol, respectively (Fig. 4), which is consistent with the findings of a study where licorice exhibited higher ERβ binding activity compared with ERα binding activity [42]. Both ERα and ERβ binding activities increased by fermentation with M. albidulus. On day 4 of fermentation, the ERα binding activity reached to the maximum level, which was 22.0% that of the positive control. Conversely, the ERβ binding activity increased up to 82.54% with the extracts of the 8-day fermented licorice. However, the increase in ER binding activity was not proportional to the liquiritigenin and isoliquiritigenin concentrations, suggesting the involvement of other components in ER binding. Licorice contains not only estrogenic agonists but also estrogenic antagonists [5].
Fig. 4.
ER binding activities of the licorice fermentation extracts. The ERα and ERβ-binding activities of licorice extracts were evaluated before and after fermentation using an in vivo assay system. ER binding activity was expressed as % relative absorbance to the 17 β- estradiol treatment whose maximum absorbance was set to 100%.
The ERα and ERβ binding activity of licorice has been reported using the MCF-7 cells; however, the binding activity was reported to be low compared with the positive control [5, 30]. In the present study, we demonstrate that fermentation with Monascus could considerably increase the ERα and ERβ binding activities of licorice. Particularly, the ERβ binding activity was almost equivalent to that of the positive control. Such an increase in the selective binding capacity of fermented licorice to ERβ could be due to increased liquiritigenin concentrations in the course of fermentation. Liquiritigenin binds selectively to ERβ [30].
According to van Patten (2002), a very high binding activity for ERα is associated with phytoestrogen-induced side effects, such as breast cancer [43]. The most commonly used phytoestrogens, soybean isoflavones, have a high binding affinity for ERα. For example, genistein exhibits approximately 60% ERα binding ability [6]. In recent years, the Japanese Food Safety Commission has limited the daily intake of isoflavone to less than 30 mg/kg in a specialized diet, which was also adopted by MFDS in Korea [1, 13, 15]. The fermented licorice in the present study exhibited an ER binding activity, preferentially to ERβ, of up to 82.5%. Therefore, fermented licorice could have an advantage over other phytoestrogens in the alleviation of menopause symptoms with low side effects.
Market demand for women's menopausal remedies is on the rise due to growing interest in women’s health. In the present study, Monascus-fermented licorice contained high levels of liquiritigenin and monacolin K and exhibited high ERβ-selective binding activity. Therefore, Monascus-fermented licorice is a promising material that could be applied in the prevention and treatment of menopausal syndrome with or without minimal side effects.
Acknowledgments
This work was supported by a grant from the National Research Foundation of Korea (No. 2017R1A2B4004748). D. H. Kim and D. Jeong are acknowledged for technical assistance.
Footnotes
Conflict of Interest
The authors have no financial conflicts of interest to declare.
REFERENCES
- 1.Allred CD, Allred KF, Ju YH, Virant SM, Helferich WG. Soy diets containing varying amounts of genistein stimulate growth of estrogen-dependent (MCF-7) tumors in a dose-dependent manner. Cancer Res. 2001;61:5045–5050. [PubMed] [Google Scholar]
- 2.Aura A-M. Microbial metabolism of dietary phenolic compounds in the colon. Phytochem. Rev. 2008;7:407–429. doi: 10.1007/s11101-008-9095-3. [DOI] [Google Scholar]
- 3.Avis NE, Stellato R, Crawford S, Bromberger J, Ganz P, Cain V, et al. Is there a menopausal syndrome? Menopausal status and symptoms across racial/ethnic groups. Soc. Sci. Med. 2001;52:345–356. doi: 10.1016/S0277-9536(00)00147-7. [DOI] [PubMed] [Google Scholar]
- 4.Blanc PJ, Laussac J, Le Bars J, Le Bars P, Loret M, Pareilleux A, et al. Characterization of monascidin A from Monascus as citrinin. Int. J. Food Microbiol. 1995;27:201–213. doi: 10.1016/0168-1605(94)00167-5. [DOI] [PubMed] [Google Scholar]
- 5.Boonmuen N, Gong P, Ali Z, Chittiboyina AG, Khan I, Doerge DR, et al. Licorice root components in dietary supplements are selective estrogen receptor modulators with a spectrum of estrogenic and anti-estrogenic activities. Steroids. 2016;105:42–49. doi: 10.1016/j.steroids.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Boever P, Demaré W, Vanderperren E, Cooreman K, Bossier P, Verstraete W. Optimization of a yeast estrogen screen and its applicability to study the release of estrogenic isoflavones from a soygerm powder. Environ. Health Perspect. 2001;109:691–697. doi: 10.1289/ehp.01109691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Depypere HT, Comhaire FH. Herbal preparations for the menopause: beyond isoflavones and black cohosh. Maturitas. 2014;77:191–194. doi: 10.1016/j.maturitas.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Endo A. Monacolin K, a new hypocholesteroemic agent produced by a Monascus species. J. Antibiot. (Tokyo). 1979;32:852–854. doi: 10.7164/antibiotics.32.852. [DOI] [PubMed] [Google Scholar]
- 9.Erdogrul L. Review of the studies on the red yeast rice (Monascus purpureus) Turkish Electro. J. Biotechnol. 2004;2:37–49. [Google Scholar]
- 10.Ettinger B. Overview of estrogen replacement therapy:a historical perspective. Proc. Soc. Exp. Biol. Med. 1998;217:2–5. doi: 10.3181/00379727-217-44198. [DOI] [PubMed] [Google Scholar]
- 11.Fox JE, Burow ME, McLachlan JA, Miller III CA. Detecting ligands and dissecting nuclear receptor-signaling pathways using recombinant strains of the yeast Saccharomyces cerevisiae. Nat. Protoc. 2008;3:637–645. doi: 10.1038/nprot.2008.33. [DOI] [PubMed] [Google Scholar]
- 12.Gong P, Madak-Erdogan Z, Li J, Cheng J, Greenlief CM, Helferich W, et al. Transcriptomic analysis identifies gene networks regulated by estrogen receptor α ( ERα) and ERβ that control distinct effects of different botanical estrogens. Nucl. Recept. Signal. 2014;12:e001. doi: 10.1621/nrs.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hankinson SE, Eliassen AH. Endogenous estrogen, testosterone and progesterone levels in relation to breast cancer risk. J. Steroid Biochem. Mol. Biol. 2007;106:24–30. doi: 10.1016/j.jsbmb.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong S-Y, Oh J-H, Lee I. Simultaneous enrichment of deglycosylated ginsenosides and monacolin K in red ginseng by fermentation with Monascus pilosus. Biosci. Biotechnol. Biochem. 2011;75:1490–1495. doi: 10.1271/bbb.110195. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh C-Y, Santell RC, Haslam SZ, Helferich WG. Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res. 1998;58:3833–3838. [PubMed] [Google Scholar]
- 16.Hwang HJ, Jeong SC, Park JP. Production of liquiritigenin with cell-based biotrasformation and its antiaging activity. Kor. Soc. Biotechnol. Bioeng. J. 2015;30:166–174. doi: 10.7841/ksbbj.2015.30.4.166. [DOI] [Google Scholar]
- 17.Writing Groups for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 18.Kao T-C, Wu C-H, Yen G-C. Bioactivity and potential helth benefits of licorice. J. Agric. Food Chem. 2014;62:542–553. doi: 10.1021/jf404939f. [DOI] [PubMed] [Google Scholar]
- 19.Kim H-J, Ji GE, Lee I. Natural occuring levels of citrinin and monacolin K in Korean Monascus fermentation products. Food Sci. Biotechnol. 2007;16:142–145. [Google Scholar]
- 20.Kim JY, Kim H-J, Oh J-H, Lee I. Characteristics of Monascus sp. isolated from Monascus fermentation products. Food Sci. Biotechnol. 2010;19:1151–1157. doi: 10.1007/s10068-010-0164-1. [DOI] [Google Scholar]
- 21.Kondo K, Shiba M, Nakamura R, Morota T, Shoyama Y. Constituent properties of licorices derived from Glycyrrhiza uralensis, G. glabra, or G. inflata identified by genetic information. Biol. Pharm. Bull. 2007;30:1271–1277. doi: 10.1248/bpb.30.1271. [DOI] [PubMed] [Google Scholar]
- 22.Krejci ME, Bretz NS, Koechel DA. Citrinin produces acute adverse changes in renal function and ultrastructure in pentobarbital-anesthetized dogs without concomitant reductions in [potassium] plasma. Toxicology. 1996;106:167–177. doi: 10.1016/0300-483X(95)03183-G. [DOI] [PubMed] [Google Scholar]
- 23.Kuba-Miyara M, Yasuda M. Bioorganic compounds produced by the fungus Monascus and their use in health sciences ans medicine. Mini-Rev. Org. Chem. 2012;9:11–19. doi: 10.2174/157019312799080071. [DOI] [Google Scholar]
- 24.Lee DS, Lee I. Development of monacolin K-enriched ganghwayakssuk (Artemisia princeps Pamp.) by fermentation with Monascus pilosus. J. Microbiol. Biotechnol. 2012;22:975–980. doi: 10.4014/jmb.1201.01016. [DOI] [PubMed] [Google Scholar]
- 25.Lim JY, Kim JJ, Lee DS, Kim GH, Shim JY, Lee I, Imm JY. Physicochemical characteristics and production of whole soymilk from Monascus fermented soybeans. Food Chem. 2010;120:255–260. doi: 10.1016/j.foodchem.2009.10.017. [DOI] [Google Scholar]
- 26.Lin YL. Biologically active components and nutraceuticals in the Monascus-fermented rice: a review. Appl. Microbiol. Biotechnol. 2008;77:965–973. doi: 10.1007/s00253-007-1256-6. [DOI] [PubMed] [Google Scholar]
- 27.Lo R, Matthews J. A new class of estrogen receptor beta-selective activators. Mol. Interv. 2010;10:133–136. doi: 10.1124/mi.10.3.3. [DOI] [PubMed] [Google Scholar]
- 28.Ma J, Li Y, Ye Q, Li J, Hua Y, Ju D, et al. Constituents of red yeast rice, a traditional Chinese food and medicine. J. Agric. Food Chem. 2000;48:5220–5225. doi: 10.1021/jf000338c. [DOI] [PubMed] [Google Scholar]
- 29.Mazur W, Adlercreutz H. Overview of naturally occuringendocrine-active substances in the human diet in relation to human health. Nutrition. 2000;16:654–687. doi: 10.1016/S0899-9007(00)00333-6. [DOI] [PubMed] [Google Scholar]
- 30.Mersereau JE, Levy N, Staub RE, Baggett LF, Leitman DC. Liquiritigenin is a plant-derived highly selective estrogen receptor beta agonist. Mol. Cell. Endocrinol. 2008;283:49–57. doi: 10.1016/j.mce.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller III CA, Tan X, Wilson M, Bhattacharyya S, Ludwig S. Single plasmids expressing human steroid hormone receptors and a reporter gene for use in yeast signaling assays. Plasmid. 2010;63:73–78. doi: 10.1016/j.plasmid.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nilsson S, Koehler KF, Gustafsson JA. Development of subtype-selective oestrogen receptor-based therapeutics. Nat. Rev. Drug Discov. 2011;10:778–792. doi: 10.1038/nrd3551. [DOI] [PubMed] [Google Scholar]
- 33.Oldenhave A, Jaszmann LJ, Haspels AA, Everaerd WTA. Impact of climacteric on well-being: a survey based on 5213 women 39 to 60 years old. Am. J. Obstet. Gynecol. 1993;168:772–780. doi: 10.1016/S0002-9378(12)90817-0. [DOI] [PubMed] [Google Scholar]
- 34.Paruthiyil S, Cvoro A, Zhao X, Wu Z, Sui Y, Staub RE, et al. Drug and cell type-specific regulation of genes with different classes of estrogen receptor β-selective agonists. PLoS One. 2009;4:e6271. doi: 10.1371/journal.pone.0006271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pisareva E, Savov V, Kujumdzieva A. Pigments and citrinin biosynthesis by fungi belonging to genus Monascus. Z. Naturforsch. C J. Biosci. 2005;60:116–120. doi: 10.1515/znc-2005-1-221. [DOI] [PubMed] [Google Scholar]
- 36.Roepke TA, Ronnekleiv OK, Kelly MJ. Physiological consequences of membrane-initiated estrogen signaling in the brain. Front. Biosci. 2011;16:1560–1573. doi: 10.2741/3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato Y, He J-X, Nagai H, Tani T, Akao T. Isoliquiritigenin, one of the antispasmodic principles of Glycyrrhiza ularensis roots, acts in the lower part of intestine. Biol. Pharm. Bull. 2007;30:145–149. doi: 10.1248/bpb.30.145. [DOI] [PubMed] [Google Scholar]
- 38.Simmler C, Hajirahimkhan A, Lankin DC, Bolton JL, Jones T, Soejarto DD, et al. Dynamic residual complexity of the isoliquiritigenin-liquiritigenin interconversion during bioassay. J. Agric. Food Chem. 2013;61:2146–2157. doi: 10.1021/jf304445p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song J, Luo J, Ma Z, Sun Q, Wu C-H, Li X. Quality and authenticity conttrol of functional red yeast rice- a review. Molecules. 2019;24(10) doi: 10.3390/molecules24101944. pii: E19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song W, Qiao X, Chen K, Wang Y, Ji S, Feng J, et al. Biosynthesis-based quantitative analysis of 151 secondary metabolites of licorice to differentiate medicinal Glycyrrhiza species and their hybrids. Anal. Chem. 2017;89:3146–3153. doi: 10.1021/acs.analchem.6b04919. [DOI] [PubMed] [Google Scholar]
- 41.Su Y-C, Wang J-J, Lin T-T, Pan T-M. Production of the secondary metabolites γ-aminobutyric acid and monacolin K by Monascus. J. Ind. Microbiol. Biotechnol. 2003;30:41–46. doi: 10.1007/s10295-002-0001-5. [DOI] [PubMed] [Google Scholar]
- 42.Tamir S, Eizenberg M, Somjen D, Izrael S, Vaya J. Estrogen-like activity of glabrene and other constituents isolated from licorice root. J. Steroid Biochem. Mol. Biol. 2001;78:291–298. doi: 10.1016/S0960-0760(01)00093-0. [DOI] [PubMed] [Google Scholar]
- 43.van Patten CL, Olivotto IA, Chambers GK, Gelmon KA, Hislop TG, Templeton E, et al. Effect of soy phytoestrogens on hot flashes in postmenopausal women with breast cancer: a randomized, controlled clinical trial. J. Clin. Oncol. 2002;20:1449–1455. doi: 10.1200/JCO.2002.20.6.1449. [DOI] [PubMed] [Google Scholar]
- 44.Wang ZY, Nixon DW. Licorice and cancer. Nutr. Cancer. 2001;39:1–11. doi: 10.1207/S15327914nc391_1. [DOI] [PubMed] [Google Scholar]
- 45.Zhang W, Jiang S, Qian D, Shang Ex, Duan Ja. Effect of liquiritin on human intestinal bacteria growth: metabolism and modulation. Biomed. Chromatogr. 2014;28:1271–1277. doi: 10.1002/bmc.3160. [DOI] [PubMed] [Google Scholar]