Abstract
Since the CD40/CD40 ligand (CD40L) interaction is involved in the regulation of macrophage production of interleukin 12 (IL-12) and T-cell production of gamma interferon (IFN-γ), effector cell functions associated with resistance to Toxoplasma gondii, the role of CD40L in immunity to this parasite was assessed. Infection of C57BL/6 mice with T. gondii results in an upregulation of CD40 expression on accessory cell populations at local sites of infection as well as in lymphoid tissues. Splenocytes from C57BL/6 mice infected with T. gondii for 5 days produced high levels of IL-12 and IFN-γ when stimulated with toxoplasma lysate antigen, and blocking CD40L did not significantly alter the production of IFN-γ or IL-12 by these cells. Similar results were observed with splenocytes and mononuclear cells isolated from the brains of chronically infected mice. Interestingly, although CD40L−/− mice infected with T. gondii produced less IL-12 than wild-type mice, they produced comparable levels of IFN-γ but succumbed to toxoplasmic encephalitis 4 to 5 weeks after infection. The inability of CD40L−/− mice to control parasite replication in the brain correlated with the ability of soluble CD40L, in combination with IFN-γ, to activate macrophages in vitro to control replication of T. gondii. Together, these results identify an important role for the CD40/CD40L interaction in resistance to T. gondii. However, this interaction may be more important in the control of parasite replication in the brain rather than the generation of protective T-cell responses during toxoplasmosis.
The induction of a TH1-type immune response is critical for resistance to many intracellular pathogens, including Toxoplasma gondii (10). The events that lead to protective immunity are dependent on the production of interleukin 12 (IL-12), which drives the development of a TH1-type response dominated by the production of gamma interferon (IFN-γ). IFN-γ is the major mediator of resistance to T. gondii (41) and is required for the activation of effector mechanisms that are essential for control of T. gondii (10). The importance of T cells in resistance to T. gondii is best illustrated by the patients with acquired immune deficiencies who develop toxoplasmic encephalitis (TE). For example, patients with certain cancers, or who are being actively immunosuppressed to allow successful transplantation, are susceptible to reactivation of toxoplasmosis (21, 36). Moreover, patients with AIDS become susceptible to TE as their T-cell counts fall (20), which correlates with a reduction in the levels of IFN-γ that they can produce (11). The common characteristic of these patients is that they have an acquired defect in their T-cell functions that cripples the ability of the immune system to control T. gondii.
Interestingly, a role for the CD40/CD40 ligand (CD40L) interaction in resistance to T. gondii is indicated by the development of TE in pediatric patients with a primary defect in this receptor-ligand interaction (24, 42). However, although the CD40/CD40L interaction is important for a number of immunological processes, it is unclear how this defect leads to susceptibility to TE. For example, the interaction of CD40L on T cells with CD40 on B cells is a critical signal required for isotype switching, and in the absence of this signal patients develop a hyper-immunoglobulin M (IgM) syndrome (26). Moreover, the CD40/CD40L interaction is important in providing costimulation for T cells, has an important role in the maturation and activation of accessory cells to produce IL-12 and upregulate costimulatory molecules, and can directly activate macrophage effector functions (7, 15, 16, 23, 27, 30, 35, 38, 39, 43, 45). Studies which have directly addressed the role of the CD40/CD40L interaction in immunity to T. gondii have shown that human macrophages infected with T. gondii upregulate their expression of CD40 and that the ability of live parasites to stimulate maximal production of IL-12 and IFN-γ by human peripheral blood mononuclear cells (PBMCs) in vitro is dependent on the CD40/CD40L interaction (40). However, studies in a murine system have demonstrated that intravenous treatment of mice with soluble toxoplasma antigens results in production of IL-12 by dendritic cells that is independent of the CD40/CD40L interaction (29). Nevertheless, it is clear that patients deficient in CD40L signaling are highly susceptible to toxoplasmosis, but the mechanism whereby this interaction mediates resistance to T. gondii is uncertain.
In order to understand the role of the CD40/CD40L interaction in immunity to T. gondii the expression of CD40 was assessed following infection of C57BL/6 mice, and the effect of anti-CD40L antibodies on production of IL-12 and IFN-γ during recall responses was measured. To address the role of this interaction in vivo, CD40L−/− mice were infected with T. gondii, and the course of the infection was followed and immune responses of these mice were analyzed. Lastly, the ability of soluble CD40L (sCD40L) to activate macrophages in vitro to control parasite replication was assessed. Together, the results obtained suggest that the CD40/CD40L interaction has a limited role in the regulation of T-cell responses associated with resistance to T. gondii. Rather, this interaction may have a more important role in the activation of the effector mechanisms that control parasite replication in the brain.
MATERIALS AND METHODS
Animals.
Female CBA/CaJ, 129/B6 and C57BL/6 mice were obtained from Jackson Laboratories. Mice were between 4 and 6 weeks of age when used for experiments. CD40L−/− mice on a 129/B6 background were either obtained from Jackson Laboratories or supplied by Immunex (Seattle, Wash.) and were bred and maintained within Thoren caging units within the animal facilities at the University of Pennsylvania.
Parasites.
Toxoplasma lysate antigen (TLA) was prepared from the RH strain tachyzoites as previously described (34). The Rh strain tachyzoites were maintained in human foreskin fibroblasts. TLA was titrated to determine the optimal concentration for splenocyte proliferation and was used at 25 to 40 μg/ml for these experiments. Cysts of the ME49 strain of T. gondii were harvested from brains of CBA/CaJ mice infected for 1 to 2 months. For experimental infections, mice were given 20 ME49 cysts intraperitoneally (i.p.) in a volume of 0.2 ml.
Histology.
At different times postinfection, samples of lung, liver, heart, spleen, and brain were removed from each mouse, fixed in 4% formaldehyde–70% ethanol–0.8 N acetic acid, and embedded in paraffin. Organs were sectioned and stained with hematoxylin and eosin for evaluation of pathological changes. Cytospin preparations of peritoneal exudate cells (PECs) were prepared as previously described and used to estimate the percentage of cells infected with T. gondii (19). Where the percentage of cells infected was less than 0.1% but parasites could still be detected, a value of 0.1% was assigned.
Reagents.
Anti-mouse CD3ɛ (145-2C11) was prepared from hybridoma supernatants. Hamster anti-murine CD40 (4C11) was provided by Bob Coffman (DNAX, Palo Alto, Calif.). IFN-γ, IL-2, and IL-4 levels were measured by using two-site enzyme-linked immunosorbent assays (ELISAs) as previously described (1, 31). IL-12 p40 levels were measured by using monoclonal antibody (MAb) C17.8 and biotinylated MAb C15.6, and IL-12 p70 levels were measured by using MAb C18.2 and biotinylated MAb C17.8 (obtained from hybridomas provided by Giorgio Trinchieri, Wistar Institute, Philadelphia, Pa.). Lipopolysaccharide was purchased from Sigma (St. Louis, Mo.). Murine IFN-γ was purchased from R & D Systems (Minneapolis, Minn.), and murine sCD40L (lot 7379-081, 14 pg of endotoxin per mg based on the kinetic chromatogenic Limulus amoebocyte lysate assay from BioWhittaker, Walkersville, Md.) was a generous gift from Immunex.
Analysis of T-cell responses.
Spleens from mice were harvested and dissociated in complete RPMI medium (10% heat-inactivated fetal calf serum [HyClone Laboratories, Logan, Utah], 1,000 U of penicillin/ml, 10 mg of streptomycin/ml, 0.25 mg of Fungizone [BioWhittaker]/ml) into single-cell suspensions as previously described (6). Cells were plated at a density of 4 × 105 cells per well in a final volume of 200 μl in 96-well plates and incubated with various stimuli. Supernatants were harvested after 48 h and assayed for the production of IL-2, IL-12, and IFN-γ. For analysis of the responses of mononuclear cells in the brains of mice, animals were first anesthetized and perfused with sterile phosphate-buffered saline to remove peripheral blood from the brain. Following excision, brains were minced with scissors and then digested for 1 h at 37°C with 300 μg of collagenase/dispase (Boehringer Mannhein, Indianapolis, Ind.) and 600 μg of DNase I (Boehringer Mannheim) per ml in complete RPMI medium. The dissociated brain tissue was pelleted at 200 × g for 10 min, resuspended in a 60% isotonic Percoll solution (Sigma), and overlaid with a 30% Percoll solution. Discontinuous gradients were centrifuged for 25 min at 1,000 × g. After removal of the myelin layer on top of the gradient, brain-associated mononuclear cells (BMNC) were harvested from the 30%/60% interphase and washed twice in complete RPMI medium before further analysis (33). The composition of the cells isolated reflected the composition observed by using immunohistochemistry for CD4, CD8, and B220.
RNAse protection assay.
Total RNA was isolated from the brains of mice by the guanidine isothiocyanate method and was assayed for cytokine mRNA levels by using the Riboquant multiprobe protection assay (RPA) system (PharMingen, San Diego, Calif.). Briefly, 10 μg of RNA from each sample was hybridized in solution with the appropriate radiolabeled antisense RNA probe set. mCK-1 (IL-4, IL-5, IL-10, IL-13, IL-15, IL-9, IL-2, IL-6, and IFN-γ) was employed for the detection of cytokine mRNA as recommended by the manufacturers. Following hybridization, free probe and remaining single-strand RNA were digested with RNases, and the protected probes were purified and resolved on 5% denaturing polyacrylamide gels by using ultrapure Sequagel reagents (National Diagnostics, Atlanta, Ga.). Dried gels were then exposed to a phosphorimaging screen (Bio-Rad) and visualized with a Bio-Rad molecular imager system.
Cytofluorometric analysis.
After dissociation and lysis of erythrocytes, cells were resuspended at a final concentration of 107/ml in fluorescence-activated cell sorting (FACS) buffer composed of 1× phosphate-buffered saline (BioWhittaker), 0.2% bovine serum albumin fraction V (Sigma), and 4 mM sodium azide. For FACS analysis, 106 cells were stained with various conjugated MAbs specific for F480 (Caltag, San Francisco, Calif.), CD40, CD4, or CD8 (PharMingen) for 20 min on ice in the presence of saturating amounts of Fc Block (PharMingen). Cells were then washed and analyzed with a FACScalibur flow cytometer (Becton Dickinson, Mountain View, Calif.). For biotinylated antibodies, cells were stained and washed as described above and then incubated with fluorescein isothiocyanate- or phycoerythrin-conjugated streptavidin (PharMingen) for 20 min on ice. Cells were then washed with FACS buffer and analyzed. Antibodies and streptavidin reagents were used at dilutions empirically determined to give optimal staining for flow cytometric analyses. Results were analyzed by using CELL Quest software (Becton Dickinson).
Analysis of macrophage functions.
Bone marrow macrophages (BMMφ) from C57BL/6 or B6/129 mice were derived from bone marrow cells grown on petri dishes (150 by 15 mm; Falcon; Becton Dickinson Labware, Paramus, New Jersey) in Dulbecco modified Eagle medium containing 20% (vol/vol) heat-inactivated fetal calf serum, 20% L-cell-conditioned medium, 100 U of penicillin/ml, and 100 μg of streptomycin per ml. After at least 6 days of incubation at 37°C and 5% CO2 in a humidified incubator, adherent cells were harvested by using ice-cold buffered saline without calcium or magnesium and washed three times in complete RPMI medium. For antitoxoplasma activity, 4 × 105 BMMφ in complete RPMI medium were incubated at 37°C and 5% CO2 in Falcon polypropylene tubes (Becton Dickinson) with medium alone or with 100 U of IFN-γ/ml, 20 μg of sCD40L/ml, or the combination of IFN-γ plus sCD40L. After 4 h, cultures were infected with RH tachyzoites at a ratio of one parasite to one macrophage for 2 h. Cells were then washed to remove free parasites, and cultures were replenished with IFN-γ, sCD40L, or the combination of these reagents. At 2 and 20 h postinfection 5 × 104 cells were removed, cytospins were prepared, and cells were stained with DiffQuik (Dade Diagnostics, Aguada, P.R.). Baseline infections and the numbers of parasites per 100 infected cells were determined microscopically.
Statistics.
INSTAT software (GraphPad, San Diego, Calif.) was used for the unpaired two-tailed Student t test, paired t test evaluations, or the Mann-Whitney nonparametric test. A P value of <0.05 was considered significant.
RESULTS
Expression of CD40 following infection with T. gondii.
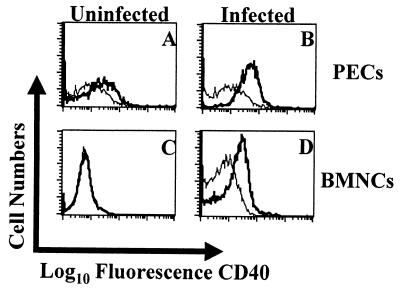
C57BL/6 mice were inoculated i.p. with 20 cysts of T. gondii, and the expression of CD40 was assessed during the early phase of infection by using FACS. By day 5 postinfection, there were increased numbers of macrophages that expressed CD40 in the peritoneum (Fig. 1A and B) or spleens (data not shown) of infected mice. In addition, since C57BL/6 mice develop TE during the chronic phase of the infection, we used immunohistochemistry to assess expression of CD40 in the brains of mice with TE. Staining for CD40 revealed that brains from uninfected mice were negative for CD40 expression, but by 4 weeks postinfection there was intense staining for CD40 associated with areas of inflammation and parasite replication (data not shown). To further characterize the cell populations that expressed CD40 during TE, mononuclear cell populations were isolated from the brains of infected mice and analyzed by using FACS. These studies revealed that F4/80-positive cells, likely macrophages/microglia, expressed elevated levels of CD40 (Fig. 1C and D). These data demonstrate that following infection with T. gondii, the expression of CD40 is upregulated during the acute and chronic phases of disease.
FIG. 1.
Expression of CD40 during toxoplasmosis. PECs were isolated from uninfected C57BL/6 mice (A) or mice that were infected for 5 days (B), and expression of CD40 (thick line) on F480+ cells was measured by FACS. The staining for the isotype control (thin line) was based on a pooled population from infected and uninfected mice. BMNC were isolated from the brains of uninfected C57BL/6 mice (C) or mice infected for 8 weeks (D), and expression of CD40 (thick line) on F480+ cells was measured by FACS. The staining for the isotype control for the individual samples is represented by the thin line. Similar results were observed in two repeat experiments.
Effect of CD40L blockade on production of IL-12 and IFN-γ during toxoplasmosis.
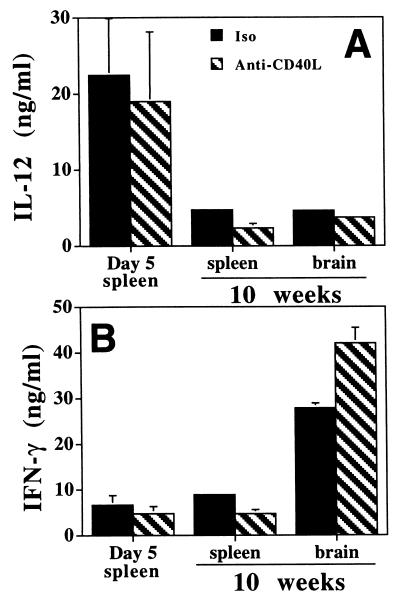
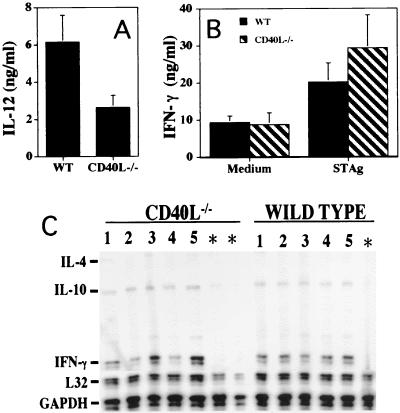
Since the CD40/CD40L interaction has been shown to regulate IL-12 production and T-cell activation, it is possible that the increased expression of CD40 observed following infection is involved in the T. gondii-induced production of IL-12 and IFN-γ. To test this hypothesis, the CD40/CD40L interaction was blocked with anti-CD40L MAb and the production of IL-12 and IFN-γ by splenocytes from C57BL/6 mice infected with T. gondii for 5 days was measured (Fig. 2). Stimulation of splenocytes from these mice with TLA resulted in the production of high levels of IFN-γ and IL-12 (Fig. 2). The addition of anti-CD40L MAb to these cultures did not result in a statistically significant reduction (based on paired t test analysis of the data from five experiments) in the levels of these cytokines, although there was a trend towards decreased production of IL-12. Splenocytes from uninfected mice stimulated with TLA did not produce appreciable levels of IFN-γ; only low levels of IL-12 were detected, and these were not affected by addition of anti-CD40L MAb to these cultures (data not shown).
FIG. 2.
Effect of anti-CD40L on production of IL-12 and IFN-γ during toxoplasmosis. Splenocytes and BMNC were isolated from C57BL/6 mice, cells were stimulated in vitro for 48 h with TLA in the presence of an isotype control antibody (Iso) or anti-CD40L, and the levels of IL-12 (A) and IFN-γ (B) were measured by ELISA. For mice infected for 5 days the results are the means plus SEs of the pooled data from five experiments with three to five mice per experiment. For mice infected for 10 weeks the data are the means plus SEs of the pooled data from three experiments.
The increased expression of CD40 in the brain during TE suggested that this interaction may be important in the regulation of the immune response in the brain. Therefore, the role of CD40L in the production of IL-12 and IFN-γ by splenocytes and BMNC from chronically infected C57BL/6 mice (10 weeks) was tested. Similar to the effects observed with splenocytes during acute infection, the inclusion of anti-CD40L MAb in these cultures did not result in a statistically significant reduction in the production of IFN-γ or IL-12 (Fig. 2). However, we did observe that, similar to the results observed with splenocytes, blockade of CD40L resulted in a trend towards lower levels of IL-12. Together, these results suggest that the CD40L interaction has a limited role in the regulation of ex vivo production of IL-12 or IFN-γ during toxoplasmosis.
CD40L is required for resistance to toxoplasmosis.
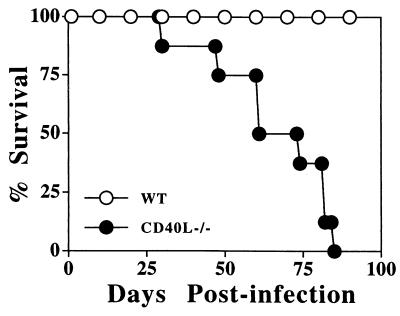
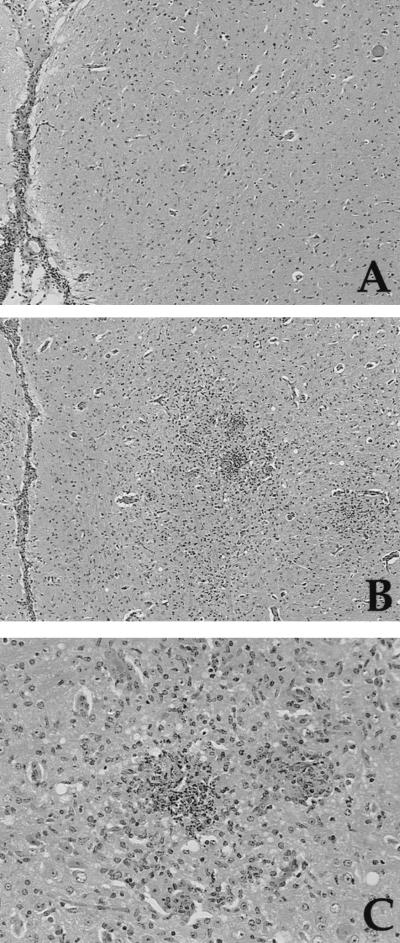
Although the studies described above show that CD40L is not required for the in vitro production of IL-12 or IFN-γ, they do not address whether CD40L is involved in the initiation of these responses in vivo. To functionally test the significance of the CD40/CD40L interaction in resistance to T. gondii in an experimental system, wild-type (WT) and CD40L−/− mice were inoculated with the ME49 strain of T. gondii and the course of infection was assessed. WT mice with a B6/129 genetic background are relatively resistant to development of TE, and when these mice were infected with T. gondii they survived for the course of these studies (3 months postinfection). Comparison of the parasite burden in WT and CD40L−/− mice at day 7 postinfection revealed no significant differences in the percentage of PECs infected following i.p. challenge with T. gondii. In a typical experiment (of four performed) the mean percentage of cells infected in WT mice was 0.6% ± 0.14% (mean ± standard error [SE]; n = 6) whereas CD40L−/− mice had 0.39% ± 0.07% of peritoneal cells infected. Statistical analysis revealed no significant difference between these experimental groups (P = 0.2). However, although the CD40L−/− mice survived the acute phase of the infection, they were susceptible to the chronic phase of infection and died within 4 to 6 weeks (Fig. 3). Analysis of the brains of mice infected for 4 weeks revealed that WT and CD40L−/− mice had a similar meningitis but the CD40L−/− mice had a more severe encephalitis associated with the presence of large numbers of cysts and areas of parasite replication (Fig. 4). In several experiments CD40L−/− mice exhibited lung pathology that was more severe than that observed in WT mice. However, this was an inconsistent observation since lung pathologies in WT and CD40L−/− mice at 4 weeks postinfection were not different in other experiments (data not shown). Thus, similar to patients with defects in CD40L signaling, CD40L−/− mice are susceptible to TE.
FIG. 3.
CD40L is required for resistance to T. gondii. Groups of eight WT 129/B6 and CD40L−/− mice were inoculated i.p. with 20 cysts of T. gondii, and survival was monitored daily. WT mice survived for at least 3 months. This experiment was repeated three times with similar results.
FIG. 4.
Histological analysis of brains of WT and CD40L−/− mice infected with T. gondii. (A) Brain of a WT mouse 4 weeks postinfection showing a mild meningitis, minimal encephalitis, and a single cyst (top right). Hematoxylin and eosin stain was used. Magnification, ×64. (B) Brain of a CD40L−/− mouse 4 weeks postinfection showing mild meningitis and severe encephalitis characterized by inflammatory foci surrounded by edematous neuropil. Hematoxylin and eosin stain was used. Magnification, ×64. (C) A higher magnification of the inflammatory cell foci in the CD40L−/− mouse showing a necrotic neutrophilic focus (left) and a smaller histiocytic focus (right) surrounded by marked gliosis. Hematoxylin and eosin stain was used. Magnification, ×200.
CD40L is not required for the generation or maintenance of TH1-type responses during toxoplasmosis.
While initial studies indicated that the CD40/CD40L interaction was not required for the ex vivo production of IFN-γ or IL-12 by cells from infected WT mice, it was still possible that the susceptibility of CD40L−/− mice to TE could be a consequence of an intrinsic defect in the production of these cytokines. Indeed, although infected CD40L−/− mice produced increased levels of IL-12 in serum and during recall responses, these levels were reduced compared with those in WT mice (Fig. 5A). However, CD40L−/− mice produced levels of IFN-γ in serum and recall responses that were comparable to those in WT mice (Fig. 5B). Thus, the defect in IL-12 production observed in the CD40L−/− mice did not have a significant effect on the infection-induced production of IFN-γ.
FIG. 5.
Analysis of IL-12 and IFN-γ production during acute and chronic phases of toxoplasmosis in WT and CD40L−/− mice. WT and CD40L−/− mice were infected with T. gondii, and groups of mice were sacrificed at 5 days or 4 weeks after infection. Serum was collected from mice infected for 5 days and assayed for levels of IL-12 (A) and IFN-γ (B). Splenocytes from infected mice were stimulated in vitro with TLA, and the levels of IL-12 or IFN-γ were measured by ELISA. Results presented are means plus SEs of the pooled data from three experiments.
Although production of IFN-γ in the periphery of infected WT and CD40L−/− mice was similar, the increased levels of inflammation present in the brains of infected CD40L−/− mice suggested that there may be differences in the intracerebral immune response between WT and CD40L−/− mice. Characterization of the inflammatory responses in the brains of these animals revealed that similar numbers of mononuclear cells were isolated from WT and CD40L−/− mice at 4 weeks postinfection (WT, 4.5 × 106 ± 1.2 × 106/brain; CD40L−/−, 3.9 × 106 ± 1.6 × 106/brain; P = 0.32). While the relative percentages of CD8+ T cells were similar (WT, 33.5% ± 7.75% [mean ± standard deviation]; CD40L−/−, 36.7% ± 6%), there was a significant increase in the percentage (mean ± standard deviation) of CD4+ T cells in the CD40L−/− mice compared with WT (WT, 27.2% ± 3.7%; CD40L−/−, 38.4% ± 7.75; P = 0.0173). These data indicate that CD40L is not required for the ability of T cells to traffic to the brain during TE. Cultured BMNC from WT and CD40L−/− mice produced high basal levels of IL-12 (Fig. 6A) that were not altered after stimulation with TLA (data not shown). However, BMNC from CD40L−/− mice produced significantly less IL-12 than BMNC from WT mice. Interestingly, BMNC from CD40L−/− mice produced levels of IFN-γ that were comparable to levels of BMNC from WT mice (Fig. 6B). These findings were further supported by RPA analysis of brain tissue from WT and CD40L−/− mice, which demonstrated that there were similar levels of IFN-γ mRNA in the brains of these mice (Fig. 6C). These data suggest that the increased susceptibility of CD40L−/− mice to TE is not a consequence of a defect in the production of IFN-γ, either in the periphery or within the brain.
FIG. 6.
BMNC were isolated from WT and CD40L−/− mice infected with T. gondii for 4 weeks, cells were stimulated in vitro with TLA for 48 h, and the levels of IL-12 (A) and IFN-γ (B) in culture supernatants were measured by ELISA. The levels of IL-12 in the cultures were not altered by the addition of TLA. (C) RPA analysis of mRNA isolated from the brains of five infected (lanes 1 to 5) and two uninfected (asterisks) CD40L−/− mice and five infected and one uninfected WT mouse (infections were for 4 weeks). Note that the brains of uninfected mice lack IFN-γ and IL-10 mRNA but these cytokines are upregulated following infection.
Stimulation through CD40 enhances the ability of macrophages to control replication of T. gondii.
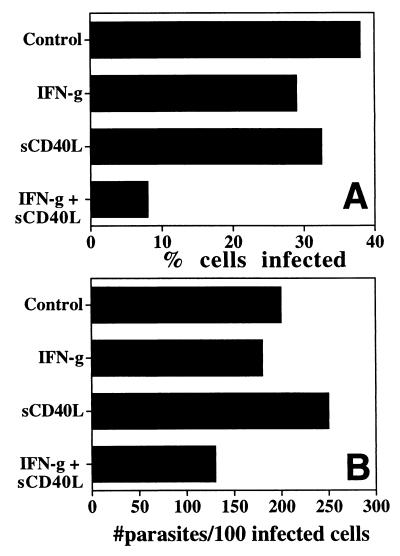
Although the production of IFN-γ during toxoplasmosis was independent of the CD40/CD40L interaction, CD40L−/− mice were still highly susceptible to TE. These results suggested that the absence of CD40L may lead to a defect in effector cell function. Since the CD40/CD40L interaction can directly activate macrophages to produce antimicrobial effector molecules (38), we assessed whether stimulation through CD40 would affect the ability of macrophages to control replication of T. gondii. BMMφ from C57BL/6 mice were used to test this hypothesis. Macrophages infected with tachyzoites of T. gondii were unable to control parasite replication, and the addition of IFN-γ (100 U/ml) or sCD40L alone did not affect the percentage of cells infected (Fig. 7A). However, when IFN-γ and sCD40L were used in combination, they significantly reduced the percentage of cells infected (n = 6; P < 0.0001) (Fig. 7A). In addition, the combination of sCD40L also resulted in a decrease in the number of parasites per 100 infected cells (Fig. 7B). A similar reduction in the percentage of cells infected and numbers of parasites per 100 infected cells was observed when we used the stimulatory anti-CD40 MAb 4C11 instead of sCD40L (data not shown). Thus, stimulation through CD40 (when used in combination with IFN-γ) is able to activate macrophages to inhibit replication of T. gondii.
FIG. 7.
Stimulation with sCD40L plus IFN-γ inhibits replication of T. gondii. BMMφ were preincubated with different stimuli and then infected with T. gondii as described in Materials and Methods. Cells were incubated in medium alone (control), IFN-γ (100 U/ml) alone, sCD40L (10 μg/ml) alone, or the combination of IFN-γ plus sCD40L, and the percentage of cells infected (A) and numbers of parasites per infected cell (B) were estimated after 18 to 20 h. Similar results were observed in five additional experiments using BMMφ from individual mice.
DISCUSSION
The CD40/CD40L interaction has been shown to be important in resistance to several intracellular parasites, including Leishmania species (4, 22, 37), Cryptosporidium parvum (8), and Pneumocystis carinii (44). In those studies, the protective effect of CD40L was attributed primarily to its immunoregulatory properties, through either regulation of IL-12 production or, alternatively, production of antibody. It is well appreciated that stimulation of macrophages or dendritic cells through CD40 will lead to the production of IL-12, which is important for resistance to many intracellular pathogens. However, stimulation through CD40 will also affect maturation of dendritic cells and upregulate the expression of B7 on accessory cells and so enhance T-cell responses (16, 45). In addition, the CD40/CD40L pathway can lead to direct stimulation of T cells (43) and has an important role in the initiation of T-cell responses and generation of effector cells (17, 39). The studies presented here identify a critical role for the CD40/CD40L interaction in immunity to the important opportunistic pathogen T. gondii. However, our studies suggest that rather than being required for the development of a cell-mediated response that leads to the production of IFN-γ, CD40L may function in resistance to T. gondii by acting in concert with IFN-γ to control parasite replication in the brain.
Recent studies have examined the role of the CD40/CD40L interaction in the generation of IL-12 and IFN-γ by PBMCs from humans seronegative for T. gondii (40). In those experiments, the production of IL-12 and IFN-γ by PBMCs from healthy control patients stimulated with suboptimal numbers of tachyzoites was almost completely blocked by the addition of anti-CD40L. Moreover, PBMCs from patients with hyper-IgM syndrome (who lack functional CD40L) were defective in their ability to produce IL-12 and IFN-γ in response to T. gondii. These results suggest that the CD40/CD40L interaction has an important regulatory role in the initiation of resistance to T. gondii. In agreement with those findings, CD40L−/− mice have a reduced ability to produce IL-12 during infection, and blockade of the CD40/CD40L interaction in recall responses from infected WT mice resulted in a trend towards decreased IL-12 production. Interestingly, the reduction in the levels of IL-12 produced by splenocytes and BMNC from CD40L−/− mice was more noteworthy than the effects of anti-CD40L on these populations from WT mice. These results suggest that the CD40/CD40L interaction has an important role in priming accessory cells to produce IL-12 rather than being directly required for ex vivo production of IL-12.
In contrast to studies with human PBMCs, the IFN-γ responses in both of these murine systems were unaffected by anti-CD40L or a complete absence of CD40L. Whether the differences in IFN-γ production observed between CD40L−/− mice and human PBMCs indicate real differences between the role of CD40L in the regulation of human and murine immune responses to T. gondii or are simply a function of different experimental approaches is not clear. Nevertheless, like the hyper-IgM patients, mice deficient in the CD40/CD40L pathway are susceptible to T. gondii as well as the opportunistic parasites P. carinii and C. parvum. Interestingly, although there are reports of disseminated tuberculosis and histoplasmosis in patients that lack CD40L (25), studies with mice infected with Mycobacterium tuberculosis (5) or Histoplasma capsulatum (48) demonstrated that the CD40/CD40L interaction is not required for resistance to these pathogens. Thus, murine models of CD40L deficiency do not always correlate with human disease.
It has long been established that IFN-γ is the major mediator of resistance to T. gondii in the periphery as well as in the brain (10). In contrast, IL-12 is required for the initiation of the protective TH1-type response but is not required for the control of TE (13). In support of those studies, the defective production of IL-12 in the brains of CD40L−/− mice did not appear to affect the production of IFN-γ at this site. However, it should be noted that although CD8+ T cells are regarded as the major source of IFN-γ during TE there was an increase in the percentage of CD4+ T cells present in the BMNC derived from the CD40L−/− mice with TE. Nevertheless, it is unlikely that the reduced levels of IL-12 produced by BMNC from CD40L−/− mice are the cause of the enhanced parasite replication and severe TE observed in the CD40L−/− mice. Therefore, if CD40L is not required for regulation of protective T-cell responses (as measured by production of IFN-γ) in the brain, what is its role in controlling T. gondii at this site? The finding that sCD40L stimulation can enhance the ability of macrophages to control parasite replication offers a possible explanation. The presence of activated T cells in the brain during TE (28) and the immunohistochemical detection of CD40L at this site (C. A. Hunter, unpublished observations) suggest that T cells could be directly involved in the local activation of antiparasite effector mechanisms. Experiments are currently ongoing to address this issue. However, these findings raise several questions about the role of CD40L and tumor necrosis factor alpha (TNF-α) in the activation of effector functions required for resistance to T. gondii. Several studies have identified TNF-α (9, 46) and inducible nitric oxide synthase (iNOS) (32) as being critical for resistance to T. gondii in the brain. The protective effect of TNF-α in the brain was attributed to the ability of TNF-α to act as a second signal for IFN-γ-activated macrophages to produce NO. Whether there are reduced levels of iNOS in the brains of mice that lack TNF or TNF signaling is controversial. Original studies in which mice were treated with anti-TNF-α MAb reported that the enhanced susceptibility to TE correlated with reduced levels of iNOS mRNA (12) and similar results were reported with mice deficient in the p55 TNF-receptor (TNF-R) (9). In contrast, studies with mice deficient in both the p55 and p75 TNF-R and infected with T. gondii reported that iNOS levels in the brains of these mice were normal (46). We have also observed that there are elevated levels of iNOS protein in the brains of p55 TNF-R−/− mice during TE (W. Walker and C. A. Hunter, unpublished data) as well as in mice treated with anti-TNF-α MAb (G. Reichmann and C. A. Hunter, unpublished data), suggesting that there is a TNF-α-independent mechanism that is operative in the central nervous system that leads to activation of iNOS. Given the similar phenotypes of the TNF-R−/− and CD40L−/− mice (both die of TE) and that CD40L and TNF-α are members of the same cytokine superfamily, use similar signaling pathways, and can enhance macrophage production of NO, it is possible that TNF-α and CD40L are both required for maximal induction of NO production during TE. Interestingly, our initial studies have found that BMNC from chronically infected CD40L−/− mice produce normal levels of NO, and immunohistochemistry has shown the presence of iNOS protein in the brains of these mice (unpublished observations). Together with the previous studies on TNF-R-deficient mice, these findings suggest the possibility that the ability of TNF-α and/or CD40L to mediate killing of T. gondii in the brain may involve NO-dependent as well as NO-independent pathways. Interestingly, Yap and Sher provided evidence for iNOS-dependent and -independent mechanisms of resistance to T. gondii in the brain (47). Further studies are needed to directly determine the mechanism whereby different TNF family members mediate resistance to T. gondii in the brain.
The regulation of protective immunity to T. gondii in the brain is complex, and the studies presented here add to our knowledge of the molecules involved in this process. It is clear that CD40L is required to control parasite replication in the brain, and recent studies support a role for CD40L in the immunopathogenesis of other neuroimmune diseases. The CD40/CD40L interaction is required for activated TH1 cells to stimulate microglia to produce IL-12 (2), and this interaction is thought to be involved in the pathogenesis of multiple sclerosis (3, 14). Moreover, in support of the findings presented here, Howard and colleagues suggested that the ability of anti-CD40 MAb to inhibit adoptive experimental allergic encephalomyelitis supports a role for CD40L in the ability of T cells to activate macrophage/microglia effector functions in the brain (18). Thus, the CD40/CD40L interaction is not restricted to the regulation of immune responses but appears to have an important role in effector functions at local sites of infection and inflammation.
ACKNOWLEDGMENTS
This work was supported by NIH grant AI 41158-01 and by the Commonwealth of Pennsylvania, Dept. of Agriculture. E.N.V. is supported by an NIH predoctoral fellowship (AI 09562); C.A.H. is a Burroughs Wellcome New Investigator in Molecular Parasitology. J.A. was on research leave sponsored by the Wellcome Trust.
We thank Thad Radzanowski for expert technical assistance and Jay Farrell for advice on the preparation of the manuscript.
REFERENCES
- 1.Abrams J S, Roncarolo M G, Yssel H, Andersson G J, Silver J E. Strategies of anti-cytokine monoclonal antibody development: immunoassay of IL-10 and IL-5 in clinical samples. Immunol Rev. 1992;127:5–24. doi: 10.1111/j.1600-065x.1992.tb01406.x. [DOI] [PubMed] [Google Scholar]
- 2.Aloisi F, Penna G, Polazzi E, Minghetti L, Adorini L. CD40-CD154 interaction and IFN-γ are required for IL-12 but not prostaglandin E2 secretion by microglia during antigen presentation to Th1 cells. J Immunol. 1999;162:1384–1391. [PubMed] [Google Scholar]
- 3.Balashov K E, Smith D R, Khoury S J, Hafler D A, Weiner H L. Increased interleukin 12 production in progressive multiple sclerosis: induction by activated CD4+ T cells via CD40 ligand. Proc Natl Acad Sci USA. 1997;94:599–603. doi: 10.1073/pnas.94.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell K A, Ovendale P J, Kennedy M K, Fanslow W C, Reed S G, Maliszewski C R. CD40 ligand is required for protective cell-mediated immunity to Leishmania major. Immunity. 1996;4:283–289. doi: 10.1016/s1074-7613(00)80436-7. [DOI] [PubMed] [Google Scholar]
- 5.Campos-Neto A, Ovendale P, Bement T, Koppi T A, Fanslow W C, Rossi M A, Alderson M R. CD40 ligand is not essential for the development of cell-mediated immunity and resistance to Mycobacterium tuberculosis. J Immunol. 1998;160:2037–2041. [PubMed] [Google Scholar]
- 6.Candolfi E, Hunter C A, Remington J S. Roles of gamma interferon and other cytokines in suppression of the spleen cell proliferative response to concanavalin A and toxoplasma antigen during acute toxoplasmosis. Infect Immun. 1995;63:751–756. doi: 10.1128/iai.63.3.751-756.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cosyns M, Tsirkin S, Jones M, Flavell R, Kikutani H, Hayward A R. Requirement for CD40-CD40 ligand interaction for elimination of Cryptosporidium parvum from mice. Infect Immun. 1998;66:603–607. doi: 10.1128/iai.66.2.603-607.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deckert-Schluter M, Bluethmann H, Rang A, Hof H, Schluter D. Crucial role of TNF receptor type 1 (p55), but not of TNF receptor type 2 (p75), in murine toxoplasmosis. J Immunol. 1998;160:3427–3436. [PubMed] [Google Scholar]
- 10.Denkers E Y, Gazzinelli R T. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev. 1998;11:569–588. doi: 10.1128/cmr.11.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gazzinelli R T, Bala S, Stevens R, Baseler M, Wahl L, Kovacs J, Sher A. HIV infection suppresses type 1 lymphokine and IL-12 responses to Toxoplasma gondii but fails to inhibit the synthesis of other parasite-induced monokines. J Immunol. 1995;155:1565–1574. [PubMed] [Google Scholar]
- 12.Gazzinelli R T, Eltoum I, Wynn T A, Sher A. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-α and correlates with the downregulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J Immunol. 1993;151:3672–3681. [PubMed] [Google Scholar]
- 13.Gazzinelli R T, Wysocka M, Hayashi S, Denkers E Y, Hieny S, Caspar P, Trinchieri G, Sher A. Parasite-induced IL-12 stimulates early IFN-γ synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol. 1994;153:2533–2543. [PubMed] [Google Scholar]
- 14.Gerritse K, Laman J D, Noelle R J, Aruffo A, Ledbetter J A, Boersma W J A, Claassen E. CD40-CD40 ligand interactions in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci USA. 1996;93:2499–2504. doi: 10.1073/pnas.93.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grewal I S, Flavell R A. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 16.Grewal I S, Foellmer H G, Grewal K D, Xu J, Hardardottir F, Baron J L, Janeway C A, Flavell R A. Requirement for CD40 ligand in costimulation induction, T cell activation, and experimental allergic encephalomyelitis. Science. 1996;273:1864–1867. doi: 10.1126/science.273.5283.1864. [DOI] [PubMed] [Google Scholar]
- 17.Grewal I S, Xu J, Flavell R A. Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature. 1996;378:617–620. doi: 10.1038/378617a0. [DOI] [PubMed] [Google Scholar]
- 18.Howard L M, Miga A J, Vanderlugt C L, Dal Canto M C, Laman J D, Noelle R J, Miller S D. Mechanisms of immunotherapeutic intervention by anti-CD40L (CD154) antibody in an animal model of multiple sclerosis. J Clin Investig. 1999;103:281–290. doi: 10.1172/JCI5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunter C A, Ellis-Neyer L, Gabriel K, Kennedy M, Linsley P, Remington J S. The role of the CD28/B7 interaction in the regulation of NK cell responses during infection with Toxoplasma gondii. J Immunol. 1997;158:2285–2293. [PubMed] [Google Scholar]
- 20.Israelski D M, Chmiel J S, Poggensee L, Phair J P, Remington J S. Prevalence of Toxoplasma infection in a cohort of homosexual men at risk of AIDS and toxoplasmic encephalitis. J Acquir Immune Defic Syndr. 1993;6:414–418. [PubMed] [Google Scholar]
- 21.Israelski D M, Remington J S. Toxoplasmosis in patients with cancer. Clin Infect Dis. 1993;17(Suppl.):S423–S435. doi: 10.1093/clinids/17.supplement_2.s423. [DOI] [PubMed] [Google Scholar]
- 22.Kamanaka M, Yu P, Yasui T, Yoshida K, Kawabe T, Horii T, Kishimoto T, Kikutani H. Protective role of CD40 in Leishmania major infection at two distinct phases of cell-mediated immunity. Immunity. 1996;4:275–281. doi: 10.1016/s1074-7613(00)80435-5. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy M K, Picha K S, Fanslow W C, Grabstein K H, Alderson M R, Clifford K N, Chin W A, Mohler M M. CD40/CD40 ligand interactions are required for T cell-dependent production of interleukin-12 by mouse macrophages. Eur J Immunol. 1996;26:370–378. doi: 10.1002/eji.1830260216. [DOI] [PubMed] [Google Scholar]
- 24.Leiva L E, Junprasert J, Hollenbaugh D, Sorensen R U. Central nervous system toxoplasmosis with an increased proportion of circulating gamma delta T cells in a patient with hyper-IgM syndrome. J Clin Immunol. 1998;18:283–290. doi: 10.1023/a:1027337923709. [DOI] [PubMed] [Google Scholar]
- 25.Levy J, Espanol-Boren T, Thomas C, Fischer A, Tovo P, Bordigoni P, Resnick I, Fasth A, Baer M, Gomez L, Sanders E A, Tabone M D, Plantaz D, Etzioni A, Monafo V, Abinun M, Hammarstrom L, Abrabamsen T, Jones A, Finn A, Klemola T, DeVries E, Sanal O, Peitsch M C, Notarangelo L D. Clinical spectrum of X-linked hyper-IgM syndrome. J Pediatr. 1997;131:47–54. doi: 10.1016/s0022-3476(97)70123-9. [DOI] [PubMed] [Google Scholar]
- 26.Notarangelo L D, Duse M, Ugazio A G. Immunodeficiency with hyper-IgM (HIM) Immunodefic Rev. 1992;3:101–121. [PubMed] [Google Scholar]
- 27.Peng X, Kasran A, Warmerdam P A, de Boer M, Ceuppens J L. Accessory signalling by CD40 for T cell activation: induction of Th1 and Th2 cytokines and synergy with interleukin-12 for interferon-γ production. Eur J Immunol. 1996;26:1621–1627. doi: 10.1002/eji.1830260732. [DOI] [PubMed] [Google Scholar]
- 28.Reichmann G, Villegas E N, Craig L, Peach R, Hunter C A. The CD28/B7 interaction is not required for resistance to Toxoplasma gondii in the brain but contributes to the development of immunopathology. J Immunol. 1999;163:3354–3362. [PubMed] [Google Scholar]
- 29.Reis e Sousa B C, Hieny S, Scharton-Kersten T, Jankovic D, Charset H, Germain R N, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saito K, Sakurai J, Ohata J, Kohsaka T, Hashimoto H, Okumura K, Abe R, Azuma M. Involvement of CD40 ligand-CD40 and CTLA4-B7 pathways in murine acute graft-versus-host disease induced by allogeneic T cells lacking CD28. J Immunol. 1998;160:4225–4231. [PubMed] [Google Scholar]
- 31.Sander B, Hoiden I, Andersson U, Moller E, Abrams J S. Similar frequencies and kinetics of cytokine producing cells in murine peripheral blood and spleen. J Immunol Methods. 1993;166:201–214. doi: 10.1016/0022-1759(93)90361-a. [DOI] [PubMed] [Google Scholar]
- 32.Scharton-Kersten T M, Yap G, Magram J, Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185:1261–1273. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sedgwick J D, Schwender S, Imrich H, Dorries R, Butcher G W, ter Meulen V. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci USA. 1991;88:7438–7442. doi: 10.1073/pnas.88.16.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma S D, Mullenax J, Araujo F G, Erlich A A, Remington J S. Western blot analysis of the antigens of Toxoplasma gondii recognized by human IgM and IgG antibodies. J Immunol. 1983;131:977–983. [PubMed] [Google Scholar]
- 35.Shu U, Kiniwa M, Wu C Y, Maliszewski C, Vezzio N, Gately M, Delespesse G. Activated T cells induce interleukin-12 production by monocytes via CD40-CD40 ligand interaction. Eur J Immunol. 1995;25:1125–1128. doi: 10.1002/eji.1830250442. [DOI] [PubMed] [Google Scholar]
- 36.Slavin M A, Meyers J D, Remington J S, Hackman R C. Toxoplasma gondii infection in marrow transplant recipients: a 20 year experience. Bone Marrow Transplant. 1994;13:549–557. [PubMed] [Google Scholar]
- 37.Soong L, Xu J-C, Grewa I S, Kima P, Sun J, Longley B J, Ruddle N H, McMahon-Pratt D, Flavell R A. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity. 1996;4:263–273. doi: 10.1016/s1074-7613(00)80434-3. [DOI] [PubMed] [Google Scholar]
- 38.Stout R D, Suttles J, Xu J, Grewal I S, Flavell R A. Impaired T cell-mediated macrophage activation in CD40 ligand-deficient mice. J Immunol. 1996;156:8–11. [PubMed] [Google Scholar]
- 39.Stuber E, Strober W, Neurath M. Blocking the CD40-CD40L interaction in vivo specifically prevents the priming of T helper 1 cells through the inhibition of interleukin 12 secretion. J Exp Med. 1996;183:693–698. doi: 10.1084/jem.183.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Subauste C S, Wessendarp M, Sorensen R U, Leiva L E. CD40-CD40 ligand interaction is central to cell-mediated immunity against Toxoplasma gondii: patients with hyper IgM syndrome have a defective type 1 immune response that can be restored by soluble CD40 ligand trimer. J Immunol. 1999;162:6690–6700. [PubMed] [Google Scholar]
- 41.Suzuki Y, Orelana M A, Schreiber R D, Remington J S. Interferon-γ: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 42.Tsuge I, Matsuoka H, Nakagawa A, Kamachi Y, Aso K, Negoro T, Ito M, Torii S, Watanabe K. Necrotizing toxoplasmic encephalitis in a child with the X-linked hyper-IgM syndrome. Eur J Pediatr. 1998;157:735–737. doi: 10.1007/s004310050925. [DOI] [PubMed] [Google Scholar]
- 43.van Essen D, Kikutani H, Gray D. CD40 ligand-transduced costimulation of T cells in the development of helper function. Nature. 1995;378:620–623. doi: 10.1038/378620a0. [DOI] [PubMed] [Google Scholar]
- 44.Wiley J A, Harmsen A G. CD40 ligand is required for resolution of Pneumocystis carinii pneumonia in mice. J Immunol. 1995;155:3525–3529. [PubMed] [Google Scholar]
- 45.Yang Y, Wilson J M. CD40 ligand-dependent T cell activation: requirement of B7-CD28 signalling through CD40. Science. 1996;273:1862–1864. doi: 10.1126/science.273.5283.1862. [DOI] [PubMed] [Google Scholar]
- 46.Yap G S, Scharton-Kersten T, Charest H, Sher A. Decreased resistance of TNF receptor p55- and p75-deficient mice to chronic toxoplasmosis despite normal activation of inducible nitric oxide synthase in vivo. J Immunol. 1998;160:1340–1345. [PubMed] [Google Scholar]
- 47.Yap G S, Sher A. Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-γ- and tumor necrosis factor (TNF)-α-dependent host resistance to the intracellular pathogen, Toxoplasma gondii. J Exp Med. 1999;189:1083–1092. doi: 10.1084/jem.189.7.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou P, Seder R A. CD40 ligand is not essential for induction of type 1 cytokine responses or protective immunity after primary or secondary infection with Histoplasma capsulatum. J Exp Med. 1998;187:1315–1324. doi: 10.1084/jem.187.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]