Abstract
This report describes a novel therapeutic technique called photo-mediated ultrasound therapy (PUT). PUT applies synchronized short pulse duration (nanosecond) laser and ultrasound burst on targeted tissue, offering high-precision localized treatment. PUT is based on controlled induction and promotion of micro-cavitation activity in the target tissue. PUT is able to safely and effectively treat retinal neovascularization in rabbits.
Clinical Relevance—
PUT can selectively remove retinal angiogenesis in clinically-relevant disease models in human-sized eyes (rabbit) without damaging surrounding tissue.
I. Introduction
Retinal and choroidal neovascularization are major causes of vision loss and blindness around the world, including in diabetic retinopathy and age-related macular degeneration (AMD). 196 million people suffer from AMD and 11.3 million people suffer from advanced AMD worldwide.1 The United States population with AMD is growing rapidly from over 7 million in 20002 to an expected 18 million patients by 2050.3,4 The burden of AMD is expected to rise with the aging of the population, with nearly half of patients older than 75 years exhibiting AMD.5
The development of new blood vessels, called choroidal neovascularization (CNV), is the leading cause of blindness due to AMD.1 Current treatment of wet AMD involves frequent (often monthly) intravitreal (inside the posterior part of the eye) injections of anti-vascular endothelial growth factor (VEGF) agents, which reduces vascular leakage and associated edema. Persistent disease activity (PDA) is common. The Comparison of AMD Treatments Trial (CATT) demonstrated persistent fluid in 53% and 71% of patients treated monthly with ranibizumab and bevacizumab, respectively.6
Frequent intravitreal anti-VEGF injections also come with the risks of infection, bleeding, glaucoma, and cataracts along with significant financial burden, costing approximately $2,000 per month in medication costs for FDA-approved treatments. In addition, monthly evaluation and treatment is a significant burden on these elderly patients and their families. Hence for the 50 to 70% of patients who respond poorly to anti-VEGF therapy, alternative therapies are urgently needed to prevent patients from becoming blind and to reduce the significant burden and risk for all patients.
This report describes a novel therapy called photo-mediated ultrasound therapy (PUT). PUT applies synchronized nanosecond laser and ultrasound burst to selectively and permanently remove angiogenesis in the eye. When a laser pulse is absorbed by a chromophore, photoacoustic vibration occurs in the chromophore. The concurrent application of ultrasound significantly increases the likelihood of micro-cavitation in the particle. The induced cavitation activities can then be further enhanced and driven by the subsequent ultrasound bursts. The combined effect of ultrasound and laser is not linear and thus allows high precision treatment at a much lower energy level that with laser or ultrasound alone. This report evaluates PUT to treat a rabbit model of retinal neovascularization.
II. METHODS
A. PUT System
A standard Nd:YAG laser (Continuum Powerlite DLS 8010, Santa Clara, CA) with a pulse repetition rate of 10 Hz (5 ns pulse width) was used as the laser source. A high intensity focused ultrasound (HIFU) transducer with a focal length 62.6 mm (center frequency 0.5 MHz, H-107, Sonic Concepts, Bothell, WA) generated ultrasound bursts. A pulse delay generator (Model DG355, Stanford Research Systems) was employed to provide triggers for the laser and ultrasound systems with a repetition rate of 10 Hz as described previously.7-11 The therapeutic transducer had a geometric focal distance of 63.2 mm, a focal depth of 21.42 mm, and a focal width of 3.02 mm. A custom-built, 3D printed cone was designed and attached to the ultrasound transducer, and filled with agar-gelatin based couplant to provide acoustic coupling. A space was left in the center of the cone for the optical lens fixation and light propagation including the treatment laser beam and the illumination light beam. The ultrasound and laser light beams were aligned in advance.
B. Animal Experiments
New Zealand white rabbits (2.5 to 3.0 kg, 3 to 4 months old, both genders) were acquired from the Center for Advanced Models for Translational Sciences and Therapeutics (CAMTraST) at the University of Michigan Medical School. The animals were housed in an air-conditioned room with a 12-hour light–dark cycle, fed standard laboratory food and allowed free access to water. All the animal handling procedures were carried out in compliance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Michigan, protocol number PRO00008567, PI Paulus, with strict adherence to the ARVO (Association for Research in Vision and Ophthalmology) Statement for the Use of Animals in Ophthalmic and Vision Research.
Five New Zealand white rabbits were anesthetized by intramuscular injection of xylazine 5mg/kg (MWI/VetOne, Boise, ID) and ketamine 40mg/kg (Par Pharmaceutical Co Inc, Spring Valley, NY). Anesthesia was maintained with 2% isoflurane and oxygen using a V-Gel® (D10004, Jorgensen Laboratories, Loveland, CO). The rabbit pupils were dilated with topical application of phenylephrine hydrochloride 2.5% and tropicamide 1% eye drops. Tetracaine hydrochloride 1% (Akorn Inc, Lake Forest, IL) was administered as a topical anesthetic prior to experiments. To evaluate anesthesia levels and temperature, continuous monitoring of the heart rate and respiratory rate was performed using a V8400D Capnograph & SpO2 Digital Pulse Oximetry (MWI Animal Health, Boise, ID). Rectal temperature was measured every 15 min and used to adjust a water-circulating heating pad (TP-700, Stryker Corporation, Kalamazoo, MI) to keep the body temperature stable.
Retinal neovascularization was created in rabbits through intravitreal injection of VEGF and DL-AAA. RNV was achieved through intravitreous injection of VEGF (Human VEGF-165, Shenandoah Biotechnology Inc, Warwick, PA) to induce RNV in the rabbits.12-13 The eyelid was retracted with a pediatric Barraquer wire speculum, and the conjunctiva was grasped with forceps to ensure stability. The conjunctiva was cleaned with 5% povidone-iodine, and an intravitreous injection was performed using a 30 G ½ inch needle inserted 3mm posterior to the limbus through the pars plana. The needle was directed at an angle of ~45 degrees to avoid touching the rabbit lens. A total of 0.1 mL of VEGF-165 (100 μg mL−1) was injected into the vitreous cavity. The conjunctiva was then irrigated with BSS, and the speculum was then removed. For DL-AAA, a single intravitreal injection with 50 μL of 0.025 M DL-AAA was injected in rabbits using the same technique as described above with VEGF.
The animal was followed serially after induction of RNV with intravitreous injection with fundus photography, red free fundus photography, and fluorescein angiography (FA) images before and following PUT treatment using a digital fundus camera (Topcon 50EX Fundus Camera and Digital Imaging System, Topcon, Tokyo, Japan). FA was performed by injecting 0.1 ml/kg of AK-FLUOR 10% 100mg/ml (Fluorescein Injection, USP, NDC 17478-253-10) intravenously (IV) into the marginal ear vein. At the end of each experiment, the rabbit was euthanized with a lethal dose of Beuthanasia-D (Merck Animal Health; Summit, NJ, USA) IV through the marginal ear vein along with the removal of a vital organ.
C. Histology and Immunohistochemistry
After euthanasia of the rabbits, the eyes were immediately enucleated and fixed in Davidson’s Solution for 24 hours, followed by transferred to 50% ethanol for 8 hours and then preserved in 70% ethanol until processing. After removal of the anterior segment and the vitreous fluid, the eyecup containing the treated area was dissected into multiple representative strips with a thickness of 5 mm. All the strips were pre-fixed in 4% agar and then embedded in paraffin. The paraffin-embedded sections (thickness 5 μm) were obtained with a microtome and stained with hematoxylin and eosin (H&E). Theses sections were analyzed under a light microscope (Olympus BX-51). Photographs were taken with a digital camera (Olympus DP70).
III. RESULTS
A. Removal of Retinal Neovascularization
Injection of VEGF and DL-AAA caused a rapid and robust development of retinal neovascularization emanating from the optic nerve and medullary ray in rabbits with leakage on eye angiography (Fig 1). There was prominent neovascularization present by 7 days after injection of VEGF, and thus 7 days was selected as the time point for treatment with PUT. PUT treatment was performed at 7 days using a laser of 30 mJ/cm2 (14% at 532nm and 86% at 1064nm) with a 3mm spot diameter and ultrasound of 0.5 MPa for 5 minutes.
Figure 1.
Fundus photography at baseline in normal rabbits, 7 days after VEGF-induced retinal neovascularization, and 1 and 3 days after PUT treatment demonstrating removal of retinal neovascularization in the treated area up to 3 days after PUT treatment with persistence of the larger normal retinal vasculature within the treated area.
Following treatment with PUT, a rapid regression of the retinal neovascularization occurred within 1 day (Fig 1). Of note, the neovascularization was mostly removed while the normal, larger retinal vasculature persisted within the treatment area. This removal of neovascularization persisted until at least 3 days following treatment (Day 10).
B. Short term effects on RNV
Additional rabbits were treated 7 days after injection of VEGF-induced retinal neovascularization with a laser fluence of 33 mJ/cm2 (14% at 532nm and 86% at 1064nm) with a 3mm spot diameter and ultrasound of 0.5 MPa for a duration of 5 minutes. These rabbits were evaluated for more acute effects within the first hour following treatment. By 7 days after VEGF injection, robust retinal neovascularization emanating from the optic nerve and medullary ray was present in rabbits (Fig 2).
Figure 2.
Fundus photography in 2 additional rabbits 7 days after VEGF-induced retinal neovascularization before, immediately after, and 1 hour after PUT treatment demonstrating remove of microvasculature in the treated area (blue circle).
Immediately following PUT treatment, a rapid regression occurred in the neovascularization within the treatment area (Fig 2). This regression persisted to 1 hour following treatment, indicating an immediate and acute effect of PUT on causing regression of the retinal neovascularization. In some cases, eg Rabbit 3, this caused the presence of a hemorrhage in the area of treatment.
C. Removal of Choroidal Vasculature
PUT treatment was also performed of normal choroidal vasculature. To remove normal choroidal vasculature, the laser fluence was increased from 30 to 75 mJ/cm2 (Fig. 3).
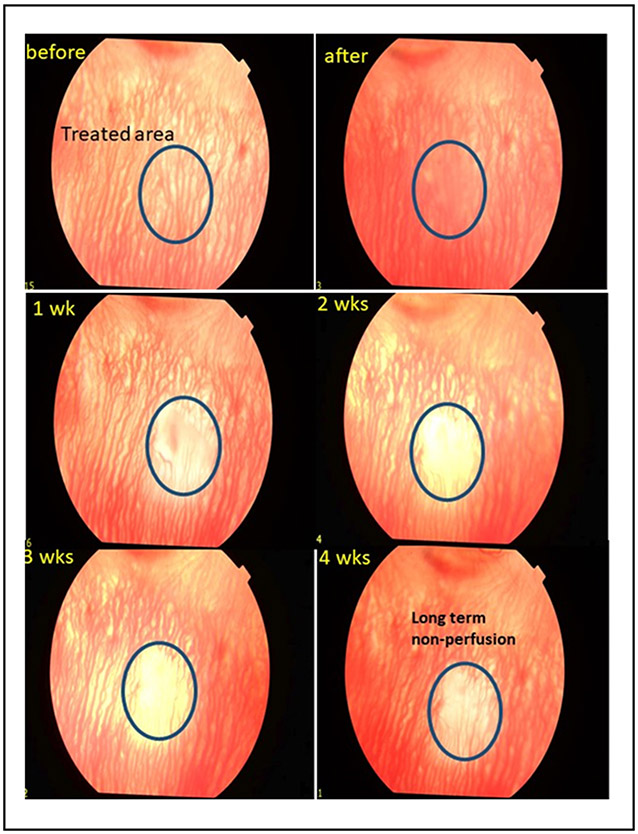
Figure 3.
Fundus photography of the choroidal vasculature at baseline, immediately after, and weekly for 4 weeks after PUT treatment demonstrating removal of the choroidal microvasculature in the treated area that persists with long term non-perfusion at 4 weeks.
Treatment of choroidal vasculature was performed in normal rabbits (without intravitreal injection) using laser fluence of 75 mJ/cm2, ultrasound of 2MPa with a total of 15 minutes of treatment. Fundus images demonstrates immediately a haziness to the choroidal vascular wall followed by removal of the choroidal vasculature that persisted to 4 weeks and resulted in long-term non-perfusion of the choroidal vasculature to 4 weeks. The treatment of the choroidal vasculature necessitated the use of higher laser fluence, ultrasound, and treatment duration than required for treatment of angiogenesis, suggesting that lower PUT treatment could selectively remove neovascularization without damaging the normal vasculature. These results suggested that PUT could result in selective removal of angiogenesis in the treated area without damaging the surrounding normal retinal and choroidal structure.
D. Histology and Immunohistochemistry safety evaluation
To ensure the safety of PUT treatment, hematoxylin and eosin (H&E) histology and immunohistochemistry were performed. The animals were sacrificed at 24 hours after PUT treatment in Dutch belted pigmented rabbits and representative histology samples are shown in Figure 4.
Figure 4.
H&E histology at 24 hours after PUT treatment using laser of 55 mJ/cm2 (left) and 75 mJ/cm2 (right) along with ultrasoun od 0.5 MPa. This demonstrates changes confined to the vasculature with no changes noted in the neurosensory retina.
This demonstrated that PUT resulted in no visible morphologic changes in the neurosensory retina at 24 hours after PUT treatment. Of note, the internal limiting membrane, ganglion cell layer, inner nuclear layer, inner plexiform layer, outer nuclear layer, outer limiting membrane, and the inner and outer segments of the photoreceptors are intact. The only changes noted are within the choroidal vasculature lumens where possible blood clots are noted on some images.
IV. Conclusion
PUT is the synchronized application of short pulse duration laser and ultrasound burst to induce and promote micro-cavitation activity in the target tissue. PUT is able to safely and effectively treat retinal neovascularization in rabbits. PUT can shut down microvessels and stop perfusion without damaging surrounding tissues in this rabbit model of retinal neovascularization with a single treatment without any exogenous contrast agent.
Acknowledgment
The authors would like to thank Dr. Yuqing Chen and the Center for Advanced Models and Translational Sciences and Therapeutics (CAMTrasST) at the University of Michigan Medical School for the generous donation of the rabbits used in this study.
Research supported by Research supported by the National Institutes of Health (NIH), National Eye Institute (NEI), Fight for Sight- International Retinal Research Foundation, the Alliance for Vision Research, and Research to Prevent Blindness.
Contributor Information
Yannis M. Paulus, Department of Ophthalmology and Visual Sciences and the Department of Biomedical Engineering, University of Michigan, Ann Arbor, MI 48105, USA.
Yu Qin, Department of Biomedical Engineering, University of Michigan, Ann Arbor, MI 48105 USA.
Yixin Yu, Department of Ophthalmology and Visual Sciences, University of Michigan, Ann Arbor, MI 48105 USA.
Julia Fu, Department of Chemistry, University of Michigan, Ann Arbor, MI 48105 USA.
Xueding Wang, Department of Biomedical Engineering, Department of Radiology, University of Michigan, Ann Arbor, MI 48105 USA.
Xinmai Yang, Institute for Bioengineering Research and Department of Mechanical Engineering, University of Kansas, Lawrence, KS 66045 USA.
References
- [1].Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014;2(2):e106–16. [DOI] [PubMed] [Google Scholar]
- [2].Friedman DS, O'Colmain BJ, Muñoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J; Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 2004; 122(4):564–72. [DOI] [PubMed] [Google Scholar]
- [3].Klein R, Klein BE. The prevalence of age-related eye diseases and visual impairment in aging: current estimates. Invest Ophthalmol Vis Sci 2013; 54(14): p. ORSF5–ORSF13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rudnicka AR, Kapetanakis VV, Jarrar Z, Wathern AK, Wormald R, Fletcher AE, Cook DG, Owen CG. Incidence of late-stage age-related macular degeneration in American whites: Systematic review and meta-analysis. Am J Ophthalmol 2015;160(1):85–93.e3. [DOI] [PubMed] [Google Scholar]
- [5].Rein DB, Wittenborn JS, Zhang X, Honeycutt AA, Lesesne SB, Saaddine J; Vision Health Cost-Effectiveness Study Group. Forecasting age-related macular degeneration through the year 2050: the potential impact of new treatments. Arch Ophthalmol 2009;127(4):533–40. [DOI] [PubMed] [Google Scholar]
- [6].Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group, Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ: Ranibizumab and bevacizumab for neovascular age-related macular degeneration. The New England Journal of Medicine 2011, 364(20):1897–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hu Z, Zhang H, Mordovanakis A, Paulus YM, Liu Q, Wang X, Yang X. High-precision, non-invasive anti-microvascular approach via concurrent ultrasound and laser irradiation. Scientific Reports 2017. Jan 11;7:40243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang H, Xie X, Li J, Qin Y, Zhang W, Cheng Q, Yuan S, Liu Q, Paulus YM, Wang X, Yang X. Removal of choroidal vasculature using concurrently applied ultrasound burst and nanosecond laser pulses. Scientific Reports 2018; 8(1):12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang W, Qin Y, Xie X, Hu Z, Paulus YM, Yang X, Wang X. Real-time Photoacoustic Sensing for Photo-mediated Ultrasound Therapy. Optics Letters 2019; 44(16): 4063–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yang X, Hu Z, Zhang H, Mordovanakis A, Paulus YM, and Wang X, "Antivascular photo-mediated ultrasound therapy," in 2016 IEEE International Ultrasonics Symposium (IUS), 2016: IEEE, pp. 1–4. [Google Scholar]
- [11].Yang X, Zhang H, Li J, Paulus Y, and Wang X, "The application of antivascular photo-mediated ultrasound therapy in removing microvessels in the eye," in 2017 IEEE International Ultrasonics Symposium (IUS), 2017: IEEE, pp. 1–1. [Google Scholar]
- [12].Wong CG, Rich KA, Liaw LHL, Hsu HT & Berns MW Intravitreal VEGF and bFGF produce florid retinal neovascularization and hemorrhage in the rabbit. Curr. Eye Res 22, 140–147 (2001). [DOI] [PubMed] [Google Scholar]
- [13].Grossniklaus HE, Kang SJ & Berglin L Animal models of choroidal and retinal neovascularization. Prog. Retin. Eye Res 29, 500–519 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]