Abstract
Objectives
Recent research suggests that the use of antibiotics could reduce the efficacy of checkpoint inhibitors, in addition to other well-known factors. It could be due to gut microbiota modification, which impact over the immune system response. However, the information available so far is contradictory. The objective of this research was to clarify whether antibiotic use influences efficacy of checkpoint inhibitors treatments in non-small cell lung cancer patients in clinical practice.
Methods
Therefore, a retrospective observational study was designed. Use of antibiotics among patients treated with atezolizumab, pembrolizumab or nivolumab was assessed within 2 months of checkpoint inhibitors treatments initiation.
Results
A total of 140 patients were included, mostly men, with good performance status (ECOG 0-1), all of them previously treated with chemotherapy. An antibiotic prescription was identified in 31% of these patients, mainly fluoroquinolones or beta-lactams. The most frequent indication was respiratory infection. Both progression-free survival and overall survival were lower for patients treated with anti-infective drugs, although this difference was not statistically significant.
Conclusion
More studies are needed to draw conclusions about the impact of antibiotics on the efficacy of immunotherapy.
Keywords: Antineoplastic agents, Immunotherapy, antibiotics, efficacy
Abstract
Objetivos
Investigaciones recientes sugieren que el uso de antibióticos podría reducir la eficacia de los inhibidores del punto de control inmunológico, además de otros factores ya conocidos. Podría deberse a la modificación de la microbiota, por su impacto en la respuesta del sistema inmune. En cualquier caso, la información disponible hasta el momento es contradictoria. El objetivo de esta investigación es esclarecer si el uso de antibióticos influye en la eficacia de los inhibidores del punto de control para el tratamiento de pacientes con carcinoma de pulmón no microcítico en la práctica clínica.
Métodos
Se diseñó un estudio observacional, retrospectivo. Se investigó el uso de antibióticos entre aquellos pacientes a tratamiento con atezolizumab, pembrolizumab o nivolumab en los 2 meses previos o posteriores a su inicio.
Resultados
Se incluyeron 140 pacientes, principalmente hombres con aceptable estado general (ECOG 0-1), todos previamente tratados con quimioterapia. Se identificó una prescripción antibiótica en el 31% de la población, principalmente fluoroquinolonas o betalactámicos. La indicación más frecuente para dicha prescripción era la infección respiratoria. Tanto la supervivencia libre de progresión con la supervivencia global fue inferior en el grupo tratado con antiinfecciosos, aunque no se alcanzó significación estadística.
Conclusiones
Son necesario más estudios para concluir acerca del impacto de los antibióticos en la eficacia de la inmunoterapia.
Keywords: agentes antineoplásicos, Inmunoterapia, antibióticos, eficacia
INTRODUCTION
Lung cancer is the leading cause of cancer deaths worldwide, being tobacco the main risk factor [1]. It is the third most frequent type of tumour in Spain, after colorectal and prostate cancer [2]. Although lung cancer has been more prevalent in men, there has been an increased incidence in women due to the change in the prevalence of tobacco use [1].
Lung cancer can be divided in two main histological groups: small cell lung carcinoma (SCLC, 15% of all lung cancers) and non-SCLC (NSCLC, 85% of all lung cancers). Among non-SCLC, the most prevalent type is adenocarcinoma (40%) followed by squamous cell carcinoma (25%) [3].
Several key factors determine the choice of initial treatment for advanced or metastatic NSCLC: tumour-related factors (histology, molecular testing), patient-related factors (age, performance status, comorbidities, patient preferences) [4]. Platinum-based regimens have been the main treatment while using conventional chemotherapy, but they are no longer in the front-line setting due to its low overall survival (less than 50% after one year of treatment) [5,6]. The incorporation of immunotherapy into clinical practice has revolutionized the management of this pathology, specially with monoclonal antibodies directed against programmed death receptor 1 (PD-1) or its ligands (PD-L1) in patients without driver mutations; although in these patients the treatment will also depend on the general condition performance status (PS) and tumor PD-L1 expression [7].
The intestinal microbiota has recently been postulated as a potential predictor or modulator of ICI response such as the expression of PD-L1 or tumor mutational burden (TMB) [8].
Routy et al. [9] showed that the gut microbiome influenced the outcome of PD-1 blockade in both mice and humans: they designed a trial in which they transplanted fecal microbiota from cancer patients who responded to immune checkpoint inhibitors (ICI) into germ-free or antibiotic-treated mice, and they observed a better PD-1 blockade, while no effect was seen when the recipient was a non-respondent patient.
This idea was reinforced by series of published cases: fecal microbiota transplantation improved colitis associated with ICI through a relative increase in the proportion of regulatory T cells in the colon mucosa [10]. Some authors suggest that it could be due to the relative abundance of different bacteria such as Akkermansia muciniphila, BIfidobacterium or Bacteroides fragilis [11].
Antibiotics are widely used in clinical practice, and it is well known that their administration produces changes in the intestinal microbiota. Based on this line of research, several studies published last years have associated poorer overall survival and an increased risk of refractory disease in cancer patients treated with ICI therapy associated with antibiotic treatment [8;12-25], when administered within 60 days prior to the start of or during ICI therapy.
However, there is controversy regarding these findings, since other authors have not observed such association in their cohorts [8].
Patients with advanced or metastatic NSCLC are especially candidates for receiving antibiotic treatment during the course of their disease or prior to their diagnosis, based on 2 reasons [8]:
- Smoking: NSCLC is widely linked to smoking (80-90% of cases). Smoking favors lung infection as it weakens local epithelial immunity and cilio-induced mucus clearance [17]. In addition, tobacco induces a pulmonary obstruction that leads to more frequent respiratory infections, with cough and chronic expectoration, which requires repeated courses of antibiotics.
- Age: the median age for the diagnosis of patients with advanced or metastatic NSCLC is between 65 and 70 years, so they could be more sensitive to infections.
This study therefore aims to analyze the influence of antibiotics over the effectiveness of immunotherapy when used for advanced or metastatic NSCLC, using progression-free survival (PFS) end overall survival (OS) of atezolizumab, nivolumab, pembrolizumab based on the exposure to antibiotics.
MATERIAL AND METHODS
Retrospective cohort study from May 2016 to May 2021 in a third-level hospital, including every patient diagnosed of metastatic NSCLC and treated with at least two doses of atezolizumab, nivolumab or pembrolizumab, regardless of its use alone or in combination with other antineoplastic agents.
Exclusion criteria included non-available primary-care medical records, single dose ICI administration, treatment interruption due to adverse effects prior to response assessment, incomplete follow-up for any reason.
A sample of 150-170 patients diagnosed with stage IIIIV NSCLC was estimated, who had received a PD1 / PD-L1 antagonist. Antibiotic use within 2 months of checkpoint inhibitors treatments initiation (2 months before or 2 months after CPI initiation) was assessed.
The primary endpoint was progression-free survival (PFS) evaluated by iRECIST criteria. Secondary endpoints included overall survival (OS).
Patients` characteristics and treatment outcomes were analyzed based on exposure or non-exposure to antibiotics, and the data were compared using the Chi-square test for categorical variables and the Student’s t test for continuous variables. A Cox model was used to estimate the hazard ratio (HR) of each endpoint associated with potential risk factors.
Ethics approval. Comité de Investigación Clínica de Cabueñes approved the study. (reference number 25052021).
RESULTS
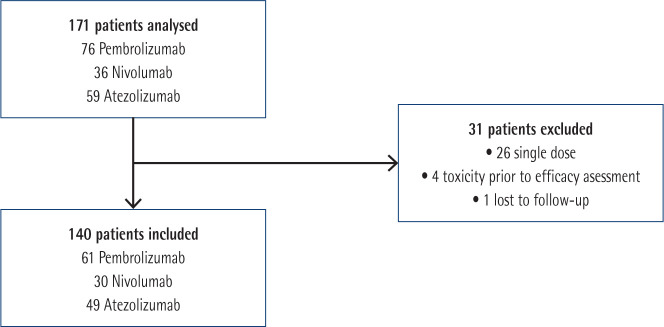
A total of 171 patients were included in the study, 140 of whom were finally analysed. Flow diagram for patients`inclusion and exclusion is showed in Figure 1.
Figure 1.
Flow diagram for inclusion and exclusion of studies.
Patients’ characteristics are described on table 1. Groups were well balanced, including predominantly stage IV NSCLC, mainly men (70%), in good general condition (ECOG 0-1) with a median age of 65.5 years at the start of treatment. ICI were used mainly in monotherapy, both in the first (46.4%) and second (47.1%) lines. The most frequently observed histology was adenocarcinoma (67.1%), with the PD-L1 marker being positive in 77% of the cases.
Table 1.
Patients’ characteristics
| Non antibiotic exposure (n=97) |
Antibiotic exposure (n=43) |
P-value | Total (N=140) | |
|---|---|---|---|---|
| Age | ||||
| Mean (SD) | 65.5 (7.35) | 65.9 (9.79) | 0.61 | 65.7 (8.15) |
| Median [Q1, Q3] | 65.8 [60.0, 71.0] | 65.1 [60.9, 73.5] | 65.5 [60.2, 71.8] | |
| Sex | ||||
| Men | 70 (72.2%) | 27 (62.8%) | 0.322 | 97 (69.3%) |
| Women | 27 (27.8%) | 16 (37.2%) | 43 (30.7%) | |
| Histology | ||||
| Adenocarcinoma | 71 (73.2%) | 23 (53.5%) | 0.013 | 94 (67.1%) |
| Squamous | 10 (10.3%) | 13 (30.2%) | 23 (16.4%) | |
| Others | 16 (16.5%) | 7 (16.3%) | 23 (16.4%) | |
| Stage | ||||
| IIIa | 1 (1.03%) | 1 (2.33%) | 0.622 | 2 (1.43%) |
| IIIb | 7 (7.22%) | 2 (4.65%) | 9 (6.43%) | |
| IV | 89 (91.8%) | 40 (93.0%) | 129 (92.1%) | |
| Drug | ||||
| Atezolizumab | 38 (39.2%) | 11 (25.6%) | 0.238 | 49 (35.0%) |
| Nivolumab | 18 (18.6%) | 12 (27.9%) | 30 (21.4%) | |
| Pembrolizumab | 41 (42.3%) | 20 (46.5%) | 61 (43.6%) | |
| ECOG | ||||
| 0 | 30 (30.9%) | 8 (18.6%) | 0.11 | 38 (27.1%) |
| 1 | 67 (69.1%) | 34 (79.1%) | 101 (72.1%) | |
| 2 | 0 (0%) | 1 (2.33%) | 1 (0.714%) | |
| Smoker | ||||
| Previous | 55 (56.7%) | 21 (48.8%) | 0.658 | 76 (54.3%) |
| Never | 5 (5.15%) | 3 (6.98%) | 8 (5.71%) | |
| Current | 37 (38.1%) | 19 (44.2%) | 56 (40.0%) | |
| PDL1 expression | ||||
| PDL1 ≥50 | 38 (39.1%) | 21 (48.8%) | 0.146 | 59 (42.1%) |
| PDL1 < 50 | 43 (44.3%) | 13 (30.2%) | 56 (40.0%) | |
ECOG: Eastern Cooperative Oncology Group
Table 2 lists the characteristics of antibiotic treatment. Antibiotic use within 2 months previous or after the start of ICI was observed in 43 patients (30.7%). The main indication was respiratory infections (80.4%), for which fluoroquinolones (47,1%) or beta-lactams (39.2%) were indicated.
Table 2.
Characteristics of the antibiotic treatment
| n | % | ||
|---|---|---|---|
| Pacients on antibiotics (n; %) | 43 | 30.71% | |
| Atezolizumab | 12 | 27.90% | |
| Nivolumab | 12 | 27.90% | |
| Pembrolizumab | 19 | 44.18% | |
| Antibiotic type (n; %) | 51* | ||
| Fluoroquinolone | 24 | 47.06% | |
| Ciprofloxacin | 4 | ||
| Levofloxacin | 18 | ||
| Moxifloxacin | 2 | ||
| Beta-Lactam | 20 | 39.22% | |
| Penicillin | 13 | ||
| Cephalosporin | 7 | ||
| Aminoglycoside | Tobramycin | 1 | 1.96% |
| Glycopeptide | Vancomycin | 1 | 1.96% |
| Lincosamide | Clindamycin | 1 | 1.96% |
| Macrolide | Azithromycin | 3 | 5.88% |
| Others | Fosfomycin | 1 | 1.96% |
| Indication (n; %) | |||
| Respiratory infection | 41 | 80.39% | |
| Urinary tract infection | 4 | 7.84% | |
| Bacteriemia | 2 | 3.92% | |
| Others | 4 | 7.84% | |
| Duration | |||
| Mean - range (days) | 8,25 | 1-15 |
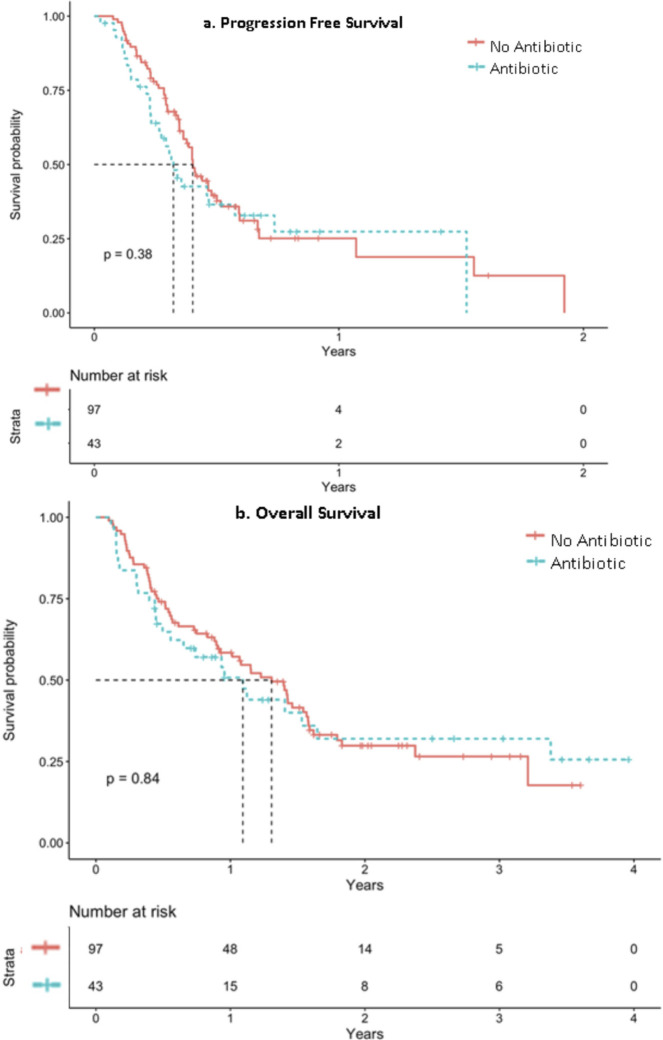
Regarding the effectivity of ICIs, figure 2 shows PFS (figure 2a) and OS (figure 2b) based on their exposure to antibiotics. Not statistically differences were observed. The median PFS was 123 days (95% IC 126.84-170.82), being higher in patients without antibiotic treatment (170 days, 95% CI 116.041-223.96) compared to those who did received (124 days, 95% CI 52.79-195.21). Regarding OS, the median was also more favorable in patients without antibiotic treatment (median 477 days 95% CI 361.07-592.93 vs 399.00 days 95% CI 227.62-570.38), without reaching statistical significance in this case either (p 0.8).
Figure 2.
Progression free survival (figure 2a) and overall survival (figure 2b) based on their exposure to antibiotics.
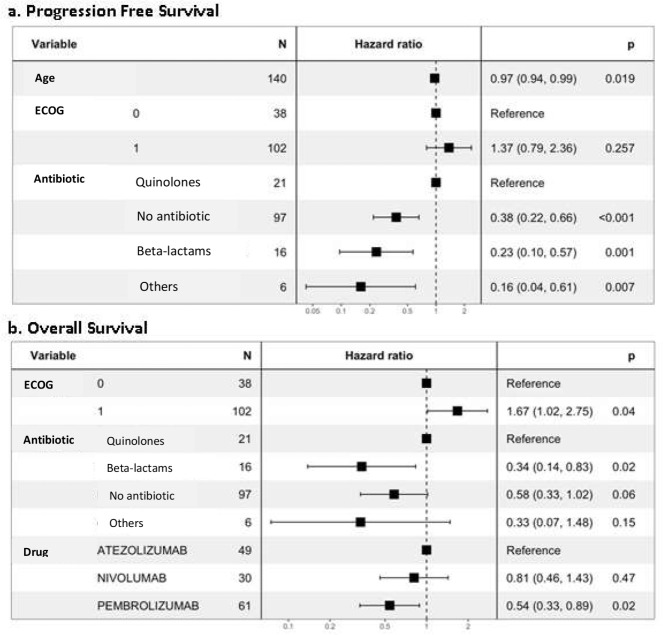
According to the subgroup analysis, both PFS and OS were worse among patients who received fluoroquinolones (figure 3). The OS was significantly better in patients receiving beta-lactams compared to those treated with fluoroquinolones (figure 3b). PFS was slightly better among patients exposed to IV antibitiotics (median PFS 167.9 days; 95% IC 54.0-NA) rather than oral antibiotics (median PFS 112 days; 95% IC 83.95-209.8). Better PFS were reached among patients on nivolumab (167.9 days; 95% IC 123.73-NA), followed by pembrolizumab (146.7 days; 95% IC 129.9-NA) and atezolizumab (140.9 days; 95% IC 108.77-182.86). The trend of better outcomes for patients without antibiotic treatment was observed regardless of ECOG value, sex or atezolizumab/pembrolizumab treatment. However, this trend was reversed in the group of patients who received nivolumab, among whom PFS was better in those exposed to antibiotics (167.90 days vs 137.97 days).
Figure 3.
Progression free survival (figure 3a) and overall survival (figure 3b): subgroup analysis.
DISCUSSION
The demographic characteristics are similar to those of other studies analysing the same effect: in the meta-analysis carried out by Lurienne et al. [8], male patients predominated (58.3%; 40-82%), with non-squamous histology (71.2%) and ECOG 0-1 (92.6%). Patients included in our study were slightly younger than the referenced.
Most patients were receiving second or third line treatment, which is consistent with the fact that ICIs were used mainly in monotherapy, since the authorized use in monotherapy is reserved for patients with progression on or after platinum-based chemotherapy, with the exception of pembrolizumab, which can also be used as monotherapy as the first-line treatment of patients with metastatic NSCLC whose tumors express PD-L1 Tumor Proportion Score ≥50% [27-29].
The prevalence of antibiotic use was around 30%, similar to that previously reported in the literature [9; 16]; although it could range between 14 and 44% depending on the selected cohort [23-24]. Antibiotic types were also similar to the other authors, predominating fluoroquinolones and beta-lactams due to their wide coverage of pathogens that cause respiratory infections. However, no study or meta-analysis has been able to analyze the influence of the type of antibiotic on the effectiveness of the treatment. Nor the effect of its duration, which appears heterogeneously collected depending on the cohort.
When analyzing the effect of antibiotics on survival, there are two key aspects to consider. The window of exposure to the antibiotic, which was set at 60 days before or after the start of treatment in our study. This period was selected based on the systematic review by Lurienne et al. [8], in which they subdivided the studies into 4 groups based on the window selected to perform the analysis, concluding that the effect of antibiotics on ICIs was greater when these were used within 60 days of the start of immunotherapy. However, despite being a critical factor that would explain the difference observed in terms of efficacy, there is no consensus on the time to analyze, varying from 30-90 days before [22-29] to more than 365 days later in some cases [25].
While analysing the influence of antibiotic exposure in survival, we obtained a difference of 1.5 months in PFS and 2.6 months in OS, without finding significant differences. These values differ from those of any other published study, probably due to their heterogeneity in terms of patients included and window of exposure to antibiotics.
This difference in survival could also be related to the limitations of the study: the bias inherent to this type of design due to its retrospective nature, the lack of controlled variables, introducing confounding factors that could influence the interpretation of the results. Other aspects to take into account are the limited number of patients, and the lack of information on other intestinal modulators such as diet, concomitant medications that can alter the bacterial flora or self-medication with over-the-counter prescriptions. Corticosteroid use, that has not been analysed, could also influence treatment response.
The use of antibiotics was associated with a reduction in PFS and OS in patients who received antibiotic treatment, not statistically different. More studies would be necessary to determine its real influence on the effectiveness of ICIs.
FUNDING
None to declare.
CONFLICT OF INTEREST
Authors declare no conflict of interest.
References
- 1.Organización Mundial de la Salud. Datos y cifras sobre el cáncer [cited 10 April 2022]. Available from: http://www.who.int/cancer/about/facts/es/
- 2.Sociedad Española de Oncología Médica. Las cifras del cáncer en España 2017 [cited 10 April 2022]. Available from: https://www.seom.org/seomcms/images/stories/recursos/Las_cifras_del_cancer_en_Esp_2017.pdf
- 3.Sos ML, Thomas RK. Genetic insight and therapeutic targets in squamous-cell lung cancer. Oncogene 2012;31:4811-14. DOI: 10.1038/onc.2011.640 [DOI] [PubMed] [Google Scholar]
- 4.Novello S, Barlesi F, Califano R, Cufer T, Ekman S, Giaj Levra M, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016. Sep; 27 (Suppl 5):v1-v27 . DOI: 10.1093/annonc/mdw326 [DOI] [PubMed] [Google Scholar]
- 5.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüs M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. DOI: 10.1056/NEJMoa1810865 [DOI] [PubMed] [Google Scholar]
- 6.Qiu Z, Chen Z, Zhang C, Zhong W. Achievements and futures of immune checkpoint inhibitors in non-small cell lung cancer. Exp Hematol Oncol. 2019;8:9. DOI: 10.1186/s40164-019-0143-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majem M, Juan O, Insa A, Reguart N, Trigo JM, Carcereny E, et al. SEOM clinical guidelines for the treatment of non-small cell lung cancer (2018). Clin Transl Oncol 2019;21:3-17. DOI: 10.1007/s12094-018-1978-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lurienne L, Cervesi J, Duhalde L, Gunzburg J, Andremont A, Zalcman G, et al. NSCLC Immunotherapy Efficacy and Antibiotic Use: A Systematic Review and Meta-Analysis. J Thorac Oncol . 2020. Jul;15(7):1147-1159. DOI: 10.1016/j.jtho.2020.03.002 [DOI] [PubMed] [Google Scholar]
- 9.Routy B, Le Chatelier E, Derosa L, Duong C, Tidjani M, Daillère R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018; 359(6371): 91-97. DOI: 10.1126/science.aan3706 [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Wienoski D, Helmink B, Gopalakrishnan V, Choi K, DuPont H, et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med 2018;24:1804-1808. DOI: 10.1038/s41591-018-0238-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schett A, Rotschild S, Curioni-Fontecedro A, Krähenbühl S, Früh M, Schmid S, et al. Predictive impact of antibiotics in patients with advanced non small-cell lung cancer receiving immune checkpoint inhibitors. Cancer chemother Pharmacol 2020;85:121-131. DOI: 10.1007/s00280-019-03993-1 [DOI] [PubMed] [Google Scholar]
- 12.Ubeda C & Pamer EG. Antibiotics, microbiota, and immune defense. Trends Immunol 2012; 33: 459-466. DOI: 10.1016/j.it.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mielgo Rubio X, Chara L, Sotelo-Lezama M, Lopez-Castro R, Rubio-Martinez J, Velastegui A, et al. Antibiotic use and PD-1 inhibitors: shorter survival in lung cancer,especially when given intravenously. Type of infection also matters. J Thorac Oncol. 2018, 13 : S389-S437. DOI: 10.1016/j.jtho.2018.08.395 [DOI] [Google Scholar]
- 14.Tinsley N, Zhou C, Tan G, Rack S, Lorigan P, Blackhall F, et al. Cumulative antibiotic use and efficacy of immune checkpoint inhibitors in patients with advanced cancer. Oncologist. 2020. Jan;25:55-63. DOI: 10.1634/theoncologist.2019-0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derosa L, Routy B, Mezquita L, Naltet C, Enot D, Fidelle M, et al. Antibiotic prescription to decrease progression-free survival (PFS) and overall survival (OS) in patients with advanced cancers treated with PD1/PDL1 immune checkpoint inhibitors. J Clin Oncol. 2017; 35(15):3015. [Google Scholar]
- 16.Derosa L, Hellman M, Spaziano M, Halpenny D, Fidelle M, Rizvi H, et al., Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol 2018; 29(6): 1437-1444. DOI: 10.1093/annonc/mdy103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet 2017;389:1931-1940. DOI: 10.1016/S0140-6736(17)31222-9 [DOI] [PubMed] [Google Scholar]
- 18.Socinski MA, Obasaju C, Gandara D, Hirsch F, Bonomi P, Bunn P, et al. Clinicopathologic Features of Advanced Squamous NSCLC. J Thorac Oncol. 2016;11:1411-1422. DOI: 10.1016/j.jtho.2016.05.024 [DOI] [PubMed] [Google Scholar]
- 19.Galli G, Triulzi T, Proto C, Signorelli D, Imbimbo M, Poggi M, et al. Association between antibiotic-immunotherapy exposure ratio and outcome in metastatic non small cell lung cancer. Lung Cancer. 2019;132:72–78. DOI: 10.1016/j.lungcan.2019.04.008 [DOI] [PubMed] [Google Scholar]
- 20.Hakozaki T, Okuma Y, Omori M, Hosomi Y. Impact of prior antibiotic use on the efficacy of nivolumab for non-small cell lung cancer. Oncol Lett. 2019;17:2946–2952. DOI: 10.3892/ol.2019.9899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huemer F, Lang D, Westphal T, Gampenrieder SP, Hutarew G, Weiss L, et al. Baseline absolute lymphocyte count and ECOG performance score are associated with survival in advanced non-small cell lung cancer undergoing PD-/PD-L1 blockade. J Clin Med. 2019;8:1014. DOI: 10.3390/jcm8071014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaderbhai C, Richard C, Fumet JD, Aarnink A, Foucher P, Coudert B, et al. Antibiotic use does not appear to influence response to nivolumab. Anticancer Res. 2017;37:3195–3200. DOI: 10.21873/anticanres.11680 [DOI] [PubMed] [Google Scholar]
- 23.Ouaknine Krief J, Helly de Tauriers P, Dumenil C, Neveux N, Dumoulin J, Giraud V, et al. Role of antibiotic use, plasma citrulline and blood microbiome in advanced non-small cell lung cancer patients treated with nivolumab. J Immunother Cancer. 2019;7:176. DOI: 10.1186/s40425-019-0658-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinato DJ, Howlett S, Ottaviani D, Urus H, Patel A, Mineo T, et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol. 2019;5:1774–1778. DOI: 10.1001/jamaoncol.2019.2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao S, Gao G, Li W, Li X, Zhao C, Jiang T, et al. Antibiotics are associated with attenuated efficacy of anti-PD-1/PD-L1 therapies in Chinese patients with advanced non-small cell lung cancer. Lung Cancer. 2019;130:10–17. DOI: 10.1016/j.lungcan.2019.01.017 [DOI] [PubMed] [Google Scholar]
- 26.Seymour, L; Bogaerts J., Perrone A, Ford R, Schwartz L, Mandrekar S, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet. Oncol. 2017. Mar; 18: e143-e152. DOI: 10.1016/S1470-2045(17)30074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Summary of product characteristics (Tecentriq®). [cited 10 April 2022]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/tecentriq
- 28.Summary of product characteristics (Keytruda®). [cited 10 April 2022]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/keytruda
- 29.Summary of product characteristics (Opdivo®). [cited 10 April 2022]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/opdivo