Abstract
Survival of symbiotic reef-building corals under global warming requires rapid acclimation or adaptation. The impact of accumulated heat stress was compared across 1643 symbiont communities before and after the 2016 mass bleaching in three coral species and free-living in the environment across ~900 kilometers of the Great Barrier Reef. Resilient reefs (less aerial bleaching than predicted from high satellite sea temperatures) showed low variation in symbioses. Before 2016, heat-tolerant environmental symbionts were common in ~98% of samples and moderately abundant (9 to 40% in samples). In corals, heat-tolerant symbionts were at low abundances (0 to 7.3%) but only in a minority (13 to 27%) of colonies. Following bleaching, environmental diversity doubled (including heat-tolerant symbionts) and increased in one coral species. Communities were dynamic (Acropora millepora) and conserved (Acropora hyacinthus and Acropora tenuis), including symbiont community turnover and redistribution. Symbiotic restructuring after bleaching occurs but is a taxon-specific ecological opportunity.
Climate change-driven mass coral bleaching restructures algal symbiont communities in some corals and in the environment.
INTRODUCTION
Corals form the structural and biological foundation of tropical reefs—among the most biodiverse ecosystems on the planet. Corals build and maintain reefs through the accretion of skeletons, underpinned by the nutritional symbiosis with photoautotrophic microbes Symbiodiniaceae (1). Many reef-building corals cannot survive without the transfer of carbon and nitrogen from their algal symbionts (2). However, this obligate relationship is threatened by rapid global warming where heat wave conditions disrupt the symbiosis and cause corals to lose their symbionts (termed coral bleaching). If heat is severe or prolonged, then coral bleaching can lead to disease or death of the animal host, affecting reefs at global scales (3). As temperatures continue to rise, extreme episodes of heat stress will increase mass coral bleaching with substantial negative impacts for reef ecosystems. Coral reef biodiversity is already changing in response to warming (4), with repeated extreme episodes of bleaching leading to the restructuring of corals (5), fish, and other invertebrate communities (6).
To better predict coral reef futures, there is a need to understand whether climate change will restructure this foundational symbiosis to a more vulnerable or more resilient assemblage (7). One mechanism of rapid acclimation or adaptation to the environment is through changes to host-associated microbial communities (8, 9). Quantifying the propensity for symbionts to change therefore enables the prediction of corals’ evolutionary trajectories during climate change (10). Corals generally associate with specific symbionts (11–15), with most Pacific coral species hosting more speciose communities compared to other regions (13, 16). Coral-symbiont associations are generally stable over time with disruption requiring substantial environmental stress (17), although specific changes can enhance host survival during short-term or prolonged heat waves (18, 19). Although damaging, bleaching can create a high-risk ecological opportunity [sensu (20)] to mitigate stress [adaptive bleaching hypothesis (ABH) sensu (21, 22)] by partnering with previously undocumented combinations of symbionts better suited to present or future conditions (i.e., those that are heat tolerant). ABH may be a mechanism for rapid acclimation to heat, indicated by host-directed expulsion (23) and evolutionary selection on symbiont communities (24) and supported by the availability of physiologically diverse symbionts free-living in the environment (25). Symbiodiniaceae include hundreds of symbiotic and free-living putative “species” (11–15). The current paradigm is that stress is ameliorated by two mechanisms that restructure the symbiont community: “shuffling” the relative abundance of existing symbionts [predominately from the genera Cladocopium to Durusdinium (1, 26)] or by “switching” to new symbionts acquired from the environment (potentially from a pool of free-living symbionts in reef sediments). To investigate the impacts of mass bleaching on coral symbioses, we quantified the temporal changes in Symbiodiniaceae communities in the surviving colonies of three abundant coral species and in the environment (reef sediments), across latitudinal and cross-shelf gradients on the Great Barrier Reef (GBR) over a 16-year period (2003–2019; total, n = 1643) and acknowledged that bleaching events were not the only disturbance during this period (e.g., cyclones and crown-of-thorns predation). There are also currently alternative methodologies for describing Symbiodiniaceae communities, each with their merit. Here, we highlight and apply multiple methods [DIV (defining intragenomic variant) and ASV (amplicon sequence variant) approaches; see Materials and Methods] to analyze data that include collections before (“pre”) and after (“post”) the 2016 mass bleaching event from 26 reefs (Fig. 1 and table S1).
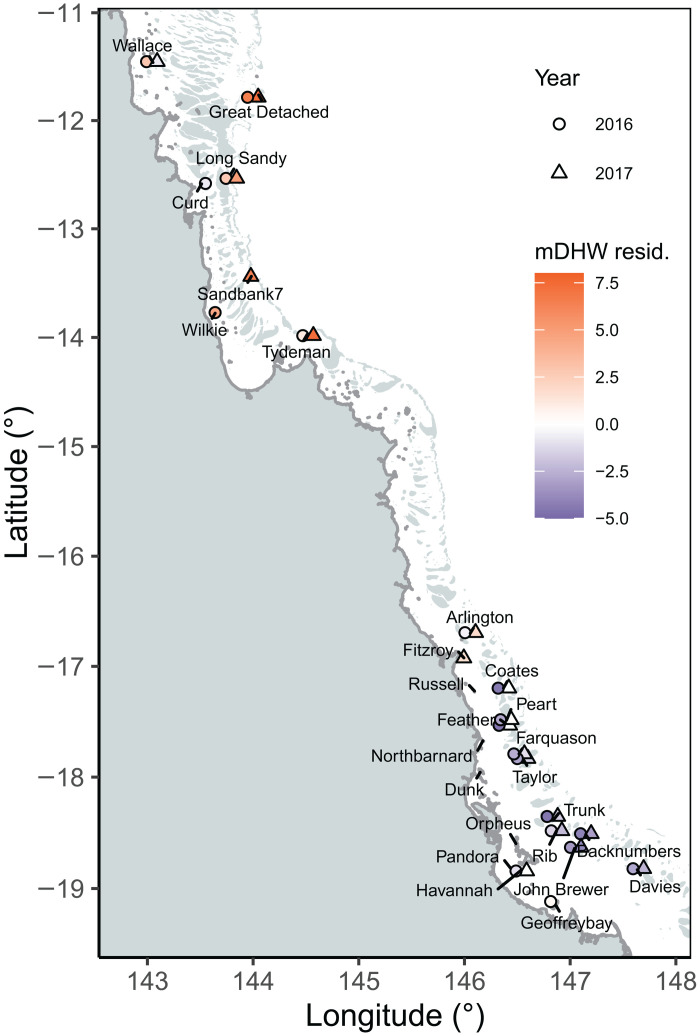
Fig. 1. Sampling of corals and the environment along reefs from the northern and central GBR.
Samples for genetic analysis of corals’ symbiotic communities included 1644 samples from individual coral colonies of A. hyacinthus, A. tenuis, and A. millepora (n = 1454) and sediments representing the environmental pool (n = 189) from 2003 to 2019. Twenty-six reefs were surveyed genetically and varied in their responses to bleaching in 2016 (circles) and 2017 (triangles). The deviance in the maximum degree heating week (mDHW) residual (red to blue) between the accumulated heat stress measured in DHWs and the aerial survey bleaching response is shown per reef surveyed.
RESULTS AND DISCUSSION
Symbiont communities varied among species and environmental gradients
The functional diversity within Symbiodiniaceae is extensive (1) and can be detected across multiple taxonomic levels (27). Here, we mainly present sequence data using the ASV approach (see Materials and Methods for information regarding Symbiodiniaceae taxonomic methods and further comparisons). Contemporary coral-algal symbioses are globally dominated by stress-sensitive members of the genus Cladocopium (1), and this pattern was evident before and after bleaching within Acropora spp. (Fig. 2 and fig. S1). Symbiont communities varied by sample group (i.e., coral host species or sediments), sector (north/central), region (inshore/offshore), and bleaching history (pre- or post-2016) [permutational multivariate analysis of variance (PERMANOVA), P < 0.001; table S2]. Variation was greatest along an inshore to offshore gradient in coral and environment samples [coefficient of determination (R2) variance explained = 0.1 to 0.39; table S3], consistent with established biogeographical patterns (14, 28, 29). In comparison, variability in symbionts was lower between the northern and central sectors and between pre- and postbleaching time points (R2 = 0.02 to 0.09). Bleaching history explained greater variation in Acropora millepora symbiont communities compared to Acropora hyacinthus or Acropora tenuis (R2 = 0.09 versus 0.07 and 0.006). We also tested multiple, independent methods on an informative subset of highly variable reefs and time points in the most variable species, A. millepora. This comparison confirmed that both approaches render comparable interpretations, specifically in which the relative proportion of Cladocopium was lower in presamples and replaced by Durusdinium in the postsamples (fig. S2).
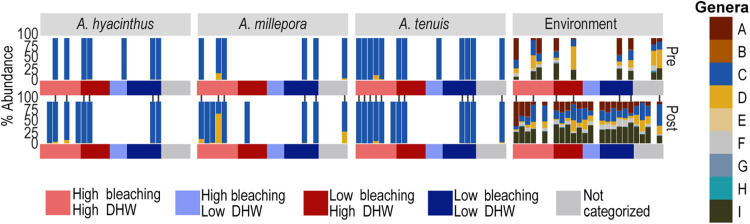
Fig. 2. Normalized relative abundances (%) of Symbiodiniaceae communities sequenced from coral and environmental samples collected along reefs from the northern and central GBR before and after the 2016 mass bleaching event.
Each bar is a separate reef. Barplots depict the variance-normalized relative abundance of the nine Symbiodiniaceae “genera” (1) (colors from “A to I”) in the three coral species and in the environment categorized by each reefs’ mDHW residual category [colors correspond to H-H (high bleaching–high mDHW), H-L (high bleaching–low mDHW), L-H (low bleaching–high mDHW), and L-L (low bleaching–low mDHW)].
When averaged per paired reef (pre versus post; Figs. 2 and 3), only a minority (13.3 to 26.8%) of individual corals sampled contained Durusdinium before the 2016 bleaching (A. tenuis, 47 of 352 colonies hosted Durusdinium; A. hyacinthus, 34 of 160; A. millepora, 44 of 164). After bleaching, only A. millepora colonies had increased Durusdinium prevalence (61.4%; fig. S1, A and B). The relative abundances of Durusdinium per reef ranged from <1% before to ~50% after 2016, and changes were highly species-specific and driven by inshore reefs (Figs. 2 and 3, fig. S1A, and table S4). One species (A. millepora) hosted the highest abundances of stress-tolerant members of the genus Durusdinium (19), with a notable difference compared to A. hyacinthus and A. tenuis colonies, which exhibited changes only at low relative abundances (<3%), mostly on northern (A. hyacinthus) or central, inshore reefs (A. tenuis) (Fig. 4A, tables S4 and S5, and fig. S1A).
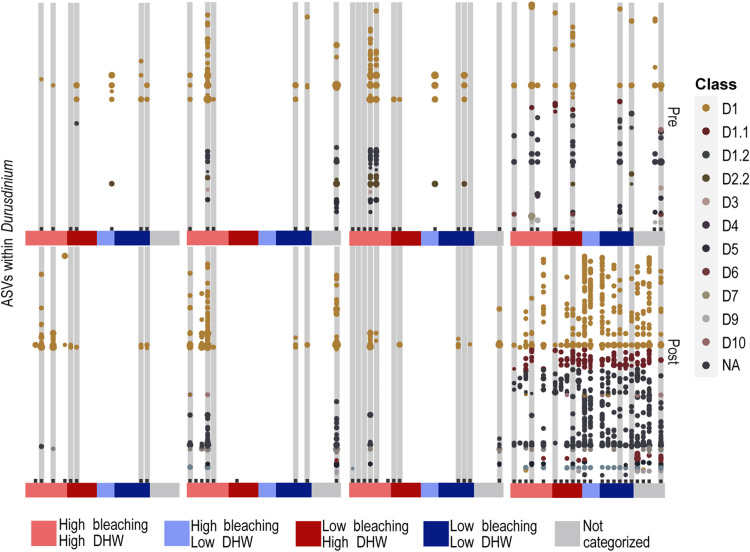
Fig. 3. Diversity of Durusdinium (“D”) Symbiodiniaceae communities sequenced from coral and environmental samples collected along reefs from the northern and central GBR before and after the 2016 mass bleaching event.
Bubble plots depict the diversity of Durusdinium at each reef before and after bleaching with each bar representing a separate reef. Bubbles represent normalized relative abundances, grouped into classes that correspond to lower taxonomic resolution (colors correspond to D1 to D10; NA = unclassified). Boxes in gray denote reefs with paired pre- and post- bleaching samples. Reefs are grouped into the three coral species and in the environment categorized by each reefs’ mDHW residual category (colors correspond to H-H, H-L, L-H, and L-L). Please note that bubble plots are mainly used for depicting diversity and not relative abundances, although dot sizes here are scaled to variance normalized relative abundances.
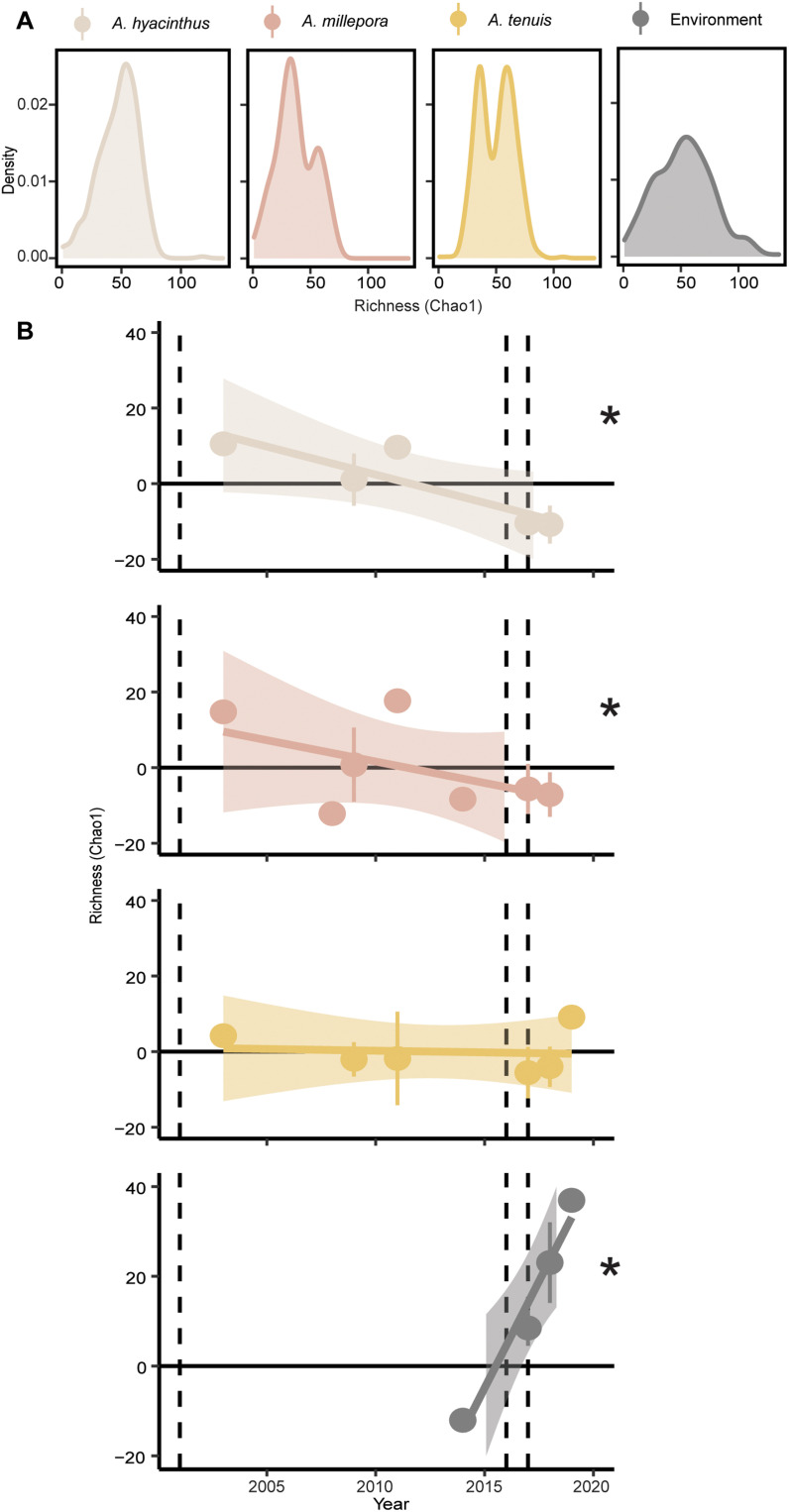
Fig. 4. Changes in Symbiodiniaceae richness in coral and environmental samples collected along reefs from the GBR before and after the 2016 mass bleaching.
(A) The Chao1 richness of ASVs, from 2003 to 2019, collected from reefs colored by sample type. The number of samples (density, y axis) corresponding to the Choa1 metric for each sample type. (B) The means ± SD of Chao1 richness shown as the linear trend of the metric through time. The dashed lines represent the 2002, 2016, and 2017 mass bleaching events. Asterisks (*) signify significant differences calculated with linear mixed effects models (lmer, P < 0.05; exact statistical values in table S7; n values in table S1) in community composition between coral species and environmental samples. The colors correspond to each of the four sample types (gray, A. hyacinthus; pink, A. millepora; yellow, A. tenuis; black, environment).
The prevalence of Durusdinium in the environment was consistently high (97.1 to 100%) compared to coral samples (pre, 21 of 21 samples; post, 34 of 35 samples). These values suggest that heat-tolerant Durusdinium was found across almost all environmental gradients and was thus available for coral uptake. The abundance of Durusdinium decreased in the environment after bleaching by >56.6%. This was not explained by sequencing read depth given the greater abundance of reads retrieved after bleaching (mean reads ± SE: pre, 48,982 ± 1493; post, 108,026 ± 1789) but could have been influenced by seasonal fluctuations or water patterns. The increased abundance of Durusdinium in corals (i.e., postbleaching uptake) mostly occurred in A. millepora (Fig. 3) and was limited in A. hyacinthus and A. tenuis. This coral species–specific pattern could have potentially been driven by host genotype or other heritable mechanisms (24, 30). Shuffling or switching to specific heat-tolerant symbiont genera may therefore be a specialized mechanism to contend with environmental change and not be as common across the coral phylogenetic tree as previously hoped (19, 31–33). High variation in changes in symbiont abundances and communities at the reef and host species levels—as shown through sequence variation (Fig. 2)—suggests that corals have several potential mechanisms to acclimate to rapid ocean warming. Understanding this variability will be essential to predicting reef vulnerability and recovery potentials.
The three coral species varied significantly in symbiont ASV community composition (PERMANOVA; table S1). The most abundant ASVs differed by coral species (in A. hyacinthus: C3k, Cspc, and C29; A. millepora: Cspc and C3k; A. tenuis: Cspc, C1m, and C3k; table S5) and may influence different physiological tolerances across corals. Free-living environmental symbiont communities were highly diverse compared to those in corals, spanning all major Symbiodiniaceae genera (Fig. 2, environment) (1) and may be a critically important reservoir of unknown but functionally important symbiont diversity for corals. Heat-tolerant Symbiodiniaceae (e.g., D1 and D1a) were abundant in the environment, as were the common and shared ASVs among all three Acropora species (C1m, Cspc, and C3k).
Leading up to 2016, other disturbances had already affected the GBR (34), including bleaching. The 2016 cutoff was selected as a feature that best represents responses to greater accumulated thermal stress over this long sampling period as opposed to the specific 2016 heat wave and acknowledges that the 2016 bleaching event represented the most extreme bleaching that had occurred before or after within our sampling time frame from 2003 to 2019 (7), with previous bleaching occurring outside our sampling window in 1998 and 2002 (35). Although 2005 to 2015 were typified by relatively low sea surface temperature and low summertime marine heat waves (35), given how bleaching impacts varied across the GBR in 2016, we also explored a subset of the data (2011–2019) to examine whether earlier warming and bleaching were driving community variability (i.e., in the years directly after the 2002 bleaching). PERMANOVA results using this subset of data (table S10) were highly similar to previous results using the full time-course data (table S2) and underscored our previous findings that symbiont communities varied by sample group (i.e., coral host species or sediments), sector (north/central), region (inshore/offshore), and bleaching history (P < 0.001; table S10).
Symbiont community richness and diversity varied through time
Network modeling suggests that corals with symbiont communities characterized by high richness are more susceptible to bleaching (36). To explore this, we examined patterns in richness (Chao1 index of ASVs) and diversity (number of ASVs) of symbiont communities in corals and in the environment, in which both metrics varied before and after the 2016 bleaching (Fig. 4). There was a general pattern of decline in corals over the sampling times, as well as when examined before and after bleaching, with variability between sampling time points. After accounting for interreef variability, richness significantly decreased after 2016 in A. hyacinthus and A. millepora but not A. tenuis (tables S6 and S7; lmer, P < 2 × 10−16, P < 0.0006, and P < 0.38, respectively). In the environmental samples, richness estimates increased in both regions by ~50%, most notably in the north (P < 2 × 10−16). Mean diversity decreased significantly in all species but not A. millepora (tables S8 and S9). During this time frame, the GBR has experienced several environmental events, including less severe bleaching and cyclones (34); therefore, these changes in abundance may be a response to greater accumulated thermal stress over this ~16-year period as opposed to the specific 2016 heat wave. Current management action often focuses on protecting coral diversity. However, this potential loss in symbiont diversity is concerning given the previous modeling that suggests protecting generalist symbionts with high heat tolerances, but not coral host diversity, contributes more to coral reef resilience (37). This highlights the importance of protecting the coral-symbiont relationship.
Changes in richness and diversity can also signal larger shifts in ecosystem functioning. As observed on Caribbean reefs (38), introduced Durusdinium can competitively displace other symbionts after repeated environmental stress, driven by the rapid spread of these tolerant “opportunists” (39) into novel locations and hosts. We detected this pattern here, where A. millepora exhibited, on average, some of the lowest symbiont richness overall paired with the highest Durusdinium abundance. Also noteworthy were the increases in Durusdinium diversity in the environmental samples after bleaching (Fig. 2). This increase may have been caused by strong selection from extreme heat waves influencing rapid diversification (e.g., adaptive radiation) of free-living symbionts or potentially other disturbances, including the 2011 freshwater influx that may have reduced free-living symbiont diversity in the pre-2016 samples. There were also substantial linkages between the species displaying the largest changes in relative abundances and richness (A. millepora) and the environmental communities. In this, 29% of Durusdinium ASVs were found and shared in A. millepora and the environment after bleaching, and two of the 10 most abundant coral symbionts were also shared. The large postbleaching shifts in the sediment symbiont pool (Figs. 5 and 6 and fig. S1) and the increasing richness and diversity in the short-term suggest similar responses to repeated disturbance as those observed in the Caribbean (38). The longer-term consequences of these changes remain unknown.
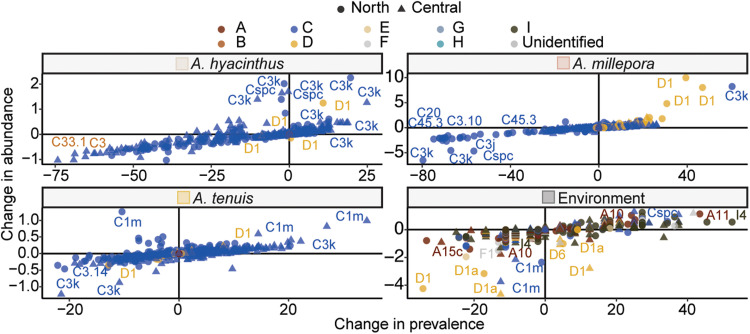
Fig. 5. Shuffling of Symbiodiniaceae communities after the 2016 bleaching on the GBR.
Symbiont dynamics were explored by quantifying the change in variance normalized relative abundances of symbionts compared to their change in prevalence (presence or absence). Shuffling of Symbiodiniaceae communities, in which ASVs that significantly changed in variance normalized relative abundances (Padj < 0.05) either before or after 2016 are colored by genus. Specific symbiont taxa are labeled. ASVs recovered from reefs from central (triangle) and northern (circle) locations are indicated.
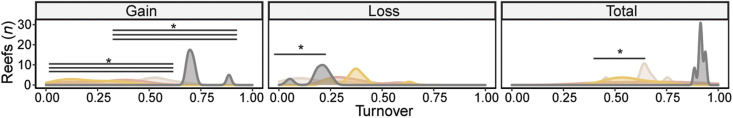
Fig. 6. Switching of Symbiodiniaceae communities after the 2016 bleaching on the GBR.
Community turnover describes the gain and loss (switching) of Symbiodiniaceae ASVs per reef that were not reported before 2016 but were detected after 2016 (gain) and were reported before 2016 but not after 2016 (loss), as well as the sum of these ASVs (total) across the sample types. Asterisks (*) signify significant differences (lmer, P < 0.05) in ASV community composition between coral species and environmental samples (P values are referenced within the text; n = values in table S1). The colors correspond to each of the four sample types (gray, A. hyacinthus; pink, A. millepora; yellow, A. tenuis; black, environmental).
Symbiodiniaceae shuffling and switching in response to mass bleaching
Symbiont communities extend the host phenotype, increasing acclimation and adaptation potential of the organism. Although generally explored only at the taxonomic resolution of genera (33), here, we quantify shuffling and switching at the more ecologically relevant level (40, 41) of ASVs and then assess the propensity for both mechanisms at the reef level (see Materials and Methods). Overall, symbiont communities were restructured after the 2016 bleaching in both coral and environmental samples, driven by changes in specific symbionts between samples collected before and after the 2016 bleaching (Figs. 5 and 6). The three Acropora species exhibited relatively more shuffling compared to switching (22.8% of A. millepora ASVs, 18% A. hyacinthus, and 36.1% A. tenuis; figs. S1A, S3, and S4 and table S4). In addition to the classic shuffling response (from Cladocopium to Durusdinium) in A. millepora, the other two coral species either shuffled or switched taxa within Cladocopium (C3k, C1m, and Cspc; Fig. 5). Shuffling occurred in the environmental samples but was relatively less common and included a greater diversity of symbiont taxa (Fig. 5 and fig. S4), decreases in Durusdinium abundance, and the appearance of new taxa after bleaching (Fig. 5).
Symbiont switching occurs when new taxa appear in the established community within the host coral, although evidence for corals to accommodate these changes remains limited (42, 43). Here, we apply a standard ecological metric, community turnover (4), to quantify the proportion of ASVs gained or lost in samples taken before and after bleaching, at the reef level (Fig. 6). Switching was prevalent across many sampled reefs and was significantly different among the three Acropora species and environmental communities even after accounting for between-reef differences (lmer, P ≤ 0.0001 to 0.0033; Fig. 6; total percentage of ASVs that changed, 68.1 ± 3% SE). Postbleaching switching of symbiont taxa was twice as high in the environment [91.6 ± 0%; mean community turnover metric (MRS), 108.5 to 354.2] compared to corals (60.9 ± 2.7%; MRS, 35.8 to 193), mostly driven by the appearance of significantly more new taxa after bleaching relative to all three coral species (72.6 ± 3.1% versus 29.5 ± 3.6%; all comparisons, P ≤ 0.0001 to 0.009). The proportion of symbionts lost was only significantly less compared to A. hyacinthus and A. millepora (P = 0.005 to 0.03). Overall, total turnover in corals was driven by significantly fewer losses of ASVs within A. hyacinthus, especially in offshore, northern reefs, compared to A. tenuis (P = 0.005 to 0.03). This indicates the relative stability of A. hyacinthus communities. Together, we conclude that symbiosis flexibility to environmental change varies across coral species, driven mostly by symbiont taxa lost, not gained.
Symbiont community changes differed depending on bleaching exposure at each reef
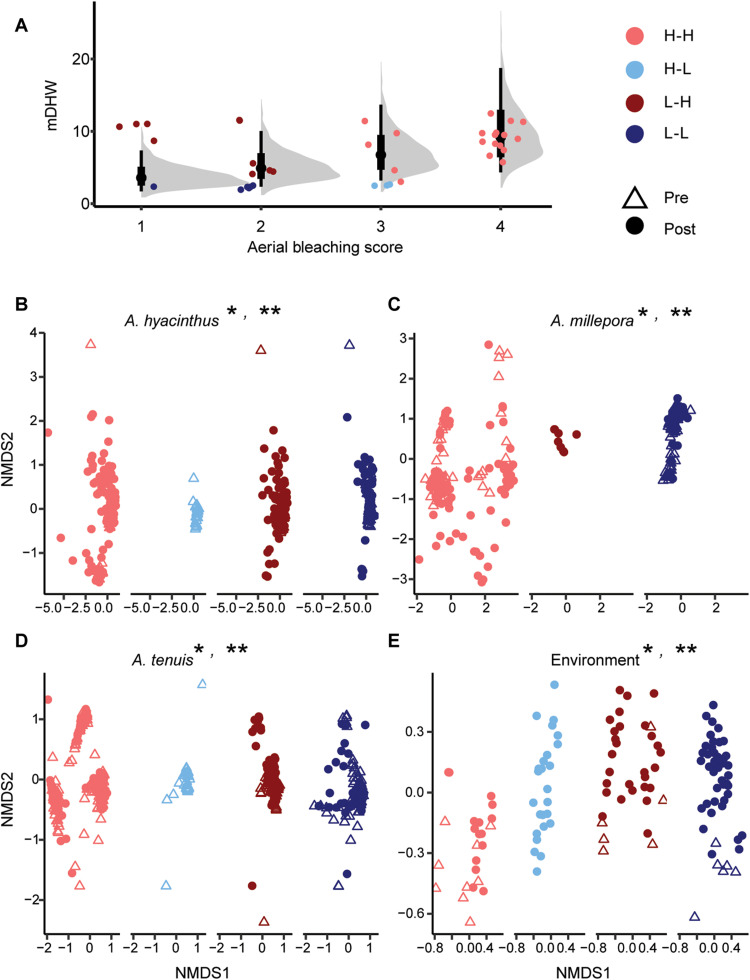
The mass coral bleaching observed in 2016 was associated with pronounced accumulated heat exposure (Figs. 1 and 7A) (5), with extreme maximum degree heating weeks (mDHWs) compared to previous events from 1985 to 2015 (35). To better understand the variability in responses across regions on the GBR, we examined the expected versus observed bleaching responses across each reef. We calculated the deviance between the expected bleaching responses given the amount of accumulated heat for each reef and the actual bleaching observed, expressed as the residuals between the observed mDHW and the Bayesian posterior predicated mean mDHW (see Materials and Methods) and classified these responses into four categories (Fig. 7A). As expected (44), greater mDHWs were associated with higher aerial bleaching scores [denoted as high bleaching–high mDHW (H-H)], high bleaching even under relatively low mDHW (H-L), low bleaching and high mDHW [low bleaching–high mDHW (L-H)], or no bleaching [low bleaching–low mDHW (L-L)]. Notably, four reefs diverged from the typical bleaching conditions associated with high temperatures and did not bleach even when exposed to high heat stress (L-H), a phenomenon also observed in some Eastern Tropical Pacific reefs (45). Symbiont communities differed between the four coral bleaching response categories (Fig. 7, B to E), mostly driven by the variability within each group. Differences, regardless of time point, in the dispersion of samples suggest variability in the responses of symbiont communities within each bleaching group (table S11). This observation is supported by significant differences in centroid and dispersion differences (adonis test; table S12).
Fig. 7. Symbiont community responses in corals and the environment on the GBR.
(A) Bleaching experienced by each reef in 2016 and 2017 compared to the accumulated heat stress experienced in mDHWs. The deviance of the relationship between mDHW and bleaching score is represented by the mDHW residual. This residual was used to classify each reef into the following four categories: H-H, H-L, L-H, and L-L. Gray densities indicate the posterior distribution of mDHW for each bleaching category, while black points and bars indicate the mean and 66% and 95% intervals for the posterior. Boxplots include the median values (center lines), upper and lower quartiles (box limits), 1.5× interquartile range (whiskers), and outliers (points). (B to E) Symbiodiniaceae communities plotted in ordination space and colored by DHW deviance categories. Nonmetric multidimensional scaling (NMDS) using Bray-Curtis distance of variance normalized ASVs for the four sample sources. Individual points represent symbiont communities categorized as either before 2016 (circle) or after 2016 (triangle) sampling. Asterisks (* and **) signify significant differences (P < 0.05) in the multivariate dispersion between the four mDHW categories (*) or differences in the combined variability of the centroids and dispersion of the four categories (**). Exact P values in tables S11 and S12; n = values in table S1.
None of the A. tenuis communities from H-L reefs were associated with Durusdinium. Communities from H-H reefs were highly variable in the three coral species and the environment, especially compared to “baselines” demonstrated by L-L reefs (Fig. 7C), supporting evidence that stress promotes variability (46, 47). Symbiont communities were less variable in L-H reefs, especially in A. tenuis, and may be indicative of resilient coral symbiont communities. H-H reefs generally had divergent communities compared to the other categories (notably in A. tenuis; Fig. 7D). This suggests that communities in some species such as A. tenuis and A. millepora were distinct before and after bleaching, not only in response to bleaching (Fig. 7). A. millepora communities from bleached reefs (H-H) had distinct communities compared to those that do not bleach (L-L) (Fig. 7C). Samples from L-H reefs followed similar patterns to L-L reefs and did have Durusdinium after heat stress (Figs. 2 and (7C). Last, there was high overlap in the shifts associated with changes in Cladocopium ASVs in coral and environmental samples, suggesting that these symbionts may contribute heavily to bleaching responses. These data demonstrate that symbiont community structure varies under different bleaching histories and outcomes.
Here, we provide key insights that the consequences of mass coral bleaching on algal symbiont communities across the GBR are of both loss and gain. The loss of dominant and background generalist symbionts can reduce corals’ ability to resist bleaching (37), limit recovery and resilience (13, 16), and have negative, ecosystem-wide effects on the modeled long-term stability of symbioses (13, 16, 41). Here, some corals, but not all, responded by increasing the abundance of heat-tolerant Durusdinium, likely from their availability in the free-living sediment community. Knowledge of the species-specific ability of corals to shuffle or switch symbionts is essential for managing and conserving these ecosystems under continual ocean warming. Although the future risk to reef building corals from climate change around the world remains, variation in corals’ responses highlights pathways for increased reef resilience.
MATERIALS AND METHODS
Experimental design
Ocean warming has driven widespread bleaching and mortality across the full length of the GBR, with recent mass bleaching concentrated at different times in the north (2016), central (2017), and southern (2006 and 2020) regions (35, 48). Widespread bleaching affects coral genera and species differently (37, 49, 50), leading to variation in recovery potentials (51). There is little understanding of how mass bleaching disrupts symbioses over long time scales (>5 years) (17) or whether these processes are driven by acclimation or adaptation of the host-symbiont partnership. This understanding is critical given that the loss of host-symbiont associations has a disproportionally greater impact on long-term reef resilience compared to the loss of individual coral species alone (37). Overall, we found that mass bleaching led to significant losses in symbiont diversity in some corals and a concomitant explosion of heat-tolerant symbionts free-living in the environment. Coral responses were both dynamic (A. millepora) and conserved (A. hyacinthus and A. tenuis), suggesting that symbiotic restructuring may be a species-specific ecological opportunity.
To investigate the impacts of mass bleaching on coral symbioses, we quantified the temporal changes in Symbiodiniaceae communities across latitudinal and water-quality gradients on the GBR. We deeply sequenced the ITS2 locus (the Internal Transcribed Spacer 2 region) of the dinoflagellate family Symbiodiniaceae to characterize this symbiotic community inside of corals and free-living in the environment. To do this, we collected environmental sediment samples (n = 189) and branch fragments from individual colonies of three common, widespread, and ecologically important coral species (A. hyacinthus, A. tenuis, and A. millepora; n = 1454). Each measurement was therefore taken from distinct samples. The sample collections spanned a 16-year period (2003–2019; total, n = 1643), and this time frame included collections before (pre) and after (post) the 2016 mass bleaching event from 26 reefs across the northern and central sectors and the inshore and offshore regions of the GBR (Fig. 1 and table S1). Although we use the 2016 mass bleaching event as a cutoff, we recognize that the patterns after 2016 may represent accumulated thermal stress as opposed to a response to the specific 2016 heat wave. We present results at two taxonomic levels: the commonly used level of genera (e.g., Durusdinium and Cladocopium) and the more “species-specific” sequence level [ASVs (52)]. We then assess the temporal turnover of Symbiodiniaceae community dynamics using metrics of symbiont taxon abundance, diversity, and richness (defined here, in order, as the total number of reads, total number of ASVs, and alpha diversity expressed as the Choa1 metric). For each reef, we calculate the propensity for corals to undertake two modes of symbiosis restructuring (shuffling and switching) to acclimate rapidly to heat stress and develop a metric for classifying reef responses. This metric is based on the reported bleaching via aerial surveys (53), and we then calculated an accumulated stress DHW deviance metric per reef derived from satellite sea surface temperatures (54).
To compare Symbiodiniaceae communities before and after mass bleaching, historical tissue samples (2003–2014) of A. hyacinthus, A. millepora, and A. tenuis stored at the Australian Institute of Marine Science were sequenced. Samples were selected, and then the corresponding reefs for which historical samples were present were revisited for new sampling from 2017 to 2019. Most collections during this 15-year period fell between September and November, with a majority of samples collected during spawning collection trips, generally at the end of October and into November. Of these new collections, coral fragments (<5 cm) were collected by divers on Self Contained Underwater Breating Apparatus (SCUBA) from healthy colonies on the forereef at <6 m in depth and >6 m apart to prevent the collection of clones. Fragments from each colony were sealed in individual plastic bags, brought to the surface, and stored in 100% ethanol at room temperature, and ethanol was later exchanged to maintain DNA integrity.
Simultaneously, sediment was collected from the surface of the benthos into three-replicate 1-liter containers [as developed in (25)]. Sediment was collected before the 2016 bleaching event from a subset of sites [2013–2014 in (25)] and after the bleaching event (2017–2019). All sediment samples were preserved by freezing at −20°C until processing, when sediments were thawed, filtered through a series of sieves (<63 μM) using 0.1-μm filtered seawater, and centrifuged to produce a pellet and frozen until processing.
DNA extraction
Following procedures previously described in (48), we identified Symbiodiniaceae by sequencing the ITS2 locus within three coral hosts: A. hyacinthus, A. millepora, and A. tenuis (table S1). DNA was extracted using the E.Z.N.A. Tissue DNA Kit (Omega Bio-tek, USA) following the manufacturer’s instruction with minor modification as follows. Approximately 20 mg of coral tissue was scraped off the skeleton using a scalpel, incubated in TL buffer and OB protease with sterile acid–washed glass beads (212 to 300 μm; Sigma-Aldrich, USA), and homogenized using three cycles of 30-s homogenizations (FastPrep-24 5G, MP Biomedicals, USA). Samples were then incubated for 1 hour at 55°C in a microarray oven (50 rpm; Model 777, SciGene, USA). The remaining steps of the protocol were left unchanged, and DNA was eluted in 60 μl of ultrapure distilled water (deoxyribonuclease/ribonuclease free, Invitrogen, Life Technologies, USA), and the pellet was frozen at −20°C until polymerase chain reaction (PCR) amplification.
DNA was extracted from sediment samples using a modified protocol from the DNeasy PowerMax Soil Kit (QIAGEN, Germany). To facilitate cell lysis, pelleted sediment samples (~5 g) were homogenized in a bead beater (3 × 20 s at 6 m s−1) and incubated for 1 hour at 65°C in a rotating oven with the initial extraction buffer. Further modifications are described in (25). DNA was further purified using ethanol precipitation, which included incubation in 0.2× volume of 5 M NaAc, followed by incubation in 2.5× volume of 100% ice-chilled ethanol at −20°C for 2 hours. DNA was then pelleted by centrifugation at 4000g for 20 min.
Amplification and sequencing of the ITS2 region from isolated DNA
A ~350–base pair (bp) region of ITS2 was amplified using Symbiodiniaceae-specific primers (55) with Illumina overhang adapter sequences for the next-generation amplicon sequencing, using procedures previously described in (48). The ITS2 region was amplified from all coral or sediment DNA samples (25, 56) with the ITS-DINO and ITS2Rev2 Symbiodiniaceae-specific primers (57) combined with Illumina adapter overhangs (25). PCR reactions were conducted in 30 μl using the AmpliTaq Gold 360 Master Mix, 0.4 μM of each primer, and 1 mM MgCl2. The amplification cycles were as follows: 95°C for 10 min; 30 to 35 cycles at 95°C for 30 s, 56°C for 30 s, 72°C for 30 s, and 72°C for 7 min. Samples that failed to amplify well with 30 cycles were amplified with 33 or 35 cycles so that all PCR products yielded an even, visible band when run on agarose gel electrophoresis. No-template PCR reactions were performed within each PCR batch as negative controls, and none of which yielded visible bands by agarose gel electrophoresis. The proceeding steps of library preparation and sequencing were performed by the Ramaciotti Centre for Genomics (University of New South Wales, Sydney, Australia) using the Illumina MiSeq (2 × 300–bp paired-end reads).
Challenge of symbiont taxonomy
Symbiodiniaceae taxonomy is currently undergoing revision (1) with substantial effort being made to understand how sequence diversity links with species, genus, and family designations and the functional significance of different symbiont taxa. Because of the extensive intragenomic and multicopy nature of Symbiodiniaceae genomes, there has been a lack of consensus on how to best interpret the next-generation sequencing molecular data that results from gene amplicon analysis. Although the next-generation sequencing has revolutionized our ability to detect this important group (58), the ability to define and identify a species and how that scales to community metrics is uncertain. To address this need, a workshop funded by the National Science Foundation [Symbiodiniaceae Diversity Workshop (59)] was convened, and consensus guidelines were prepared to aid in the standardization of data interpretation across studies. Briefly, there are two main approaches currently in use: the ASV clustering and DIV methods. ASV approaches rely on collapsing sequence length variation into ASV using clustering algorithms (40), whereas the DIV approach separates by genus/clade and groups by minimum entropy decomposition to determine DIVs (60). Within these guidelines, there are pros and cons presented for both ASV and DIV interpretations (59). In general, ASVs may inflate diversity metrics, whereas the DIV approach may cause a loss of diversity but result in less false positives and therefore may be a better indication of species-level diversity in known or low-diversity systems. Environmental samples pose challenges for both methods due to the lack of a reference database for many of these novel sequences (ASVs) or methodological issues of novel sequences detection (DIVs).
Here, the use of the ASV approach was selected as it provides higher resolution at the sequence level for exploration of potential novel diversity at both high and low abundances. We do acknowledge that some of the ASVs found may be spurious sequence variants and may not represent actual Symbiodiniaceae species per se. However, although the ASV clustering approach may overestimate species diversity, it should overestimate it in a consistent manner across sampling points and types, and, therefore, the ecological conclusions drawn here are unlikely to vary. Therefore, we acknowledge the assumptions associated with the data and highlight that other methods and interpretations exist and are open to whether interpretations would change the ecological relevance of the study (59). Upon reanalysis of a subset of the data using both methods, we found that the ecological interpretations hold (results in Fig. 2 and fig. S2). In this analysis, we tested these independent methods, including the SymPortal pipeline described in (60) on an informative subset of highly variable reefs and highly variable time points in the most variable species, A. millepora. The other species were not tested given their lower overall variability (and therefore less likely to pick up differences between methods if they existed) or the challenge of interpretation for both methods (environmental samples).
Sequence processing
Sequences were processed using demultiplexing, read quality inspection, filtering, and variance normalization using a protocol and code fully described and reviewed in (40, 61). ITS2 sequence variants were identified via the DADA2 pipeline in R statistical software (52). High-confidence sequence variants were identified via Blast search of a custom database of Symbiodiniaceae ITS2 sequences (40). Intragenomic ITS2 sequence variants (i.e., sequence variants within a single symbiont, ASVs) were collapsed into “genotypes” via clustering with the LULU algorithm (62). As described above, these methods follow previous publications (40) and have been discussed in the National Science Foundation Symbiodiniaceae Diversity Workshop (2021).
Statistical analysis
All statistical analysis was performed in R (v.4.1.2). As previously described in (48), Symbiodiniaceae ITS2 communities within samples from different sample sources (three Acropora spp., environmental sediment samples), GBR regions (north or central), reef sector (inshore and offshore), bleaching (pre and post), and all interaction terms were analyzed using PERMANOVA on Bray-Curtis distances using 9999 permutations with the package vegan (63). Given the large, significant differences in communities between sample sources (table S1), further analyses investigated community differences within region and sector differences within each source type. PERMANOVA for each sample source included effects of GBR region, reef sector, and bleaching on Bray-Curtis distances using the same parameter settings as the larger analysis. Communities were visualized using nonmetric multidimensional scaling (NMDS) ordinations. Richness metrics of ITS2 ASVs were summarized using the Chao1 estimator where ASV diversity per sample was calculated as the sum of the number of observed ASVs and the ratio of the number of singletons squared two times the number of doubletons. The Chao1 metric aptly describes microbial community data with a high number of low-abundance taxa with an additional variance normalization step in addition to those in our workflow. The Chao1 richness metric was calculated using the physloseq, plot_richness function (64). Metrics were averaged per ASV, per sample, to account for differences in sampling depth across reefs and sequencing artifacts [a common bias of the ITS2 Symbiodiniaceae marker (55, 65)]. These metrics are only presented on the level of “reef” as the same colonies were not measured before and after bleaching. Statistical significance tests for richness and turnover were run in lmerTest (66) using the lmer function with bleaching and sector set as interactive fixed effects and reef as a random effect. Test of symbiont community diversity, as measured in changes in the number of ASVs per sample, was run using the glmer function in lme4 (67), with region and bleaching as fixed effects and reef as a random effect using a Poisson distribution.
Coral bleaching and DHW exposure
Coral bleaching was observed from the air by researchers from the Australian Research Council Centre of Excellence for Coral Reef Studies at James Cook University during the mass bleaching events of 2016 and 2017 (53). Bleaching was categorized on a scale of “0 to 4,” from minimal (0) to intense bleaching (4). We acknowledge the assumption that the reef-wide metrics (DHW, aerial bleaching surveys) may not accurately reflect the individual coral species-specific responses used in this study. For example, low aerial bleaching scores may have been driven by other species not targeted in this present study. However, more species specific, in-water surveys during this time and region show that all three species were affected significantly [>80% mean bleaching severity; sensu (68)], suggesting that reef-wide metrics may provide some indication of impact at the species level.
The mDHWs were downloaded from the National Oceanic and Atmospheric Administration Coral Reef Watch annual thermal history database (v.2.1) (54). The closest mDHW value to the reef location was identified by minimizing latitudinal and longitudinal distance between the DHW observations and reef locations. mDHW was modeled in a Bayesian framework using a generalized linear mixed model with bleaching score as a fixed effect (mDHW~BleachingScore) in the package brms (69). The model used a gamma likelihood with a log link function. Gaussian priors [N(0, 1)] were used for intercepts and slopes, while a gamma prior was used for the shape parameter of the Gamma likelihood [gamma(1, 0.001)]. The model consisted of two chains of 2000 warmup iterations and 8000 Markov Chain Monte Carlo sampling iterations. Chains were inspected using trace plots for convergence and autocorrelation plots. Given the large variance in mDHW for bleaching score categories 1 and 2, several points had to be excluded from the model. Furthermore, because the 2016 and 2017 bleaching events were extreme, there is only one reef in bleaching category 1 in the dataset after excluding the outliers. To generate a continuous measure to compare reefs, the residual between the observed mDHW and posterior predicted mean mDHW was calculated. Positive residuals indicate that reefs experienced more mDHW than other reefs in a given bleaching category, while negative residuals indicate that a reef experienced less mDHW than other reefs in the same bleaching category. Therefore, reefs with positive residuals experienced mDHW that typically led to more intense bleaching on other reefs. To assess the variability across the four mDHW categories (L-L, L-H, H-H, and H-L), NMDS plots were colored using the four categories, and an analysis of variance (ANOVA)–like permutation test to check for homogeneity of multivariate dispersions (betadisper permutest) was run, followed by an ANOVA using distance matrices (adonis) using the vegan package. Adonis was a secondary test to examine whether differences between groups were due to combined differences in centroid distances and dispersion.
Acknowledgments
We thank the Traditional Owners whose Sea Country these samples were collected from. The samples were collected under permit nos. G12/35236.1 and G41597.1. Animal ethics permits are not required for corals in Australia as they are not classified as “animals” under the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (seventh edition, 2004). We would like to thank C. Nicholas, G. Matthews, S. Noonan, C. Alvarez Roa, J. Mayr, A. Terrell, M. Piu, C. Baird, S. Threlfall, D. Skilton, I. Nicetic, M. Flores, H. Kiff, A. Macadam, J. Randle, D. Yacoub, J. Coote, and R. Py for help in the field or processing of the sediment samples.
Funding: This project is supported with funding from the Australian government’s National Environmental Science Program (NESP) and the Australian Institute of Marine Science.
Author contributions: Conceptualization and design: K.M.Q. and L.K.B. Collection of data with the assistance of other Australian Institute of Marine Science divers and volunteers: V.J.L.M., L.K.B., and K.M.Q. Methodology: K.M.Q., V.J.L.M., and J.H. Investigation: K.M.Q., P.L., and B.R. Visualization: K.M.Q., P.L., and B.R. Writing—original draft: K.M.Q. Writing—review and editing: K.M.Q., V.J.L.M., J.H., B.R., L.K.B., and P.L.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. All correspondence should be directed to K.M.Q. (katemarie.quigley@my.jcu.edu.au). All raw data from this paper can be found in the Sequence Read Archive (SRA) under the BioProject accession PRJNA765385. All code available from K.M.Q.’s project specific Github (https://github.com/LaserKate/MassBleaching_SymbiontsNESP) and archived on https://zenodo.org/badge/latestdoi/560226527 (DOI: 10.5281/zenodo.6100726).
Supplementary Materials
This PDF file includes:
Figs. S1 to S4
Tables S1 to S12
REFERENCES AND NOTES
- 1.LaJeunesse T. C., Parkinson J. E., Gabrielson P. W., Jeong H. J., Reimer J. D., Voolstra C. R., Santos S. R.,Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr. Biol. 28,2570–2580.e6 (2018). [DOI] [PubMed] [Google Scholar]
- 2.G. Muller-Parker, C. F. D’Elia, C. B. Cook, Interactions between corals and their symbiotic algae, in Coral Reefs in the Anthropocene, C. Birkeland, Ed. (Springer, 2015), pp. 99–116. [Google Scholar]
- 3.Hughes T. P., Anderson K. D., Connolly S. R., Heron S. F., Kerry J. T., Lough J. M., Baird A. H., Baum J. K., Berumen M. L., Bridge T. C. L., Claar D. C., Eakin C. M., Gilmour J. P., Graham N. A. J., Harrison H. B., Hobbs J.-P. A., Hoey A. S., Hoogenboom M. O., Lowe R. J., McCulloch M. T., Pandolfi J. M., Pratchett M. S., Schoepf V., Torda G., Wilson S. K.,Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359,80–83 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Shimadzu H., Dornelas M., Magurran A. E.,Measuring temporal turnover in ecological communities. Methods Ecol. Evol. 6,1384–1394 (2015). [Google Scholar]
- 5.Hughes T. P., Kerry J. T., Baird A. H., Connolly S. R., Dietzel A., Eakin C. M., Heron S. F., Hoey A. S., Hoogenboom M. O., Liu G., McWilliam M. J., Pears R. J., Pratchett M. S., Skirving W. J., Stella J. S., Torda G.,Global warming transforms coral reef assemblages. Nature 556,492–496 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Stuart-Smith R. D., Brown C. J., Ceccarelli D. M., Edgar G. J.,Ecosystem restructuring along the Great Barrier Reef following mass coral bleaching. Nature 560,92–96 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Hughes T. P., Kerry J. T., Connolly S. R., Baird A. H., Eakin C. M., Heron S. F., Hoey A. S., Hoogenboom M. O., Jacobson M., Liu G., Pratchett M. S., Skirving W., Torda G.,Ecological memory modifies the cumulative impact of recurrent climate extremes. Nat. Clim. Chang. 9,40–43 (2019). [Google Scholar]
- 8.Kolodny O., Schulenburg H.,Microbiome-mediated plasticity directs host evolution along several distinct time scales. Philos. Trans. R. Soc. Lond. B Biol. Sci. 375,20190589 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webster N. S., Reusch T. B. H.,Microbial contributions to the persistence of coral reefs. ISME J. 11,2167–2174 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Logan C. A., Dunne J. P., Ryan J. S., Baskett M. L., Donner S. D.,Quantifying global potential for coral evolutionary response to climate change. Nat. Clim. Chang. 11,537–542 (2021). [Google Scholar]
- 11.Finney J. C., Pettay D. T., Sampayo E. M., Warner M. E., Oxenford H. A., LaJeunesse T. C.,The relative significance of host–habitat, depth, and geography on the ecology, endemism, and speciation of coral endosymbionts in the genus Symbiodinium. Microb. Ecol. 60,250–263 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Bongaerts P., Riginos C., Ridgway T., Sampayo E. M., van Oppen M. J. H., Englebert N., Vermeulen F., Hoegh-Guldberg O.,Genetic divergence across habitats in the widespread coral Seriatopora hystrix and its associated Symbiodinium. PLOS ONE 5,e10871 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fabina N. S., Putnam H. M., Franklin E. C., Stat M., Gates R. D.,Symbiotic specificity, association patterns, and function determine community responses to global changes: Defining critical research areas for coral-Symbiodinium symbioses. Glob. Chang. Biol. 19,3306–3316 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Quigley K. M., Willis B. L., Bay L. K.,Heritability of the Symbiodinium community in vertically-and horizontally-transmitting broadcast spawning corals. Sci. Rep. 7,8219 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaJeunesse T. C., Pettay D. T., Sampayo E. M., Phongsuwan N., Brown B., Obura D. O., Hoegh-Guldberg O., Fitt W. K.,Long-standing environmental conditions, geographic isolation and host–symbiont specificity influence the relative ecological dominance and genetic diversification of coral endosymbionts in the genus Symbiodinium. J. Biogeogr. 37,785–800 (2010). [Google Scholar]
- 16.Fabina N. S., Putnam H. M., Franklin E. C., Stat M., Gates R. D.,Transmission mode predicts specificity and interaction patterns in coral-Symbiodinium networks. PLOS ONE 7,e44970 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thornhill D. J., LaJeunesse T. C., Kemp D. W., Fitt W. K., Schmidt G. W.,Multi-year, seasonal genotypic surveys of coral-algal symbioses reveal prevalent stability or post-bleaching reversion. Mar. Biol. 148,711–722 (2006). [Google Scholar]
- 18.Claar D. C., Starko S., Tietjen K. L., Epstein H. E., Cunning R., Cobb K. M., Baker A. C., Gates R. D., Baum J. K.,Dynamic symbioses reveal pathways to coral survival through prolonged heatwaves. Nat. Commun. 11,6097 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berkelmans R., van Oppen M. J. H.,The role of zooxanthellae in the thermal tolerance of corals: A “nugget of hope” for coral reefs in an era of climate change. Proc. Royal Soc. B 273,2305–2312 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker A. C.,Reef corals bleach to survive change. Nature 411,765–766 (2001). [DOI] [PubMed] [Google Scholar]
- 21.R. W. Buddemeier, A. C. Baker, D. G. Fautin, J. R. Jacobs, The adaptive hypothesis of bleaching, in Coral Health and Disease (Springer, 2004), pp. 427–444. [Google Scholar]
- 22.Ware J. R., Fautin D. G., Buddemeier R. W.,Patterns of coral bleaching: Modeling the adaptive bleaching hypothesis. Ecol. Model. 84,199–214 (1996). [Google Scholar]
- 23.Bieri T., Onishi M., Xiang T., Grossman A. R., Pringle J. R.,Relative contributions of various cellular mechanisms to loss of algae during cnidarian bleaching. PLOS ONE 11,e0152693 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quigley K. M., Bay L. K., Willis B. L.,Leveraging new knowledge of Symbiodinium community regulation in corals for conservation and reef restoration. Mar. Ecol. Prog. Ser. 600,245–253 (2018). [Google Scholar]
- 25.Quigley K. M., Bay L. K., Willis B. L.,Temperature and water quality-related patterns in sediment-associated Symbiodinium communities impact symbiont uptake and fitness of juveniles in the genus Acropora. Front. Mar. Sci. 4,00401 (2017). [Google Scholar]
- 26.Dilworth J., Caruso C., Kahkejian V. A., Baker A. C., Drury C.,Host genotype and stable differences in algal symbiont communities explain patterns of thermal stress response of Montipora capitata following thermal pre-exposure and across multiple bleaching events. Coral Reefs 40,151–163 (2021). [Google Scholar]
- 27.Voolstra C. R., Valenzuela J. J., Turkarslan S., Cárdenas A., Hume B. C. C., Perna G., Buitrago-López C., Rowe K., Orellana M. V., Baliga N. S., Paranjape S., Banc-Prandi G., Bellworthy J., Fine M., Frias-Torres S., Barshis D. J.,Contrasting heat stress response patterns of coral holobionts across the Red Sea suggest distinct mechanisms of thermal tolerance. Mol. Ecol. 30,4466–4480 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Tonk L., Sampayo E. M., Chai A., Schrameyer V., Hoegh-Guldberg O.,Symbiodinium (Dinophyceae) community patterns in invertebrate hosts from inshore marginal reefs of the southern Great Barrier Reef, Australia. J. Phycol. 53,589–600 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Cooper T. F., Berkelmans R., Ulstrup K. E., Weeks S., Radford B., Jones A. M., Doyle J., Canto M., O’Leary R. A., van Oppen M. J. H.,Environmental factors controlling the distribution of Symbiodinium harboured by the coral Acropora millepora on the Great Barrier Reef. PLOS ONE 6,e25536 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuller Z. L., Mocellin V. J. L., Morris L. A., Cantin N., Shepherd J., Sarre L., Peng J., Liao Y., Pickrell J., Andolfatto P., Matz M., Bay L. K., Przeworski M.,Population genetics of the coral Acropora millepora: Toward genomic prediction of bleaching. Science 369,eaba4674 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Bay L. K., Doyle J., Logan M., Berkelmans R.,Recovery from bleaching is mediated by threshold densities of background thermo-tolerant symbiont types in a reef-building coral. R. Soc. Open Sci. 3,160322 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones A. M., Berkelmans R., van Oppen M. J. H., Mieog J. C., Sinclair W.,A community change in the algal endosymbionts of a scleractinian coral following a natural bleaching event: Field evidence of acclimatization. Proc. Royal Soc. B 275,1359–1365 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baker A. C.,Flexibility and specificity in coral-algal symbiosis: Diversity, ecology, and biogeography of Symbiodinium. Annu. Rev. Ecol. Evol. Syst. 34,661–689 (2003). [Google Scholar]
- 34.De’ath G., Fabricius K. E., Sweatman H., Puotinen M.,The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc. Natl. Acad. Sci. U.S.A. 109,17995–17999 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeCarlo T. M., Harrison H. B.,An enigmatic decoupling between heat stress and coral bleaching on the Great Barrier Reef. PeerJ. 7,e7473 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swain T. D., Lax S., Backman V., Marcelino L. A.,Uncovering the role of Symbiodiniaceae assemblage composition and abundance in coral bleaching response by minimizing sampling and evolutionary biases. BMC Microbiol. 20,124 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams S. D., Patterson M. R.,Resistance and robustness of the global coral–symbiont network. Ecology 101,e02990 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pettay D. T., Wham D. C., Smith R. T., Iglesias-Prieto R., LaJeunesse T. C.,Microbial invasion of the Caribbean by an Indo-Pacific coral zooxanthella. Proc. Natl. Acad. Sci. U.S.A. 112,7513–7518 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LaJeunesse T. C., Smith R. T., Finney J., Oxenford H.,Outbreak and persistence of opportunistic symbiotic dinoflagellates during the 2005 Caribbean mass coral “bleaching” event. Proc. Royal Soc. B 276,4139–4148 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quigley K. M., Willis B. L., Kenkel C. D.,Transgenerational inheritance of shuffled symbiont communities in the coral Montipora digitata. Sci. Rep. 9,13328 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ziegler M., Eguíluz V. M., Duarte C. M., Voolstra C. R.,Rare symbionts may contribute to the resilience of coral–algal assemblages. ISME J. 12,161–172 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boulotte N. M., Dalton S. J., Carroll A. G., Harrison P. L., Putnam H. M., Peplow L. M., van Oppen M. J. H.,Exploring the Symbiodinium rare biosphere provides evidence for symbiont switching in reef-building corals. ISME J. 10,2693–2701 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewis C. L., Coffroth M. A.,The acquisition of exogenous algal symbionts by an octocoral after bleaching. Science 304,1490–1492 (2004). [DOI] [PubMed] [Google Scholar]
- 44.Hughes T. P., Kerry J. T., Connolly S. R., Álvarez-Romero J. G., Eakin C. M., Heron S. F., Gonzalez M. A., Moneghetti J.,Emergent properties in the responses of tropical corals to recurrent climate extremes. Curr. Biol. 31,5393–5399.e3 (2021). [DOI] [PubMed] [Google Scholar]
- 45.Romero-Torres M., Acosta A., Palacio-Castro A. M., Treml E. A., Zapata F. A., Paz-García D. A., Porter J. W.,Coral reef resilience to thermal stress in the Eastern Tropical Pacific. Glob. Chang. Biol. 26,3880–3890 (2020). [DOI] [PubMed] [Google Scholar]
- 46.Quigley K. M., Willis B. L., Bay L. K.,Maternal effects and Symbiodinium community composition drive differential patterns in juvenile survival in the coral Acropora tenuis. R. Soc. Open Sci. 3,160471 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wright R. M., Kenkel C. D., Dunn C. E., Shilling E. N., Bay L. K., Matz M. V.,Intraspecific differences in molecular stress responses and coral pathobiome contribute to mortality under bacterial challenge in Acropora millepora. Sci. Rep. 7,2609 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.K. M. Quigley, B. Ramsby, P. Laffy, V. Mocellin, J. Harris, L. Bay, The Genetic Traits of Corals that Survived Recent Bleaching Events (Report to the National Environmental Science Program. Reef and Rainforest Research Centre Limited, Cairns, 2021).
- 49.Loya Y., Sakai K., Yamazato K., Nakano Y., Sambali H., van Woesik R.,Coral bleaching: The winners and the losers. Ecol. Lett. 4,122–131 (2001). [Google Scholar]
- 50.van Woesik R., Sakai K., Ganase A., Loya Y.,Revisiting the winners and the losers a decade after coral bleaching. Mar. Ecol. Prog. Ser. 434,67–76 (2011). [Google Scholar]
- 51.Pratchett M. S., McWilliam M. J., Riegl B.,Contrasting shifts in coral assemblages with increasing disturbances. Coral Reefs 39,783–793 (2020). [Google Scholar]
- 52.Callahan B. J., McMurdie P. J., Rosen M. J., Han A. W., Johnson A. J. A., Holmes S. P.,DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13,581–583 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hughes T. P., Kerry J. T., Álvarez-Noriega M., Álvarez-Romero J. G., Anderson K. D., Baird A. H., Babcock R. C., Beger M., Bellwood D. R., Berkelmans R., Bridge T. C., Butler I. R., Byrne M., Cantin N. E., Comeau S., Connolly S. R., Cumming G. S., Dalton S. J., Diaz-Pulido G., Eakin C. M., Figueira W. F., Gilmour J. P., Harrison H. B., Heron S. F., Hoey A. S., Hobbs J.-P. A., Hoogenboom M. O., Kennedy E. V., Kuo C.-y., Lough J. M., Lowe R. J., Liu G., Mc Culloch M. T., Malcolm H. A., Mc William M. J., Pandolfi J. M., Pears R. J., Pratchett M. S., Schoepf V., Simpson T., Skirving W. J., Sommer B., Torda G., Wachenfeld D. R., Willis B. L., Wilson S. K.,Global warming and recurrent mass bleaching of corals. Nature 543,373–377 (2017). [DOI] [PubMed] [Google Scholar]
- 54.K. Saha, X. Zhao, H.-m. Zhang, K. S. Casey, D. Zhang, Y. Zhang, S. Baker-Yeboah, J. M. Relph, A. Krishnan, T. Ryan, The Coral Reef Temperature Anomaly Database (CoRTAD) Version 6 - Global, 4 km Sea Surface Temperature and Related Thermal Stress Metrics for 1982 to 2019 (NOAA National Centers for Environmental Information, 2018).
- 55.Pochon X., Putnam H. M., Burki F., Gates R. D.,Identifying and characterizing alternative molecular markers for the symbiotic and free-living dinoflagellate Genus Symbiodinium. PLOS ONE 7,e29816 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cunning R., Yost D. M., Guarinello M. L., Putnam H. M., Gates R. D.,Variability of Symbiodinium communities in waters, sediments, and corals of thermally distinct reef pools in American Samoa. PLOS ONE 10,e0145099 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pochon X., Pawlowski J., Zaninetti L., Rowan R.,High genetic diversity and relative specificity among Symbiodinium-like endosymbiotic dinoflagellates in soritid foraminiferans. Mar. Biol. 139,1069–1078 (2001). [Google Scholar]
- 58.Quigley K. M., Davies S. W., Kenkel C. D., Willis B. L., Matz M. V., Bay L. K.,Deep-sequencing method for quantifying background abundances of Symbiodinium types: Exploring the rare Symbiodinium biosphere in reef-building corals. PLOS ONE 9,e94297 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.S. Davies, M. H. Gamache, L. I. Howe-Kerr, N. G. Kriefall, A. C. Baker, A. T. Banaszak, L. K. Bay, A. J. Bellantuono, D. Bhattacharya, C. X. Chan, D. C. Claar, M. A. Coffroth, R. Cunning, S. K. Davy, Javier del Campo, E. M. Diaz-Almeyda, J. C. Frommlet, L. E. Fuess, R. A. Gonzalez-Pech, T. L. Goulet, K. D. Hoadley, E. J. Howells, B. C. C. Hume, D. W. Kemp, Carly, D. Kenkel, S. A. Kitchen, Todd C. La Jeunesse, S. Lin, S. M. Ilroy, R. M. Minds, M. R. Nitschke, C. A. Oakley, R. S. Peixoto, C. Prada, H. M. Putnam, K. Quigley, H. G. Reich, J. D. Reimer, M. Rodriguez-Lanetty, S. Rosalas, O. S. Saad, E. M. Sampayo, S. Santos, E. Shoguchi, E. G. Smith, M. Stat, T. G. Stephens, M. E. Strader, D. J. Suggett, T. D. Swain, C. Tran, N. Traylor-Knowles, C. R. Voolstra, M. E. Warner, V. M. Weis, R. Wright, T. Xiang, H. Yamashita, M. Ziegler, A. M. S. Correa, J. E. Parkinson, Building consensus around the assessment and interpretation of Symbiodiniaceae diversity. [Preprints]. 21 June 2022. 10.20944/preprints202206.0284.v1. [DOI]
- 60.Hume B. C. C., Smith E. G., Ziegler M., Warrington H. J. M., Burt J. A., LaJeunesse T. C., Wiedenmann J., Voolstra C. R.,SymPortal: A novel analytical framework and platform for coral algal symbiont next-generation sequencing ITS-2 profiling. Mol. Ecol. Resour. 19,1063–1080 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Howe-Kerr L. I., Bachelot B., Wright R. M., Kenkel C. D., Bay L., Correa A. M. S.,Symbiont community diversity is more variable in corals that respond poorly to stress. Glob. Chang. Biol. 26,2220–2234 (2020). [DOI] [PubMed] [Google Scholar]
- 62.Frøslev T. G., Kjøller R., Bruun H. H., Ejrnæs R., Brunbjerg A. K., Pietroni C., Hansen A. J.,Algorithm for post-clustering curation of DNA amplicon data yields reliable biodiversity estimates. Nat. Commun. 8,1188 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.J. Oksanen, F. G. Blanchet, R. Kindt, P. Legendre, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, H. Wagner, Package ‘vegan.’ Community Ecology Package, Version, in R Package Version 2.2-1 (2013), vol. 2.
- 64.McMurdie P. J., Holmes S.,phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLOS ONE 8,e61217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi T., Niu G., Kvitt H., Zheng X., Qin Q., Sun D., Tchernov D.,Untangling ITS2 genotypes of algal symbionts in zooxanthellate corals. Mol. Ecol. Resour. 21,137–152 (2021). [DOI] [PubMed] [Google Scholar]
- 66.Kuznetsova A., Brockhoff P. B., Christensen R. H. B.,lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 82,1–26 (2017). [Google Scholar]
- 67.D. Bates, M. Maechler, B. Bolker, S. Walker, lme4: Linear mixed-effects models using Eigen and S4, in R Package Version (2014), vol. 1.
- 68.Hoogenboom M. O., Frank G. E., Chase T. J., Jurriaans S., Álvarez-Noriega M., Peterson K., Critchell K., Berry K. L. E., Nicolet K. J., Ramsby B., Paley A. S.,Environmental drivers of variation in bleaching severity of Acropora species during an extreme thermal anomaly. Front. Mar. Sci. 376,00376 (2017). [Google Scholar]
- 69.Bürkner P.-C.,brms: An R package for Bayesian multilevel models using Stan. J. Stat. Softw. 80,1–28 (2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S4
Tables S1 to S12