Abstract
Introduction
The primary outcome of the study was to evaluate the effect on 30 day mortality of the combination ceftazidime/avibactam + fosfomycin in the treatment of bloodstream infections (BSIs) caused by KPC-producing Klebsiella pneumoniae (KPC-Kp).
Materials and methods
From October 2018 to March 2021, a retrospective, two-centre study was performed on patients with KPC-Kp BSI hospitalized at Sapienza University (Rome) and ISMETT-IRCCS (Palermo) and treated with ceftazidime/avibactam-containing regimens. A matched cohort (1:1) analysis was performed. Cases were patients receiving ceftazidime/avibactam + fosfomycin and controls were patients receiving ceftazidime/avibactam alone or in combination with in vitro non-active drugs different from fosfomycin (ceftazidime/avibactam ± other). Patients were matched for age, Charlson comorbidity index, ward of isolation (ICU or non-ICU), source of infection and severity of BSI, expressed as INCREMENT carbapenemase-producing Enterobacteriaceae (CPE) score.
Results
Overall, 221 patients were included in the study. Following the 1:1 match, 122 subjects were retrieved: 61 cases (ceftazidime/avibactam + fosfomycin) and 61 controls (ceftazidime/avibactam ± other). No difference in overall mortality emerged between cases and controls, whereas controls had more non-BSI KPC-Kp infections and a higher number of deaths attributable to secondary infections. Almost half of ceftazidime/avibactam + fosfomycin patients were prescribed fosfomycin without MIC fosfomycin availability. No difference in the outcome emerged after stratification for fosfomycin susceptibility availability and dosage. SARS-CoV-2 infection and ICS ≥ 8 independently predicted 30 day mortality, whereas an appropriate definitive therapy was protective.
Conclusions
Our data show that fosfomycin was used in the treatment of KPC-Kp BSI independently from having its susceptibility testing available. Although no difference was found in 30 day overall mortality, ceftazidime/avibactam + fosfomycin was associated with a lower rate of subsequent KPC-Kp infections and secondary infections than other ceftazidime/avibactam-based regimens.
Introduction
Bloodstream infections (BSIs) caused by KPC-producing Klebsiella pneumoniae (KPC-Kp) have a high fatality rate, ranging from 40% to 50%, especially if appropriate antibiotic therapy is started >24 h from index blood culture (BC) collection.1 Nowadays, the treatment of KPC-Kp infections is mainly based on ceftazidime/avibactam, which undoubtedly represented a therapeutic advance in the field and contributed to the reduction of mortality rates compared with traditional therapies.2 Nevertheless, its efficacy may be reduced in particular conditions, such as pneumonia, continuous renal replacement therapy,3 delayed source control4 and septic thrombosis,5 where a risk of underexposure and, therefore, clinical failure exists, raising the question of the optimal use of this drug.
Despite no significant difference in mortality and microbiological cure rates between ceftazidime/avibactam alone or in combination was observed, ceftazidime/avibactam has been mostly used as part of combination therapy in real life.6–10 As a matter of fact, several in vitro studies showed ceftazidime/avibactam to display a high level of synergism with different antimicrobials, including carbapenems, fosfomycin and tigecycline.11–14
Fosfomycin is an old drug that has gained renewed interest in the last years due to its extreme versatility and the high level of in vitro and clinical synergism with different antimicrobials against several difficult-to-treat organisms, including KPC-Kp.7
Indeed, given its high tissue penetration and unique mechanism of action, which makes the development of cross-resistance with other agents very unlikely, fosfomycin is a potentially effective agent for combination therapy.
To the best of our knowledge, no clinical studies specifically evaluating the potential mortality benefit of the ceftazidime/avibactam + fosfomycin combination for the treatment of KPC-Kp BSI have been performed so far.
Therefore, the primary aim of the study was to investigate the effect on 30 d mortality of the ceftazidime/avibactam + fosfomycin combination in the treatment of BSIs caused by KPC-Kp. Secondary aims were: (i) to correlate the mode of fosfomycin prescription with the availability of in vitro susceptibility testing; and (ii) to evaluate the recurrence of KPC-Kp BSI, new non-bacteraemic KPC-Kp and secondary infection rates in subjects treated with ceftazidime/avibactam + fosfomycin versus ceftazidime/avibactam ± other.
Materials and methods
From October 2018 to March 2021, a retrospective, two-centre study was performed on patients with KPC-Kp BSI hospitalized at Azienda Policlinico Umberto I, Sapienza University of Rome and at ISMETT-IRCCS of Palermo and treated with ceftazidime/avibactam-containing regimens. Patients were further divided into those receiving ceftazidime/avibactam + fosfomycin and those receiving ceftazidime/avibactam in monotherapy or in combination with agents other than fosfomycin. Afterwards, a matched cohort (1:1) analysis was performed, with cases being patients with KPC-Kp BSI receiving ceftazidime/avibactam + fosfomycin, and controls being patients with KPC-Kp BSI receiving ceftazidime/avibactam alone or in combination with in vitro non-active drugs different from fosfomycin (ceftazidime/avibactam ± other). For every case, one matched control was randomly selected from patients who suffered from KPC-Kp BSI but did not receive fosfomycin in addition to ceftazidime/avibactam. Patients were matched for age, Charlson comorbidity index (CCI),15 ward of isolation (ICU, or non-ICU), source of infection and severity of BSI, expressed as INCREMENT carbapenemase-producing Enterobacteriaceae (CPE) score (ICS).16 Data collection for cases was blinded for the outcome.
Since an exact match for each of the five variables in all the patients was not possible, taking into account the type of the considered variables and their clinical and prognostic meanings, we matched each variable as follows: (i) age was matched according to decades; (ii) ward of isolation (ICU or non-ICU) and source of infection were exactly matched; (iii) in the case that the values of CCI were not exactly the same, we decided to consider the exact value ± 1; and (iv) the same for the severity of BSI (expressed as ICS), where we considered the exact value ± 2. In particular, given the clinical meaning of a low-risk ISC versus a high-risk ICS, we exactly matched the patients with ICS values of 6–7 and 8–9, whereas we were more confident that matching the exact value ± 2 in values of <6 or >9 would have influenced the results less and, at the same time, would have given us the possibility to include more patients in the study.16
For each subject, clinical data were collected and recorded anonymously in an electronic database.
The primary outcome was all-cause mortality 30 days after BSI onset. Secondary outcomes included: (i) the development of KPC-Kp BSI recurrence or new, non-bacteraemic KPC-Kp infections within 30 days after the end of treatment; (ii) the occurrence of secondary infections within 30 days from the start of antibiotic treatment and their related mortality; and (iii) the development of ceftazidime/avibactam-resistant strains during hospitalization.
Inclusion/exclusion criteria
Inclusion criteria were: (i) ≥ 1 positive BC for KPC-Kp in adult patients between October 2018 and March 2021; and (ii) the receipt of IV treatment with ceftazidime/avibactam for at least 48 h.
Exclusion criteria were: (i) not receiving ceftazidime/avibactam for the treatment of KPC-Kp BSI; (ii) ceftazidime/avibactam duration of treatment <48 h; (iii) BSI due to carbapenem-resistant Kp other than a KPC producer (i.e. OXA, MBL producers); (iv) polymicrobial BSI, with the exception of CoNS isolation, which has been considered as contamination; (v) patients treated with ceftazidime/avibactam ± other if the pathogen was tested susceptible to the companion drug; and (vi) no availability of clinical and microbiological data.
Data collection
The following information was reviewed: demographics, burden of comorbidities (expressed as CCI),15 clinical and laboratory findings, ICU admission, source of infection, severity of infection (expressed by means of ICS),16 microbiological data (including availability of fosfomycin susceptibility testing), antibiotic regimens (ceftazidime/avibactam + fosfomycin versus ceftazidime/avibactam ± other), dosage of fosfomycin, therapeutic appropriateness, development of ceftazidime/avibactam resistance, clinical cure, 7, 14, 30 day mortality, recurrence of BSI KPC-Kp, development of new, non-bacteraemic KPC-Kp infections, development of secondary infections and duration of hospital stay after BSI onset.
Definitions
Infections were defined according to the standard definitions of the ECDC.17 KPC-Kp BSI was defined when KPC-Kp was isolated from BCs in the presence of clinical signs of infection and BSI onset was defined as the date of collection of the index BC.
The likely or ascertained source of infection was indicated by the attending physician or by the Infectious Diseases consultant in the medical record and defined in accordance with guidelines.17,18 Primary BSI was defined as BSI occurring in patients without a recognized source of infection. Central line-related BSI (CLRBSI) was defined if the semiquantitative culture of the catheter tip was positive for the same KPC-Kp isolated from the blood.19
The burden of underlying comorbidities was evaluated by means of CCI.15 Immunosuppression was defined as either steroid therapy with prednisone (or its equivalent) at a dose >0.5 mg/kg/day for at least 1 month or the receipt of chemotherapy, TNF-α inhibitors, cyclophosphamide, azathioprine, methotrexate or mycophenolate mofetil in the previous 90 days.
Infections were classified as hospital acquired if the index BC had been collected >48 h after hospital admission and no signs or symptoms of infection had been noted at admission. Healthcare-associated BSIs were defined if positive BCs were obtained at hospital admission or within 48 h from admission and if the patient had attended a hospital or a haemodialysis centre, was hospitalized in an acute care hospital for ≥2 days in the previous 90 days or was a resident in a nursing home or long-term care facility.20
Severity of infection was determined by using ICS calculated at the time of infection onset.16 Pitt bacteraemia score was also calculated for each patient.21 Sepsis and septic shock were defined in accordance with the SEPSIS-3 criteria.22
Antimicrobial treatment evaluation
Patients were evaluated by Infectious Diseases consultants referring to a well-established consultation system at Policlinico Umberto I, Sapienza University of Rome23,24 and at ISMETT-IRCCS of Palermo.
Early (within 24 h) in vitro active therapy was classified as appropriate if at least one administered antibiotic exhibited in vitro activity, according to the breakpoints established by EUCAST.1,25
Definitive antibiotic therapy (defined as the definitive antimicrobial treatment administered after the availability of susceptibility results) was considered appropriate if KPC-Kp was susceptible to ceftazidime/avibactam and ceftazidime/avibactam was administered within 48–72 h from the index BC collection.
Early (within 48–72 h) clinical improvement was defined as at least one of the following: weaning from vasopressors; fever disappearance >48 h; procalcitonin reduction by >80%;26 and C-reactive protein reduction by >75%.27
Clinical cure was defined as clinical response to treatment with resolution of symptoms/signs of the infection upon discontinuation of antimicrobials.6
Microbiological response was defined as the negativity of follow-up blood cultures (FUBCs) (when performed) under treatment with ceftazidime/avibactam at three different timepoints (72 h and 7 and 14 days from BSI onset).
KPC-Kp BSI recurrence was defined as the onset of a second microbiologically documented KPC-Kp BSI in the 30 days after the end of treatment in a patient who had previously achieved clinical cure and microbiological response.
New, non-bacteraemic KPC-Kp infection was considered as isolation of KPC-Kp causing infections other than BSI in the 30 days after the end of treatment after achieving clinical cure (defined as above).
Secondary infection was defined as an infection [i.e. urinary tract infection (UTI), pneumonia, bacteraemia, candidaemia] caused by a microorganism other than KPC-Kp in the 30 days after the start of treatment.
Emergence of a ceftazidime/avibactam-resistant isolate was defined as the occurrence of a KPC-Kp strain exhibiting in vitro resistance to ceftazidime/avibactam, according to the EUCAST breakpoints.25
All-cause mortality was re-collected at 7, 14 and 30 days from BSI onset.
Mortality was considered related to secondary infections if the cause of death was considered to be the secondary infections and no other conditions; for instance, the subject died with BSI other than KPC-Kp and/or candidaemia.
Length of stay from BSI onset was considered as the number of days from the date of BSI to the date of discharge or death.
Microbiology
According to routine the Hospitals’ Microbiology Laboratory protocol implemented to speed up the diagnostic procedures, bacterial pellets obtained from positive BCs were used for bacterial identification by MALDI-TOF MS (Bruker Daltonics). Subsequent molecular analysis for the search of the blaKPC gene was performed by the GeneXpert® System (Cepheid). Antimicrobial susceptibility testing was performed with the VITEK 2 automated system (bioMérieux, Marcy l’Étoile, France) or the Sensititre™ system (Thermo Fisher Scientific). The determination of fosfomycin susceptibility, when available for the clinicians, was performed with MicroScan panels that use the broth dilution method, officially not suitable for drug categorization. Indeed, no categorization was provided by the laboratory in the final report for the clinicians.
Statistical analysis
The data, unless otherwise stated, were given as medians with IQRs for continuous variables and as simple frequencies, proportions and percentages for categorical variables. Mann–Whitney test was used for unpaired samples. Dichotomous variables were compared using Fisher’s exact tests or chi-squared test statistics, as appropriate. Survival was analysed by Kaplan–Meier curves and the statistical significance of the differences between the two groups was assessed using the log-rank test. Multivariable Cox regression model was performed to tease out the independent predictors for 30 day mortality. Multivariate analysis was constructed using a forward stepwise procedure, entering all variables deemed clinically significant. P value analyses were two-sided and a P value of less than 0.05 was considered statistically significant. All statistical analyses were performed with STATA/IC (StataCorp, version 15) or Statistical Program for the Social Sciences (SPSS, version 22, SPSS Inc., Chicago, IL, USA) software packages.
Ethics
The study was approved by the local Ethical Committees (no. 0069/2022); informed consent was waived due to the retrospective nature of the research. The study was conducted according to the guidelines of the Declaration of Helsinki.
Results
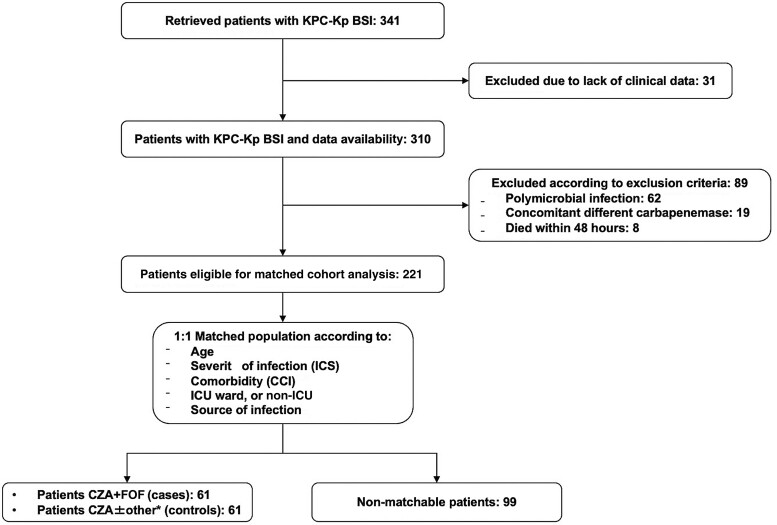
Our search retrieved 341 subjects hospitalized in Policlinico Umberto I and at ISMETT-IRCCS during the study period and with demonstrated growth of KPC-Kp from at least one BC sample. Thirty-one were excluded due to the lack of essential clinical and therapeutic data, while 89 were excluded according to exclusion criteria: 62 had a polymicrobial BSI, 19 were prescribed ceftazidime/avibactam for a Kp BSI using a non-KPC mechanism and 8 died in the first 48 h of treatment. Finally, 221 patients were included in the analysis. Sixty-one of them had received treatment with ceftazidime/avibactam + fosfomycin and thus were all 1:1 matched with 61 who received ceftazidime/avibactam monotherapy or ceftazidime/avibactam with a non-active companion drug different from fosfomycin (ceftazidime/avibactam ± other) according to the match criteria. The remaining 99 could not be matched and were further excluded from the matched cohort study (Figure 1).
Figure 1.
Enrolment flowchart. CZA: ceftazidime/avibactam; FOF: fosfomycin. *, ‘other’ includes CZA monotherapy and CZA associated with one antibiotic among gentamicin, tigecycline or colistin.
Whole-population analysis
Descriptive analysis of the whole population (n = 122) is reported in Table 1. Median age was 68 years and male gender accounted for 68.9%; almost one-third of the population (29.5%) was hospitalized in the ICU at the time of index BC collection and 79.5% had previously known rectal colonization by KPC-Kp.
Table 1.
Characteristics of the matched cohort population
| Variables | Matched population (N = 122) | Cases and controlsa | ||
|---|---|---|---|---|
| Cases (N = 61) | Controls (N = 61) | P value | ||
| Gender male, n (%) | 84 (68.9) | 44 (72.1) | 40 (65.6) | 0.558 |
| Age, years, median (IQR) | 68.0 (57.0–78.0) | 67.0 (56.0–77.5) | 69.0 (58.5–79.0) | 0.603 |
| ICU, n (%) | 36 (29.5) | 18 (29.5) | 18 (29.5) | 1.0 |
| SARS-CoV-2 coinfection, n (%) | 9 (7.4) | 5 (8.2) | 4 (6.6) | 1.0 |
| CCI, median (IQR) | 6 (5–9) | 6 (4–9) | 6 (5–9) | 0.730 |
| CCI ≥ 3, n (%) | 106 (86.9) | 50 (82.0) | 56 (91.8) | 0.179 |
| Diabetes, n (%) | 31 (25.4) | 16 (26.2) | 15 (24.6) | 1.0 |
| Cerebral and/or cardiovascular disease, n (%) | 77 (63.1) | 34 (55.7) | 43 (70.5) | 0.133 |
| COPD, n (%) | 38 (31.1) | 20 (32.8) | 18 (29.5) | 0.845 |
| Chronic kidney disease, n (%) | 31 (25.4) | 15 (24.6) | 16 (26.2) | 1.0 |
| Pre-admission dialysis, n (%) | 4 (3.3) | 2 (3.3) | 2 (3.3) | 1.0 |
| Chronic liver disease, n (%) | 17 (13.9) | 10 (16.4) | 7 (11.5) | 0.602 |
| Cancer, n (%) | 41 (33.6) | 21 (34.4) | 20 (32.8) | 1.0 |
| Immunosuppression,bn (%) | 38 (31.1) | 23 (37.7) | 15 (24.6) | 0.171 |
| Solid organ transplant, n (%) | 13 (10.7) | 9 (14.8) | 4 (6.6) | 0.240 |
| Rectal KPC-Kp colonization, n (%) | 97 (79.5) | 50 (82.0) | 47 (77.0) | 0.654 |
| Serum lactate (mmol/L), median (IQR) | 2.2 (1.5–3.6) | 2.0 (1.4–3.5) | 2.4 (1.7–4.0) | 0.511 |
| C-reactive protein (mg/dL), median (IQR) | 10.2 (5.4–17.4) | 10.2 (5.6–18.1) | 10.5 (4.8–16.3) | 0.415 |
| Procalcitonin (ng/dL), median (IQR) | 4.5 (0.8–28.9) | 3.6 (0.8–28.1) | 4.8 (1.0–30.8) | 0.595 |
| Continuous renal replacement therapy, n (%) | 14 (11.5) | 7 (11.5) | 7 (11.5) | 1.0 |
| Hospital acquired Healthcare acquired |
101 (82.8) 21 (17.2) |
47 (77.0) 14 (23.0) |
54 (88.5) 7 (11.5) |
0.149 |
| Septic shock, n (%) | 25 (20.5) | 14 (23.0) | 11 (18.0) | 0.654 |
| Pitt score, median (IQR) | 2.0 (1.0–4.0) | 3.0 (2.0–4.0) | 2.0 (1.0–4.0) | 0.173 |
| ICS, median (IQR) | 6 (3–8) | 6 (3–8) | 6 (3–8) | 0.682 |
| ICS ≥ 8, n (%) | 39 (32.0) | 20 (32.8) | 19 (31.1) | 1.0 |
| Available FOF MIC | 75 (61.5) | 29 (47.5) | 46 (75.4) | 0.003 |
| Source of infection, n (%) | ||||
| Primary bacteraemia | 34 (27.9) | 17 (27.9) | 17 (27.9) | — |
| CLRBSI | 4 (3.3) | 2 (3.3) | 2 (3.3) | |
| Urinary tract | 36 (29.5) | 18 (29.5) | 18 (29.5) | |
| Intra-abdominal | 26 (21.3) | 13 (21.3) | 13 (21.3) | |
| Lower respiratory tract [including VAP] | 22 (18.0) [14 (63.6)] | 11 (18.0) [7 (63.6)] | 11 (18.0) [7 (63.6)] | |
| CZA MIC, median (IQR) | 2 (2–4) | 2 (2–4) | 2 (2–4) | 0.813 |
| Antimicrobial regimens, n (%) | ||||
| CZA + MEM | 40 (32.8) | — | 40 (65.6) | — |
| CZA + FOF | 61 (50.0) | 61 (100) | — | |
| CZA + otherc | 8 (6.5) | — | 8 (13.1) | |
| CZA monotherapy | 13 (10.7) | — | 13 (21.3) | |
| FOF dose (g/day), median (IQR) | — | 16 (12–24) | — | — |
| FOF dose ≥ 16 g/day, n (%) | — | 43 (70.5) | — | — |
| Early active therapy,dn (%) | 53 (43.4) | 32 (52.5) | 21 (34.4) | 0.067 |
| Appropriate definitive therapy,en (%) | 113 (92.6) | 56 (91.8) | 57 (93.4) | 1.0 |
| CZA prolonged infusion, n (%) | 52 (42.6) | 32 (52.5) | 20 (32.8) | 0.044 |
| Performed source control,fn (%) | 60 (64.8) | 25 (60.7) | 35 (68.9) | 0.168 |
| Duration of definitive treatment, median (IQR) | 14 (11–19) | 14.0 (11.0–17.0) | 15 (11.5–19.5) | 0.348 |
CZA, ceftazidime/avibactam; FOF, fosfomycin; MEM, meropenem. Bold type indicates statistical significance.
Cases were patients with KPC-Kp BSI receiving CZA + FOF; controls were patients with KPC-Kp BSI receiving CZA alone or in combination with in vitro non-active drugs different from FOF (CZA ± other).
Immunosuppression was defined as either steroid therapy with prednisone (or its equivalent) at a dose of >0.5 mg/kg/day for at least 1 month or the receipt of chemotherapy, TNF-α inhibitors, cyclophosphamide, azathioprine, methotrexate or mycophenolate mofetil in the previous 90 days.
‘Other’ includes CZA in association with one antibiotic among gentamicin, tigecycline or colistin.
Early active therapy was classified as appropriate if at least one administered antibiotic exhibited in vitro activity within 24 h.
Definitive antibiotic therapy (defined as the definitive antimicrobial treatment administered after the availability of susceptibility results) was considered appropriate if KPC-Kp was susceptible to CZA, and CZA was administered within 48–72 h from the index BC collection.
The percentage of source control was calculated only on the patients for whom it was considered necessary.
Primary bacteraemia accounted for 34 episodes (27.9%), UTIs for 36 (29.5%), lower respiratory tract for 22 (18.0%), including 14 (63.6%) ventilator-associated pneumonia (VAP), whereas an intra-abdominal source was identified in 26 (21.3%) and a CLRBSI in 4 (3.3%).
The initiation of empirical therapy generally coincided with the collection of BCs (median time lag of 0 days), but only 43.4% of empirical regimens could be classified as appropriate according to study protocol. Definitive treatment was considered appropriate in 92.6% and continued for a median of 14 days.
Ceftazidime/avibactam + fosfomycin and the combination of ceftazidime/avibactam with meropenem were the most frequently prescribed regimens for KPC-Kp BSI, followed by ceftazidime/avibactam monotherapy (10.7%) and ceftazidime/avibactam with another companion drug (6.5%).
As for the outcomes (Table 2), early improvement was found in 80.3% of the patients and clinical cure was obtained by 68.0%. Recurrence of KPC-Kp BSI occurred in 10 individuals (8.2%); likewise, a new non-bacteraemic KPC-Kp infection was detected in 13 (10.7%), mainly represented by urinary catheter-associated infections. In the setting of BSI recurrence or new non-bacteraemic infection, the offending KPC-Kp revealed an increased MIC of ceftazidime/avibactam compared with the initial isolate in six patients (4.9%); in two of them the microorganism achieved clear resistance (MIC = 16 mg/L) to ceftazidime/avibactam.25
Table 2.
Clinical outcomes of the matched population
| Variables | Matched population (N = 122) | Cases and controlsa | ||
|---|---|---|---|---|
| Cases (N = 61) | Controls (N = 61) | P value | ||
| CZA MIC increase [CZA resistance development], n (%) | 6 (4.9) [2 (33.0)] | 3 (4.9) [0] | 3 (4.9) [2 (66.0)] | 0.185 |
| Early clinical improvement,bn (%) | 98 (80.3) | 52 (85.2) | 46 (75.4) | 0.255 |
| Clinical cure,cn (%) | 83 (68.0) | 46 (75.4) | 37 (60.7) | 0.120 |
| KPC-Kp BSI recurrence,dn (%) | 10 (8.2) | 4 (6.6) | 6 (9.8) | 0.509 |
| New KPC-Kp infection (BSI excluded),en (%) | 13 (10.7) | 3 (4.9) | 10 (16.4) | 0.039 |
| Source of new KPC-Kp infection, n (%) | ||||
| Urinary tract | 9 (69.2) | 3 (100.0) | 6 (60.0) | 0.332 |
| Intra-abdominal | 1 (7.7) | 0 | 1 (10.0) | |
| Lower respiratory tract | 2 (15.4) | 0 | 2 (20.0) | |
| Bone and joint | 1 (7.7) | 0 | 1 (10.0) | |
| Secondary infections,fn (%) | 42 (34.4) | 17 (27.9) | 25 (41.0) | 0.182 |
| Source of secondary infection, n (%) | ||||
| Urinary tract | 6 (14.3) | 2 (11.8) | 4 (16.0) | 0.746 |
| Intra-abdominal | 1 (2.4) | 0 | 1 (4.0) | |
| Lower respiratory tract | 4 (9.5) | 1 (5.9) | 3 (12.0) | |
| C. difficile | 4 (9.5) | 1 (5.9) | 3 (12.0) | |
| Bacteraemia | 17 (40.5) | 9 (52.9) | 8 (32.0) | |
| Candidaemia | 10 (23.8) | 4 (23.5) | 6 (24.0) | |
| Negative KPC-Kp FUBCs 72 h after treatment start, n (%) [performed in n (%)] | 68 (85.0) [80 (65.8)] | 33 (76.7) [43 (70.5)] | 35 (94.6) [37 (60.7)] | 0.026 |
| Negative KPC-Kp FUBCs 7 days after treatment start, n (%) [performed in n (%)] | 58 (92.1) [63 (51.6)] | 30 (88.2) [34 (55.7)] | 28 (96.6) [29 (47.5)] | 0.224 |
| Negative KPC-Kp FUBCs 14 days after treatment start, n (%) [performed in n (%)] | 42 (93.3) [45 (36.9)] | 25 (96.2) [26 (42.6)] | 17 (89.5) [19 (31.2)] | 0.375 |
| Cumulative mortality at 7 days from BSI onset, n (%) | 6 (4.9) | 3 (4.9) | 3 (4.9) | 1.0 |
| Cumulative mortality at 14 days from BSI onset, n (%) | 11 (9.0) | 6 (9.8) | 5 (8.2) | 0.752 |
| Cumulative mortality at 30 days from BSI onset, n (%) | 20 (16.4) | 9 (14.8) | 11 (18.0) | 0.807 |
| Overall in-hospital mortality, n (%) | 39 (32.0) | 20 (32.8) | 19 (31.1) | 1.0 |
| Death associated to secondary infection (KPC-Kp excluded), n (%) | 8 (6.6) | 1 (1.6) | 7 (11.5) | 0.020 |
| Length of stay from BSI onset (days), median (IQR) | 29 (15–47) | 28 (14–48) | 29 (16–47) | 0.544 |
CZA, ceftazidime/avibactam. Bold type indicates statistical significance.
Cases were patients with KPC-Kp BSI receiving CZA + FOF; controls were patients with KPC-Kp BSI receiving CZA alone or in combination with in vitro non-active drugs different from FOF (CZA ± other).
Early (within 48–72 h) clinical improvement was defined as at least one of the following: weaning from vasopressors; fever disappearance >48 h; procalcitonin reduction by >80%; C-reactive protein reduction by >75%.
Clinical cure was defined as clinical response to treatment with resolution of symptoms/signs of the infection upon discontinuation of antimicrobials.
KPC-Kp BSI recurrence was defined as the onset of a second microbiologically documented KPC-Kp BSI in a patient who had previously achieved clinical cure.
New, non-bacteraemic KPC-Kp infection was considered as isolation of KPC-Kp causing infections other than BSI after achieving clinical cure.
Secondary infection was defined as an infection (i.e. UTI, pneumonia, bacteraemia, candidaemia) caused by a microorganism other than KPC-Kp in the 30 days after the start of treatment.
Secondary infections occurred in 42 patients (34.4%) after a median time of 12 days. Out of these, 17 (40.5%) were bacteraemia and 10 (23.8%) were candidaemia.
All-cause mortality at 7, 14 and 30 days from BSI onset was 4.9% (6/122), 9.0% (11/122) and 16.4% (20/122), respectively. The median length of stay from index BCs collection was 29 days. The death of eight individuals (6.6%) was considered attributable to the secondary infections.
Matched cohort analysis
The 61 patients who received ceftazidime/avibactam + fosfomycin (cases) were compared with 61 subjects who were treated with ceftazidime/avibactam monotherapy or ceftazidime/avibactam associated with in vitro non-active drugs different from fosfomycin (ceftazidime/avibactam ± other, controls), after being paired according to match criteria (Table 1). There was no difference in the timing from BC collection to start of empirical therapy. Prolonged ceftazidime/avibactam infusion and early active therapy prescription were significantly more applied to ceftazidime/avibactam + fosfomycin patients, although the latter without statistical significance.
Overall, new non-bacteraemic infections from KPC-Kp were more frequent among controls (4.9% versus 16.4%, P = 0.039) as well as the percentage of secondary infections, albeit not statistically significant (27.9% versus 41.0%, P = 0.182). In this regard, we found that 45% (18/40) of patients treated with ceftazidime/avibactam + meropenem developed secondary infections and that ceftazidime/avibactam + meropenem accounted for the majority (18/25, 72%) of secondary infections in the group ceftazidime/avibactam + other.
Although FUBCs performed at 72 h yielded more frequently negative results among controls, no difference was detected at 7 and 14 days (Table 2).
A higher number of deaths attributable to secondary infections in the control group (11.5% versus 1.6%, P = 0.020) was also found.
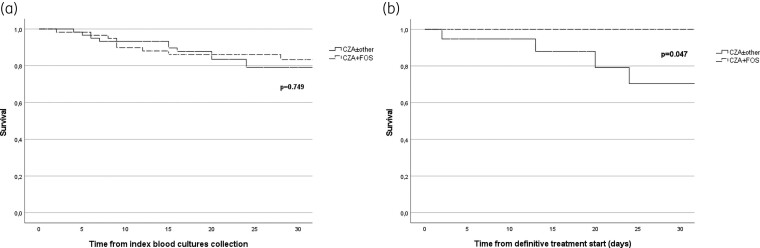
As reported in Figure 2(a), no difference in overall 30 day mortality emerged between cases and controls. Differently, controls had significantly reduced survival in terms of deaths attributable to secondary infections [Figure 2(b)].
Figure 2.
Kaplan–Meier curve for 30 day overall survival (a) and for 30 day secondary infections attributable deaths from (b) from index BC collection in the matched population.
Utilization of fosfomycin
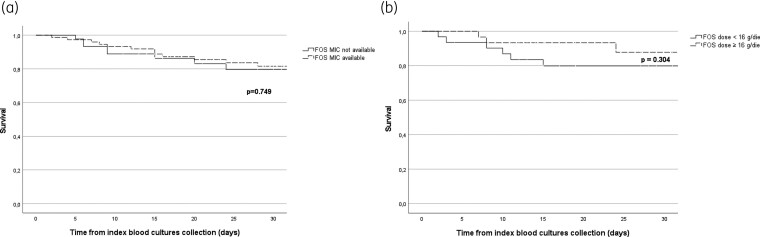
Overall, fosfomycin antimicrobial susceptibility was available in 75 (61.5%) of the matched population (Table 1) and for a significantly higher percentage in the population that did not receive fosfomycin (P = 0.003). Notably, 52.5% of the ceftazidime/avibactam + fosfomycin group members were prescribed fosfomycin without fosfomycin susceptibility availability. Of the 61 subjects treated with ceftazidime/avibactam + fosfomycin, 70.5% received a fosfomycin daily dose ≥16 g and, in general, the 24 g/day schedule was the most frequently prescribed (40.9%).
In the ceftazidime/avibactam + fosfomycin group, no difference in the final outcome emerged after stratification for the availability of fosfomycin susceptibility [Figure 3(a)] and for the administration of a fosfomycin dose ≥16 g [Figure 3(b)].
Figure 3.
(a) Kaplan–Meier curve for 30 day overall mortality from index BC collection in the matched population according to the availability (dashed line) or unavailability (solid line) of FOF MIC. (b) Kaplan–Meier curve for 30 day overall mortality from index BC collection in the CZA + FOF population according to the administration of a high dose (≥16 g/day, dashed line) or low dose (<16 g/day, solid line) of FOF. CZA, ceftazidime/avibactam; FOF, fosfomycin.
Risk factors for 30 day mortality of the matched population
Multivariable analysis showed that SARS-CoV-2 coinfection (HR 3.36, 95% CI 1.04–10.87, P = 0.042) and a high ICS (≥8) (HR 3.24, 95% CI 1.27–8.22, P = 0.013) independently predicted 30 day mortality, whereas the appropriateness of definitive therapy (HR 0.16, 95% CI 0.04–0.55, P = 0.004) was a protective factor (Table 3).
Table 3.
Analysis of risk factors for 30 day mortality in patients with BSI caused by KPC-Kp
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Casesa (versus controls) | 0.84 (0.34–2.03) | 0.70 | 0.72 (0.28–1.85) | 0.504 |
| Gender male, n (%) | 0.844 (0.33–2.11) | 0.71 | — | — |
| Age ≥ 65 years, median (IQR) | 1.43 (0.57–3.60) | 0.43 | 1.14 (0.32–3.94) | 0.835 |
| ICU, n (%) | 1.96 (0.81–4.73) | 0.13 | — | — |
| SARS-CoV-2 coinfection, n (%) | 3.81 (1.27–11.44) | 0.017 | 3.36 (1.04–10.87) | 0.042 |
| CCI ≥ 5 | 1.24 (0.41–3.72) | 0.694 | 2.34 (0.49–11.12) | 0.835 |
| Immunosuppression,bn (%) | 1.77 (0.72–4.34) | 0.213 | — | — |
| Continuous renal replacement therapy, n (%) | 3.90 (1.49–10.17) | 0.005 | — | — |
| Septic shock, n (%) | 5.65 (2.34–13.65) | <0.0001 | — | — |
| Pitt score, median (IQR) | 1.25 (1.00–1.55) | 0.044 | — | — |
| ICS, median (IQR) | 1.23 (1.08–1.40) | 0.001 | — | — |
| ICS ≥ 8, n (%) | 3.48 (1.42–8.53) | 0.006 | 3.24 (1.27–8.22) | 0.013 |
| Available FOF MIC | 0.87 (0.35–2.14) | 0.771 | — | — |
| FOF dose ≥ 16 g/day, n (%) | 0.60 (0.17–2.05) | 0.420 | — | — |
| Early active therapy,cn (%) | 0.72 (0.28–1.82) | 0.495 | — | — |
| Definitive active therapy,dn (%) | 0.30 (0.10–0.90) | 0.032 | 0.16 (0.04–0.55) | 0.004 |
| CZA prolonged infusion, n (%) | 1.11 (0.46–2.67) | 0.816 | — | — |
| Source of infection (UTI versus others) | 0.24 (0.05–1.06) | 0.062 | 0.26 (0.05–1.27) | 0.098 |
| Source control, n (%) | 1.08 (0.56–2.07) | 0.806 | — | — |
CZA, ceftazidime/avibactam; FOF, fosfomycin. Bold type indicates statistical significance.
Cases were patients with KPC-Kp BSI receiving CZA + FOF; controls were patients with KPC-Kp BSI receiving CZA alone or in combination with in vitro non-active drugs different from FOF (CZA ± other).
Immunosuppression was defined as either steroid therapy with prednisone (or its equivalent) at a dose of >0.5 mg/kg/day for at least 1 month or the receipt of chemotherapy, TNF-α inhibitors, cyclophosphamide, azathioprine, methotrexate or mycophenolate mofetil in the previous 90 days.
Early active therapy was classified as appropriate if at least one administered antibiotic exhibited in vitro activity within 24 h.
Definitive antibiotic therapy was considered appropriate if KPC-Kp was susceptible to CZA and CZA was administered within 48–72 h from index BC collection.
Discussion
We presented a matched cohort analysis of 122 patients affected by KPC-Kp BSI who received a ceftazidime/avibactam-based therapy, 61 treated with ceftazidime/avibactam + fosfomycin and 61 controls receiving ceftazidime/avibactam alone or in combination with a non-active companion drug selected through match criteria. We found that, although overall 30 day survival did not differ between the two groups, patients receiving ceftazidime/avibactam + fosfomycin had a lower rate of subsequent non-bacteraemic KPC-Kp infections and, albeit not significantly, secondary infections than those receiving other ceftazidime/avibactam-based regimens, with a significantly lower mortality related to secondary infections than controls. Furthermore, the outcome was not influenced by the availability of fosfomycin susceptibility.
To date, most of the evidence on ceftazidime/avibactam usage comes from the largest currently available post-marketing series,6 which included, amongst others, 391 cases of KPC-Kp BSI. Compared with the group of Tumbarello et al., we found a similar median age of the patients (68 years) and gender distribution, with a male predominance (68.9%); on the other hand, our population was more frequently admitted to the ICU (29.5% versus 24.5%) and had a higher prevalence of COPD (31.1% versus 15.6%) and immunodeficiency (31.1% versus 8.2%). If the percentages of individuals with CCI ≥ 3 (86.9% versus 86.2%) were similar, that of patients with ICS ≥ 8 (32.0% versus 27.8%) was mildly higher in our series.6
Currently, several systematic reviews and meta-analyses published in the literature have shown no benefit of combinatory treatment over ceftazidime/avibactam monotherapy for KPC-Kp infections.7–9 National and international guidelines do not recommend combinations as no conclusive data are available,28,29 while, on the other hand, the risk of increased costs, adverse events and Clostridioides difficile infection remains on the prowl. Nevertheless, the use in ‘real life’ that appears from observational studies seems plainly inconsistent with this evidence, with a clear prevalence of combination treatments. Here, ceftazidime/avibactam monotherapy was applied in only 10.7% of cases, a lower value than that found by Tumbarello et al. (28.9%). This trend to prescribe combination regimens could reflect the increasing frequency of isolation of ceftazidime/avibactam-resistant strains,30 which pushes the Infectious Diseases consultants to somehow reinforce prescriptions, often directed to severely ill patients. Curiously, more than half of controls (65.6%) received the ceftazidime/avibactam + meropenem combination. Given the retrospective nature of the study, it was very difficult to explain the exact reason for this choice for all the patients; however, the possible reasons were clinical conditions where a possible risk of ceftazidime/avibactam underexposure existed or the prevention of emergence of KPC variants, a condition which has been widely described in our hospital.30
An increase of ceftazidime/avibactam MIC following treatment was in fact displayed by six isolates (4.9%) of the matched population, with two (1.6%, both in the controls group) achieving frank resistance. Zhang et al.31 found 3.7% resistance to ceftazidime/avibactam by testing strains collected in China during 2017, demonstrating ceftazidime/avibactam resistance pre-existing before its commercialization. In 2018, a retrospective study reported a 10% emergence of resistance in patients treated with ceftazidime/avibactam,3 while more recent studies showed an incidence of 3.6%.6 We identified a BSI recurrence rate slightly lower than those previously reported (8.2% versus 10.7%).6
The overall 30 day mortality was 16.4% in the matched population, lower than that reported by Tumbarello et al.6 (26.3%) and Zheng et al.10 (24.4% in the combination arm). This difference could be speculatively explained by the higher prevalence of UTIs in our series, which represented almost one third of cases. Indeed, urinary tract-related BSIs were more frequent in survivors than in non-survivors, although at the multivariate analysis it showed only a trend towards having a protective role.
Although the 30 day mortality did not differ between cases and controls, we could show a possible advantage of the ceftazidime/avibactam + fosfomycin combination in terms of reduced new non-bacteraemic KPC-Kp infections, which were mostly represented by UTIs (63.2%). Therefore, also taking into account that the source of KPC-Kp BSI was predominantly the urinary tract and considering the high level of fosfomycin concentration in the urine,32 we could consider that patients with KPC-Kp BSI originating from the urinary tract may benefit more from the addition of fosfomycin to ceftazidime/avibactam. We believe that additional studies are needed in order to investigate the potential benefit of adding fosfomycin in KPC-Kp BSIs other than those originating from the urinary tract.
FUBCs tested positive in a larger proportion of cases when performed at 72 h; this difference was not maintained at the subsequent 7 and 14 days follow-up. Although it is possible that the ceftazidime/avibactam + fosfomycin combination has a slower bactericidal power than the preponderant alternative combination of ceftazidime/avibactam + meropenem, it must also be considered a possible bias related to the fact that FUBCs were performed more often among cases at all timepoints.
We showed that mortality was independently associated with BSI severity, expressed by high ICS, thus confirming its firm predictive power.33 Although representing only a small part of the entire matched population, the strong effect of SARS-CoV-2 concurrent infection on mortality was also evident.34
One of our most interesting findings regarded the relation between fosfomycin prescription modality and fosfomycin susceptibility availability. In fact, we could demonstrate that in more than half of cases (52.5%) fosfomycin was prescribed without susceptibility reports availability and that, despite this, patients treated with ceftazidime/avibactam + fosfomycin did not suffer a worse outcome compared with others. This finding has important clinical implications, since the gold standard for fosfomycin susceptibility, the agar diffusion method, is time-consuming and therefore cannot be routinely implemented in clinical practice, while different susceptibility assessment methods demonstrated poor performance.35,36 Indeed, we were aware that the used method did not provide accurate results owing to unacceptable major error (ME) and very major error (VME) percentages even when performed as intended by the guidelines;35 accordingly, no categorization was provided by the laboratory in the final report for the clinicians.
The development of bacterial and fungal secondary infections was lower, albeit not significantly, in cases than in controls (27.9% versus 41.0%, respectively); however, when we looked more in depth, we found that 45% (18/40) of patients treated with ceftazidime/avibactam + meropenem developed secondary infections and that ceftazidime/avibactam + meropenem accounted for the majority (72%) of secondary infections in the ceftazidime/avibactam + other group. These interesting results, together with the observed favourable effect on mortality related to secondary infections, could be explained considering the lower ecological impact of fosfomycin compared with other ceftazidime/avibactam ‘companion drugs’, in particular the most frequently prescribed, meropenem. In the systematic review by Zimmerman et al.37 on gut microbiota, fosfomycin caused a reduction of Enterococcus species and an increase of Enterobacterales, without any impact on anaerobic and fungal compartments. The bacterial abundance returned to pre-antibiotic values in 12–14 days after the end of treatment. Differently, the administration of carbapenems caused a reduction of Enterobacterales, an increase of Enterococcus, a significant decrease of anaerobic species and an increase of Candida species. These alterations take 28 days to recover.37 In addition, an antimicrobial treatment with anti-anaerobic activity has been recognized as a risk factor for candidaemia.38,39 The administration of meropenem, in particular, was associated with loss of microbial diversity in the gut microbiota, greater than for other anti-anaerobic antibiotics such as piperacillin/tazobactam.40 Furthermore, meropenem administration was in some cases directly associated with subsequent candidaemia.41,42
This study undoubtedly has some limitations, mainly related to the retrospective nature of data collection. Furthermore, the sample size was limited and allowed us to conduct a match analysis of only a part of the population. A design-specific limitation was the choice to include patients treated with both ceftazidime/avibactam monotherapy and ceftazidime/avibactam with non-fosfomycin combination therapy, although these groups did not differ according to the match criteria; nevertheless, to overcome this bias we excluded from the final analysis those patients treated with a companion drug active towards the isolated KPC-Kp. Furthermore, we are aware that a sample of such patients may be biased toward clinical success and immortal time bias may also be present, as patients need to survive long enough to receive fosfomycin.43
In addition, we could not perform the gold standard method for fosfomycin susceptibility and, accordingly, we could not evaluate the association of fosfomycin MIC with the outcome. Nevertheless, this was not the primary aim of the study; rather, we aimed to correlate the mode of fosfomycin prescription with the availability of in vitro susceptibility testing, irrespective of the MIC values that were present in the final microbiology report. A possible source of error may have been represented by the occurrence of the SARS-CoV-2 pandemic in Italy, which introduced a relevant comorbidity in the middle of the observation period and impacted at an organizational level on the management of MDR pathogens.
Despite these limitations, our study gives important insights into the treatment of KPC-Kp BSI by (i) confirming the low usage of ceftazidime/avibactam in monotherapy in the real-life setting; (ii) showing no difference in mortality between ceftazidime/avibactam + fosfomycin and other combinations; and (iii) highlighting the possible ecological advantage of ceftazidime/avibactam + fosfomycin over other treatment choices, with obvious consequences in terms of antimicrobial stewardship programmes.
Conclusions
Our data show that fosfomycin was used in the treatment of KPC-Kp BSI independently from having its susceptibility testing available. Although no difference was found in 30 day overall mortality, the matched cohort study detected a lower rate of new, non-bacteraemic KPC-Kp infections and of secondary infection-related death in the ceftazidime/avibactam + fosfomycin group. This study describes treatment and outcomes of ‘real life’ patients hospitalized with KPC-Kp BSI in two hospitals with large catchment areas. Nevertheless, these data need a prospective reassessment that could confirm fosfomycin as a valid ‘carbapenem sparing’ alternative in the increasingly complex fight against MDR pathogens.
Acknowledgements
We thank all medical and nurse staff for their contribution in the clinical management of the patients.
Contributor Information
A Oliva, Department of Public Health and Infectious Diseases, Sapienza University of Rome, Piazzale Aldo Moro 5, Rome 00185, Italy.
L Volpicelli, Department of Public Health and Infectious Diseases, Sapienza University of Rome, Piazzale Aldo Moro 5, Rome 00185, Italy.
S Di Bari, Department of Public Health and Infectious Diseases, Sapienza University of Rome, Piazzale Aldo Moro 5, Rome 00185, Italy.
A Curtolo, Department of Public Health and Infectious Diseases, Sapienza University of Rome, Piazzale Aldo Moro 5, Rome 00185, Italy.
C Borrazzo, Department of Medico-Surgical Sciences and Biotechnologies, Sapienza University of Rome, Piazzale Aldo Moro 5, Rome 00185, Italy.
F Cogliati Dezza, Department of Public Health and Infectious Diseases, Sapienza University of Rome, Piazzale Aldo Moro 5, Rome 00185, Italy.
A Cona, Unit of Infectious Diseases, ISMETT-IRCCS Istituto Mediterraneo per i Trapianti e Terapie ad Alta Specializzazione, Via E. Tricomi, 5, Palermo 90127, Italy.
S Agrenzano, Unit of Infectious Diseases, ISMETT-IRCCS Istituto Mediterraneo per i Trapianti e Terapie ad Alta Specializzazione, Via E. Tricomi, 5, Palermo 90127, Italy.
A Mularoni, Unit of Infectious Diseases, ISMETT-IRCCS Istituto Mediterraneo per i Trapianti e Terapie ad Alta Specializzazione, Via E. Tricomi, 5, Palermo 90127, Italy.
M Trancassini, Department of Public Health and Infectious Diseases, Sapienza University of Rome, Piazzale Aldo Moro 5, Rome 00185, Italy.
F Mengoni, Department of Public Health and Infectious Diseases, Sapienza University of Rome, Piazzale Aldo Moro 5, Rome 00185, Italy.
S Stefani, Department of Biomedical and Biotechnological Sciences. Policlinico Hospital, University of Catania, Via Androne 81, Catania 95124, Italy.
G Raponi, Department of Public Health and Infectious Diseases, Sapienza University of Rome, Piazzale Aldo Moro 5, Rome 00185, Italy.
M Venditti, Department of Public Health and Infectious Diseases, Sapienza University of Rome, Piazzale Aldo Moro 5, Rome 00185, Italy.
Funding
This study was carried out as part of our routine work. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Transparency declarations
None to declare.
Data availability
Data are available upon request from corresponding author.
References
- 1. Falcone M, Bassetti M, Tiseo Get al. Time to appropriate antibiotic therapy is a predictor of outcome in patients with bloodstream infection caused by KPC-producing Klebsiella pneumoniae. Crit Care 2020; 24: 29. 10.1186/s13054-020-2742-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Karaiskos I, Daikos GL, Gkoufa Aet al. Ceftazidime/avibactam in the era of carbapenemase-producing Klebsiella pneumoniae: experience from a national registry study. J Antimicrob Chemother 2021; 76: 775–83. 10.1093/jac/dkaa503 [DOI] [PubMed] [Google Scholar]
- 3. Shields RK, Nguyen MH, Chen Let al. Pneumonia and renal replacement therapy are risk factors for ceftazidime-avibactam treatment failures and resistance among patients with carbapenem-resistant Enterobacteriaceae infections. Antimicrob Agents Chemother 2018; 62: e02497-17. 10.1128/AAC.02497-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tiseo G, Falcone M, Leonildi Aet al. Meropenem-vaborbactam as salvage therapy for ceftazidime-avibactam-, cefiderocol-resistant ST-512 Klebsiella pneumoniae-producing KPC-31, a D179Y variant of KPC-3. Open Forum Infect Dis 2021; 8: ofab141. 10.1093/ofid/ofab141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oliva A, Curtolo A, Volpicelli Let al. Synergistic meropenem/vaborbactam plus fosfomycin treatment of KPC producing K. pneumoniae septic thrombosis unresponsive to ceftazidime/avibactam: from the bench to the bedside. Antibiotics (Basel) 2021; 10: 781. 10.3390/antibiotics10070781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tumbarello M, Raffaelli F, Giannella Met al. Ceftazidime-avibactam use for Klebsiella pneumoniae carbapenemase-producing K. pneumoniae infections: a retrospective observational multicenter study. Clin Infect Dis 2021; 73: 1664–76. 10.1093/cid/ciab176 [DOI] [PubMed] [Google Scholar]
- 7. Meini S, Viaggi B, Tascini C. Mono vs. combo regimens with novel beta-lactam/beta-lactamase inhibitor combinations for the treatment of infections due to carbapenemase-producing Enterobacterales: insights from the literature. Infection 2021; 49: 411–21. 10.1007/s15010-021-01577-x [DOI] [PubMed] [Google Scholar]
- 8. Onorato L, Di Caprio G, Signoriello Set al. Efficacy of ceftazidime/avibactam in monotherapy or combination therapy against carbapenem-resistant Gram-negative bacteria: a meta-analysis. Int J Antimicrob Agents 2019; 54: 735–40. 10.1016/j.ijantimicag.2019.08.025 [DOI] [PubMed] [Google Scholar]
- 9. Fiore M, Alfieri A, Di Franco Set al. Ceftazidime-avibactam combination therapy compared to ceftazidime-avibactam monotherapy for the treatment of severe infections due to carbapenem-resistant pathogens: a systematic review and network meta-analysis. Antibiotics (Basel) 2020; 9: E388. 10.3390/antibiotics9070388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zheng G, Zhang J, Wang Bet al. Ceftazidime-avibactam in combination with in vitro non-susceptible antimicrobials versus ceftazidime-avibactam in monotherapy in critically ill patients with carbapenem-resistant Klebsiella pneumoniae infection: a retrospective cohort study. Infect Dis Ther 2021; 10: 1699–713. 10.1007/s40121-021-00479-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ojdana D, Gutowska A, Sacha Pet al. Activity of ceftazidime-avibactam alone and in combination with ertapenem, fosfomycin, and tigecycline against carbapenemase-producing Klebsiella pneumoniae. Microb Drug Resist 2019; 25: 1357–64. 10.1089/mdr.2018.0234 [DOI] [PubMed] [Google Scholar]
- 12. Mikhail S, Singh NB, Kebriaei Ret al. Evaluation of the synergy of ceftazidime-avibactam in combination with meropenem, amikacin, aztreonam, colistin, or fosfomycin against well-characterized multidrug-resistant Klebsiella pneumoniae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 2019; 63: e00779-19. 10.1128/AAC.00779-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Romanelli F, De Robertis A, Carone Get al. In vitro activity of ceftazidime/avibactam alone and in combination with fosfomycin and carbapenems against KPC-producing Klebsiella pneumoniae. New Microbiol 2020; 43: 136–8. [PubMed] [Google Scholar]
- 14. Papalini C, Sabbatini S, Monari Cet al. In vitro antibacterial activity of ceftazidime/avibactam in combination against planktonic and biofilm carbapenemase-producing Klebsiella pneumoniae isolated from blood. J Glob Antimicrob Resist 2020; 23: 4–8. 10.1016/j.jgar.2020.07.028 [DOI] [PubMed] [Google Scholar]
- 15. Charlson ME, Pompei P, Ales KLet al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 16. Gutiérrez-Gutiérrez B, Salamanca E, de Cueto Met al. Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect Dis 2017; 17: 726–34. 10.1016/S1473-3099(17)30228-1 [DOI] [PubMed] [Google Scholar]
- 17. ECDC . EU case definitions. https://www.ecdc.europa.eu/en/surveillance-and-disease-data/eu-case-definitions.
- 18. CDC . National Healthcare Safety Network (NHSN). Bloodstream infection (BSI) events.https://www.cdc.gov/nhsn/psc/bsi/index.html.
- 19. Cleri DJ, Corrado ML, Seligman SJ. Quantitative culture of intravenous catheters and other intravascular inserts. J Infect Dis 1980; 141: 781–6. 10.1093/infdis/141.6.781 [DOI] [PubMed] [Google Scholar]
- 20. Lenz R, Leal JR, Church DLet al. The distinct category of healthcare associated bloodstream infections. BMC Infect Dis 2012; 12: 85. 10.1186/1471-2334-12-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Korvick JA, Bryan CS, Farber Bet al. Prospective observational study of Klebsiella bacteremia in 230 patients: outcome for antibiotic combinations versus monotherapy. Antimicrob Agents Chemother 1992; 36: 2639–44. 10.1128/AAC.36.12.2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Singer M, Deutschman CS, Seymour CWet al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315: 801–10. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oliva A, Bianchi A, Russo Aet al. Effect of N-acetylcysteine administration on 30-day mortality in critically ill patients with septic shock caused by carbapenem-resistant Klebsiella pneumoniae and Acinetobacter baumannii: a retrospective case-control study. Antibiotics (Basel) 2021; 10: 271. 10.3390/antibiotics10030271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Volpicelli L, Venditti M, Ceccarelli Get al. Place in therapy of the newly available armamentarium for multi-drug-resistant Gram-negative pathogens: proposal of a prescription algorithm. Antibiotics (Basel) 2021; 10: 1475. 10.3390/antibiotics10121475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. EUCAST. Clinical breakpoints—bacteria. https://www.eucast.org/clinical_breakpoints/.
- 26. de Jong E, van Oers JA, Beishuizen Aet al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis 2016; 16: 819–27. 10.1016/S1473-3099(16)00053-0 [DOI] [PubMed] [Google Scholar]
- 27. von Dach E, Albrich WC, Brunel A-Set al. Effect of C-reactive protein-guided antibiotic treatment duration, 7-day treatment, or 14-day treatment on 30-day clinical failure rate in patients with uncomplicated gram-negative bacteremia: a randomized clinical trial. JAMA 2020; 323: 2160–9. 10.1001/jama.2020.6348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tiseo G, Brigante G, Giacobbe DRet al. Diagnosis and management of infections caused by multidrug-resistant bacteria: guideline endorsed by the Italian Society of Infection and Tropical Diseases (SIMIT), the Italian Society of Anti-Infective Therapy (SITA), the Italian Group for Antimicrobial Stewardship (GISA), the Italian Association of Clinical Microbiologists (AMCLI) and the Italian Society of Microbiology (SIM). Int J Antimicrob Agents 2022; 60: 106611. 10.1016/j.ijantimicag.2022.106611 [DOI] [PubMed] [Google Scholar]
- 29. Paul M, Carrara E, Retamar Pet al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin Microbiol Infect 2022 Apr; 28: 521–47. 10.1016/j.cmi.2021.11.025 [DOI] [PubMed] [Google Scholar]
- 30. Carattoli A, Arcari G, Bibbolino Get al. Evolutionary trajectories toward ceftazidime-avibactam resistance in Klebsiella pneumoniae clinical isolates. Antimicrob Agents Chemother 2021; 65: e0057421. 10.1128/AAC.00574-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang P, Shi Q, Hu Het al. Emergence of ceftazidime/avibactam resistance in carbapenem-resistant Klebsiella pneumoniae in China. Clin Microbiol Infect 2020; 26: 124.e1–e4. 10.1016/j.cmi.2019.08.020 [DOI] [PubMed] [Google Scholar]
- 32. López-Montesinos I, Horcajada JP. Oral and intravenous fosfomycin in complicated urinary tract infections. Rev Esp Quimioter 2019; 32Suppl 1: 37–44. [PMC free article] [PubMed] [Google Scholar]
- 33. Cano A, Gutiérrez-Gutiérrez B, Machuca Iet al. Risks of infection and mortality among patients colonized with Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: validation of scores and proposal for management. Clin Infect Dis 2018; 66: 1204–10. 10.1093/cid/cix991 [DOI] [PubMed] [Google Scholar]
- 34. Adalbert JR, Varshney K, Tobin Ret al. Clinical outcomes in patients co-infected with COVID-19 and Staphylococcus aureus: a scoping review. BMC Infect Dis 2021; 21: 985. 10.1186/s12879-021-06616-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cottell JL, Webber MA. Experiences in fosfomycin susceptibility testing and resistance mechanism determination in Escherichia coli from urinary tract infections in the UK. J Med Microbiol 2019; 68: 161–8. 10.1099/jmm.0.000901 [DOI] [PubMed] [Google Scholar]
- 36. Mojica MF, De La Cadena E, Hernández-Gómez Cet al. Performance of disk diffusion and broth microdilution for fosfomycin susceptibility testing of multidrug-resistant clinical isolates of Enterobacterales and Pseudomonas aeruginosa. J Glob Antimicrob Resist 2020; 21: 391–5. 10.1016/j.jgar.2020.01.003 [DOI] [PubMed] [Google Scholar]
- 37. Zimmermann P, Curtis N. The effect of antibiotics on the composition of the intestinal microbiota—a systematic review. J Infect 2019; 79: 471–89. 10.1016/j.jinf.2019.10.008 [DOI] [PubMed] [Google Scholar]
- 38. Cugno C, Cesaro S. Epidemiology, risk factors and therapy of candidemia in pediatric hematological patients. Pediatr Rep 2012; 4: e9. 10.4081/pr.2012.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang H, Wu D-W, Han Het al. Antibiotics exposure, risk factors, and outcomes with Candida albicans and non-Candida albicans candidemia. Results from a multi-center study. Saudi Med J 2014; 35: 153–8. [PubMed] [Google Scholar]
- 40. Ravi A, Halstead FD, Bamford Aet al. Loss of microbial diversity and pathogen domination of the gut microbiota in critically ill patients. Microb Genom 2019; 5: e000293. 10.1099/mgen.0.000293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ruiz GO, Osorio J, Valderrama Set al. Risk factors for candidemia in non-neutropenic critical patients in Colombia. Med Intensiva 2016; 40: 139–44. 10.1016/j.medin.2015.08.001 [DOI] [PubMed] [Google Scholar]
- 42. Caparó Ingram E, Vásquez Vega M, Norero Xet al. Risk factors and lethality associated with neonatal candidemia in a neonatal unit. Rev Chil Pediatr 2019; 90: 186–93. 10.32641/rchped.v90i2.717 [DOI] [PubMed] [Google Scholar]
- 43. Lévesque LE. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ 2010; 340: b5087. 10.1136/bmj.b5087 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request from corresponding author.