Abstract
Several phthalate acid esters (PAEs), often called phthalate esters or phthalates, are substances classified as harmful due to their carcinogenic and mutagenic properties, and moreover, as dangerous for humans because they interfere with the endocrine system. In general, phthalic esters are used as plasticizers for different polymers and more other consumer products. In the present study, we describe a simple method to quantify PAEs in coffee brew using a liquid‐liquid extraction without purification processes through analysing the obtained organic phase by GCMS in the single ion monitoring mode. The totals of single PAEs, in coffee brew samples analysed by us, are in the range of 159–5305 μg L−1. Considering that, on average, a person drinks three cups (total 90 mL) of the aforementioned drink per day, this will lead to the uptake of a total 14 to 477 μg of phthalates.
Keywords: coffee, GC-MS, phthalates, selected ion monitoring, trace analysis
Several phthalate acid esters (PAEs) are classified as harmful due to their carcinogenic and mutagenic properties and are dangerous for humans because they interfere with the endocrine system. The present study describes a simple method to quantify PAEs in coffee brew using a liquid‐liquid extraction without purification processes through analysing the obtained organic phase by GCMS in the single ion monitoring mode.

Introduction
Phthalic acid esters (PAEs), often simple identified as phthalates, have lipophilic properties. To increase the flexibility of plastic polymer, several phthalates, in articles intended for consumption, can be present up to 40 %. They are also present in other objects and products for common use: cosmetics, perfumes, paints, inks, glues and lubricants[ 1 , 2 , 3 , 4 , 5 , 6 , 7 ] and, even, in contact lenses. [8] Phthalates can easily migrate from plastic materials, for example from household items, to foods (especially if rich in fats) and to environmental matrices (air, water, soil) because, chemically, they are not covalently linked to polymers. Being weakly linked to polymeric materials employed in packaging, in articles intended for construction, in the insulation of cables and in items for electrical systems, phthalates are ubiquitous environmental contaminants. Traces of several PAEs are contained in common foods (vegetable oils, fatty, fruits, sea food, comprising roasted meat, grilled and, smoked fish and common brews (tea and coffee).[ 7 , 9 ] Many phthalates are included among the toxicologically dangerous substances due to their mutagenic, carcinogenic properties. Moreover, they have been shown to damage the endocrine system (EDC).[ 10 , 11 , 12 , 13 , 14 ] The body can absorb the components of this class of chemicals which can be absorbed by ingestion, inhalation and absorption through the skin. Studies [15] on toxicological aspects have shown that low molecular weight phthalates such as, for example, diethyl phthalate (DEP), can lead to eye, nasal and throat irritation. It has been hypothesized, that in addition to causing damage to the endocrine system, some phthalates may be carcinogenic to humans; in particular, they can affect the liver, kidneys and the reproductive system. Generally, humans are not exposed to a single phthalate because in the matrices (air, water, food, etc.) with which they come into contact there is always a mixture of non‐constant qualitative and quantitative composition of this class of substances as also the case of polycyclic aromatic hydrocarbons. [16] Despite many international researchers having studied the occurrence of phthalate compounds in several consumer products such as food and packaging materials, very little scientific data exist regarding the existence of these chemicals in food products sold in Italy and the average amount of phthalates ingested by the Italian people.[ 9 , 17 ] Food contaminated with PAEs has become a matter of public concern in recent years due to the use of plastics as food containers and packaging. However, the reports for monitoring PAEs were mainly focused on the relatively simple samples, such as the contaminated water from plastic packaging.[ 18 , 19 , 20 ] The diffusion of PAEs from plastic packaging into complex samples such as food was rarely determined due to the complicated sample matrix and low level of PAEs. Therefore, it is imperative to have a sensitive, reliable and fast method for analysing PAEs in complex samples. In the present study, considering that the world average daily consumption of coffee is about one and a half cups, while in America the majority of the population consumes more than four cups, we have optimized an analytical method to quantify the levels of PAEs in beverage samples prepared at home in order to evaluate total amount of human exposure to this class of hazardous substances. In this paper, we report an analytical method for the PAEs determination in coffee brew, because, as solid coffee is not directly ingested by the consumer, it is more pertinent to estimate the PAEs concentrations in the brew samples prepared using Moka coffee maker and ground coffee available on the market and not pre‐packaged pods or capsules. The determination of phthalates in food samples is challenging in analytical chemistry due considering complex matrix and requires a technique with both high sensitivity and selectivity. So far, a number of methodologies have been developed for the extraction of PAEs in different foodstuffs and, to our knowledge, there are few publications about the difficulty of PAEs extraction in complex matrices as coffee when hundreds of substances have been generated during thermal processes. In this study we report a simple and rapid method for PAEs (Table 1) quantification in coffee brew, based on liquid–liquid extraction with small volumes of hexane, and without a purification process since we analyse the extract by gas chromatography with mass spectrometric detectors in the single ion monitoring mode (SIM). The advantage of SIM over full scan spectral acquisition is the increase in sensitivity and in selectivity.
Table 1.
Phthalates and their abbreviations, internal and surrogate deuterated standards (in italic), quantification and qualitative ions confirmation (m/z).
|
Compound |
Abbr. |
Quantification and qualitative ions confirmation (m/z) |
|---|---|---|
|
Dimethyl phthalate |
DMP |
163‐194 |
|
Diethyl phthalate |
DEP |
121‐149‐177‐222 |
|
Dibutyl phthalate |
DBP |
149‐150‐29‐41 |
|
Bis(2‐ethylhexyl) phthalate |
DEHP |
113‐149‐167 |
|
Di‐n‐octyl phthalate |
DnOP |
149‐150 |
|
Benzyl butyl phthalate |
BBP |
91‐(149)‐206‐238 |
|
Diethyl phthalate‐d4 |
DEP d4 |
153 |
|
Bis(2‐ethylhexyl)phthalate‐d4 |
BEHP d4 |
153 |
|
Di‐n‐hexyl‐phthalate‐d4 |
DHXP d4 |
153 |
Results and Discussion
The concentrations of the single analyte in the blank solutions are always below the quantification limits (from 5 to 20 μg L−1). The best analytical results (recoveries 83±5 %) were obtained using hexane for the liquid−liquid extraction. For each sample, the extraction yield percentage was calculated by the surrogate standard solution containing known concentrations of di‐n‐hexyl‐phthalate‐d4, spiked to the samples previously to perform analysis. Considering the results of all the analysed brew samples, extraction yields percentages were always higher than 78 % and in most cases almost 100 %. Three replicates of all samples were analysed and the calculated precision (% RSD) of individual phthalate ranged from 6 to 15 %. The method optimized was used to quantify PAEs in coffee brew samples prepared by us. In Table 2, the single PAE concentrations (μg L−1) obtained from the samples are reported.
Table 2.
Single (μg L−1) PAEs in the analysed samples.
|
Sample |
DMP |
DEP |
DBP |
BzBP |
DEHP |
DnOP |
Total |
|---|---|---|---|---|---|---|---|
|
S |
10 |
48 |
250 |
20 |
18 |
9 |
352 |
|
LCG |
10 |
38 |
120 |
20 |
5 |
9 |
197 |
|
LS |
10 |
99 |
260 |
20 |
14 |
9 |
411 |
|
LD |
10 |
18 |
90 |
20 |
12 |
9 |
159 |
|
AL |
10 |
21 |
170 |
20 |
53 |
9 |
280 |
|
MO |
10 |
17 |
140 |
20 |
30 |
9 |
227 |
|
GL |
10 |
10 |
10 |
20 |
940 |
9 |
995 |
|
WHI |
10 |
3100 |
10 |
203 |
2 |
9 |
5305 |
|
GR |
10 |
510 |
10 |
20 |
460 |
9 |
1022 |
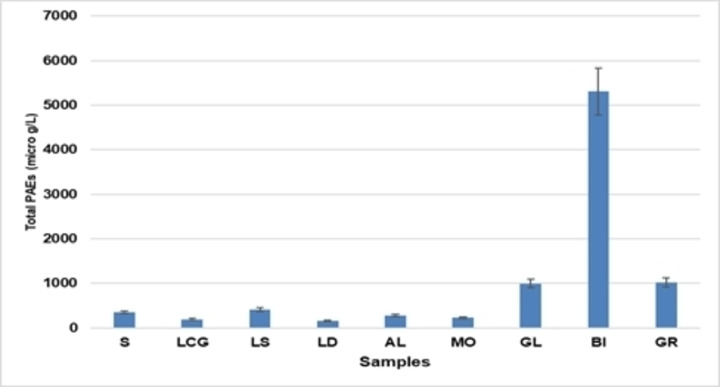
In coffee brew samples, the total concentrations of phthalates, calculated as sum of concentrations (∑PAE), are in the range 159–5300 μg L−1 (Figure 1). Only three phthalates (DEP, DEHP and DnOP) were detected in relevant amounts among the investigated compounds.
Figure 1.
Total concentrations (μg L−1) of PAEs in the analysed samples.
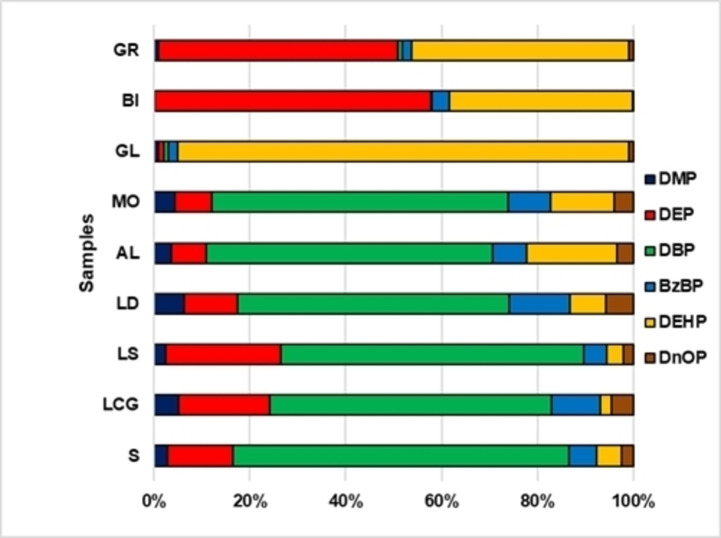
Figure 2 shown the distributions (%) for single phthalates. Di‐butyl phthalate was identified in six coffee brew samples in amounts ranging from 10 to 260 μg L−1. Several authors[ 21 , 22 , 23 ] state that exposure to BPD during the growth of male children may be associated, in adults, with lower production of testicular testosterone, decreased anogenital distance, hypospadias, reduced sperm quality and, consequently, decreased fertility. In other epidemiologic studies, several of these illnesses along with other harmful effects on human health have also been observed. DEP, at high concentration (3100 μg L−1), was found in only one of the coffee brew samples (BI), while in the other samples, the range varies from 10 μg L−1 (LOQ) (GL sample) to 510 μg L−1 (GR sample). In the body, DEP decomposes into other toxicologically dangerous compounds. [24]
Figure 2.
Distributions (%) of single PAEs in the samples.
Diethyl phthalate and its breakdown products will be eliminated within about 2 days from the body, mainly through the urine. Only low amounts of DEP and its metabolites accumulate in the kidneys and liver.[ 23 , 24 ] No inhalation minimal risk levels were reported in literature for DEP.[ 23 , 24 ] In sample GL, DEHP (940 μg L−1) predominates. The International Agency for Research on Cancer [25] considered DEHP carcinogenic to mice and rats but not to humans. DEHP alters the normal functions of Sertoli and Leydig cells by impairing spermatogenesis and testosterone production in rats. [26] Phthalates, in the European Union, are classified in category 2 (suspected carcinogen). Other researchers state that high doses of DEHP can alter sperm quality, to have effects on the human reproduction, on development of children and cause endocrine disorders. [27] In all analysed samples, DMP and DnOP are at trace levels and similar to LOQ values. In our samples, benzyl butyl phthalate (BzBP) ranged from 20 to 203 μg L−1. The European Union (EU) has listed the compound as suspected to produce endocrine alterations and identified the maximum concentrations of tolerable specific migration limits (SML) and the employed of phthalates on plastic materials that come into contact with food (Directive2002/72/EC as amended), limiting five phthalates: dibutyl phthalate (DBP), di(2‐ethylhexyl)phthalate (DEHP), butyl benzyl phthalate (BBP), di‐isononyl phthalate (DINP) and di‐isodecyl phthalate (DIDP) (Commission Directive 2007/19/EC, amending Directive 2002/72/EC).[ 27 , 28 , 29 , 30 ]
Conclusion
In this paper, a reliable and simple analytical traces method for the quantification of six PAEs in coffee brew samples is described. The sensibility, accuracy and versatility of this method makes it useful for analytical fast quality control as well as for research and development in the food and industry laboratories. In fact, this method could eventually be used to investigate similar PAEs in foods with similar characteristics. In the literature, some studies have determined the presence of phthalates in the drink obtained from instant coffee, capsules and other pre‐packed coffee transferred to plastic cups.[ 9 , 22 ] In our work, after optimizing the analytical method in relation to the quality parameters, we quantified the analyte directly in the prepared drink, as happens in most Italian families, using ground coffee and the classic Moka coffee maker. Considering that, on average, a person drinks three cups (total 90 mL) of the aforementioned drink per day, this will lead to the uptake of a total 14 to 477 μg of phthalates. These qualities naturally vary according to the brand of the roasted coffee used. The large range of phthalates amount (relative standard deviation on total phthalates was 166 %) found in the analysed samples indicates heterogeneous composition of raw coffees and processes of roasting and packaging of the product and in the materials present in the machinery used in the different stages of production starting from the green coffee bean. Some researchers, [8] assuming a daily consumption of two cup of espresso coffees (80 mL), calculated an overall intake of phthalates ranging from 34.4 μg to 136 μg, depending on the capsule employed.
Experimental Section
Laboratory material
PAEs analysis presents very critical points due to analytical blank problems. [20] All the materials (instruments, glassware, etc.) used during the analysis were well washed with surfactant solution and subsequently rinsed with Milli‐Q water and RP grade acetone. Moreover, these were heated at 120 °C for 14 h. Different glassware and syringes were used to inject calibration, extracted from coffee brew samples and quality check solutions to avoid possible cross contaminations.
Chemicals
Organic solvents used during all the procedure were of HPLC quality and employed in the commercial form without purification. Water was obtained by a Milli‐Q system. A mix of PAEs standard solution containing six analytes was used: dimethyl phthalate (DMP), diethyl phthalate (DEP), di‐n‐butyl phthalate (DnBP), benzyl butyl phthalate (BBZP), bis(2‐ethylhexyl) phthalate (DEHP) and di‐n‐octyl phthalate (DnOP) (1865–1911 mg L−1) (Mixture EPA Phthalate Esters Mix, Catalog no. 48231) were bought from Supelco (Milano). Solutions used to obtain calibration curve are reported below. In detail, standard solutions used had following concentration: 0.45, 1.35, 4.05, 8.1, 16.2, and mg L−1 and were prepared by serial dilution from the concentrate stock standard solution with appropriate volumes of a solution containing internal standards. Two deuterated phthalates were employed as internal and surrogate standard: diethyl phthalate‐d4 and bis(2 ethylhexyl) phthalate‐d4 acquired from Sigma Aldrich. In order to avoid different instrumental drift response, the deuterated internal standard was spiked to both samples and standards at the same concentration. All phthalates solutions were kept in a refrigerator at −18 °C in the dark.
Coffee Samples
Toasted coffee powder samples have been acquired in Palermo supermarkets but refer to Italian brands. The coffee brew solutions were prepared by an aluminium Moka device using 8 g of roasted coffee sample and 80 mL of water.
Apparatus and materials
In the present study, sample analyses were done by using a gas‐chromatograph (GCMS‐QP5000) coupled with mass spectrometer detector (Shimadzu mod.GC‐17A) and an acquisition data system (Shimadzu, CLASS 5000). A fused‐silica capillary column SLB5 (30 m 0.25 i.d. 0.5 μm) (5 % diphenyl 95 % dimethyl siloxane) from Supelco (Milano, Italy) was used for all the chromatographic separations. In the Selected Ion Monitoring mode (SIM) the data were acquired. As carrier gas was used Helium (99.99 %) at 21 mL min−1. The splitless mode was used to inject by hand all the solutions (samples, standards, etc.). The retention times of the components eluted from the unknown solutions were compared with those obtained from the mixtures of the standard phthalates solutions, analysed under the same instrumental conditions in order to identify the individual analyte in the brews samples. By corresponding of the quantification and confirmation m/z values of spectra of the single phthalates with those stored in the NIST library were confirmed the presence of single compound in the analysed samples. The amounts of each single phthalate, in the analysed brew samples, were calculated comparatively to the deuterated phthalates added to the dry residue. Calibration lines were repeated every 6–8 days using 5 standard solutions containing phthalates at known concentrations.
Analysis
Using several different solvents and their mix, we carried out preliminary different recovery experiments, before to apply the optimized analytical method to coffee brew samples. Being not commercially available a reference certifies material of coffee brew containing PAEs, after the total liquid‐liquid extraction of the PAEs of a sample (the absence of deuterated PAEs was established by GC‐MS analysis), we added a known volume of deuterated PAEs solution. These tests have allowed us to verify precision, recovery and accuracy of method. The detection (LOD) and quantification (LOQ) limits for each analyte were calculated by means of IUPAC (International Union of Pure and Applied Chemistry) criterions: as 3 r (three times the background noise) and 10 r (ten times the background noise) respectively. To evaluate the quality of the analytical method, the results of the GC‐MS instrument was tested every morning using a reference standards phthalates. In Table 1, the list of the phthalates, the deuterated standards and the quantification and confirmation ions relevant to this study have been summarised.
Sample extraction
A known volume (150 μL) of the surrogate standard phthalates solution was added to 15 mL of coffee brew. All the samples were extracted, for three times, in liquid‐liquid mode using reparatory funnels with 10 mL of hexane. By a rotavapor, operating at T=36±1 °C, the unified extracts were evaporated to small volume and successively dried under a weak nitrogen flow. To this residue were added 150 μL of a solution containing the deuterated hexanic internal standards solution (10 μg L−1). Every 4–5 analyses of brew samples, a blank experiment using uncontaminated water was carried out to increase quality data and evaluate any contamination problems.
Conflict of interest
The authors declare no conflict of interest.
1.
D. Amorello, R. Indelicato, S. Barreca, S. Orecchio, S. Orecchio, ChemistryOpen 2022, 11, e202200082.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1. Orecchio S., Indelicato R., Barreca S., Environmental Geochem. Health 2013, 35, 613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Orecchio S., Indelicato R., Barreca S., Microchem. J. 2013, 114, 187–191. [Google Scholar]
- 3. Barreca S., Indelicato R., Orecchio S., Pace A., Microchem. J. 2014, 114, 192–196. [Google Scholar]
- 4. Api A. M., Food and Chem. Toxicol. 2001, 39, 97–108. [DOI] [PubMed] [Google Scholar]
- 5. Bonini M., Errani E., Zerbinati G., Ferri E., Girotti S., Microchem. J. 2008, 90, 9031–9036. [Google Scholar]
- 6. Koniecki D., Wang R., Moody R. P., Zhu J., Environmental Res. 2011, 111, 329–336. [DOI] [PubMed] [Google Scholar]
- 7. Jarosova A., Czech J. Food Sci. 2006, 24, 223–231. [Google Scholar]
- 8. Perez-Feas C., Barciela-Alonso M. C., Bermejo-Barrera P., Microchem. J. 2001, 99, 108–113. [Google Scholar]
- 9. De Toni L., Tisato F., Seraglia R., Roverso M., Gandin V., Marzano C., Padrini R., Foresta C., Toxicol. Rep. 2017, 4, 234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Katsikantami I., Sifakis S., Tzatzarakis M. N., Vakonak E. I., Kalantzi O. I., Tsatsakis A. M., Rizos K., Environ. Int. 2016, 97, 212–236. [DOI] [PubMed] [Google Scholar]
- 11. Bornehag C. G., Sundell J., Weschler C. J., Sigsgaard T., Lundgren B., Hasselgren M., Environmental Health Perspect. 2004, 112, 1393–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nordkap L., Joensen U. N., Blomberg Jensen M., Jørgensen N., Mol. Cell. Endocrinol. 2012, 355, 221–230. [DOI] [PubMed] [Google Scholar]
- 13. Kolalik B., Naydenov K., Larsson M., Bornehag C. G., Sundell J., Environmental Health Perspect. 2008, 116, 98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thankamony A., Pasterski V., Ong K. K., Acerini C. L., Hughes I. A., Andrology 2016, 4, 616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim S., Lee J., Park J., Kim H. J., Cho G., Kim G. H., Eun S. H., Lee J. J., Choi G., Suh E., Choi S., Kim S., Kim Y. D., Kim S. K., Kim S. Y., Kim S., Eom S., Moon H. B., Kim S., Choi K., Sci. Total Environ. 2015, 508, 13–19. [DOI] [PubMed] [Google Scholar]
- 16.Ronald G. Harvey in The Handbook of Environmental Chemistry Vol.3I (ed. A. H. Neilson), Springer, Berlin, 1998, pp. 2–10.
- 17. Di Bella G., Potortì A. G., Lo Turco V., Saitta M., Dugo G., Food Control 2014, 41, 185–192. [Google Scholar]
- 18. Zare J. M., Rastkari N., Ahmadkhaniha R., Yunesian M., Food Res. Int. 2015, 69, 256–265. [Google Scholar]
- 19. Kayali N., Fernando L., Tamayo G., Polo-Díeza M., Talanta 2006, 69, 1095–1099. [DOI] [PubMed] [Google Scholar]
- 20. Fankhauser-Noti A., Grob K., Anal. Chim. Acta 2007, 582, 353–360. [DOI] [PubMed] [Google Scholar]
- 21. Swan S. H., Environmental Res. 2008, 108, 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agency for Toxic Substances and Disease Registry, Division of Toxicology (ATSDR), Toxicological Profile for Di-n-butyl Phthalate, Atlanta, GA, 2001 https://www.atsdr.cdc.gov/toxprofiles/tp135.pdf. [PubMed]
- 23.NRC (National Research Council), Phthalates and cumulative risk assessment; the task ahead, National Academies Press (U. S.), Washington, DC, 2008. [PubMed]
- 24.Agency for Toxic Substances and Disease Registry (ATSDR), Division of Toxicology, Toxicological Profile for Diethyl Phthalate (DEP), Atlanta, GA, 1995 https://www.atsdr.cdc.gov/ToxProfiles/tp73.pdf. [PubMed]
- 25.Agency for Toxic Substances and Disease Registry (ATSDR), Division of Toxicology, Toxicological Profile for Di 2-ethylhexyl)phthalate (DEHP), Atlanta, GA, 2022 https://www.atsdr.cdc.gov/ToxProfiles/tp9.pdf. [PubMed]
- 26.G. W. Wolfe, K. A. Layton, Multigeneration reproduction toxicity study in rats diethylhexyl phthalate: multigenerational reproductive assessment by continuous breeding when administered to Sprague–Dawley rats in the diet (TRC Study no. 7244–200), The Immune Research Corporation, Gaithersburg, Maryland, 2003.
- 27. Api A. M., Food Chem. Toxicol. 2001, 39, 97–108. [DOI] [PubMed] [Google Scholar]
- 28.T. Nakajima, B. Hopf Nancy, A. Schulte Paul, Di(2-ethylhexyl) phthalate (DEHP), Identification of Research Needs to Resolve the Carcinogenicity of High-priority IARC Carcinogens, Views and Expert Opinions of an IARC/NORA Expert Group Meeting, Lyon, France, 30 June–2 July 2009, IARC Technical Publication, No. 42, IARC, Lyon, 2010, pp. 183–196, http://monographs.iarc.fr/ENG/Publications/techrep42/TR42-18.pdf.
- 29. Mendiola J., Meeker J. D., Jørgensen N., Andersson Liu A. M. F., Calafat A. M., Redmon J. B., Drobnis E. Z., Sparks A. E., Wang C., Hauser R., Swan S. H., J. Androl. 2012, 33, 488–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mohamed M. A., Ammar A. S., Am. J. Food Technol. 2008, 3, 341–346. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.