Abstract
Background:
Older adults may have difficulty meeting the Physical Activity (PA) Guidelines. A favorable balance between PA and sedentary time (SED) is an important determinant of physical performance in older adults. Our objective was to explore associations of PA/SED with physical performance across mid-older age in adults without overt mobility disability.
Methods:
Framingham Offspring Study participants free of mobility disability with accelerometry and physical performance data (gait speed, chair stand time, and handgrip strength), were studied in cross-sectional analysis (n = 1352). We regressed physical performance on PA level, measured using steps, moderate to vigorous (MV) PA and SED. We stratified by age groups, adjusted for covariates, and modelled MVPA and SED separately and together as predictors.
Results:
Only 38% of adults 50–64 years and 15% of adults ≥75 years met the PA Guidelines (i.e., 150 min MVPA per week). Individuals achieving at least 5 min/day of MVPA had 0.062 ± 0.013 m/s greater gait speed and better chair stands and handgrip strength (in women) than those with < 5 min/day of MVPA (p < 0.01) across mid-older age. SED was associated with poorer performance on gait speed and chair stand tests, but results were not significant after adjusting for MVPA (p > 0.05). For adults ≥75 years, every 5000 more steps/day related to ~0.045 m/s greater gait speed (p = 0.006).
Conclusion:
Our cross-sectional study demonstrated that, across mid-older adulthood, MVPA related to better physical performance, but in adults ≥75 years, total steps walked associated with better gait speed. These data warrant future research on the impact of PA on physical performance and health outcomes in older age.
Keywords: Epidemiology, Physical function, Sedentary, Accelerometry, Gait speed
1. Introduction
The decline of physical function predicts disability and mortality in older adults (Studenski et al., 2011; Hardy et al., 2011). Over the next two decades, it is expected that > 15 million older adults in the United States will be living with mobility disability (defined as inability to walk 1/4 mile), potentially adding an estimated $42 billion to annual health care costs (Hardy et al., 2011; Cummings et al., 2014). One of the leading factors related to years lived with physical disability is physical inactivity (The State of US Health, 2013). By the same token, intervention programs to increase physical activity (PA) have emerged as a potentially effective prevention strategy for decreasing incident mobility disability (Pahor et al., 2014; Espeland et al., 2017) (Physical Activity Guidelines Advisory Committee, 2008). Objective accelerometry data from nationally representative samples have revealed that few older adults meet the traditional 150 min (min)/week of moderate to vigorous PA (MVPA) Guidelines for Americans from 2008 (Physical Activity Guidelines Advisory Committee, 2008; Tucker et al., 2011). New national guidelines for PA released in 2018 recommend incremental PA for older adults who are not able to meet the MVPA Guideline and suggest that some PA is better than none (US Department of Health and Human Services, 2018). However, exact intensities and durations of PA associated with mobility and physical performance in older age have not been well characterized.
Recent data suggests that maintaining a favorable balance of more light intensity PA and less sedentary time (SED) may decrease mortality in older adults (Dohrn et al., 2018; Hamer et al., 2014). In older age, PA begins to decline and SED increases (Martin et al., 2014). These lifestyle changes have also been associated with worse self-reported mobility and health status (Buman et al., 2010; Gennuso et al., 2016; Marques et al., 2014a; Marques et al., 2014b). There is also prior evidence regarding the association between objective measures of PA and SED with objective physical performance measures (Pahor et al., 2014; Keevil et al., 2016; Santos et al., 2012; Rosenberg et al., 2016; Bann et al., 2015; Dunlop et al., 2015; Hall et al., 2017). Previous studies have suggested that SED may actually be a separate risk factor for disability, regardless of the amount of PA obtained (Dunlop et al., 2015), possibly through independent mechanisms involving the influence of SED on metabolic regulation in muscle tissue (Bey and Hamilton, 2003). Few prior studies have excluded participants with mobility disability (Pahor et al., 2014; Bann et al., 2015; Hall et al., 2017), who have significantly higher SED, even after adjusting for MVPA; (Dunlop et al., 2015) therefore, it is unclear whether reverse causality (i.e., the effect of a severe mobility disability on PA and SED behavior) may have influenced prior results.
For the current investigation, we examined whether PA and SED were associated with physical performance in cross-sectional analyses, at a time before participants developed overt mobility disability in a community-based cohort study. Our design minimizes, but does not remove the potential influence of reverse causality. Secondarily, we assessed whether these associations differed by age group. We hypothesized that the total volume of PA (measured as total steps per day) and lower SED would correlate with physical performance in older adulthood. The ability to distinguish PA durations and intensities that are related to physical performance outcomes across middle and older age ranges may provide evidence supporting the establishment of more attainable goals for total PA and SED in the PA Guidelines for older Americans, which currently focus on achieving MVPA.
2. Methods
The Framingham Offspring Study (N = 5124) is an ongoing longitudinal cohort study that began in 1971 (Kannel et al., 1979). In 1994, the Omni Cohort 1 study was initiated, consisting of residents of Framingham who identified as members of a minority group (N = 507) (Quan et al., 1997). The present study includes participants who attended Offspring examination cycle 9 (N = 2430) or Omni Cohort 1 examination cycle 4 (N = 301) in 2011 to 2014, completed the self-reported mobility questionnaire and objectively measured physical performance battery, and agreed to wear the accelerometer to measure objective PA (N = 1845). Participants were excluded if they wore the accelerometer for < 4 valid days (n = 390), were < 50 years of age (n = 6), self-reported having a mobility disability (n = 82) or if they were missing covariate data (n = 15). The final sample size was 1352 (approximately 50% of attendees at the 2011–2014 examinations). Mobility disability was defined as self-reported inability to walk one half mile or climb one flight of stairs. Although exclusion for self-reported mobility disability likely did not completely remove individuals with disabilities, our goal was to limit potential bias toward self-exclusion and to limit the effect of reverse causation in our results, which cannot be completely eliminated. Analyses showing demographics and PA behavior in participants excluded for mobility disability are reported in Supplemental Table 1. All participants provided written informed consent, and the institutional review board at Boston University Medical Center approved the study protocols.
2.1. Physical performance
We assessed gait speed using the faster of two trials on a 4-meter (m) course walked at usual pace (Studenski et al., 2011; Studenski et al., 2003; Guralnik et al., 2000). We also assessed lower body strength by measuring the ability and time taken to stand five times from the sitting position in a straight-backed chair with arms folded, termed the chair stand test/task (Riskowski et al., 2012). Handgrip strength was assessed by measuring the highest force generated (in kilograms [kg]) by participants asked to squeeze a JAMAR dynamometer (Sammons Preston/JLW Instruments, Chicago, IL, USA) as hard as possible, three times in each hand (Murabito et al., 2017).
2.2. Physical activity accelerometry
All participants were asked to wear an omnidirectional accelerometer (Actical model no. 198-0200-00; Philips Respironics) on the hip for 8 days during all hours throughout the day and night (except when bathing) (Glazer et al., 2013). Recorded signals (within 0.5–3 Hz and accelerations/decelerations within 0.05–2 g) were grouped into “counts” or “steps” at 30 s (s) intervals and averaged over 1 min intervals. Data were processed for quality control and analyzed at the Framingham Heart Study using customized software (Kinesoft, version 3.3.63), during which the first day of wear was removed from the dataset (Glazer et al., 2013). The measurement of steps by Actical devices has been externally validated (Esliger et al., 2007).
Accelerometer data was considered valid if the device was worn for ≥10 h per day for at least 4 days, not including the first day of wear on which the device was given out. Non-wear time was removed during data processing (defined as 60 consecutive min of zero counts, allowing for 2-min interruption periods). SED was defined as < 200 cpm and light intensity PA was defined as 200–1486 cpm (Glazer et al., 2013). SED and light intensity PA were only considered during wear time occurring between 6 AM-10 PM, and were reported as a percentage of wear time (%SED and %Light Intensity PA). Due to high correlation between %SED and %Light Intensity PA (r = −0.90, p < 0.0001, Supplemental Table 2), only %SED results were displayed in the main document. MVPA was defined as > 1486 cpm (Crouter and Bassett Jr., 2008; Heil, 2006). MVPA and total steps per day were considered during any time of the day.
2.3. Covariates
Covariates included body mass index (BMI), current smoking, stage II hypertension (using the 2017 American College of Cardiology/American Heart Association Hypertension Guideline, systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mm Hg) (Whelton et al., 2017), diabetes mellitus (fasting blood glucose ≥126 mg/dL, or diabetes medications), prevalent cardiovascular disease (CVD), and prevalent cancer.
2.4. Statistical analysis
Descriptive statistics were presented overall and by age group as follows: means and standard deviations for continuous variables and frequencies for the categorical variables (Table 1). Medians and quartiles 1 and 3 are also presented in Supplemental Table 3. Chair stand time and MVPA were log-transformed for the analysis. For each outcome (gait speed, chair stand time, and sex-specific handgrip strength) and each of the following predictors (SED, MVPA, and steps), two sets of linear regression models were performed. The first set was adjusted for age, sex, cohort, season, wear time and residence in New England (vs. elsewhere). The second set was additionally adjusted for BMI, current smoking, hypertension, diabetes mellitus, CVD, and cancer. For the MVPA and SED models, a third model was added, additionally adjusting SED models for MVPA and MVPA models for SED to test which PA component was the more predominant factor related to physical performance. Each set of models was repeated stratifying by age group (50–64, 65–74, and ≥75 years). Partial Pearson correlation coefficients between the physical activity variables adjusting for age, sex, cohort, season, wear time and residence in New England were also presented. For primary analyses, an alpha value of 0.05 was our threshold for statistical significance. For the interactions, alpha of 0.10 was set as the threshold.
Table 1.
Study sample characteristics.
| Overall (n = 1352) |
Age 50–64 (n = 406) |
Age 65–74 (n = 662) |
Age ≥ 75 (n = 284) |
|
|---|---|---|---|---|
| Age, years | 68.6 ± 7.5 | 60.0 ± 3.4 | 69.2 ± 2.8 | 79.2 ± 3.7 |
| Women, % | 54 | 55 | 54 | 52 |
| Body mass index, kg/m2 | 27.6 ± 4.6 | 27.7 ± 5.1 | 27.6 ± 4.5 | 27.1 ± 4.1 |
| Current smoking, % | 5 | 7 | 5 | 2 |
| Hypertension, % | 56 | 42 | 58 | 71 |
| Diabetes, % | 11 | 7 | 12 | 14 |
| Cardiovascular disease, % | 12 | 6 | 12 | 19 |
| Cancer, % | 13 | 7 | 12 | 24 |
| Physical activity variables | ||||
| Wear time, min/day during 6 am-10 pm, a 16 h day |
749 ± 71 | 761 ± 69 | 747 ± 72 | 737 ± 71 |
| Sedentary time, % wear time (standardized to a 16 h day, h) |
84.3 ± 6.3 (13.5 ± 1.0) |
82.1 ± 6.2 (13.1 ± 1.0) |
84.1 ± 6.0 (13.5 ± 1.0) |
87.8 ± 5.4 (14.0 ± 0.9) |
| Light intensity PA, % wear time (standardized to a 16 h day, h) |
13.3 ± 5 (2.1 ± 0.8) |
14.7 ± 4.9 (2.4 ± 0.8) |
13.6 ± 5.0 (2.2 ± 0.8) |
10.8 ± 4.5 (1.7 ± 0.7) |
| Steps/day | 6927 ± 3678 | 7952 ± 3636 | 6877 ± 3625 | 5577 ± 3410 |
| MVPA time, min/day Mean ± SD and (min, max) |
19 ± 22 (0, 254) |
25 ± 25 (0, 254) |
18 ± 20 (0, 173) |
12 ± 17 (0, 96) |
| Achieved MVPA guidelines (≥150 min/week), % | 27 | 38 | 25 | 15 |
| % with < 5 min MVPA per day | 27 | 10 | 27 | 50 |
| Physical performance variables | ||||
| Gait speed, m/s Mean ± SD and (min, max) |
1.17 ± 0.19 (0.47, 1.84) | 1.23 ± 0.18 (0.47, 1.84) |
1.18 ± 0.18 (0.67, 1.75) |
1.08 ± 0.17 (0.71, 1.59) |
| Chair stands time, s Mean ± SD and (min, max) |
9.9 ± 2.6 (4.4, 29.8) |
9.3 ± 2.5 (4.6, 29.8) |
9.8 ± 2.4 (4.4, 19.5) |
11.2 ± 3.0 (5.1, 25.3) |
| Hand grip strength for men, kg Mean ± SD and (min, max) |
39.1 ± 8.7 (16, 68) |
42.9 ± 9.2 (19, 68) |
39.2 ± 7.8 (16, 59) |
33.8 ± 6.8 (16, 54) |
| Hand grip strength for women, kg Mean ± SD and (min, max) |
23.3 ± 5.7 (6, 44) |
26.1 ± 5.3 (12, 39) |
22.9 ± 5.2 (6, 44) |
20.0 ± 5.1 (10, 34) |
Abbreviations: physical activity (PA); moderate-to-vigorous physical activity (MVPA); kilograms (kg); meters (m); seconds (s); minutes (min).
3. Results
Only 38% of adults aged 50–64 years achieved the PA Guidelines of performing 150 min of MVPA per week (calculated using the average MVPA min/day multiplied by 7). Guideline achievement dropped to 15% in participants ≥75 years (Table 1). Approximately 50% of participants ≥75 years achieved < 5 min of MVPA per day (Table 1 and Fig. 1). In data standardized to a 16-h day, average SED increased across our age categories by almost one hour due to decreases in MPVA (by ~13 min/day) and light intensity PA (by ~42 min/day). Average gait speed decreased from 1.23 to 1.08 m/s across age groups. The percent of participants in each age group achieving PA and physical performance categories are displayed in Fig. 1 and Supplemental Fig. 1. By comparison, for participants that were excluded from the main results of this investigation due to self-reported mobility disability (mean age 73.7 ± 8.3 years), only 2% achieved the PA Guidelines, 80% did not even achieve 5 min MVPA/day, and physical performance of the lower body (gait speed and chair stand time) were poorer than those included in the investigation (Supplemental Table 1).
Fig. 1.
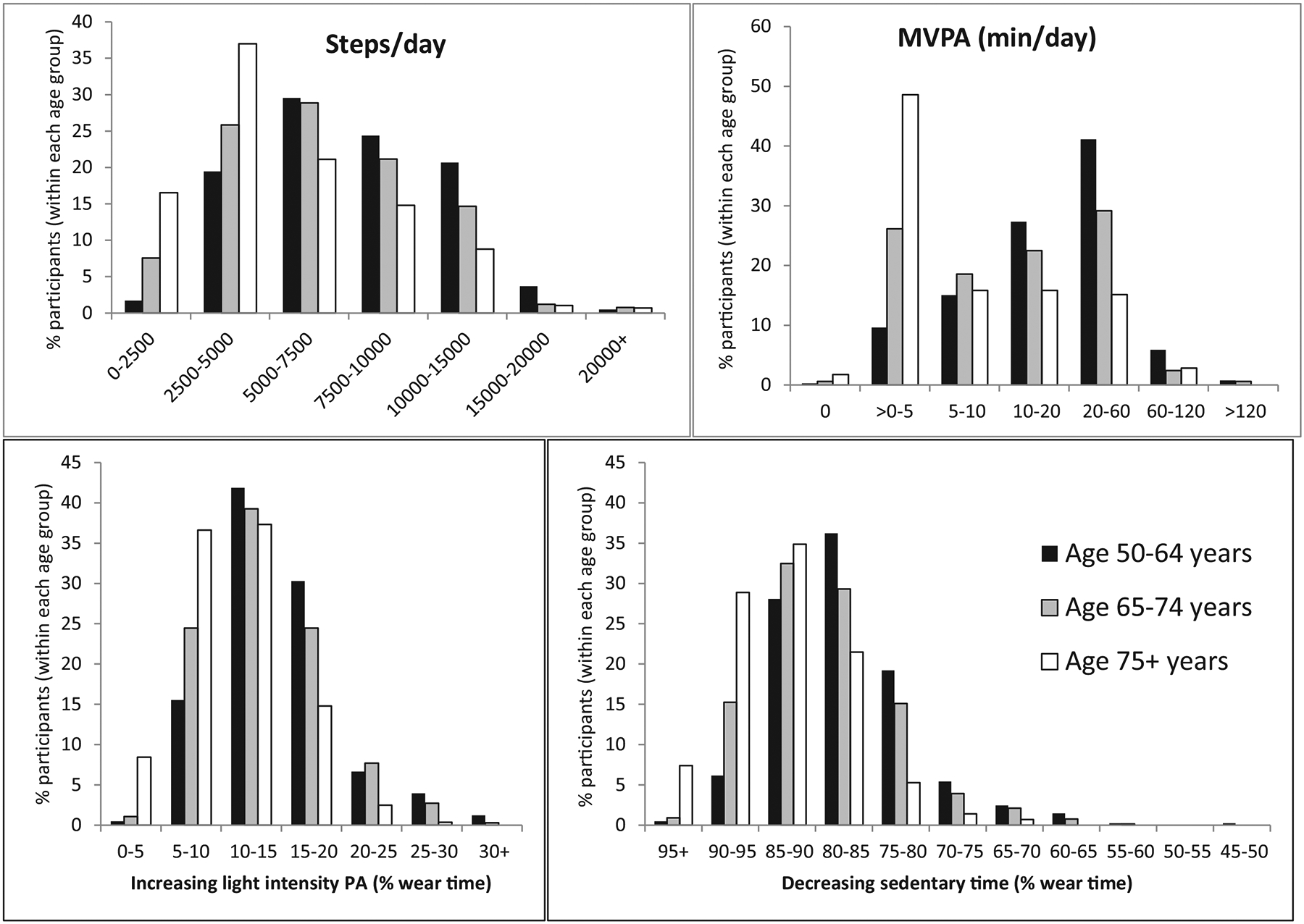
Percent of participants in each age group achieving physical activity categories.
Abbreviations: physical activity (PA); moderate-to-vigorous physical activity (MVPA); minutes (min).
In participants without mobility disability, MVPA was associated with a higher gait speed, lower time to complete five chair-stands (β = 0.041 ± 0.006 and β = −0.044 ± 0.008, both p < 0.0001, Table 2) and higher handgrip strength in men (β = 1.25 ± 0.42, p = 0.003) and women (β = 1.03 ± 0.26, p < 0.0001) in multi-variable models including adjustment for SED. No statistical interactions by age group were detected for the association of MVPA with any physical performance variable. Similarly, the number of steps taken per day was also associated with better performance on the chair-stand task (lower time) in the full sample (β = −0.007 ± 0.002, p = 0.0002, Table 3), without evidence of a statistical age interaction.
Table 2.
Relations of MVPA to physical performance measures.
| Physical activity variable | Model | Gait speed (m/s) | ln(Chair Stand) | Handgrip strength (men) | Handgrip strength (women) | ||||
|---|---|---|---|---|---|---|---|---|---|
| B est. ± SE | p | B est. ± SE | p | B est. ± SE | p | B est. ± SE | p | ||
| Log MVPA | 1 | 0.048 ± 0.005 | < 0.0001 | −0.057 ± 0.006 | < 0.0001 | 0.58 ± 0.34 | 0.090 | 0.64 ± 0.19 | 0.0008 |
| 2 | 0.041 ± 0.005 | < 0.0001 | −0.047 ± 0.007 | < 0.0001 | 0.56 ± 0.37 | 0.125 | 0.81 ± 0.20 | < 0.0001 | |
| 2 + SED(%) | 0.041 ± 0.006 | < 0.0001 | −0.044 ± 0.008 | < 0.0001 | 1.25 ± 0.42 | 0.003 | 1.03 ± 0.26 | < 0.0001 | |
| INT × age | 0.783 | 0.155 | 0.866 | 0.289 | |||||
| MVPA (< 5 min vs. ≥ 5 min/day) | 2 + SED(%) | 0.062 ± 0.013 | < 0.0001 | −0.079 ± 0.017 | < 0.0001 | 1.51 ± 0.91 | 0.098 | 2.05 ± 0.50 | < 0.0001 |
Abbreviations: moderate to vigorous physical activity (MVPA); sedentary time as a percent of wear time (SED [%]); standard error (SE); interaction (INT).
The following variables were natural log transformed: chair stand time and MVPA. Beta estimate (B est.) is in units of physical performance variable per physical activity variable difference. For the outcomes gait speed and handgrip strength, higher is better. For chair stand time, lower is better.
Model 1: adjusted for age, sex, cohort, wear time, season of physical activity monitor worn, residence in New England or other.
Model 2 (in addition to Model 1 adjustments): body mass index, diabetes, cardiovascular disease, hypertension, current smoking, cancer.
Interaction significance was tested only for model 1. Significant p-values were bolded for significance (p < 0.05 for regression and p < 0.1 for interaction analysis).
Table 3.
Relations of physical activity to physical performance, by age group.
| Phys.Perf. | Physical activity variable | Model | Total sample | P for INT by age | Age 50–64 (n = 408) |
Age 65–74 (n = 665) |
Age ≥ 75 (n = 287) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B est. ± SE | p | B est. ± SE | p | B est. ± SE | p | B est. ± SE | p | ||||
| Gait speed (m/s) | Steps (1000 steps) | 1 | 0.006 ± 0.001 | 0.0001 | 0.032 | 0.004 ± 0.003 | 0.148 | 0.004 ± 0.002 | 0.037 | 0.011 ± 0.003 | 0.0005 |
| 2 | 0.003 ± 0.001 | 0.018 | – | 0.001 ± 0.002 | 0.612 | 0.002 ± 0.002 | 0.359 | 0.009 ± 0.003 | 0.006 | ||
| SED | 1 | −0.005 ± 0.001 | < 0.0001 | 0.027 | −0.003 ± 0.001 | 0.031 | −0.004 ± 0.001 | 0.002 | −0.008 ± 0.002 | < 0.0001 | |
| (1% or ~10 min) | 2 | −0.004 ± 0.001 | < 0.0001 | – | −0.002 ± 0.001 | 0.127 | −0.003 ± 0.001 | 0.021 | −0.007 ± 0.002 | 0.0003 | |
| 2 + MVPA | 0.0002 ± 0.001 | 0.876 | – | 0.001 ± 0.002 | 0.518 | 0.001 ± 0.001 | 0.505 | −0.002 ± 0.002 | 0.327 | ||
| Chair stand time (log) | Steps (1000 steps) | 1 | −0.010 ± 0.002 | < 0.0001 | 0.266 | – | – | – | – | – | – |
| 2 | −0.007 ± 0.002 | 0.0002 | – | – | – | – | – | – | – | ||
| SED | 1 | 0.006 ± 0.001 | < 0.0001 | 0.071 | 0.005 ± 0.002 | 0.011 | 0.005 ± 0.002 | 0.004 | 0.010 ± 0.003 | 0.0004 | |
| (1% or ~10 min) | 2 | 0.005 ± 0.001 | < 0.0001 | – | 0.003 ± 0.002 | 0.147 | 0.004 ± 0.002 | 0.013 | 0.009 ± 0.003 | 0.002 | |
| 2 + MVPA | 0.001 ± 0.001 | 0.596 | – | −0.002 ± 0.002 | 0.406 | 0.0003 ± 0.002 | 0.861 | 0.005 ± 0.004 | 0.221 | ||
| Handgrip (kg) men | Steps (1000 steps) | 1 | −0.16 ± 0.09 | 0.077 | 0.016 | −0.54 ± 0.19 | 0.004 | −0.07 ± 0.13 | 0.570 | 0.22 ± 0.16 | 0.163 |
| 2 | −0.19 ± 0.09 | 0.043 | −0.54 ± 0.20 | 0.007 | −0.14 ± 0.13 | 0.302 | 0.20 ± 0.16 | 0.223 | |||
| SED | 1 | 0.09 ± 0.05 | 0.088 | 0.034 | 0.27 ± 0.10 | 0.010 | −0.01 ± 0.07 | 0.844 | 0.08 ± 0.10 | 0.444 | |
| (1% or ~10 min) | 2 | 0.10 ± 0.05 | 0.049 | – | 0.26 ± 0.11 | 0.019 | 0.01 ± 0.07 | 0.861 | 0.11 ± 0.11 | 0.280 | |
| 2 + MVPA | 0.19 ± 0.06 | 0.001 | – | 0.41 ± 0.12 | 0.0008 | 0.04 ± 0.09 | 0.671 | 0.30 ± 0.12 | 0.013 | ||
| Handgrip (kg) women | Steps (1000 steps) | 1 | 0.09 ± 0.06 | 0.125 | 0.711 | – | – | – | – | – | – |
| 2 | 0.11 ± 0.06 | 0.078 | – | – | – | – | – | – | |||
| SED | 1 | −0.05 ± 0.04 | 0.133 | 0.025 | −0.03 ± 0.06 | 0.682 | −0.001 ± 0.05 | 0.992 | −0.25 ± 0.09 | 0.004 | |
| (1% or ~10 min) | 2 | −0.05 ± 0.04 | 0.147 | – | −0.01 ± 0.06 | 0.883 | 0.02 ± 0.05 | 0.775 | −0.27 ± 0.09 | 0.003 | |
| 2 + MVPA | 0.06 ± 0.05 | 0.165 | – | 0.06 ± 0.08 | 0.421 | 0.01 ± 0.07 | 0.053 | −0.14 ± 0.12 | 0.233 | ||
Abbreviations: Physical performance measure (Phys. Perf.); moderate to vigorous physical activity (MVPA); sedentary time as a percent of wear time (SED [%]); standard error (SE); interaction (INT).
The following variables were log-transformed: chair stand time and MVPA. Beta estimate (B est.) is in units of physical performance variable per physical activity variable difference. For the outcomes gait speed and handgrip strength, higher is better. For chair stand time, lower is better.
Model 1: adjusted for age, sex, cohort, wear time, season of physical activity monitor worn, residence in New England or other.
Model 2 (in addition to Model 1 adjustments): body mass index, diabetes, cardiovascular disease, hypertension, current smoking, cancer.
Interaction significance was tested only for model 1. Significant p-values were bolded for significance (p < 0.05 for regression and p < 0.1 for interaction analysis).
Interactions by age were observed for the relations of steps and SED to many of the physical performance measures (p < 0.10, Table 3). Higher total activity (measured by steps/day) and lower SED were associated with higher gait speed in participants ≥75 years (β = 0.009 ± 0.003, p = 0.006; β = −0.007 ± 0.002, p = 0.0003), but the relation with SED was no longer significant after adjusting for MVPA (β = −0.002 ± 0.002, p = 0.327). Similarly, associations of SED with worse performance on chair stand (higher time) and handgrip strength (in women only), were no longer significant after adjustment for MVPA (p > 0.20). In contrast, in men, there were unexpected relations of lower steps and higher SED to higher handgrip strength in middle age (50–64 years: β = −0.54 ± 0.20, p = 0.007; β = 0.420 ± 0.123, p = 0.0008) and in older age (≥75 years: β = 0.303 ± 0.120, p = 0.013).
Individuals achieving at least 5 min/day of MVPA have 0.062 ± 0.013 m/s greater gait speed than those with < 5 min/day of MVPA (p < 0.0001) across middle and older age (Fig. 2 and Table 2). For adults ≥75 years, the magnitude of the association for every 5000 more steps related to approximately 0.045 m/s higher gait speed (Table 3 displays β for every 1000 steps), which was slightly smaller than the magnitude of the association of achieving ≥5 min/day MVPA. Similarly, for participants ≥75 years old, the relation of SED to gait speed was equivalent to ~0.04 m/s for every hour less SED per day (~0.007 m/s for every 10 min less SED), but after adjusting for MVPA these relations were no longer significant. Fig. 2 also demonstrates that ≥5 min/day MVPA is associated with approximately ~1 s lower chair stand time, better performance than achieving < 5 min/day MVPA (after reversing the log-transformation on chair stand time, Table 2, p < 0.0001).
Fig. 2.
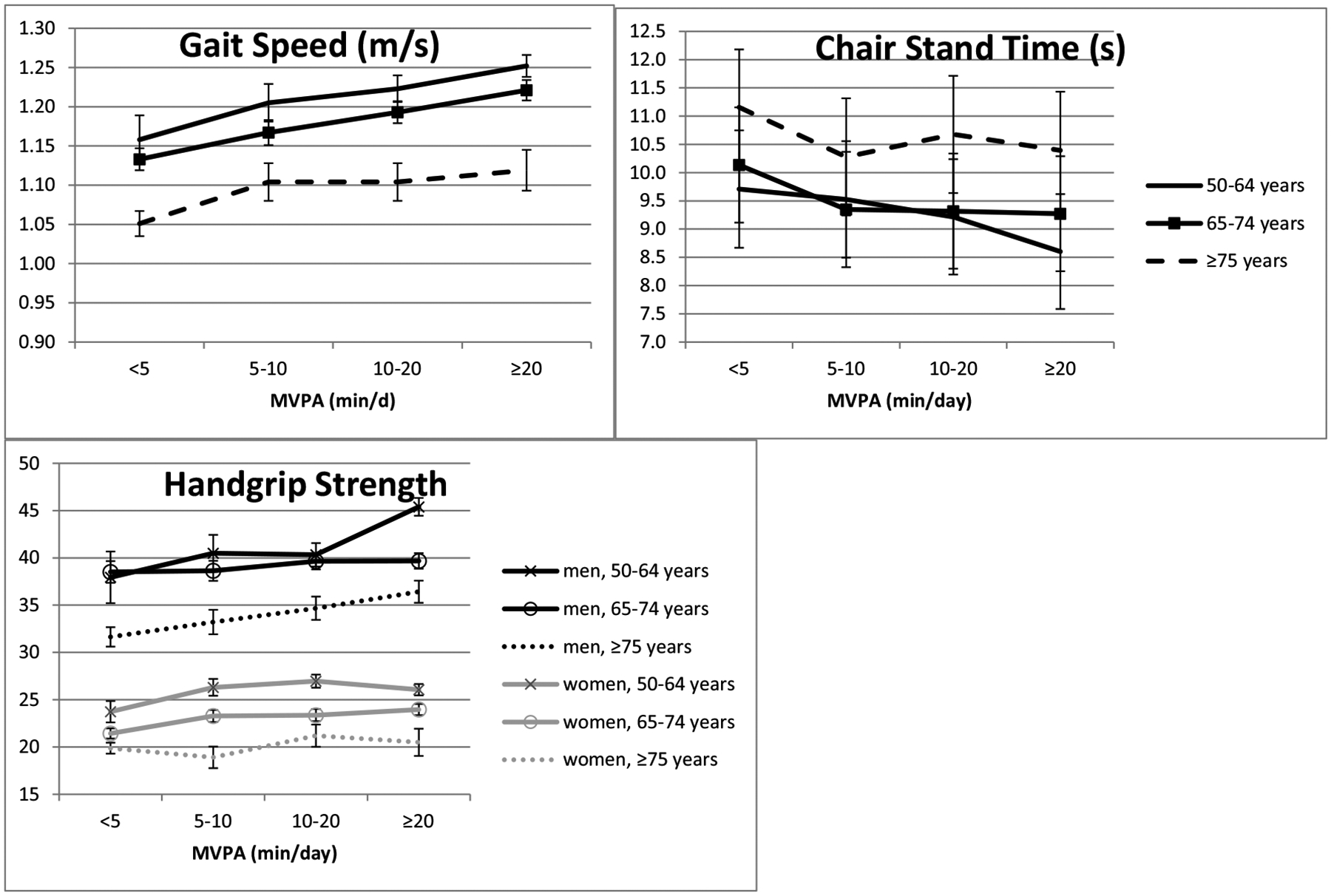
Adjusted means of physical performance measures by categories of MVPA and age group.
Abbreviations: moderate to vigorous physical activity (MVPA). Adjustment model: age, sex, cohort, wear time, season of physical activity monitor worn, residence in New England or other, body mass index, diabetes, cardiovascular disease, hypertension, current smoking, cancer, %SED.
4. Discussion
Our community-based study of middle-aged and older adults free of mobility disability demonstrates several important findings with respect to PA and physical function. First, both total PA (step accumulation) and MVPA were related to physical performance of the lower body, including gait speed and the chair stand task. For the association of total PA with gait speed, significance was only observed in persons ≥75 years. MVPA was also positively associated with handgrip strength. Second, we observed associations of SED with physical performance of the lower body, which did not remain significant after adjustment for MVPA. The 2018 PA Guidelines (2nd edition) continue to focus on achievement of MVPA goals for adults (≥150 min MVPA/week or ≥ 21.4 min MVPA/day), young and old alike, based on a consensus from the literature and experts (US Department of Health and Human Services, 2018). But these new guidelines now also include advice for achieving incremental PA, stating that “even 5 minutes of PA has real health benefits.” (US Department of Health and Human Services, 2018) The data we present supports these current recommendations, identifying associations of the total volume of PA, measured by step count, and much lower MVPA levels (achieving just 5 min MVPA per day) with physical performance. Our cross-sectional data suggests that even incremental differences in PA volume and intensity may play a role in physical function, and vice versa.
We reported that the relative importance of achieving small amounts of MVPA or taking more steps appears to be more prominent than achieving a favorable balance in the proportion of time spent in SED in terms of the association with gait speed. To put our results into context, previous studies have identified that a PA intervention can improve gait speed (Espeland et al., 2017) and that small changes in gait speed (0.1 m/s increments) were associated with better survival (Studenski et al., 2011). Small differences in accelerometer-determined light intensity activity and MVPA were also related to lower mortality rates in 3-year follow-up analysis in the Women’s Health Initiative (LaMonte et al., 2018). Furthermore, change in gait speed at even smaller increments (0.03–0.05 m/s) have been estimated to be clinically meaningful in subjective self-assessments (Kwon et al., 2009). In our investigation, achieving at least 5 min/day of MVPA was associated with 0.062 m/s greater gait speed, across the age ranges. For older adults (≥75 years), every 5000 more steps/day achieved was related to approximately 0.045 m/s greater gait speed. Because our population of adults ≥75 years take only 5577 steps/day on average, doubling one’s total PA to make a modest clinical impact on gait speed may not be an attainable goal.
Although the size of the association with SED appeared robust for participants ≥75 years old (~0.04 m/s for every hour less SED), after adjusting for MVPA these relations were no longer significant. However, it is important to remember that we have only presented data from participants not reporting mobility disability and may have higher physical function. Our goal was to limit the effect of reverse causation by participants with severe mobility disability, although reverse causation likely still does play some role in our findings. More research is needed with data from longitudinal design to further understand the role of reverse causality. Previous literature suggests that SED is strongly related to functional/mobility disability (Dunlop et al., 2015; Gennuso et al., 2013), which we confirmed in the current investigation. We observed higher SED and much lower PA in participants with mobility disability (excluded from our main investigation, but presented in supplemental materials). We were interested in assessing associations of PA and SED with physical performance at a time before participants developed mobility disability.
Recent studies have identified an association of SED with gait speed (Rosenberg et al., 2016) and other measures of physical function (Santos et al., 2012; Rosenberg et al., 2016), which remained after adjusting for MVPA. Although investigators in these studies adjusted for wear time rather than index SED to wear time as in our investigation, neither previous studies nor the current study are able to account for residual confounding by wear time. It is clear that wear time has a major influence on total SED (Keevil et al., 2016). Additionally, the study that observed an association of SED with gait speed was in much older adults (mean age 84 years) living in retirement communities with low levels of MVPA (mean 8.7 min/day) and very poor performance on gait speed task (mean 0.83 m/s) (Rosenberg et al., 2016). Therefore, although a large proportion of our oldest sample had low MVPA (i.e., 50% of adults our sample ≥ 75 years had < 5 min/day MVPA), our full investigation is not completely comparable to the retirement communities study. Instead, our results are in agreement with a separate investigation in a British cohort by Keevil et al., demonstrating that the association of the proportion of time spent in SED relates to poorer gait speed, but in interaction analyses they observed an association of SED with gait speed, chair stand time, and handgrip strength in those achieving < 19 min MVPA/day (Keevil et al., 2016). We must also recognize that individuals with mobility disability were not excluded from this study, but were excluded from our study, possibly contributing to slightly different results observed in our investigation. An important future direction may be to understand the relation of SED to physical performance in participants with low MVPA.
We also reported an unexpected result that we are unable to explain, which was that lower total step count and higher SED related to higher handgrip strength in middle-aged men. One explanation may stem from the known association of BMI with both higher handgrip strength and higher SED (Bann et al., 2015; Fried et al., 2001). Therefore, although we adjusted for BMI, our observations could be a result of residual confounding by body composition or due to weight lifting or other non-ambulatory activities that are not assessed by accelerometry. Other studies similar to ours did not observe these significant associations in their study samples, but also did not test for interactions by age group (Keevil et al., 2016; Bann et al., 2015). We may have identified a novel association of SED with handgrip strength in middle-aged men, which could theoretically be due to behavioral or demographic determinants of SED, possibly including the sedentary nature of certain occupations or other hobbies/interests. These relationships require further investigation.
It is clear that there is a shift toward a less active and a more SED lifestyle in older age but more studies are needed to understand whether the pattern and duration of SED has an impact on healthy aging and physical function. It will also be important to understand potential causes of increased SED behavior in older age that may be unrelated to mobility, such as changes in employment status and social behavior. Furthermore, our decision to standardize wear time to a 16 h day may introduce some bias because SED may not be equally distributed throughout the day. However, it was important to standardize wear time because of the strong correlation of SED with device wear time that we reported in the supplemental material. We must also acknowledge the potential influence of selection bias (including survival bias), which limits our analysis to individuals who have survived and agreed to participate in the ninth Offspring examination. Individuals that participate in accelerometry studies are typically more healthy than those who opt out (Spartano et al., 2017).
The current investigation was a cross-sectional observational study and, therefore, precludes inferences of causality or temporality. The interpretation that most likely explains our results is a bi-directional association, in which declining PA and physical function both have causal pathways that impact one another (Keevil et al., 2016). While the large sample size allowed us to account for covariates, others, such as chronic obstructive pulmonary disease, arthritis, and other pain, may account for some of the observed associations. This study may also lack generalizability to individuals of non-European ancestry. Individuals of non-European ancestry are included in the Omni cohort, but these numbers are too small to analyze separately.
It is also important to mention that the ranges of steps we observed in our study were higher than study samples of similar ages (Tudor-Locke et al., 2013). Average reported steps/day for the National Health and Nutrition Examination Survey (NHANES, 2005–2006) were consistently > 2500 steps/day lower for each age group (NHANES ages 60–64 years: 5444 steps/day; age 65–74 years: 4030 steps/day; ages 75–79: 2519 steps/day; ages 80–84 years: 1928 steps/day) (Tudor-Locke et al., 2013). NHANES and other studies have used different data censoring methods (Tudor-Locke et al., 2013; Tudor-Locke et al., 2009; Chomistek et al., 2017) and different accelerometer devices, which have different sensitivity to very low walking speeds (low frequency movement) (Feito et al., 2012); thus, influencing absolute levels of step accumulation, which should, therefore, be considered cautiously. Actical devices have been demonstrated to be more sensitive to low frequency movement than other popular accelerometers, recording a higher percentage of steps accumulated at slower walking speeds (Feito et al., 2012). Therefore, Actical devices may be more suited to older adults with slower walking speeds. Notably, our average steps/day were similar to those reported by the Physical Performance Across the Life-span Study (Hall et al., 2017).
It is also difficult to compare physical function across different large studies due to differences in testing protocols (Rikli and Jones, 1999). Comparative physical performance measures were similar or slightly faster for gait speed and chair stand time compared to other reported reference values by age group including NHANES (Hall et al., 2017; Steffen et al., 2002; Ostchega et al., 2000), but our study sample had lower hand grip strength than other reference groups (Yoshimura et al., 2011).
The PA Guidelines for Americans have historically focused predominantly on MVPA (Physical Activity Guidelines Advisory Committee, 2008; US Department of Health and Human Services, 2018). Among Framingham Offspring Study participants ≥75 years old, only 15% were meeting the PA Guidelines and 50% were not even performing an average of 5 min of MVPA per day. If we focus only on MVPA, it appears that older Americans are inactive, a behavior which is associated with increased mortality rates (Koster et al., 2012). However, it is possible that the assessment of PA intensity in older adults requires more sensitive investigation. MVPA is defined as any PA performed at a work rate ≥3 metabolic equivalents (METs) and is most commonly defined as activity above the 1400–2500 cpm accelerometer cutpoint in young and middle aged adults (Glazer et al., 2013; Crouter and Bassett Jr., 2008; Heil, 2006; Freedson et al., 1998). In contrast, older adults often have lower fitness and may achieve 3 METs of work at much lower counts of accelerometer movement (Ainsworth et al., 2000; Corbett et al., 2016). Therefore, PA performed at a lower accelerometer count threshold, may be more appropriate to determine the proportion of older adults meeting PA MET requirements (Martin et al., 2014; Gorman et al., 2014; Schrack et al., 2018). This is an active area of research and other groups are exploring different cutpoints (LaMonte et al., 2018), but for the current study we chose to keep a consistent MVPA count threshold across all age groups to test associations related to different intensities of movement rather than modifying cutpoints that would define MVPA for each age group. Instead, our analysis of total step count may provide a less biased measure that accumulates total PA regardless of intensity.
5. Conclusions
Our cross-sectional study across middle and older ages demonstrated consistent associations of higher MVPA, even just 5 min/day MVPA, with better physical performance and some association of total step accumulation with physical performance. This result is in agreement with the new PA Guidelines which promote the concept that some PA is better than none. Associations of SED with physical performance of the lower body did not remain significant after adjustment for MVPA. These data warrant future research on the influence of different intensities of PA on physical function and health outcomes in the elderly.
Supplementary Material
Funding
The work was supported by funding from the National Institutes of Health (NIH) (N01-HC25195, HHSN268201500001I; R01-AG047645; R01-HL131029; R56-AG029451); and American Heart Association (15GPSGC24800006 and 16MCPRP30310001). Dr. Vasan is supported in part by the Evans Medical foundation and the Jay and Louis Coffman Endowment, Department of Medicine, BUSM.
Abbreviations:
- min
minutes
- PA
physical activity
- MVPA
moderate to vigorous PA
- SED
sedentary time
- kg
kilograms
- BMI
body mass index
- CVD
cardiovascular disease
- NHANES
National Health and Nutrition Examination Survey
- METs
Metabolic equivalents
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.exger.2019.02.003.
References
- Ainsworth BE, Haskell WL, Whitt MC, et al. , 2000. Compendium of physical activities: an update of activity codes and MET intensities. Med. Sci. Sports Exerc 32 (9 Suppl), S498–S504 (Epub 2000/09/19.). [DOI] [PubMed] [Google Scholar]
- Bann D, Hire D, Manini T, et al. , 2015. Light Intensity physical activity and sedentary behavior in relation to body mass index and grip strength in older adults: cross-sectional findings from the Lifestyle Interventions and Independence for Elders (LIFE) study. PLoS One 10 (2), e0116058 (Epub 2015/02/04. doi: 10.1371/journal.-pone.0116058.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bey L, Hamilton MT, 2003. Suppression of skeletal muscle lipoprotein lipase activity during physical inactivity: a molecular reason to maintain daily low-intensity activity. J Physiol. 551 (Pt 2), 673–682 (Epub 2003/06/20. doi: 10.1113/jphysiol.2003.045591.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buman MP, Hekler EB, Haskell WL, et al. , 2010. Objective light-intensity physical activity associations with rated health in older adults. Am J Epidemiol. 172 (10), 1155–1165 (Epub 2010/09/17. doi: 10.1093/aje/kwq249.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomistek AK, Yuan C, Matthews CE, et al. , 2017. Physical activity assessment with the actigraph gt3x and doubly labeled water. Med. Sci. Sports Exerc 49 (9), 1935–1944 (Epub 2017/04/19. doi: 10.1249/mss.0000000000001299.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett DB, Valiani V, Knaggs JD, Manini TM, 2016. Evaluating walking intensity with hip-worn accelerometers in elders. Med. Sci. Sports Exerc 48 (11), 2216–2221 (Epub 2016/10/19. doi: 10.1249/mss.0000000000001018.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouter SE, Bassett DR Jr., 2008. A new 2-regression model for the Actical accelerometer. Br. J. Sports Med 42 (3), 217–224 (Epub 2007/09/01. doi: 10.1136/bjsm.2006.033399.). [DOI] [PubMed] [Google Scholar]
- Cummings SR, Studenski S, Ferrucci L, 2014. A diagnosis of dismobility–giving mobility clinical visibility: a Mobility Working Group recommendation. JAMA 311 (20), 2061–2062 (Epub 2014/04/26. doi: 10.1001/jama.2014.3033.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrn IM, Sjostrom M, Kwak L, Oja P, Hagstromer M, 2018. Accelerometer-measured sedentary time and physical activity-a 15 year follow-up of mortality in a Swedish population-based cohort. Aust. J. Sci. Med. Sport 21 (7), 702–707 (Epub 2017/11/13. doi: 10.1016/j.jsams.2017.10.035.). [DOI] [PubMed] [Google Scholar]
- Dunlop DD, Song J, Arnston EK, et al. , 2015. Sedentary time in US older adults associated with disability in activities of daily living independent of physical activity. J. Phys. Act. Health 12 (1), 93–101 (Epub 2014/02/11. doi: 10.1123/jpah.2013-0311.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esliger DW, Probert A, Connor Gorber S, Bryan S, Laviolette M, Tremblay MS, 2007. Validity of the Actical accelerometer step-count function. Med. Sci. Sports Exerc 39 (7), 1200–1204. 10.1249/mss.0b013e3804ec4e9. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Lipska K, Miller ME, et al. , 2017. Effects of physical activity intervention on physical and cognitive function in sedentary adults with and without diabetes. J. Gerontol. A Biol. Sci. Med. Sci 72 (6), 861–866 (Epub 2016/09/04. doi: 10.1093/gerona/glw179.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feito Y, Bassett DR, Thompson DL, Tyo BM, 2012. Effects of body mass index on step count accuracy of physical activity monitors. J. Phys. Act. Health 9 (4), 594–600 (Epub 2011/09/29.). [DOI] [PubMed] [Google Scholar]
- Freedson PS, Melanson E, Sirard J, 1998. Calibration of the computer science and applications, Inc. accelerometer. Med. Sci. Sports Exerc 30 (5), 777–781 (Epub 1998/05/20.). [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, et al. , 2001. Frailty in older adults: evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci 56 (3), M146–M156 (Epub 2001/03/17.). [DOI] [PubMed] [Google Scholar]
- Gennuso KP, Gangnon RE, Matthews CE, Thraen-Borowski KM, Colbert LH, 2013. Sedentary behavior, physical activity, and markers of health in older adults. Med. Sci. Sports Exerc 45 (8), 1493–1500 (Epub 2013/03/12. doi: 10.1249/MSS.0b013e318288a1e5.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennuso KP, Thraen-Borowski KM, Gangnon RE, Colbert LH, 2016. Patterns of sedentary behavior and physical function in older adults. Aging Clin. Exp. Res 28 (5), 943–950 (Epub 2015/05/30. doi: 10.1007/s40520-015-0386-4.). [DOI] [PubMed] [Google Scholar]
- Glazer NL, Lyass A, Esliger DW, et al. , 2013. Sustained and shorter bouts of physical activity are related to cardiovascular health. Med. Sci. Sports Exerc 45 (1), 109–115 (doi: 10.1249/MSS.0b013e31826beae5.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman E, Hanson HM, Yang PH, Khan KM, Liu-Ambrose T, Ashe MC, 2014. Accelerometry analysis of physical activity and sedentary behavior in older adults: a systematic review and data analysis. Eur Rev Aging Phys Act. 11, 35–49. 10.1007/s11556-013-0132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik JM, Ferrucci L, Pieper CF, et al. , 2000. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J. Gerontol. A Biol. Sci. Med. Sci 55 (4), M221–M231 (Epub 2000/05/16.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall KS, Cohen HJ, Pieper CF, et al. , 2017. Physical performance across the adult life span: correlates with age and physical activity. J Gerontol A Biol Sci Med Sci. 72 (4), 572–578 (Epub 2016/07/01. doi: 10.1093/gerona/glw120.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M, de Oliveira C, Demakakos P, 2014. Non-exercise physical activity and survival: English longitudinal study of ageing. Am. J. Prev. Med 47 (4), 452–460 (Epub 2014/07/23. doi: 10.1016/j.amepre.2014.05.044.). [DOI] [PubMed] [Google Scholar]
- Hardy SE, Kang Y, Studenski SA, Degenholtz HB, 2011. Ability to walk 1/4 mile predicts subsequent disability, mortality, and health care costs. J. Gen. Intern. Med 26 (2), 130–135 (Epub 2010/10/26. doi: 10.1007/s11606-010-1543-2.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil DP, 2006. Predicting activity energy expenditure using the Actical activity monitor. Res. Q. Exerc. Sport 77 (1), 64–80 (Epub 2006/05/02.). [DOI] [PubMed] [Google Scholar]
- Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP, 1979. An investigation of coronary heart disease in families. The Framingham offspring study. Am. J. Epidemiol 110 (3), 281–290 (Epub 1979/09/01.). [DOI] [PubMed] [Google Scholar]
- Keevil VL, Cooper AJ, Wijndaele K, et al. , 2016. Objective sedentary time, moderate-to-vigorous physical activity, and Physical capability in a British cohort. Med. Sci. Sports Exerc 48 (3), 421–429 (Epub 2015/10/27. doi: 10.1249/mss.0000000000000785.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster A, Caserotti P, Patel KV, et al. , 2012. Association of sedentary time with mortality independent of moderate to vigorous physical activity. PLoS One 7 (6), e37696. 10.1371/journal.pone.0037696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon S, Perera S, Pahor M, et al. , 2009. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study). J Nutr Health Aging 13 (6), 538–544 Epub 2009/06/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMonte MJ, Buchner DM, Rillamas-Sun E, et al. , 2018. Accelerometer-measured physical activity and mortality in women aged 63 to 99. J. Am. Geriatr. Soc 66 (5), 886–894 (Epub 2017/11/17. doi: 10.1111/jgs.15201.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques EA, Baptista F, Santos DA, Silva AM, Mota J, Sardinha LB, 2014a. Risk for losing physical independence in older adults: the role of sedentary time, light, and moderate to vigorous physical activity. Maturitas. 10.1016/j.maturitas.2014.06.012. [DOI] [PubMed] [Google Scholar]
- Marques EA, Baptista F, Santos DA, Silva AM, Mota J, Sardinha LB, 2014b. Risk for losing physical independence in older adults: the role of sedentary time, light, and moderate to vigorous physical activity. Maturitas 79 (1), 91–95 (Epub 2014/07/16. doi: 10.1016/j.maturitas.2014.06.012.). [DOI] [PubMed] [Google Scholar]
- Martin KR, Koster A, Murphy RA, et al. , 2014. Changes in daily activity patterns with age in U.S. men and women: National Health and Nutrition Examination Survey 2003–04 and 2005–06. J. Am. Geriatr. Soc 62 (7), 1263–1271. 10.1111/jgs.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murabito JM, Rong J, Lunetta KL, et al. , 2017. Cross-sectional relations of whole-blood miRNA expression levels and hand grip strength in a community sample. Aging Cell 16 (4), 888–894 (Epub 2017/06/10. doi: 10.1111/acel.12622.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostchega Y, Harris TB, Hirsch R, Parsons VL, Kington R, Katzoff M, 2000. Reliability and prevalence of physical performance examination assessing mobility and balance in older persons in the US: data from the Third National Health and Nutrition Examination Survey. J. Am. Geriatr. Soc 48 (9), 1136–1141 (Epub 2000/09/13.). [DOI] [PubMed] [Google Scholar]
- Pahor M, Guralnik JM, Ambrosius WT, et al. , 2014. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA 311 (23), 2387–2396. 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Physical Activity Guidelines Advisory Committee, 2008. Physical Activity Guidelines Advisory Committee Report, 2008. US Department of Health and Human Services, Washington, DC. [Google Scholar]
- Quan SF, Howard BV, Iber C, et al. , 1997. The sleep heart health study: design, rationale, and methods. Sleep 20 (12), 1077–1085 (Epub 1998/03/11. [PubMed] [Google Scholar]
- Rikli RE, Jones CJ, 1999. Functional fitness normative scores for community-residing older adults, ages 60–94. J. Aging Phys. Act 7 (2), 162–181. [Google Scholar]
- Riskowski JL, Hagedorn TJ, Dufour AB, Hannan MT, 2012. Functional foot symmetry and its relation to lower extremity physical performance in older adults: the Framingham Foot Study. Journal of Biomechanics 45 (10), 1796–1802 (Epub 2012/05/09. doi: 10.1016/j.jbiomech.2012.04.019.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg DE, Bellettiere J, Gardiner PA, Villarreal VN, Crist K, Kerr J, 2016. Independent associations between sedentary behaviors and mental, cognitive, physical, and functional health among older adults in retirement communities. J Gerontol A Biol Sci Med Sci. 71 (1), 78–83 (Epub 2015/08/15. doi: 10.1093/gerona/glv103.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos DA, Silva AM, Baptista F, et al. , 2012. Sedentary behavior and physical activity are independently related to functional fitness in older adults. Exp. Gerontol 47 (12), 908–912 (Epub 2012/08/14. doi: 10.1016/j.exger.2012.07.011.). [DOI] [PubMed] [Google Scholar]
- Schrack JA, Leroux A, Fleg JL, et al. , 2018. Using heart rate and accelerometry to define quantity and intensity of physical activity in older adults. J Gerontol A Biol Sci Med Sci. 73 (5), 668–675 (Epub 2018/03/07. doi: 10.1093/gerona/gly029.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services, 2018. Physical Activity Guidelines for Americans, 2nd edition. US Department of Health and Human Services, Washington, DC. [Google Scholar]
- Spartano NL, Stevenson MD, Xanthakis V, et al. , 2017. Associations of objective physical activity with insulin sensitivity and circulating adipokine profile: the Framingham Heart Study. Clinical obesity 7 (2), 59–69 (Epub 2017/01/24. doi: 10.1111/cob.12177.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen TM, Hacker TA, Mollinger L, 2002. Age- and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Phys. Ther 82 (2), 128–137 (Epub 2002/02/22.). [DOI] [PubMed] [Google Scholar]
- Studenski S, Perera S, Wallace D, et al. , 2003. Physical performance measures in the clinical setting. J. Am. Geriatr. Soc 51 (3), 314–322 (Epub 2003/02/18.). [DOI] [PubMed] [Google Scholar]
- Studenski S, Perera S, Patel K, et al. , 2011. Gait speed and survival in older adults. JAMA 305 (1), 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The State of US Health, 2013. 1990–2010: burden of diseases, injuries, and risk factors. JAMA 310 (6), 591–608 (Epub 2013/07/12. doi: 10.1001/jama.2013.13805.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JM, Welk GJ, Beyler NK, 2011. Physical activity in U.S.: adults compliance with the Physical Activity Guidelines for Americans. Am. J. Prev. Med 40 (4), 454–461 (Epub 2011/03/17. doi: 10.1016/j.amepre.2010.12.016.). [DOI] [PubMed] [Google Scholar]
- Tudor-Locke C, Johnson WD, Katzmarzyk PT, 2009. Accelerometer-determined steps per day in US adults. Med. Sci. Sports Exerc 41 (7), 1384–1391 (Epub 2009/06/12. doi: 10.1249/MSS.0b013e318199885c.). [DOI] [PubMed] [Google Scholar]
- Tudor-Locke C, Schuna JM Jr., Barreira TV, et al. , 2013. Normative steps/day values for older adults: NHANES 2005–2006. J. Gerontol. A Biol. Sci. Med. Sci 68 (11), 1426–1432 (Epub 2013/08/06. doi: 10.1093/gerona/glt116.). [DOI] [PubMed] [Google Scholar]
- Whelton PK, Carey RM, Aronow WS, et al. , 2017. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and Management of High Blood Pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J. Am. Coll. Cardiol 2017 (Epub 2017/11/18. doi: 10.1016/j.jacc.2017.11.006.). [DOI] [PubMed] [Google Scholar]
- Yoshimura N, Oka H, Muraki S, et al. , 2011. Reference values for hand grip strength, muscle mass, walking time, and one-leg standing time as indices for locomotive syndrome and associated disability: the second survey of the ROAD study. J. Orthop. Sci 16 (6), 768–777 (Epub 2011/10/07. doi: 10.1007/s00776-011-0160-1.). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


