Abstract
Recent studies have synchronized transcranial magnetic stimulation (TMS) application with pre-defined brain oscillatory phases showing how the effect of a perturbation depends on the brain state. However, none have investigated if phase-dependent TMS can possibly modulate connectivity with homologous distant brain regions belonging to the same network. In the framework of network-targeted TMS, we investigated whether stimulation delivered at a specific phase of ongoing brain oscillations might favor stronger cortico-cortical (c-c) synchronization of distant network nodes connected to the stimulation target. Neuronavigated TMS pulses were delivered over the primary motor cortex (M1) during ongoing electroencephalography recording in twenty-four healthy individuals over two repeated sessions 1-month apart. Stimulation effects were analyzed considering whether the TMS pulse was delivered at the time of a positive (peak) or negative (trough) phase of μ-frequency oscillation, which determines c-c synchrony within homologous areas of the sensorimotor network. Diffusion Weighted Imaging was used to study c-c connectivity within the sensorimotor network and identify contralateral regions connected with the stimulation spot. Depending on when during the μ-activity the TMS-pulse was applied (peak or trough), its impact on inter-hemispheric network synchrony varied significantly. Higher M1-M1 phase-lock synchronization with after the TMS-pulse (0-200ms) in the μ-frequency band was found for trough compared to peak stimulation trials in both study visits. Phase-dependent TMS delivery might be crucial not only to amplify local effects but also to increase magnitude and the reliability of the response to the external perturbation, with implications for interventions aimed at engaging more distributed functional brain networks.
Keywords: TMS-EEG, structural connectivity, μ-rhythm, cortico-cortical connectivity, brain-state dependent effect
INTRODUCTION
In the last two decades transcranial magnetic stimulation (TMS) has been extensively tested for the treatment of several neurological and psychiatric disorders (Connolly et al., 2012). Even though TMS provides a safe and well tolerated therapeutic option for several neuropsychiatric conditions, and several TMS devices have been cleared by the Food and Drug Administration and other regulatory bodies, findings often show high variability and sometimes small effect sizes reflecting only moderate clinical improvement (López-Alonso et al., 2014). It is well-known that the impact of a TMS pulse on the neural system is not determined only by the properties of that stimulus but also on the initial brain state of the perturbed region (Silvanto & Pascual-Leone, 2008). Brain states can be non-invasively measured via electroencephalography (EEG) and the underlying cortical activity can be characterized for example by ongoing oscillations (Buzsáki, 2006). Such oscillations represent a fundamental mechanism enabling brain network communication at multiple temporal and spatial scales which sustain both sensory processing and higher order coordination of motor and cognitive functions (Buzsáki & Draguhn, 2004; Uhlhaas & Singer, 2010; Akam & Kullmann, 2014). A compelling way to explore the neurophysiologic significance of such oscillations is to combine TMS and EEG (Thut & Pascual-Leone, 2010; Voineskos et al., 2010). Indeed, the effects of controlled and well-timed perturbations induced by TMS pulses can be measured by EEG which assess both local induction of brain activity and its millisecond-level propagation throughout the brain (Momi et al., 2020). It is also possible to study the impact of specific brain states at the time of stimulation on the TMS-induced effects (Schaworonkow et al., 2019; Bergmann et al., 2019) and relate those to cognitive or behavioral consequences. For this reason, in the last years multiple efforts have aimed to individualize the treatment of several disorders taking into account for example spontaneous brain oscillation (Drysdale et al., 2017) and neural excitability (Perera et al., 2016), in order to investigate the contribution of these components to the variability in the TMS interventions. In this context, recent studies have started to deliver individual or trains of external pulses during pre-defined brain oscillatory phases, reporting promising results within the motor system in healthy individuals (Stefanou et al., 2018) and stroke patients (Hussain et al., 2020). Specifically, phase-dependent TMS applied during the negative peak (trough) of the sensorimotor μ-frequency band (8-13 Hz) increased corticospinal behavioral output (Zrenner et al., 2018) and brain TMS evoked-potentials (TEPs) (Desideri et al., 2019) to a larger extent than TMS applied irrespective of this phase. Such results are based on the fact that the trough of the μ-oscillation represents a state where the dendritic trees of pyramidal neurons are closer to the firing threshold and, therefore, more likely to generate action potentials in response to a TMS pulse (Buzsáki et al., 2012). However, it is worth to mention that another research group haven’t found any consistent modulatory effect of mu-phase on corticospinal excitability using brain-state informed TMS targeting the peak and trough (Madsen et al., 2019). Such discrepancy in the findings between the two groups might be due to several differences in the experimental approach such as the number of trials per condition, the inter-stimulus interval (ISI) between the TMS pulses and the statistical model employed for the analyses.
Despite the aforementioned studies have evaluated the behavioral and brain evoked-potentials outcomes following phase-dependent TMS application, none so far have investigated possible changes in c-c synchronization of homologous brain regions belonging to the same network. In this study we used image-guided TMS-EEG to selectively perturb the primary motor cortex (M1) and further investigate whether c-c connectivity is modulated by the phase of the ongoing sensorimotor μ-rhythm. Given that the aforementioned studies have demonstrated how the trough of the μ-rhythm represents a high-excitability state (Zrenner et al., 2018; Desideri et al., 2019), we hypothesized that external perturbation at the negative peak would be able to induce higher c-c synchrony between the stimulated target region and homologous regions of the same network compared to stimulation over the peak. It is important to mention that that the focused of our analysis was on the contralateral homologous motor regions connected to the stimulated spot via transcallosal white matter fibers in accordance with recent publications (Zrenner et al., 2018; Desideri et al., 2019; Bortoletto et al., 2021; Zazio et al., 2021). In order to identify the individual sensorimotor network, the Schaefer’s atlas (Schaefer et al., 2018) was employed which divided the brain into 7 Networks.
Finally, control analyses were conducted to investigate if microstructural properties of the white matter were related to c-c synchronization. Importantly, considering the quest for data reproducibility, the same analyses were repeated on data collected on the same sample of healthy individuals across two separate study visits one month apart.
MATERIAL AND METHODS
Participants
The study was approved by the Institutional Review Board of the Beth Israel Deaconess Medical Center (2016P000351). Each participant was asked to provide written informed consent conformed to the Declaration of Helsinki and was remunerated for the entire study. Twenty-four right-handed (Oldfield, 1971) healthy volunteers (mean age= 32± 10 years, ranging from 19 to 49 years) with normal neurological and psychiatric evaluation and no history of drugs acting on the central nervous system were recruited through flyers and on-line advertisement. A pre-TMS MRI assessment was carried out comprehensive of a T1-weighted (T1w) anatomical, a resting state fMRI and a DWI scans. Such imaging assessment was carried out in order to extract individualized networks maps (Figure 1A). Each participant subsequently carried out two TMS visits, separated by one month, where 120 single pulses (for each visit) were delivered every 5-8s (random jitter) over M1 (Figure 1B). TMS spot was personalized based on individual resting motor threshold (RMT), defined as the lowest stimulation intensity necessary to evoke a MEP (~ 50 uV) in at least 50% of the trials (Rossini et al., 2015). The “hotspot” of stimulation was therefore determined as the cortical hand region where MEPs were larger and more consistent in the right first dorsal interosseous (FDI) muscle, as compared to abductor pollicis brevis (APB) muscle (Rothwell et al., 1999). During the stimulation application, participants were asked to wear earplugs (Rossi et al., 2020) where auditory white noise was played to minimize the impact of the TMS click (ter Braack et al., 2015). A thin layer of foam was placed under the TMS coil to minimize somato-sensory contamination of the TMS-evoked EEG potentials. The stimulation intensity was set at the 120% of the RMT with randomly jittered (5000-8000ms) inter stimulus intervals.
Figure 1. Methodological workflow and metrics extraction.
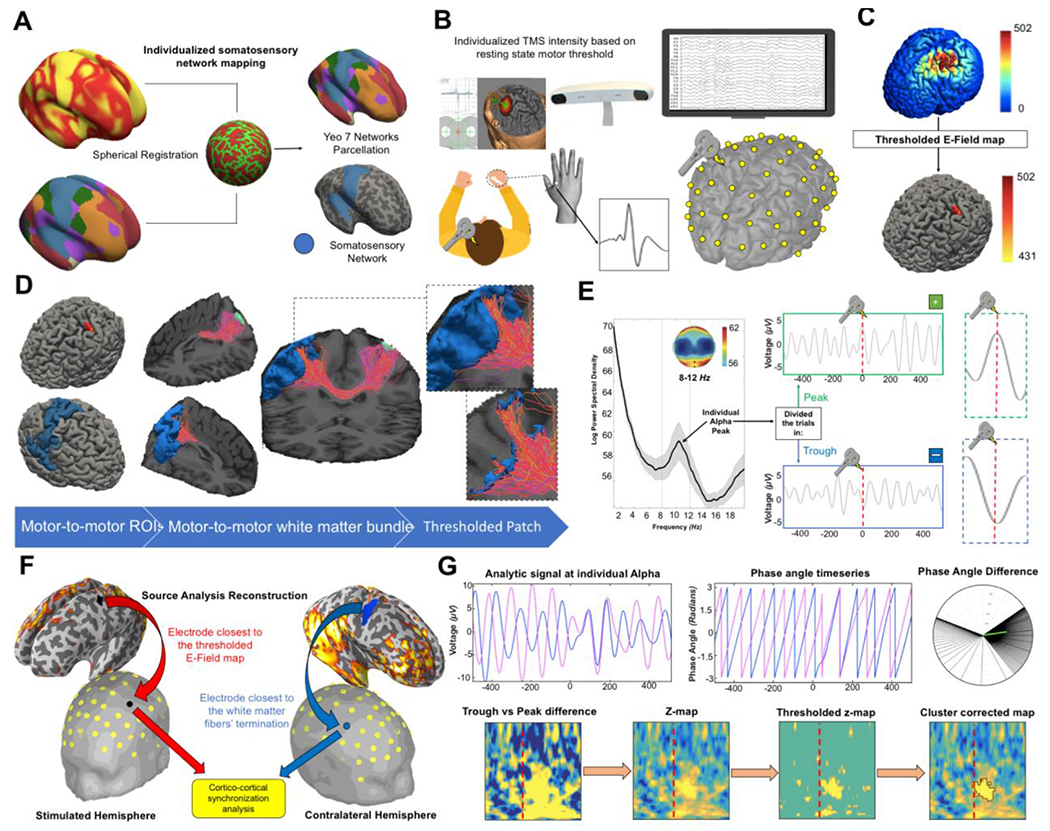
(A) In order to identify the contralateral motor network, the 7-network functional cortical atlas (Yeo et al., 2011) was projected onto subject’s cortical surface using the spherical registration implemented in Freesurfer software. (B) TMS was applied to the individual left primary motor area (M1) “hotspot” determined as the cortical hand region where MEPs were larger and more consistent in the right first dorsal interosseous (FDI) muscle, as compared to abductor pollicis brevis (APB) muscle [Rothwell et al., 1999]. Once the “hotspot” was identified by evaluating MEPs responses, anatomical MRI were used for the neuronavigation of the TMS spot while hd-EEG with 64 channels was simultaneously recorded. (C) TMS-induced electric field was modelled with SimNIBS (Thielscher et al., 2015). The point with maximal electric field (E-field) was then defined and from there a sphere of radius 0.5cm was created. (D) Seed-based anatomically constrained tractography (Smith et al., 2012) was performed in order to extrapolate the white matter bundle connecting the E-field map and the contralateral sensorimotor network. The individual sensorimotor network map was thresholded using the reconstructed tract where only the vertices reached by a streamline were retailed. The remaining vertices represent the cortical regions structurally connected with the stimulation spot. (E) The timeseries from the electrode closest to the stimulation spot were extracted and filtered using the individual peak μ-frequency (Corcoran et al., 2018). Then, trials were classified in peak and trough depending on whether the TMS pulse was delivered at the positive or the negative peak respectively. In order to investigate how much the results would have depended on how close the TMS pulse was to the actual peak of the phase, TMS stimuli were classified as landing on a peak or trough using several sensitivity windows ranging from 5ms (very accurate) to 40ms (more liberal). TMS pulses that did not land within a given sensitivity window were not included in that analysis (see Methods for further details). (F) As for the connectivity analysis at source level, metrics were extracted from the white matter fibers’ terminations of the tract connecting the stimulated grey matter portion. As for electrode level analysis, the Euclidian distance was computed from every electrode and the centroid of the thresholded E-field map (stimulated hemisphere) and the white matter fibers’ terminations (contralateral hemisphere). The closest electrodes for the left and right hemisphere were then selected for the TMS-EEG connectivity analysis. (G) Phase Locking Value (PLV) (Lachaux et al., 1999; Mormann et al., 2000) was calculated as measure of connectivity for both source and electrode level analyses. The raw difference between the PLV of trough and peak trials was calculated for all the frequencies between 3Hz and 40Hz. Then, 1000 permutation t-test were performed in which the surrogated trough vs peak difference was computed after each iteration and statistically compared with the real difference (Pernet et al., 2015). Finally, the cluster threshold was determined as the 95th percentile of the cluster’s surrogate distribution.
Modelled and image-guided selection of electrodes
In order to perform c-c connectivity analysis, the portions of grey matter mainly affected by the TMS pulses were selected based on electric field (E-field) modelling (left hemisphere) and tractography (right hemisphere). TMS-induced E-field was modelled with SimNIBS (Thielscher et al., 2015) to identify the cortical region directly engaged by the external perturbation (Figure 1C). Given that in literature there is no consensus on how selectively identify only the neural tissue recruited by the TMS pulse (Romero et al., 2019), we defined the point with maximal E-field and from there created a sphere of radius 0.5 cm and used as seed for connectivity analysis in source space. As for electrode level analysis, the Euclidian distance was computed from every electrodes of the stimulated hemisphere and the closest to the centroid of the thresholded E-field map was picked for connectivity analysis. Following, in order to identify the contralateral motor network, the 7-network functional cortical atlas (Yeo et al., 2011) was projected onto subject’s cortical surface using the spherical registration implemented in Freesurfer software (Figure 1A). The resulting maps were then resampled to native structural T1w MRIs. Seed-based anatomically constrained tractography (Smith et al., 2012) was performed in order to extrapolate the white matter bundle connecting the E-field map and the contralateral sensorimotor network (Figure 1D). The reconstructed tract was used to threshold the sensorimotor network map where only the vertices reached by a streamline were retailed. The remaining vertices represent the cortical regions structurally connected with the stimulation spot and were used to extract connectivity metrics in source space. As for electrode level analysis, the Euclidian distances between the largest area of the white matter fibers’ terminations cluster and the electrodes of the right hemisphere were computed and the closest electrode was picked. For further details on TMS-induced electric field modelling please see Supplementary Information.
EEG trial partitioning in peak and trough
Based on previous studies which reported higher corticospinal excitability when the TMS pulse was delivered at the negative peak of sensorimotor μ-oscillation (Zrenner et al., 2018; Desideri et al., 2019), we first identified the individual sensorimotor rhythm (Figure 1E). To this purpose, individual peak alpha frequency (PAF) and center of gravity (CoG) were calculated from resting-state power spectra, which had been previously smoothed using a Savitzky-Golay filter (Corcoran et al., 2018).
A surface Laplacian spatial band-pass filter was then applied to the preprocessed EEG in order to control for volume conduction effects, attenuating activity from distant sources or activity that is highly spatially distributed and temporal coherent (Tenke & Kayser, 2012). The timeseries from the electrode closest to the stimulation spot (see dedicated section for details on electrode selection) were extracted and filtered using the individual PAF (filter width of ± 2Hz). Thus, trials were classified in peak and trough depending on whether the TMS pulse was delivered at the positive or the negative peak respectively.
In order to evaluate the specificity of the phase-dependent TMS effects, different window sizes ranging from 5ms (very accurate) to 40ms (more liberal) were used during the classification of the trials into peak or trough. Specifically, pulses were classified as landing on a peak or trough if they fell within the sensitivity window (i.e. ± 5ms) of the peak or trough of the ongoing oscillation. For instance, if the employed window size was 5ms, the trials were classified into peak (or trough) if and only if the pulse was delivered ±5ms near the positive (or negative) phase peak. On the other hand, if the pulse of a given trial was delivered >5ms distant from the peak, that trial was discarded and not included in subsequent analyses. Such differentiation was performed in order to evaluate how much the results depend on how close the TMS pulse was to the actual peak of the phase. Importantly the peak/trough ratio was included in all the statistical analyses as covariate of nuisance.
EEG source reconstruction and metrics
All TMS evoked EEG source reconstruction was performed in Minimum-norm estimate (MNE) software (Gramfort et al., 2013) (https://mne.tools/stable/index.html) running in Python 3.6 (Figure 1F). First, the watershed algorithm was used to generate the inner skull, the outer skull and the outer skin surfaces triangulations. Then the EEG forward solution was calculated using a three compartment boundary-element model (Gramfort et al., 2010). Noise covariance was estimated from individual trials using the pre TMS (−500 0) time window as baseline. The inverse model solution of the cortical sources was performed using the dynamic Statistical Parametric Mapping (dSPM) method with current density (Hämäläinen & Ilmoniemi, 1994) and constraining source dipoles to the cortical surface. The resulting output of EEG source reconstruction was the dSPM current density time series for each cortical vertex. In order to investigate inter-hemisphere c-c synchronization differences between trough and peak trial, whole-brain Phase Locking Value (PLV) was computed from the thresholded individual E-field map for different frequencies (ranging from 3Hz to 40Hz). Specifically PLV was calculated as the length of the average of phase angle differences between electrodes over time (Lachaux et al., 1999; Mormann et al., 2000), resulting from the convolution between a complex Morlet wavelet and the data.
where n is the number of time points and and are phase angles from electrodes b and a at frequency f for each time point t. By subtracting these phase angle vectors and computing their average length the result will be between 0 for absent coupling and 1 for perfect synchrony.
The final PLV maps for trough and peak trial were then corrected for the baseline using the decibel conversion (Cohen, 2017) in order to account for both limitations of raw values and electrode-specific idiosyncratic characteristics.
where the horizontal bar over baseline indicates the mean across the baseline time period, and t and f are time and frequency respectively. Finally, individual PLV maps were morphed to fsaverage average brain for group analysis where both permutation testing and cluster correction were implemented in order to compare trough vs peak trials.
EEG phase-based connectivity analysis
In order to investigate whether if the connectivity results found at the source level were replicable also at electrode level (Figure 1G), PLV was also computed between the electrodes affected by the TMS pulse (see dedicated section for details on the electrodes selection).
The baseline corrected time-frequency matrices for peak and trough were statistically compared using both a condition-wise permutation testing and a cluster-based thresholding (Pernet et al., 2015) as a correction for multiple comparisons. Specifically, the permutation test transformed the difference between the trough and the peak trials into a z-values with respect to a null distribution of surrogate conditions difference values, obtained by swapping condition labels at each of 1000 permutations. The resulting z-scores were thresholded at p < .05. With an additional 1000 iterations permutation test, a distribution of cluster sizes of contiguous significant points under the null hypothesis of no difference was computed, and only the time-frequency clusters that exceeded the 95th percentile of this distribution was retained.
DWI metrics computation
White matter fiber orientation distributions (FODs) was computed using MRtrix3Tissue (https://3tissue.github.io/) for each participant via “single-shell 3-tissue constrained spherical deconvolution” (SS3T-CSD) (Dhollander & Connelly, 2016) using a group averaged response function for each tissue type (white matter (WM), gray matter (GM) and cerebrospinal fluid (CSF)) (Raffelt et al., 2012). Following these processing steps, we calculated the three standard fixel-based metrics for FBA (Raffelt et al., 2017):
Fiber density (FD): a microstructural metric that serves as a proxy for axonal density or packing;
Fiber cross-section (FC): a macrostructural metric that approximates relative fiber bundle diameter or size;
Fiber density and cross-section (FDC): the product of FD and FC, which encapsulates changes to both micro- and macro-structure.
MRI data acquisition
The MRI evaluation was performed on a 3T scanner (GE Healthcare, Ltd., United Kingdom). The T1w was used for neuronavigation using the Brainsight™ TMS Frameless Navigation system (Rogue Research Inc., Montreal, Canada) as discussed below. The T1w was acquired using a 3D spoiled gradient echo sequence: 166 axial-oriented slices for whole-brain coverage; 240mm isotropic field-of-view; 0.937-mm × 0.937mm × 1mm native resolution; flip angle = 15°; TE/TR ≥ 2.9/6.9ms; duration ≥ 432s. DWI sequence were collected using a single-shot echo planar imaging (slices = 71; matrix size = 256 × 256 × 71; voxel size = 0.8mm × 0.8mm × 2.2mm; repetition time = 8500ms, time echo= 79ms; 30 non-colinear directions, b-value = 1000s/mm2).
DWI data preprocessing
A customized pipeline running in Ubuntu 18.04 LTS was used for the preprocessing of DWI images using tools in FMRIB Software Library (FSL 5.0.3; www.fmrib.ox.ac.uk/fsl) (Jenkinson et al., 2012), Mrtrix3 (mrtrix.readthedocs.io/en/latest/) (Tournier et al., 2012), FreeSurfer (Fischl et al., 2004) and ANTs (stnava.github.io/ANTs/) (Avants et al., 2011). All images were denoised (Veraart et al., 2016), preprocessed via FSL’s EDDY (Andersson & Sotiropoulos, 2016), and bias field corrected (Zhang et al., 2001). The response function for a single fiber population was estimated using spherical deconvolution Tournier algorithm (Tournier et al., 2007). Simultaneously, the T1w images were coregistered to the b0 volume and then segmented using FAST algorithm (Zhang et al., 2001). Two subjects were excluded from the analysis given poor quality in their DWI data.
TMS
TMS was delivered using a figure-of-eight shaped coil with dynamic fluid cooling (Magspro 75mm cool B-65, Magpro A/S., Denmark) attached to a MagPro X-100 stimulator (MagVenture A/S, Denmark). T1w anatomical images were imported into the Brainsight™ TMS Frameless Navigation system (Rogue Research Inc., Montreal, Canada), and a coregistration procedure was performed using scalp landmarks (nasion, vertex, and the two preauricular points) in order to monitor the coil position. Motor evoked potentials (MEPs) were recorded with active electrodes positioned on the right FDI and the right APB muscles, while the reference electrode was placed over the metacarpophalangeal joint of the index finger. EMG data were amplified and digitized using a Powerlab 4/25T data acquisition system (ADInstruments) at a sampling rate of 4000 Hz (bandpass filtered at 10Hz to 2000Hz). EMG signals were continuously streamed by using LabChart software (LabChart 8.0) to monitor MEPs and epochs were recorded with a 150ms window length covering from 50 ms before to 100 ms after TMS pulse. The individual T1w was used to localize the anatomical cortical hand region before the TMS visit. This served as a starting point location for identifying the “hotspot” which corresponded to the scalp location where TMS intensity was sufficient to evoke a motor response (∼ 50 uV) in the FDI muscle in at least 50% of the trials.
EEG
Whole scalp 64-channel EEG data was collected with a TMS-compatible amplifier system (actiCHamp system, Brain Products GmbH, Munich, Germany) and labeled in accordance with the extended 10–20 international system. EEG data were online referenced to Fp1 electrode. Electrode impedances were maintained below 5kΩ at a sampling rate of 1000 Hz. EEG signals were digitized using a BrainCHamp DC amplifier and linked to BrainVision Recorder software (version 1.21) for online monitoring. Digitized EEG electrode locations on the scalp are also co-registered to individual MRI scans using Brainsight™ TMS Frameless Navigation system.
EEG data processing
A customized script running in Matlab R2017b (Math-Works Inc., USA) was used for offline data preprocessing mainly performed by EEGLAB 14.1 toolbox (Delorme & Makeig, 2004). A single block of 120 trials was first created by merging the two single blocks of 60 trials each, and then segmented into epochs of 1500ms each (from −500ms (pre-pulse) to 1000ms (post-pulse)). Baseline correction was performed using an amplitude of the mean pre-pulse (−500ms to −100ms) signal and raw data were visually inspected to then remove noisy channels. Zero-padding was applied on a window of −2ms to 14ms to reject early TMS pulse artefact and noisy epochs were then removed based on the voltage (≥100 μV), kurtosis (≥ 3), joint probability (single channel-based threshold ≥ 3.5sd) and visual inspection. In order to minimize overfitting and noise components, the dimensionality of the data was firstly reduced to 60 components via principal component analyses (PCA). Subsequently, a first round of fast independent component analysis (fICA) (Hyvärinen & Oja, 1997) was run specifically aimed at removing remaining early TMS-evoked and EMG artefacts. A linear interpolation was used to interpolate the zero-padded time window and the EEG data were then band pass filtered using a forward-backward 4th order Butterworth filter from 1 to 100Hz, notch filtered between 57 and 63Hz, and referenced to global average. A second PCA was further employed to reduce the data dimensionality into 57 components before removing remaining artefact (e.g. eye movement/blink, muscle noise (EMG), single electrode noise, TMS evoked muscle, cardiac beats, auditory evoked potentials) with a second round of fICA (Rogasch et al., 2017). During both fICA, the components were visually inspected where a semi-automated artefact detection algorithm incorporated into the open source TMS-EEG Signal Analyzer (TESA v0.1.0-beta; https://nigelrogasch.github.io/TESA) was used (Rogasch et al., 2017). Finally, a low pass filtered with a 4th order Butterworth filter at 60Hz was employed and previously removed channels were spherically interpolated.
Control analyses
In order to test potential differences in the peak/trough ratio for visit 1 and visit 2, a 2×5 ANOVA was run with the factor VISIT (2 levels: visit 1 and visit 2) and TIME (5 levels: 40ms, 30ms, 20ms, 10ms, 5ms). No significant interaction VISIT*TIME (F(1,23)=0.55, p=0.46) or main effect of VISIT (F(1,23)=3.83, p=0.06) and TIME (F(1,23)=0.76, p=0.39) were found.
Morever, in order to control the specificity of the TMS-induced c-c phase-dependent connectivity for trough trials, 3 minutes of resting-state EEG collected during eyes open were analyzed. Specifically, after data preprocessing, surrogate epochs were created with time windows compared to the TMS-evoked data. Afterwards, timeseries from the same electrode were extracted and filtered using the same individual peak μ-frequency. Then, trials were categorized as peak or trough depending on whether the surrogate event occurred at the time of the positive or the negative peak respectively. Topoplots cosine similarity was computed between resting-state and TMS-EEG data for both peak and trough trials.
Moreover, c-c connectivity of the resting-state period (from −500ms to 0ms) preceding TMS were also extracted and compared to the post-TMS window for both peak and trough trials. To make this comparison, a 2×2×4 analysis of variance (ANOVA) was performed with a (within subjects) factor “TRIAL” (2 levels: Peak; Trough), a (within subjects) factor “TIME” (2 levels: Pre-TMS; Post-TMS) as well as a (within subjects) factor “ FREQUENCY “ (4 levels: Theta, Alpha, Beta, Gamma). The critical p-value was then adjusted using Bonferroni correction to account for multiple comparisons (*0.05 Bonferroni corrected). We hypothesized that TMS would induce higher c-c synchrony between structurally connected brain regions compared to conventional resting-state EEG and specifically for trough trials.
RESULTS
Source level phase-based connectivity between left and right hemisphere
In order to investigate c-c synchronization within the motor network, source-level individual PLV maps were computed using the thresholded E-field map as seed separately for trough and peak trials. As shown in Figure 2A, higher PLV value at individual μ-band was found with the contralateral somato-motor network for trough (visit 1: average PLV=0.78; visit 2: average=0.63) compared to peak (visit 1: average PLV=0.33; visit 2: average=0.21) trials. As shown in Figure 2B, individual PLV scores extracted from thresholded streamline maps were significantly higher for trough compared to peak trials for both visit 1 (t=4.58, p<0.001) and visit 2 (t=5.94, p<0.001). Given that in the preprocessing steps the first 14 ms after the pulse were zero-padded and the interpolated, connectivity analysis was re-run leaving out the first 15ms after the pulse in order to control for this confounding factor. Results were substantially similar and are reported in the Supplementary Results.
Figure 2. Source level PLV whole-brain connectivity with the stimulated region.
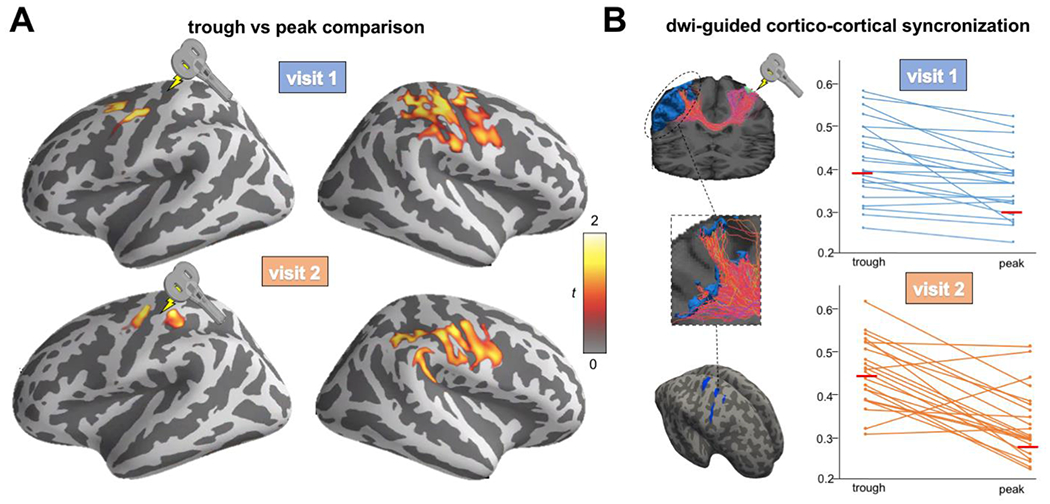
(A) Significant cluster comparing PLV maps for trough vs peak trials. Higher synchronization between the stimulated region and the contralateral hemisphere was found for both visit 1 (top) and visit 2 (bottom) (B) Individual PLV between the stimulated region and the thresholded streamline map. Higher synchronization was found for trough compared to peak trials for both visit 1 (top; 1 (t=4.58, p<0.001) and visit 2 (bottom; t=5.94, p<0.001).
Electrodes selection and trough/peak ratio
Over the stimulated hemisphere (Figure 3A left), the electrodes closest to the centroid of the thresholded E-Field map were C3 (visit 1: 19/24; visit 2: 19/24), C1 (visit 1: 2/24; visit 2: 2/24), FC1 (visit 1: 1/24; visit 2: 0/24), FC3 (visit 1: 2/24; visit 2: 2/24), C5 (visit 1: 0/24; visit 2: 1/24). Over the contralateral hemisphere (Figure 3A right), the electrodes closest to the white matter fibers’ terminations area were C4 (visit 1: 13/24; visit 2: 13/24), FC4 (visit 1: 1/24; visit 2: 2/24), C2 (visit 1: 7/24; visit 2: 4/24), CP4 (visit 1: 2/24; visit 2: 1/24), FC2 (visit 1: 1/24; visit 2: 2/24), C6 (visit 1: 0/24; visit 2: 1/24), P4 (visit 1: 0/24; visit 2: 1/24). For further details on the electrodes employed for the analysis for each subject and for each visit please see supplementary Table S1.
Figure 3. Time-frequency PLV results comparing trough vs peak for 5ms thresholds of sensitivity.
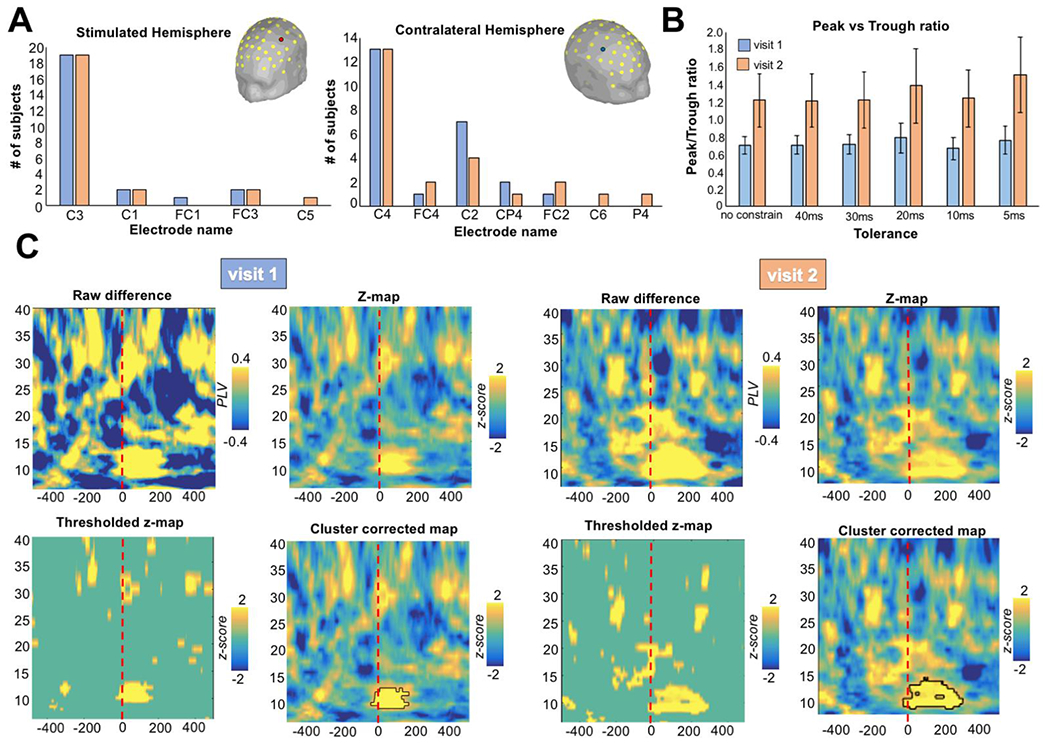
(A) In the majority of the subject and across visits, the electrode directly mostly affected by the TMS pulse was C3 (n: 19/24) for stimulated hemisphere and C4 (n: 13/24) for the contralateral hemisphere. (B) Ratio of trials classified as peak or trough considering different degree of temporal sensitivity in the trial split selection (from no constraint to a minimum of a 5ms window). (C) The time-frequency PLV for the thresholds of sensitivity of 5ms is shown. Raw difference plot represents the subtraction between trough vs peak trials. Then the time-frequency matrices for peak and trough were transformed into z-values (z-map plot) and statistically with respect to a null distribution of surrogate conditions difference values, obtained by swapping condition labels at each of 1000 permutations. The resulting z-scores were thresholded at p < .05 (thresholded z-map). Finally, a distribution of cluster sizes of contiguous significant points under the null hypothesis of no condition difference was computed, and only the time-frequency clusters that exceeded the 95th percentile of this distribution was retained (cluster corrected-map). Trough trials were observed to induce greater synchronization between the selected electrode in the μ-band compared to peak trials for both visit 1 and visit 2.
For the peak/trough ratio (Figure 3B), we found a rate around 1 regardless of the temporal thresholds of sensitivity in the trial subdivision, meaning that the same number of peak and trough trials were found: unconstraint (visit 1: mean=0.70, SEM=0.10; visit 2: mean=1.22, SEM=0.30), 40ms (visit 1: mean=0.71, SEM=0.10; visit 2: mean=1.22, SEM=0.30), 30ms (visit 1: mean=0.72, SEM=0.11; visit 2: mean=1.23, SEM=0.32), 20ms (visit 1: mean=0.79, SEM=0.17; visit 2: mean=1.39, SEM=0.43), 10ms (visit 1: mean=0.67, SEM=0.12; visit 2: mean=1.24, SEM=0.33), 5ms (visit 1: mean=0.76, SEM=0.16; visit 2: mean=1.52, SEM=0.44).
Electrode level phase-based connectivity between left and right hemisphere
As for 5ms thresholds of sensitivity in splitting the trials (Figure 3C), the time-frequency PLV showed a greater synchronization between the selected electrode after the TMS-pulse in the μ-band for the trough compared to peak trials for both visit 1 (average frequency=11.35Hz) and visit 2 (average frequency=11.23Hz).
Interestingly, such result was independent from the thresholds of temporal sensitivity used to subdivide the trials (Figure 4). Indeed, for both visit the same higher synchronization between the selected electrode in the μ-band was evident for trough relative to peak trials: unconstraint (visit 1: average frequency=12.97; visit 2: average frequency=11.54), 40ms (visit 1: average frequency=12.84; visit 2: average frequency=11.52), 30ms (visit 1: average frequency=12.72; visit 2: average frequency=11.34), 20ms (visit 1: average frequency=12.59; visit 2: average frequency=11.73), 10ms (visit 1: average frequency=12.44; visit 2: average frequency=11.67), 5ms (visit 1: average frequency=12.53; visit 2: average frequency=12.02).
Figure 4. Time-frequency PLV results comparing trough vs peak for difference ms thresholds of sensitivity.
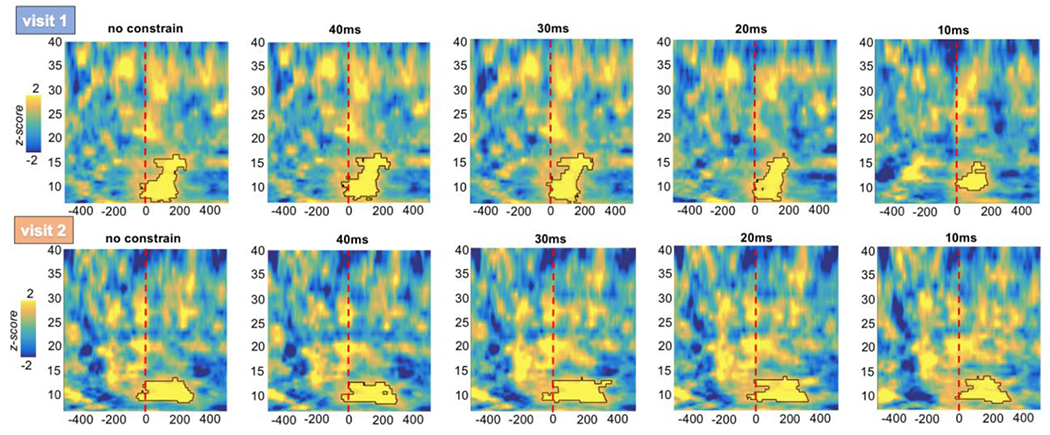
The time-frequency PLV for different thresholds of sensitivity in redistributing trough and peak trials is shown. For both visits, the same higher synchronization between the selected electrode in the μ-band was evident for trough relative to peak trials.
Control analyses
As shown in Figure 5A, EEG trials categorization was reliably performed both using resting-state and TMS-EEG datasets. This demonstrates that even though it is known to be notoriously challenging to identify phase properly in the presence of TMS-related (residual) artifacts and evoked responses (Zrenner et al., 2020), such TMS-induced contamination of the signal do not compromise the overall ability to categorize the trials accurately. For further details on the similarity between resting-state and TMS-EEG topoplots for both peak and trough, please see supplementary Table S2.
Figure 5. Control analyses.
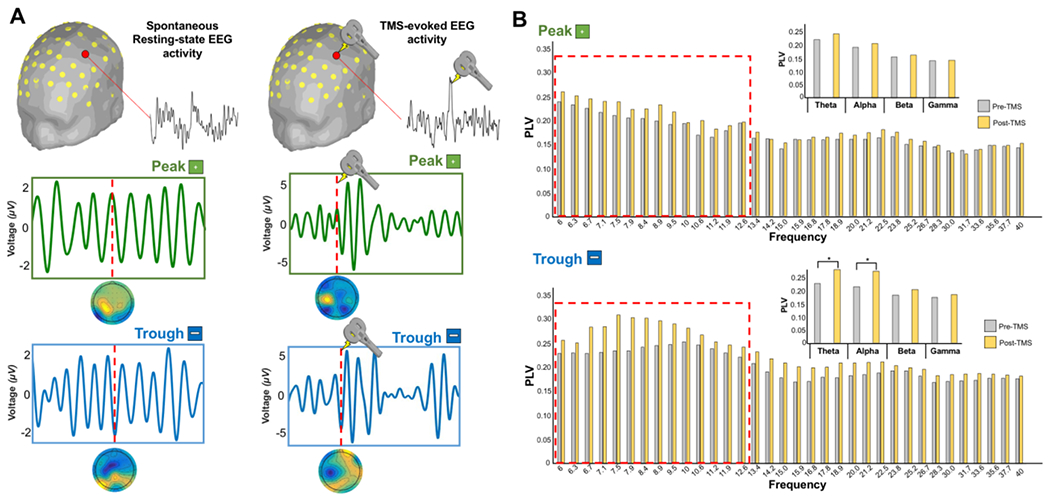
(A) Peak and trough trials categorization were performed using EEG data collected during resting-state eyes open. The resulting topomaps have shown comparable pattern to the TMS-EEG data demonstrating that TMS-related (residual) artifacts do not compromise the overall ability to categorize the trials accurately. (B) Gran mean average of PLV extracted for all the frequencies for both pre-TMS and post-TMS time window. A significant difference (red square boxes) was found in the Theta (Post-TMS > Pre-TMS: t-value = 3.06, p = 0.002) and Alpha (Post-TMS > Pre-TMS: t-value = 2.14, p = 0.003) frequency bands for trough compared to peak trials.
As shown in Figure 5B, a significant interaction TIME*TRIAL*FREQUENCY was found in the 2×2×4 ANOVA (F(2,42)=8.17, p=0.003) with a significant interaction TIME*FREQUENCY (F(1,21)=9.87, p=0.002) and TIME*TRIAL (F(1,21)=11.45, p<0.001). Post-hoc level comparisons revealed that such pattern was driven by trough trials were a significant difference was found in the Theta (Post-TMS > Pre-TMS: t-value = 3.06, p = 0.002) and Alpha (Post-TMS > Pre-TMS: t-value = 2.14, p = 0.003) frequency bands.
Relationship between DWI metric and synchronization
To investigate the association between the significant trough vs peak synchronization in the μ-band and brain structure, separate non-parametric permutation GLM analyses with individual PLV as dependent variable and FD, FC, or FDC as independent variables were run. As shown in Figure 6, a significant positive association between the amount of synchronization for both peak and trough trials and the FDC metric (p < 0.05, FWE-corrected) was found for the transcallosal corpus callosum fibers connecting the primary motor cortices.
Figure 6. Microstructural predictors of cortico-cortical synchronization.
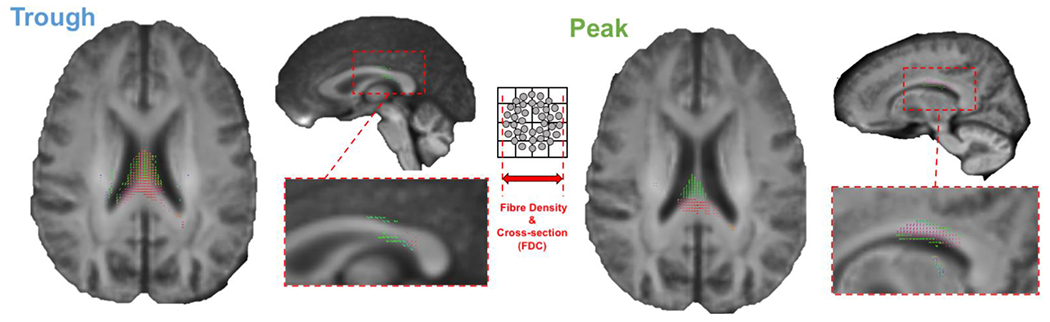
A significant positive association was found between the amount of synchronization for both peak and trough trials and the FDC metric (p < 0.05, FWE-corrected) was found for the transcallosal corpus callosum fibers connecting the primary motor cortices.
DISCUSSION
Previous studies have shown how TMS protocols synchronized with the phase of spontaneous brain oscillations result in long-term changes of excitability of the stimulated neural circuit, at both brain (Desideri et al., 2019) and behavioral level (Zrenner et al., 2018). Here, we further expand this concept demonstrating how external perturbation of the motor network delivered at the negative peak of ongoing μ-oscillations seems able to induce stronger c-c synchronization between the stimulated area and contralateral homologous regions belonging to the same network, with results being particularly relevant for the engagement and coupling of other non-motor networks of the brain. Finally, the microstructural nature of the stimulated white matter bundle was significatively related to the amount of synchronization, suggesting that axonal properties are relevant for the propagation of action potentials regardless of the phase moment when the external perturbation was delivered.
TMS at trough induces higher cortico-cortical synchronization
In line with our hypothesis, external perturbations delivered at the negative peak of spontaneous μ-oscillations induced higher c-c synchrony between the stimulated area and the contralateral homologous brain region of the same network, compared to the positive peak. Moreover, our control analyses reveled that such result was specific for theta and alpha frequency bands and importantly was not driven by pre-TMS differences in the c-c synchronization values across the conditions. Previous single cell recording on the rat have shown that stimulated pyramidal cells discharge mostly on the negative phase, corresponding to the time when the soma is least hyperpolarized (Buzsáki et al., 1983; Fox et al., 1986). In this framework, recent TMS-EEG studies in humans have shown how corticospinal excitability is enhanced at the negative compared to the positive peak of the μ-oscillation as measured via MEPs (Zrenner et al., 2018) and TEPs (Desideri et al., 2019) amplitude. Indeed, following TMS-induced E-field modelling, external perturbation over motor hand area excites fibers in the pre and postcentral gyrus parallel to the magnetic field (Laakso et al., 2014; Bungert et al., 2017). Such input leads to transsynaptic activation of the apical dendritic trees of pyramidal cells (Amassian et al., 1987), which has been indicated as the main generator of negative deflections in the surface EEG (Buzsáki et al., 2012). For this reason, external perturbation delivered at the negative peak of the μ-rhythm reaches the dendritic trees of pyramidal cells at a time when they are closer to the firing threshold, leading to a higher fraction of them being recruited (Buzsáki et al., 1983; Kamondi et al., 1998). In this context, our retrospective analyses provide the first evidence that stimulation-induced connectivity changes within the targeted brain networks depend on the phase of the ongoing endogenous brain oscillations at the time of stimulation. In line with the aforementioned physiological studies, we have shown that the EEG negative peak of the μ-oscillation represents the phase instant to induce higher c-c connectivity in homologous brain areas belonging to the same networks at source level, and not only a high-excitability state of corticospinal neurons as previously demonstrated (Zrenner et al., 2018). Importantly, the same result was obtained at electrode level where the spatial resolution is lower compared to source-based reconstruction, even though we have tried to overcome this issue using multimodal neuroimaging approach for the electrodes selection. Such procedure overcome the fundamental limitations of individual modalities and should be potentially extended to other cognitive domains where the variability in the brain anatomy and geometry is higher.
Early experimental evidence in cats found that interhemispheric oscillatory synchronization between homologous neural assemblies in primary visual cortex is fundamental for establishing relationship between distributed features in the two visual hemi-fields (Engel et al., 1991). Moreover, in the motor system, anatomical tracers’ work in non-human primates has demonstrated that the main callosal connections of the primary motor cortex (M1) are with homologous regions of the contralateral hemisphere (Rouiller et al., 1994; Dancause et al., 2007). From a functional perspective, whilst β-frequency transiently synchronize the two hemispheres during bimanual and unimanual motor tasks (Murthy & Fetz, 1996), μ-band constitutes the dominant rhythm in the frequency spectrum between homologous areas of the sensorimotor cortex at rest (Gastaut & Bert, 1954).
Overall, in line with the state-dependency effect of TMS (Silvanto & Pascual-Leone, 2008), our findings demonstrate that the effect of a given stimulus on the brain is highly dependent on the brain oscillation phase at that instant and not simply by the nature of the stimulus itself. Thus, given the high variability in outcome of the TMS application for the treatment of brain disorders, future studies should test whether stimuli synchronized with the individual patient’s instantaneous brain state would improve the outcome of the intervention.
Neural predictors of cortico-cortical synchronization
Apart from estimating the impact of a given intervention, identifying specific features predicting the likelihood of higher or lower responsiveness to a given treatment or therapy is becoming crucial in clinical and non-clinical settings (Drysdale et al., 2017). We highlighted a very interesting, yet preliminary, predictor of TMS-induced c-c synchronization in the FDC of the transcallosal fibers of the corpus callosum. Several studies have reported a positive relationship between microstructure of fiber tracts connecting bilateral primary motor cortices and the strength of interhemispheric inhibition, as measured in adults with short interval interhemispheric inhibition (SIHI) (Wahl et al., 2007; Fling et al., 2011). Furthermore, recent biophysical models of axon conduction have demonstrated a linear relationship between the delay of a simulated local field potential (LFP) and the structural properties of transcallosal corpus callosum fibers (i.e. length and g-ratio) (Berman et al., 2019).
In this framework, our findings demonstrate that microstructural properties (both axonal density and bundle size) of the white matter tract connecting the two primary motor cortices was linearly related with the amount of synchronization. The fact that such result was found for both peak and trough trials might suggest that an external perturbation would engage the same neuronal population and white matter fibers regardless of the phase period when the stimulus is delivered. Conversely, it is the amount of information conveyed by the white matter tract that is highly depend on the phase moment when the TMS pulse is applied. Prospective studies including a predefined assignment to a low and high corpus callosum fibers FDC group are needed to causally validate this hypothesis.
Limitations of the study and future directions
Our results must be interpreted considering the low spatial resolution of the EEG. Despite we selected the electrode based on individual white matter tracts obtained via a high spatial resolution DWI acquisition, a more precise spatial mapping of TMS-induced network effects would be better captured by ideally combining TMS-EEG with concurrent fMRI acquisitions (Peters et al., 2020).
As for the time-frequency EEG analysis, one important limitation concerns the fact that we haven’t tested potential changes in c-c connectivity of others frequency band oscillations. However, selecting the μ-rhythm was well grounded on previous research (Hari, 2006; Jensen & Mazaheri, 2010; Haegens et al., 2011; Stefanou et al., 2020), while results on other oscillation bands have been demonstrated to be contradictory (Guerra et al., 2016; Raco et al., 2016). Moreover, in addition to being previously reported, using the μ-rhythm made the classification of the trial in negative and positive peak easier than other bands with higher frequency.
It is important to mention that the statistical analysis at the electrode level was performed treating the two visits as independent. Indeed, the main goal of the study was to investigate differences between condition (peak and trough) across the two visits. For this reason, a condition-wise (instead of visit-wise) permutation testing was performed in order to control for type I error (Maris & Oostenveld, 2007). Furthermore, a cluster- based thresholding (Pernet et al., 2015) was performed retaining only clusters that exceeded the 95th percentile of the distributions of contiguous significant points obtained for all the permutations.
Furthermore, as for the diffusion signal, future studies might employ quantitative MRI techniques combined with advanced biophysical models to measure microstructural features of white matter, such as axon diameter, the g-ratio and the overall tract length (Duval et al., 2017). In addition to this, future studies should integrate diffusion and functional MRI to constrain the resolution of the EEG inverse problem in order to evaluate the transfer of information through the white matter on a millisecond scale (Deslauriers-Gauthier et al., 2019).
Finally, future studies should be conducted to explore whether such phase-dependent effect of TMS can be extended to other resting state networks (RSNs) involved in high cognitive functioning.
CONCLUSION
Our retrospective analyses expand on previous studies by demonstrating how TMS pulses delivered at specific phase instant result in differential long-distance connectivity values within the stimulated network, depending on the phase of the targeted oscillation. Moreover, such c-c synchronization changes are linearly predicted by the microstructure of the white matter tract that connect the two brain regions, regardless of the phase state when the stimulus was delivered. Findings can be used to tailor –or predict the impact of— interventions targeting other sensory as well as cognitive brain networks outside the motor system such as the Default Mode or Dorsal Attention Networks.
Supplementary Material
KEY POINTS.
Synchronized TMS pulses with pre-defined brain oscillatory phases allows to evaluate the impact of brain states on TMS effects.
TMS pulses over M1 at the negative peak of μ-frequency band induces higher phase-lock synchronization with interconnected contralateral homologous regions.
Cortico-cortical synchronization changes are linearly predicted by the fiber density and cross-section of the white matter tract that connect the two brain regions.
Phase-dependent TMS delivery might be crucial not only to amplify local effects but also to increase magnitude and reliability of within-network synchronization.
Acknowledgements
We are grateful to the gracious funding from the MIT-Harvard Broad institute (6600024-5500000895) directly supporting the study and our line of research on brain plasticity biomarkers. The authors would like to thank all participants who took part in the study and for their efforts. Dr. Santarnecchi is supported by the Defense Advanced Research Projects Agency (DARPA) via HR001117S0030, the NIH (P01 AG031720-06A1, R01 MH117063-01, R01 AG060981-01), and ADDF (ADDF-FTD GA201902–2017902). Dr. Shafi is supported by the Football Players Health Study (FPHS) at Harvard University, and the NIH (R01 MH115949, R01AG060987, P01 AG031720-06A1). Dr. Pascual-Leone is supported by grants from the National Institutes of Health (P01 AG031720; R24AG06142; R01AG059089), National Science Foundation, and La Caixa. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of Harvard University and its affiliated academic health care centers or the National Institutes of Health.
Footnotes
CRediT author contribution statement
Davide Momi: designed the study, conceptualization, conceptualized the framework, Data curation, collected the data, preprocessed the TMS-EEG data, Formal analysis, Writing - original draft, preprocessed and analyzed TMS-EEG data and wrote the first draft. Recep A. Ozdemir: data curation, collected the data. Ehsan Tadayon: formal analysis and define individual TMS targets. Pierre Boucher: data curation, collected the data, preprocessed the TMS-EEG data. Alberto Di Domenico: writing - review & editing. All authors critically reviewed the manuscript for content and approve the final version for publication. Mirco Fasolo: writing - review & editing. Alvaro Pascual-Leone: designed the study, writing - review & editing, oversaw study conduction and edited the first draft. Mouhsin M. Shafi: designed the study, Conceptualization, conceptualized the framework. overviewed the selection of stimulation sites, writing - review & editing, oversaw study conduction and edited the first draft. Emiliano Santarnecchi: Designed the study, Conceptualization, conceptualized the framework. Overviewed the selection of stimulation sites. Design data analysis. Writing - review & editing, oversaw study conduction and edited the first draft. Finalized final version of the manuscript. All authors critically reviewed the manuscript for content and approve the final version for publication.
Competing interests
Dr. Pascual-Leone Disclosure is a co-founder of Linus Health and TI Solutions AG; serves on the scientific advisory boards for Starlab Neuroscience, Neuroelectrics, Magstim Inc., Nexstim, Cognito, and MedRhythms; and is listed as an inventor on several issued and pending patents on the real-time integration of noninvasive brain stimulation with electroencephalography and magnetic resonance imaging. Dr. Santarnecchi serves on the scientific advisory boards of Neurocare, EBneuro; and is listed as an inventor on several issued and pending patents on the application of brain stimulation in patients with Alzheimer’s Disease and brain tumors. The authors declare no competing financial interests.
Data availability statement
Data cannot be shared as participants were informed that their data would be stored confidentially, in accordance with the rules of the local ethics committee. Code for generating the E-field modeling maps, the DWI and the EEG metrics is available in http://www.tmslab.org/netconlab-macroscale.php
REFERENCES
- Akam T & Kullmann DM (2014). Oscillatory multiplexing of population codes for selective communication in the mammalian brain. Nat Rev Neurosci 15, 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amassian VE, Stewart M, Quirk GJ & Rosenthal JL (1987). Physiological basis of motor effects of a transient stimulus to cerebral cortex. Neurosurgery 20, 74–93. [PubMed] [Google Scholar]
- Andersson JLR & Sotiropoulos SN (2016). An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage 125, 1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A & Gee JC (2011). A Reproducible Evaluation of ANTs Similarity Metric Performance in Brain Image Registration. NeuroImage 54, 2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann TO, Lieb A, Zrenner C & Ziemann U (2019). Pulsed Facilitation of Corticospinal Excitability by the Sensorimotor μ-Alpha Rhythm. J Neurosci 39, 10034–10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman S, Filo S & Mezer AA (2019). Modeling conduction delays in the corpus callosum using MRI-measured g-ratio. NeuroImage 195, 128–139. [DOI] [PubMed] [Google Scholar]
- Bortoletto M, Bonzano L, Zazio A, Ferrari C, Pedullà L, Gasparotti R, Miniussi C & Bove M (2021). Asymmetric transcallosal conduction delay leads to finer bimanual coordination. Brain Stimulat 14, 379–388. [DOI] [PubMed] [Google Scholar]
- ter Braack EM, de Vos CC & van Putten MJAM (2015). Masking the Auditory Evoked Potential in TMS-EEG: A Comparison of Various Methods. Brain Topogr 28, 520–528. [DOI] [PubMed] [Google Scholar]
- Bungert A, Antunes A, Espenhahn S & Thielscher A (2017). Where does TMS Stimulate the Motor Cortex? Combining Electrophysiological Measurements and Realistic Field Estimates to Reveal the Affected Cortex Position. Cereb Cortex N Y N 1991 27, 5083–5094. [DOI] [PubMed] [Google Scholar]
- Buzsáki G (2006). Rhythms of the Brain. Oxford University Press. Available at: 10.1093/acprof:oso/9780195301069.001.0001/acprof-9780195301069 [Accessed August 13, 2020]. [DOI] [Google Scholar]
- Buzsáki G, Anastassiou CA & Koch C (2012). The origin of extracellular fields and currents — EEG, ECoG, LFP and spikes. Nat Rev Neurosci 13, 407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G & Draguhn A (2004). Neuronal Oscillations in Cortical Networks. Science 304, 1926–1929. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Lai-Wo SL & Vanderwolf CH (1983). Cellular bases of hippocampal EEG in the behaving rat. Brain Res Rev 6, 139–171. [DOI] [PubMed] [Google Scholar]
- Cohen MX (2017). Rigor and replication in time-frequency analyses of cognitive electrophysiology data. Int J Psychophysiol 111, 80–87. [DOI] [PubMed] [Google Scholar]
- Connolly KR, Helmer A, Cristancho MA, Cristancho P & O’Reardon JP (2012). Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an academic medical center. J Clin Psychiatry 73, e567–573. [DOI] [PubMed] [Google Scholar]
- Corcoran AW, Alday PM, Schlesewsky M & Bornkessel-Schlesewsky I (2018). Toward a reliable, automated method of individual alpha frequency (IAF) quantification. Psychophysiology 55, e13064. [DOI] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Mahnken JD & Nudo RJ (2007). Interhemispheric connections of the ventral premotor cortex in a new world primate. J Comp Neurol 505, 701–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A & Makeig S (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134, 9–21. [DOI] [PubMed] [Google Scholar]
- Desideri D, Zrenner C, Ziemann U & Belardinelli P (2019). Phase of sensorimotor μ-oscillation modulates cortical responses to transcranial magnetic stimulation of the human motor cortex. J Physiol 597, 5671–5686. [DOI] [PubMed] [Google Scholar]
- Deslauriers-Gauthier S, Lina J-M, Butler R, Whittingstall K, Gilbert G, Bernier P-M, Deriche R & Descoteaux M (2019). White matter information flow mapping from diffusion MRI and EEG. NeuroImage 201, 116017. [DOI] [PubMed] [Google Scholar]
- Dhollander T & Connelly A (2016). A novel iterative approach to reap the benefits of multi-tissue CSD from just single-shell (+b=0) diffusion MRI data. 8. [Google Scholar]
- Drysdale AT et al. (2017). Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med 23, 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval T, Stikov N & Cohen-Adad J (2017). Modeling white matter microstructure. Funct Neurol 31, 217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Kreiter AK, Konig P & Singer W (1991). Synchronization of oscillatory neuronal responses between striate and extrastriate visual cortical areas of the cat. Proc Natl Acad Sci 88, 6048–6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B & Dale AM (2004). Automatically parcellating the human cerebral cortex. Cereb Cortex N Y N 1991 14, 11–22. [DOI] [PubMed] [Google Scholar]
- Fling BW, Benson BL & Seidler RD (2011). Transcallosal sensorimotor fiber tract structure-function relationships. Hum Brain Mapp 34, 384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SE, Wolfson S & Ranck JB (1986). Hippocampal theta rhythm and the firing of neurons in walking and urethane anesthetized rats. Exp Brain Res 62, 495–508. [DOI] [PubMed] [Google Scholar]
- Gastaut HJ & Bert J (1954). EEG changes during cinematographic presentation (Moving picture activation of the EEG). Electroencephalogr Clin Neurophysiol 6, 433–444. [DOI] [PubMed] [Google Scholar]
- Gramfort A, Luessi M, Larson E, Engemann DA, Strohmeier D, Brodbeck C, Goj R, Jas M, Brooks T, Parkkonen L & Hämäläinen M (2013). MEG and EEG data analysis with MNE-Python. Front Neurosci; DOI: 10.3389/fnins.2013.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramfort A, Papadopoulo T, Olivi E & Clerc M (2010). OpenMEEG: opensource software for quasistatic bioelectromagnetics. Biomed Eng OnLine 9, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra A, Pogosyan A, Nowak M, Tan H, Ferreri F, Di Lazzaro V & Brown P (2016). Phase Dependency of the Human Primary Motor Cortex and Cholinergic Inhibition Cancelation During Beta tACS. Cereb Cortex N Y N 1991 26, 3977–3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S, Nácher V, Luna R, Romo R & Jensen O (2011). α-Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proc Natl Acad Sci U S A 108, 19377–19382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen MS & Ilmoniemi RJ (1994). Interpreting magnetic fields of the brain: minimum norm estimates. Med Biol Eng Comput 32, 35–42. [DOI] [PubMed] [Google Scholar]
- Hari R (2006). Action-perception connection and the cortical mu rhythm. Prog Brain Res 159, 253–260. [DOI] [PubMed] [Google Scholar]
- Hussain SJ, Hayward W, Fourcand F, Zrenner C, Ziemann U, Buch ER, Hayward MK & Cohen LG (2020). Phase-dependent transcranial magnetic stimulation of the lesioned hemisphere is accurate after stroke. Brain Stimulat 13, 1354–1357. [DOI] [PubMed] [Google Scholar]
- Hyvärinen A & Oja E (1997). A Fast Fixed-Point Algorithm for Independent Component Analysis. Neural Comput 9, 1483–1492. [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW & Smith SM (2012). FSL. NeuroImage 62, 782–790. [DOI] [PubMed] [Google Scholar]
- Jensen O & Mazaheri A (2010). Shaping Functional Architecture by Oscillatory Alpha Activity: Gating by Inhibition. Front Hum Neurosci; DOI: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamondi A, Acsády L, Wang XJ & Buzsáki G (1998). Theta oscillations in somata and dendrites of hippocampal pyramidal cells in vivo: activity-dependent phase-precession of action potentials. Hippocampus 8, 244–261. [DOI] [PubMed] [Google Scholar]
- Laakso I, Hirata A & Ugawa Y (2014). Effects of coil orientation on the electric field induced by TMS over the hand motor area. Phys Med Biol 59, 203–218. [DOI] [PubMed] [Google Scholar]
- Lachaux J-P, Rodriguez E, Martinerie J & Varela FJ (1999). Measuring phase synchrony in brain signals. Hum Brain Mapp 8, 194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Alonso V, Cheeran B, Río-Rodríguez D & Fernández-del-Olmo M (2014). Inter-individual Variability in Response to Non-invasive Brain Stimulation Paradigms. Brain Stimul Basic Transl Clin Res Neuromodulation 7, 372–380. [DOI] [PubMed] [Google Scholar]
- Madsen KH, Karabanov AN, Krohne LG, Safeldt MG, Tomasevic L & Siebner HR (2019). No trace of phase: Corticomotor excitability is not tuned by phase of pericentral mu-rhythm. Brain Stimulat 12, 1261–1270. [DOI] [PubMed] [Google Scholar]
- Maris E & Oostenveld R (2007). Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 164, 177–190. [DOI] [PubMed] [Google Scholar]
- Momi D, Ozdemir RA, Tadayon E, Boucher P, Shafi MM, Pascual-Leone A & Santarnecchi E (2020). Network-level Macroscale Structural Connectivity Predicts Propagation of Transcranial Magnetic Stimulation. NeuroImage117698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormann F, Lehnertz K, David P & Elger CE (2000). Mean phase coherence as a measure for phase synchronization and its application to the EEG of epilepsy patients. Phys D 144, 358–369. [Google Scholar]
- Murthy VN & Fetz EE (1996). Oscillatory activity in sensorimotor cortex of awake monkeys: synchronization of local field potentials and relation to behavior. J Neurophysiol 76, 3949–3967. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Perera T, George MS, Grammer G, Janicak PG, Pascual-Leone A & Wirecki TS (2016). The Clinical TMS Society Consensus Review and Treatment Recommendations for TMS Therapy for Major Depressive Disorder. Brain Stimulat 9, 336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet CR, Latinus M, Nichols TE & Rousselet GA (2015). Cluster-based computational methods for mass univariate analyses of event-related brain potentials/fields: A simulation study. J Neurosci Methods 250, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JC, Reithler J, Graaf TA de, Schuhmann T, Goebel R & Sack AT (2020). Concurrent human TMS-EEG-fMRI enables monitoring of oscillatory brain state-dependent gating of cortico-subcortical network activity. Commun Biol 3, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raco V, Bauer R, Tharsan S & Gharabaghi A (2016). Combining TMS and tACS for Closed-Loop Phase-Dependent Modulation of Corticospinal Excitability: A Feasibility Study. Front Cell Neurosci; DOI: 10.3389/fncel.2016.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffelt D, Tournier J-D, Rose S, Ridgway GR, Henderson R, Crozier S, Salvado O & Connelly A (2012). Apparent Fibre Density: a novel measure for the analysis of diffusion-weighted magnetic resonance images. NeuroImage 59, 3976–3994. [DOI] [PubMed] [Google Scholar]
- Raffelt DA, Tournier J-D, Smith RE, Vaughan DN, Jackson G, Ridgway GR & Connelly A (2017). Investigating white matter fibre density and morphology using fixel-based analysis. NeuroImage 144, 58–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogasch NC, Sullivan C, Thomson RH, Rose NS, Bailey NW, Fitzgerald PB, Farzan F & Hernandez-Pavon JC (2017). Analysing concurrent transcranial magnetic stimulation and electroencephalographic data: A review and introduction to the open-source TESA software. NeuroImage 147, 934–951. [DOI] [PubMed] [Google Scholar]
- Romero MC, Davare M, Armendariz M & Janssen P (2019). Neural effects of transcranial magnetic stimulation at the single-cell level. Nat Commun 10, 2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S et al. (2020). Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clin Neurophysiol; DOI: 10.1016/j.clinph.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM et al. (2015). Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol Off J Int Fed Clin Neurophysiol 126, 1071–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P & Paulus W (1999). Magnetic stimulation: motor evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl 52, 97–103. [PubMed] [Google Scholar]
- Rouiller EM, Babalian A, Kazennikov O, Moret V, Yu XH & Wiesendanger M (1994). Transcallosal connections of the distal forelimb representations of the primary and supplementary motor cortical areas in macaque monkeys. Exp Brain Res 102, 227–243. [DOI] [PubMed] [Google Scholar]
- Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo X-N, Holmes AJ, Eickhoff SB & Yeo BTT (2018). Local-Global Parcellation of the Human Cerebral Cortex from Intrinsic Functional Connectivity MRI. Cereb Cortex N Y N 1991 28, 3095–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaworonkow N, Triesch J, Ziemann U & Zrenner C (2019). EEG-triggered TMS reveals stronger brain state-dependent modulation of motor evoked potentials at weaker stimulation intensities. Brain Stimulat 12, 110–118. [DOI] [PubMed] [Google Scholar]
- Silvanto J & Pascual-Leone A (2008). State-Dependency of Transcranial Magnetic Stimulation. Brain Topogr 21, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RE, Tournier J-D, Calamante F & Connelly A (2012). Anatomically-constrained tractography: improved diffusion MRI streamlines tractography through effective use of anatomical information. NeuroImage 62, 1924–1938. [DOI] [PubMed] [Google Scholar]
- Stefanou M-I, Desideri D, Belardinelli P, Zrenner C & Ziemann U (2018). Phase Synchronicity of μ-Rhythm Determines Efficacy of Interhemispheric Communication Between Human Motor Cortices. J Neurosci 38, 10525–10534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanou M-I, Galevska D, Zrenner C, Ziemann U & Nieminen JO (2020). Interhemispheric symmetry of μ-rhythm phase-dependency of corticospinal excitability. Sci Rep 10, 7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenke CE & Kayser J (2012). Generator localization by current source density (CSD): Implications of volume conduction and field closure at intracranial and scalp resolutions. Clin Neurophysiol Off J Int Fed Clin Neurophysiol 123, 2328–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielscher A, Antunes A & Saturnino GB (2015). Field modeling for transcranial magnetic stimulation: A useful tool to understand the physiological effects of TMS? In 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), pp. 222–225. IEEE, Milan. Available at: http://ieeexplore.ieee.org/document/7318340/ [Accessed March 2, 2020]. [DOI] [PubMed] [Google Scholar]
- Thut G & Pascual-Leone A (2010). Integrating TMS with EEG: How and What For? Brain Topogr 22, 215–218. [DOI] [PubMed] [Google Scholar]
- Tournier J-D, Calamante F & Connelly A (2007). Robust determination of the fibre orientation distribution in diffusion MRI: Non-negativity constrained super-resolved spherical deconvolution. NeuroImage 35, 1459–1472. [DOI] [PubMed] [Google Scholar]
- Tournier J-D, Calamante F & Connelly A (2012). MRtrix: Diffusion tractography in crossing fiber regions. Int J Imaging Syst Technol 22, 53–66. [Google Scholar]
- Uhlhaas PJ & Singer W (2010). Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci 11, 100–113. [DOI] [PubMed] [Google Scholar]
- Veraart J, Novikov DS, Christiaens D, Ades-Aron B, Sijbers J & Fieremans E (2016). Denoising of diffusion MRI using random matrix theory. NeuroImage 142, 394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos AN, Farzan F, Barr MS, Lobaugh NJ, Mulsant BH, Chen R, Fitzgerald PB & Daskalakis ZJ (2010). The role of the corpus callosum in transcranial magnetic stimulation induced interhemispheric signal propagation. Biol Psychiatry 68, 825–831. [DOI] [PubMed] [Google Scholar]
- Wahl M, Lauterbach-Soon B, Hattingen E, Jung P, Singer O, Volz S, Klein JC, Steinmetz H & Ziemann U (2007). Human motor corpus callosum: topography, somatotopy, and link between microstructure and function. J Neurosci Off J Soc Neurosci 27, 12132–12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, Fischl B, Liu H & Buckner RL (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106, 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zazio A, Miniussi C & Bortoletto M (2021). Alpha-band cortico-cortical phase synchronization is associated with effective connectivity in the motor network. Clin Neurophysiol; DOI: 10.1016/j.clinph.2021.06.025. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M & Smith S (2001). Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging 20, 45–57. [DOI] [PubMed] [Google Scholar]
- Zrenner C, Desideri D, Belardinelli P & Ziemann U (2018). Real-time EEG-defined excitability states determine efficacy of TMS-induced plasticity in human motor cortex. Brain Stimulat 11, 374–389. [DOI] [PubMed] [Google Scholar]
- Zrenner C, Galevska D, Nieminen JO, Baur D, Stefanou M-I & Ziemann U (2020). The shaky ground truth of real-time phase estimation. NeuroImage 214, 116761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data cannot be shared as participants were informed that their data would be stored confidentially, in accordance with the rules of the local ethics committee. Code for generating the E-field modeling maps, the DWI and the EEG metrics is available in http://www.tmslab.org/netconlab-macroscale.php


