Abstract
The effectiveness of quinolone prophylaxis in high-risk hematological pediatric patients is controversial. A systematic review was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, including studies that involved children and young adults undergoing chemotherapy for acute leukemia or hematopoietic stem cell transplantation (HSCT) who received quinolone prophylaxis compared with no prophylaxis. A meta-analysis was performed on bloodstream infections and neutropenic fever. Data regarding the impact of prophylaxis on overall survival, antibiotic exposure, antibiotic-related adverse effects, antibiotic resistance, Clostridium difficile infections, fungal infections, length of hospitalization, and costs were reviewed in the descriptive analysis. Sixteen studies were included in the qualitative analysis, and 10 of them met the criteria for quantitative analysis. Quinolone prophylaxis was effective in reducing the rate of bloodstream infections and neutropenic fever in pediatric acute leukemia compared with no prophylaxis, but it had no significant effect in HSCT recipients. Prophylaxis was associated with a higher rate of bacterial resistance to fluoroquinolones and higher antibiotic exposure.
Keywords: acute leukemia, meta-analysis, pediatric, quinolone prophylaxis, stem cell transplantation
Infective complications represent the leading cause of morbidity and mortality among pediatric patients with cancer receiving intensive chemotherapy and undergoing hematopoietic stem cell transplantation (HSCT) [1]. Particularly, the presence of a central line, the relatively high frequency of blood draws and transfusions, the intestinal mucositis, and the therapy-induced neutropenia expose these patients to a higher risk of bloodstream infections (BSIs) [2, 3]. BSIs lead to longer hospitalizations with a consequent increase in health care costs, extensive exposure to antibiotics, more systemic complications, and higher mortality [4]. Antibiotic prophylaxis (PPX) represents a potential preventive strategy for BSI, and several prophylactic regimens have been historically proposed for cancer patients consisting of oral absorbable and nonabsorbable compounds as well as intravenous antibiotics. However, oral nonabsorbable antibiotics have been generally abandoned for poor tolerance and compliance, whereas most of the oral absorbable antibiotics, such as trimethoprim-sulfamethoxazole, have failed to demonstrate a significant difference in mortality [5]. In this context, quinolones have been historically commonly used as prophylactic agents, given their broad spectrum of antimicrobial activity, their capacity to preserve the anaerobic flora, their good tolerability, and their low myelosuppression [5–7]. In a meta-analysis of studies published before 2010 including adult patients with hematological and nonhematological malignancies undergoing chemotherapy, antibiotic prophylaxis was associated with reduced all-cause mortality, fewer febrile episodes, and gram-negative bacillus BSI, most significantly when assessing prophylaxis with quinolones [5]. However, a meta-analysis of studies published during 2006–2014 did not confirm a reduction in mortality on fluoroquinolone prophylaxis but still showed lower rates of BSI and of episodes of fever during neutropenia [8, 9]. The most recent meta-analysis, comprising adult and pediatric studies, found that levofloxacin PPX during intensive chemotherapy for acute leukemia significantly reduced febrile neutropenia, bacteremia, and microbiologically documented infection rates, but did not improve the death rate [10]. While the effectiveness of PPX in adult patients has been addressed in several studies, data regarding pediatric patients are limited [11]. A narrative review by Calitri and colleagues raised questions about antibacterial prophylaxis in children with leukemia, highlighting the lack of strong evidence for its use in the pediatric population [12]. Moreover, new evidence demonstrated the association of extended-spectrum antibiotic use with the emergence of multidrug-resistant bacteria and gut dysbiosis [13, 14], further suggesting a clear risk-benefit assessment. Among pediatric cancers, patients with acute leukemia (AL) or receiving HSCT are at higher risk of developing BSI due to long-lasting neutropenia combined with a higher presence of mucositis [15]. The current guideline does not recommend routine antibacterial PPX for pediatric patients with AL receiving intensive chemotherapy or with neutropenia during the pre-engraftment stage of HSCT based on the low level of evidence [11]. Moreover, the possible benefits should be weighed against potential harm, including Clostridium difficile infection (CDI) risk, drug-related side effects, and association with colonization or infection with fluoroquinolone- or multidrug-resistant strains [8, 16]. Recent data also highlighted the detrimental role of antibiotic prophylaxis on the gut microbiota, which results in a disruption of eubiosis associated with bacterial dominance and a higher rate of immune-mediated complications [14, 17]. We here conducted a systematic review and meta-analysis of the available studies comparing quinolone PPX vs no PPX in pediatric patients with AL undergoing HSCT.
METHODS
Literature Search
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [18]. Electronic databases, including PubMed, TRIP, and CINAHL, were searched up to January 20, 2022. The search strategy and the search string used to perform the browsing are reported in the Supplementary Data. The search was restricted to English-language studies involving children and young adults (age >30 days and <23 years) receiving chemotherapy for AL or undergoing HSCT who received a quinolone-based antibacterial PPX compared with no PPX. Two reviewers (D.L. and N.A.) independently identified potentially eligible studies by title/abstract screening. The same authors assessed the full texts of potentially relevant studies for inclusion and consulted the references of previously published primary and secondary papers, including reviews and meta-analyses, to manually search for additional relevant papers. Any disagreement regarding eligibility and inclusion in the systematic review was resolved through discussion and consensus between the 2 authors. If consensus was not reached, the opinion of a third author (E.M.), who acted as the final arbiter, was requested. Investigators and corresponding authors were contacted to obtain additional information about studies with incomplete data.
Data Extraction and Meta-analysis
We used the same methodology for data extraction, performed independently by the same 2 reviewers (D.L. and N.A.) under the supervision of a third author (E.M.). Data were summed and analyzed using Microsoft Office Excel for Mac 2022 (Microsoft, Redmond, WA, USA) and Stata 13 (StataCorp, College Station, TX, USA). Only papers reporting outcomes related to the total number of patients were included in the quantitative synthesis. Subsequently, we performed a meta-analysis considering the primary outcome, incidence of BSI and neutropenic fever (NF), considering the number of patients with at least 1 episode of BSI/NF. Primary outcomes were selected in consideration of the main aim of prophylactic antibiotic treatments. Secondary outcomes, in order, were overall survival, antibiotic exposure, antibiotic-related adverse effects, antibiotic resistance, Clostridium difficile infections, fungal infections, length of hospitalization, and health care costs. BSIs have been defined as any infection caused by a recognized pathogen that was isolated from ≥1 blood culture in the context of a compatible clinical illness. Even if there is no consensus yet, febrile neutropenia has been consistently defined as a core body temperature ≥38.3°C or ≥38°C for ≥1 hour in the context of neutropenia, defined as an absolute neutrophil count (ANC) ≤500/mmc [19, 20]. We analyzed statistical heterogeneity to determine the feasibility of summing the results of the different studies considered eligible for the meta-analysis. We assessed heterogeneity by graphic funnel plots and by calculating the I2 statistic, which represents the percentage of the variance in effect estimates that is caused by heterogeneity rather than by sampling bias (chance). An I2 statistic >40% was considered significantly heterogeneous. When the number of studies was <5 or studies were substantially heterogeneous, we used a random-effects model in accordance with the Cochrane Handbook for Systematic Reviews of Interventions [21]. We followed the method of DerSimonian and Laird [22] to compute the random effects estimates for the corresponding statistics. We chose to use forest plots to graphically show effect estimates with 95% CIs for individual trials and pooled results. We carried out the meta-analysis using RevMan, version 5.3 (https://revman.cochrane.org). Sensitivity analyses were also performed by removing studies separately based on the chosen criteria, namely, quality of the study, type of study, and type of country in which the study was performed.
Quality Assessment
Quality assessment was performed independently by 2 authors (D.L. and N.A.), and any disagreement was resolved through discussion and consensus between the 2 authors. We used the Cochrane Tool for Quality Assessment for evaluating RCTs and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement to assess the quality of the observational studies included in the meta-analysis. The Cochrane tool allows for the analysis of 7 types of bias: sequence generation and allocation concealment (both within the domain of selection bias or allocation bias), blinding of participants and personnel (performance bias), blinding of outcome assessors (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and an auxiliary domain, “other bias” [23]. For each type of bias, it was possible to assign a value of “high,” “low,” or “unclear” risk of bias when it was not specified whether a specific type of bias was present. Each bias judgment aids in assigning a global assessment to every RCT (good, fair, or poor) according to the Agency for Healthcare Research and Quality standards [24]. The STROBE statement is a 22-item tool specifically designed to evaluate the quality of cohort studies [25]. Items are associated with different sections of an article, such as title and abstract (item 1), introduction (items 2 and 3), methods (items 4–12), results (items 13–17), discussion (items 18–21), and other information (item 22 for funding). Eighteen items are identical for 3 different study designs, whereas 4 items (items 6, 12, 14, and 15) are differentially intended for a specific study type (ie, cohort or case–control study). The STROBE statement does not provide scoring stratification. As a general rule, the higher the score, the higher the quality of the study. Thus, we created 3 score thresholds corresponding to 3 levels of quality: 0–14 was considered low quality; 15–25, intermediate quality; 26–33, high quality.
RESULTS
Literature Search and Population
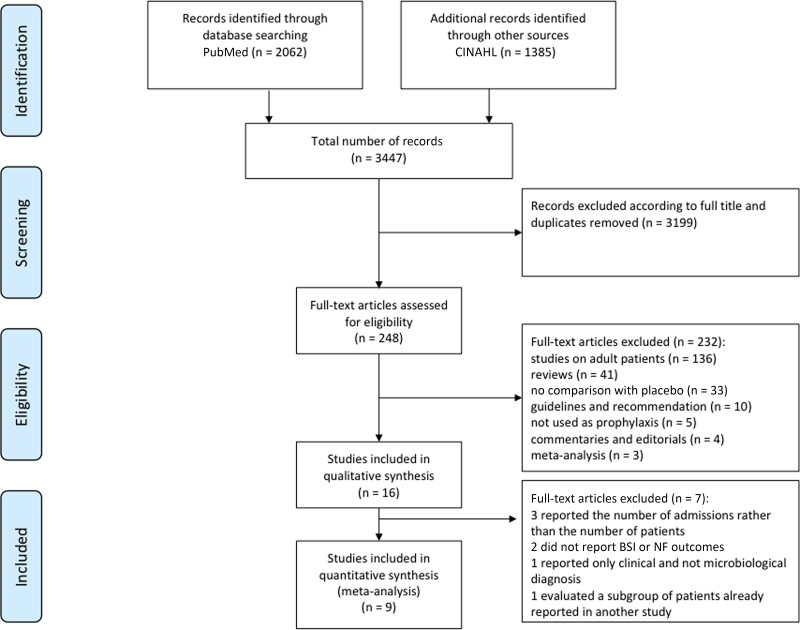
The literature search strategy identified a total of 3447 references (2062 in PubMed, 1385 in CINAHL) (Figure 1). A total of 3199 records were excluded according to the full title, and the duplicates were removed. The resulting 248 records were assessed by full text: 136 studies were excluded because they concerned adult patients, 33 because they did not compare quinolone PPX with no PPX, and 5 because quinolones were not used in a PPX setting. Reviews (41), meta-analyses (3), guidelines and recommendations (10), and complementary articles and editorials (4) were excluded as well. Of the 16 studies included in the qualitative synthesis, 7 were excluded [26–32]. Detailed reasons for exclusion are reported in Supplementary Table 1 and Figure 1. The total number of patients included in the quantitative synthesis was 2254. Among the 9 studies selected for the meta-analysis, 6 were retrospective single-center studies [33–38] and 3 were prospective studies [39–41], 2 of which were randomized [40, 41]; 1 was also multicentric [41]. Two studies included only patients with acute lymphoblastic leukemia (ALL) [39, 40], 1 only patients with acute myeloid leukemia (AML) [37], 2 both ALL and AML patients [33, 42], 1 only patients undergoing autologous HSCT (auto-HSCT) [35], 2 both auto- and allogenic HSCT (allo-HSCT) [34, 36], and 1 all groups of patients [41]. Two of the 6 studies on ALL patients studied relapsed ALL (rALL) [33, 41]. Detailed data on AL patients in the studies included in the meta-analysis are reported in Table 1. Levofloxacin (LVX) [33, 39, 41] and ciprofloxacin (CPFX) [37, 39, 40, 42] were both used in the AL setting, whereas HSCT studies investigated only the use of LVX PPX [34–36, 41]. The quality of the included studies in the meta-analysis was assessed as described in Methods and reported in Table 2.
Figure 1.
PRISMA flow diagram of the search strategy and included studies. The relevant number of papers at each point is given. Abbreviation: BSI, bloodstream infection; NF, neutropenic fever; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1.
Characteristics of AL Patients Included in the Meta-analysis
| Study | Population | Chemotherapy Phase and/or Criteria for FLQ PPX |
|---|---|---|
| Alexander et al. [41] | rALL, any AML (de novo, relapsed, or secondary AML, AL of ambiguous lineage treated with standard AML therapy) | 2 consecutive cycles of intensive chemotherapy, defined as regimens that are predicted to cause neutropenia (ANC <200/mm3) for >7 d |
| Davis et al. [33] | rALL, AML, other AL who received AML-type chemotherapy | Phase not specified—chemotherapy expected to lead to prolonged severe neutropenia (ANC <200/mm3) |
| Laoprasopwattana et al. [40] | ALL, lymphoma—not specified if newly diagnosed and/or relapse | Either induction or consolidation |
| Wolf et al. [39] | Newly diagnosed ALL | Induction |
| Yeh et al. [43] | Newly diagnosed ALL, newly diagnosed nonacute promyelocytic leukemia AML | Induction, consolidation, or reinduction (ALL); induction, postremission high dose and modest dose (AML)—expected prolonged neutropenia (ANC ≤500/mm3 for >7 d) |
| Yeh et al. [37] | Newly diagnosed AML (no Down syndrome, acute promyelocytic leukemia, or therapy-related AML) | Induction, postremission high dose, postremission modest dose |
Abbreviations: AL, acute leukemia; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ANC, absolute neutrophil count; FLQ, fluoroquinolone; PPX, prophylaxis; rALL, relapsed ALL.
Table 2.
Summary of Studies Included in the Meta-analysis
| First Authors | Year | Study Design | Type of Patients | Total Patients | PPX Group | No PPX Group | Type of PPX | Quality Assessment |
|---|---|---|---|---|---|---|---|---|
| Alexander et al. [41] | 2018 | Randomized, open-label, multicenter | rALL, AML auto-HSCT, allo-HSCT |
195, 418 | 96, 210 | 99, 208 | Levofloxacin | Good qualityb |
| Davis et al. [33] | 2022 | Retrospective, single center | rALL, AML | 135 | 72 | 63 | Levofloxacin | High qualitya |
| Gardner et al. [34] | 2021 | Retrospective, single center | Auto/allo-HSCT | 443 | 227 | 216 | Levofloxacin | High qualitya |
| Hafez et al. [35] | Retrospective, single center | Auto-HSCT | 96 | 50 | 46 | Levofloxacin | Intermediate qualitya | |
| Laoprasopwattana et al. [40] | 2013 | Randomized, open-label, single center | ALL | 95 | 45 | 50 | Ciprofloxacin | Fair qualityb |
| Lopes et al. [36] | 2014 | Retrospective, single center | Auto/allo-HSCT | 378 | 233 | 145 | Levofloxacin | Low qualitya |
| Wolf et al. [39] | 2017 | Prospective, observational, single center | ALL | 253 | 80 | 173 | Levofloxacin (69), ciprofloxacin (11) | High qualitya |
| Yeh et al. [37] | 2021 | Retrospective, single center | AML | 90 | 28 | 62 | Ciprofloxacin | High qualitya |
| Yeh et al. [43] | 2014 | Retrospective, single center | ALL, AML | 151 | 65 | 86 | Ciprofloxacin | Intermediate qualitya |
Quality assessment was carried out as specified in the Methods.
Abbreviations: AL, acute leukemia; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; auto, autologous; allo, allogeneic; HSCT, hematopoietic stem cell transplantation; PPX, prophylaxis; rALL, relapsed ALL.
Quality assessed using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement for prospective cohorts.
Quality assessed using the Cochrane Tool for Quality Assessment for randomized controlled trials.
Bloodstream Infections
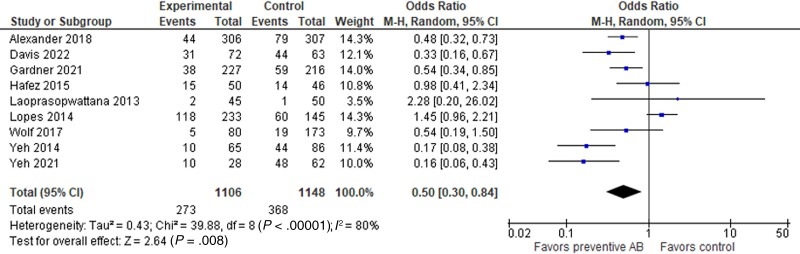
Nine studies were included in the meta-analysis for the impact of quinolone PPX on BSI [33–37, 39–41, 43]. Among these, 6 studies were conducted in patients with AL [33, 37, 39–41, 43] and 4 in the HSCT setting [34–36, 41]. One study included both AL and HSCT patients but considered separately the incidence of BSI in patients with AL and those undergoing HSCT [34]. Analyzing the data together, the incidence of BSI was significantly lower in the PPX groups compared with non-PPX (273 of 1106 vs 368 of 1148), with an odds ratio (OR) of 0.50 (95% CI, 0.30–0.84; P = .008). Heterogeneity among the studies was 88% (Figure 2).
Figure 2.
Forest plot showing the association between the use of quinolone PPX and the incidence of BSI in pediatric patients with AL or receiving HSCT. Abbreviations: AB, antibiotics; AL, acute leukemia; BSI, bloodstream infection; HSCT, hematopoietic stem cell transplantation; PPX, prophylaxis.
Dissecting the analysis between patients with AL and those receiving HSCT, the results were different. In the AL group, the incidence of BSI was still significantly lower in PPX compared with non-PPX (79 of 386 vs 199 of 533), with an OR of 0.31 (95% CI, 0.22–0.43; P < .001). Heterogeneity was lower in this subanalysis (35%) (Figure 3).
Figure 3.
Forest plot showing the association between the use of quinolone PPX and the incidence of BSI in pediatric patients with AL. Abbreviations: AB, antibiotics; AL, acute leukemia; BSI, bloodstream infection; PPX, prophylaxis.
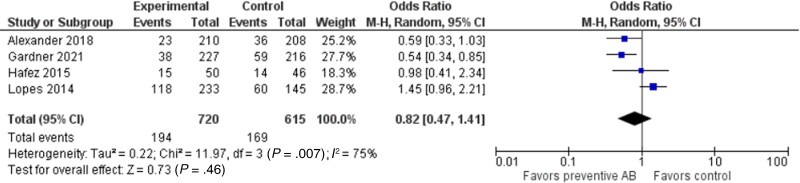
Analyzing the studies reporting outcomes for patients receiving HSCT, the incidence of BSI was comparable between the 2 groups (194 of 720 vs 169 of 615), with an OR of 0.82 (95% CI, 0.47–1.41; P = .46). Heterogeneity among these studies was higher, reaching 75% (Figure 4).
Figure 4.
Forest plot showing the association between the use of quinolone PPX and the incidence of BSI in pediatric patients with HSCT. Abbreviations: AB, antibiotics; BSI, bloodstream infection; HSCT, hematopoietic stem cell transplantation; PPX, prophylaxis.
We then performed a sensitivity analysis for the quality of studies and type of studies, and the data were comparable (Supplementary Figures 1, 2, and 3). A sensitivity analysis for countries' incomes was performed as well, showing no effect on the incidence of BSI (Supplementary Figure 4).
Six papers on children with AL did not meet the inclusion criteria and were not included in the quantitative synthesis. McCormick et al. retrospectively compared the incidence of BSI for each hospital admission in which no PPX or quinolone PPX was used. They reported a BSI incidence of 26.2% and 8.9% in the non-PPX and PPX groups, respectively [26]. Felsenstein et al. compared the incidence of BSI in 153 chemotherapy courses for AML in pediatric patients receiving or not receiving CPFX PPX. They found no statistically significant difference in the 2 groups (odds ratio, 1.1; 95% CI, 0.6–2.1; P = .80) [27]. In a paper by Yousef et al., the incidence of culture-positive bacteremia per delayed intensification (DI) chemotherapy cycle in children with ALL was retrospectively analyzed [28]. The authors found a reduction in the rate of positive blood cultures from 22% in the control population to 9% in the study group in which PPX with CPFX was administered (P = .028). Widjajanto et al. analyze the role of CPFX PPX in the frequency of bacterial infection and toxic death during induction treatment of childhood ALL in a middle/low-income country; this study was excluded from the meta-analysis because it did not consider the microbiological diagnosis. The authors found that the CPFX arm had a modestly greater risk for clinical sepsis compared with the placebo group (50.0% vs 38.5%; P = .22) [31]. Interestingly, fluoroquinolone PPX seems to reduce infections due to gram-negative bacteria having a small influence on gram-positive BSI both in AL [27, 28, 37, 41] and HSCT settings [35, 36, 41].
Neutropenic Fever
Four of the 9 studies included in the meta-analysis reported the incidence of neutropenic fever (NF) among patients receiving fluoroquinolone PPX or placebo [39–41, 43]. All studies included patients with AL. Alexander et al. reported the overall number of patients with at least 1 episode of neutropenic fever, without considering separately patients with AL and those undergoing HSCT [41]. Overall, the incidence of NF was significantly lower in the PPX group than in the control group (191 of 300 vs 351 of 459), with an OR of 0.44 (95% CI, 0.31–0.62; P < .001). Heterogeneity was 37% (Figure 5).
Figure 5.
Forest plot showing the association between the use of quinolone PPX and the incidence of NF in pediatric patients with AL and HSCT. Abbreviations: AB, antibiotics; AL, acute leukemia; HSCT, hematopoietic stem cell transplantation; NF, neutropenic fever; PPX, prophylaxis.
Removing the study by Alexander et al. and considering only papers including patients with AL exclusively, the incidence of NF was still significantly lower in the PPX group than in the control group (36 of 83 vs 144 of 207), with an OR of 0.31 (95% CI, 0.16–0.59; P < .001). Heterogeneity was 22% (Figure 6). We then performed a sensitivity analysis by type of study, and the data were comparable (Supplementary Figure 5).
Figure 6.
Forest plot showing the association between the use of quinolone PPX and the incidence of NF in pediatric patients with AL. Abbreviations: AB, antibiotics; AL, acute leukemia; NF, neutropenic fever; PPX, prophylaxis.
The paper by Yeh et al. reported the incidence of NF in AML patients but was excluded because it reported an outcome related to the number of chemotherapy courses rather than the number of patients [37]. The frequencies of NF were reduced significantly during the PPX period, namely, in induction from 99% to 78%, in high-dose chemotherapy from 94% to 64%, and in modest-dose chemotherapy from 58% to 27% (all P < .001). However, a lower cumulative incidence of NF using Kaplan–Meier analysis was observed only in induction (P = .037) or modest-dose chemotherapy (P < .001) during the PPX period. Widjajanto et al. [31] found that patients who received CPFX PPX had more fever compared with those who did not receive PPX (50.0% vs 32.7%), even if significance was not achieved [31].
Overall Survival
Clinical outcomes were described in 8 studies reporting variable parameters and results [27, 28, 33–35, 37, 41, 43]; outcome results are summarized in Table 3. Data regarding mortality were reported too heterogeneously to allow for a meta-analysis. In the acute leukemia setting, overall mortality was not associated with CPFX PPX in the univariate analysis of the study by Felsenstein et al. [27], whereas it was reduced after the introduction of quinolone PPX in 2 other studies [33, 42]. When infection-related mortality was specifically analyzed, no difference was reported with FLQ PPX in 2 studies [28, 33], while it was significantly reduced in the 2 subsequent studies by Yeh et al. [37, 43]. Notably, in these last 2 papers, antifungal PPX was associated with quinolone PPX, possibly biasing these results. Regarding HSCT, no difference in survival outcomes was reported by studies investigating survival and mortality [34, 35, 41]. Gardner et al. reported a significantly higher rate of acute graft-vs-host disease in the PPX group [34].
Table 3.
Summary of Outcome Results of Studies Included in the Systematic Review
| Study | Patients | FLQ Prophylaxis | Outcome Results |
|---|---|---|---|
| Felsenstein et al. [27] | AML | Ciprofloxacin | No association between all-cause mortality and PPX, expressed as number of chemotherapy cycles treated with PPX (OR, 0.99; P = .96) and total days of PPX exposure (OR, 1.1; P = .85). |
| Yousef et al. [28] | ALL | Ciprofloxacin | No infection-related deaths in either the controls or the PPX patients. PPX group experienced a greater induction failure rate (31.0% vs 25.0%; 95% CI, 0.58–3.12; P = .48) and higher toxic death rate (18.9% vs 5.8%; 95% CI, 0.92–13.80; P = .05). |
| Davis et al. [33] | ALL, AML | Levofloxacin | Death during PPX was significantly reduced (RR, 0.58; 95% CI, 0.36–0.95; P = .04) but not bacterial infection–associated death (RR, 0.38; 95% CI, 0.05–2.79; P = .63). |
| Yeh et al. [42] | ALL, AML | Ciprofloxacin + voriconazole or micafungin | In AML patients, overall mortality rate in the pre-PPX and PPX periods was 25% and 7%, EFS rate was 50 and 55%, and OS rate was 60% and 68%, respectively, a median of 7 months after the completion of intensive chemotherapy. Infection-related deaths during PPX were significantly reduced (7/24 vs 0/14; P = .03). In ALL patients, overall mortality rate in the pre-PPX and PPX periods was 6.5% and 2%, EFS rate was 78% and 87%, and OS rate was 86% and 98%, respectively, a median of 21 months after the completion of intensive chemotherapy. Infection-related deaths during PPX were not significantly reduced (1/62 vs 0/51; P = .55). |
| Yeh et al. [37] | AML | Ciprofloxacin + voriconazole or micafungin | Infection-related deaths decreased from 21% (13/62 patients) during the pre-PPX period to 4% (1/28 patients) in the PPX period. 5-year OS rate increased from 54.8% (42.5% to 67.1%) to 78.6% (63.3% to 93.9%), and 5-year EFS rate increased from 51.6% (39.3% to 63.9%) to 70.6% (53.4% to 87.8%) with the introduction of PPX. |
| Alexander et al. [41] | rALL, AML auto-HSCT, allo-HSCT |
Levofloxacin | No infection-related deaths. |
| Gardner et al. [34] | Auto/allo-HSCT | Levofloxacin | Higher rate of graft-vs-host disease by day 100 in the PPX group (11.7% vs 4.2%, P = .01). No difference in mortality in the first 100 days (4% vs 8%; P = .16) and in the first 12 months post-transplant (18.1% vs 23.6%; P = .16) in PPX group and non-PPX group. No difference in nonrelapse mortality in the first 12 months post-transplant (11.5% vs 14%; P = .48) in PPX group and non-PPX group. |
| Hafez et al. [35] | Auto-HSCT | Levofloxacin | No difference in infection-related mortality between PPX group and control group (0/50 vs 2/46; P = .227). |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; auto, autologous; allo, allogeneic; FLQ, fluoroquinolone; HSCT, hematopoietic stem cell transplantation; OR, odds ratio; PPX, prophylaxis; rALL, relapsed ALL; RR, relative risk.
Antibiotic Exposure
The impact of fluoroquinolone PPX on antimicrobial exposure, namely, the number of days on which a specific antimicrobial was administered, was analyzed by 7 studies, including both leukemia and HSCT settings [27, 34–37, 39, 41]; the results are summarized in Table 4. Among children with AL, 2 studies reported a significantly greater exposure to antimicrobials used for PPX with a concomitant significantly lower exposure to antibiotics and/or antifungal agents administered for the empirical therapy of infections in the fluoroquinolone group compared with the control group [37, 39]. Similarly, in the study by Felsenstein et al., PPX with CPFX significantly decreased the duration of antibiotic treatment both overall and specifically of aminoglycoside therapy, primarily because of fewer gram-negative infections in the PPX group [27]. In the transplantation setting, LVX PPX significantly reduced the duration of empiric antibiotic administration [34, 35]. Lopes et al. described a marked increase in the use of LVX during the PPX period; conversely, the use of systemic treatment antibiotics was similar before and after the introduction of LVX PPX [36]. Finally, in the research by Alexander et al., in children with AL and undergoing HSCT, total duration of exposure and any exposure to aminoglycosides, third- and fourth-generation cephalosporins, and antibiotics commonly used for empirical therapy for fever and neutropenia were lower in the LVX group compared with the no PPX group [41].
Table 4.
Summary of Antibiotic Exposure Results of Studies Included in the Systematic Review
| Study | Patients | FLQ Prophylaxis | Antibiotic Exposure |
|---|---|---|---|
| Yeh et al. [36] | AML | Ciprofloxacin + voriconazole or micafungin | Patients receiving PPX had greater exposure to ciprofloxacin, vancomycin, and voriconazole and lower exposure to carbapenem, amikacin, amphotericin B, and caspofungin compared with those receiving no prophylaxis (all P < .001). |
| Wolf et al. [38] | ALL | Levofloxacin, ciprofloxacin | Antibiotic exposure and cumulative antibiotic exposure were greater in patients receiving any PPX (P < .001). Patients receiving levofloxacin PPX had less exposure to cefepime/ceftazidime, vancomycin, meropenem, or aminoglycosides when compared with those receiving no PPX (all P < .01) or other PPX (all P < .05). |
| Felsenstein et al. [27] | AML | Ciprofloxacin | Longer exposure to treatment antibiotics overall in the control group (PPX: median [IQR], 15 [5–21] days; no PPX: median [IQR], 19 [12–30.5] days; P < .01). Ciprofloxacin PPX did not impact duration of meropenem use per CC (PPX: median [IQR], 10 [4.2–19] days; no PPX: median [IQR], 11 [3.5–22.5] days; P = .62) or duration of vancomycin use per CC (PPX: median [IQR], 2 [2–9] days; no PPX: median [IQR], 4 [4–8] days; P = .43). However, it decreased duration of aminoglycoside use per CC (PPX: median [IQR], 0 [0–0] days; no PPX: median [IQR], 2 [0–4] days; P < .01). |
| Hafez et al. [35] | Auto-HSCT | Levofloxacin | The median duration of empiric antibiotic use in the PPX group was 11 days compared with 14 days in the control group (P < .001). The frequency of empirical antifungal use was higher in the control group compared with the PPX group (98% vs 46%; P < .001). |
| Gardner et al. [34] | Auto/allo-HSCT | Levofloxacin | Higher average number of antibiotic days (mean, 47 vs 35 days; P < .0019) and greater meropenem (mean, 4.2 vs 2.5 days; P = .02), metronidazole (mean, 1.4 vs 0.25 days; P < .001), and cefepime use (mean, 19.6 vs 14.9 days; P < .001) in the control group than in the PPX group. |
| Lopes et al. [36] | Auto/allo-HSCT | Levofloxacin | Increase in the use of levofloxacin in the PPX period from 19.4 to 166.6 DDD per 1000 patient-days. Slight increase in meropenem use from 4.59 to 5.33 DDD per 1000 patient-days and decrease in cefepime use from 3.75 to 3.32 DDD per 1000 patient-days in the PPX period. |
| Alexander et al. [41] | rALL, AML auto-HSCT, allo-HSCT |
Levofloxacin | The mean antibiotic exposure days per 30 patient-days was 1.2 vs 2.3 (adjusted RR, 0.49; 95% CI, 0.33–0.73; P = .001) for aminoglycosides, 5.3 vs 7.1 (adjusted RR, 0.74; 95% CI, 0.60–0.92; P = .006) for third- and fourth-generation cephalosporins, 9.6 vs 13.1 (adjusted RR, 0.72; 95% CI, 0.63–0.83; P < .001) for antibiotics commonly used empirically for fever and neutropenia (defined as imipenem, meropenem, cefepime, ceftazidime, or piperacillin-tazobactam), and 5.3 vs 6.1 (adjusted RR, 0.87; 95% CI, 0.7–1.06; P = .17) for gram-positive agents (defined as vancomycin, linezolid, daptomycin, or quinupristin/dalfopristin) in LVX group and no PPX group, respectively. |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; auto, autologous; allo, allogeneic; CC, chemotherapy cycle; DDD, defined daily dose; FLQ, fluoroquinolone; HSCT, hematopoietic stem cell transplantation; IQR, interquartile range; LVX, levofloxacin; PPX, prophylaxis; rALL, relapsed ALL; RR, relative risk.
Antibiotic Resistance
Eight of the included studies reported data on the impact of fluoroquinolone PPX on the development of antibiotic resistance in colonizing microorganisms and/or in bacteria isolated from blood [29, 32, 33, 36, 37, 40, 41, 43]; the results are summarized in Table 5. Among patients with AL receiving fluoroquinolone PPX, 3 studies found an increased incidence of gram-negative bacteria resistant to fluoroquinolone in intestinal microflora [29, 40] and isolated from blood [33]. Similarly, in the study by Margolis et al., the prevalence of topoisomerase point mutations, known to confer fluoroquinolone resistance, increased during induction chemotherapy for ALL in participants receiving LVX but not those receiving no PPX [32]. Conversely, in the 2 works of Yeh et al., CPFX [43] and amikacin [37] resistance to the most common gram-negative bacilli at the study institution was significantly reduced during the PPX period, with a concomitant rising of cefuroxime and imipenem resistance [37]. Among patients undergoing HSCT, a significant increase in quinolone resistance throughout LVX PPX compared with the pre-PPX period was demonstrated in 1 study [36]. Finally, Alexander et al. found that the proportion of selected intestinal organisms with newly detected resistance to LVX, cefepime, and imipenem from baseline to follow-up was low, reaching a maximum of 9.3% for LVX resistance among patients with AL receiving PPX, and not significantly different between the LVX PPX and control groups for both patients with AL and those undergoing HSCT [41].
Table 5.
Summary of Antibiotic Resistance Results of Studies Included in the Systematic Review
| Study | Patients | FLQ Prophylaxis | Sample | Antibiotic Resistance |
|---|---|---|---|---|
| Tunyapanit et al. [29] | ALL and lymphoma | Ciprofloxacin | Rectal swab cultures | The percentage of ciprofloxacin susceptibility of E. coli and K. pneumoniae before intervention and at the third week of the study decreased in PPX group (83.9% vs 4.5%) and improved in the placebo group (70.6% vs 100%). After the study, the MIC50s of ciprofloxacin were significantly higher in the PPX group than in the placebo group. Although the susceptibility rates to ceftazidime were not different between the PPX and placebo groups after the study, the MIC50s were significantly higher in the PPX group compared to the placebo group; moreover, the MIC50s significantly increased in PPX group (from 0.12 μg/mL before intervention to 0.19–0.38 μg/mL after 1–3 weeks), but significantly decreased in placebo group (from 0.12 μg/mL to 0.12–0.09 μg/mL; all P < .01). |
| Laoprasopwattana et al. [40] | ALL and lymphoma | Ciprofloxacin | Rectal swab cultures | In the first and second weeks after intervention, ciprofloxacin susceptibility was lower in PPX group compared with placebo group, in both E. coli (first week 5.1% vs 75.0%, second week, 2.9% vs 77.3%, all P < .001) and K. pneumoniae (first week 0% vs 65.5%; P = .002). |
| Davis et al. [33] | AML, rALL | Levofloxacin | Blood cultures | Incidence of bacteremia due to gram-negative rods nonsusceptible to levofloxacin increased during the PPX period (RR, 3.38; P < .001). |
| Yeh et al. [42] | ALL, AML | Ciprofloxacin + voriconazole or micafungin | Not specified | During the PPX period, a reduction was observed in the ciprofloxacin resistance of E. coli (from 21% to 19%), K. pneumoniae (from 17% to 10%), P. aeruginosa (from 33% to 28%), and S. marcescens (from 41% to 30%; all P < .01). Ciprofloxacin resistance of A. baumannii, E. cloacae, P. mirabilis, Salmonella spp. did not change. |
| Yeh et al. [37] | AML | Ciprofloxacin + voriconazole or micafungin | Not specified | During the PPX period, cefuroxime susceptibility of E. coli or K. pneumonia decreased (P = .027, P = .01, respectively); imipenem susceptibility of E. cloacae or A. baumannii decreased (P = .009, and P = .002, respectively). Amikacin susceptibility of E. coli, K. pneumoniae, E. cloacae, P. aeruginosa, C. freundii improved during the PPX period (P = .042, P = .001, P = .007, P = .003, P = .001, respectively). Ampicillin/sulbactam, linezolid, teicoplanin, and vancomycin resistance of Enterococcus spp. decreased during the PPX period (P = .001, P = .027, P = .001, P = .001, respectively). Values are presented as the median number of infection episodes from any site of the body per year. |
| Margolis et al. [32] | ALL | Levofloxacin | Fecal samples | Prevalence of topoisomerase point mutations increased from baseline to follow-up (completion of induction and completion of consolidation therapy) in the PPX group (10.4%; 95% CI, 3.2%–25.4%; after induction; vs 3.7%; 95% CI, 0.2–22.5; at baseline) but not in the no-PPX group (0% vs 0%; P < .0001). Acquisition of specific fluoroquinolone resistance genes was too infrequent for any effect of PPX to be detected; the estimated prevalence remained low, reaching a maximum of 10.4% after the completion of induction in participants who received LVX, and increasing to 15.1% after the 8-week consolidation phase of chemotherapy, when the fluoroquinolone pressure had been removed. A significant increase in the relative abundance of aminoglycoside and multidrug resistance genes was seen regardless of PPX. Vancomycin resistance genes and β-lactam resistance genes did not change significantly. |
| Lopes et al. [36] | Auto/allo-HSCT | Levofloxacin | Different samples | An increase in quinolone resistance during the PPX period compared with pre-PPX was observed for all bacteria isolated (46.0% vs 76.5%; P = .0002) and for gram-negatives (21.4% vs 60.7%; P = .0163) and gram-positives (55.6% vs 82.9%; P = .0025) separately. Considering the single species, that is, Enterobacteriaceae, E. coli, P. aeruginosa, and coagulase-negative staphylococci, the increase in resistance was not statistically significant. |
| Alexander et al. [41] | rALL, AML auto-HSCT, allo-HSCT |
Levofloxacin | Fecal samples | In the AL setting, newly detected resistance to levofloxacin among S. mitis, E. coli, K. pneumoniae, and P. aeruginosa in follow-up specimens was 9.3% vs 8.9% in the PPX and placebo groups, respectively. Newly detected resistance to cefepime was 2.3% vs 8.9% and resistance to imipenem was 0% vs 6.7% in the PPX and placebo groups. In the HSCT setting, newly detected resistance to levofloxacin among the same species in follow-up specimens was 1.7% vs 0.8% in the PPX and placebo groups, respectively. Newly detected resistance to cefepime was 2.5% vs 2.5% and resistance to imipenem was 0.9% vs 0% in the PPX and placebo groups. The overall proportion of newly detected resistance to any of the selected pathogens was low and not significantly different between the levofloxacin prophylaxis and control groups for patients with acute leukemia (5 of 43 vs 7 of 45; P = .59) or patients undergoing HSCT (4 of 118 vs 4 of 120; P = .98). |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; auto, autologous; allo, allogeneic; FLQ, fluoroquinolone; HSCT, hematopoietic stem cell transplantation; MIC, minimal inhibitory concentration; PPX, prophylaxis; rALL, relapsed ALL; RR, relative risk.
Antibiotic-Related Adverse Effects
Quinolones have been commonly associated with several side effects, mainly consisting of gastrointestinal symptoms, such as dyspepsia, nausea and vomiting, and central nervous system reactions such as dizziness, insomnia, headache, and musculoskeletal adverse events [44, 45]. Details of the main side effects associated with quinolone prophylaxis are listed in Table 6. The study of Karol et al. specifically investigated the association between fluoroquinolone during induction therapy for ALL and the development of neuropathic pain and vincristine-induced neuropathy, reporting no significant association between antibiotic exposure and neurotoxicity [30]. Rare skin allergic reactions associated with quinolones were reported in 2 studies [27, 40]. Three studies evaluated musculoskeletal side effects in the 2 cohorts and did not find any differences [26, 27, 40]. Gardner et al. investigated the impact of PPX with LVX on cardiac function by evaluating baseline and follow-up electrocardiogram (ECG) and reporting no difference in terms of QTc prolongation between the 2 groups (21/93 vs 16/106; P = .20) [34]. In the 2 studies evaluating the impact of combined antibacterial and antifungal PPX, increased liver enzyme levels were reported in patients receiving PPX, but this side effect was mainly related to micafungin and voriconazole administration [37, 43].
Table 6.
Summary of Side Effects Associated With Quinolone Prophylaxis
| Study | Patients | FLQ Prophylaxis | Side Effects |
|---|---|---|---|
| Karol et al. [30] | ALL | Ciprofloxacin, levofloxacin | No significant correlation between fluoroquinolone exposure during the induction phase for ALL and vincristine-induced peripheral neurotoxicity (neuropathic pain, neuropathy, combined pain/neuropathy; hazard ratio, 0.8; 95% CI, 0.5–1.04; P = 0.08) and high-grade neuropathy (hazard ratio, 1.1; 95% CI, 0.4–2.2; P = 0.87). The lack of association was maintained adjusting for race and age and after restriction to early onset symptoms. Considering specific drug, no significant increase in neuropathy or neuropathic pain was shown when comparing levofloxacin with ciprofloxacin or no fluoroquinolone. |
| Laoprasopwattana et al. [40] | ALL and lymphoma | Ciprofloxacin | Similar numbers of patients in the ciprofloxacin (45) and placebo (50) groups developed minor side effects, including skin rash (2 vs 0), nausea/vomiting (12 vs 11), diarrhea (0 vs 1), abdominal pain (2 vs 5), and arthralgia/arthritis (1 vs 1; P = .05). Only 1 skin rash in a patient presented a definite association with the drug (subsequently discontinued), whereas all other adverse events were associated with chemotherapy or the underlying disease. |
| Felsenstein et al. [27] | AML | Ciprofloxacin | 1/64 patients receiving prophylaxis developed an allergic skin rash attributed to ciprofloxacin with discontinuation. No musculoskeletal side effects in any patient who received prophylaxis. |
| Yousef et al. [28] | ALL | Ciprofloxacin | No musculoskeletal side effects were noted in the placebo group or PPX group. |
| Alexander et al. [1] | rALL, AML auto-HSCT, allo-HSCT |
Levofloxacin | No significant differences in musculoskeletal side effects at 2 months (11.4% vs 16.3%; risk difference, 4.8%; 95% CI, −1.6% to 11.2%; P = .15) or 12 months (10.1% vs 14.4%; risk difference, 4.3%; 95% CI, −3.4% to 12.0%; P = .28) between the levofloxacin and control groups. |
| Gardner et al. [34] | Auto/allo-HSCT | Levofloxacin | No difference in terms of cardiac function evaluated by QTc prolongation at ECG. 17/216 patients (7.9%) in the no prophylaxis group had a prolonged QTc interval at baseline, compared with 15/227 patients (6.6%) in the prophylaxis group (P = .46). At follow-up ECG, a prolonged QTc interval was found in 21 patients in the no prophylaxis group and 16 in the prophylaxis group (P = .20). |
| Yeh et al. [58] | ALL, AML | Ciprofloxacin + voriconazole or micafungin | Hepatotoxiciy with elevated transaminase levels (related to micafungin and voriconazole) |
| Yeh et al. [37] | AML | Ciprofloxacin + voriconazole or micafungin | Hepatotoxiciy with elevated transaminase levels (related to micafungin and voriconazole) |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; auto, autologous; allo, allogeneic; ECG, electrocardiogram; FLQ, fluoroquinolone; HSCT, hematopoietic stem cell transplantation; IFI, invasive fungal infection; PPX, prophylaxis; rALL, relapsed ALL.
Fungal Infections
The detrimental role of quinolones on intestinal flora has been reported to be associated with fungal overgrowth and ultimately with fungal infections [46]. Six studies investigated the modification of fungal infection rate in patients receiving and not receiving PPX with fluoroquinolones [27, 28, 34, 37, 41, 43]; details are reported in Table 7. In the study of Felsenstein et al., after the introduction of CPFX PPX, fungemia occurred significantly more frequently (P = .01) [27]. An opposite trend was described in the study by Yousef et al., in which all 3 fungal septicemias occurred in the control group, while no episode was reported in the PPX group [28]. Of note, a significant decrease in invasive fungal infection rate was also reported in the 2 studies by Yeh et al., but it has to be considered that the intervention adopted in the study included a combined antibacterial and antifungal PPX with CPFX and micafungin or voriconazole [37, 43]. In patients with AL and undergoing HSCT randomized to receive LVX or no PPX, there were no significant differences in invasive fungal disease (2.9% vs 2.0%; risk difference, −1.0%; 95% CI, −3.4% to 1.5%; P = .41) [41]. In the transplanted patients in the study of Gardner et al., there was no significant difference between the PPX and control groups regarding fungal infection rate (1/227 [0.4%] vs 4/216 [2%]; P = .21) [34].
Table 7.
Summary of Fungal Infection Results of Studies Included in the Systematic Review
| Study | Patients | FLQ Prophylaxis | Fungal Infections |
|---|---|---|---|
| Felsenstein et al. [27] | AML | Ciprofloxacin | Fungemia occurred more frequently in the PPX group (5 vs 0 episodes in the PPX and no PPX groups; P = .01). Fungi isolated from blood were A. versicolor (1), C. krusei, (2), C. lipolytica (1), C. parapsilosis (1). No difference in all proven, probable, and possible IFIs considered combined between the PPX and no PPX groups. |
| Yousef et al. [28] | ALL | Ciprofloxacin | Fungemia occurred only in the no PPX group (0 vs 3 episodes in the PPX and no PPX groups). All isolates were Candida spp. |
| Yeh et al. [58] | ALL, AML | Ciprofloxacin + voriconazole or micafungin | All episodes of IFI (fungi isolated body fluid culture or histology of infected tissue) occurred in the no PPX period (22 vs 0 episodes in the PPX and no PPX groups). Candida species were the leading pathogens (15/22 episodes, 68%), followed by Aspergillus species (6/22 episodes, 27%). 12 episodes occurred in patients with AML, due to C. glabrata (2), C. albicans (1), C. tropicalis (1), Aspergillus spp. (5), Rhodotorula spp. (1), other (2). 10 episodes occurred in patients with ALL, due to C. albicans (2), C. tropicalis (4), C. parapsilosis (1), Aspergillus spp. (1), other (2). |
| Yeh et al. [37] | AML | Ciprofloxacin + voriconazole or micafungin | All episodes of IFI occurred in the no PPX period (17 vs 0 episodes in the PPX and no PPX groups; P = .003), due to Aspergillus spp. (9*), Candida spp. (6*), other (2). *Two microorganisms were isolated concomitantly |
| Alexander et al. [41] | rALL, AML auto-HSCT, allo-HSCT |
Levofloxacin | No differences in invasive fungal disease (9/306 [2.9%] vs 6/307 [2.0%] patients in the PPX and no PPX groups; risk difference, −1.0%; 95% CI, −3.4% to 1.5%; P = .41). |
| Gardner et al. [34] | Auto/allo-HSCT | Levofloxacin | No difference in fungal infection rate (1/227 [0.4%] vs 4/216 [2%] patients in the PPX and no PPX groups; risk difference; P = .21) |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; auto, autologous; allo, allogeneic; FLQ, fluoroquinolone; HSCT, hematopoietic stem cell transplantation; IFI, invasive fungal infection; PPX, prophylaxis; rALL, relapsed ALL.
Clostridium difficile Infections
The impact of antibiotic PPX on Clostridium difficile infection rate was evaluated in 3 studies [33, 39, 41]. In the post hoc analyses of the RCT by Alexander et al., patients receiving levofloxacin were less likely to have a positive test result for Clostridium difficile (7.8% vs 14.0%; P = .02) [41]. One study showed a significant reduction in cumulative incidence of Clostridium difficile infection in patients receiving PPX with LVX compared with those receiving no PPX during induction therapy for newly diagnosed pediatric ALL (from 9.8% to 0%; adjusted odds ratio, 0.03; 95% CI, 0.01–0.24; P < .001 on multivariate logistic regression analysis) [39]. Another paper in the leukemia setting performed only a descriptive analysis regarding Clostridium difficile infections, showing no difference between the 2 groups (19.0% vs 19.4%) [33].
Length of Hospitalization
The impact of antibiotic PPX on the duration of hospitalization was assessed in only 2 studies concerning the HSCT setting [28, 35]. The duration of hospitalization was significantly shorter in patients receiving PPX, from 28 to 24 days in 1 study (P < .01) [35] and from 10 to 6 days in a second study (P = .001) [28].
Health Care Costs
Two studies on AL reported a decrease in health care costs with the administration of PPX [26, 43]. A multicenter retrospective study evaluated epidemiologic data regarding LVX use as prophylaxis in children with AML and specifically evaluated the cost-effectiveness of this strategy by using a decision analysis model. Cost-effectiveness was defined as cost per bacteremia episode, intensive care unit (ICU) admission, and avoidance of death in children undergoing LVX PPX compared with no PPX. PPX decreased the absolute risk of bacteremia by 17%, with a cost of $1464 compared with no PPX, thus resulting in a PPX cost of $8491 per bacteremia episode prevented. This approach was determined to be cost-effective, considering that an episode of bacteremia added an average hospital cost of $119.478. The percentage of ICU admission and mortality reduction due to PPX was lower, resulting in lower cost-effectiveness of PPX regarding these outcomes. PPX also decreased ICU admission risk by 2.1% with a cost of $81 609 per ICU admission avoided compared with an average added hospital cost of $94 181 per ICU admission. Finally, PPX reduced mortality attributed to bacteremia risk by 0.7%, costing $220 457 per death avoided. A probabilistic and sensitivity analysis was also performed to evaluate model uncertainty, and PPX remained cost-effective in 94.6% of simulations [26]. Another study on AL evaluated the total costs of antibiotics and antifungal agents in patients receiving and not receiving PPX, comparing the preprophylaxis and prophylaxis periods. A significant reduction of antimicrobial costs during the PPX period was observed in both ALL and AML patients [43].
DISCUSSION
Antibiotic PPX represents an important approach to reducing bacterial infections and their consequences. Among these, quinolones have been widely used as prophylactic agents in pediatric patients, mainly due to the observed efficacy in adults [47]. Other prophylactic regimens have been proposed in the literature; however, quinolones have been more commonly used in current practice [9, 48]. Notably, the use of antibiotic PPX may be associated with several drawbacks, such as toxicity or emergence of antibiotic-resistant bacterial strains, and thus a balance between risks and benefits should be considered. However, this fine-tuning is difficult as data in the pediatric setting are scarce and heterogenous, and recommendation, therefore, relies on a low level of evidence [11].
To our knowledge, this is the first meta-analysis on the effectiveness of quinolone PPX in pediatric patients with AL or undergoing HSCT. Our data suggest that quinolone PPX is effective at reducing the number of BSIs in pediatric leukemia patients but does not seem to be as effective in the HSCT setting. Moreover, PPX seems to reduce the incidence of NF in the AL setting. However, these results should be interpreted specifically in the AL setting. AL studies are heterogeneous, comprising different pathologies—that is, ALL and AML—within which exist different classes of risk and corresponding chemotherapy protocols, as well as different phases of chemotherapy. Moreover, the newly diagnosed acute leukemia setting is different from the relapse or refractory setting. Most of the included studies analyzed the use of FLQ PPX during a period of intensive chemotherapy, expected to lead to prolonged neutropenia (Table 1). Due to the small number and heterogeneity between studies, it was not possible to carry out subgroup analysis in the AL setting. Unfortunately, the data regarding mortality are heterogeneous, and we could not include them in the quantitative synthesis. Thus, it is not possible to draw meaningful conclusions regarding the impact of quinolone PPX on mortality. This should be a major focus of future research to understand if the observed reduced incidence of infectious complications translates into improved survival for the patients.
The occurrence of side effects is a frequent concern in the pediatric population, also considering that antibacterial agents used for treatment and prophylaxis are often not licensed in children. Interestingly, the safety profile of LVX was confirmed in all the reported studies, showing no differences in drug-related adverse events in patients undergoing or not undergoing prophylaxis.
A reduction in the length of hospitalization and health care costs was reported in patients undergoing PPX [26, 28, 35, 43]. These results need to be confirmed in larger cohorts but are certainly of interest. First, prophylaxis can prevent febrile episodes, potentially leading to a reduced length of hospital stay, with a relevant positive effect on the quality of life of patients and caregivers. Moreover, shorter hospitalization can contribute to the potential cost-effectiveness of the prophylaxis approach.
Although studies reported variable results, an increase in fluoroquinolone resistance was generally reported in patients undergoing PPX [41]. Interestingly, this increase seems to be related to the acquisition of topoisomerase mutations known to confer resistance to fluoroquinolones [32]. Furthermore, the emergence of new antibiotic resistance in bacterial microflora was noted in some studies, reporting an increase in ceftazidime and cefuroxime resistance [29, 37]. This effect is particularly relevant for patients, potentially affecting the efficacy of fourth-grade cephalosporins as first-line antibiotic therapy in acute leukemia and HSCT settings [11]. The emergence of multidrug-resistant gram-negative bacteria has also been reported in adults undergoing quinolone prophylaxis, representing a major threat in neutropenic patients [8]. However, it must be considered that data on antibiotic susceptibility are highly dependent on local epidemiology, which may represent a bias in interpreting the results. Surveillance of bacterial resistance and colonization is mandatory to guide the appropriate clinical management of these patients.
Regarding Clostridium difficile infections or antibiotic exposure, some inconsistencies between the studies are reported, suggesting a nonsignificant role for quinolone PPX.
Gut microbiota modifications were studied by Margolis et al., focusing on resistome modifications after PPX and providing evidence that LVX can increase the risk of colonization with resistant bacteria. Nonetheless, considering that new important evidence reported a detrimental role of antibiotic-mediated dysbiosis [49–51], this should be addressed in future studies, with a particular focus on understanding the modifications associated with adverse effects such as antibiotic-associated diarrhea, risk of BSI with resistant bacteria, Clostridium difficile infections, and NF [52].
Finally, the occurrence of breakthrough infections has not been reported in the included studies, and specific analyses have not been systematically performed [39]. An increase in breakthrough infections with resistant organisms in patients receiving prophylaxis could represent a relevant concern for clinicians considering the poor outcomes of MDR infections [53, 54]. The possible emergence of potentially severe breakthrough infections in this category of patients certainly needs to be considered in future studies.
This meta-analysis presents several limitations. Patients receiving allo-HSCT were few and often mixed with autologous transplantation, and the AL population included in this study is heterogeneous. Infectious risk in these different categories is significantly different, depending on various factors. The incidence of bacterial infections is higher in patients with AML than ALL and is also higher in the induction therapy phase than in the consolidation phase [38, 39]. Moreover, this risk is significantly higher in relapsed patients [9]. Among the meta-analyzed studies on AL patients, in all cases PPX was prescribed during a period of intensive chemotherapy, differently but quite consistently defined, namely, during induction for ALL [39, 40] or during regimens that are predicted to cause prolonged neutropenia [32, 33, 41, 42]. Moreover, 4 of 6 studies included patients with AML [33, 37, 41, 42] and 2 of 6 included patients with rALL [33, 41]. Future studies should focus on the benefit of PPX in these specific subpopulations to better define its clinical impact. Local epidemiology and resistance patterns change year by year. The results of LVX PPX in adult patients seem to change based on the time span considered [7, 8]. From the results of this meta-analysis, including patients from January 2005 [42] to February 2021 [33], temporal changes cannot be clearly seen because of the small number and different designs of the analyzed studies. Future evaluations could show temporal changes in PPX effectiveness and downsides. The effect of prophylaxis seems to be different in lower-income countries than in higher-income countries. Among the included studies, 3 [35, 36, 40] were performed in low- and middle-income countries according to the most recent World Bank and Organisation for Economic Co-operation and Development classification [55]. We performed a sensitivity analysis on this topic, and we found no effect of prophylaxis in reducing the incidence of BSI. Nevertheless, we observed that none of these papers reached statistical significance in the end points that we considered for the quantitative synthesis and that none of them was rated as high or good quality. It is therefore certainly an issue to be considered in future studies to generalize the present results for countries with lower income.
CONCLUSIONS
To our knowledge, this is the first meta-analysis on the effectiveness of quinolone PPX in pediatric patients with leukemia or undergoing HSCT. Our results seem to confirm the positive effect of quinolone PPX on reduction of the risk of infections during chemotherapy courses for ALs. No significant effect was reported in HSCT setting. The main limitation of our study is the impossibility of defining the effect of PPX on the different risk classes of AL and the phase of treatment. Further larger randomized studies will help better define its exact effectiveness and indications. Moreover, future studies on the impact of antibiotic PPX on the gut microbiota are highly awaited [56, 57].
Supplementary Material
Acknowledgments
Financial support. The work reported in this publication was funded by the Italian Ministry of Health, RC-2022-2774259.
Patient consent. This work does not include factors necessitating patient consent due to design of the study.
Ethical approval. The study was conducted in accordance with the Declaration of Helsinki. Ethical review and approval were waived for this study due to the design of the study.
Contributor Information
Davide Leardini, Pediatric Oncology and Hematology Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Edoardo Muratore, Pediatric Oncology and Hematology Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Nicoletta Abram, Pediatric Oncology and Hematology Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Francesco Baccelli, Pediatric Oncology and Hematology Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Tamara Belotti, Pediatric Oncology and Hematology Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Arcangelo Prete, Pediatric Oncology and Hematology Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Davide Gori, Department of Biomedical and Neuromotor Sciences (DIBINEM), University of Bologna, Bologna, Italy.
Riccardo Masetti, Pediatric Oncology and Hematology Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy; Department of Medical and Surgical Sciences (DIMEC), University of Bologna, Bologna, Italy.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Dandoy CE, Kelley T, Gaur AH, et al. Outcomes after bloodstream infection in hospitalized pediatric hematology/oncology and stem cell transplant patients. Pediatr Blood Cancer 2019; 66:e27978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lehrnbecher T, Robinson P, Fisher B, et al. Guideline for the management of fever and neutropenia in children with cancer and hematopoietic stem-cell transplantation recipients: 2017 update. J Clin Oncol 2017; 35:2082–94. [DOI] [PubMed] [Google Scholar]
- 3. Ibrahim KY, Pierrotti LC, Freire MP, et al. Health care-associated infections in hematology-oncology patients with neutropenia: a method of surveillance. Am J Infect Control 2013; 41:1131–3. [DOI] [PubMed] [Google Scholar]
- 4. Sung L, Lange BJ, Gerbing RB, Alonzo TA, Feusner J. Microbiologically documented infections and infection-related mortality in children with acute myeloid leukemia. Blood 2007; 110:3532–39. [DOI] [PubMed] [Google Scholar]
- 5. Gafter-Gvili A, Fraser A, Paul M, et al. Antibiotic prophylaxis for bacterial infections in afebrile neutropenic patients following chemotherapy. Cochrane Database Syst Rev 2012; 1:CD004386. [DOI] [PubMed] [Google Scholar]
- 6. Egan G, Robinson PD, Martinez JPD, et al. Efficacy of antibiotic prophylaxis in patients with cancer and hematopoietic stem cell transplantation recipients: a systematic review of randomized trials. Cancer Med 2019; 8:4536–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bucaneve G, Micozzi A, Menichetti F, et al. Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. N Engl J Med 2005; 353:977–87. [DOI] [PubMed] [Google Scholar]
- 8. Mikulska M, Averbuch D, Tissot F, et al. Fluoroquinolone prophylaxis in haematological cancer patients with neutropenia: ECIL critical appraisal of previous guidelines. J Infect 2018; 76:20–37. [DOI] [PubMed] [Google Scholar]
- 9. Carreras E, Dufour C, Mohty M, Kröger N. The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies. Springer International Publishing AG; 2019. [PubMed] [Google Scholar]
- 10. Owattanapanich W, Chayakulkeeree M. Efficacy of levofloxacin as an antibacterial prophylaxis for acute leukemia patients receiving intensive chemotherapy: a systematic review and meta-analysis. Hematology 2019; 24:362–8. [DOI] [PubMed] [Google Scholar]
- 11. Lehrnbecher T, Averbuch D, Castagnola E, et al. 8th European Conference on Infections in Leukaemia: 2020 guidelines for the use of antibiotics in paediatric patients with cancer or post-haematopoietic cell transplantation. Lancet Oncol 2021; 22:e270–80. [DOI] [PubMed] [Google Scholar]
- 12. Calitri C, Ruberto E, Castagnola E. Antibiotic prophylaxis in neutropenic children with acute leukemia: do the presently available data really support this practice? Eur J Haematol 2018; 101:721–7. [DOI] [PubMed] [Google Scholar]
- 13. Romick-Rosendale LE, Haslam DB, Lane A, et al. Antibiotic exposure and reduced short chain fatty acid production after hematopoietic stem cell transplant. Biol Blood Marrow Transplant 2018; 24:2418–24. [DOI] [PubMed] [Google Scholar]
- 14. Masetti R, Muratore E, Leardini D, et al. Gut microbiome in pediatric acute leukemia: from predisposition to cure. Blood Adv 2021; 5:4619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garrido MM, Garrido RQ, Cunha TN, Ehrlich S, Martins IS. Comparison of epidemiological, clinical and microbiological characteristics of bloodstream infection in children with solid tumours and haematological malignancies. Epidemiol Infect 2019; 147:e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Muratore E, Baccelli F, Leardini D, et al. Antimicrobial stewardship interventions in pediatric oncology: a systematic review. J Clin Med 2022; 11:4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ingham AC, Kielsen K, Mordhorst H, et al. Microbiota long-term dynamics and prediction of acute graft-versus-host disease in pediatric allogeneic stem cell transplantation. Microbiome 2021; 9:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. BMJ 2009; 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haeusler GM, Phillips RS, Lehrnbecher T, Thursky KA, Sung L, Ammann RA. Core outcomes and definitions for pediatric fever and neutropenia research: a consensus statement from an international panel. Pediatr Blood Cancer 2015; 62:483–9. [DOI] [PubMed] [Google Scholar]
- 20. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2011; 52:e56–93. [DOI] [PubMed] [Google Scholar]
- 21. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–88. [DOI] [PubMed] [Google Scholar]
- 23. Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's Tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration; 2011. [Google Scholar]
- 25. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014; 12:1495–9. [DOI] [PubMed] [Google Scholar]
- 26. McCormick M, Friehling E, Kalpatthi R, Siripong N, Smith K. Cost-effectiveness of levofloxacin prophylaxis against bacterial infection in pediatric patients with acute myeloid leukemia. Pediatr Blood Cancer 2020; 67:e28469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Felsenstein S, Orgel E, Rushing T, Fu C, Hoffman JA. Clinical and microbiologic outcomes of quinolone prophylaxis in children with acute myeloid leukemia. Pediatr Infect Dis J 2015; 34:e78–84. [DOI] [PubMed] [Google Scholar]
- 28. Yousef AA, Fryer CJH, Chedid FD, Abbas AAH, Felimban SK, Khattab TM. A pilot study of prophylactic ciprofloxacin during delayed intensification in children with acute lymphoblastic leukemia. Pediatr Blood Cancer 2004; 43:637–43. [DOI] [PubMed] [Google Scholar]
- 29. Tunyapanit W, Chelae S, Laoprasopwattana K. Does ciprofloxacin prophylaxis during chemotherapy induce intestinal microflora resistance to ceftazidime in children with cancer? J Infect Chemother 2018; 24:358–62. [DOI] [PubMed] [Google Scholar]
- 30. Karol SE, Sun Y, Tang L, et al. Fluoroquinolone prophylaxis does not increase risk of neuropathy in children with acute lymphoblastic leukemia. Cancer Med 2020; 9:6550–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Widjajanto P, Sumadiono S, Cloos J, Purwanto I, Sutaryo S, Veerman A. Randomized double blind trial of ciprofloxacin prophylaxis during induction treatment in childhood acute lymphoblastic leukemia in the WK-ALL protocol in Indonesia. J Blood Med 2013; 4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Margolis EB, Hakim H, Dallas RH, et al. Antibiotic prophylaxis and the gastrointestinal resistome in paediatric patients with acute lymphoblastic leukaemia: a cohort study with metagenomic sequencing analysis. Lancet Microbe 2021; 2:e159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Davis A, Stevens AM, Brackett J, et al. Levofloxacin prophylaxis for pediatric leukemia patients: longitudinal follow-up for impact on health care-associated infections. Pediatr Blood Cancer 2022; 69:e29525. [DOI] [PubMed] [Google Scholar]
- 34. Gardner JC, Courter JD, Dandoy CE, Davies SM, Teusink-Cross A. Safety and efficacy of prophylactic levofloxacin in pediatric and adult hematopoietic stem cell transplantation patients. Transplant Cell Ther 2022; 28:167.e1–5. [DOI] [PubMed] [Google Scholar]
- 35. Hafez HA, Yousif D, Abbassi M, Elborai Y, Elhaddad A. Prophylactic levofloxacin in pediatric neutropenic patients during autologous hematopoietic stem cell transplantation. Clin Transplant 2015; 29:1112–8. [DOI] [PubMed] [Google Scholar]
- 36. Lopes LAA, Veroneze I, Burgardt CI, Stier CJN. Prophylaxis with levofloxacin: impact on bacterial susceptibility and epidemiology in a hematopoietic stem cell transplant unit. Rev Bras Hematol Hemoter 2014; 36:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yeh TC, Oreská S, Špiritović M, et al. Effectiveness and antimicrobial susceptibility profiles during primary antimicrobial prophylaxis for pediatric acute myeloid leukemia. Sci Rep 2021; 11:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yeh T-C, Liu H-C, Hou J-Y, et al. Severe infections in children with acute leukemia undergoing intensive chemotherapy can successfully be prevented by ciprofloxacin, voriconazole, or micafungin prophylaxis. Cancer 2014; 120:1255–62. [DOI] [PubMed] [Google Scholar]
- 39. Wolf J, Tang L, Flynn PM, et al. Clinical infectious diseases levofloxacin prophylaxis during induction therapy for pediatric acute lymphoblastic leukemia. Clin Infect Dis 2017; 65:1790–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Laoprasopwattana K, Khwanna T, Suwankeeree P, Sujjanunt T, Tunyapanit W, Chelae S. Ciprofloxacin reduces occurrence of fever in children with acute leukemia who develop neutropenia during chemotherapy. Pediatr Infect Dis J 2013; 32:e94–8. [DOI] [PubMed] [Google Scholar]
- 41. Alexander S, Fisher BT, Gaur AH, et al. Effect of levofloxacin prophylaxis on bacteremia in children with acute leukemia or undergoing hematopoietic stem cell transplantation. JAMA 2018; 320:995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yeh T-C, Liu H-C, Hou J-Y, et al. Severe infections in children with acute leukemia undergoing intensive chemotherapy can successfully be prevented by ciprofloxacin, voriconazole, or micafungin prophylaxis. Cancer 2014; 120:1255–62. [DOI] [PubMed] [Google Scholar]
- 43. Yeh T-C, Liu H-C, Hou J-Y, et al. Severe infections in children with acute leukemia undergoing intensive chemotherapy can successfully be prevented by ciprofloxacin, voriconazole, or micafungin prophylaxis. Cancer 2014; 120:1255–62. [DOI] [PubMed] [Google Scholar]
- 44. Norrby SR. Side-effects of quinolones: comparisons between quinolones and other antibiotics. Eur J Clin Microbiol Infect Dis 1991; 10:378–83. [DOI] [PubMed] [Google Scholar]
- 45. Egan G, Robinson PD, Martinez JPD, et al. Efficacy of antibiotic prophylaxis in patients with cancer and hematopoietic stem cell transplantation recipients: a systematic review of randomized trials. Cancer Med 2019; 8:4536–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Samonis G, Kofteridis DP, Maraki S, et al. Levofloxacin and moxifloxacin increase human gut colonization by Candida species. Antimicrob Agents Chemother 2005; 49:5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bucaneve G, Micozzi A, Menichetti F, et al. Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. N Engl J Med 2005; 353:977–87. [DOI] [PubMed] [Google Scholar]
- 48. Zama D, Masetti R, Baccelli F, et al. Antibiotic prophylaxis and management of infections in pediatric hematopoietic stem cell transplantation: a survey from the Stem Cell Transplant and the Infectious Disease Working Groups of the AIEOP Network [published online ahead of print October 07, 2022]. Bone Marrow Transplant. 10.1038/s41409-022-01793-5. [DOI] [PubMed] [Google Scholar]
- 49. D'Amico F, Soverini M, Zama D, et al. Gut resistome plasticity in pediatric patients undergoing hematopoietic stem cell transplantation. Sci Rep 2019; 9:5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Masetti R, Biagi E, Zama D, et al. Early modifications of the gut microbiome in children with hepatic sinusoidal obstruction syndrome after hematopoietic stem cell transplantation. Sci Rep 2021; 11:14307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zama D, Bossu G, Leardini D, et al. Insights into the role of intestinal microbiota in hematopoietic stem-cell transplantation. Ther Adv Hematol 2020; 11:2040620719896961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Masetti R, D’Amico F, Zama D, et al. Febrile neutropenia duration is associated with the severity of gut microbiota dysbiosis in pediatric allogeneic hematopoietic stem cell transplantation recipients. Cancers (Basel) 2022; 14:1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kim HS, Park BK, Kim S, et al. Clinical characteristics and outcomes of Pseudomonas aeruginosa bacteremia in febrile neutropenic children and adolescents with the impact of antibiotic resistance: a retrospective study. BMC Infect Dis 2017; 17:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Micozzi A, Gentile G, Minotti C, et al. Carbapenem-resistant Klebsiella pneumoniae in high-risk haematological patients: factors favouring spread, risk factors and outcome of carbapenem-resistant Klebsiella pneumoniae bacteremias. BMC Infect Dis 2017; 17:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. OECD/European Commission . Cities in the World: A New Perspective on Urbanisation. City Worlds. OECD Publishing, 2020. [Google Scholar]
- 56. Masetti R, Zama D, Leardini D, et al. The gut microbiome in pediatric patients undergoing allogeneic hematopoietic stem cell transplantation. Pediatr Blood Cancer 2020; 67:e28711. [DOI] [PubMed] [Google Scholar]
- 57. Masetti R, Zama D, Leardini D, et al. Microbiome-derived metabolites in allogeneic hematopoietic stem cell transplantation. Int J Mol Sci 2021; 22:1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yeh TC, Liu H-C, Hou J-Y, et al. Severe infections in children with acute leukemia undergoing intensive chemotherapy can successfully be prevented by ciprofloxacin, voriconazole, or micafungin prophylaxis. Cancer 2014; 120:1255–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.