Abstract
Background
Traumatic peripheral nerve injury is common and incurs significant cost to individuals and society. Healing following direct nerve repair or repair with autograft is slow and can be incomplete. Several bioengineered nerve wraps or devices have become available as an alternative to direct repair or autologous nerve graft. Nerve wraps attempt to reduce axonal escape across a direct repair site and nerve devices negate the need for a donor site defect, required by an autologous nerve graft. Comparative evidence to guide clinicians in their potential use is lacking. We collated existing evidence to guide the clinical application of currently available nerve wraps and conduits.
Objectives
To assess and compare the effects and complication rates of licensed bioengineered nerve conduits or wraps for surgical repair of traumatic peripheral nerve injuries of the upper limb.
To compare effects and complications against the current gold surgical standard (direct repair or nerve autograft).
Search methods
We used standard, extensive Cochrane search methods. The latest search was 26 January 2022. We searched online and, where not accessible, contacted societies' secretariats to review abstracts from the British Surgical Society of the Hand, International Federation of Surgical Societies of the Hand, Federation of European Surgical Societies of the Hand, and the American Society for Peripheral Nerve from October 2007 to October 2018.
Selection criteria
We included parallel group randomised controlled trials (RCTs) and quasi‐RCTs of nerve repair in the upper limb using a bioengineered wrap or conduit, with at least 12 months of follow‐up.
Data collection and analysis
We used standard Cochrane procedures. Our primary outcomes were 1. muscle strength and 2. sensory recovery at 24 months or more. Our secondary outcomes were 3. British Medical Research Council (BMRC) grading, 4. integrated functional outcome (Rosén Model Instrument (RMI)), 5. touch threshold, 6. two‐point discrimination, 7. cold intolerance, 8. impact on daily living measured using the Disability of Arm Shoulder and Hand Patient‐Reported Outcome Measure (DASH‐PROM), 9. sensory nerve action potential, 10. cost of the device, and 11. adverse events (any and specific serious adverse events (further surgery)). We used GRADE to assess the certainty of the evidence.
Main results
Five studies involving 213 participants and 257 nerve injuries reconstructed with wraps or conduits (129 participants) or standard repair (128 participants) met the inclusion criteria. Of those in the standard repair group, 119 nerve injuries were managed with direct epineurial repair, and nine autologous nerve grafts were performed. One study excluded the outcome data for the repair using an autologous nerve graft from their analysis, as it was the only autologous nerve graft in the study, so data were available for 127 standard repairs. There was variation in the functional outcome measures reported and the time postoperatively at which they were recorded.
Mean sensory recovery, assessed with BMRC sensory grading (range S0 to S4, higher score considered better) was 0.03 points higher in the device group (range 0.43 lower to 0.49 higher; 1 RCT, 28 participants; very low‐certainty evidence) than in the standard repair group (mean 2.75 points), which suggested little or no difference between the groups, but the evidence is very uncertain. There may be little or no difference at 24 months in mean touch thresholds between standard repair (0.81) and repair using devices, which was 0.01 higher but this evidence is also very uncertain (95% confidence interval (CI) 0.06 lower to 0.08 higher; 1 trial, 32 participants; very low‐certainty evidence).
Data were not available to assess BMRC motor grading at 24 months or more. Repair using bioengineered devices may not improve integrated functional outcome scores at 24 months more than standard techniques, as assessed by the Rosén Model Instrument (RMI; range 0 to 3, higher scores better); the CIs allow for both no important difference and a better outcome with standard repair (mean RMI 1.875), compared to the device group (0.17 lower, 95% CI 0.38 lower to 0.05 higher; P = 0.13; 2 trials, 60 participants; low‐certainty evidence). Data from one study suggested that the five‐year postoperative outcome of RMI may be slightly improved after repair using a device (mean difference (MD) 0.23, 95% CI 0.07 to 0.38; 1 trial, 28 participants; low‐certainty evidence). No studies measured impact on daily living using DASH‐PROM.
The proportion of people with adverse events may be greater with nerve wraps or conduits than with standard techniques, but the evidence is very uncertain (risk ratio (RR) 7.15, 95% CI 1.74 to 29.42; 5 RCTs, 213 participants; very low‐certainty evidence). This corresponds to 10 adverse events per 1000 people in the standard repair group and 68 per 1000 (95% CI 17 to 280) in the device group. The use of nerve repair devices may be associated with a greater need for revision surgery but this evidence is also very uncertain (12/129 device repairs required revision surgery (removal) versus 0/127 standard repairs; RR 7.61, 95% CI 1.48 to 39.02; 5 RCTs, 256 nerve repairs; very low‐certainty evidence).
Authors' conclusions
Based on the available evidence, this review does not support use of currently available nerve repair devices over standard repair. There is significant heterogeneity in participants, injury pattern, repair timing, and outcome measures and their timing across studies of nerve repair using bioengineered devices, which make comparisons unreliable. Studies were generally small and at high or unclear risk of bias. These factors render the overall certainty of evidence for any outcome low or very low. The data reviewed here provide some evidence that more people may experience adverse events with use of currently available bioengineered devices than with standard repair techniques, and the need for revision surgery may also be greater. The evidence for sensory recovery is very uncertain and there are no data for muscle strength at 24 months (our primary outcome measures). We need further trials, adhering to a minimum standard of outcome reporting (with at least 12 months' follow‐up, including integrated sensorimotor evaluation and patient‐reported outcomes) to provide high‐certainty evidence and facilitate more detailed analysis of effectiveness of emerging, increasingly sophisticated, bioengineered repair devices.
Plain language summary
Use of nerve repair devices in the arm, forearm, and hand
What is nerve injury and how is it repaired?
Injuries to the nerves of the arm, forearm, and hand are common and have a lasting effect on a person's ability to move and their sensation. Usually surgeons stitch injured nerves together (we call this standard repair). Sometimes they also use a nerve graft, which means taking a nerve from another area of the body to bridge a gap between the ends of a damaged nerve. Standard methods of repair are not always successful and even when successful, the healing process can be incomplete and slow. Obtaining nerve for grafting requires extra surgery and can cause discomfort. Other types of nerve repair involve use of a wrap (a device used to support the nerve repair), or a conduit (a device used to bridge the gap). Various natural and synthetic nerve repair wraps and conduits are available.
What did we want to find out?
We wanted to compare repair of injured nerves using a wrap or conduit to standard repair (with or without a nerve graft). We were particularly interested in how well a person's muscle strength and sensation returned, and how often they experienced problems (complications) from the surgery.
What did we do?
The review authors collected all relevant studies to answer this question and found five studies. It takes at least 12 months for nerves to grow back after surgery, so we only included studies that measured the effects of surgery from 12 months after the injury.
What did we find?
The studies involved 213 people with 257 nerve injuries. Conduits or wraps ('devices') were used for 129 injuries and standard repair for 128 injuries. Four studies took place in Europe and one in the US. Two studies were sponsored by the company who made the device, reflecting a potential source of bias.
A known challenge of nerve repair studies is the lack of a single reliable measure to assess the effects of treatment (outcome measure). We found that studies in the review used a range of different outcome measures and methods, which made them difficult to compare.
Key results and certainty of the evidence
There may be little or no difference in grades of recovery of sensation in people with nerve injuries 24 months or more after nerve repair using a device compared to standard repair, but the evidence is very uncertain (1 trial, 28 participants). Other methods used to test touch sensation were not always good enough to measure recovery. The studies did not report on muscle strength (according to British Medical Research Council grading) 24 months after surgery. Results indicated that there may be little or no difference in upper limb function 24 months after nerve repair with a device compared to standard repair (2 trials, 60 people). Five years after treatment, upper limb function may be slightly better after use of a device compared to standard repair, but this is very uncertain.
We found no studies that allowed people to report how they felt about the effects of surgery in relation to their activities and needs.
More complications may occur with the use of wraps and conduits, and surgery may have to be redone more often than after standard nerve repair, although these findings are also very uncertain. There was a need for unplanned surgery to remove devices due to complications in 12 of 129 devices placed; none of the 127 standard repairs required revision.
This review found no clear evidence of benefit to people with nerve injuries from use of wraps or conduits over standard surgical repair but the evidence is very uncertain. Nerve repair devices may increase complications and the need for another operation, although this evidence is also uncertain.
What are the limitations of the evidence?
We could not reliably compare hand or nerve function between studies, and a major finding of this review is that we need standardised study designs. We will need well planned studies of new nerve repair devices to inform safe future use.
The evidence is up to date to January 2022.
Summary of findings
Summary of findings 1. Bioengineered devices compared to standard techniques for peripheral nerve repair of the upper limb.
| Repair using bioengineered devices versus standard techniques | ||||||
| Patient or population: people undergoing peripheral nerve repair of the upper limb Setting: upper limb peripheral nerve injury Intervention: bioengineered devices Comparison: standard repair | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard repair | Risk with bioengineered devices | |||||
| Muscle strength at ≥ 24 months assessed with: BMRC Grading (manual muscle testing, score 0–5, where 0 = no movement, 5 = normal) | Not reported | |||||
| Sensory recovery at ≥ 24 months assessed with: BMRC Grading (score S0–S4, where S0 = no sensation, S4 = normal) Follow‐up: 2 years | The mean sensory recovery assessed with BMRC sensory grading in the standard repair group at 2 years was 2.75 points | The mean sensory recovery assessed with BMRC sensory grading at 2 years with bioengineered devices was 0.03 points higher (0.43 lower to 0.49 higher) | — | 28 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | There may be no difference in therapeutic effect on sensory recovery with bioengineered devices compared to standard repair at 24 months, but the evidence is very uncertain. |
|
Integrated functional outcome at ≥ 24 months
assessed with: RMI (scale from 0 to 3, higher score better) Follow‐up: 2 years |
The mean integrated functional outcome (RMI score) in the standard repair group was 1.875 | The mean integrated functional outcome (RMI score) with bioengineered devices was 0.17 lower (0.38 lower to 0.05 higher) | — | 60 (2 RCTs) | ⊕⊕⊝⊝ Lowc,d | There may be little or no difference in RMI with bioengineered devices compared to standard repair at 24 months to 5 years. At 5 years, the RMI may be slightly better after device repair than standard repair (MD 0.23, 95% CI 0.07 to 0.38; 1 RCT, 28 participants). |
|
Touch threshold
assessed with: Semmes‐Weinstein monofilament (score 0–1, where higher score is better) Follow‐up: 24 months |
Mean touch threshold score in the standard repair group was 0.81 | The mean touch threshold score with bioengineered devices was 0.01 higher (0.06 lower to 0.08 higher) |
— | 32 (1 RCT) | ⊕⊝⊝⊝ Very lowa,e | There may be little or no difference in touch threshold measured by Semmes‐Weinstein monofilament test with bioengineered nerve conduits compared to standard repair at 24 months. Semmes‐Weinstein monofilament test contributed to RMI data in 2 studies at 12 months. 1 further study planned to use this outcome measure but found it to be imprecise and did not report data. |
| Impact on daily living assessed with: DASH PROM Scale from: 0 (good) to 100 (poor) Follow‐up: 24 months | No studies employed DASH PROM. | |||||
|
Adverse events assessed as: adverse events (serious and non‐serious) Follow‐up: range 3 months to 5 years |
10 per 1000 | 68 per 1000 (17 to 280) |
RR 7.15 (1.74 to 29.42) |
213 participants (5 RCTs) | ⊕⊝⊝⊝ Very lowf,g,h | Use of bioengineered devices may increase adverse events compared to standard repair techniques, but the evidence is very uncertain. 2 studies included in this analysis had no adverse events. 1 study provided no information on adverse events in the standard repair group. |
|
Specific serious adverse events: further surgery (device removal or revision)i assessed as: any unplanned secondary surgery to remove device Follow‐up: range 3 months to 5 years |
12/129 devices required further surgery (device removal) in the bioengineered devices group; 0/127 procedures required further surgery in the standard repair group | RR 7.61 (1.48 to 39.02) | 256 repairs (5 RCTs) | ⊕⊝⊝⊝ Very lowf,h | The use of bioengineered devices may require more revision (device removal or revision) than standard repair but the evidence is uncertain. Unplanned removal of 12/44 devices (1/21 poly(DL‐lactide‐caprolactone) (Neurolac) devices, 8/17 silicone devices and 3/6 polyglycolic acid devices. 2 studies included in this analysis required no device removal. |
|
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMRC: British Medical Research Council; CI: confidence interval; DASH PROM: Disability of Arm Shoulder and Hand Patient‐Reported Outcome Measure; MD: mean difference; RCT: randomised controlled trial; RMI: Rosén Model Instrument; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded twice for imprecision, because of the very small sample size. bDowngraded once for study limitations; outcome assessor blinding was broken beyond the first follow‐up year, representing a high risk of bias, and we judged two domains, including allocation concealment, at unclear risk of bias. cDowngraded once for imprecision because of the small sample size and the CIs did not rule out an effect (in favour of standard repair). dDowngraded once for study limitations; in one study, outcome assessor blinding was broken beyond the first follow‐up year, representing a high risk of bias. Across both studies, multiple domains, including allocation concealment in both studies, were at unclear risk of bias. eDowngraded once for indirectness due to subjective nature of the test, and one study found the test results too heterogeneous to be reported. fDowngraded twice for very serious imprecision because of the wide CIs. gDowngraded once for indirectness. We planned to report serious adverse events, but the studies did not classify adverse events as serious or non‐serious. hDowngraded once for study limitations. All trials were either at high risk of bias or unclear risk of bias in multiple domains. iWe added secondary surgeries for unplanned device removal to the summary of findings table as a change from protocol, as this outcome is important in decision‐making.
Background
Traumatic peripheral nerve injury occurs in domestic, industrial or military trauma, and can also occur during birth. The estimated frequency is 1 per 1000 in Europe, with the greatest prevalence in working age adults (Wilson 2003). Traumatic injuries can be lacerating, crushing, or stretching in nature and are most commonly sustained by males, with more than 50% of these occurring in the workplace (Thorsén 2012). Major peripheral nerve trauma has significant socioeconomic costs, and outcomes remain very poor in terms of pain, time to achieve plateau outcome, psychosocial impact, and return of function (Kretschmer 2009; Lundborg 2003; Rosberg 2005).
The current clinical standards of epineurial repair and nerve autograft were reaching widespread adoption by 1975 (Lundborg 2005; Smith 1964; Terzis 1975). Some closed injuries can recover without surgery, but when nerves are divided, ruptured, or severely compressed they may require decompression, repair, or reconstruction. The current gold standard technique is direct, tension‐free microsurgical repair, with use of nerve autografts when segmental defects arise (Kalomiri 1994; Millesi 1990). Despite considerable refinements in microsurgical technique, nerve healing is slow and extended periods of denervation result in muscle atrophy and trophic skin changes. Misdirection of regenerating axons results in failure to re‐innervate target organs and can lead to painful neuroma formation. The overwhelming majority of people do not achieve complete functional recovery, as current strategies for peripheral nerve repair and reconstruction fail to adequately address the neurobiology of injury and of nerve regeneration (Hart 2011; Lundborg 2000; Lundborg 2005).
An extensive preclinical literature has documented translationally relevant strategies to enhance nerve regeneration (Faroni 2015; Gaudin 2016). However, to date, clinical studies have been restricted to the use of nerve wraps and nerve conduits. The purpose of nerve wraps is to minimise suture‐associated fibrosis, reduce axonal escape, and provide narrow gaps known to facilitate neurite bridging across repair sites. If conduits can be used it would remove the need for nerve autograft harvest, along with the associated donor site scarring, sensory loss, pain, and risk of symptomatic neuroma (Hallgren 2013; Martin 2014; Murphy 2019; Wiberg 2003).
Description of the condition
The peripheral nervous system is a complex network of afferent (sensory) and efferent (motor) axons that connect cell bodies located in the central nervous system with peripheral (sensory input) and effector organs (such as muscles). Axons are situated within the endoneurium of peripheral nerves, which is formed by the extracellular matrix produced by Schwann cells. Schwann cells ensheath one or more axons depending upon whether they myelinate the axons they ensheath. They myelinate a single larger axon serving motor supply, proprioception, and fine touch sensation, and ensheath multiple unmyelinated axons in Remak bundles (Salzer 2012). Other specialised connective tissue layers provide support and mechanical protection, and guide regeneration after axons cross the site of an injury. The perineurium surrounds several axons and endoneurial tissue forming a fascicle, and the outermost layer, the epineurium, envelopes several fascicles to form the nerve bundle.
Peripheral nerve injury has been classified according to severity, to assist in making prognosis and management decisions (Lundborg 2005; Seddon 1942; Sunderland 1951). Under the widely used Seddon classification, neurapraxia is interruption of conduction without loss of axonal integrity and full recovery is expected. Axonotmesis is interruption of axonal continuity, with preservation of epineurium and perineurium structure, following which there is Wallerian degeneration of the axon distal to the site of injury. Axonal regeneration is possible following axonotmesis, as the connective tissue scaffold remains to provide topographical guidance. Recovery time is lengthy, since axons regrow at approximately 1 mm/day. Neurotmesis is complete disruption of the axon and connective tissue layers. In neurotmesis, loss of distal motor and sensory function is complete and surgery is necessary to approximate the two ends of the injury and facilitate recovery. We will consider only neurotmesis in this review.
Following neurotmesis, the distal nerve stump undergoes Wallerian degeneration, a co‐coordinated debris‐clearing event. Schwann cells dedifferentiate, proliferate, and migrate, forming bands of Büngner, as they prepare to guide future axonal outgrowth from the proximal stump (Allodi 2012; Hart 2011; Lundborg 1994). Loss of axonal continuity causes the retrograde axonal transport system to fail, leading to a cascade of molecular and genetic changes within the injured neurons. Axonal transport failure culminates either in neuronal cell death, or in the adoption of a regenerative phenotype and the extension of axons into the site of injury (Hart 2011; Terenghi 2011).
Description of the intervention
Current microsurgical methods employ epineurial sutures to approximate nerve ends with minimal tension, with or without the use of human fibrin glue (Dahlin 2008). The use of vein grafts, and other autologous tissue, to wrap the repair site has been described, but is not common practice. Where there is a gap defect, the surgeon interposes nerve autograft. The autograft is obtained by excision of functionally less important sensory nerves, creating a donor defect. Sensory nerve grafts are not a perfect system to promote motor nerve regeneration (Brushart 1993). Autologous donor nerve availability may be insufficient in large proximal injuries, such as brachial plexus injury (Millesi 2007). Even under optimal experimental conditions, less than 50% of regenerating axons successfully cross the site of surgical repair (Welin 2008).
The interventions considered here are alternative approaches to neurosyntheses, which work by approximating nerve stumps to one another within a tubular nerve conduit or by wrapping a sheet of material around the stumps to entubulate the repair site. A nerve conduit can also be used instead of an autologous nerve graft to bridge a gap defect. Designed to encourage directed regeneration and prevent axonal escape (Hart 2011), these constructs are not biologically functionalised. Preclinical research indicates that future functionalisation (by patterning, cellularisation, or the incorporation of bioactive molecules) could enhance the regenerative ability of the ingrowing cells (Dahlin 2001). These products are beginning to be used in clinical trials and practice. However, there is a paucity of data to examine their efficacy and little comparative outcome data.
Nerve wrap
A nerve wrap is a form of direct neurosynthesis, using a sheet of material that is formed into a tube around the approximated nerve stumps. The composition and manufacturer of the nerve wrap, and mechanism of securing it (e.g. sutures or glue) vary, as do the injury mechanism, preoperative delay, intraoperative details, concomitant injuries, and postoperative care. These were taken into account in this review if possible.
Nerve conduit
A nerve conduit involves reconstruction of a gap defect by the placement of proximal and distal nerve stumps into a tubular repair construct. The composition and manufacturer of the nerve conduit, and mechanism of securing it (e.g. sutures or glue) vary, as do the injury mechanism, preoperative delay, intraoperative details, concomitant injuries, and postoperative care. In addition, the length of nerve gap (and therefore, the length of the conduit employed to bridge the defect) is potentially important to the outcome of the repair.
How the intervention might work
Nerve wraps and tubular conduits present means by which to approximate nerve stumps. These can provide a way to biologically enhance the nerve regeneration microenvironment, and may minimise fibrosis and the potential for ingrowth of external scar tissues. If preclinical studies are translated, future products may be able to provide directional growth cues, and prevent dissipation of proregenerative trophic and tropic factors away from the repair site. If bioengineering design is optimised these constructs could be used to reduce axonal escape or misdirection, improve regeneration into the distal nerve, and enhance functional recovery. Tubular conduits also offer the possibility of avoiding nerve autograft harvest, and the associated morbidity of that procedure (Hallgren 2013). If any bioengineered conduit is to be safely used it should be well tolerated and provoke minimal inflammatory response and no significant adverse effects.
Why it is important to do this review
Around three million peripheral nerve repairs are performed each year (Life Science Intelligence 2009). Conduits and nerve wraps carry a significantly higher cost per item than microsurgical sutures for nerve repair. Widespread adoption of these products potentially presents a considerable economic challenge to healthcare services, and if the evidence for functional benefit is uncertain, inequalities of access could ensue. Patients will be worse off if complication profiles prove worse for these products than for standard treatments, and if the indications for their use widen uncritically.
Several inert nerve conduits and wraps, manufactured from a variety of materials, are now clinically licensed. However, we lack comparative clinical trial data comparing functional outcomes and complication profiles between products (Kehoe 2012; Meek 2008), and data comparing these techniques with nerve autograft are limited. This review aims to provide a valuable resource to clinicians and patients in identifying and synthesising the evidence on the potential role of bioengineered conduits and wraps in the management of peripheral nerve injury.
A number of factors are known to influence nerve healing including gap length, type of nerve, age, smoking status, graft type, and delay to surgery (Birch 2015; Camara 2015; Hart 2011; Hundepool 2015). We will also attempt to explore the effect of these factors on nerve repair or graft success in a subgroup analysis.
A well‐planned review of the performance of these devices compared to the current clinical gold standard is necessary to aid the clinician in identifying the potential role of bioengineered conduits and wraps in the management of peripheral nerve injury. Furthermore, critical analysis of current research will inform the design of future studies. This review will focus on nerve repair and reconstruction in the upper limb. However, we anticipate that our findings will have broader application to several other areas of surgical nerve repair, including, but not limited to, head and neck, lower limb, urological, and composite tissue allotransplantation procedures.
Objectives
To assess and compare the effects and complication rates of licensed nerve conduits or wraps for surgical repair of traumatic peripheral nerve injuries of the upper limb.
To compare effects and complications against the current gold surgical standard (direct repair or nerve autograft).
Methods
Criteria for considering studies for this review
Types of studies
Parallel group randomised controlled trials (RCTs) and quasi‐RCTs of nerve repair in the upper limb using a bioengineered wrap or conduit, with at least 12 months of follow‐up were eligible.
We included studies reported as full text, those published as abstract only, and conference reports, and applied no restrictions on the language of publication.
Types of participants
We considered studies of adults and children with a peripheral nerve transection for inclusion. We considered the influence of participant age on nerve healing, and if data had been available, we would have evaluated it via subgroup analysis (four groups: aged less than 12 years, 12 to 25 years, 26 to 40 years, over 40 years), as it has been well documented that outcomes following nerve injury decline with advancing age.
We noted details of comorbidities. Participants with the following comorbidities or characteristics, which have a significant impact on nerve healing were not eligible (Kalomiri 1994):
pre‐existing peripheral neuropathy of any type;
previous nerve injury to the peripheral nerve being repaired;
multilevel nerve injury;
metabolic conditions, drug therapy, or other concomitant conditions known to impair nerve healing, such as diabetes (Stenberg 2014), thyroid disease, autoimmune disease, allo‐transplant recipients, malignancy, HIV/AIDS, or chemotherapy.
Types of interventions
Direct neurosyntheses (i.e. no gap)
Trials comparing peripheral nerve repair using different nerve wraps or conduit products, or comparing one product against the current gold standard of direct microsurgical nerve repair were eligible for inclusion.
Gap reconstruction of peripheral nerve injury
Trials comparing different nerve conduits for the reconstruction of equivalent gaps, or comparing nerve conduit with autologous nerve graft (the current gold standard of autologous nerve graft with microsurgical repair onto the proximal and distal nerve stumps) were eligible for inclusion.
We evaluated studies for data considering subgroups of length of gap defect, based on critical gap lengths in previous animal and human studies (Camara 2015; Hart 2011; Ruijs 2005), as follows:
no gap;
3 cm gap or less;
greater than 3 cm gap.
Trials in which participants received co‐interventions were eligible if co‐interventions were provided to each group equally.
Types of outcome measures
Primary and secondary outcome measures were defined according to current practice and literature search. It is recognised that more detailed and higher resolution understanding of peripheral nerve healing and regeneration is becoming technically possible and future updates of this review may employ imaging, specific patient‐reported outcomes, and biomarkers of regeneration. The outcomes listed here were not eligibility criteria for this review, but were outcomes of interest within the studies we included.
Primary outcomes
-
British Medical Research Council (BMRC) grading at 24 months or more for:
muscle strength, using manual muscle testing (MMT);
sensory recovery.
For motor function, we sought to report the BMRC grades of abductor pollicis brevis to assess median nerve function and abductor digiti minimi to assess ulnar nerve function, as these are both commonly used to assess motor function of the hand in clinical trials. Studies indicate that MMT correlates well with functional outcome and electrophysiological assessments (Brandsma 1995; Şahin 2014).
For details of BMRC grading see Appendix 1.
Secondary outcomes
-
BMRC grading at 12 to 24 months for:
muscle strength by MMT; and
sensory recovery.
Integrated functional outcome measured using the Rosén Model Instrument (RMI), a validated measure of integrated upper limb function, which evaluates function across four parameters, namely motor, sensory, dexterity, and pain/discomfort at 12 to 24 months and 24 months or more (Rosén 2000; Appendix 1).
Touch threshold, measured using Semmes Weinstein monofilament (SWM) testing as described by Bell‐Krotoski 1995, using five monofilament probes to apply forces and evaluate the lightest perceived force at 12 to 24 months and 24 months or more.
Two‐point discrimination(2‐PD) (moving and static), a commonly used measure at 12 to 24 months and 24 months or more (Aberg 2007).
Cold intolerance, measured using the Cold Intolerance Symptom Severity score, a reliable and validated questionnaire at 12 to 24 months and 24 months or more (Carlsson 2008).
Impact on daily living, measured using the Disabilities of the Arm, Shoulder and Hand (DASH) questionnaire, a validated and widely utilised participant‐reported outcome scale at 12 to 24 months and 24 months or more (Chemnitz 2013; Gummesson 2003).
Sensory nerve action potential (SNAP): amplitude across the site of the nerve repair in the upper limb, measured using sensory neurography. Given the expected paucity of eligible studies, we reported SNAPs at any time point from three months after injury, giving primacy to results obtained over 12 months from injury. We recorded the maximal response value obtained (microvolts) and, where provided, the methodology used (e.g. orthodromic or antidromic) at 12 to 24 months and 24 months or more.
Cost of the device.
-
Adverse events:
any adverse event
serious adverse events – any serious adverse event including infection requiring antibiotics, extrusion of device, further surgery (device removal or revision), donor site pain, donor site neuroma, donor site slow healing, and donor site revision (scar or neuroma excision).
Search methods for identification of studies
Appendix 2 describes our planned approach to cohort studies, if we had not found RCTs.
Electronic searches
On 26 January 2022, the Cochrane Neuromuscular Information Specialist searched the following databases using the search strategies in the appendices:
Cochrane Neuromuscular Specialised Register via the Cochrane Register of Studies (CRS‐Web) (to 26 January 2022; Appendix 3);
Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies (CRS‐Web) (to 26 January 2022; Appendix 4);
MEDLINE (OvidSP; 1946 to 25 January 2022; Appendix 5);
Embase (OvidSP; 1974 to week 3 2022; Appendix 6);
ClinicalTrials.gov (to 26 January 2022; Appendix 7);
World Health Organization (WHO) International Clinical Trials Registry Portal (ICTRP) (26 January 2022; Appendix 8).
We imposed no restriction on language or date of publication.
Searching other resources
We reviewed reference lists of all primary studies and review articles for additional studies. We searched relevant manufacturers' websites and clinical trials registries for trial information. We conducted online searches and, where not accessible, we contacted societies' secretariats to obtain abstracts from the British Surgical Society of the Hand (BSSH), International Federation of Surgical Societies of the Hand (IFSSH), Federation of European Surgical Societies of the Hand (FESSH), and the American Society for Peripheral Nerve (ASPN) from October 2008 to October 2018.
We collected data on the direct and indirect cost of the use of the conduit from studies to contextualise the efficacy and safety data. We did not perform a systematic search for economic studies or undertake a formal economic analysis. We contacted manufacturers to enquire about current device purchase costs (first contact made in October/November 2018, with two further attempts in case of no response in January and May 2019).
Data collection and analysis
Selection of studies
Two review authors (SET and NN) independently screened the titles and abstracts of all the potential studies identified as a result of the search. We coded studies as 'retrieve' (eligible, potentially eligible, or unclear) or 'do not retrieve'. We retrieved full‐text study reports and two review authors (SET and NN) independently screened them to identify studies meeting the inclusion criteria. The review authors recorded reasons for exclusion of the ineligible studies in the Characteristics of excluded studies table. We resolved any discrepancies through discussion or consultation with a third review author (AH, MR, or PK). We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report was the unit of interest in the review. We recorded the selection process in a PRISMA flow diagram (Figure 1) and the Results of the search section.
1.
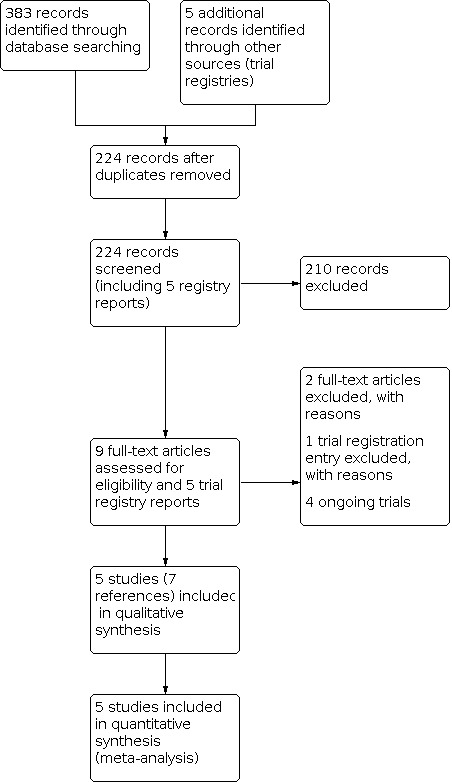
Study PRISMA flow diagram.
Where review authors were authors of included studies, they did not assess their own studies.
Data extraction and management
Two review authors (SET and NN) employed a piloted data extraction form to extract and record the study characteristics and outcome data. We extracted the following study characteristics.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and location, study setting, withdrawals, and date of study.
Participants: total number per treatment arm, mean age, age range, gender, occupation, hand dominance, mechanism of injury, severity of condition, length of gap, operative delay, level of injury, concomitant injury, smoking status, comorbidities, intraoperative detail (suture or splints), postoperative care including physiotherapy (yes or no, and any details of duration and intensity), diagnostic criteria, baseline characteristics, inclusion criteria, and exclusion criteria.
Interventions: nerve wrap or conduit (type, delay from injury until intervention), comparison (direct repair, no wrap or autologous nerve graft), concomitant surgery.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial, and notable conflicts of interest of trial authors.
Two review authors (NN and SET) independently extracted outcome data from included studies. Where studies did not report data in a useable format, we recorded that information in the Characteristics of included studies table. One review author (NN) transferred the data into the Cochrane authoring and statistical software, Review Manager 5 (Review Manager 2020). A second review author (SET) checked the outcome data entries and spot‐checked study characteristics for accuracy against the trial report.
We found no reports requiring translation but if any reports had required translation, the translator would have extracted data directly using a data extraction form, or the review authors would have extracted data from the translation provided. Where possible, a review author would have checked numerical data in the translation against the study report.
Where review authors were authors of included studies, they did not assess their own studies.
Assessment of risk of bias in included studies
Two review authors (NN and SET) independently assessed risk of bias for each study using the criteria outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021), according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as high, low, or unclear and provided a quote from the study report together with a justification in the risk of bias table. We would have resolved any disagreements by discussion or by involving another review author (AH, MR, or PK). We summarised the risk of bias judgements across different studies for each of the domains listed in Figure 2. We considered blinding separately for different key outcomes where necessary (e.g. where those performing outcome assessments were blinded to the treatment arm, the risk of bias is less when considering objective measures such as neurophysiology). Investigators provided us with raw data from some studies. We did not identify risk of bias related to unpublished data or correspondence with a trialist, if we had, we would have noted this in the risk of bias table.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
We considered the risk of bias judgement when evaluating treatment effects using the GRADE approach.
Where review authors were authors of included studies, they did not assess their own studies.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol (Thomson 2017), and any deviations from it are reported in the Differences between protocol and review section of this systematic review.
Measures of treatment effect
We analysed dichotomous data as risk ratios (RRs) with 95% confidence intervals (CIs) and continuous data as mean differences (MDs), or standardised mean differences (SMDs) with 95% CIs for results across studies with outcomes that were conceptually the same but measured in different ways. We presented data as a scale with a consistent direction of effect.
We undertook meta‐analyses only where this was meaningful, that is, if the treatments, participants, and the underlying clinical question were similar enough for pooling to make sense. Only studies employing the same outcome measures were pooled in meta‐analysis. In particular, the use of variations in BMRC grading prevented pooling of data and reflects subjectivity and inter‐rater variation inherent in this technique.
We narratively described skewed data reported as medians and interquartile ranges (IQR).
Unit of analysis issues
There were no instances of three or more treatment groups in a single study. If more than two study groups had been suitable for inclusion in a meta‐analysis, we planned to divide the sample size and event rate of the control group, so that the participants randomised to placebo or control intervention were not double counted. We would only have considered relevant intervention groups, that is, those eligible under our selection criteria (Cochrane Handbook for Systematic Reviews of Interventions; Higgins 2021).
We considered bilateral cases or situations with more than one graft to be unlikely but found that they occurred in several included studies. Where more than one graft is present the possibility of codependency exists. Where more than one graft is used and participants are randomised to the first graft and an alternative used for the second, we planned to take this into account; however, in practice this detail was not available. If it had been, we had planned to extract outcomes taking into account the paired nature of the data by seeking information on paired statistics and estimate standard errors as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). When a correlation coefficient was not provided to derive the appropriate adjusted estimate, we would have employed a correlation of 0.5 for the standard analysis, and we would have used two other extreme values of 0.1 and 0.9 in a sensitivity analysis.
We performed separate analyses at 12 to 24 months and 24 months or more if data were available, to avoid errors arising from repeated observations. See Differences between protocol and review for changes to prespecified time points.
We did not identify any cluster‐randomised trials or cross‐over studies. We did not plan to perform a multiple treatments meta‐analysis, which would be the subject of a further paper to formally compare interventions across studies if transitivity hypotheses were fulfilled.
Dealing with missing data
We contacted investigators to verify key study characteristics and obtained missing numerical outcome data where possible. If missing data had introduced serious bias, we would have explored the effects of including such studies in a sensitivity analysis. However, we found few trials and most were at high or unclear risk of bias in several domains.
Assessment of heterogeneity
We evaluated the clinical heterogeneity of studies prior to performing any meta‐analysis, with attention to the distribution of individuals belonging to the groups defined in Subgroup analysis and investigation of heterogeneity between treatment arms.
We used the I² statistic calculated on Review Manager 5 to measure statistical heterogeneity among the trials in each analysis (Higgins 2003; Review Manager 2020). There was substantial unexplained heterogeneity. Due to limited available comparable data prespecified subgroup analysis was not possible. Additional methods described in protocol are detailed in Appendix 2.
Where review authors were authors of included studies, they did not assess their own studies.
Assessment of reporting biases
Had we been able to pool more than 10 trials, we would have created a funnel plot to explore possible small‐study biases. We searched international trial registries for completed and recruiting trials.
Data synthesis
We analysed data using Review Manager 5 (Review Manager 2020). We included all eligible trials in the initial analysis, and we based our summary of findings table on the most clinically relevant data set. In most cases it was not possible to pool data. In the instances where we pooled data, we used a fixed‐effect analysis model.
Subgroup analysis and investigation of heterogeneity
It was not possible to perform subgroup analyses due to lack of comparable data. Further details of planned subgroup analyses are provided in Appendix 2.
Sensitivity analysis
It was not possible to conduct the planned sensitivity analyses. Details of planned analyses are available in Appendix 2.
Summary of findings and assessment of the certainty of the evidence
We created a summary of findings table and applied the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of a body of evidence (studies that contribute data for the prespecified outcomes). We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021) and GRADEpro GDT software (GRADEpro GDT). We downgraded evidence from 'high certainty' by one level if a GRADE consideration was present to a serious degree, or by two levels if very serious. We justified all decisions to downgrade or upgrade the certainty of the evidence using footnotes, and comments are available to aid readers' understanding of the review where necessary.
We did not report device cost in the summary of findings table, but instead provided details in the Cost of devices table (Table 2).
1. Cost of devices.
| Device trade name | Material | Cost for device to repair 10 mm gap, 2 mm diameter |
| NeuroTube | Polyglycolic acid | GBP 580 exc of VAT (November 2018) |
| Neurogen PNG220 | Type I collagen | GBP 689.26 exc of VAT (Nov 2018) |
| Neurolac (Polyganics) | poly(DL‐lactide‐ε‐caprolactone) | No reply November 2018 sales@neurolac.com and info@polyganics emailed further 7 January 2019 further 18 May 2019 |
| Salubridge | Polyvinyl alcohol | No reply November 2018 info@salumedica.com emailed further 7 January 2019 further 18 May 2019 |
| Axoguard | Porcine small intestine submucosa | USD 1000 equivalent to GBP 789.04 (April 2019) |
| Avance Axogen | Decellularised cadaveric nerve | USD 1800 equivalent to GBP 1420.28 (April 2019) |
| RevoInerv (NG02‐0203) | Porcine Type I and III collagen, bovine Type I | GBP 348 exc of VAT (January 2019) |
exc: exclusive; GBP: Great British pounds; USD: United States dollars; VAT: value added tax.
| Outcomes for inclusion in the summary of findings table | Score |
| Muscle strength, measured by BMRC grading in selected muscles (abductor digiti minimi for ulnar nerve, palmar abduction for median nerve) | Score 0–5 (0 = no movement, 5 = normal) |
| Sensory recovery, measured by BMRC grading | Score S0–S4 (S0 = no sensation, S4 = normal) |
| Integrated functional outcome, measured by Rosén Model Instrument | Score 0 to 3 (0 = no demonstrable function, 3 = normal) |
| Touch threshold, measured by Semmes‐Weinstein monofilament | Score 0–15 (0 = no sensory function, 5 = normal) |
| Impact on daily living, measured by DASH PROM | Score 0–100 (0 = no disability, 100 = most severe disability) |
| Adverse events | Proportion of participants experiencing an adverse event per group |
| Serious adverse events: further surgery (device removal or revision) | Proportion of participants requiring further surgery (device removal or revision) |
In order to maximally inform decision‐makers, we selected the time point of the summary of findings table to present the most clinically relevant data set. We originally prespecified 24‐month time points for outcomes in the summary of findings table. We revised the outcome definition to '24 months or more' to allow for longer follow‐up. If studies provided data at both 24 months and longer time points, we reported the data closest to 24‐months in summary of findings table.
Results
Description of studies
We searched for RCTs comparing surgical repair of nerve gaps using bioengineered conduits to standard surgical managements.
Results of the search
Database searches retrieved 383 individual records and five trials registry entries, which removal of duplicates reduced to 224 records (including the trial registry entries). We excluded 210 articles based on abstract screening and retrieved nine full texts. Of these, five studies (reported in seven references) met the inclusion criteria and were subject to data extraction (Figure 1). Of the five potentially relevant trials identified by trial registration screening, four met inclusion criteria (see Ongoing studies) and we listed one in excluded studies (see Excluded studies).
Included studies
Five studies met the criteria for inclusion (Aberg 2009; Bertleff 2005; Boeckstyns 2013; Lundborg 2004; Weber 2000). Two studies were multicentre (Bertleff 2005; Weber 2000). The included studies evaluated 213 participants with 257 upper limb nerve injuries. Three studies enrolled participants with injury to the median nerve, ulnar nerve, or both, at the level of the forearm (Aberg 2009; Boeckstyns 2013; Lundborg 2004), and two included participants with injury distal to the wrist crease (Bertleff 2005; Weber 2000). Four studies included participants with more than one nerve repair (Aberg 2009; Bertleff 2005; Boeckstyns 2013; Weber 2000).
The studies compared nerve repair using five engineered nerve repair devices (129 participants) to direct epineurial (119 participants) or autologous nerve graft (nine participants). The nerve repair devices used were poly(R)‐3‐hydroxybutyrate (PHB) wrap sealed with fibrin glue (six participants; Aberg 2009), poly(DL‐lactide‐caprolactone) (Neurolac) nerve guide (21 participants; Bertleff 2005), polyglycolic acid conduit (62 participants; Weber 2000), type I bovine collagen conduit (23 participants; Boeckstyns 2013), and silicone tube (17 participants; Lundborg 2004).
One study used a single autologous nerve graft in the standard repair group, but the trial authors excluded the participant from analysis, as the other 20 standard repairs used end‐to‐end suture (Boeckstyns 2013). Two studies included digital nerves (Bertleff 2005; Weber 2000), whilst the others focussed on median or ulnar nerve injuries, or both.
Participant age ranged from 15 to 75 years. The studies included 154 males and 47 females. Sex was not recorded for 12 participants.
Follow‐up time for most studies ranged from one to five years. Studies used a wide range of sensory, motor, and integrated outcome measures. All the studies included device removal and adverse events as a study outcome. Our predefined outcomes of sensory and motor recovery as measured by BMRC grading were the most commonly employed sensory outcome measures.
Details of study methodology, including outcome measures used, are provided in the Characteristics of included studies table.
Excluded studies
We excluded one study as the data presented did not meet our inclusion criteria of a minimum follow‐up of 12 months (Neubrech 2018). See Characteristics of excluded studies table.
Ongoing studies
There are currently five relevant ongoing RCTs investigating the use of bioengineered nerve conduits (see Characteristics of ongoing studies table). The biomaterials used to fabricate the conduits are fibrin, collagen, chitosan, polyethylene glycol, and poly(DL‐lactide‐caprolactone) (Neurolac). None of these RCTs evaluate conduits that incorporate cell or small molecule biofunctionalisation. Only one ongoing RCT, investigating chitosan devices, has a follow‐up of 24 months or greater (NCT02372669), with others being limited to six months or one year. There are currently no active RCTs evaluating the effect of processed nerve allograft on nerve repair. One RCT commenced evaluating processed nerve allograft to standard epineurial suture, however, was stopped early, citing slow recruitment and insufficient patient compliance, and has been excluded from this review as not results have been posted (NCT02459015).
We reviewed online resources for the four large upper limb and peripheral nerve meetings (FESSH, IFSSH, BSSH, and ASPN) from 2008 to 2018, and, where data were unavailable, we contacted the secretariat of the society. Several small cohort studies were presented at expert meetings, alongside increasing data from decellularised nerve allograft studies. No additional RCTs were presented. Whilst this information is important, the outcomes of a well‐planned prospective randomised trial comparing outcomes of repair using decellularised nerve allografts with those achievable through standard repair is awaited.
Risk of bias in included studies
See risk of bias assessments in the Characteristics of included studies table and Figure 2 for further details.
Allocation
All trials opened an envelope concealing the treatment group allocation in the operating theatre. We considered three trials at low risk of bias for allocation concealment (Aberg 2009; Bertleff 2005; Lundborg 2004); Weber 2000 and Boeckstyns 2013 were at unclear risk because nerves or participants were allocated after exploration of the wound. One study described random sequence generation, and we judged it at low risk of bias (Aberg 2007). The other four trials were at unclear risk of bias.
Blinding
It was impossible to blind surgeons to the selected intervention.
Three studies describe personnel blinding, and we judged them at low risk of bias (Aberg 2009; Boeckstyns 2013; Weber 2000). Blinding of participants and personnel was unclear in Bertleff 2005 and Lundborg 2004 resulting in an unclear risk of bias. Bertleff 2005 described the study as blinded but did not provide details as to the assessors' awareness of treatment, and we judged it at unclear risk of bias for both blinding of personnel and participants, and blinding of outcome assessment.
Four studies described assessor blinding (Aberg 2009; Boeckstyns 2013; Lundborg 2004; Weber 2000). Other than Lundborg 2004, we judged them at low risk of bias. Due to interim publication after one‐year follow‐up, blinding was not possible thereafter in Lundborg 2004, and we judged the trial at high risk of bias. This has been considered in the interpretation of the findings.
Incomplete outcome data
Four studies clearly stated the number of participants returning to follow‐up at each time point and provided the numbers who failed to attend. One study did not provide the number of participants attending for assessment at each follow‐up time point, and we judged the risk of bias as unclear (Bertleff 2005). In Aberg 2009, we judged the risk of bias as unclear because one of six (17%) participants did not have the primary outcome measured at 18 months as protocol demanded.
There were equal numbers of dropouts from standard treatment and intervention groups across all trials, as detailed in Characteristics of included studies risk of bias tables.
Only two participants were lost to follow‐up in Lundborg 2004, one from each of the treatment arm, and investigators performed an intention‐to‐treat analysis. We judged the risk of bias as low for this study and Boeckstyns 2013, which employed blinding of participants and evaluators, plus adequately objective outcome measures. Boeckstyns 2013 had one participant randomised to a cable sural nerve graft (the others had direct repair) and the trial investigators excluded data from this participant from analysis. The impact of this unplanned exclusion was unclear but may have conferred better overall outcomes in the standard (non‐device) treatment group. There was attrition from both treatment arms (4 of 22 participants from the conduit group; 8 of 21 participants from the direct repair group) but since there was no reported systematic difference in the way both groups were followed up, we attributed an unclear risk of bias.
Weber 2000 measured static and moving 2‐PD at 3, 6, 9, and 12 months postoperatively. Where the participant failed to attend follow‐up, the last measurement taken was included in the final presented data and analysis. We deemed this a high risk of bias, as it could have led to misinterpretation of results, as other studies demonstrated ongoing improvement in sensibility following direct or conduit repair over this time period (Lundborg 2004).
Selective reporting
Studies reported safety and adverse events data, alongside a range of one to nine sensory, motor, and global function outcome measures. Results for planned outcomes were generally well reported, and we judged four studies at low risk of bias (Bertleff 2005; Boeckstyns 2013; Lundborg 2004; Weber 2000). Aberg 2009 was at high risk as the trial authors provided data only for statistically significant results and excluded median nerve data.
Aberg 2009 employed a comprehensive suite of motor, sensory, and integrated outcome measures and reported all results narratively, providing the data only on those demonstrating a significant difference between groups. Planned results were reported. The trial authors provided an explanation for missing results (inability to evaluate results from neurophysiology and mechanical thresholds due to low numbers attending later follow‐up time points and lack of consistency in standardising the test protocol across the multicentre trial). Data were not presented for those undergoing median nerve repair, as the report stated that limited data were collected from median nerve injuries.
Bertleff 2005 assessed static and moving one‐point discrimination (1‐PD) and 2‐PD tests. All planned outcome measures were reported.
Boeckstyns 2013 used the Rosén scale and electrophysiological assessment at 3, 6, 12, 18, and 24 months. The compound motor action potential from wrist to abductor pollicis brevis or abductor digiti minimi at 24 months was selected as the primary efficacy parameter following multicentre discussion. It is unclear whether this discussion was before or after the results were known. The report provided extended data for electrophysiological examination and Rosén test at 12 and 24 months. One participant's data were excluded from analysis as they had a cabled sural nerve graft.
Lundborg 2004 employed the modified BMRC score, RMI, and electrophysiological assessment, all planned outcome measures were reported, and the included paper provided longitudinal data over a five‐year postoperative period.
Weber 2000 measured static and moving 2‐PD at 3, 6, 9, and 12 months postoperatively, all planned outcome measures were reported. Subgroup analysis was performed on gaps less than 4 mm and there were statistical differences in outcomes; it is unclear whether this was a planned subgroup analysis.
Other potential sources of bias
We judged three studies at unclear risk from other sources of bias due to possible unit‐of‐analysis issues (Bertleff 2005; Boeckstyns 2013; Weber 2000).
Bertleff 2005 randomised participants with the same treatment applied to eligible nerves from the same participant. Four participants in the intervention group had two nerve grafts, and it is unclear how the paired data impacted the results. One participant in Boeckstyns 2013 underwent two nerve repairs (participants were randomised). It is unclear how trial authors dealt with non‐independence of data in their analysis. One participant underwent sural nerve graft and the data were excluded from analysis. It is unclear if there was a unit of analysis bias in Weber 2000, in which nerves were randomised, as there was no apparent adjustment for non‐independence of multiple nerve repairs in the same participant. The other two studies did not have other sources of bias, and we graded them at low risk (Aberg 2009; Lundborg 2004).
Effects of interventions
See: Table 1
See Table 1.
Specific protocols varied between studies and did not allow meta‐analysis; instead, we performed a narrative synthesis.
Two studies reported outcomes of the Integrated RMI 24 months postoperatively, allowing meta‐analysis (Boeckstyns 2013; Lundborg 2004).
Primary outcomes
Muscle strength at 24 months or more
Comparable raw data were not available to enable a meta‐analysis of BMRC motor grading at 24 months or more.
Sensory recovery at 24 months or more
Comparable raw data were not available for meta‐analysis of BMRC sensory grading at 24 months or more. Only Lundborg 2004 provided data.
Lundborg 2004 (30 participants) reported the MacKinnon modification of BMRC classification of sensory recovery five years postoperatively. All participants achieved S2 or better sensory recovery, with little or no difference between the groups. In the standard repair group, scores ranged from S2 to S3+. One participant undergoing tubular repair had normal sensation (S4) at five‐year follow‐up (MD 0.03, 95% CI −0.43 to 0.49; 1 RCT, 28 participants; very low‐certainty evidence; Analysis 1.1). We downgraded the evidence twice for imprecision and once for study limitations.
1.1. Analysis.

Comparison 1: Repair using bioengineered device versus standard nerve repair, Outcome 1: Sensory recovery at ≥ 24 months
Secondary outcomes
Muscle strength at 12 to 24 months
One study, with 11 participants, reported BMRC muscle strength following use of a nerve repair device compared to standard repair of forearm level median and ulnar nerve injuries, 18 months postoperatively (Analysis 1.2; very low‐certainty evidence; Aberg 2009). We downgraded the evidence for very serious imprecision and study limitations. Five participants underwent PHB repair; of this group, three scored BMRC grade 2 movement, one achieved BMRC grade 1, and one had no palpable contraction (BMRC grade 0). Six participants had direct suture repair and all reached BMRC grade 1 movement.
1.2. Analysis.

Comparison 1: Repair using bioengineered device versus standard nerve repair, Outcome 2: Muscle strength, assessed with BMRC motor grading at 12–24 months
Two studies analysed manual motor testing as part of the RMI, reported below under integrated functional outcome measured using the RMI (Boeckstyns 2013; Lundborg 2004; Analysis 1.3).
1.3. Analysis.

Comparison 1: Repair using bioengineered device versus standard nerve repair, Outcome 3: Motor Rosén at 12–24 months
Sensory recovery at 12 to 24 months
Each study used a different modification of the scale. Outcome data were reported at times varying from three to 18 months (one study reporting a single figure for grouped results of three months to one year; Weber 2000).
Aberg 2009 (12 participants) reported six participants' injuries were repaired using PHB conduits; one achieved normal sensory recovery at 18 months, one achieved BMRC grade S3, and three achieved BMRC grade 2. Five participants in the direct repair group scored BMRC grade S2 and one had no sensory recovery (Analysis 1.4).
1.4. Analysis.

Comparison 1: Repair using bioengineered device versus standard nerve repair, Outcome 4: Sensory recovery, assessed with BMRC sensory grading at 12–24 months
Bertleff 2005 (30 participants) reported only graphical data of sensory recovery one year postoperatively, with "no significant difference" detected between participants undergoing nerve repair using poly(DL‐lactide‐caprolactone) (Neurolac) conduits versus standard repair.
Weber 2000 (98 participants) reported subgroup analysis of sensory return using BMRC grading over 3 to 12 months following repair. Time points were grouped (e.g. some participants only had data at three months postoperatively whereas others at one year postoperatively). The trial authors then subgrouped these data into three groups according to defect length (gaps less than 4 mm, gaps 5 mm to 7 mm, and gaps greater than 8 mm). The authors did not provide specific data values for BMRC sensory grading.
The certainty of the evidence for sensory recovery was very low due to study design heterogeneity, study limitations, low participant numbers, and differences between desired and measured outcome both in reporting and time frame.
Integrated functional outcome measured using the Rosén Model Instrument
Two studies reported integrated RMI scores (Boeckstyns 2013; Lundborg 2004), with one study presenting results up to five years postoperatively (Lundborg 1994). We requested raw data, which facilitated meta‐analysis at the 24‐month time point. The RMI score indicated little or no difference when repair devices were used compared to standard repair at 24 months (MD −0.17, 95% CI −0.38 to 0.05; I2 = 0%, P = 0.13; 2 RCTs, 60 participants; low‐certainty evidence; Analysis 1.5; Figure 3). We analysed results using a fixed‐effect model as planned in our protocol. We downgraded the certainty of evidence from high to low because of low participant numbers and study limitations.
1.5. Analysis.

Comparison 1: Repair using bioengineered device versus standard nerve repair, Outcome 5: Integrated functional outcome, assessed with Rosén Model Instrument
3.

Forest plot of comparison: 1 Repair using bioengineered device versus standard nerve repair, outcome: 1.5 Integrated functional outcome, assessed with Rosén Model Instrument.
One study found that the long‐term outcome of RMI (five years postoperatively) was probably improved following repair using a device (MD 0.23, 95% CI 0.07 to 0.38; P = 0.005; 1 RCT, 28 participants; low‐certainty evidence; Analysis 1.5; Figure 3; Lundborg 2004). The difference in RMI score of 0.23 at five years is small but likely to be clinically significant. No other studies investigated outcomes for this duration. We downgraded the certainty of evidence to low because of low participant numbers (imprecision) and study limitations.
Touch threshold, measured using Semmes Weinstein Monofilament testing
Lundborg 2004 and Boeckstyns 2013 found that there may be little or no difference in touch threshold between standard nerve repair and repair using a conduit at 12 and 24 months, but the evidence is very uncertain (12 months: MD 0.05, 95% CI −0.07 to 0.17; I2 = 66%; P = 0.09; 2 RCTs, 65 participants; very low‐certainty evidence; 24 months: MD 0.01, 95% CI −0.06 to 0.08; P = 0.78; 1 RCT, 19 participants; very low‐certainty evidence; Analysis 1.6). SWM was not used as an individual test, but contributed to the Rosén sensory domain scoring in two studies, and we obtained raw data from the trial authors. Aberg 2009 used von Frey filaments to test sensory thresholds, but found them to be imprecise and did not report results.
1.6. Analysis.

Comparison 1: Repair using bioengineered device versus standard nerve repair, Outcome 6: Touch threshold, measured by Semmes‐Weinstein Monofilament
We downgraded the certainty of evidence to very low because of low participant numbers (imprecision), study limitations, and indirectness in the SWM testing, which did not provide reliable data in one study. The 12‐month data showed substantial heterogeneity.
Two‐point discrimination
Due to variations in methodology and reporting it was not possible to perform meta‐analysis across the studies that measured 1‐PD and 2‐PD (Bertleff 2005; Boeckstyns 2013; Lundborg 2004; Weber 2000).
Weber 2000 reported data for static and moving 2‐PD. Measurements were taken at mixed time points postoperatively (3 to 12 months) precluding meaningful clinical comparison when considering the biology of nerve regeneration; we did not calculate the mean and 95% CI. Mean moving 2‐PD was 6.9 mm (SD 3.9; 46 participants) in the PGA conduit and 7 mm (SD 4; 56 participants) in the standard repair group (P = 0.89). Mean static 2‐PD was 10.3 mm (SD 4.7; 46 participants) in the PGA conduit and 9.3 mm (SD 3.6; 56 participants) in the standard repair group (P = 0.26).
Bertleff 2005 reported 2‐PD in graphical form with raw data unavailable. The other two studies reported it as part of the RMI (Boeckstyns 2013; Lundborg 2004).
None of the studies reported any difference in this outcome measure between intervention and control groups. The evidence was very uncertain due to indirectness, imprecision, and study limitations.
Cold intolerance
Three studies reported cold intolerance; none reported a difference between treatment alternatives at 24 months (Aberg 2009; Boeckstyns 2013; Lundborg 2004). Results for this outcome were reported as part of different composite tests (Sollerman and Rosén, Rosén 1994; Rosén 2001; Sollerman 1995), and presented as transformed data, precluding direct comparison of the three studies. There were raw data for one study (32 participants) (Boeckstyns 2013; Analysis 1.7). The certainty of the evidence was low because of imprecision and study limitations.
1.7. Analysis.

Comparison 1: Repair using bioengineered device versus standard nerve repair, Outcome 7: Cold intolerance
One study measured cold intolerance five years after repair, in which cold‐related pain was less severe in the silicone nerve wrap group compared to the direct epineurial repair group (Lundborg 2004). This finding should be interpreted with caution as there was high attrition. Raw data for the 28 of 30 participants followed up were not available; the measure was presented as part of the RMI.
Impact on daily living, measured using the Disabilities of the Arm, Shoulder and Hand questionnaire
No included study used the DASH PROM.
Sensory nerve action potential
Three studies measured motor and sensory electrophysiology (Aberg 2009; Boeckstyns 2013; Lundborg 2004). One study was unable to compare electrophysiological outcomes between treatment groups due to missing data (Aberg 2009). The other two studies detected little or no difference in electrophysiological evaluation of sensory or motor function between treatment groups at 24‐ and 60‐month follow‐up (Analysis 1.8; Boeckstyns 2013; Lundborg 2004). The certainty of the evidence was very low due to substantial heterogeneity (I2 = 61%), imprecision, and study limitations.
1.8. Analysis.

Comparison 1: Repair using bioengineered device versus standard nerve repair, Outcome 8: Sensory nerve action potential (SNAP)
At five years, there was little or no difference in SNAP between device repair (median 1.5, IQR 1 to 3; 10 participants) and direct repair (median 1.0, IQR 1.2 to 2; 4 participants) (Lundborg 2004).
Cost of the device
The mean cost of a device required to repair a 10 mm gap in a 2 mm diameter nerve was GBP 765.32 excluding tax (range GBP 348 to GBP 1420.28), based on responses by manufacturers of five devices (Table 2).
Adverse events
Three studies reported adverse events, but only one study differentiated between serious or non‐serious, and, as such, we performed an analysis for any adverse events to ensure reporting of clinically important information. This necessitated divergence from the study protocol. Two studies had no adverse events (Aberg 2009; Boeckstyns 2013). When they occurred, adverse events were more common in the device repair group than the standard repair group (RR) 7.15, 95% CI 1.74 to 29.42; I2 = 0%, P = 0.006; 5 RCTs, 213 participants; very low‐certainty evidence; Analysis 1.9; Figure 4). This corresponds to 10 adverse events per 1000 people in the standard repair group and 68 per 1000 (95% CI 17 to 280) in the device group. We analysed results using a fixed‐effect model and downgraded the evidence from high to very low certainty due to study limitations, very serious imprecision (wide CI), and for indirectness, as our outcome is serious adverse events.
1.9. Analysis.

Comparison 1: Repair using bioengineered device versus standard nerve repair, Outcome 9: Adverse events
4.

Forest plot of comparison: 1 Repair using bioengineered device versus standard nerve repair, outcome: 1.9 Adverse events.
Specific serious adverse events: further surgery (device removal or revision)
There may be a greater need for revision surgery after use of nerve repair devices; 12 of 129 devices required further surgery (device removal) in the bioengineered devices group; 0 of 127 procedures required further surgery in the standard repair group (RR 7.61, 95% CI 1.48 to 39.02; I2 = 0%, P = 0.01; 5 RCTs, 256 nerve repairs, fixed‐effect analysis; very low‐certainty evidence; Analysis 1.10; Figure 5). We downgraded the evidence to very low certainty; twice for very serious imprecision and once for study limitations.
1.10. Analysis.

Comparison 1: Repair using bioengineered device versus standard nerve repair, Outcome 10: Device removal or revision
5.

Forest plot of comparison: 1 Repair using bioengineered device versus standard nerve repair, outcome: 1.10 Device removal or revision.
Subgroup analysis
Due to relatively low study numbers and heterogeneous data reporting, it was not possible to undertake subgroup analyses. The timing of intervention from injury varied from less than 48 hours to more than 20 days and nerve gaps varied from no gap to less than 20 mm (precise gap length not detailed in one study).
Discussion
Summary of main results
Five RCTs, including 257 nerve injuries, met the inclusion criteria for this review.
A significant finding was that the methodologies and outcome measure reporting employed by studies varied widely, limiting direct comparison across many desirable parameters.
For the primary outcome, measured at 24 months, we found no evidence for the effects of repair using bioengineered devices compared to standard repair on muscle strength assessed by BMRC grading. The evidence for sensory recovery (BMRC grading) 24 months after surgery and touch sensation measured using SWM testing suggested little or no difference between repair using a device and standard repair, but was very uncertain. The standardisation in reporting of the RMI facilitated meta‐analysis of data collected between two studies, and low heterogeneity (within each time point) supported its use as a reliable outcome measure (Figure 3). An MD of 0.2 points in RMI score would be considered a small but clinically significant difference and to provide clinical perspective, an improvement of 0.8 points is expected over the 24 months following direct end‐to‐end repair of a sharply transected median nerve at the level of the wrist in a healthy adult; correlating well with improved ability in activities of daily living (Rosén 2000). Repair using standard techniques or conduits probably delivered a similar RMI integrated functional outcome score 24 months postoperatively; we judged this finding to be of low certainty due to study limitations and relatively low participant numbers in each study, with a CI that did not rule out a better outcome with standard repair. At five years, based on data from a single study, there may be a slight improvement in RMI outcome score for repairs using a silicone conduit, although it should be noted that 8 of 17 silicone conduits required a secondary surgery for device removal over this time frame (Lundborg 2004). Five‐year data were not available for other studies to allow comparison.
The studies did not report participant‐reported outcome measure data. Several hand and upper limb measures are available (including DASH, i‐HaND); peripheral nerve specific outcome questionnaires have been developed more recently (Ashwood 2018).
Two studies measured cold intolerance as part of the RMI and combined data from these trials suggested little or no difference between the conduit and standard repair groups. Lundborg 2004 reported with low certainty that cold intolerance scores continued to improve over five‐year follow‐up.
Nerve conduit use may cause more adverse events compared to standard repair (Figure 4), leading to unplanned revision surgery (in the included studies, removal of 12 devices (1 poly(DL‐lactide‐caprolactone) (Neurolac) devices, 8 silicone devices, and 3 PGA devices)). We judged the certainty of this evidence very low, despite the number of events, due to serious imprecision, study limitations, and indirectness (Higgins 2021). Only one study differentiated between serious and non‐serious adverse events and, as a result, we detailed all adverse events in our analysis, deviating from our original protocol (see Differences between protocol and review).
The devices costed several hundred GBPs (Table 2), which may limit their uptake in an economy‐focussed healthcare system; however, a full cost analysis would be required to investigate this further, taking into account socioeconomic impact and the cost of additional operating theatre time and secondary surgeries.
Overall completeness and applicability of evidence
This review encompasses all long‐term follow‐up studies of nerve repair in the upper limb that compared repair using bioengineered devices versus standard treatment.
All included participants had injuries to upper limb nerves; three studies included injuries to mixed nerves in the forearm, the other two studies focussed on injuries to digital nerves.
The longest follow‐up for any RCT was five years (one study). Our review evaluated functional, integrated, and self‐reported outcome measures, in addition to adverse events and device cost. There was significant variation in study populations, outcome measures, and reporting scales across the five included studies. The trials included very few nerve‐gap repairs, nine autologous nerve grafts, and most of the data were for direct nerve repair with or without nerve wrap devices. It is uncertain how these findings may be extrapolated to nerve gap injuries. There is a demonstrable trend in clinical practice towards higher level nerve reconstruction and, in particular, nerve grafting after injury (Karsy 2017); large‐scale trials should reflect this.
An important technical consideration is that in Bertleff 2005, gap defects of up to 20 mm were described in the control group without any reported use of autologous nerve grafts, indicating that the control group probably deviated from standard microsurgical practice (this is a risk that nerves were directly coapted under tension instead of using an autologous nerve graft).
Studies included predominantly males (22% female, 72% male, 6% unreported); sex has previously been identified as having an impact on nerve healing in vivo (Stenberg 2014).
For our primary outcomes, no trial provided data on muscle strength at 24 months and we could not draw conclusions from the small trial that reported sensory recovery at 24 months. No studies reported a DASH score, a validated participant‐reported outcome measure and one of our predetermined secondary outcome measures. The importance of longer‐term follow‐up is reflected in our inclusion criteria and remains important (Lohmeyer 2014).
No RCTs are currently available that directly compared the use of autografts against decellularised allografts or other devices, but we await the results of two studies (NCT01526681; NCT01809002) (see Table 3). Ongoing studies are investigating allograft seeded with autologous bone marrow aspirate concentrate in upper limb defects (NCT03964129), or allograft nerve repair versus no repair following robotic radical prostatectomy (NCT00953277; NCT01770340). Other ongoing trials investigating bioengineered nerve grafts in the upper limb include CONNECT (ISRCTN97234566) and POLYNERVE (NCT02970864), and are detailed in Table 3.
2. Registered studies evaluating bioengineered nerve wraps/conduits.
| Conduit/name | Study detail | Outcomes measured | Status | Study design |
| Registry of Avance Nerve Graft Evaluating utilization and outcomes for the Reconstruction of peripheral nerve discontinuities (RANGER) Avance, Axogen Inc. NCT01526681 |
Avance vs standard practice (epineurial suture or autologous nerve graft). Aim 5000 participants, 36 months' follow‐up |
Adverse events, "improvement in function, return of meaningful recovery" | Recruiting, estimated completion December 2020, extended to December 2025 | Observational retrospective registry |
| Polynerve University of Manchester (UK) NCT02970864 |
Polynerve repair of nerve gaps 5–20 mm Aim 16 participants, 12 months' follow‐up |
Adverse reactions (Clavien‐Dindo classification), 2‐PD, SWM | Recruitment complete, 17 participants, estimated completion August 2019 | Prospective observational cohort |
| Fibrin wrap or conduit University Hospital Basel (Switzerland) NCT01573650 |
Fibrin wrap or conduit vs standard practice (epineurial suture or autologous nerve graft) direct repair or > 5 mm gap digital nerves. Aim 48 participants, 6 months' follow‐up |
2‐PD, SWM, electroneurography | Recruiting, estimated completion December 2022 | Interventional case control |
| Hydrophilic polymers at repair site Vanderbilt University (Nashville, Tennessee) NCT02359825 |
Repair and topical polyethylene glycol (MiraLAX (MERCK) at repair site vs repair alone (epineurial suture or autologous nerve graft)). Within 48 hours of injury. Aim 18 participants 12 months' follow‐up |
Return of nerve function as measured by BMRC classification. | Recruiting, estimated completion March 2020 | Interventional RCT |
| Reaxon Siemers, Medovent, GmBH (Germany) NCT02459015 |
Reaxon vs standard practice (epineurial suture or autologous nerve graft) < 26 mm gap digital nerves. Within 3 months of injury. Aim 76 participants, study terminated |
2‐PD, cold intolerance, Hoffmann‐Tinel‐Test, adverse reactions | Recruiting, estimated completion December 2018. January 2019 update Terminated (study was stopped due to slow participant recruitment and insufficient participant compliance) |
Interventional RCT |
| Comparison of processed nerve allograft and collagen nerve cuffs for peripheral nerve repair (RECON) Axogen Inc. NCT01809002 |
Human nerve allograft vs bovine collagen repair cuff Aim 220 participants 12 months' follow‐up |
2‐PD | Recruiting Estimated completion November 2021 |
Interventional RCT |
| A multicentre prospective observational study of nerve repair and reconstruction associated with major extremity trauma Johns Hopkins (Baltimore, Maryland) NCT02718768 |
Partial or complete upper extremity nerve injury, all repair types. Aim 250 participants 24 months' follow‐up |
Extensive list of primary and secondary outcome measures with 2‐year follow‐up period – detail available | Active, not recruiting Estimated completion September 2022 |
Prospective observational cohort |
| Chitosan nerve tube for primary repair of traumatic sensory nerve lesions of the hand (CNT) BG Unfallklinik (Frankfurt, Germany) NCT02372669 |
Chitosan nerve tube vs standard repair sensory nerves of the hands Aim 100 participants 24 months' follow‐up |
2‐PD, DASH, grip strength, range of motion, pain, cold intolerance, hypersensitivity, existence of neuromas, adverse events | Recruiting Last update July 2017 |
Interventional RCT |
| Mid‐term effect observation of biodegradable conduit small gap tubulisation repairing peripheral nerve injury Peking University People's Hospital (Beijing, China) NCT03359330 |
Repair of peripheral nerve injury in the upper extremities using a biodegradable conduit Aim 150 participants 36 months' follow‐up |
BMRC grading SHEN Ning‐jiang score |
Active Estimated completion December 2021 |
Prospective observational cohort |
| Preliminary evaluation of the clinical safety and effectiveness of the bionic nerve scaffold Xijing Hospital (China) NCT03780855 |
Preliminary evaluation of the clinical safety and effectiveness of the bionic nerve scaffold Aim 10 participants 6 months' follow‐up |
2‐PD, joint position sense and haematological tests | Recruiting Last update December 2018 |
Prospective observational cohort |
| Pilot study to evaluate the reconstruction of digital nerve defects in humans using an implanted silk nerve guide Klinik für Plastische Chirurgie und Handchirurgie – UniversitätsSpital Zürich (Switzerland) NCT03673449 |
Prospective, unblinded, single‐group assignment silk nerve guide Aim 15 participants 12 months' follow‐up |
Adverse events, sensory recovery 2‐PD, VAS, patient satisfaction Patient Global Impression of Change questionnaire | Recruiting Estimated completion March 2021 |
Prospective observational cohort |
| CoNNECT (Conduit Nerve approximation versus Neurorrhaphy Evaluation of Clinical Outcome Trial): a study of sutureless nerve repair Queen Elizabeth Hospital Birmingham (UK) ISRCTN97234566 |
Digital nerve injuries in upper limb, direct repair vs poly(DL‐lactide‐caprolactone) (Neurolac) nerve guide sutured vs Neurolac nerve guide no sutures Aim 240 participants 12 months' follow‐up |
Static and moving 2‐PD, monofilament pressure testing, DASH, EQ‐5D, Tinel sign, pain, cold intolerance, hyperaesthesia, site, and quality of repair | Active Estimated completion January 2021 |
Interventional RCT |
| Expanded access for single patient treatment of autologous human Schwann cells (ahSC) for peripheral nerve repair NCT02480777 |
Autologous, culture expanded Schwann cells seeded in Duragen collagen matrix used to repair a sciatic nerve defect | Not provided | Single participant study, 5 years' follow‐up | Single participant study |
| BMAC Nerve Allograft Study NCT03964129 |
Decellularised cadaveric nerve graft combined with unexpanded autologous bone marrow cells. Aim 15 participant recruitment, comparison to historical outcome measures obtained for Avance nerve graft. | Adverse events, Rosén Model Instrument, motor and sensory nerve conduction studies, pinch and grip strength, 1‐PD and 2‐PD | Recruiting | Single group, interventional clinical trial |
1‐PD: 1‐point discrimination; 2‐PD: 2‐point discrimination; DASH: disability of the arm, shoulder and hand; EQ‐5D: Euro‐Qol 5 Dimension; BMRC: British Medical Research Council; RCT: randomised controlled trial; SWM: Semmes‐Weinstein monofilament; VAS: visual analogue scale.
This review did not evaluate additive treatments to the repair site, such as pharmacotherapy, support cells, or electrical stimulation, although studies are ongoing in these areas of research. We identified several preclinical studies delivering expanded Schwann cells, adipose cells, or bone marrow‐derived cells to the site of injury during production of this review. One single‐participant study used expanded autologous Schwann cells in combination with a collagen matrix for sural nerve repair with further trials registered (NCT02480777). One phase 1 human safety study evaluating decellularised nerve graft seeded with autologous bone marrow aspirate cells is recruiting (NCT03964129).
One cohort study using collagen conduits alongside nerve transfers to reconstruct motor nerves of the brachial plexus reported no difference between collagen conduit augmented repair versus standard repair (Wolfe 2012), but we did not include it in this review, which does not address the role of bioengineered nerve conduits to augment nerve transfers in the upper limb.
Certainty of the evidence
The five included studies were RCTs. There was significant variation in selection of outcome measures, reflecting lack of a single useful objective measurement of nerve healing following surgical repair.
GRADE certainty of the evidence ranged from low to very low. Relatively low participant numbers resulted in imprecision, and we downgraded the certainty of evidence accordingly at least once for all outcomes. A specific challenge was comparing studies that used different modifications of the same outcome measure or different time points for assessment, resulting in downgrading for imprecision and inconsistency. These methodological inconsistencies were particularly evident in sensory testing using the BMRC grading score between 12 and 24 months. This resulted in a change from the protocol (see Differences between protocol and review).
Results from the RMI were consistent with neurophysiology studies 12 and 24 months following surgery (Boeckstyns 2013).
We downgraded all outcomes once for study limitations. Three studies had one high risk of bias assessment in addition to one or more unclear judgements, and the other two were at unclear risk of bias in multiple domains.
Allocation concealment was generally well addressed but the method of random sequence generation was generally poorly described. In studies with multiple follow‐up points or where device removal was required, it was uncertain how this impacted assessor blinding. Follow‐up attrition placed one study at high risk of bias and three studies at unclear risk. One study was at high risk of bias from selective outcome reporting; the others were low risk. Several studies had unit‐of‐analysis issues; one randomised nerves but did not provide information on whether clustering (within participants) had been taken into account. Two other studies were randomised at the participant level, with a few participants contributing more than one nerve to analyses. It is unclear whether these issues would have an important effect on the results.
Studies did not generally report serious adverse events separately and so we reported any adverse event, only an indirect measure of the prespecified outcome. Evidence for serious adverse events and device removal were both very low certainty because of serious imprecision (very wide CIs) and study limitations.
One trial investigating decellularised nerve devices was stopped due to poor recruitment and patient compliance (NCT02459015). There are no reports from this trial, which may be a source of publication bias; however, we did not believe this required further downgrading of the evidence.
Potential biases in the review process
Every effort was made to include all relevant RCTs with 12‐month follow‐up periods. We searched multiple platforms and two review authors reviewed all abstracts during the selection process. No restriction was placed on language of publication, and we searched the historical records of the major hand and reconstructive surgical meeting abstracts. We adhered to the inclusion and exclusion criteria published in our protocol in order to minimise subjectivity (Thomson 2017). We approached trial authors to request raw data where required. Two of the co‐authors of this review were authors of included studies; in neither case did the authors assess their own studies. The search and study selection criteria were transparent. The Cochrane Neuromuscular Information Specialist performed the searches and independent review authors screened search results (SET and NY). Two studies were funded or in part conducted by the manufacturer. Reviews with relatively few included trials are subject to limitations when considering infrequent events, such as adverse events.
Agreements and disagreements with other studies or reviews
This review used robust inclusion and exclusion criteria to collate evidence of the highest available quality for best practice in the application of bioengineered devices to aid nerve repair in the upper limb. Five studies met inclusion criteria comparing standard microsurgical repair to repair using bioengineered devices following forearm, hand, and digital nerve injuries. Paprottka 2013 performed a systematic review of the available evidence for choice of technique for digital nerve repair. They identified five RCTs investigating surgical interventions, two of which we included in this review. The other three studies did not meet our inclusion criteria (two studies had follow‐up periods of less than one year, the third study compared a bioengineered conduit to a vein graft (Rinker 2011)). In agreement with Paprottka 2013, we found significant heterogeneity in the studies investigating nerve repair strategies. Paprottka and colleagues comment on the low‐certainty evidence for benefit of Neurotube reconstruction over standard repair in gaps of less than 4 mm and greater than 8 mm based on the study by Weber 2000. However, as the functional outcome scores reported by this study grouped several time points for analysis, which represents a significant methodological bias (Weber 2000), the data do not support use of nerve conduits over direct repair or autograft in clinical practice.
Braga Silva 2017 identified 10 RCTs in their systematic review, which included the five studies in this Cochrane Review, and two studies with follow‐up periods less than 12 months. In addition, it considered outcomes from Lundborg 2004 twice, and included a study comparing nerve conduit to vein grafts. Braga Silva 2017 also included techniques out of the scope of this Cochrane Review, for example, one included study compared direct repair of a 3 cm gap defect in the median nerve by mesh‐assisted splinting under tension to standard microsurgical nerve graft reconstruction (Bertelli 2011).
Mauch 2019 compared outcomes across different studies that employed allograft, collagen conduits, direct nerve repair, or nerve autograft for the management of digital nerve injury. Interestingly, they found nerve allografts had fewer adverse events than collagen conduits (they reported two infections in 66 allografts, but of 101 collagen nerve conduits, seven required surgical removal and one extruded). There were no infections or reoperations in the standard microsurgical repair groups; however, the authors reported that a symptomatic donor site neuroma occurred in 4 of 70 medial antebrachial cutaneous nerve donors. In agreement with the current review, Mauch 2019 highlighted the need for standardised outcome measures and further study.
Braga Silva 2021 reassessed people from the cohort reported by Braga Silva 2017 for the longer‐term outcome of digital nerve injuries. The authors found no overall effect of the repair technique used upon static or moving 2‐PD; however, subgroup analysis for gap length showed that standard autologous graft outperformed conduits (2‐PD primary outcome measure) for defects greater than 11 mm. They detected no differences in shorter nerve gap lengths. This meta‐analysis also commented on the significant heterogeneity in study design and outcome measure reporting.
Authors' conclusions
Implications for practice.
This review assessed all relevant clinical studies with appropriate long‐term outcome reporting, and details ongoing trials investigating the use of bioengineered nerve conduits in upper limb peripheral nerve repair.
As yet there is no clinically significant evidence of benefit to support the use of nerve repair devices in preference to standard microsurgical techniques (nerve repair or autograft).
This review identifies risks in the use of existing licensed bioengineered nerve repair devices, but identified no clinically meaningful benefit sufficient to mitigate those risks when compared against standard microsurgical nerve repair techniques (including autograft). It does not support the use of currently available nerve repair devices when standard repair methods are possible.
Implications for research.
This Cochrane Review provides an overview of the meaningful nerve regeneration outcome measures that have been used in existing randomised controlled trials (RCTs) to study the use of bioengineered nerve repair devices, and the range of injuries in which use of these devices has been meaningfully studied. While these may be incorporated into future study methodologies, the review very clearly highlights the need for larger cohorts, patient‐reported outcome measures, and methodological standardisation across studies to support both critical appraisal and future meta‐analysis. The Rosén Model Instrument is a reliable outcome measure, as demonstrated by low heterogeneity, and agreeing standardised postoperative time points for measurement would facilitate meta‐analysis of data between studies of different devices.
This Cochrane Review highlights the clear need for further RCTs to critically assess emerging nerve repair technologies, and for those studies to have improved consistency and precision in trial methodology and reporting. It is encouraging that the protocols of recently registered Ongoing studies detail more comprehensive outcome measure suites and longer follow‐up periods than historical studies.
Agreeing a minimum duration, outcome measure dataset, and definition of nerve lesion to be studied should be a priority for new clinical trials evaluating new bioengineered devices for peripheral nerve repair; global collaborative registries would further support the development of peripheral nerve repair technologies. This would facilitate evidence‐based practice and the safe clinical translation of findings, plus support future systematic reviews and meta‐analysis.
History
Protocol first published: Issue 3, 2017
Acknowledgements
The review authors would like to acknowledge the support of the Medical Research Council, which funded the lead author's PhD Fellowship (L70085). We would also like to acknowledge the ongoing support throughout the review process of Ruth Brassington, Cochrane Neuromuscular and Information Specialists of Cochrane Neuromuscular, Angela Gunn and Farhad Shokraneh, who developed the search strategy in consultation with the review authors.
The Methods section of this review is based on a template developed by Cochrane Neuromuscular from an original created by the Cochrane Airways Group.
Appendices
Appendix 1. Types of outcome measurement
Outcome measurements that currently prevail in the literature are British Medical Research Council (BMRC) motor and sensory grading system, Semmes Weinstein Monofilament (SWM) test, and static or moving 2‐point discrimination (s/m2PD). Neurophysiological and magnetic resonance imaging (MRI) assessment modalities are not routinely employed in the postoperative period at present (Chemnitz 2015). The Rosén Model Instrument is becoming increasingly recognised as a comprehensive assessment tool of integrated function, but is yet to be adopted throughout the literature. Ideally, following peripheral nerve surgery a holistic evaluation of outcome would be made. This would be performed at regular and defined postoperative periods and comprise an objective, sensitive analysis of integrative function (e.g. The Model Instrument, Rosén 2000), accurate biodynamic outcome analysis (Pruszynski 2014), or vibrotactile perception (Dahlin 2015). Combined with participant‐reported outcome measurements investigating the individual's perception of performance over a series of tasks, this would allow more detailed evaluation. Currently, resource limitations and clinical practicalities necessitate the pragmatic use of less rigorous measurements of outcome in most studies.
The BMRC Motor Grade assesses motor function on a 6‐point scale, with ability to overcome gravity and comparison to the contralateral limb providing important benchmarks.
| BMRC Motor Grade | Muscle strength |
| M0 | No movement is observed |
| M1 | Trace or flicker of movement or fasciculations seen |
| M2 | Muscle can exert movement when gravity is eliminated |
| M3 | Muscle can exert movement against gravity |
| M4 | Muscle strength is reduced but some movement against resistance |
| M5 | Normal movement |
The BMRC sensory grade assesses sensory recovery on a 7‐point scale, where S0 is absence of any sensory recovery and S4 is recovery of sensibility and two‐point discrimination (2‐PD) of 3–6 mm (S2 and S3 are further subdivided into S2+ and S3+) (Zachary 1954). Weighted pins (e.g. 5–10 g) may be used for testing pressure and pain, Von Frey's hairs are used to assess tactile sensation; these are available in various calibres, and 2‐PD is assessed with a fine caliper. The sensory scale will be interpreted critically and detailed methodology provided.
| BMRC Sensory Grade | Sensory recovery |
| S0 | Absence of sensibility in the autonomous area |
| S1 | Recovery of deep cutaneous pain sensibility |
| S2 | Return of some degree of some degree of superficial cutaneous pain and tactile sensibility within the autonomous area of the nerve |
| S3 | Return of some degree of some degree of superficial cutaneous pain and tactile sensibility within the autonomous area of the nerve with disappearance of any previous over reactivity |
| S3+ | Return of sensibility as in Stage 3 with the addition that there is recovery of 2‐PD within the autonomous area |
| S4 | Complete recovery |
The Model Instrument is one of the most well recognised and widely applied composite assessment tools for assessment of peripheral nerve function following repair at the distal forearm and wrist (Rosén 2000). This standardised tool correlates well with the Disability of the Arm Shoulder and Hand (DASH), a validated participant‐reported outcome measurement of perceived ability to carry out activities of daily living (Beaton 2001; Gummesson 2003). A detailed protocol of the components of the test has been published, allowing inter‐operator standardisation. Briefly, a quantitative assessment of:
Motor recovery – manual muscle testing and Jamar dynamometer to test motor innervation and grip strength;
Sensory recovery – Semmes Weinstein Monofilaments (SWM) are used to assess touch/pressure thresholds, 2‐PD;
Tactile gnosis and dexterity – shape texture identification (STI) to assess as well as 3 selected tasks from the Sollerman grip test (Sollerman 1995);
Pain/discomfort – 4‐point scale questionnaire focusing on cold sensitivity, hyperaesthesia, and allodynia is performed.
The score for each subtest is normalised by dividing by a 'normal' result – this may be predetermined or obtained by testing the uninjured, contralateral arm. The mean score is calculated for each domain and the sum of these 3 scores generates the overall score which is plotted against 'average results' and over time. This allows recording of functional recovery and a prediction of recovery, by comparison to values generated in participants with similar injuries ('average results'). As the Rosén method encompasses a spectrum of tests it requires availability of assessment tools, experienced staff, and protected time periods to conduct. This ideally would form part of ongoing routine assessment by a dedicated and experienced hand surgery team, unfortunately this limits its use in some settings. As such, although desirable, lack of this reporting outcome will not be used as exclusion criteria.
Appendix 2. Additional methods described in the protocol but not used in the review
Our protocol described additional methods that were not required for the review (Thomson 2017).
Types of studies
Where RCTs are unavailable, we will provide narrative discussion of large cohort studies that satisfy minimum quality criteria, namely adequate description of the following:
injury (mixed/motor/sensory nerve, adult/child/neonate, mechanism, location, size, and concomitant injuries sustained);
surgical procedure (gap length to be reconstructed, length of inserted nerve conduit or graft);
neurosynthesis technique (e.g. suture or fibrin glue);
outcome assessment (timing relative to injury, blinding measures used, detail of measure(s) used and how applied);
rate of dropout from study.
We will not perform a formal non‐randomised studies meta‐analysis and we will describe the non‐randomised studies in the discussion section only.
Subgroup analysis and investigation of heterogeneity
Ideally, subgroup analysis would be performed evaluating the role of the clinical factors detailed below on outcome. However, it is anticipated that this may be limited by heterogeneity within studies that meet the inclusion criteria.
We will evaluate the clinical heterogeneity of studies prior to performing any meta‐analysis, with attention to the distribution of individuals belonging to the above subgroups between treatment arms. We will assess statistical heterogeneity using the I2 statistic (value > 50% represents substantial heterogeneity) and Chi2 test (significance level 0.1). In the case of low level of heterogeneity (I2 < 50% or P > 0.1), we will perform a meta‐analysis. In the case of significant heterogeneity among included trials, we will provide a systematic narrative synthesis instead.
We selected the following subgroup analyses as predicted and proven factors influencing nerve healing (Birch 2015; Camara 2015; Hart 2011; Hundepool 2015).
Length of nerve gap (none, less than 3 cm, greater than 3 cm) (Grinsell 2014).
Nerve type (sensory, motor, or mixed) (Lundborg 1986).
Delay from time of injury until repair (less than 48 hours, 48 hours to 2 weeks, 2 weeks to 2 months, over 2 months) (British Orthopaedic Association) (Hart 2008).
Participant age in years (less than 12, 12 to 25, 26 to 40, and greater than 40). Younger age confers increased regenerative capacity and younger individuals may be less able to comply with assessment; therefore, where possible we will perform subgroup analysis to consider those under the age of 12 years and over the age of 40 years (Chemnitz 2013).
Smoking status at time of surgery (smoker versus non‐smoker) (Hundepool 2015).
Biological versus synthetic scaffold for wrap or conduit (Hart 2011).
Gender (Stenberg 2014).
We will use the outcomes selected for inclusion in the summary of findings table in subgroup analyses.
We will use the formal test for subgroup interactions in Review Manager 5 (Review Manager 2020). If subgroup data were available a simple significance test to investigate differences between two or more subgroups can be performed (Borenstein 2013). This procedure consists of undertaking a standard test for heterogeneity across subgroup results rather than across individual study results and using the fixed‐effect model calculating an I2 statistic.
It is not expected that controlled studies would include participants with obstetric brachial plexus palsy; however, if they had done, we would have reported this, and considered such participants as a separate subgroup.
Sensitivity analysis
We plan to carry out the following sensitivity analyses to ensure results are robust and meaningful.
Repeat the analysis excluding any unpublished studies.
Repeat the analysis excluding studies at high risk of bias (non‐blinded trials, questionable randomisation methods, significant difference between treatment groups, non‐gold‐standard management in control group).
Appendix 3. Cochrane Neuromuscular Specialised Register via CRS‐Web search strategy
1 nerve* adj5 (wrap* or cuff* or tube or tubes or tubular or repair* or graft* or allograft* or autograft*) AND INREGISTER 41
2 nerve* and conduit* AND INREGISTER 7
3 #1 or #2 41
4 upper limb* or arm* or arms or hand or hands or finger* or upper extremit* AND INREGISTER 1247
5 MeSH DESCRIPTOR Upper Extremity Explode All AND INREGISTER 125
6 MeSH DESCRIPTOR Brachial Plexus Explode All AND INREGISTER 154
7 #4 or #5 or #6 1340
8 peripheral nerve injur* AND INREGISTER 49
9 injury or innervation:MH AND INREGISTER 388
10 #8 or #9 390
11 #3 and #7 and #10 22
12 INCENTRAL AND INREGISTER 7221
13 #11 NOT #12 0
Appendix 4. Cochrane Central Register of Controlled Trials (CENTRAL) via CRS‐Web search strategy
1 nerve* adj5 (wrap* or cuff* or tube or tubes or tubular or repair* or graft* or allograft* or autograft*) AND INREGISTER 41
2 nerve* and conduit* AND INREGISTER 7
3 #1 or #2 41
4 upper limb* or arm* or arms or hand or hands or finger* or upper extremit* AND INREGISTER 1247
5 MeSH DESCRIPTOR Upper Extremity Explode All AND INREGISTER 125
6 MeSH DESCRIPTOR Brachial Plexus Explode All AND INREGISTER 154
7 #4 or #5 or #6 1340
8 peripheral nerve injur* AND INREGISTER 49
9 injury or innervation:MH AND INREGISTER 388
10 #8 or #9 390
11 #3 and #7 and #10 22
12 INREGISTER 7877
13 #11 NOT #12 0
Appendix 5. MEDLINE (OvidSP) search strategy
Database: Ovid MEDLINE(R) ALL <1946 to January 25, 2022>
1 ((Randomized Controlled Trial or Controlled Clinical Trial or Pragmatic Clinical Trial).pt. or (Randomi?ed or Randomly or Placebo or Trial or Groups).ab. or Drug Therapy.fs.) not (Animals not (Humans and Animals)).sh. (4579543)
2 ((nerve*1 adj5 (wrap* or cuff* or tube or repair* or graft* or allograft* or autograft*)) or (nerve*1 and conduit*1)).mp. (13687)
3 (upper limb* or arm*1 or hand*1 or finger*1 or upper extremit*).mp. or exp upper extremity/ or exp Brachial plexus/in (903633)
4 Peripheral Nerve Injuries/ or (in or ir).fs. (394474)
5 1 and 2 and 3 and 4 (146)
6 limit 5 to ed=20201212‐20221231 (4)
7 limit 5 to dt=20201212‐20221231 (1)
8 6 or 7 (4)
Appendix 6. Embase (OvidSP) search strategy
Database: Embase <1974 to 2022 week 03>
1 Randomized controlled trial/ (691698)
2 Controlled clinical study/ (464804)
3 random$.ti,ab. (1744987)
4 randomization/ (92766)
5 intermethod comparison/ (278912)
6 placebo.ti,ab. (335163)
7 (compare or compared or comparison).ti. (554914)
8 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. (2430077)
9 (open adj label).ti,ab. (93908)
10 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. (252422)
11 double blind procedure/ (191507)
12 parallel group$1.ti,ab. (28712)
13 (crossover or cross over).ti,ab. (114316)
14 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. (370900)
15 (assigned or allocated).ti,ab. (436768)
16 (controlled adj7 (study or design or trial)).ti,ab. (397303)
17 (volunteer or volunteers).ti,ab. (263950)
18 human experiment/ (561857)
19 trial.ti. (348228)
20 or/1‐19 (5632908)
21 (random$ adj sampl$ adj7 ("cross section$" or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.) (8833)
22 Cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.) (296038)
23 (((case adj control$) and random$) not randomi?ed controlled).ti,ab. (19353)
24 (Systematic review not (trial or study)).ti. (197153)
25 (nonrandom$ not random$).ti,ab. (17508)
26 "Random field$".ti,ab. (2632)
27 (random cluster adj3 sampl$).ti,ab. (1402)
28 (review.ab. and review.pt.) not trial.ti. (957287)
29 "we searched".ab. and (review.ti. or review.pt.) (39966)
30 "update review".ab. (119)
31 (databases adj4 searched).ab. (47817)
32 (rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/ (1134805)
33 Animal experiment/ not (human experiment/ or human/) (2381946)
34 or/21‐33 (3870082)
35 20 not 34 (4993846)
36 ((nerve*1 adj5 (wrap* or cuff* or tube or tubes or tubular or repair* or graft* or allograft* or autograft*)) or (nerv* and conduit*)).mp. (19638)
37 (upper limb* or arm*1 or hand*1 or finger*1 or upper extremit*).mp. or exp upper limb/ (1301168)
38 peripheral nerve injury/ or (nerve*1 adj5 injur*3).mp. (76405)
39 35 and 36 and 37 and 38 (199)
40 limit 39 to (conference abstracts or embase) (162)
41 limit 40 to em=202050‐202203 (14)
Appendix 7. ClinicalTrials.gov search strategy
Advanced search
Condition: Nerve Injury
Study Type: select Interventional/clinical trial
Intervention: Wrap OR Tube OR Scaffold OR Cuff OR Conduit OR Bridge
First posted on or after 12/13/2020
2 studies found
Appendix 8. WHO ICTRP search strategy
On 13 December 2020, this resource was inaccessible. The following strategy is for previous search:
Advanced search
Nerve Injury in the Condition
Wrap OR Tube OR Scaffold OR Cuff OR Conduit OR Bridge in the Intervention
Recruitment status: select ALL
8 records for 8 trials found
Data and analyses
Comparison 1. Repair using bioengineered device versus standard nerve repair.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Sensory recovery at ≥ 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 At 5 years | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.43, 0.49] |
| 1.2 Muscle strength, assessed with BMRC motor grading at 12–24 months | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.2.1 At 18 months | 1 | 11 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.38, 1.18] |
| 1.3 Motor Rosén at 12–24 months | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.3.1 At 24 months | 2 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.24, 0.05] |
| 1.3.2 At 12 months | 1 | 35 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.18, ‐0.12] |
| 1.4 Sensory recovery, assessed with BMRC sensory grading at 12–24 months | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.4.1 At 18 months | 1 | 11 | Mean Difference (IV, Random, 95% CI) | 0.93 [‐0.09, 1.95] |
| 1.5 Integrated functional outcome, assessed with Rosén Model Instrument | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.5.1 At 5 years | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [0.07, 0.38] |
| 1.5.2 At 24 months | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.38, 0.05] |
| 1.5.3 At 12 months | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐2.29 [‐2.49, ‐2.09] |
| 1.6 Touch threshold, measured by Semmes‐Weinstein Monofilament | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.6.1 At 24 months | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.06, 0.08] |
| 1.6.2 At 12 months | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.07, 0.17] |
| 1.7 Cold intolerance | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.7.1 At 24 months | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.08, 0.30] |
| 1.8 Sensory nerve action potential (SNAP) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.8.1 At 24 months | 2 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐1.89, 1.73] |
| 1.8.2 At 12 months | 2 | 61 | Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.58, 1.03] |
| 1.9 Adverse events | 5 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.15 [1.74, 29.42] |
| 1.10 Device removal or revision | 5 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.61 [1.48, 39.02] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aberg 2009.
| Study characteristics | ||
| Methods | Study type: RCT Follow‐up: 18 months |
|
| Participants | Participants: 12 (6 intervention, 6 control) Injury: < 1 week following injury, complete median or ulnar nerve injury, or both, at the wrist/forearm level Age range (years): 15–58 Sex: 1 female, 11 male |
|
| Interventions | Intervention: PHB wrap (6 participants, total number of nerves unclear) Control: epineural end‐to‐end suturing (6 participants, total number of nerves unclear) |
|
| Outcomes | Outcomes measured at 3, 6, 9, 12, and 18 months Motor recovery (MMT, grip and pinch strength, motor neurography, EMG) Sensory recovery (BMRC score S0–S4, thermal threshold, 2‐PD, sensory neurography, morphological assessment of sensory neuropeptides of skin biopsies) Functional recovery (sensorimotor test, Sollerman hand function test, 4 question form) Safety (adverse events, complications) |
|
| Funding | Study sponsored by AstraTech AB, Sweden, manufacturers of PHB wrap | |
| Conflicts of interest | Stated no conflicts | |
| Notes | Results from sensory neurography were excluded from the final analysis due to missing data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Sealed envelope, opened at time of surgery. |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation with a block size of 10 participants. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All assessors and participants were unaware of the type of treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All assessors and participants were unaware of the type of treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 participant did not have the primary outcome (BMRC sensory recovery) measured at 18‐month follow‐up. Detail was provided of incomplete data sets. The discussion advised careful interpretation of results in which missing data resulted in < 5 participants. |
| Selective reporting (reporting bias) | High risk | All predetermined outcome measures commented on. Report provided data only on those demonstrating a significant difference between groups and data were discarded for median nerve injuries in 2 participants who had > 1 nerve repaired. |
| Other bias | Low risk | No other specific risks of bias. |
Bertleff 2005.
| Study characteristics | ||
| Methods | Study type: multicentre RCT Follow‐up: 12 months |
|
| Participants | Participants: 30 (17 intervention, 13 control) Injury: unknown time from injury, complete nerve injury distal to the wrist Age range (years): 18–75 Sex: 7 female, 23 male |
|
| Interventions | Intervention: poly(DL‐lactide‐caprolactone) (Neurolac) nerve guide (21 nerve repairs in 17 participants) Control: end‐to‐end suturing (13 nerve repairs in 13 participants). |
|
| Outcomes | Outcomes measured at 3, 6, 9, and 12 months Sensory recovery (static and moving 1‐ and 2‐PD) Safety (adverse events, complications) |
|
| Funding | Study supported by Polyganics, manufacturers of Neurolac nerve guide | |
| Conflicts of interest | Awarded funding support by company who makes conduits. | |
| Notes | Nerve lesions were subgrouped according to defect length (< 4 mm, 4–8 mm, and 8–20 mm). Each subgroup had its own randomisation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Allocation made in operating theatre after exploration of wound, nurse opened concealed envelope. |
| Random sequence generation (selection bias) | Unclear risk | Detail of sequence generation not provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants may have been aware of intervention following allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessors may have been aware of treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Detail not provided of incomplete data, number of participants at time points not provided. No comment on number who attended follow‐up. |
| Selective reporting (reporting bias) | Low risk | Results for all expected outcome measures were reported. |
| Other bias | Unclear risk | Randomisation of participants (with nerves from same participant receiving the same treatment) producing a unit‐of‐analysis error. 4 participants had 2 nerve grafts, and it is unclear how these paired data impacted the results. |
Boeckstyns 2013.
| Study characteristics | ||
| Methods | Study type: RCT Follow‐up period: 24 months |
|
| Participants | Participants: 43 participants (22 intervention, 21 control) Injury: < 72 hours following injury, complete nerve laceration of the median or ulnar nerves or both in the distal third of the forearm Age range (years): 21–66 Sex: 9 female, 22 male (demographics provided for those attending 2‐year follow‐up) |
|
| Interventions | Intervention: collagen nerve guide conduit (23 nerves in 22 participants) Control: end‐to‐end suturing or nerve grafting (22 nerves in 22 participants, 21 direct repairs and 1 sural nerve graft). Note: data for sural nerve graft subsequently excluded from analysis. |
|
| Outcomes | Outcomes measured at 3, 6, 12, 18, and 24 months RMI Motor action potential Sensory action potential Safety (adverse events and complications) |
|
| Funding | No funding source declared | |
| Conflicts of interest | Nerve conduit company employee featured on authorship | |
| Notes | Nerve gap > 20 mm was an exclusion criterion. Trialists performed only 1 nerve grafting, which was excluded from the final analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Quote: "We opened the randomization envelopes at the time of surgery after having measured the nerve gap and found the lacerations suitable for direct end‐to‐end suture or implantation of a short nerve graft." |
| Random sequence generation (selection bias) | Unclear risk | Details of sequence generation not provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The participants and postoperative personnel were blinded to the treatment and subjective outcomes used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The evaluators were blinded to the treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 11 participants did not have follow‐up at 24 months. 1 died, and the others did not attend follow‐up. Therefore, data were analysed for 18/22 participants in the conduit repair group and 13/21 participants in the direct suture group at 24 months. However, we judged the risk of bias unclear because there was no reported systematic difference in the way participant groups were followed up. There was only 1 participant with a nerve gap injury repaired using autologous nerve graft and the data were excluded from analysis. We deemed this a low risk of attrition bias in itself. We graded the risk of attrition bias overall as unclear as the reasons for loss to follow‐up were not detailed. |
| Selective reporting (reporting bias) | Low risk | All outcome measures were reported. |
| Other bias | Unclear risk | Nerve gaps were compared to direct repair. Length of defects repaired in the conduit group unclear. Quote: "We measured the nerve gaps to ensure that they did not exceed 20 mm but we did not record the measurements for further analysis." Comment: this could conceivably bias results to favour direct repair but we did not consider this a major concern. 1 participant underwent 2 nerve repairs (unit of randomisation was the participant). It is unclear how authors dealt with non‐independence of data in analysis. |
Lundborg 2004.
| Study characteristics | ||
| Methods | Study type: RCT Follow‐up: 60 months |
|
| Participants | Participants: 30 participants (17 intervention, 13 control) Injury: < 48 hours following injury, complete transection of the median or ulnar nerve at wrist or distal forearm (< 10 cm from the wrist) Age range (years): 12–72 Sex: 4 female, 26 male |
|
| Interventions | Intervention: silicone tube (17 nerve repairs in 17 participants) Control: epineural end‐to‐end suturing (13 nerve repairs in 13 participants) |
|
| Outcomes | Outcomes measured at 3, 6, 12, 24, 36, 48, and 60 months BMRC grading for sensory recovery Sensory and motor neurophysiology RMI |
|
| Funding | Supported by grants from the Swedish Research Council, Swedish Brain Foundation, Faculty of Medicine, Lund University | |
| Conflicts of interest | Did not state any conflict of interest. | |
| Notes | 17 participants underwent neurophysiological assessment. We requested raw data, which was provided and facilitated meta‐analysis at the 24‐month time point. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Concealment was performed with sealed envelopes. It is uncertain at what point this was opened; however, all surgeries were performed within the first 48 hours following injury. |
| Random sequence generation (selection bias) | Unclear risk | Detail of sequence generation not provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is unclear if participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The examiner was actively blinded during the first follow‐up year; however, due to small‐study size and close follow‐up, blinding was broken by 5‐year follow‐up. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2/30 participants failed to make the 5‐year follow‐up, 1 from the conduit repair and 1 from the standard repair group. All analysis was performed as intention‐to‐treat and there were no deviations from random allocation. |
| Selective reporting (reporting bias) | Low risk | Results for all expected outcomes were reported. |
| Other bias | Low risk | No other specific areas of risk of bias. |
Weber 2000.
| Study characteristics | ||
| Methods | Study type: multicentre RCT Follow‐up: 12 months |
|
| Participants | Participants: 98 participants with 136 nerve transections (62 intervention, 74 control) Injury: complete transection of a sensory nerve distal to the distal wrist crease (common or proper digital nerves) Age range (years): 17–65 Sex: 26 female, 72 male Timing of repair: varied < 72 hours (112 nerves), 4–20 days (15 nerves), > 20 days (9 nerves) |
|
| Interventions | Intervention: polyglycolic acid conduit (62 nerves in 54 participants) Control: end‐to‐end suturing or nerve grafting (74 nerves in 52 participants) |
|
| Outcomes | Outcomes measured at 3, 6, 9, and 12 months Sensory recovery (static and moving 2‐PD) Safety (adverse events, complications) |
|
| Funding | No funding source declared. | |
| Conflicts of interest | RA Weber and colleagues have no stated conflicts of interest. | |
| Notes | Each nerve transection was randomised individually. Nerve gap, if any, was ≤ 3 cm Reporting of patient functional outcomes were grouped based on last follow‐up time (i.e. 3, 6, or 9 months); as such, data were not in a useable format for meta‐analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was performed with sealed envelopes at time of surgery after exploration of wound. |
| Random sequence generation (selection bias) | Unclear risk | Detail of sequence generation not provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomisation performed in theatre following exploration. Each nerve was randomised individually. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The therapist making the assessments was blinded to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Postoperative sensory measurements were obtained detailing 46/62 nerves in the conduit participant group and 54/74 nerves in the standard repair group. For participants who did not return for the complete 12‐month follow‐up, the result of their last visit, whether at 3, 6, or 9 months was carried forward and used to determine their outcome. |
| Selective reporting (reporting bias) | Low risk | Results for all expected outcomes were reported. |
| Other bias | Unclear risk | Randomisation of nerves. It is unclear if there is unit‐of‐analysis bias. There was no apparent adjustment for non‐independence of multiple nerve repairs in same participant. Number of nerve surgeries per participant:
|
1‐PD: 1‐point discrimination; 2‐PD: 2‐point discrimination; BMRC: British Medical Research Council; EMG: electromyography; MMT: manual muscle test; PHB: poly(R)‐3‐hydroxybutyrate; RCT: randomised controlled trial; RMI: Rosén Model Instrument.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| NCT02459015 | Insufficient patient compliance and data collection led to study termination. No published data. |
| Neubrech 2016 | Methodology did not meet inclusion criteria. Time points for follow‐up different for device repair and standard repair cohorts. |
| Neubrech 2018 | Follow‐up limited to 6 months. |
Characteristics of ongoing studies [ordered by study ID]
ISRCTN97234566.
| Study name | CoNNECT: a study of sutureless nerve repair |
| Methods | Interventional 3‐arm RCT powered for equivalence. Computer randomisation. Participants and observers blinded. |
| Participants | Patients aged 16–75 years with a traumatic complete digital nerve injury between the wrist and middle of the affected finger < 10 days old |
| Interventions | Stitching injured nerve ends directly together Stitching nerve ends directly together and placing a nerve conduit around it Placing the injured nerve ends together without stitches and using the nerve conduit to maintain their position and heal |
| Outcomes |
Primary outcome measure Sensory recovery using static and moving 2‐PD (tactile gnosis) for each repaired nerve. The comparable area on the opposite hand will be tested for static and moving 2‐PD to act as a baseline for assessment of recovery. These measurements will allow the modified Weber score to be calculated. This will be assessed at weeks 2, 6, 12, 26, and 52. Secondary outcome measures Monofilament pressure thresholds (innervation density), assessed using the WEST Monofilaments Upper extremity disability and symptoms, assessed using the DASH score Self‐rated health, assessed using the EQ‐5D Nerve irritation, assessed using differential Tinel's sign Pain, assessed using a VAS Cold intolerance, assessed using a VAS Hyperaesthesia, assessed using a VAS Site of repair, measured in mm from the hyponychium of the same digit (the duration of each repair will be recorded) For suture repairs, the quality of the repair will be recorded using the visual grading scale for suture‐only nerve repair For common digital nerve repair, the outcome for each digital nerve territory will be recorded Each assessed at weeks 2, 6, 12, 26, and 52 |
| Starting date | 1 February 2017 |
| Contact information | dominic.power@uhb.nhs.uk |
| Notes | Retrospectively registered |
NCT01809002.
| Study name | Comparison of processed nerve allograft and collagen nerve cuffs for peripheral nerve repair (RECON) |
| Methods | Multicentre, prospective, randomised, participant‐ and evaluator‐blinded comparative study of nerve cuffs and Avance nerve graft evaluating recovery outcomes for the repair of nerve discontinuities |
| Participants | 220 participants enrolled, aged 18–65 years |
| Interventions | Intervention: processed nerve allograft (human) Active comparator: collagen nerve cuff |
| Outcomes | Recovery of static 2‐PD assessed by discriminator (in mm) (time frame: 12 months) |
| Starting date | June 2015 |
| Contact information | L Scott Levin, University of Pennsylvania |
| Notes |
NCT02359825.
| Study name | Nerve repair using hydrophilic polymers to promote immediate fusion of severed axons and swift return of function |
| Methods | Randomised, single‐blind, parallel‐group |
| Participants | Planned recruitment: 18 People with diagnosis of Sunderland Class 5 traumatic neuropathy (transection injury) of a digital nerve in the upper extremity who are candidates for immediate surgical repair, within 72 hours of injury or 48 hours if the injury requires nerve grafting. Participants are required to have no significant comorbidities to prevent immediate repair and be willing to comply with treatment and evaluation schedule. People with peripheral nerve injuries complicated by significant vascular or orthopaedic damage were eligible. Exclusion criteria: gross contamination of injuries, inadequate soft tissue coverage, or planned staged repair; diabetes, diagnosed neuromuscular disease, undergoing chemotherapy, radiotherapy, or other treatments known to affect the growth of the neural and vascular system; people enrolled in another investigational study, those unlikely to complete the normal regimen of occupational therapy; time of injury outside study parameters |
| Interventions | 3 'no intervention' groups (no medication used)
3 experimental groups (with PEG‐assisted axonal fusion technique)
Quote: "For the control groups, epineural repair or interposition grafting will be undertaken in the standard end‐to‐end fashion using interrupted nylon suture after irrigation of the wound with normal saline as deemed necessary by the operating surgeon. For the experimental group, the nerve(s) will be repaired using standard suture neurorrhaphy techniques and a 149.25 mM (50%) solution of PEG 3.35 kD in sterile water will then be irrigated onto the neurorrhaphy site for one minute. Following this, the approximated nerve ends will be irrigated with sterile water gently for 2 minutes. All wounds will be closed in the fashion deemed appropriate by the operating surgeon." |
| Outcomes | Return of nerve function as measured by (Medical Research Council Classification) (time frame: 12 months) |
| Starting date | 1 September 2015 |
| Contact information | Wesley Thayer, Julia Yao, Vanderbilt University Medical Centre |
| Notes |
NCT02372669.
| Study name | Chitosan nerve tube for primary repair of traumatic sensory nerve lesions of the hand (CNT) |
| Methods | To evaluate whether the additional use of a chitosan nerve tube in primary microsurgical repair of traumatic sensory nerve lesions of the hand has an effect on convalescence and functional results. |
| Participants | Adults aged 18–67 years with a sensory nerve defect in the hand |
| Interventions | Chitosan nerve tube Gold‐standard repair |
| Outcomes | Static 2‐PD of injured finger measured with compasses 6 months after intervention (primary outcome) Static 2‐PD of injured finger/sensibility (checking participants' ability to recognise filaments of different calibres) at other follow‐ups (3, 6, 12, and 24 months after intervention). DASH‐score (at 3, 6, 12, and 24 months after intervention). Patients' individual disability in activities of daily living will be measured with the DASH questionnaire. Grip strength (at 3, 6, 12, and 24 months after intervention). Grip strength of both hands will be measured with a dynamometer and will be compared to the opposite side. Range of motion of the injured finger (at 3, 6, 12, and 24 months after intervention). Range of motion of the injured finger measured with a goniometer for small joints and will be compared to the opposite side. Pain (VAS) (at 3, 6, 12, and 24 months after intervention). Participants will self‐report pain on VAS, ranged from 0 (no pain) up to 10 (maximum of pain) Cold intolerance (grades: 0 = hinders function; 1 = disturbing; 2 = moderate; 3 = none/minor) (at 3, 6, 12, and 24 months after intervention). The examiner will question the participant about cold intolerance (grades: 0 = hinders function; 1 = disturbing; 2 = moderate; 3 = none/minor) Hypersensitivity (grades: 0 = hinders function; 1 = disturbing; 2 = moderate; 3 = none/minor) (at 3, 6, 12, and 24 months after intervention). The examiner will stroke the dysfunctional area and question the participant about cold hypersensitivity (grades: 0 = hinders function; 1 = disturbing; 2 = moderate; 3 = none/minor) Existence of neuromas (at 3, 6, 12, and 24 months after intervention). The existence of a neuroma will be assessed clinically and by neurosonography. |
| Starting date | 1 July 2015 |
| Contact information | florian.neubrech@bgu‐ludwigshafen.de |
| Notes |
2‐PD: 2‐point discrimination; DASH: Disabilities of the Arm, Shoulder and Hand; EQ‐5D: Euro‐Qol 5 Dimension; PEG: polyethylene glycol; VAS: visual analogue scale; WESY: Weinstein Enhanced Sensory Test.
Differences between protocol and review
We made the following changes from our protocol (Thomson 2017).
Types of studies. Our protocol described plans for narrative discussion of large cohort studies if RCTs were unavailable. We identified RCTs and this was not necessary.
No subgroup analysis was possible due to heterogeneity, methodological variation, and relatively low participant numbers per trial. There were no instances of three or more treatment groups per trial and there were no cases when participants were randomised to one graft and an alternative used for a second graft.
We prespecified 24 months' follow‐up for the primary outcomes in our summary of findings tables and also 12‐month time point for secondary outcomes. We changed them to '24 months or more' and '12 to 24 months' to allow for extended follow‐up times, given their relevance for decision‐makers, and to maximise the use of data from 12 to 24 months. In the protocol, we specified a minimum follow‐up duration of 12 months in included studies, which we applied in the review. In keeping with this, we made a correction to the outcomes section in the review to remove references to outcome measurement at six months.
The review protocol specified that we would report serious adverse events. Only one study made the distinction between serious and non‐serious device‐related adverse events (Aberg 2009), and did not find either. Other studies did not make a distinction between serious and non‐serious adverse events. We reported all adverse events mentioned in the included studies in order to adequately capture benefits and harms.
Data on cost of bioengineered devices were complex and we did not report them in the summary of findings table as planned in the protocol. Instead, the authors provided detail in a separate table, for clarity of presentation.
We removed a planned sensitivity analysis on the advice of the group methodologist: 'Repeat the analysis excluding any large studies to establish how much they dominate the results.'
Contributions of authors
SET, MR, AH, and PK conceived the review.
SET and NN drafted the protocol, and MR, AH, PK, LD, and MW revised the draft. All authors approved publication of the protocol.
All authors developed the search strategy with assistance from Cochrane team.
SET and NN: screened titles and abstracts and full‐text reports performed data extraction and assessed risk of bias.
AH, MR, or PK: resolved differences in study selection and risk of bias assessment.
NN entered data into Review Manager 5 and SET checked data entry.
All authors commented on and approved the final draft of the review.
Sources of support
Internal sources
No sources of support provided
External sources
-
Medical Research Council, UK
Clinical Research Training Fellowship (SET)
-
National Institute of Health Research (NIHR), Queen Square Centre for Neuromuscular Diseases, UK
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Neuromuscular. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health. Cochrane Neuromuscular is also supported by the Queen Square Centre for Neuromuscular Diseases.
Declarations of interest
SET: recently complete PhD clinical research training fellowship (MRC 70085). She collaborates with NHS Blood & Transplant (NHSBT) on peripheral nerve repair projects with no financial links and no current research output. She holds a Tenovus Scotland Grant actively researching nerve regeneration strategies in collaboration with the Centre for the Cellular Microenvironment at the Advanced Research Centre, University of Glasgow. She works clinically on surgery of the peripheral nerve and has published and presented translationally relevant research on the topic. She serves on the British Surgical Society of the Hand (BSSH) Overseas Trainee Committee as research lead and contributes to global research in the field of upper limb surgery.
NYBN: is an Orthopaedic Surgery Registrar, NHS Greater Glasgow and Clyde.
MOR: University Of Glasgow (MRC Fellowship (MR/L017741/1) held by Suzanne Thomson: grant/contract) (July 2014 to July 2017). He has given talks on multiple occasions in the UK (Strathclyde, University College London, Aberdeen, King's College London), posted on Twitter (@morenorse), and contributed to scientific publications and commentary. He is a member of the British Neuroscience Association, British Society for Cell Biology and of the Federation of European Neuroscience Societies.
PJK: his former institution is the patent holder for a peripheral nerve growth conduit (patent US20160082149A1) and a peripheral nerve growth scaffold including poly‐E‐caprolactone (patent GB2490269A), for which he has received no personal payment.
LBD: has received payment from AxoGen for membership of an advisory board on a practical course in nerve repair and membership of a Data Safety and Monitoring Board (November 2019). LBD was a member of an EU consortium, financially supported by an EU grant, in which Medovent AG (manufacturer of chitosan nerve guides) was also a member. He has received no financial support from this company. Medovent Inc provides chitosan conduits in the project. He has an active research collaboration with Vibrosense Inc concerning evaluation of vibrotactile sense (Dahlin 2015), which is supported by the foundation VINNOVA, Sweden. He has received no financial support from the company. He has been consultant for AxoGen Inc, Gainsville, Florida, USA 2003 to 2004. AxoGen Inc produce Avance (R) Nerve Graft, Axoguard(R) Nerve Connector, and Axoguard(R) Nerve Protector. LBD has been a principal investigator in a clinical trial of Neurocap(R) (Protect Neuro) for neuroma treatment financed by Polyganics B.V. LBD is also a board member of Scania Hand Center AB. He was involved in an included study that had university and Swedish Medical Research Council funding (Lundborg 2004).
MW: works as a hand surgeon at Umeå University Hospital. He was involved in a study eligible for the review funded as previously in receipt of an AstraTech research funding for the investigation of poly(R)‐3‐hydroxybutyrate (PHB) as a material for use in peripheral nerve repair from 1992 to 2008, a consulting fee 2002 to 2008, and funding for a prospective randomised clinical controlled trial of the use of PHB material for wraparound repair of peripheral nerve injuries. The research has been concluded and there are no ongoing connections by AstraTech (Aberg 2009).
AMH: is in receipt of a stipend from the British Association of Plastic and Reconstructive Surgery (BAPRAS) for his role as Editor of the Journal of Plastic Reconstructive & Aesthetic Surgery (JPRAS). He receives payment from BMI and Nuffield hospitals for small volume private practice as a Consultant Hand & Plastic Surgeon, which (rarely) includes peripheral nerve reconstruction. His institution receives grants from the following: NHSBT – provision of clinical and academic advice on product development (potential decellularised nerve allograft product) and research options, and ethical/regulatory dataset requirements; Ossur – clinical trial lead, contract start January 2021; and Bobby Charlton Foundation – clinical trial lead for the first in human testing of a nanokicked osteogenic stem cell therapy. He has worked as a hand and plastic surgeon in the National Health Service, New Zealand, and Sweden for over 20 years, as a consultant since around 2006. His honorary post in the University of Glasgow is focused on peripheral nerve injury and tissue engineering research. He has subspecialty clinical practice in major peripheral nerve injury (adult and paediatric). He has published, presented, supervised research, and given expert advice in the field of peripheral nerve regeneration and reconstruction as part of normal clinical and academic roles. He is a member of British Association of Plastic, Reconstructive and Aesthetic Surgeons (BAPRAS) and the BSSH, in addition to other professional bodies. BSSH and, to a lesser extent, BAPRAS have been actively involved in the field of peripheral nerve research, and AMH was involved in the BSSH‐funded James Lind Alliance project to identify research priorities, which included the field of peripheral nerve injury.
New
References
References to studies included in this review
Aberg 2009 {published data only}
- Aberg M, Ljungberg C, Edin E, Millqvist H, Nordh E, Theorin A, et al. Clinical evaluation of a resorbable wrap-around implant as an alternative to nerve repair: a prospective, assessor-blinded, randomised clinical study of sensory, motor and functional recovery after peripheral nerve repair. Journal of Plastic, Reconstructive & Aesthetic Surgery 2009;62(11):1503-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bertleff 2005 {published data only}
- Bertleff MJ, Meek MF, Nicolai JP. A prospective clinical evaluation of biodegradable Neurolac nerve guides for sensory nerve repair in the hand. Journal of Hand Surgery (Edinburgh, Scotland) 2005;30(3):513-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Boeckstyns 2013 {published data only}
- Boeckstyns ME, Krarup C, Rosen B, Fores J, Sorensen AI, Navarro X. A collagen conduit versus microsurgical neurorrhaphy 2-year follow-up of a prospective blinded clinical and electrophysiological multicenter RCT. Journal of Hand Surgery (Edinburgh, Scotland) 2013;38(10 Suppl 1):e3-4. [DOI] [PubMed] [Google Scholar]
- Boeckstyns ME, Sørensen AI, Viñeta JF, Rosén B, Navarro X, Archibald SJ, et al. Collagen conduit versus microsurgical neurorrhaphy: 2-year follow-up of a prospective, blinded clinical and electrophysiological multicenter randomized, controlled trial. Journal of Hand Surgery (Edinburgh, Scotland) 2013;38(12):2405-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lundborg 2004 {published data only}
- Lundborg G, Rosén B, Dahlin L, Holmberg J, Rosén I. Tubular repair of the median or ulnar nerve in the human forearm: a 5-year follow-up. Journal of Hand Surgery (Edinburgh, Scotland) 2004;29(2):100-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Lundborg G, Rosen B, Dahlin L, Danielsen N, Holmberg J. Tubular versus conventional repair of median and ulnar nerves in the human forearm: early results from a prospective, randomized, clinical study. Journal of Hand Surgery (Edinburgh, Scotland) 1997;22(1):99-106. [DOI] [PubMed] [Google Scholar]
Weber 2000 {published data only}
- Weber RA, Breidenbach WC, Brown RE, Jabaley ME, Mass DP. A randomized prospective study of polyglycolic acid conduits for digital nerve reconstruction in humans. Plastic and Reconstructive Surgery 2000;106(5):1036-45. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
NCT02459015 {published data only}
- NCT02459015. Performance study of an artificial nerve guide (Reaxon® nerve guide) to treat digital nerve lesions. www.clinicaltrials.gov/ct2/show/NCT02459015 (first received 1 June 2015).
Neubrech 2016 {published data only}
- Neubrech N, Heider S, Otte M, Hirche C, Kneser U, Kremer T. Nerve tubes for the repair of traumatic sensory nerve lesions of the hand: review and planning study for a randomised controlled multicentre trial CNO – CN-0145953. Handchirurgie, Mikrochirurgie, Plastische Chirurgie 2016;48(3):148-54. [DOI] [PubMed] [Google Scholar]
Neubrech 2018 {published data only}
- Neubrech N, Sauerbier M, Moll W, Seegmüller J, Sina Heider S, Harhaus L, et al. Enhancing the outcome of traumatic sensory nerve lesions of the hand by additional use of a chitosan nerve tube in primary nerve repair: a randomized controlled bicentric trial. Plastic and Reconstructive Surgery 2018;142(2):415-24. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN97234566 {published data only}
- ISRCTN97234566. CoNNECT: a study of sutureless nerve repair. www.isrctn.com/ISRCTN97234566 (first received 24 July 2018).
NCT01809002 {published data only}
- NCT01809002. Comparison of processed nerve allograft and collagen nerve cuffs for peripheral nerve repair (RECON). www.clinicaltrials.gov/ct2/show/NCT01809002 (first received 12 March 2013).
NCT02359825 {published data only}
- NCT02359825. Nerve repair using hydrophilic polymers to promote immediate fusion of severed axons and swift return of function. www.clinicaltrials.gov/ct2/show/NCT02359825 (first received 10 February 2015).
NCT02372669 {published data only}
- NCT02372669. Chitosan nerve tube for primary repair of traumatic sensory nerve lesions of the hand (CNT). www.clinicaltrials.gov/ct2/show/NCT02372669 (first received 26 February 2015).
Additional references
Aberg 2007
- Aberg M, Ljungberg C, Edin E, Jenmalm P, Millqvist H, Nordh E, et al. Considerations in evaluating new treatment alternatives following peripheral nerve injuries: a prospective clinical study of methods used to investigate sensory, motor and functional recovery. Journal of Plastic, Reconstructive and Aesthetic Surgery 2007;60(2):103-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Allodi 2012
- Allodi I, Udina E, Navarro X. Specificity of peripheral nerve regeneration: interactions at the axon level. Progress in Neurobiology 2012;98(1):16-37. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ashwood 2018
- Ashwood M, Jerosh-Herold C, Shepstone L. Development and Validation of a new patient-reported outcome measure for peripheral nerve disorders of the hand, the I-HaND Scale. Journal of Hand Surgery, European Volume 2018;43(8):864-74. [DOI] [PubMed] [Google Scholar]
Beaton 2001
- Beaton DE, Katz JN, Fossel AH, Wright JG, Tarasuk V, Bombardier C. Measuring the whole or the parts? Validity, reliability, and responsiveness of the Disabilities of the Arm, Shoulder and Hand outcome measure in different regions of the upper extremity. Journal of Hand Therapy 2001;14(2):128-46. [PMID: ] [PubMed] [Google Scholar]
Bell‐Krotoski 1995
- Bell-Krotoski JA, Fess EE, Figarola JH, Hiltz D. Threshold detection and Semmes-Weinstein monofilaments. Journal of Hand Therapy 1995;8(2):155-62. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bertelli 2011
- Bertelli JA, Kechele PR, Ghizoni MF, Fröde TS. Mesh epineurial splinting for late median nerve repair in older patients: a preliminary report. Microsurgery 2011;31(6):441-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Birch 2015
- Birch R. Timing of surgical reconstruction for closed traumatic injury to the supraclavicular brachial plexus. Journal of Hand Surgery, European Volume 2015;40(6):562-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Borenstein 2013
- Borenstein M, Higgins JP. Meta-analysis and subgroups. Prevention Science 2013;14:134-43. [DOI] [PubMed] [Google Scholar]
Braga Silva 2017
- Braga Silva J, Marchese GM, Cauduro CG, Debiasi M. Nerve conduits for treating peripheral nerve injuries: a systematic literature review. Hand Surgery & Rehabilitation 2017;36(2):71-85. [DOI: 10.1016/j.hansur.2016.10.212] [DOI] [PubMed] [Google Scholar]
Braga Silva 2021
- Braga Silva J, Leal BL, Magnus GA, De Souza Stanham V, Mattiello R, Wolff CG. Comparison of nerve conduits and nerve graft in digital nerve regeneration: a systematic review and meta-analysis. Hand Surgery & Rehabilitation 2021;40(6):715-21. [DOI: 10.1016/j.hansur.2021.08.006] [DOI] [PubMed] [Google Scholar]
Brandsma 1995
- Brandsma JW, Schreuders TA, Birke JA, Piefer A, Oostendorp R. Manual muscle strength testing: intraobserver and interobserver reliabilities for the intrinsic muscles of the hand. Journal of Hand Therapy 1995;8(3):185-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Brushart 1993
- Brushart TM. Motor axons preferentially reinnervate motor pathways. Journal of Neuroscience 1993;13(6):2730-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Camara 2015
- Camara J, Griessenauer CJ. Volume 2: pain, treatment, injury, disease and future directions. Part VII: the future of peripheral nerve injury. In: Tubbs RS, Rizk E, Shoja MM, Loukas M, Barbaro N, Spinner RJ, editors(s). Nerves and Nerve Injuries. 1st edition. London (UK): Academic Press, 2015. [Google Scholar]
Carlsson 2008
- Carlsson I, Cederlund R, Höglund P, Lundborg G, Rosén B. Hand injuries and cold sensitivity: reliability and validity of cold sensitivity questionnaires. Disability and Rehabilitation 2008;30(25):1920-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chemnitz 2013
- Chemnitz A, Björkman A, Dahlin LB, Rosén B. Functional outcome thirty years after median and ulnar nerve repair in childhood and adolescence. Journal of Bone and Joint Surgery, American Volume 2013;95(4):329-37. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chemnitz 2015
- Chemnitz A, Weibull A, Rosén B, Andersson G, Dahlin LB, Björkman A. Normalized activation in the somatosensory cortex 30 years following nerve repair in children: an fMRI study. European Journal of Neuroscience 2015;42(4):2022-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dahlin 2001
- Dahlin LB, Lundborg G. Use of tubes in peripheral nerve repair. Neurosurgery Clinics of North America 2001;12(2):341-52. [PMID: ] [PubMed] [Google Scholar]
Dahlin 2008
- Dahlin LB. Techniques of peripheral nerve repair. Scandinavian Journal of Surgery 2008;97(4):310-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dahlin 2015
- Dahlin LB, Güner N, Elding Larsson H, Speidel T. Vibrotactile perception in finger pulps and in the sole of the foot in healthy subjects among children or adolescents. PLOS One 2015;10(3):e0119753. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Faroni 2015
- Faroni A, Mobasseri SA, Kingham PJ, Reid AJ. Peripheral nerve regeneration: experimental strategies and future perspectives. Advanced Drug Delivery Reviews 2015;82-3:160-7. [DOI] [PubMed] [Google Scholar]
Gaudin 2016
- Gaudin R, Knipfer C, Henningsen A, Smeets R, Heiland M, Hadlock T. Approaches to peripheral nerve repair: generations of biomaterial conduits yielding to replacing autologous nerve grafts in craniomaxillofacial surgery. BioMed Research International 2016;2016:3856262. [DOI: 10.1155/2016/3856262] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed September 2022. Available at gradepro.org.
Grinsell 2014
- Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. BioMed Research International 2014;2014:698256. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gummesson 2003
- Gummesson C, Atroshi I, Ekdahl C. The Disabilities of the Arm, Shoulder and Hand (DASH) outcome questionnaire: longitudinal construct validity and measuring self-rated health change after surgery. BMC Musculoskeletal Disorders 2003;4:11. [PMC165599] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hallgren 2013
- Hallgren A, Björkman A, Chemnitz A, Dahlin LB. Subjective outcome related to donor site morbidity after sural nerve graft harvesting: a survey in 41 patients. BMC Surgery 2013;13:39. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hart 2008
- Hart AM, Terenghi G, Wiberg M. Neuronal death after peripheral nerve injury and experimental strategies for neuroprotection. Neurological Research 2008;30(10):999-1011. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hart 2011
- Hart A, Terenghi G, Wiberg M. Tissue engineering for peripheral nerve regeneration. In: Pallua N, Suschek CV, editors(s). Tissue Engineering. Berlin (Germany): Springer Verlag, 2011:245-62. [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Hundepool 2015
- Hundepool CA, Ultee J, Nijhuis TH, Houpt P, Research Group 'ZERO', Hovius SER. Prognostic factors for outcome after median, ulnar, and combined median-ulnar nerve injuries: a prospective study. Journal of Plastic, Reconstructive & Aesthetic Surgery 2015;68(1):1-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kalomiri 1994
- Kalomiri DE, Soucacos PN, Beris AE. Nerve grafting in peripheral nerve microsurgery of the upper extremity. Microsurgery 1994;15(7):506-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
Karsy 2017
- Karsy M, Watkins R, Jensen M, Guan J, Mahan M. Trends in upper extremity nerve injury using the national inpatient sample database. Journal of Neurosurgery 2017;126(4):A1439. [DOI: 10.3171/2017.4.JNS.AANS2017abstracts] [DOI] [Google Scholar]
Kehoe 2012
- Kehoe S, Zhang XF, Boyd D. FDA approved guidance conduits and wraps for peripheral nerve injury: a review of materials and efficacy. Injury 2012;43(5):553-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kretschmer 2009
- Kretschmer T, Ihle S, Antoniadis G, Seidel JA, Heinen C, Börm W, et al. Patient satisfaction and disability after brachial plexus surgery. Neurosurgery 2009;65(4 Suppl):A189-96. [PMID: ] [DOI] [PubMed] [Google Scholar]
Life Science Intelligence 2009
- Life Science Intelligence. Worldwide markets and emerging technologies for tissue engineering and regenerative medicine. LSI Reports 2009;1-714-847-3540:1-262.
Lohmeyer 2014
- Lohmeyer JA, Kern Y, Schmauss D, Paprottka F, Stang F, Siemers F, et al. Prospective clinical study on digital nerve repair with collagen nerve conduits and review of literature. Journal of Reconstructive Microsurgery 2014;30(4):227-34. [DOI: 10.1055/s-0033-1358788] [DOI] [PubMed] [Google Scholar]
Lundborg 1986
- Lundborg G, Dahlin LB, Danielsen N, Nachemson AK. Tissue specificity in nerve regeneration. Scandinavian Journal of Plastic and Reconstructive Surgery 1986;20(3):279-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lundborg 1994
- Lundborg G, Dahlin LB, Danielson N, Zhao Q. Trophism, tropism, and specificity in nerve regeneration. Journal of Reconstructive Microsurgery 1994;10(5):345-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lundborg 2000
- Lundborg G. A 25-year perspective of peripheral nerve surgery: evolving neuroscientific concepts and clinical significance. Journal of Hand Surgery, America 2000;25(3):391-414. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lundborg 2003
- Lundborg G. Richard P. Bunge Memorial Lecture. Nerve injury and repair – a challenge to the plastic brain. Journal of the Peripheral Nervous System 2003;8(4):209-26. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lundborg 2005
- Lundborg G. Nerve Injury and Repair: Regeneration, Reconstruction, and Cortical Remodeling. 2nd edition. London (UK): Churchill Livingstone, 2005. [Google Scholar]
Martin 2014
- Martin C, Dejardin T, Hart A, Riehle MO, Cumming DR. Directed nerve regeneration enabled by wirelessly powered electrodes printed on a biodegradable polymer. Advanced Healthcare Materials 2014;3(7):1001-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mauch 2019
- Mauch JT, Bae A, Shubinets V, Lin IC. A systematic review of sensory outcomes of digital nerve gap reconstruction with autograft, allograft, and conduit. Annals of Plastic Surgery 2019;82:166. [DOI: 10.1097/SAP.0000000000001851] [DOI] [PubMed] [Google Scholar]
Meek 2008
- Meek MF, Coert JH. US Food and Drug Administration /Conformit Europe – approved absorbable nerve conduits for clinical repair of peripheral and cranial nerves. Annals of Plastic Surgery 2008;60(1):110-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Millesi 1990
- Millesi H. Peripheral nerve surgery today: turning point or continuous development? Journal of Hand Surgery 1990;15(3):281-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Millesi 2007
- Millesi H. Bridging defects: autologous nerve grafts. Acta Neurochirurgica Supplement 2007;100:37-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Murphy 2019
- Murphy R, Faroni A, Wong J, Reid A. Protocol for a phase I trial of a novel synthetic polymer nerve conduit 'Polynerve' in participants with sensory digital nerve injury (UMANC). F1000Research 2019;8:959. [DOI: 10.12688/f1000research.19497.1] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00953277
- NCT00953277. Study of nerve reconstruction using AVANCE in subjects who undergo robotic assisted prostatectomy for treatment of prostate cancer. clinicaltrials.gov/ct2/show/NCT00953277 (first received 6 August 2009).
NCT01526681
- NCT01526681. Registry of Avance® nerve graft's utilization and recovery outcomes post peripheral nerve reconstruction (RANGER®) [A multicenter, registry study of Avance® nerve graft utilization, evaluations and outcomes in peripheral nerve injury repair]. clinicaltrials.gov/ct2/show/NCT01526681 (first received 6 February 2012).
NCT01573650
- NCT01573650. Optimization of peripheral nerve reconstruction: a non-inferiority trial. www.clinicaltrials.gov/ct2/show/study/NCT01573650 (first received 9 April 2012).
NCT01770340
- NCT01770340. Nerve grafting with an allograft during radical prostatectomy – extended follow-up in a prospective randomized trial. www.clinicaltrials.gov/ct2/show/NCT01770340 (first received 17 January 2013).
NCT02480777
- NCT02480777. Expanded access for single patient treatment of autologous human Schwann cells (ahSC) for peripheral nerve repair. www.clinicaltrials.gov/ct2/show/NCT02480777 (first received 25 June 2015).
NCT02718768
- NCT02718768. Study of nerve repair and reconstruction associated with major extremity trauma (nerve). www.clinicaltrials.gov/ct2/show/NCT02718768 (first received 24 March 2016).
NCT02970864
- NCT02970864. A phase I trial of a novel synthetic polymer nerve conduit 'Polynerve' in participants with sensory digital nerve injury (UMANC). www.clinicaltrials.gov/ct2/show/NCT02970864 (first received 22 November 2016).
NCT03359330
- NCT03359330. Mid-term effect observation of biodegradable conduit small gap tublization repairing peripheral nerve injury. www.clinicaltrials.gov/ct2/show/NCT03359330 (first received 2 December 2017).
NCT03673449
- NCT03673449. Evaluate the reconstruction of digital nerve defects in humans using an implanted silk nerve guide. www.clinicaltrials.gov/ct2/show/NCT03673449 (first received 17 September 2018).
NCT03780855
- NCT03780855. Preliminary evaluation of the clinical safety and effectiveness of the bionic nerve scaffold. www.clinicaltrials.gov/ct2/show/NCT03780855 (first received 19 December 2018).
NCT03964129
- NCT03964129. BMAC nerve allograft study. www.clinicaltrials.gov/ct2/show/NCT03964129 (first received 28 May 2019).
Paprottka 2013
- Paprottka FJ, Wolf P, Harder Y, Kern Y, Paprottka PM, Machens HG, et al. Sensory recovery outcome after digital nerve repair in relation to different reconstructive techniques: meta-analysis and systematic review. Plastic Surgery International 2013;2013:704589. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pruszynski 2014
- Pruszynski JA, Johansson RS. Edge-orientation processing in first-order tactile neurons. Nature Neuroscience 2014;17(10):1404-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Rinker 2011
- Rinker B, Liao J. A prospective randomized study comparing woven polyglycolic acid and autogenous vein conduits for reconstruction of digital nerve gaps. Journal of Hand Surgery (Edinburgh, Scotland) 2011;36(5):775-81. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rosberg 2005
- Rosberg HE, Carlsson KS, Höjgård S, Lindgren B, Lundborg G, Dahlin LB. Injury to the human median and ulnar nerves in the forearm-analysis of costs for treatment and rehabilitation of 69 patients in southern Sweden. Journal of Hand Surgery (Edinburgh, Scotland) 2005;30(1):35-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rosén 1994
- Rosén B, Lundborg B, Dahlin LB, Holmberg J, Karlson B. Nerve repair: correlation of restitution of functional sensibility with specific cognitive capacities. Journal of Hand Surgery (Edinburgh, Scotland) 1994;19(4):452-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rosén 2000
- Rosén B, Lundborg G. A model instrument for the documentation of outcome after nerve repair. Journal of Hand Surgery (Edinburgh, Scotland) 2000;25(3):535-43. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rosén 2001
- Rosén B, Lundborg G. The long term recovery curve in adults after median or ulnar nerve repair: a reference interval. Journal of Hand Surgery (Edinburgh, Scotland) 2001;26(3):196-200. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ruijs 2005
- Ruijs AC, Jaquet JB, Kalmijn S, Giele H, Hovius SE. Median and ulnar nerve injuries: a meta-analysis of predictors of motor and sensory recovery after modern microsurgical nerve repair. Plastic and Reconstructive Surgery 2005;116(2):484-94; discussion 495-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Şahin 2014
- Şahin F, Atalay NŞ, Akkaya N, Ercidoğan Ö, Başakçi B, Kuran B. The correlation of neurophysiological findings with clinical and functional status in patients following traumatic nerve injury. Journal of Hand Surgery, European volume 2014;39(2):199-206. [PMID: ] [DOI] [PubMed] [Google Scholar]
Salzer 2012
- Salzer JL. Axonal regulation of Schwann cell ensheathment and myelination. Journal of the Peripheral Nervous System 2012;17(Suppl 3(0 3)):14-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Seddon 1942
- Seddon HJ. A classification of nerve injuries. British Medical Journal 1942;2(4260):237-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 1964
- Smith JW. Microsurgery of peripheral nerves. Plastic and Reconstructive Surgery 1964;33:317-29. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sollerman 1995
- Sollerman C, Ejeskär A. Sollerman hand function test. A standardised method and its use in tetraplegic patients. Scandinavian Journal of Plastic and Reconstructive Surgery and Hand Surgery 1995;29(2):167-76. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stenberg 2014
- Stenberg L, Dahlin LB. Gender differences in nerve regeneration after sciatic nerve injury and repair in healthy and in type 2 diabetic Goto-Kakizaki rats. BMC Neuroscience 2014;15:107. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sunderland 1951
- Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain 1951;74(4):491-516. [PMID: ] [DOI] [PubMed] [Google Scholar]
Terenghi 2011
- Terenghi G, Hart AM, Wiberg M. The nerve injury and the dying neurons: diagnosis & prevention. Journal of Hand Surgery, European Volume 2011;36(9):730-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Terzis 1975
- Terzis J, Faibisoff B, Williams B. The nerve gap: suture under tension vs. graft. Plastic and Reconstructive Surgery 1975;56(2):166-70. [PMID: ] [PubMed] [Google Scholar]
Thorsén 2012
- Thorsén F, Rosberg HE, Steen Carlsson K, Dahlin LB. Digital nerve injuries: epidemiology, results, costs, and impact on daily life. Journal of Plastic Surgery and Hand Surgery 2012;46(3-4):184-90. [DOI] [PubMed] [Google Scholar]
Welin 2008
- Welin D, Novikova LN, Wiberg M, Kellerth JO, Novikov LN. Survival and regeneration of cutaneous and muscular afferent neurons after peripheral nerve injury in adult rats. Exploratory Brain Research 2008;186(2):315-23. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wiberg 2003
- Wiberg M, Terenghi G. Will it be possible to produce peripheral nerves? Surgical Technology International 2003;11:303-10. [PMID: ] [PubMed] [Google Scholar]
Wilson 2003
- Wilson A, Hart A, Brannstrom T, Wiberg M, Terenghi G. Primary sensory neuronal rescue with systemic acetyl-L-carnitine following peripheral axotomy. A dose–response analysis. British Journal of Plastic Surgery 2003;56(8):732-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wolfe 2012
- Wolfe S, Strauss H, Garg R, Feinberg J. Use of bioabsorbable nerve conduits as an adjunct to brachial plexus neurorrhaphy. Journal of Hand Surgery 2012;37(10):1980-5. [DOI] [PubMed] [Google Scholar]
Zachary 1954
- Zachary RB. Results of nerve suture. In: Seddon HJ, editors(s). Peripheral Nerve Injuries. Medical Research Council Special Report. London (UK): HMSO, 1954:354-88. [PubMed] [Google Scholar]
References to other published versions of this review
Thomson 2017
- Thomson SE, Ng NYB, Riehle MO, Kingham PJ, Dahlin LB, Wiberg M, et al. Bioengineered nerve conduits and wraps for peripheral nerve repair of the upper limb. Cochrane Database of Systematic Reviews 2017, Issue 3. Art. No: CD012574. [DOI: 10.1002/14651858.CD012574] [DOI] [PMC free article] [PubMed] [Google Scholar]


