Abstract
A mixture of well-defined recombinant antigens together with an adjuvant that preferentially stimulates specific gamma interferon (IFN-γ)-secreting helper type 1 CD4+ T cells (Th1 cells) presents a rational option for a vaccine against leishmaniasis. The potential of this approach was investigated in murine infections with Leishmania mexicana, which are characterized by the absence of a parasite-specific Th1 response and uncontrolled parasite proliferation. A mixture of three antigens (glycoprotein 63, cysteine proteinases, and a membrane-bound acid phosphatase), which are all expressed in amastigotes, the mammalian stage of the parasite, were used for the immunization of C57BL/6 mice in combination with six adjuvants (interleukin 12 [IL-12], Detox, 4′-monophosphoryl lipid A, QS-21, Mycobacterium bovis BCG, and Corynebacterium parvum). All six vaccine formulations containing the mixture of recombinant antigens were protective against challenge infections with promastigotes, the insect stage of the parasite, in that mice controlled and healed infections but developed transient and, in certain cases, accentuated disease. The most effective adjuvants were IL-12 followed by Detox. Further studies using these two adjuvants showed that a similar protective effect was observed with a mixture of the corresponding native proteins, and mice which had controlled the infection showed a preponderance of IFN-γ-secreting CD4+ T cells in the lymph nodes draining the lesion. Using the recombinant proteins individually, it is shown that the relatively abundant cysteine proteinases and glycoprotein 63, but not the acid phosphatase, are able to elicit a protective response. The results are discussed in comparison to previous studies with subunit vaccines and with respect to cell biological aspects of antigen presentation in Leishmania-infected macrophages.
The solid immunity observed following recovery from cutaneous leishmaniasis in humans has stimulated numerous attempts for the development of a prophylactic vaccination against this widespread (sub)tropical disease (12, 16, 19, 35, 36). Leishmania major, the etiological agent of Old World cutaneous leishmaniasis, has been the most popular species both in studies of murine infections and in human trials. In the Middle East, the deliberate infection with L. major was a common and effective practice for immunization against subsequent infections, but a fraction of the vaccinated persons produced lesions that required medical treatment. A vaccine based on killed promastigotes, the insect stage of the parasite, and Mycobacterium bovis BCG was recently shown to be ineffective in a controlled trial with several thousand volunteers in Iran (37). Mice have been used in a range of vaccination protocols against infection by L. major. Representative examples include: attenuated but live parasites (23, 29, 34, 48, 59); subunit vaccines delivered by live carriers such as BCG expressing the surface proteinase GP63 of L. major (11); vaccinia virus expressing the glycoprotein GP46/M-2 (20, 30); GP63 expressed in attenuated Salmonella typhimurium (72, 73); purified recombinant or native proteins formulated with an adjuvant such as LACK (Leishmania-homologue of receptors for activated C kinase) plus interleukin-12 (IL-12 [40]), PSA-2, or protein dp72 plus Corynebacterium parvum (20, 43); T-cell epitopes plus Poloxamer 407 as adjuvant (27); and vaccination with DNA encoding GP63 (63, 70, 71), LACK (17, 18), or PSA-2 (54). In general, these vaccination protocols elicited partial protection against L. major. Resistant mouse strains (e.g., C57BL/6) produced smaller, more rapidly healing lesions and susceptible strains (e.g., BALB/c) showed a reduced rate of parasite growth compared to unimmunized mice.
There are significantly fewer reports of vaccination attempts against the South American parasite, L. mexicana. Convit et al. (13, 14) successfully used killed promastigotes plus BCG for immunotherapy of patients in Venezuela. In mice, irradiated L. major amastigotes conferred protection against infection with L. mexicana (5). GP63 had a protective effect when formulated either in liposomes (50) or expressed in BCG (11). A 46-kDa membrane glycoprotein (M-2), a member of a family of proteins related to the PSA-2 family in L. major mentioned above, was used in combination with C. parvum for immunization against the related parasite L. amazonensis (10). In a recent study, infection of C57BL/6 mice with an attenuated strain lacking cysteine proteinases showed partial protection against subsequent challenge infection with wild-type L. mexicana (6). We have performed similar experiments with a deletion mutant for a mitogen-activated kinase homologue which is essential for growth of amastigotes (65). Mice abortively infected with promastigotes of this mutant were not protected against subsequent infection with the wild type (M. Wiese, unpublished results). This disappointing result may indicate that genetically attenuated, live parasites will not be of use as a vaccine against New World cutaneous leishmaniasis.
The development of protective immunity to L. major in resistant mouse strains is dependent on the ability to mount an IL-12-driven CD4+ type 1 helper T-cell (Th1) response (46). Infections with parasites of the L. mexicana complex are characterized by a strongly reduced ability to mount a Th1 response (4). Most mouse strains develop progressive, nonhealing lesions that may metastasize to the extremities or the viscera (49). In C57BL/6 mice this phenotype appears to be dependent on the production of IL-4, since IL-4-deficient mice develop no lesions at all when infected with L. mexicana amastigotes (52) or transient, self-healing lesions when infected with promastigotes (P. Overath, D. Harbecke, and W. Müller, unpublished data). Therefore, the development of a subunit vaccine against L. mexicana provides a particular challenge. One must not only find protective antigens but also adjuvants capable of eliciting a type 1-dominated T-cell response.
The present study describes vaccination experiments against murine infections with L. mexicana designed on the basis of two criteria. First, the antigens used should be known to be expressed in the disease-causing mammalian stage of the parasite, namely, the amastigote. We chose three amastigote proteins and used these either singly or in combination, as well as both in E. coli-expressed recombinant and in native form. Second, the adjuvants selected for the immunizations should elicit a cellular, Th1-biased immune response and should, in principle, be applicable for use in humans.
MATERIALS AND METHODS
Animals and parasites.
Specific-pathogen-free female C57BL/6 mice were purchased from Charles River (Sulzfeld, Germany), maintained in the animal facility of the institute, and used at 8 to 16 weeks of age. L. mexicana subsp. mexicana MNYC/BZ/62/M379 promastigotes were cultivated as described by Menz et al. (33) or obtained as amastigotes from lesions of infected BALB/c or CBA mice (24).
Adjuvants.
Murine recombinant IL-12 was kindly provided by M. Gateley, Hoffman-La Roche, Inc., Nutley, N.J. Live BCG (ATCC 27289) was a gift of S. Daugelat and S. H. E. Kaufmann, Universität Ulm, Ulm, Germany. After a passaging in mice, the bacteria were grown in Dubos medium (Difco) with 10% bovine albumin (Difco) and stored in aliquots at −70°C. Before use, the bacteria were centrifuged and washed once in phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, 1.4 mM KH2PO4 [pH 7.2]). QS-21 was provided by C. R. Kensil, Aquila Biopharmaceuticals, Farmingham, Mass. Monophosphoryl lipid A (MPL; 0.5 mg/ml, solubilized in 0.1% triethylamine; lot K1093A) and Detox (Detox; Squalane Stable Emulsion-MPLR/CWS, 10×; Lot 337-251) were gifts of T. Ulrich, Ribi ImmunoChem Research, Inc., Hamilton, Mont. Killed C. parvum was a product of Wellcome Biotechnology, Ltd., Beckenham, England.
Recombinant antigens.
The expression and purification of a 323-amino-acid fragment (rCP5) corresponding to the cDNA of lmcpb, a member of the gene family encoding the major cysteine proteinases of L. mexicana amastigotes, has been described elsewhere (69).
Plasmid pSKE5 (kindly provided by E. Medina-Acosta, The Rockefeller University, New York, N.Y., and D. Russell, Washington School of Medicine, St. Louis, Mo.) contains the complete open reading frame of a member of the C1 subclass of genes coding for GP63 cloned into the EcoRI restriction site of the vector pBluescript SK(+) (31). Because in L. mexicana amastigotes transcription is restricted to this subfamily (31), the GP63-related polypeptides purified from the amastigotes (see below) are considered to be products of the C1 genes. Plasmid pSKE5 DNA was digested with AspEI, which cuts in the region corresponding to the putative pro-region of the protein and in the 3′-untranslated region. A 1,770-bp fragment was blunt-end ligated into the BamHI-site of the vector pQE-11 (Qiagen, Hilden, Germany), yielding the recombinant plasmid pSQ1, which encoded a polypeptide with the sequence NH2-MRGSHHHHHHGI-(vector)-TSPG------LPT-COOH (rGP63) and a predicted molecular mass of 60.6 kDa. A protein of this size was strongly expressed in E. coli XL1-Blue (Stratagene, Heidelberg, Germany) induced for 5 h with 1 mM isopropyl-β-d-thiogalactoside. rGP63 was purified from inclusion bodies on a Ni-nitriloacetic acid (NTA)-agarose affinity column (42). The protein eluted from the column with a linear gradient of imidazole remained soluble after dialysis against water. Upon sodium dodecyl sulfate-polyacrylamide gel electrophoresis and staining with Coomassie blue, the purified protein gave rise to a single band corresponding to 61 kDa and negligible amounts of degradation products. In immunoblots, the 61-kDa protein reacted strongly and the degradation products very weakly with antibodies against promastigote GP63 (42).
Recombinant membrane-bound acid phosphatase (rMBAP) was prepared by using the same strategy as rGP63. Plasmid pB-MBAP containing the lmmbap gene and flanking sequences in the vector pBluescript SKII(+) (66) was digested with Bsp1407I and BamHI, yielding a fragment containing the lmmbap sequence from codon 4 to the termination codon and an additional 90 bp of untranslated region. The ends of the fragment were filled in using Klenow polymerase, and the blunt-ended product was cloned into the expression vector pQE8 (Qiagen) linearized by digestion with BamHI. The recombinant gene predicted the sequence NH2-MRGSHHHHHHGS (vector)-YKVEL-------AIIV-COOH (rMBAP). A protein of the expected size (55 kDa) was purified from E. coli inclusion bodies by Ni-NTA-agarose affinity column chromatography. The reading frame of the recombinant protein was verified by immunoblots with an antiserum against a peptide corresponding to the COOH terminus of MBAP (66, 67). The purified protein was dialyzed against PBS, which resulted in aggregation. Immediately before use the material was dispersed in solution by sonication, and aliquots corresponding to the required amounts of protein were removed. The lipopolysaccharide (LPS) content of the recombinant proteins was determined using the Limulus amebocyte assay (BioWhittaker, Verviers, Belgium) and was below <100 pg/μg of protein for rCP5 and rGP63, while rMBAP contained 10 ng/μg.
Native antigens.
Cysteine proteinases (CP) were purified from lesion-derived amastigotes or from the supernatant of lesion homogenates (24, 25). The isolation of GP63-related proteins (GP63) from amastigotes and a truncated form of MBAP from the culture supernatant of promastigotes has been described before (24, 68).
Immunizations.
The following adjuvants were prepared for injection into mice: QS-21 reconstituted at 1 mg/ml in 10 mM Na-phosphate buffer (pH 6) and diluted in PBS to give 100 μg/ml (20 μg/dose), Detox diluted with 9 volumes of PBS, MPL diluted with 1 volume of PBS (50 μg/dose), 1 mg of C. parvum in 2 ml PBS (100 μg/dose), 5 × 106 CFU BCG/ml in PBS (106/dose), and IL-12 diluted to yield 5 μg/ml in PBS containing 1% normal mouse serum (1 μg/dose). The adjuvants plus antigens contained a mixture of the recombinant proteins rCP5, rGP63 and rMBAP, each at a concentration of 12.5 μg/ml (2.5 μg/dose). In some experiments, the native antigens CP, GP63 and MBAP were substituted for the recombinant proteins at the same concentration.
Mice were immunized by two injections 1 week apart with 200 μl of either adjuvant alone or adjuvants containing Leishmania proteins as indicated. Injections were given subcutaneously at the tailbase with the exception of C. parvum, which was injected intraperitoneally.
Restimulation of antigen-specific T cells in vitro.
Single cell suspensions were prepared from the spleens of vaccinated mice. The splenocytes (2 × 105/well) were stimulated in triplicate in round-bottom 96-well tissue culture plates (Falcon; Becton Dickinson, Heidelberg, Germany) with rMBAP (4 μg/ml), rCP5 (5 μg/ml), or rGP63 (5 μg/ml). In controls, the cells were stimulated with a freeze-thawed lysate of L. mexicana promastigotes (equivalent to 106 parasites/ml). The cultures were incubated for 5 days in Dulbecco modified Eagle medium (CCpro, Neustadt, Germany) supplemented with 1% heat-inactivated mouse serum, 2 mM l-glutamine, 1% nonessential amino acids (Gibco, Eggenstein, Germany), and 50 μM β-mercaptoethanol at 37°C in a humidified atmosphere of 5% CO2 in air. Cultures were then pulsed with [3H]thymidine (0.5 μCi/well) for 16 h, and incorporation was analyzed on a β-plate reader (Pharmacia-LKB, Freiburg, Germany). Stimulation indices were calculated by dividing the total radioactivity incorporated in the presence of the antigens by the radioactivity incorporated in medium alone.
The total parasite-specific CD4+ T-cell response was analyzed in mice with progressive or healed infections after vaccination. Lymph nodes draining the site of infection were removed, and single cell suspensions were prepared by mechanical disruption and passage through a steel mesh in balanced salt solution (BSS-EDTA, cf. reference 62). Cells were passed through cotton wool plugs and washed with BSS-EDTA. CD4+ cells were enriched to more than 90% by depletion of CD8α+ CD11b+ CD16-CD32+ CD45R+ cells on a MACS separation column according to the manufacturer's instructions (Miltenyi Biotech, Bergisch Gladbach, Germany). CD4+ T lymphocytes (2 × 105/well) were restimulated in 96-well round-bottom microtiter plates (Falcon) for 48 h by mixing with irradiated syngeneic low-density spleen cells (2 × 105/well) from naive mice and culturing in the presence or absence of freeze-thaw lysed L. mexicana promastigotes (equivalent to 106 parasites/ml) or concanavalin A (2.5 μg/ml) in medium. Gamma interferon (IFN-γ) production in response to parasite antigens was measured by determining the concentration of the cytokine in the culture supernatants by bioassay as described earlier (2).
Blood was collected from individual mice by retroorbital bleeding and then coagulated; serum was prepared by centrifugation. Sera were diluted in PBS–5% milk powder containing 0.05% Tween 20, and 100 μl of the diluted samples was incubated in duplicate in microtiter plates (Microtest III, Falcon; Becton Dickinson, Oxnard, Calif.) coated with native CP at 2 μg/ml in 50 mM NaHCO3–100 mM NaCl (pH 8.2). Bound antibodies were detected by the use of goat anti-mouse immunoglobulin polyclonal antibodies conjugated to alkaline phosphatase (Sigma, Deisenhofen, Germany) and p-nitrophenylphosphate as substrate.
RESULTS
Choice of antigens and adjuvants.
Considering that in infections by sandflies only small numbers of metacyclic promastigotes are injected, antigens important for the immunological control of the disease by the host must be expressed in amastigotes. Therefore, we choose three L. mexicana antigens. The first was a family of CPs (24 to 27 kDa) that is abundantly expressed in the lysosomes of amastigotes (3.5% of the cellular protein corresponding to 1.2 × 106 molecules/cell) but not in promastigotes. These enzymes are encoded by the lmcpb gene family (24, 25, 38, 39). The second was a zinc metalloproteinase, a glycoprotein of 63 kDa (GP63) carrying a glycosylphosphatidylinositol (GPI) membrane anchor, which is the major surface protein of promastigotes (0.5 to 1% of the cellular protein corresponding to about 5 × 105 molecules/cell); this protein is present in promastigotes of all Leishmania species investigated (53). In contrast, L. mexicana amastigotes from mouse lesion tissue express only very small amounts of GP63 on their surface (32). Instead, a soluble form of the protease encoded by subclass C of the gp63-gene family lacking a GPI anchor addition signal is located in the lysosomes of the parasites (approximately 0.3 to 0.4% of the cellular protein corresponding to about 50 000 molecules/cell [8, 25, 31]). The third was MBAP, a transmembrane glycoprotein (70 to 72 kDa) encoded by the single copy gene (lmmbap), which is expressed in both promastigotes and amastigotes of L. mexicana and is localized in membrane structures close to the flagellar pocket (33, 66, 68). This enzyme is present in only about 7,300 copies/amastigote (0.066% of the cellular protein [68]). All three antigens were prepared both in their recombinant and in their native forms (see Materials and Methods). Previous immunization experiments using each of these proteins in recombinant form and complete Freund adjuvant have shown that they all elicit a T-cell response in mice (42, 68, 69).
Six adjuvants were used. The first was IL-12. This lymphokine is nature's main adjuvant, required for the stimulation of a Th1 response. Its efficacy for eliciting a protective immune response in the murine Leishmania model has been demonstrated repeatedly (3, 21, 58). The second was MPL. This compound is derived by mild acid hydrolysis from LPS of Salmonella enterica serovar Typhimurium R595 and is considered to be a stimulant of both the humoral and the cellular immune responses (60, 61). The third was Detox, an investigational adjuvant consisting of cell wall skeleton from Mycobacterium phlei and MPL emulsified in squalane, which has been designed as a replacement of complete Freund adjuvant. Detox has been used in both animals and humans to enhance immunological responses to vaccine antigens (28, 47). The fourth was QS-21, a triterpene glycoside from Quillaja saponaria Molina cortex, which stimulates both cellular (Th1) and humoral immune responses and has been used in human vaccination trials with a variety of antigens (44, 45, 51). The fifth was live M. bovis BCG, an adjuvant known to elicit long-lasting cellular and humoral immune responses, which has been used in human vaccination trials against leishmaniasis (9, 37, 57). The sixth was killed C. parvum, an adjuvant used in several experimental immunizations against leishmaniasis (10, 20, 26, 43, 55).
Antigen-specific T-cell responses in mice immunized with antigens in combination with different adjuvants.
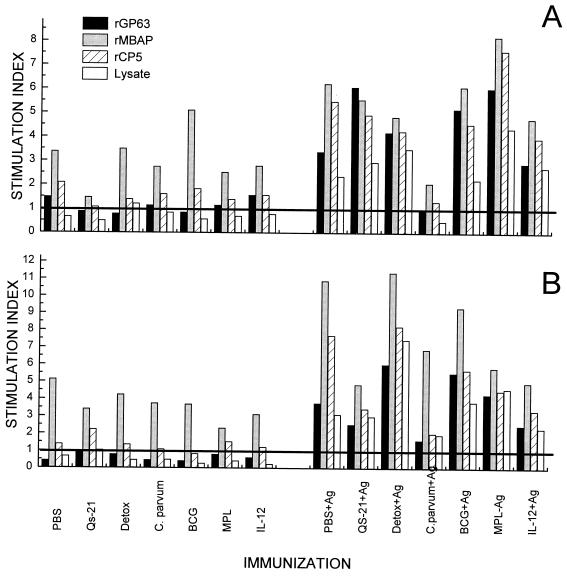
C57BL/6 mice were immunized with a mixture of the three recombinant L. mexicana proteins, rCP5, rMBAP, and rGP63, in combination with the six adjuvants or PBS. Single cell suspensions of spleens and draining lymph nodes of immunized animals were prepared 7 days after the first immunization and restimulated in vitro (Fig. 1). Immunization effected a specific proliferative response with each antigen or a promastigote cell lysate. In general, the responses were low, with stimulation indices ranging from 2 to 11; however, responses were also detectable in the spleen, a site distant from the injection site (compare Fig. 1A and B). Cells from mice treated only with adjuvants proliferated in response to the rMBAP preparation, which may have been due to a contamination by LPS. The culture supernatants were also analyzed for the presence of IFN-γ and IL-4, lymphokines indicative for type 1 and type 2 T-helper-cell activation, respectively. Low levels of IFN-γ (0.4 to 1 U/ml) were detected only in cell culture supernatants from mice immunized with the antigen mixture plus adjuvants (results not shown). In contrast, no IL-4 could be detected in any of the culture supernatants (detection limit, 0.1 U/ml). These results suggested that T cells specific for each antigen were induced and that these lymphocytes can produce IFN-γ that is normally associated with a type 1 immune response.
FIG. 1.
Antigen-specific T cells are induced in mice by immunization with a mixture of recombinant proteins and different adjuvants. The mice were immunized with formulations containing all three recombinant proteins and the adjuvants as indicated in the figure (see Materials and Methods). Single cell suspensions of spleens (A) and lymph nodes (B) of vaccinated mice were restimulated in vitro in the presence of rMBAP, rCP5, rGP63 or a freeze-thawed lysate of L. mexicana as indicated. Cell proliferation was assessed by measuring [3H]thymidine incorporation. Antigen-specific cell proliferation is expressed by stimulation indices calculated from the incorporated radioactivity in the presence of antigen divided by the activity incorporated in cells cultured in medium only (background 5 × 103 to 2.6 × 104 cpm/culture well). Data represent the arithmetic mean of triplicate determinations.
Vaccination with the antigen mixture protects C57BL/6 mice from uncontrolled L. mexicana infection.
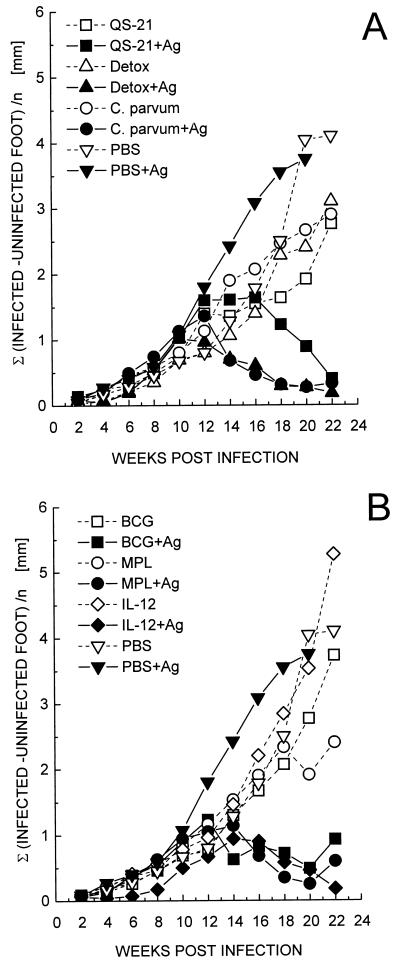
C57BL/6 mice were immunized with two injections of the recombinant antigen mixture in combination with the different adjuvants or with adjuvants alone. Immunized animals were subsequently challenged by injection of 4 × 105 L. mexicana promastigotes into the left hind foot 1 week after the last immunizing injection. Lesion development was scored biweekly and is depicted in Fig. 2. Infections progressed similarly in vaccinated and control mice for the first 3 months. Thereafter, lesion size progressed in control mice, whereas the majority of animals vaccinated with the antigen mixture and adjuvants healed. This behavior was defined as protection. The most effective adjuvant was IL-12 followed by Detox: all five mice in each of these groups had healed by the end of the experiment. Animals treated with QS-21, Detox, or C. parvum in combination with the antigens showed slightly accelerated lesion development on average compared to PBS-treated controls. While all mice immunized with antigen mixed with Detox or C. parvum healed, groups immunized with QS-21, BCG, or MPL plus antigens did not heal uniformly, with only three of five mice healing in the latter two groups and four of five mice in the QS-21-immunized animals. Cured mice did not show any recurrence of the disease for over a year. In the groups treated solely with QS-21, Detox, or C. parvum, one of five animals showed a tendency to heal by 6 months after infection.
FIG. 2.
Efficacy of different vaccine formulations containing rMBAP, rCP5, and rGP63. C57BL/6 mice were injected twice at a weekly interval into the lower back with 200 μl of vaccine containing 2.5 μg of each protein mixed with the indicated adjuvants. One week after the boosting injection, 4 × 105 L. mexicana promastigotes were injected into the hind left footpad. Footpad swelling was measured every second week, and the data represent the mean increase in thickness compared to the uninfected foot of five mice per group. One animal in the groups treated only with QS-21, Detox, and C. parvum showed a tendency to heal at the end of the experiment. However, the averaged values include all mice per group. The plots are arranged in two graphs: lesion development in groups immunized with QS-21, Detox, or C. parvum (A) and in groups immunized with BCG, MPL, or IL-12 (B). PBS controls are shown in both graphs.
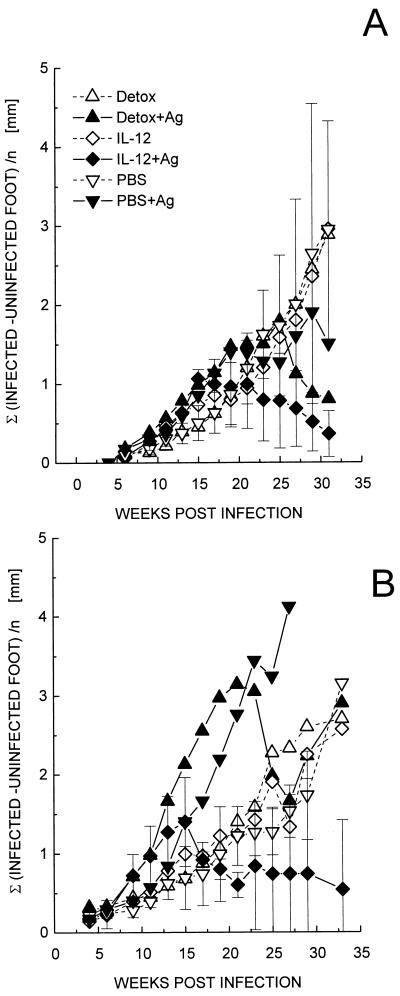
Based on these results, IL-12 and Detox were selected for use in comparing native and recombinant proteins, as well as for testing the efficacy of these adjuvants at a higher challenge dose of parasites. Mice immunized with the mixture of native antigens formulated with either IL-12 or Detox were challenged with L. mexicana promastigotes. Like the recombinant-protein-based vaccine, both formulations were protective (Fig. 3). Mice immunized with IL-12 and antigens, however, developed only small lesions, and healing occurred uniformly in all animals, while mice treated with Detox and antigens developed more-severe lesions (peak mean lesion size, 2.18 mm), and only four out of five animals healed. This suggests that IL-12 provides a more potent adjuvant effect.
FIG. 3.
Protective efficacy of vaccines containing native antigens and IL-12 or Detox as adjuvants. C57BL/6 mice (five per group) were vaccinated with two doses of 2.5 μg each of CP, GP63, and MBAP mixed with IL-12 or Detox as described in the text. Immunized animals were infected with 4 × 105 L. mexicana promastigotes. Lesion development was monitored as outlined in Fig. 2. In the group treated with antigen (Ag) and Detox, two of five animals still had active lesions at the end of the experiment, while one of five mice showed a tendency to heal in the groups treated only with IL-12 or Detox or PBS-antigen (PBS-Ag). Error bars are shown for one group only for better clarity and correspond to the mean ± the standard deviation (SD), including all mice, and are representative for all groups.
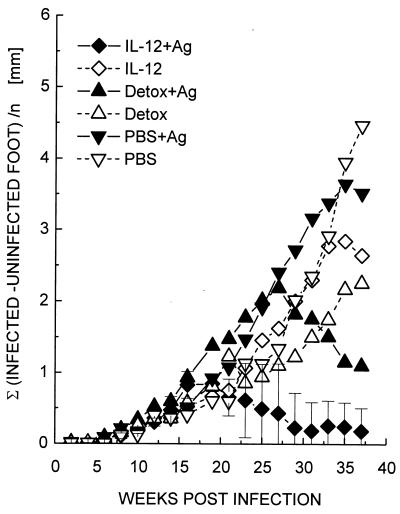
Increasing the challenge dose clearly revealed the superiority of IL-12 as an adjuvant in this system. Mice were immunized again with the mixture of recombinant antigens and IL-12 or Detox as adjuvants and subsequently were challenged with either 4 × 105 (Fig. 4A) or 2 × 106 promastigotes (Fig. 4B). At the lower challenge dose, both vaccines were protective; however, animals immunized with Detox plus antigens and challenged with the higher dose of L. mexicana were unable to contain the infection, and eventually all mice developed uncontrolled lesions and metastases. In the group immunized with IL-12, only one of five animals retained a detectable lesion at the end of the experiment. Animals in this group still harbored persisting parasites at this time point at the original site of infection as determined by culture of homogenized footpad tissue.
FIG. 4.
Protective efficacy of vaccines in mice challenged with different doses of L. mexicana. C57BL/6 mice were vaccinated with a mixture of the three recombinant antigens and IL-12 or Detox as adjuvants as described in Fig. 2 and challenged with 4 × 105 (A) or 2 × 106 (B) L. mexicana promastigotes. Lesions were scored as in Fig. 2, and data represent the average values of five mice per group. Error bars are shown for representative groups and correspond to the mean ± the SD.
In summary, vaccine formulations containing each of the six adjuvants but not the purified antigens alone were able to confer protection against uncontrolled disease caused by a low-dose challenge with L. mexicana promastigotes. Whether the vaccines contained native or recombinant forms of the three antigens was not relevant in regard to the protective effect. IL-12 was the most potent of the five adjuvants and conferred protection superior to Detox or the frequently used C. parvum or BCG.
CD4+ T cells from cured mice produce higher amounts of IFN-γ.
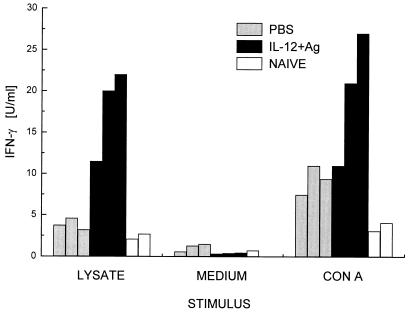
Healing in experimental murine leishmaniasis caused by L. major is mediated primarily by CD4+ Th1 cells secreting IFN-γ. C57BL/6 mice were either treated with PBS (control) or immunized with the mixture of rCP5, rGP63, and rMBAP plus IL-12 as adjuvant (vaccinated) and challenged with L. mexicana promastigotes (cf. Fig. 2). Six months after the challenge infection, CD4+ T cells were purified from the draining lymph nodes of both groups of mice. For control purposes, CD4+ T cells were also prepared from uninfected, age-matched mice. Leishmania-specific cells were restimulated in vitro with a freeze-thawed lysate of L. mexicana promastigotes. Alternatively, cells were polyclonally activated with concanavalin A. CD4+ T cells from vaccinated mice secreted significantly higher levels of IFN-γ in response to leishmanial antigens (Fig. 5). In contrast, the IFN-γ concentration in supernatants of T cells from nonvaccinated mice was as low as that in supernatants from cells of naive mice. IL-4 secretion in these cultures was below the sensitivity of the bioassay used (limit, 0.1 U/ml). The observation that vaccinated mice showed a type 1-biased antiparasite immune response was corroborated by the analysis of parasite lysate- and cysteine proteinase-specific serum antibodies. In animals vaccinated with the antigen mixture and IL-12, immunoglobulin G2a (IgG2a) titers against the lysate and cysteine proteinases were 10 and 100 times higher, respectively, than in nonvaccinated controls (data not shown).
FIG. 5.
Comparison of IFN-γ secretion by CD4+ T cells of protected and nonprotected C57BL/6 mice. CD4+ T cells were prepared from lymph nodes draining the site of infection of cured mice vaccinated with IL-12 and rMBAP, rCP, and rGP63 (see Fig. 2) or of PBS mock-treated mice with progressive disease. For control purposes, CD4+ T cells were also enriched from corresponding lymph nodes of naive, noninfected animals. The enriched cell populations were stimulated with a freeze-thawed lysate of L. mexicana promastigotes presented by syngeneic splenocytes in vitro. Antigen-specific IFN-γ production was determined in the supernatants and compared to the production of this cytokine in cultures treated with concanavalin A or medium alone. Values represent the mean of duplicate cultures of CD4+ cells from individual mice (three animals of each infected group and two naive controls). The lymphokine content varied by less than 10% between duplicates. Antigen-specific IFN-γ production by CD4+ cells from vaccinated mice was significantly higher than that by cells from mock-treated mice (P ≤ 0.05; in a Wilcox ranking test).
Only two of the three antigens are protective.
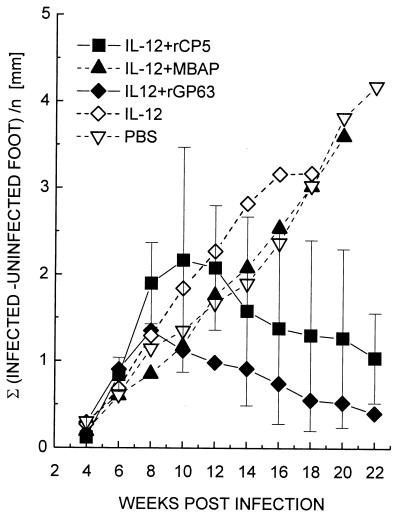
In order to test which of the three antigens confers protection, C57BL/6 mice were immunized with a single recombinant antigen (rCP5, rGP63, or rMBAP) mixed with IL-12. The mice were challenged with L. mexicana, and the course of the infection was monitored (Fig. 6). rGP63 corresponding to the gp63 genes expressed in amastigotes was protective. Mice immunized with rCP5 healed, although they developed, on average, larger lesions. In contrast, mice immunized with rMBAP as a single subunit vaccine were not protected from a subsequent challenge infection and produced progressive lesions indistinguishable from those observed in nonimmunized mice.
FIG. 6.
Efficacy of rMBAP, rCP5, or rGP63 in combination with IL-12 as single-component subunit vaccines. C57BL/6 mice (five per group) were vaccinated with two doses of 5 μg of the individual proteins mixed with IL-12. Control mice were mock immunized with IL-12 in PBS or with PBS alone. Mice were challenged 1 week later with 4 × 105 L. mexicana promastigotes. Lesion development was monitored as outlined in Fig. 2. Error bars are shown for the two protected groups and correspond to the mean ± the SD.
DISCUSSION
In humans, simple cutaneous lesions caused by infection with L. mexicana mexicana (Chiclero's ulcer) usually heal spontaneously within 1 to 2 years, but chronic nonhealing lesions, recurrence of infection, and general dissemination are not uncommon (64). Because most mouse strains cannot control an infection with this parasite, the mouse provides a model for the severe manifestation of the disease. Studies with the closely related parasite L. mexicana amazonensis have traced this phenotype to the inability to mount an effective Th1 response (4). It is clear from the experiments presented in this study that immunization with a cocktail of three proteins and one of six different adjuvants can modulate the immune response in such a way that a Th1 response develops and, as a consequence, C57BL/6 mice can control the infection. Whether the mechanism of protection relies solely on CD4+ cells or involves also CD8+ cells as in other systems (18, 43) remains to be tested.
It is not surprising that IL-12 was the most effective adjuvant given the fact that this molecule is the likely endogenous mediator of the immunomodulatory substances included here. A recent study, however, suggests that immunity induced by IL-12 plus leishmanial protein is relatively short-lived (17); furthermore, IL-12 is presently not considered a safe adjuvant for use in humans because of significant side effects even at low doses (7). The second most efficient adjuvant was Detox; however, human trials of a malaria sporozoite vaccine have shown that this formulation also has considerable side effects (23). Although in the group immunized with BCG only a fraction of animals healed, it is possible that this acceptable adjuvant would be more effective in humans than in mice.
The efficacy of the cocktail of three antigens in combination with IL-12 was also tested under more stringent conditions, i.e., in the vaccination of BALB/c and CBA/J mice. Thus far, we have not been successful in protecting these mice from a challenge infection with L. mexicana (not shown). This may be achieved by varying the vaccination protocol because GP63 and a homologue of cysteine proteinases from L. pifanoi were shown to protect CBA and/or BALB/c mice to a certain degree against a challenge with parasites of the L. mexicana complex (11, 50, 55). All vaccine formulations are, however, potentially protective, as indicated by their efficacy in C57BL/6 mice. Yet none of them prevented infection. This is a feature observed for most experimental vaccines against leishmaniasis tested to date (see reference 50 for an exception). We consider the expression of an antigen in the amastigote to be a prerequisite for the promotion to a vaccine candidate. The number of promastigotes injected by sandflies is several orders of magnitude smaller than in our experimental infections. Thus, promastigote antigens may not be available at the quantities necessary to boost a memory population of CD4+ Th1 cells and, even if presented by infected macrophages, will disappear quickly due to the high turnover of major histocompatibility complex (MHC) class II molecules on this cell type. This can explain the failure to protect GP63-immunized BALB/c mice against challenge with L. major because it is controversial whether this antigen is expressed at all by amastigotes of this species (see reference 11 for more discussion). In contrast, the intracellular proteins used in this study are expressed in amastigotes, albeit in different amounts. It is therefore of considerable significance that the two abundant proteins (CP and GP63) when used alone elicit a protective response, while a much rarer protein (MBAP) does not. GP63 may be more effective against an experimental challenge because it is also an abundant surface protein in promastigotes and could thus be presented almost immediately after infection. Overall, the results tally well with experiments in vitro (68, 69), which showed that macrophages infected with live amastigotes cannot present intracellular antigens in the context of MHC class II molecules. Once the amastigotes are killed and degraded in the macrophages, peptides derived from abundant antigens such as CP are efficiently presented to specific T-cell lines, while peptides derived from minor proteins such as MBAP are not. The correlation between the previous experiments in cell culture and the immunizations reported here suggests that, first, during resolution of a lesion, antigen presentation from activated macrophages is important and, second, that upon degradation of amastigotes abundant antigens are more effective in restimulating specific T cells primed by immunization.
We have recently attempted to provide an explanation for the fact that infection is contained by Th1 cells only after disease has developed (41). In the early phase of the infection, leishmanicidal activity is not triggered in resting macrophages becoming parasitized. Therefore, infected macrophages will not present antigens to immune Th1 cells because MHC class II expression is not upregulated. Parasites will replicate unhindered, and the lesion will expand. In the course of the infection focal activation of individual infected macrophages may occur. These cells will be able to present amastigote-antigens to parasite-specific Th1 cells. The interacting Th1 cells will be reciprocally stimulated and, due to their secretion of IFN-γ and tumor necrosis factor alpha, will activate neighboring infected macrophages. There is evidence from immunohistological studies that focal activation of macrophages is a feature of resolving leishmanial infections (56). Eventually, the density of infected macrophages will decline, and the lesions will resolve. In this phase the process becomes inefficient and will lead to the persistent state of the infection where there is a balance between parasite destruction and replication (1, 56). This scenario could equally well apply to parasite antigen-specific type 1 CD8+ T cells that secrete IFN-γ and which may contribute in resolving active lesions.
The two abundant amastigote proteins used in this study can be readily produced from bacteria in large amounts and in highly purified form. In combination with a third protein, which should be expressed in amastigotes, they could provide an affordable polypeptide cocktail for either prophylactic or immunotherapeutic immunization against American tegumental leishmaniasis. Considering alternatives such as immunization with attenuated and/or killed promastigotes or, perhaps even more problematic, with DNA (15), the use of well-characterized proteins and an acceptable adjuvant deserves serious consideration.
ACKNOWLEDGMENTS
We thank Monika Demar and Dorothee Harbecke for expert technical assistance and Nathan Goehring for correcting the manuscript.
REFERENCES
- 1.Aebischer T. Recurrent cutaneous leishmaniasis: a role for persistent parasites? Parasitol Today. 1994;10:25–28. doi: 10.1016/0169-4758(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 2.Aebischer T, Morris L, Handman E. Intravenous injection of irradiated Leishmania major into susceptible BALB/c mice: immunization or protective tolerance. Internat Immunol. 1994;6:1535–1543. doi: 10.1093/intimm/6.10.1535. [DOI] [PubMed] [Google Scholar]
- 3.Afonso L C C, Scharton T M, Vieira L Q, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1994;263:235–237. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 4.Afonso L C C, Scott P. Immune response associated with susceptibility of C57BL/10 mice to Leishmania amazonensis. Infect Immun. 1993;61:2952–2959. doi: 10.1128/iai.61.7.2952-2959.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexander J. A radioattenuated Leishmania major vaccine markedly increases the resistance of CBA mice to subsequent infection with Leishmania mexicana mexicana. Trans R Soc Trop Med Hyg. 1982;76:646–649. doi: 10.1016/0035-9203(82)90232-2. [DOI] [PubMed] [Google Scholar]
- 6.Alexander J, Coombs G H, Mottram J. Leishmania mexicana cysteine proteinase-deficient mutants have attenuated virulence for mice and potentiate a Th1 response. J Immunol. 1998;161:6794–6801. [PubMed] [Google Scholar]
- 7.Atkins M B, Robertson M J, Gorodon M, Lotze M T, DeCoste M, DuBois J S, Ritz J S, Sandler A B, Edington A B, Garzone P D, Mier J W, Canning C M, Battiato L, Tahara H, Sherman M L. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin Cancer Res. 1997;3:409–417. [PubMed] [Google Scholar]
- 8.Bahr V, Stierhof Y-D, Ilg T, Demar M, Quinten M, Overath P. Expression of lipophosphoglycan, high-molecular-weight phosphoglycan and glycoprotein 63 in promastigotes and amastigotes of Leishmania mexicana. Mol Biochem Parasitol. 1993;58:107–122. doi: 10.1016/0166-6851(93)90095-f. [DOI] [PubMed] [Google Scholar]
- 9.Castes M, Blackwell J, Trujillo D, Formica S, Cabrera M, Zorrilla G, Rodas A, Castellanos P L, Convit J. Immune response in healthy volunteers vaccinated with killed leishmanial promastigotes plus BCG. I: Skin-test reactivity, T-cell proliferation and interferon-γ production. Vaccine. 1994;12:1041–1051. doi: 10.1016/0264-410x(94)90342-5. [DOI] [PubMed] [Google Scholar]
- 10.Champsi J, McMahon-Pratt D. Membrane glycoprotein M-2 protects against Leishmania amazonensis infection. Infect Immun. 1988;52:3272–3279. doi: 10.1128/iai.56.12.3272-3279.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connell N D, Medina-Acosta E, McMaster W R, Bloom B R, Russell D. Effective immunization against cutaneous leishmaniasis with recombinant bacille Calmette-Guérin expressing the Leishmania surface proteinase gp63. Proc Natl Acad Sci USA. 1993;90:11473–11477. doi: 10.1073/pnas.90.24.11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Convit J. Leishmaniasis: immunological and clinical aspects and vaccines in Venezuela. Clin Dermatol. 1996;14:479–487. doi: 10.1016/0738-081x(96)00042-9. [DOI] [PubMed] [Google Scholar]
- 13.Convit J, Castellanos P L, Ulrich M, Castes M, Rondon A, Pinardi M E, Rodriguez N, Bloom B R, Formica S, Valecillos L. Immunotherapy of localized, intermediate and diffuse forms of American cutaneous leishmaniasis. J Infect Dis. 1989;160:104–115. doi: 10.1093/infdis/160.1.104. [DOI] [PubMed] [Google Scholar]
- 14.Convit J, Castellanos P L, Rondon A, Pinardi M E, Ulrich M, Castes M U M, Bloom B, Garcia L. Immunotherapy versus chemotherapy in localized cutaneous leishmaniasis. Lancet. 1987;i:401–405. doi: 10.1016/s0140-6736(87)90116-4. [DOI] [PubMed] [Google Scholar]
- 15.Donnelly J J, Ulmer J B, Shiver J W, Liu M A. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 16.Grimaldi G., Jr Meetings on vaccine studies towards the control of leishmaniasis. Mem Inst Oswaldo Cruz. 1995;90:553–556. [PubMed] [Google Scholar]
- 17.Gurunathan S, Prussin C, Sacks D L, Seder R A. Vaccine requirements for sustained cellular immunity to an intracellular parasitic infection. Nat Med. 1998;4:1409–1415. doi: 10.1038/4000. [DOI] [PubMed] [Google Scholar]
- 18.Gurunathan S, Sacks D L, Brown D R, Reiner S L, Charest H, Glaichenhaus N, Seder R A. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J Exp Med. 1997;186:1137–1147. doi: 10.1084/jem.186.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Handman E. Leishmania vaccines: old and new. Parasitol Today. 1997;13:236–238. doi: 10.1016/s0169-4758(97)01060-0. [DOI] [PubMed] [Google Scholar]
- 20.Handman E, Symons F M, Baldwin T M, Curtis J M, Scheerlinck J-P Y. Protective vaccination with promastigote surface antigen 2 from Leishmania major is mediated by a Th1 type of immune response. Infect Immun. 1995;63:4261–4267. doi: 10.1128/iai.63.11.4261-4267.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heinzel F P, Schoenhaut D S, Rerko R M, Rosser L E, Gately M K. Recombinant interleukin 12 cures mice infected with Leishmania major. J Exp Med. 1993;177:1505–1509. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman S L, Edelman R, Bryan J P, Schneider I, Davis J, Sedegah M, Gordon D, Church P, Gross M, Siverman C, et al. Safety, immunogenicity, and efficacy of a malaria sporozoite vaccine administered with monophosphoryl lipid A, cell wall skeleton of mycobacteria, and squalane as adjuvant. Am J Trop Med Hyg. 1994;51:603–612. doi: 10.4269/ajtmh.1994.51.603. [DOI] [PubMed] [Google Scholar]
- 23.Howard J G, Nicklin S, Hale C, Liew F Y. Prophylactic immunization against experimental leishmaniasis. I. Protection induced in mice genetically vulnerable to fatal Leishmania tropica infection. J Immunol. 1982;129:2002–2012. [PubMed] [Google Scholar]
- 24.Ilg T, Fuchs M, Gnau V, Wolfram M, Harbecke D, Overath P. Distribution of parasite cysteine proteinases in lesions of mice infected with Leishmania mexicana amastigotes. Mol Biochem Parasitol. 1994;67:193–203. doi: 10.1016/0166-6851(94)00126-x. [DOI] [PubMed] [Google Scholar]
- 25.Ilg T, Harbecke D, Overath P. The lysosomal gp63-related protein in Leishmania mexicana amastigotes is a soluble metalloproteinase with an acidic pH optimum. FEBS Lett. 1993;327:103–107. doi: 10.1016/0014-5793(93)81049-6. [DOI] [PubMed] [Google Scholar]
- 26.Jaffe C L, Rachamim N, Sarfstein R. Characterization of two proteins from Leishmania donovani and their use for vaccination against visceral leishmaniasis. J Immunol. 1990;144:699–706. [PubMed] [Google Scholar]
- 27.Jardim A, Alexander J, Teh H S, Ou D, Olafson R W. Immunoprotective Leishmania major synthetic T cell epitopes. J Exp Med. 1990;172:645–648. doi: 10.1084/jem.172.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanza P, Moss R B, Giermakowska W, Hancock R B, Richieri S P, Theofa G, Jensen F C, Salk P L, Carlo D J. Whole-killed gp120-depleted HIV-1 antigen in a murine model for prophylactic vaccination. Vaccine. 1998;16:727–731. doi: 10.1016/s0264-410x(97)00256-9. [DOI] [PubMed] [Google Scholar]
- 29.Liew F Y, Hale C, Howard J G. Prophylactic immunization against experimental leishmaniasis. IV. Subcutaneous immunization prevents the induction of protective immunity against fatal Leishmania major infection. J Immunol. 1985;135:2095–2101. [PubMed] [Google Scholar]
- 30.McMahon-Pratt D, Rodriguez D, Rodriguez J-R, Zhang Y, Manson K, Bergman C, Rivas L, Rodriguez J F, Lohman K L, Ruddle N H, Esteban M. Recombinant vaccinia viruses expressing GP46/M-2 protect against Leishmania infection. Infect Immun. 1993;61:3351–3359. doi: 10.1128/iai.61.8.3351-3359.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medina-Acosta E, Karess R E, Russell D G. Structurally distinct genes for the surface protease (gp63) of Leishmania mexicana are developmentally regulated. Mol Biochem Parasitol. 1993;57:31–46. doi: 10.1016/0166-6851(93)90241-o. [DOI] [PubMed] [Google Scholar]
- 32.Medina-Acosta E, Karess R E, Schwarz H, Russell D G. The promastigote surface protease (gp63) of Leishmania is expressed but differentially processed and localized in the amastigote stage. Mol Biochem Parasitol. 1989;37:263–274. doi: 10.1016/0166-6851(89)90158-8. [DOI] [PubMed] [Google Scholar]
- 33.Menz B, Winter G, Ilg T, Lottspeich F, Overath P. Purification and characterization of a membrane-bound acid phosphatase of Leishmania mexicana. Mol Biochem Parasitol. 1991;47:101–108. doi: 10.1016/0166-6851(91)90152-v. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell G F, Handman E. Heterologous protection in murine cutaneous leishmaniasis. Immunol Cell Biol. 1987;65:387–392. doi: 10.1038/icb.1987.44. [DOI] [PubMed] [Google Scholar]
- 35.Modabber F. Experience with vaccines against cutaneous leishmaniasis: of men and mice. Parasitology. 1989;98:S49–S60. doi: 10.1017/s0031182000072243. [DOI] [PubMed] [Google Scholar]
- 36.Modabber F. Vaccines against leishmaniasis. Ann Trop Med Parasitol. 1995;89:83–88. doi: 10.1080/00034983.1995.11813017. [DOI] [PubMed] [Google Scholar]
- 37.Momeni A Z, Jalayer T, Emamjomeh M, Khamesipour A, Zicker F, Ghassemi R L, Dowlati Y, Sharifi I, Aminjavaheri M, Shafiei A, Alimohammadian M H, Hashemi-Fesharki R, Nasseri K, Godal T, Smith P G, Modabber F. A randomized, double-blind, controlled trial of a killed L. major vaccine plus BCG against zoonotic cutaneous leishmaniasis in Iran. Vaccine. 1998;17:466–472. doi: 10.1016/s0264-410x(98)00220-5. [DOI] [PubMed] [Google Scholar]
- 38.Mottram J C, Books D R, Coombs G H. Roles of cysteine proteinases of trypanosomes and Leishmania in host-parasite interactions. Curr Opin Microbiol. 1998;1:455–460. doi: 10.1016/s1369-5274(98)80065-9. [DOI] [PubMed] [Google Scholar]
- 39.Mottram J C, Souza A E, Hutchison J E, Carter R, Frame M J, Coombs G H. Evidence from disruption of the lmcpb gene array of Leishmania mexicana that cysteine proteinases are virulence factors. Proc Natl Acad Sci USA. 1996;93:6008–6013. doi: 10.1073/pnas.93.12.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mougneau E, Altare F, Wakil A E, Zheng S, Coppola T, Wang Z-E, Waldmann R, Locksley R M, Glaichenhaus N. Expression cloning of a protective Leishmania antigen. Science. 1995;268:563–566. doi: 10.1126/science.7725103. [DOI] [PubMed] [Google Scholar]
- 41.Overath, P., and Aebischer, T. 1999. Antigen presentation by macrophages harboring intravesicular pathogens. Parasitol. Today 15:325–332. [DOI] [PubMed]
- 42.Patzer S. Leishmania mexicana: Expression in E. coli und Reinigung des Amastigoten-spezifischen GP63 und Isolierung der membrangebundenen sauren Phosphatase. Diploma thesis. Universität T; 1994. übingen, Tübingen, Germany. [Google Scholar]
- 43.Rachamim N, Jaffe C L. Pure protein from Leishmania donovani protects mice against both cutaneous and visceral leishmaniasis. J Immunol. 1993;150:2322–2331. [PubMed] [Google Scholar]
- 44.Read Kensil C. Saponins as vaccine adjuvants. Crit Rev Ther Drug Carrier Syst. 1996;13:1–55. [PubMed] [Google Scholar]
- 45.Read Kensil C, Kammer R. QS-21: a water-soluble triterpene glycoside adjuvant. Exp Opin Investig Drugs. 1998;7:1475–1482. doi: 10.1517/13543784.7.9.1475. [DOI] [PubMed] [Google Scholar]
- 46.Reiner S L, Locksley R M. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 47.Rickman L S, Gordon D M, Wistar R, Jr, Krzych U, Gross M, Hollingdale M R, Egan J E, Chulay J D, Hoffman S L. Use of adjuvant containing mycobacterial cell-wall skeleton, monophosphoryl lipid A, and squalane in malaria circumsporozoite protein vaccine. Lancet. 1991;337:998–1001. doi: 10.1016/0140-6736(91)92659-p. [DOI] [PubMed] [Google Scholar]
- 48.Rivier D, Shah R, Bovay P, Mauel J. Vaccine development against cutaneous leishmaniasis. Subcutaneous administration of radioattenuated parasites protects CBA mice against virulent Leishmania major challenge. Parasitol Immunol. 1993;15:75–84. doi: 10.1111/j.1365-3024.1993.tb00587.x. [DOI] [PubMed] [Google Scholar]
- 49.Roberts M, Alexander J, Blackwell J M. Influence of Lsh, H-2, and an H-11-linked gene on visceralization and metastasis associated with Leishmania mexicana infection in mice. Infect Immun. 1989;57:875–881. doi: 10.1128/iai.57.3.875-881.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Russell D G, Alexander J. Effective immunization against cutaneous leishmaniasis with defined membrane antigens reconstituted into liposomes. J Immunol. 1988;140:1274–1279. [PubMed] [Google Scholar]
- 51.Sasaki S, Sumino K, Hamajima K, Fukushima J, Ishii N, Kawamoto S, Mohri H, Read Kensil C, Okuda K. Induction of systemic and mucosal immune responses to human immunodeficiency virus type 1 by a DNA vaccine formulated with QS-21 saponin adjuvant via intramuscular and intranasal routes. J Virol. 1998;72:4931–4939. doi: 10.1128/jvi.72.6.4931-4939.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Satoskar A, Bluethmann H, Alexander J. Disruption of the murine interleukin-4 gene inhibits disease progression during Leishmania mexicana infection but does not increase control of Leishmania donovani infection. Infect Immun. 1995;63:4894–4899. doi: 10.1128/iai.63.12.4894-4899.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schneider P, Bordier C, Etges R. Membrane proteins and enzymes of Leishmania. Sub-cell Biochem. 1992;18:39–72. doi: 10.1007/978-1-4899-1651-8_2. [DOI] [PubMed] [Google Scholar]
- 54.Sjölander A, Baldwin T M, Curtis J M, Handman E. Induction of a Th1 immune response and simultaneous lack of activation of a Th2 response are required for generation of immunity to leishmaniasis. J Immunol. 1998;160:3949–3957. [PubMed] [Google Scholar]
- 55.Soong L, Monroe Duboise S, Kima P, McMahon-Pratt D. Leishmania pifanoi amastigote antigens protect mice against cutaneous leishmaniasis. Infect Immun. 1995;63:3559–3566. doi: 10.1128/iai.63.9.3559-3566.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stenger S, Donhauser N, Thüring H, Röllinghoff M, Bogdan C. Reactivation of latent leishmaniasis by inhibition of inducible nitric oxide synthase. J Exp Med. 1996;183:1501–1514. doi: 10.1084/jem.183.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stover C K. Recombinant vaccine delivery systems and encoded vaccines. Curr Opin Immunol. 1994;6:568–571. doi: 10.1016/0952-7915(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 58.Sypek J P, Chung C L, Mayor S E H, Subramanyam J M, Goldman S J, Sieburth D S, Wolf S F, Schaub R G. Resolution of cutaneous leithmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177:1797–1802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Titus R G, Gueiros-Filho F J, de Freitas L A R, Beverley S M. Development of a safe live Leishmania vaccine line by gene replacement. Proc Natl Acad Sci USA. 1995;92:10267–10271. doi: 10.1073/pnas.92.22.10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ulrich J R, Cantrell J L, Gustafson G L, Myers K R, Rudbach J A, Hiernaux J R. The adjuvant activity of monophosphoryl lipid A. In: Spriggs D R, Koff W C, editors. Topics in vaccine adjuvant research. Boca Raton, Fla: CRC Press; 1990. pp. 137–143. [Google Scholar]
- 61.Ulrich J T, Myers K R. Monophosphoryl lipid A as an adjuvant. Past experiences and new directions. Pharmacol Biotechnol. 1995;6:495–524. [PubMed] [Google Scholar]
- 62.Vremec D, Zorbas M, Scollay R, Saunders D J, Aradavin C F, Wu L, Shortman K. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med. 1992;176:47–58. doi: 10.1084/jem.176.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walker P S, Scharton-Kersten T, Rowton E D, Hengge U, Bouloc A, Udey M C, Vogel J C. Genetic immunization with glycoprotein 63 cDNA results in a helper T cell type 1 immune response and protection in a murine model of leishmaniasis. Hum Gene Ther. 1998;9:1899–1907. doi: 10.1089/hum.1998.9.13-1899. [DOI] [PubMed] [Google Scholar]
- 64.Walton B C. American cutaneous and mucocutaneous leishmaniasis. In: Peters W, Killick-Kendrick R, editors. The leishmaniases in biology and medicine. 2. Clinical aspects and control. London, England: Academic Press; 1987. pp. 637–664. [Google Scholar]
- 65.Wiese M. A mitogen-activated protein (MAP) kinase homologue of Leishmania mexicana is essential for parasite survival in the infected host. EMBO J. 1998;17:2619–2628. doi: 10.1093/emboj/17.9.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wiese M, Berger O, Stierhof Y-D, Wolfram M, Fuchs M, Overath P. Gene cloning and cellular localization of a membrane-bound acid phosphatase of Leishmania mexicana. Mol Biochem Parasitol. 1996;82:153–165. doi: 10.1016/0166-6851(96)02729-6. [DOI] [PubMed] [Google Scholar]
- 67.Wolfram M. Antigenpräsentation durch Leishmania mexicana-infizierte Makrophagen. Ph.D. dissertation. Universität T; 1996. übingen, Tübingen, Germany. [Google Scholar]
- 68.Wolfram M, Fuchs M, Wiese M, Stierhof Y-D, Overath P. Antigen presentation by Leishmania mexicana-infected macrophages: activation of helper T cells by a model parasite antigen secreted into the parasitophorous vacuole or expressed on the amastigote surface. Eur J Immunol. 1996;26:3153–3162. doi: 10.1002/eji.1830261248. [DOI] [PubMed] [Google Scholar]
- 69.Wolfram M, Ilg T, Mottram J C, Overath P. Antigen presentation by Leishmania mexicana-infected macrophages: activation of helper T cells specific for amastigote cysteine proteinases requires intracellular killing of the parasites. Eur J Immunol. 1995;25:1094–1100. doi: 10.1002/eji.1830250435. [DOI] [PubMed] [Google Scholar]
- 70.Xu D, Liew F Y. Genetic vaccination against leishmaniasis. Vaccine. 1994;12:1534–1536. doi: 10.1016/0264-410x(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 71.Xu D, Liew F Y. Protection against leishmaniasis by injection of DNA encoding a major surface glycoprotein, gp63, of L. major. Immunology. 1995;84:173–176. [PMC free article] [PubMed] [Google Scholar]
- 72.Xu D, McSorley S J, Chatfield S N, Dougan G, Liew F Y. Protection against Leishmania major infection in genetically susceptible BALB/c mice by GP63 delivered orally in attenuated Salmonella typhimurium (AroA− AroD−) Immunology. 1995;85:1–7. [PMC free article] [PubMed] [Google Scholar]
- 73.Yang D M, Fairweather N, Button L L, McMaster W R, Kahl L P, Liew F Y. Oral Salmonella typhimurium (AroA−) vaccine expressing a major leishmanial surface protein (gp63) preferentially induces T helper 1 cells and protective immunity against leishmaniasis. J Immunol. 1990;145:2281–2285. [PubMed] [Google Scholar]