Abstract
Introduction
Dementia as an inevitable aging consequence has been challenged and underscores the need for investigations of the factors that confer resilience. We examine whether the functionally advantageous KL‐VS variant of the putative aging suppressor KLOTHO gene attenuates age‐related cognitive decline and deleterious biomolecular changes.
Methods
Trajectories of change in memory and executive function (N = 360; 2–12 visits) and cerebrospinal fluid (CSF) Alzheimer's disease (AD) biomarkers—amyloid beta (Aβ)42, total tau (t‐tau), phosphorylated tau (p‐tau) (N = 112; 2–4 samplings)—were compared between KL‐VS non‐carriers and heterozygotes in middle‐aged and older adults from the Wisconsin Registry for Alzheimer's Prevention and the Wisconsin Alzheimer's Disease Research Center studies.
Results
Memory and executive function declined (p’s 0.001) and CSF t‐tau, p‐tau, t‐tau/Aβ42, and p‐tau/Aβ42 levels increased (all p’s 0.004) with age. The rate of p‐tau accumulation was attenuated for KL‐VS heterozygotes (p = 0.03).
Discussion
KL‐VS heterozygosity may confer resilience to AD‐associated biomolecular changes.
Keywords: Alzheimer's disease, CSF, biomarkers, memory, executive function, tau
1. BACKGROUND
Alzheimer's disease (AD), a progressive and debilitating neurological disorder of old age, is currently the sixth leading cause of death in the developed world. 1 AD is clinically hallmarked by memory loss and neuropathologically by accumulation of plaques and tangles in the brain. 2 The neuropathological changes are believed to begin years or perhaps even decades before diagnosis. 3 , 4
Age is the single biggest risk factor for developing AD. 5 The long‐standing belief that dementia is an inevitable consequence of aging has been challenged on multiple fronts in recent years. Not only is it possible to age successfully 6 , but even some individuals at genetic risk for AD 7 or harboring AD neuropathology are able to remain cognitively normal as they age. 8 , 9 This has refocused research away from risk and onto potentially protective or compensatory factors in hopes of reducing disability and disease incidence. 10
Klotho, dubbed an anti‐aging and longevity factor encoded by the KLOTHO gene, is a transmembrane protein and a circulating factor that plays a key role in cellular metabolism and body homeostasis. 11 , 12 Although the mechanisms of KLOTHO action are still not well understood, the available evidence indicates that it enhances synaptic and cognitive functions, protects from neurodegeneration, 12 and may also play a role in central nervous system maturation and aging. 13 Neurons pre‐treated with klotho can be rescued in the presence of amyloid beta (Aβ) and glutamate toxicity, suggesting a potential target for therapeutic approaches that might protect against further deterioration and positively affect the outcome for patients with AD. 14 , 15 , 16
Two KLOTHO single‐nucleotide polymorphisms (SNPs; rs9536314 and rs9527025) segregate to form a functional haplotype, KL‐VS, which modulates klotho secretion in humans. 17 , 18 , 19 Extant meta‐analyses of human studies suggest that the functionally advantageous KL‐VS heterozygosity (KL‐VSHET) is associated with various favorable outcomes, including longevity, better cardiovascular health, and better cognitive function, to name a few. 18 , 19 , 20 , 21 , 22 , 23 , 24 The KLOTHO literature related to AD and its biomarkers is still nascent. The evidence available thus far suggests that KL‐VSHET is associated with lesser Aβ burden 17 , 25 and lower AD risk in apolipoprotein E (APOE) ε4 carriers. 25
Our group recently reported cross‐sectional findings indicating that age‐related alterations in cerebrospinal fluid (CSF) biomarkers, and memory and executive function, were attenuated in late‐middle‐aged, cognitively normal KL‐VSHET individuals enrolled in the Wisconsin Registry for Alzheimer's Prevention (WRAP) and the Wisconsin Alzheimer's Disease Research Center (W‐ADRC). 26 We now leverage longitudinal data from these same risk‐enriched cohorts to examine whether KLOTHO attenuates the rate of prospective changes in cognition, specifically decline in memory and executive function, given their sensitivity to incipient AD, 27 and in CSF biomarkers of AD (Aβ42, total tau [t‐tau], phosphorylated tau [p‐tau], and their respective ratios to Aβ42). We hypothesized that the expected adverse effect of age on the rates of change in both cognitive performance and AD CSF biomarkers will be attenuated in carriers of the functionally advantageous genotype of KLOTHO (KL‐VSHET).
2. METHODS
2.1. Participants
Inclusion of participants in this report was based on the availability of the KL‐VS genotype and longitudinal CSF and neuropsychological data, and being characterized as cognitively normal based on standardized, multidisciplinary, consensus conferences. 17 , 28 This resulted in a sample of 360 individuals (age range 45–65 years at study entry; 68% female) who had two or more visits with available neuropsychological data, each visit occurring biannually. Of these, 112 (69% female) had two or more lumbar puncture procedures to date, which occurred ≈2 years apart. Additional information regarding the WRAP and the W‐ADRC's IMPACT (Investigating Memory in Preclinical AD–Causes and Treatments) cohorts have been published previously. Both cohorts are enriched with risk factors for AD, namely parental history and APOE ε4 genotype. 17 , 28
2.2. Standard protocol approvals, registrations, and patient consent
Study procedures were approved by the University of Wisconsin Institutional Review Board and all participants signed written consent.
2.3. Genotyping
DNA was extracted from blood using the PUREGENE DNA Isolation Kit (Gentra Systems, Inc, Minneapolis, MN). DNA concentrations were quantified using ultraviolet spectrophotometry (DU 530 Spectrophotometer, Beckman Coulter, Fullerton, CA). LGC Genomics (Beverly, MA) performed genotyping for APOE (rs429358 and rs7412) and KLOTHO (rs9536314 and rs9527025) using competitive allele‐specific polymerase chain reaction (PCR)–based KASP genotyping assays. Previously published quality‐control procedures are deemed satisfactory. 17 , 29 Consistent with HapMap and the literature, 17 , 18 , 25 , 26 rs9536314 and rs9527025 were also in perfect linkage disequilibrium in our study population.
2.4. CSF assessment
Lumbar puncture was performed in the morning after a 12‐hour fast with a Sprotte 24‐ or 25‐gauge spinal needle at L3‐4 or L4‐5 with extraction into polypropylene syringes. Each sample consisted of 22 mL CSF, which was then combined, mixed, and centrifuged at 2000g for 10 minutes. Supernatants were frozen in 0.5 mL aliquots in polypropylene tubes and stored at −80°C. The samples were immunoassayed for Aβ42, t‐tau, and p‐tau181 with INNOTEST ELISAs (Fujirebio, Ghent, Belgium) in two batches as described previously, 17 , 30 with a subset of samples re‐assayed. Statistical conversion models were developed based on this subset and applied to adjust for the batch differences and harmonize the values across batches. 31
2.5. Neuropsychological testing
Participants completed a comprehensive cognitive test battery at each visit. 28 , 32 , 33 The assessment spans five cognitive domains: episodic memory, attention, executive function, language, and visuospatial ability. Here we focus primarily on measures of episodic memory (Rey Auditory Verbal Learning Test [RAVLT]) 35 and visual search and processing speed, mental flexibility, and executive function (Trail Making Test [TMT], Parts A & B) 35 given their sensitivity to incipient AD, 27 and also because these tests are common to both WRAP and the W‐ADRC batteries. For the RAVLT, we focused on Total Learning (sum of Trials 1–5) and Long Delay Free Recall, whereas for the TMT we analyzed time to test completion.
RESEARCH IN CONTEXT
Systematic review: The authors performed a traditional literature review related to Alzheimer's disease (AD) biomarkers, cognition, and the KLOTHO gene. There is evidence that KLOTHO heterozygosity is associated with better cognition and lesser AD biomarker burden. Whether KLOTHO heterozygosity also attenuates age‐related cognitive decline and the rates of deleterious biomolecular changes is currently unknown.
Interpretation: Overall, our results suggest that KLOTHO heterozygosity attenuates deleterious age‐related changes in risk markers for AD within a cohort of non‐demented middle‐aged and older adults enriched for AD risk. Given the lack of disease‐modifying therapies, the identification of new genetic variants that modify AD risk can potentially uncover novel targets for therapeutic interventions.
Future directions: Additional studies with larger and more diverse samples and CSF biomarker data spanning longer periods would be beneficial to confirm the current findings. In addition, longitudinal prospective studies of older adults with mild cognitive impairment or AD are warranted.
HIGHLIGHTS
KLOTHO is considered a putative aging suppressor gene.
The functionally advantageous KL‐VS variant is linked to favorable health outcomes.
Memory and executive function decline did not differ based on KLOTHO status.
Phosphorylated tau accumulation is attenuated in KL‐VS heterozygotes.
KL‐VS heterozygosity may confer resilience to deleterious age‐related changes.
2.6. Statistical analyses
All analyses were done in SPSS, v. 26.0 (IBM, Armonk, NY). Participants were split into two groups based on KL‐VS status (non‐carriers [KL‐VSNC] vs heterozygotes [KL‐VSHET]) for analytical purposes. KL‐VS homozygosity, associated with decreased longevity and worse cognition, is rare; hence, five homozygotes in our sample were omitted from the analysis.
We compared the groups on demographic characteristics either using chi‐square (χ2) or independent‐samples t‐tests. With age as the time scale, we used linear mixed‐effects regression models to investigate longitudinal changes in our neuropsychological (N = 360) and biomarker (N = 112) outcomes. Covariates included were APOE ε4 status, sex, and parental history of AD. Education was additionally covaried in the neuropsychological models. To ascertain whether the observed trajectories varied as a function of KLOTHO genotype, we refitted the models while including KLOTHO and age*KLOTHO as additional covariates. The age*KLOTHO term was of primary interest, as it would indicate whether interindividual trajectories in the modeled outcome differed between KL‐VSHET and KL‐VSNC.
3. RESULTS
3.1. Sample characteristics
Characteristics of both the larger sample with multiple neuropsychological visits and the smaller sub‐sample with two or more lumbar punctures are detailed in Table 1. Overall, participants were predominantly White (96%) and female (68%), with a mean age ± SD of 67 ± 8 and 16 years of education. The sample was enriched for AD risk; 38% are APOE ε4 carriers and 74% have a parental history of dementia. There were no significant differences between the larger sample with multiple neuropsychological visits and the sub‐sample with two or more lumbar punctures, or between KL‐VSHET and KL‐VSNC, within either the CSF or the cognitive sample, in any of the aforementioned characteristics (all p’s ≥ 0.05), except for sex in the CSF biomarker sub‐sample whereby 73% of KL‐VSNC but only 54% of KL‐VSHET were female (p = 0.03).
TABLE 1.
Background characteristics of study participants
|
Sample with repeat Neuropsychological test measures |
Sub‐sample with repeat CSF measures |
|||||||
|---|---|---|---|---|---|---|---|---|
| VARIABLE |
TOTAL (N = 360) |
KL‐VSNC (N = 260) |
KL‐VSHET (N = 100) |
p |
TOTAL (N = 112) |
KL‐VSNC (N = 89) |
KL‐VSHET (N = 23) |
p |
| Age, mean (SD) | 66.59 (7.97) | 62.31 (6.64) | 61.39 (5.98) | 0.37 | 62.12 (6.49) | 62.31 (6.64) | 61.39 (5.98) | 0.54 |
| Education, mean (SD) | 16.03 (2.5) | 16.00 (2.56) | 16.17 (2.35) | 0.41 | 16.42 (2.51) | 16.35 (2.56) | 16.42 (2.51) | 0.59 |
| MMSE | 29.23 (1.03) | 29.29 (0.87) | 29.10 (1.33) | 0.22 | 29.5 (0.72) | 29.58 (0.67) | 29.21 (0.83) | 0.14 |
| Females, N (%) | 244 (68) | 179 (69) | 65 (65) | 0.59 | 78 (69) | 65 (73) | 13 (54) | 0.03 |
| White, N (%) | 347 (96) | 252 (97) | 95 (95) | 0.36 | 106 (95) | 85 (95) | 21 (92) | 0.60 |
| APOE ε4+, N (%) | 137 (38) | 97 (37) | 40 (40) | 0.72 | 45 (40) | 35 (40) | 10 (42) | 0.81 |
| Parental history of AD, N (%) | 267 (74) | 197 (76) | 70 (70) | 0.28 | 89 (80) | 71 (80) | 18 (86) | 0.54 |
| Aβ42 positive, N (%) a | 46 (12) | 37 (14) | 9 (9) | 0.14 | 12 (10) | 10 (11) | 2 (9) | 0.21 |
| t‐Tau positive, N (%) a | 53 (14) | 38 (14) | 15 (15) | 0.49 | 13 (11) | 12 (13) | 1 (5) | 0.73 |
| p‐Tau positive, N (%) a | 53 (14) | 36 (13) | 17 (17) | 0.25 | 10 (9) | 9 (10) | 1 (5) | 0.64 |
| Number of visits | ||||||||
| 1 | 360 | 260 | 100 | — | 112 | 89 | 23 | — |
| 2 | 355 | 257 | 98 | — | 112 | 89 | 23 | — |
| 3 | 346 | 253 | 93 | — | 20 | 15 | 5 | — |
| 4 | 328 | 237 | 91 | — | 7 | 4 | 3 | — |
| 5 | 276 | 206 | 70 | — | — | — | — | — |
| 6 | 190 | 140 | 50 | — | — | — | — | — |
| 7 | 71 | 49 | 22 | — | — | — | — | — |
| 8 | 29 | 17 | 12 | — | — | — | — | — |
| 9 | 11 | 6 | 5 | — | — | — | — | — |
| 10 | 1 | 0 | 1 | — | — | — | — | — |
| Total Visits | 1967 | 1425 | 542 | 251 | 197 | 54 | ||
Abbreviations: KL‐VSNC = KL‐VS non‐carriers; KL‐VSHET = KL‐VS heterozygotes; MMSE = Mini‐Mental State Examination; APOE ε4+ = APOE ε4 carrier; Aβ42 = amyloid beta 42; t‐Tau = total tau; p‐Tau = phosphorylated tau; mean (SD) = mean (standard deviation).
Positive based on our center's derived cut point for CSF AD biomarkers.27
We also assessed how many of the participants in each sample would be considered positive (i.e., abnormal) based on our center's derived cut points for CSF AD biomarkers (Aβ42, ≤471; p‐tau, ≥59.5; and t‐tau, ≥461. 26 , 36 Although most of the sample was negative for AD biomarkers (>88%), the number of biomarker‐positive participants at baseline (<14%) did not differ significantly between KL‐VSHET and KL‐VSNC (see Table 1): 14% of KL‐VSNC versus 9% of KL‐VSHET were considered positive (p = 0.14) for Aβ42; 14% of KL‐VSNC versus 15% of KL‐VSHET were considered positive (p = 0.49) for t‐tau; and 13% of KL‐VSNC versus 17% of KL‐VSHET were considered positive (p = 0.25) for p‐tau.
3.2. Trajectories of neuropsychological performance tapping memory and executive function in KL‐VS non‐carriers and heterozygotes
Performance on neuropsychological tests tapping memory (RAVLT trials 1–5 and long delay) and executive function (TMT B) declined significantly with age across both KLOTHO genotypes taken together (all p’s < 0.001), with no significant genotype differences in longitudinal rates of performance decline (Table 2).
TABLE 2.
Trajectories of change in cognitive outcomes and CSF biomarkers of AD as a function of the KLOTHO KL‐VS polymorphism
| VARIABLE | ESTIMATE | SE | t | p | |
|---|---|---|---|---|---|
| COGNITIVE OUTCOMES a | |||||
| RAVLT Trials 1–5 | Age | –0.12 | 0.03 | –3.45 | 0.001 |
| KLOTHO | –0.32 | 0.82 | –0.39 | 0.69 | |
| Age x KLOTHO | –0.07 | 0.07 | 1.09 | 0.27 | |
| RAVLT Long Delay | Age | –0.04 | 0.01 | –3.20 | 0.001 |
| KLOTHO | –0.34 | 0.28 | –1.23 | 0.22 | |
| Age x KLOTHO | –0.0006 | 0.02 | –0.03 | 0.97 | |
| TMT A | Age | –0.002 | 0.04 | –0.07 | 0.95 |
| KLOTHO | –0.03 | 0.90 | –0.03 | 0.97 | |
| Age x KLOTHO | –0.03 | 0.07 | –0.39 | 0.70 | |
| TMT B | Age | 0.64 | 0.09 | 6.42 | <0.001 |
| KLOTHO | 1.22 | 2.56 | 0.48 | 0.63 | |
| Age x KLOTHO | –0.16 | 0.19 | –0.85 | 0.39 | |
| CSF BIOMARKERS b | |||||
| Aβ42 | Age | –4.16 | 2.22 | –1.79 | 0.07 |
| KLOTHO | –26.52 | 35.99 | –0.74 | 0.46 | |
| Age x KLOTHO | –5.46 | 5.57 | –0.99 | 0.33 | |
| t‐Tau | Age | 5.35 | 1.31 | 4.08 | <0.001 |
| KLOTHO | –33.35 | 24.17 | –1.38 | 0.17 | |
| Age x KLOTHO | –4.09 | 3.06 | –1.34 | 0.18 | |
| p‐Tau | Age | 0.49 | 0.18 | 2.93 | 0.004 |
| KLOTHO | –1.78 | 2.56 | –0.69 | 0.49 | |
| Age x KLOTHO | –1.78 | 0.40 | –2.26 | 0.02 | |
| t‐Tau/Aβ42 | Age | 0.01 | 0.002 | 5.33 | <0.001 |
| KLOTHO | –0.06 | 0.05 | –1.23 | 0.22 | |
| Age x KLOTHO | –0.006 | 0.006 | –1.03 | 0.31 | |
| p‐Tau/Aβ42 | Age | 0.001 | 0.0003 | 4.23 | <0.001 |
| KLOTHO | –0.004 | 0.005 | –0.76 | 0.45 | |
| Age x KLOTHO | –0.001 | 0.001 | –1.42 | 0.16 | |
KLOTHO denotes the estimated mean difference at age 0 (mean age for the whole sample) in the outcome of interest between KL‐VSNC and KL‐VSHET. Age indicates the annual rate of change in the outcome of interest for KL‐VSNC. Age x KLOTHO denotes the difference in the annual rate of change between KL‐VSNC and KL‐VSHET (this estimate must be added to the estimate for age to determine annual rate of change for KL‐VSHET).
Abbreviations: KL‐VSNC = KL‐VS non‐carriers; KL‐VSHET = KL‐VS heterozygotes; RAVLT = Rey Auditory Verbal Learning Test; TMT = Trail Making Test, Aβ42 = amyloid beta 42; t‐Tau = total tau; p‐Tau = phosphorylated tau; SD = standard deviation.
Covariates: APOE ε4, sex, parental history of AD, education.
Covariates: APOE ε4, sex, parental history of AD.
3.3. Trajectories of CSF biomarkers in KL‐VS non‐carriers and heterozygotes
Except for a non‐significant trend for Aβ42 (p = 0.07), all biomarkers investigated showed significant longitudinal change with age in both groups taken together (all p’s ⩽ 0.004). Genotype differences in rates of longitudinal accumulation (Table 2) were observed for p‐tau only, with that rate being slower for KL‐VSHET (t 131.9 = –2.26, p = 0.03; Figure 1).
FIGURE 1.
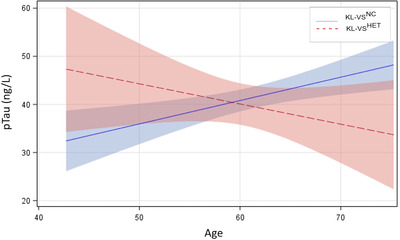
Estimated trajectories of change in phosphorylated tau p‐tau as a function of KL‐VS status. Rate of cerebrospinal fluid (CSF) p‐tau accumulation decreases in KL‐VSHET but increases in KL‐VSNC with age (p = 0.03)
4. DISCUSSION
We report that in a late‐middle‐aged cohort enriched for AD risk, unfavorable accumulation of p‐tau with age was attenuated in carriers of a functionally favorable KL‐VS genotype. Specifically, KL‐VSNC exhibited a steeper rate of expected age‐related alterations in CSF p‐tau compared to KL‐VSHET.
In a prior cross‐sectional study from this same cohort, we reported that KL‐VSNC exhibited an expected age‐related pattern of associations with CSF biomarkers, memory, and executive function, which were all attenuated in KL‐VSHET. 26 Our current study expands on those previous findings and further adds to the literature by demonstrating for the first time that the favorable effects of KL‐VSHET extend to prospective trajectories of age‐related changes in a core AD biomarker (i.e., p‐tau accumulation). Further confirmation of our findings is needed in larger samples given the collinearity between p‐tau and t‐tau. 36 , 37 , 38 It is important to note, however, that these and other reports of correspondence of p‐tau and t‐tau results are largely cross‐sectional in nature, including our own previous report in this cohort, whereby we observed both lesser p‐tau and t‐tau burden at baseline in carriers of a functionally advantageous KLOTHO variant. 26
The filamentous core of neurofibrillary tangles (NFTs), one of the two histopathologic hallmarks of AD, is composed of highly phosphorylated forms of tau. 39 , 40 In addition to being a key neuropathological feature of AD and central to NFT formation, p‐tau accumulation is related to synaptic impairment, neuronal dysfunction, and impairment in mitochondrial transport. 41 , 42 , 43 There is substantial evidence that NFTs and neuropil threads are significantly lower in people without cognitive impairment compared to those with mild cognitive impairment (MCI) or AD, and further, that NFTs correlate with episodic memory performance, 43 although the exact mechanisms by which tau phosphorylation affects cognitive function are still under investigation. Our finding of attenuated age‐related accumulation in CSF p‐tau in KL‐VSHET has direct implications for klotho as a potential target against age‐related cognitive decline and AD neuropathology.
The role for KLOTHO in longevity 11 , 12 , 19 , 20 , 21 , 22 , 23 , 24 is well‐established, and the evidence in support of preserved brain integrity and cognitive,, 23 , 26 , 44 , 45 , 46 , 47 as well as slower cognitive decline, 45 in KL‐VSHET within the context of aging is mounting. Moreover, KL‐VSHET exhibits better memory 26 , 47 and better executive function 26 , 44 in conjunction with greater dorsolateral prefrontal cortex volume 44 and greater intrinsic connectivity in functional brain networks vulnerable to deleterious effects of aging. 46 Although we previously reported that KL‐VSHET in this cohort performed significantly better on tests of memory and executive function at baseline, 26 this favorable genotype‐related outcome does not seem to extend to trajectories of memory and executive function performance with age.
Our sample is predominantly White, highly educated, and has a higher prevalence of both parental history of AD and APOE ε4 carriers than what is normally observed in the general population, which may potentially limit the generalizability of our findings. Another potential limitation is the arguably modest sample size of our CSF analyses. In addition, a recent study in bonobos and chimpanzees reported that while both sexes show an age‐related decline in soluble alpha‐klotho, females tend to have higher levels throughout life. 48 Although our findings could be influenced by 68% of our sample being female, sex was controlled for in all of our analyses. Nonetheless, the correlation between levels of soluble klotho protein in CSF and plasma across KL‐VS variants should be established for males and females separately going forward and replicated in other cohorts. The foregoing caveats, however, should not undermine the unique strengths of our study, namely the longitudinal examination of multimodal AD‐relevant outcomes using data from extensively characterized cohorts that have been followed prospectively for many years.
Overall our results suggest that KL‐VS heterozygosity attenuates deleterious age‐related changes in risk markers for AD, namely memory, executive function, and CSF p‐tau. In addition to being the single greatest risk factor for AD, age is the most robust determinant of CSF biomarker changes and cognitive decline in the absence of diagnosis. Here, we offer a glimpse into how one genetic factor, KLOTHO, offers resilience against age‐related changes in cognition and CSF tau. The importance of identifying factors that confer resilience is gaining increased recognition given the current lack of curative therapies for AD. 49 The identification of new genetic variants that modify AD risk will potentially uncover novel molecular targets. This line of research is poised to identify complementary pathways for curbing AD progression and delaying symptom onset.
CONFLICTS OF INTEREST
H.Z. has served at scientific advisory boards for Alector, Eisai, Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics, Nervgen, AZTherapies, and CogRx; has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, and Biogen; and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). K.B. has served as a consultant, on advisory boards, or on data‐monitoring committees for Abcam, Axon, Biogen, JOMDD/Shimadzu. Julius Clinical, Lilly, MagQu, Novartis, Prothena, Roche Diagnostics, and Siemens Healthineers; and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). All other authors have no disclosures to report (see Supporting Information).
Supporting information
SUPPORTING INFORMATION
ACKNOWLEDGEMENTS
We would like to acknowledge the researchers and staff of the Clinical Neurochemistry Laboratory, Institute of Neuroscience and Physiology, the Sahlgrenska Academy at the University of Gothenburg, Sweden, where CSF was assayed. We also thank the staff and study participants of the Wisconsin Registry for Alzheimer's Prevention and the Wisconsin Alzheimer's Disease Research Center, without whom this work would not be possible.
KLOTHO is the subject of a pending international patent application held by the Regents of the University of California. All authors report no disclosures relevant to the manuscript.
This work was supported by National Institute on Aging grants K23 AG045957 (O.C.O.), R21 AG051858 (O.C.O.), R01 AG027161 (S.C.J.), P50 AG033514 (S.A.), and P30 AG062715 (S.A.); by National Institute of Neurological Disorders and Stroke grant R01 NS092918 (D.B.D.); and a Clinical and Translational Science Award (UL1RR025011) to the University of Wisconsin, Madison. Portions of this research were supported by the Extendicare Foundation; Alzheimer's Association; Wisconsin Alumni Research Foundation; Veterans Administration, including facilities and resources at the Geriatric Research Education and Clinical Center of the William S. Middleton Memorial Veterans Hospital, Madison, WI; European Research Council; the Swedish Research Council, Swedish Brain Foundation (#FO2017‐0243); the Swedish Alzheimer Foundation (#AF‐742881), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF‐agreement (#ALFGBG‐715986), and the Knut and Alice Wallenberg Foundation.
Driscoll I, Ma Y, Lose SR, et al. AD‐associated CSF biomolecular changes are attenuated in KL‐VS heterozygotes. Alzheimer's Dement. 2022;14:e12383. 10.1002/dad2.12383
REFERENCES
- 1. Alzheimer's Association . 2018 Alzheimer's disease facts and figures. Alzheimers Dement 2018;14:367‐429. [Google Scholar]
- 2. Van Cauwenberghe C, Van Broeckhoven C, Sleegers K. The genetic landscape of Alzheimer disease: clinical implications and perspectives. Genet Med. 2016;18:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Briggs R, Kennelly SP, O'Neill D. Drug treatments in Alzheimer's disease. Clin Med (Lond). 2016;16(3):247‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lane CA, Hardy J, Schott JM. Alzheimer's disease. Eur J Neurol. 2018;25:59‐70. [DOI] [PubMed] [Google Scholar]
- 5. Hou Y, Dan X, Babbar M, et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol 2019;15:565‐581. [DOI] [PubMed] [Google Scholar]
- 6. Stern Y, Barnes CA, Grady C, Jones RN, Raz N. Brain reserve, cognitive reserve, compensation, and maintenance: operationalization, validity, and mechanisms of cognitive resilience. Neurobiol Aging. 2019;83:124‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gallagher M, Okonkwo OC, Resnick SM, Jagust WJ, Benzinger TLS, Rapp PR. What are the threats to successful brain and cognitive aging? Neurobiol Aging. 2019;83:130‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Driscoll I, Resnick SM, Troncoso JC, An Y, O'Brien R, Zonderman AB. Impact of Alzheimer's pathology on cognitive trajectories in nondemented elderly. Ann Neurol. 2006;60(6):688‐695. [DOI] [PubMed] [Google Scholar]
- 9. Driscoll I, Troncoso J. Asymptomatic Alzheimer's disease: a prodrome or a state of resilience? Curr Alzheimer Res. 2011;8(4):330‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Singer B, Friedman E, Seeman T, Fava GA, Ryff CD. Protective environments and health status: cross‐talk between human and animal studies. Neurobiol Aging. 2005;S26:S113‐118. [DOI] [PubMed] [Google Scholar]
- 11. Chateau MT, Araiz C, Descamps S, Galas S. Klotho interferes with a novel FGF‐signaling pathway and insulin/Igf‐like signalling to improve longevity and stress resistance in Caenorhabditis elegans. Aging. 2010;2:567‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829‐1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim JH, Hwang KH, Park KS, Kong ID, Cha SK. Biological role of anti‐aging protein klotho. J Lifestyle Med. 2015;5(1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuang X, Chen YS, Wang LF, et al. Klotho upregulation contributes to the neuroprotection of ligustilide in an Alzheimer's disease mouse model. Neurobiol Aging. 2014;35(1):169‐178. [DOI] [PubMed] [Google Scholar]
- 15. Semba RD, Moghekar AR, Hu J, et al. Klotho in the cerebrospinal fluid of adults with and without Alzheimer's disease. Neurosci Lett. 2014; 558:37‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dubal DB, Zhu L, Sanchez PE, et al. Life extension factor klotho prevents mortality and enhances cognition in hAPP transgenic mice. J Neurosci. 2015;35(6):2358‐2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Erickson CM, Schultz SA, Oh JM, et al. KLOTHO heterozygosity attenuates APOE4‐related amyloid burden in preclinical AD. Neurology. 2019;92(16):e1878‐e1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dubal DB, Yokoyama JS, Zhu L, et al. Life extension factor klotho enhances cognition. Cell Rep. 2014;7:1065‐1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arking DE, Krebsova A, Macek M, et al. Association of human aging with a functional variant of klotho. Proc Natl Acad Sci USA. 2002;99:856‐861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheikhi A, Barchowsky A, Sahu A, et al. Klotho: An elephant in aging research. J Gerontol A Biol Sci Med Sci. 2019;74(7):1031‐1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Di Bona D, Accardi G, Virruso C, Candore G, Caruso C. Association of Klotho polymorphisms with healthy aging: a systematic review and meta‐analysis. Rejuvenation Res. 2014;17:212‐216. [DOI] [PubMed] [Google Scholar]
- 22. Montesanto A, Dato S, Bellizzi D, Rose G, Passarino G. Epidemiological, genetic and epigenetic aspects of the research on healthy ageing and longevity. Immunity Ageing. 2012, 9:6‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Revelas M, Thalamuthu A, Oldmeadow C, et al. Review and meta‐analysis of genetic polymorphisms associated with exceptional human longevity. Mech Ageing Dev. 2018;175:24‐34. [DOI] [PubMed] [Google Scholar]
- 24. Vo HT, Laszczyk AM, King GD. Klotho, the key to healthy brain aging? Brain Plast. 2018;3(2):183‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Belloy ME, Napolioni V, Han SS, Le Guen Y, Greicius MD, for the Alzheimer's Disease Neuroimaging Initiative . Associations of Klotho‐KS heterozygosity with risk of Alzheimer's disease in individuals who carry APOE4. JAMA Neurol. 2020;77(7):849‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Driscoll I, Ma Y, Gallagher CL, Johnson SC, et al. Age‐related tau burden and cognitive deficits are attenuated in KLOTHO KL‐VS heterozygotes. J Alzheimers Dis. 2021;79(3):1297‐1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blacker D, Lee H, Muzikansky A, et al. Neuropsychological measures in normal individuals that predict subsequent cognitive decline. Arch Neurol. 2007;64(6):862‐871. [DOI] [PubMed] [Google Scholar]
- 28. Johnson SC, Koscik RL, Jonaitis EM, et al. The Wisconsin Registry for Alzheimer's Prevention: a review of findings and current directions. Alzheimers Dement (Amst). 2018;10:130‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Darst BF, Koscik RL, Racine AM, et al. Pathway‐specific polygenic risk scores as predictors of amyloid‐beta deposition and cognitive function in a sample at increased risk for Alzheimer's disease. J Alzheimers Dis. 2017;55:473‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palmqvist S, Zetterberg H, Blennow K, et al. Accuracy of brain amyloid detection in clinical practice using cerebrospinal fluid beta‐amyloid 42: a cross‐validation study against amyloid positron emission tomography. JAMA Neurol. 2014;71:1282‐1289. [DOI] [PubMed] [Google Scholar]
- 31. Ma Y, Norton DL, Van Hulle CA, et al. Measurement batch differences and between‐batch conversion of Alzheimer's disease cerebrospinal fluid biomarker values. Alzheimers Dement (Amst). 2021;13(1):e12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sager MA, Hermann B, La Rue A. Middle‐aged children of persons with Alzheimer's disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer's Prevention. J Geriatr Psychiatry Neurol. 2005;18(4):245‐249. [DOI] [PubMed] [Google Scholar]
- 33. Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23(2):91‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schmidt M. Rey Auditory Verbal Learning Test: A Handbook. Western Psychological Services; 1996. [Google Scholar]
- 35. Reitan R, Wolfson D. The Halstead‐Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Neuropsychology Press; 1993. [Google Scholar]
- 36. Blom ES, Giedraitis V, Zetterberg H, et al. Rapid progression from mild cognitive impairment to Alzheimer's disease in subjects with elevated levels of tau in cerebrospinal fluid and the APOE epsilon4/epsilon4 genotype. Dement Geriatr Cogn Disord. 2009;27(5):458‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sämgård K, Zetterberg H, Blennow K, Hansson O, Minthon L, Londos E. Cerebrospinal fluid total tau as a marker of Alzheimer's disease intensity. Int J Geriatr Psychiatry. 2010;25(4):403‐410. [DOI] [PubMed] [Google Scholar]
- 38. Wattmo C, Blennow K, Hansson O. Cerebro‐spinal fluid biomarker levels: phosphorylated tau (T) and total tau (N) as markers for rate of progression in Alzheimer's disease. BMC Neurol. 2020;20(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grundke‐Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule‐associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA. 1986;83:4913‐4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goedert M, Wischik CM, Crowther RA, Walker JE, Klug A. Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: identification as the microtubule‐associated protein tau. Proc Natl Acad Sci USA. 1988;85:4051‐4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stoothoff WH, Johnson GVW. Tau phosphorylation: physiological and pathological consequences. Biochim Biophys Acta (BBA) ‐ Mol Basis Dis. 2005;1739(2‐3):280‐297. [DOI] [PubMed] [Google Scholar]
- 42. Lee G, Leugers CJ. Tau phosphorylation: physiological and pathological consequences. In Teplow DB (ed). Tau and Tauopathies. In: Progress in Molecular Biology and Translational Science. Academic Press; 2012;107:263‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mitchell TW, Mufson EJ, Schneider JA, et al. Parahippocampal tau pathology in healthy aging, mild cognitive impairment, and early Alzheimer's disease. Ann Neurol. 2002;51(2):182‐189. [DOI] [PubMed] [Google Scholar]
- 44. Yokoyama JS, Sturm VE, Bonham LW, et al. Variation in longevity gene KLOTHO is associated with greater cortical volumes. Ann Clin Transl Neurol. 2015;2:215‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. de VriesCF, Staff RT , Se Harris, et al. Klotho, APOEepsilon4, cognitive ability, brain size, atrophy and survival: a study in Aberdeen Birth Cohort of 1936. Neurobiol Aging. 2017;55:91‐98. [DOI] [PubMed] [Google Scholar]
- 46. Yokoyama JS, Marx G, Brown JA, et al. Systemic klotho is associated with KLOTHO variation and predicts intrinsic cortical connectivity in healthy human aging. Brain Imaging Behav. 2017;11(2):391‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Neitzel J, Franzmeier N, Rubinski A, et al. KL‐VS heterozygosity is associated with lower amyloid‐dependent tau accumulation and memory impairment in Alzheimer's disease. Nat Commun. 2021;12(1):3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Behringer V, Stevens JMG, Deschner T, Sonnweber R, Hohmann G. Aging and sex affect soluble alpha klotho levels in bonobos and chimpanzees. Front Zool. 2018;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Okonkwo OC, Vemuri P. Stemming the Alzheimer tsunami: introduction to the special issue on reserve and resilience in Alzheimer's disease. Brain Imaging Behav. 2017;11(2):301‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kuro‐o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45‐51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION


