Sir,
At the present time, there are no recommendations on the management of SARS-CoV-2 pneumonia in immunocompromised patients or on the usefulness of antiviral treatment beyond the first days of illness in patients without viral clearance. We describe the good response to nirmatrelvir/ritonavir in an oncohematologic patient, who is in his fifth month of infection with SARS-CoV-2 pneumonia and in his fifth admission due to recurrent pneumonia attributed to the infection itself.
68-year-old male diagnosed with diffuse large B-cell lymphoma in 2009 and treated with three cycles of chemotherapy that was terminated due to infectious complications. In 2014 and 2019 he presented relapses of the disease that required new treatment. He has been in complete remission for two years and with bimonthly maintenance rituximab (RTX) (end of treatment 11/8/21). Vaccinated with 3 doses against SARSCoV-2 (Pfizer-BioNTech®, last dose 11/27/21).
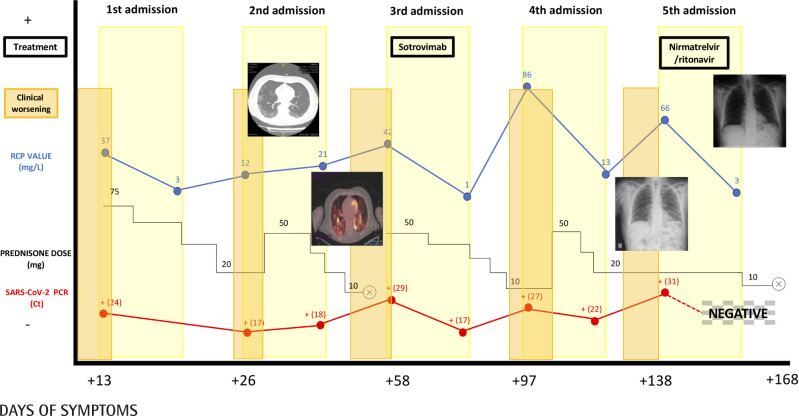
On 1/6/22 he started febrile symptoms and cough, and was diagnosed with COVID-19 by detection in nasopharyngeal exu-date sample of SARS-CoV-2 RNA by real-time PCR (Simplexa™-COVID-19 & Flu A/B Direct Kit, Diasorin®) and associated dyspnea on day +13. In analytical tests, C reactive protein (CRP) 37.70 mg/L (1.00-10.00), procalcitonin (PCT) 0.09 ng/mL (0.0-0.1), ferritin 464 ng/mL (20-300), absence of lymphopenia and a faint bilateral infiltrate on chest X-ray (CXR). He was admitted and received 60 mg/24h of methylprednisolone (MTP) for 6 days, with good clinical, analytical and radiological evolution. On admission, a serological study of IgG was performed by chemiluminescent microparticle assay (CMIA) against SARS-CoV 2 protein S (SARSCoV-2 IgG II Quant, Alinity, Abbott®) and IgM against SARS-CoV-2 protein S by chemiluminescence assay (CLIA) (LIAISON® SARS-CoV-2 IgM, Diasorin®), both results being negative. At discharge, prednisone (PDN) was prescribed in a descending regimen.
After 10 days and being on 20 mg of PDN daily, he consulted again for dyspnea and cough, with the appearance of new infiltrates on CXR. Both SARSCoV-2 antigen and PCR were positive (Ct=17), without detecting IgG antibodies against SARS-CoV-2 protein S. High-resolution computed axial tomography (HRCT) was performed and revealed the presence of ground glass in the periphery of both lower lung lobes. No clinical improvement was observed until the corticosteroid dose was increased to 40 mg of MTP daily, and he was discharged on day 12 with 30 mg of PDN. At discharge, COVID-19 CRP positive (Ct=18) (day +36 since onset of symptoms).
Coinciding with the decrease to 10 mg of PDN, he presented again dyspnea and fever (38ºC), for which he was admitted (day +58 of symptoms), having completed the tapering corticosteroid regimen. Previously, a PET-CT scanner was performed with marked bilateral pulmonary hypermetabolism compatible with an active infectious process. On admission he presented lymphopenia, discrete elevation of CRP and bilateral peripheral interstitial involvement on chest peripheral interstitial involvement on chest X-ray. He still did not develop IgG against S protein and PCR was still positive (Ct=29). Fibrobronchoscopy was performed, in which only the detection by RT-PCR of SARSCoV-2 (E and Y genes) (Xpert®Xpress SARS-CoV-2/Flu/ RSV, Cepheid®Xpress RSV, Cepheid®) in the bronchioalveolar lavage was outstanding (Ct=17). Corticosteroids were reintroduced and a single dose of sotrovimab 500 mg was administered. With this he showed immediate improvement. However, at discharge the SARS-CoV-2 PCR remained positive (Ct=20) and a further decrease in corticosteroids was scheduled.
With the decrease of PDN, he presented respiratory symptoms requiring admission, similar to the three previous admissions: reappearance of radiological infiltrates with positive PCR of SARS-CoV-2 (Ct=27). After a few days of admission and in the absence of improvement, corticotherapy was increased (40 mg MTP) with resolution of clinical, analytical and radiological alterations. At discharge PDN 20 mg was maintained and the SARS-CoV-2 PCR was positive (Ct=22).
After 9 days of PDN 20 mg daily, the previous symptoms reappeared. He was admitted on day +138 of symptoms. At this time, radiographic worsening with positive SARS-CoV-2 antigen and PCR (Ct=31). After verbal consent from the patient, nirmatrelvir/ritonavir was administered off-label for 5 days and the PDN 20 mg dose was maintained. This resulted in immediate clinical improvement as well as analytical and radiological normalization. The first negative PCR result for SARSCoV-2 since the onset of the disease was obtained.
After discharge from this fifth and last hospitalization, corticosteroids were progressively reduced until they were withdrawn without relapse of the disease. He is currently at day +75 since taking nirmatrelvir/ritonavir and the patient remains asymptomatic. The patient’s evolution is summarized in Figure 1.
Figure 1.
Clinical, analytical and radiological evolution of the patient
Corticosteroid therapy could contribute to reduce viral clearance [1] and cases with persistence of viral activity and relapse of pneumonia are beginning to be described in patients with immunosuppressive treatment, especially with RTX (anti-CD20 monoclonal antibody) [2-4]. After a review of the literature, we found the description of 3 cases in which the administration of antiviral treatment beyond the first days of symptoms in patients treated with anti-CD20 drugs and with evidence of prolonged persistence of viral activity [5-7]. In all of them, the antiviral used was remdesivir, and although the symptoms subsided, later relapse of the disease occurred with PCR positivization. In these cases, remdesivir seems to contribute to attenuate the infection but not to eliminate it completely [5-7]. In contrast, we found no similar experience with nirmatrelvir/ritonavir. Therefore, the present work describes the first experience to date of an exceptional clinical response to late treatment with nirmatrelvir/ritonavir in an immunosuppressed patient with anti-CD20 drug and evidence of prolonged SARSCoV-2 infection.
FUNDING
None to declare
CONFLICTS OF INTEREST
IPC has participated in several scientific meetings paid for by the Gilead laboratory on the antiviral treatment of COVID-19 during the current year 2022. The rest of the authors declare that they have no conflict of interest directly or indirectly related to the contents of the manuscript.
References
- 1.Tang X, Feng YM, Ni JX, Zhang JY, Liu LM, Hu K, et al. Early Use of Corticosteroid May Prolong SARS-CoV-2 Shedding in Non-Intensive Care Unit Patients with COVID-19 Pneumonia: A Multicenter, Single-Blind, Randomized Control Trial. Respiration. 2021;100(2):116-126. doi: 10.1159/000512063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morel A, Imbeaud S, Scemla A, Péré H, Fourgeaud J, Amrouche L, et al. Severe relapse of SARS-CoV-2 infection in a kidney transplant recipient with negative nasopharyngeal SARS-CoV-2 RT-PCR after rituximab. Am J Transplant. 2022. Feb 12:10.1111/ajt.17000. doi: 10.1111/ajt.17000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prasad RM, Srivastava S, Wang E, Liu JZ, Gami R, Abdelgadir A, et al. Effect of Immunosuppressive Diseases and Rituximab Infusions on Allowing COVID-19 Infection to Relapse. Perm J. 2021. Oct 29;26(1):123-131. doi: 10.7812/TPP/21.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martínez-Chinchilla C, Vazquez-Montero L, Palazón-Carrión N, Fernández-Román IM, López-Barba J, de la Cruz-Merino L, et al. Persistence of SARS-CoV-2 Infection in Severely Immunocom-promised Patients With Complete Remission B-Cell Lymphoma and Anti-CD20 Monoclonal Antibody Therapy: A Case Report of Two Cases. Front Immunol. 2022. Apr 14;13:860891. doi: 10.3389/fimmu.2022.860891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baang JH, Smith C, Mirabelli C, Valesano AL, Manthei DM, Bach-man MA, et al. Prolonged Severe Acute Respiratory Syndrome Coronavirus 2 Replication in an Immunocompromised Patient. J Infect Dis. 2021. Jan 4;223(1):23-27. doi: 10.1093/infdis/jiaa666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi B, Choudhary MC, Regan J, Sparks JA, Padera RF, Qiu X, et al. Persistence and Evolution of SARS-CoV-2 in an Immunocom-promised Host. N Engl J Med. 2020. Dec 3;383(23):2291-2293. doi: 10.1056/NEJMc2031364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camprubí D, Gaya A, Marcos MA, Martí-Soler H, Soriano A, Mosquera MDM, et al. Persistent replication of SARS-CoV-2 in a severely immunocompromised patient treated with several courses of remdesivir. Int J Infect Dis. 2021. Mar;104:379-381. doi: 10.1016/j.ijid.2020.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]