Abstract
Antioxidant (AOX) capacity assays are important analytical tools, used worldwide to measure the AOX capacities of various food commodities. Although numerous protocols have been published to ascertain AOX capacities, there are increasing concerns about the reliability of many of these assays. Poor correlation of results between various assays, as well as problems with reproducibility, consistency, and accuracy, is to blame. Published AOX assays also differ markedly from each other by employing different reaction conditions, using different extracting solvents, and applying dissimilar quantification methods. In this study, AOX capacities of a range of fruit, vegetables, and spices, commonly consumed and of commercial importance in Australia and worldwide, were measured in both hydrophilic and lipophilic solvents by using two different assay systems. As the polyphenolic compounds present in any sample matrix are the main contributors to its AOX properties, the commodities were also analysed for total phenolic content (TPC), again using both solvent systems. Analysis of the results from the current study with values from the published literature exposed the challenges that make direct comparison of any quantitative results difficult. However, a strong mutual correlation of our assay results facilitated a meaningful comparison of the data within the laboratory. Concurrent use of lipophilic and hydrophilic solvents made the results more reliable and understandable. Findings from this study will aid to address the existing challenges and bring a more rational basis to the AOX capacities. This unique analytical approach also provided a platform to build an internal reference database for the commonly consumed and commercially important food commodities with the potential to broaden the scope into a database for similar food matrices.
1. Introduction
Antioxidants (AOXs), the molecules that fight against harmful free radicals and protect cells, are considered essential for the survival of all living things. This in turn has led to a rising awareness within the scientific community of the need to develop simple but effective assays that measure the antioxidant (AOX) properties of foods. AOX capacity assays are important analytical tools, used worldwide to measure the AOX capacities of various food commodities. In principle, the assay involves a chemical reaction which allows AOXs present in the sample to react with a set concentration of the assay reagent. The course of the reaction is monitored and the consumption of the assay reagent by the sample extract is measured instrumentally. The subsequent readings can then be interpreted as AOX capacity values. Though the procedure appears simple in principle, most assays involve many complex and diverse reaction mechanisms [1, 2]. One of the main challenges in the development of a universal AOX capacity assay is that, within any sample matrix, there are numerous AOXs, which can react with a particular assay reagent in different ways based on their molecular structures and physicochemical properties [1]. Examples of chemical reactions between AOXs and an assay reagent are hydrogen atom transfer (HAT), single electron transfer (SET), and the chelation with transition metals, which are largely influenced by assay reaction conditions such as time, pH, temperature, and reaction medium (solvent) [1–3].
To study the AOX capacity of the commonly consumed fruit, vegetable, and spices, the commonly employed AOX assay systems are Oxygen Radical Absorbance Capacity (ORAC), Total Radical Trapping AOX Parameter (TRAP), 2,2-diphenyl-1-picrylhydrazyl (DPPH), Vitamin C Equivalent AOX Capacity (VCEAC), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), Trolox Equivalent AOX Capacity (TEAC), Ferric Ion Reducing AOX Power (FRAP), and Cupric Ion Reducing AOX Capacity (CUPRAC). Having a specific or combined AOX target within a sample matrix, each AOX assay system exhibits its own distinct advantages and disadvantages [1, 2]. Clearly, matching assay reagent and system characteristics to the AOX reaction mechanisms are critical in the selection of appropriate AOX assay methods. Furthermore, AOX compounds present in food-based matrices can be either water-soluble (hydrophilic) or fat-soluble (lipophilic) [2–4]. So, it is very important that the selected assay can incorporate the determination of both the lipophilic and hydrophilic components if a meaningful assessment of the total AOX capacity is to be made. Because multiple reaction mechanisms are usually involved, no single assay will accurately reflect all AOXs in a mixed or complex sample. It must be understood from the outset that no two assays will necessarily produce the same value for a given AOX compound [5] and that there is no simple universal method by which AOX capacity can be measured accurately and quantitatively [1]. It is difficult to compare the quantitative results obtained by different AOX assay methods because of the diverse range of mechanisms, solvents used, and reaction conditions of the various assays [2]. Compounding the issue is the expression of results in different units (μmol, mmol, or mg of the reference standard equivalent per g, 100 g, or kg of the sample on a fresh or dry weight basis). Since there is no single universal method to unequivocally measure AOX capacities, the technical report of International Union of Pure and Applied Chemistry (IUPAC) [2] highly recommends measuring the AOX capacity of any given sample by two independent assay systems to ensure meaningful comparisons are made within a laboratory.
It is also recommended by most researchers that the total phenolic content (TPC) be ascertained to complement the AOX capacity assay due to the belief that the polyphenolics present in any food-based sample matrix are the main contributors to its AOX properties [4, 6]. However, the conventional Folin-Ciocalteu total polyphenolic content (FC-TPC) assay [7] traditionally used to measure the TPC is not compatible with lipophilic polyphenolic molecules because increased concentrations of lipophilic extracting solvents like ethanol can lead to the precipitation of the assay reagent [3, 8, 9]. Thus, the use of hydrophilic solvents alone can result in the exclusion of lipophilic polyphenols and their contribution in the TPC assay.
Assay systems addressing the above-mentioned shortcomings in the application of the AOX capacity and TPC assays were not available at the time of the current study. It is essential to have reliable methods established in any analytical laboratory to utilise these useful assays to produce meaningful results. It is also important to establish a reference database for a wide range of sample matrices to make meaningful comparisons.
Therefore, the aim of this work was to overcome the shortcomings in applying the AOX capacity and TPC assays, to address challenges that prevent meaningful comparisons with the published values, and to apply the improved assay conditions to a range of fruit, vegetables, and spices commonly consumed in Australia and worldwide (sample details are given under Section 2.1). The authors of this study chose a modified ABTS assay by Ozgen et al. [10] with assay reaction conditions at pH 4.5 (this has the advantage of increased stability for the assay reagents as well as the AOXs and ascorbic acid (AA) reference standards), a CUPRAC assay based on Apak et al. [11] (which has the advantages of better reagent stability than radical reagents like ABTS, a neutral pH of 7.0 that is close to the biological systems, and compatibility towards hydrophilic and lipophilic solvents), and a modified FC-TPC assay based on Pereira et al. [12] with reaction conditions at pH 11.5 (which has the benefit of better compatibility towards lipophilic solvent systems than the conventional method by Singleton and Rossi [7]). Ultrapure (Milli-Q) water was selected as the hydrophilic extracting solvent, whereas 40% ethanol in Milli-Q water was employed as the lipophilic extracting solvent. Suitability of the selected assays within our laboratory conditions was investigated. Using the selected methodologies and solvent systems, the AOX capacity and TPC of fruit, vegetables, and spices were evaluated. The scope of expansion of results to a reference database was also investigated.
2. Materials and Methods
2.1. Materials
The samples (collected between October 2020 and January 2021) consisted of (1) fruit–duplicate punnets of blueberries (CO_OP Ltd, OzGroup), blackberries (Driscoll's), raspberries (the berry collective raspberries), and cherries (Cherry Isle, Tasmania PLU, 4045) purchased from various supermarkets in Brisbane, Queensland, Australia; pale and red coloured strawberry varieties (identified as MRF, 2020, 217-230 and MRF, 2020, 2017-139, respectively) from Maroochy Research Facility (MRF) of the Queensland Department of Agriculture and Fisheries (DAF); a plum variety (identified as ARF, 2020, 401-43, a relative of Queen Garnet plum) from Applethorpe Research Facility (ARF) of DAF; and a commercial sample of Queen Garnet plum freeze-dried powder from Nutrafruit Pty Ltd, Australia (purchased in June 2018); (2) fruit waste–papaya seeds separated from fruit purchased from a local supermarket and pomegranate seeds and husks separated from fruit (POM and SunnyGem, 3127 USA) purchased from a local supermarket; (3) vegetables–green, yellow, and red capsicums (Australian Mini Capsicums) purchased in duplicate from various local supermarkets; pumpkin (Kent cut, Australian Grown) purchased in duplicate from local supermarkets; and fresh whole tomatoes purchased from local markets; and (4) spices–turmeric powder, ginger powder, cocoa powder, and green tea (Twinings of London) bags, all purchased from various local stores. Specific information about the varieties of fruit, vegetables, and spices sourced from supermarkets was not available.
Analytical grade reference standards of L-ascorbic acid (AA), gallic acid (GA), and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) were purchased from Sigma-Aldrich, Australia. All other reagents and chemicals used (copper (II) chloride dihydrate, neocuproine, ABTS, potassium persulfate, methyl-beta-cyclodextrin (mβCD), sodium acetate, ammonium acetate, Folin-Ciocalteu (FC) reagent, sodium carbonate, and ethanol) were of high-quality analytical grade obtained from Sigma-Aldrich, Australia, and the water used was ultrapure Milli-Q from Merck-Millipore.
2.2. Methods
2.2.1. Sample Preparation
Samples were prepared for extraction based on the collective information gathered from multiple references listed in Table 1. The edible portions of the berries, capsicum, cherry, plum, and pumpkin were separately hand chopped into small pieces, freeze-dried for seven days, and finally milled in a ball mill (Retsch MM400) to get uniform powders. Samples of papaya seeds, pomegranate seeds, and husks were separately dried in an oven (Gallenkamp, Oven 300) at 40°C for 24 hours and milled in a ball mill (Retsch MM400) to get uniform powders. Green tea, turmeric powder, ginger powder, cocoa powder, and commercial Queen Garnet plum freeze-dried powder were used as received in commercial packaging. Fresh whole tomatoes were pureed using a domestic blender to get a consistent sample. All the resulting samples were quickly transferred to 50 mL centrifuge tubes and stored at -80°C till the time of analysis.
Table 1.
Extraction solvents as presented in the references.
| Reference | Samples tested | Extraction solvents |
|---|---|---|
| 2 | Green tea | Steeping in boiling water |
| 16 | Blueberry, blackberry, raspberry, strawberry | Not given |
| 18 | Blueberry | Acidified methanol |
| 19 | Blueberry, blackberry, raspberry | Aqueous methanol-acetone extraction followed by evaporation, reextraction in diethyl ether-ethyl acetate, final evaporation, and redissolution in methanol |
| 20 | Blueberry, blackberry, raspberry | Multiple extractions using methanol |
| 21 | Blueberry, blackberry, raspberry, cherry, strawberry | 50 : 50 methanol-water extraction followed by 70 : 30 acetone-water extraction |
| 22 | Blueberry, blackberry, strawberry | 80% methanol |
| 23 | Blueberry | 60 : 40 ethanol-water |
| 24 | Raspberry, strawberry, plum, tomato | Methanol, n-hexane, varying solvents |
| 25 | Plum | Multiple extractions using 90% methanol |
| 26 | Plum | Methanolic extraction followed by 80% methanol, evaporation, and redissolution in methanol |
| 29 | Pumpkin | Multiple extractions using 80% methanol |
| 30 | Pumpkin | Various solvents, solvent combinations, and conditions |
| 32 | Tomato | Multiple extractions using 75% methanol in water |
| 33 | Tomato, capsicum | Multiple extractions using 75% methanol in water |
| 34 | Capsicum | Multiple extractions using various solvents and conditions |
| 35 | Papaya seeds | Absolute methanol and 80 : 20 methanol-water |
| 36 | Papaya seeds | Subcritical water extraction and conventional soxhlet extraction using water |
| 37 | Pomegranate husks | Multiple extractions using 75% aqueous methanol including 0.1% (v/v) formic acid |
| 38 | Pomegranate husks | Compared many different extractions solvents, their combinations and conditions |
| 39 | Papaya seeds | Multiple extractions of 95% ethanolic extract after partitioning with water, ethanol extract with petroleum ether, and ethyl acetate |
| 40 | Pomegranate husks | Decoction in boiling water |
| 41 | Pomegranate seeds, pomegranate husks | Multiple extractions using 80% methanol in water, evaporated and separate hydrolysis followed by extraction with 100% methanol containing 0.1% formic acid |
| 42 | Pomegranate husks | 96% ethanol |
| 47 | Turmeric powder | Boiling water |
| 48 | Turmeric powder, ginger powder | Water and 50% ethanol-water at various conditions |
| 49 | Turmeric powder | Methanol, water, and ethanol |
| 50 | Turmeric | Expressed juice |
| 51 | Turmeric powder | Separate multiple extractions using 80% ethanol, 80% methanol, 80% acetone, and water |
| 52 | Turmeric powder | Dimethyl formamide |
| 53 | Ginger powder | 80% methanol |
| 54 | Ginger powder | Separate extractions using 80% ethanol and water followed by solvent removal |
| 56 | Ginger powder | Multiple extractions using methanol followed by evaporation and redissolution in methanol |
| 57 | Cocoa powder | Separate extractions using water and 70% methanol after removing fat with n-hexane |
| 58 | Green tea | 98°C boiling water |
| 59 | Green tea | Steeping in hot water at different temperatures |
| 60 | Green tea | Steeping in boiling water |
| 61 | Green tea | Steeping in freshly boiled water |
| 62 | Green tea | Multiple extractions using 80% methanol |
2.2.2. Moisture Content
The moisture content of the prepared samples was determined from the weight loss after drying to constant weight at 70°C under vacuum (AOAC) [13]. Duplicates of approximately 0.2 g of the samples (except 2.0 g for fresh tomato) were tested in this manner. The percentage difference in weight loss after drying was expressed as the moisture content in g/100 g sample.
2.2.3. Sample Extraction
Samples were extracted based on the collective information gathered from multiple references listed in Table 1. Milli-Q water was selected as the hydrophilic extracting solvent, whereas 40% ethanol in Milli-Q water was employed as the lipophilic extracting solvent. mβCD is used as a solubility enhancer to bring hydrophilic and lipophilic components together.
Triplicates of known weights of the prepared samples were separately extracted in different solvent systems that were proven to be suitable for the hydrophilic and lipophilic components present in the samples and compatible with the three assay systems.
Hydrophilic solvent extraction (marked as Milli-Q water for solvents in tables): approximately 0.2 g of sample was accurately weighed into a 15 mL centrifuge tube. 10 mL of Milli-Q water was added and the tubes were vortexed for 8-10 seconds for homogenous mixing. The resulting mixture was sonicated for 10 min with occasional manual shaking and centrifuged for 5 min at 4000 rpm at 20°C
Lipophilic solvent extraction (marked as 40% ethanol for solvents in tables): approximately 0.2 g of sample was accurately weighed into a 15 mL centrifuge tube. 10 mL of 40% ethanol was added and the tubes were vortexed for 8-10 seconds for homogenous mixing. The resulting mixture was sonicated for 10 min with occasional manual shaking and centrifuged for 5 minutes at 4000 rpm at 20°C
Extracts in the presence of methyl-beta-cyclodextrin (marked as mβCD for solvents in Tables 2 and 3): the lipophilic extract in 40% ethanol was modified by diluting it 1 : 1 with 7% w/v mβCD in Milli-Q water. mβCD is used as a solubility enhancer to bring hydrophilic and lipophilic components together
Table 2.
Range of AOX and TPC values of the samples in different solvent systems in comparison with the reported values after unit conversions.
| Sample | Solvent | ABTS μmol AA E/g DW | ɸ ABTS μmol AAE/g DW (∗reference) | CUPRAC μmol TE/g DW | ɸ CUPRAC μmol TE/g DW (∗reference) | FC-TPC μmol GAE/g DW | ɸ FC-TPC μmol GAE/g DW (∗reference) |
|---|---|---|---|---|---|---|---|
| Blueberry | Milli-Q water | 65.7-73.3 | 134-231 [14] 370.8 [15] 269.8 [16] 67.9 [17] 212.9 [18] 180.4 [19] |
56.8-62.2 | 100-120 [20] | 50.9-56.1 | 9.3-163.7 [21] 107-272 [14] 84.1 [15] 114.9 [16] 145.9 [17] 55.5 [18] 143-179 [20] 179-206 [20] |
| 40% ethanol | 131.1-157.9 | 148.3-168.5 | 72.4-85.4 | ||||
| mßCD | 209.2-240.9 | 152.0-172.4 | 57.9-73.5 | ||||
|
| |||||||
| Blackberry | Milli-Q water | 174.3-192.2 | 683.1 [15] 225.4 [16] 155.6 [17] 163.1 [18] |
157.9-204.5 | _ | 96.2-114.3 | 2.9-40.6 [21] 106.5 [15] 99.9 [16] 413.9 [17] 32.8 [18] |
| 40% ethanol | 207.6-228.6 | 197.6-233.6 | 70.4-89.9 | ||||
| mßCD | 248.4-298.4 | 197.6-257.5 | 61.7-84.1 | ||||
|
| |||||||
| Raspberry | Milli-Q water | 75.4-92.1 | 472.3 [15] 297.5 [16] 8.2 [17] |
60.9-86.5 | 184 [22] | 50.2-62.2 | 6.7-107.1 [21] 34.0 [15] 118.4 [16] 184.5 [17] 89 [22] |
| 40% ethanol | 112.5-142.4 | 118.3-153.0 | 54.0-63.7 | ||||
| mßCD | 124.6-162.1 | 118.7-169.6 | 42.3-55.4 | ||||
|
| |||||||
| Cherry | Milli-Q water | 80.2-96.4 | 92.5 [17] | 65.2-94.2 | _ | 56.3-70.1 | 136.2 [17] |
| 40% ethanol | 94.7-135.7 | 109.4-126.3 | 52.7-67.7 | ||||
| mßCD | 108.9-141.4 | 113.3-135.8 | 36.9-57.7 | ||||
|
| |||||||
| Strawberry; MRF, 2020, 217-230 | Milli-Q water | 121.6-130.2 | 153.2 [17] 63.1 [18] 103.5 [19] |
106.1-140.4 | 259 [22] | 95.0-99.4 | 18.6-42.1 [21] 500.8 [17] 16.0 [18] 129 [22] |
| 40% ethanol | 147.4-149.2 | 144.6-153.0 | 82.8-90.2 | ||||
| mßCD | 210.3-225.0 | 146.5-155.4 | 70.3-79.1 | ||||
| Strawberry; MRF, 2020, 2017-139 | Milli-Q water | 138.6-163.8 | 130.5-131.8 | 89.4-111.5 | |||
| 40% ethanol | 176.4-183.6 | 283.7-304.8 | 89.1-99.8 | ||||
| mßCD | 235.5-243.5 | 259.7-289.8 | 86.7-91.9 | ||||
|
| |||||||
| Queen Garnet plum commercial FDP | Milli-Q water | 215.2-223.1 | 140.8 [19] | 201.0-220.2 | 182 [16] 65-206 [23] |
164.9-171.4 | 121 [22] 25-62 [23] |
| 40% ethanol | 286.1-296.1 | 336.4-364.3 | 163.3-165.2 | ||||
| mßCD | 352.3-366.2 | 265.2-334.6 | 144.6-148.5 | ||||
| Queen Garnet plum sibling ARF, 2020, 401-43 | Milli-Q water | 199.5-211.1 | 175.7-180.5 | 133.8-140.9 | |||
| 40% ethanol | 257.8-267.2 | 267.1-273.9 | 131.5-134.0 | ||||
| mßCD | 314.2-325.3 | 274.6-303.7 | 113.4-115.6 | ||||
|
| |||||||
| Green capsicum | Milli-Q water | 41.1-62.5 | 29.4-295 [24] | 35.2-74.2 | 93.6-98.8 [25] 64.8-188.4 [24] 65.7 [25] |
61.8-86.0 | 63.7-94.5 [25] 23.5-130.4 [24] |
| 40% ethanol | 55.8-69.1 | 55.8-89.4 | 47.6-65.1 | ||||
| mßCD | 47.1-65.6 | 46.7-91.7 | 42.8-62.2 | ||||
| Yellow capsicum | Milli-Q water | 103.3-117.7 | 101.8-110.1 | 114.2-128.1 | |||
| 40% ethanol | 100.8-110.6 | 95.1-114.8 | 74.6-89.8 | ||||
| mßCD | 114.2-123.1 | 86.1-106.5 | 101.8-109.1 | ||||
| Red capsicum | Milli-Q water | 88.4-99.7 | 84.1-100.9 | 97.8-105.6 | |||
| 40% ethanol | 77.9-92.1 | 76.1-107.2 | 51.8-73.6 | ||||
| mßCD | 79.3-111.9 | 89.2-108.4 | 67.4-99.8 | ||||
|
| |||||||
| Pumpkin | Milli-Q water | 19.7-26.2 | 4.5-7.2 [26] 3.2-4.19 [26] |
11.2-24.1 | 3.97-10.25 [27] | 19.6-27.4 | 5.49-13.98 [26] 5.48-10.98 [26] |
| 40% ethanol | 21.5-26.0 | 11.2-18.3 | 12.9-17.5 | ||||
| mßCD | 18.4-23.1 | 9.1-19.0 | 5.8-8.9 | ||||
|
| |||||||
| Tomato | Milli-Q water | 30.8-33.2 | 24.5 [28] | 22.9-23.6 | 16.6 [28] | 22.1-25.0 | 27.8 [25] 27.1 [22] 16.1 [28] |
| 40% ethanol | 32.0-33.6 | 19.8-20.3 | 22.2-23.8 | ||||
| mßCD | 45.3-48.0 | 19.3-19.9 | 22.1-24.7 | ||||
|
| |||||||
| Papaya seeds | Milli-Q water | 35.5-41.1 | _ | 267.7-272.8 | _ | 24.0-27.1 | 56.5-426.5 [29, 30] 16.3 [31] 66.5 [31] |
| 40% ethanol | 32.1-34.0 | 277.9-283.0 | 15.8-16.8 | ||||
| mßCD | 33.4-33.9 | 207.8-226.5 | 21.3-26.2 | ||||
|
| |||||||
| Pomegranate seeds | Milli-Q water | 10.6-11.0 | _ | 13.7-19.5 | 239 [32] | 3.0-9.5 | 82 [32] |
| 40% ethanol | 16.8-17.8 | 17.0-18.6 | 9.1-9.4 | ||||
| mßCD | 15.3-16.4 | 17.2-20.3 | 4.5-4.8 | ||||
|
| |||||||
| Pomegranate husks | Milli-Q water | 1381.9-1502.6 | _ | 936.4-1126.3 | 2080-8090 [33] 1118 [32] 663-3428 [34] 3756 [35] |
477.2-517.3 | 623-1545 [33] 423 [32] 175-1060 [34] 497 [36] |
| 40% ethanol | 1532.8-1636.4 | 1440.2-1493.0 | 433.5-578.9 | ||||
| mßCD | 1905.8-2035.2 | 1230.2-1267.5 | 314.9-372.5 | ||||
|
| |||||||
| Turmeric powder | Milli-Q water | 45.3-49.1 | _ | 7.6-7.8 | 600-800 [37] | 15.8-16.9 | 24.6 [38] 4.5 [39] 39.9 [40] 4.0 [41] 1011.6 [42] 22.3 [42] |
| 40% ethanol | 327.0-334.2 | 154.6-166.9 | 132.7-137.8 | ||||
| mßCD | 124.5-136.8 | 124.5-136.8 | 173.1-207.4 | ||||
|
| |||||||
| Ginger powder | Milli-Q water | 37.7-46.9 | 130.6-398.6 [43] | 32.2-34.7 | 229.3-487.0 [43] | 5.6-7.7 | 490.2-794.8 [43] 310.6-808.5 [44] 13.5-99.2 [45] 4.0 [39] |
| 40% ethanol | 50.7-57.3 | 48.2-54.4 | 119.2-152.4 | ||||
| mßCD | 64.9-65.1 | 49.5-58.8 | 133.6-152.6 | ||||
|
| |||||||
| Cocoa powder | Milli-Q water | 425.5-436.6 | _ | 328.8-333.9 | _ | 184.2-191.5 | 17-199 [46] |
| 40% ethanol | 437.8-448.7 | 403.7-421.1 | 189.3-193.4 | ||||
| mßCD | 385.8-422.7 | 414.5-450.0 | 292.0-301.1 | ||||
|
| |||||||
| Green tea | Milli-Q water | 2127.3-2148.8 | _ | 1305.0-1326.0 | 1000 [2] 50-4410 [47] 1010 [48] |
609.8-618.5 | 870-1481.3 [49] 305.7 - 687.7 [50] 336.6-605.5 [51] |
| 40% ethanol | 1671.6-1675.3 | 1240.3-1257.9 | 555.6-560.7 | ||||
| mßCD | 1029.8-1122.2 | 772.2-813.4 | 579.1-630.1 | ||||
ɸ Reported values after unit conversions. ∗Reference.
Table 3.
Correlation of three assays in three different solvent systems (∗).
(a).
| MQ-water | 40% ethanol | mßCD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ABTS | CUPRAC | TPC | ABTS | CUPRAC | TPC | ABTS | CUPRAC | TPC | ||
| MQ-water | ABTS | 1.0000 | ||||||||
| CUPRAC | 0.9852 | 1.0000 | ||||||||
| TPC | 0.9779 | 0.9742 | 1.0000 | |||||||
|
| ||||||||||
| 40% ethanol | ABTS | 0.9758 | 0.9701 | 0.9677 | 1.0000 | |||||
| CUPRAC | 0.9411 | 0.9679 | 0.9526 | 0.9773 | 1.0000 | |||||
| TPC | 0.9634 | 0.9511 | 0.9615 | 0.9844 | 0.9571 | 1.0000 | ||||
|
| ||||||||||
| mßCD | ABTS | 0.8578 | 0.8813 | 0.8887 | 0.9376 | 0.9634 | 0.9154 | 1.0000 | ||
| CUPRAC | 0.8727 | 0.9127 | 0.9075 | 0.9376 | 0.9822 | 0.9172 | 0.9815 | 1.0000 | ||
| TPC | 0.9230 | 0.8900 | 0.9011 | 0.9065 | 0.8428 | 0.9402 | 0.7463 | 0.7761 | 1.0000 | |
(b).
| MQ-water | 40% ethanol | mßCD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ABTS | CUPRAC | TPC | ABTS | CUPRAC | TPC | ABTS | CUPRAC | TPC | ||
| MQ-water | ABTS | 1.0000 | ||||||||
| CUPRAC | 0.7953 | 1.0000 | ||||||||
| TPC | 0.8892 | 0.7215 | 1.0000 | |||||||
|
| ||||||||||
| 40% ethanol | ABTS | 0.8309 | 0.5649 | 0.6864 | 1.0000 | |||||
| CUPRAC | 0.8138 | 0.8834 | 0.7282 | 0.7835 | 1.0000 | |||||
| TPC | 0.7514 | 0.4628 | 0.6529 | 0.8632 | 0.6691 | 1.0000 | ||||
|
| ||||||||||
| mßCD | ABTS | 0.8492 | 0.6086 | 0.7806 | 0.9399 | 0.8286 | 0.8419 | 1.0000 | ||
| CUPRAC | 0.8844 | 0.8767 | 0.7774 | 0.8202 | 0.9825 | 0.6999 | 0.8708 | 1.0000 | ||
| TPC | 0.7628 | 0.4862 | 0.5845 | 0.8551 | 0.6219 | 0.9277 | 0.7346 | 0.6566 | 1.0000 | |
n = 20. ∗The values in (b) are the correlations taken (n = 18) without considering green tea and pomegranate husk sample results due to the limited sample numbers and their very high assay results.
Similarly, triplicate 0.2 g samples of the commodities that contain carotenoids (capsicum, tomato, and pumpkin) were separately extracted in both 100% ethanol and dichloromethane due to their high hydrophobic nature.
All the sample extracts were further diluted with matching solvents to obtain acceptable absorbance values that fell within the reference standard concentration range of the respective assays.
2.2.4. Assay Conditions
(1) ABTS Assay. The assay was based on Ozgen et al. [10]. To a disposable glass tube was added 1 mL of the ABTS working solution and allowed to stabilise at 28°C in a water bath for 10 min. 50 μL of diluted sample extract or AA standard solution (freshly prepared in Milli-Q water immediately before the addition, with concentrations ranging from 100 μM to 700 μM) was added to the tube, vortexed, and allowed to incubate for 60 min at 28°C. The absorbance at 734 nm was recorded using a Shimadzu UV 1280 spectrophotometer (Shimadzu, Australia). A standard calibration curve was constructed by plotting absorbance versus the concentration of the AA standards. The AOX capacity of the sample was calculated from the slope of the calibration curve using equations (1) and (2) and expressed as μmol AA equivalents (AAE)/g of sample.
| (1) |
| (2) |
where y is the absorbance at 734 nm after blank correction, m is the gradient of the graph, x is the μM AAE per mL of standard or sample in the assay, C is the y-intercept of the calibration curve, DF is the dilution factor, Vi is the initial sample volume (mL), and Ws is the weight of sample (g).
The parameter estimates of “m” and “C” were obtained from the standard calibration curve, and 1000 is used for M to mol conversion.
The stability of the ABTS assay reagents and the assay incubation time were verified in our laboratory by (1) measuring the absorbance of the ABTS working solution at 734 nm before and after assay procedures, (2) monitoring the stability of the range of AA standard solutions over time by measuring their absorbance values, and (3) optimising the assay incubation time by measuring the AOX capacity of a representative sample over time.
(2) CUPRAC Assay. The assay was based on Apak et al. [11]. To a 15 mL centrifuge tube were added 1 mL each of 1.0 × 10−2 M copper (II) chloride dihydrate, 7.5 × 10−3 M neocuproine, and 1 M pH 7.0 ammonium acetate buffer solution. A known volume (x mL) of diluted sample extract or Trolox standard solution (prepared in 96% ethanol with concentrations ranging from 100 μM to 1200 μM) and solvent (Milli-Q water or 40% ethanol (1.1–x mL)) were added to the initial mixture to make the final volume 4.1 mL. The tubes were vortexed for homogenous mixing and incubated for 30 min at ambient temperature. The absorbance at 450 nm was recorded against a reagent blank using a Shimadzu UV 1280 spectrophotometer. A standard calibration curve was constructed by plotting absorbance versus the concentration of the Trolox standards in the assay and the molar absorption coefficient determined. The AOX capacity of the sample was calculated using equation (3) and expressed as mmol Trolox equivalents (TE)/g of sample.
| (3) |
where Abs is the absorbance at 450 nm after blank correction, Vf is the final volume of assay solution (mL), DF is the dilution factor, Vi is the initial sample volume (mL), ε is the molar absorption coefficient, calculated from standard calibration curve, Vs is the volume of sample extract used in assay (mL), and Ws is the weight of sample (g).
Assay results obtained for the samples in mmol TE/g were then converted to μmol TE/g by applying unit conversions.
The assay conditions were verified in our laboratory by (1) monitoring the stability of the range of Trolox standard solutions over time by measuring their absorbance values and (2) optimising the assay incubation time based on these results.
(3) FC-TPC Assay. The assay was based on Pereira et al. [12]. To a disposable glass tube was added 100 μL of diluted sample extract or GA standard solution (prepared in Milli-Q water or 40% ethanol with concentrations ranging from 50 μg/mL to 250 μg/mL) followed by 100 μL of diluted FC reagent (diluted 1 : 1 in Milli-Q water) and 800 μL of 5% sodium carbonate. The tubes were vortexed for consistent mixing and incubated for 20 min at ambient temperature. The absorbance at 750 nm was recorded using a Shimadzu UV 1280 spectrophotometer. A standard calibration curve was constructed by plotting absorbance versus the concentration of the GA standards. The concentration of the unknown AOX was calculated from the slope of the calibration curve using equations (4), (5), and (6) and expressed as mg GA equivalents (GAE)/100 g sample.
| (4) |
| (5) |
| (6) |
where, y is the absorbance at 750 nm after blank correction, m is the gradient of the graph, x is the μg GAE per mL of standard or sample in the assay, C is the y-intercept of the calibration curve, DF is the dilution factor, Vi is the initial sample volume (mL), and Ws is the weight of sample (g).
The parameter estimates of “m” and “C” were obtained from the standard calibration curve, and 10 is the correction factor to convert the results in μg to mg and adjusted for 100 g.
Assay results obtained for the samples in mg GAE/100 g are then converted to μmol GAE/g by taking the molecular weight of GA into account and by applying unit conversions.
The FC-TPC assay conditions were verified in our laboratory by (1) monitoring the stability of the GA standard solutions in 40% ethanol at two separate time points by measuring their absorbance values, (2) monitoring the behaviour of the GA standards in two different solvent systems (Milli-Q water and 40% ethanol) at two different absorption wavelengths (750 nm and 760 nm), and (3) optimising the incubation time by measuring the absorbances of the range of GA standards at different time points.
3. Results and Discussion
3.1. Moisture Content
The moisture contents of the prepared samples which were either fresh, freeze-dried, or oven-dried are given in Table 4. The moisture results were used to convert the results of the individual assays (from equations (2), (3), and (6)) to dry weight basis facilitating comparison with the published literature values for similar food products.
Table 4.
Moisture content of the freeze-dried/oven-dried/fresh samples analysed.
| Sample | Sample condition | Moisture content (g/100g) |
|---|---|---|
| Blueberry | Freeze-dried powder | 4.0 |
| Blackberry | Freeze-dried powder | 3.2 |
| Raspberry | Freeze-dried powder | 3.5 |
| Cherry | Freeze-dried powder | 3.2 |
| Strawberry; MRF, 2020, 217-230 | Freeze-dried powder | 3.1 |
| Strawberry; MRF, 2020, 2017-139 | Freeze-dried powder | 2.8 |
| Queen Garnet plum; commercial FDP | Dry powder ∗ | 2.6 |
| Queen Garnet plum sibling; ARF, 2020, 401-43 | Freeze dried powder | 1.5 |
| Green capsicum | Freeze dried powder | 2.5 |
| Yellow capsicum | Freeze dried powder | 4.1 |
| Red capsicum | Freeze dried powder | 4.9 |
| Pumpkin | Freeze dried powder | 3.1 |
| Tomato | Fresh puree | 93.5 |
| Papaya seeds | Oven-dried powder | 4.2 |
| Pomegranate seeds | Oven-dried powder | 4.1 |
| Pomegranate husk | Oven-dried powder | 7.4 |
| Turmeric powder | Dry powder ∗ | 7.7 |
| Ginger powder | Dry powder ∗ | 10.5 |
| Cocoa powder | Dry powder ∗ | 6.0 |
| Green tea | Dry leaves∗ | 4.6 |
∗Commercial sample used as received.
3.2. Verification of Assay Conditions
3.2.1. ABTS Assay Verification
Absorbance of the ABTS working solution at 734 nm was monitored before and after assay procedures and recorded as absorbance units (AU): beginning of the experiment: absorbance of working ABTS = 0.999 AU and end of the experiment: absorbance of working ABTS = 0.998 AU.
The results indicated that the ABTS working solution (in pH 4.5 acetate buffer) was stable (without any decomposition) throughout the assay eliminating the chances of absorbance measurement inaccuracies due to breakdown of the ABTS.
Stability of the AA standards was monitored over a range of concentrations (in triplicate) by measuring their absorbance values and plotting their average against a range of time points (see Figure 1(a)). A close examination of the values confirmed that the working AA standards were stable for the first three hours of the assay (see Figure 1(b)), thereby avoiding absorbance measurement inaccuracies due to the decomposition of standards in the assay system.
Figure 1.
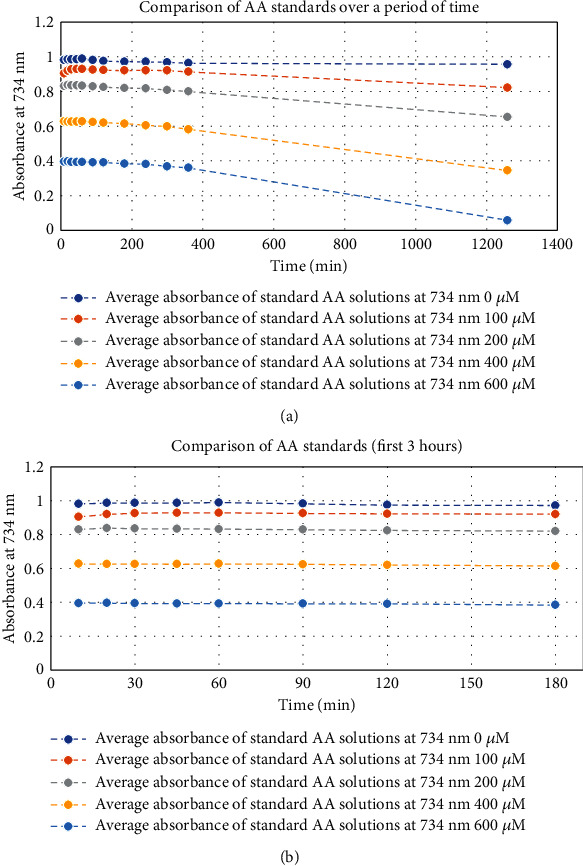
(a) Stability of AA working standards over time. (b) Stability of AA working standards for the first 3 hours.
The assay incubation time was optimised based on the graphical plot of the AOX capacity results for a representative sample obtained at a range of time points. Tangents were drawn to predict the linearity of the graph at 3 different sets of time points based on their linear trend in the first 60 minutes (min), the next 5 hours, and from there to the next 15 hours (see Figure 2).
Figure 2.
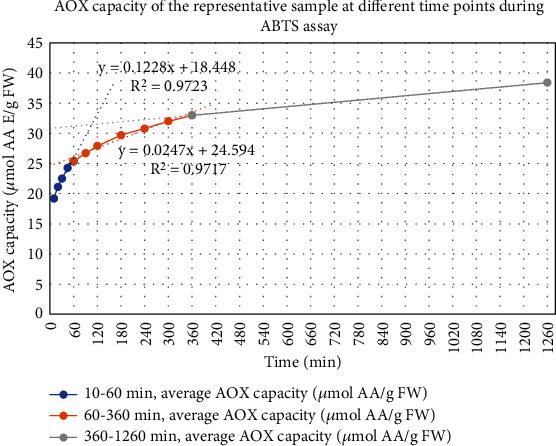
AOX capacities of the same sample at different incubation time points.
The assay tends to a steady state after 60 min, in line with the findings of Ozgen et al. [10]. Consequently, the optimal incubation time was set at 60 min.
3.2.2. CUPRAC Assay Verification
Stability of the Trolox standards was monitored over a range of concentrations (in triplicate) by measuring their absorbance values and plotting their average against a range of time points (see Figure 3).
Figure 3.
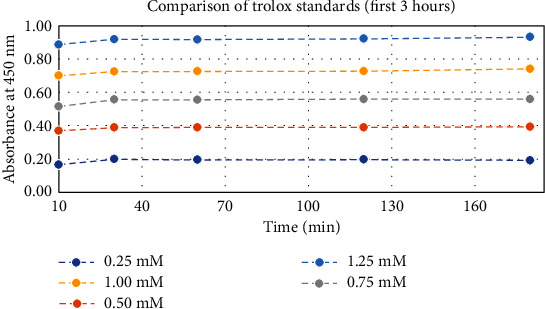
Stability of Trolox working standards over time.
It was confirmed that the working Trolox standards were stable for the first three hours of the assay (see Figure 3), thereby removing any absorbance measurement inaccuracies due to the decomposition of standards in the assay system. As evident from Figure 3, the assay reaches a stable value after 30 min and remains stable for the first three hours. So, 30 min was selected as the assay incubation time, agreeing with that reported by Gulcin [6].
3.2.3. FC-TPC Assay Verification
Absorbances of GA standard solutions with concentrations (in triplicate) ranging from 0 to 250 μg/mL, in 40% ethanol and in Milli-Q water, were separately measured after 20 min and 90 min of incubation and at two wavelengths, 750 nm and 760 nm. The calibration curves obtained by plotting their respective average absorbance values against concentration (μg/mL) of GA standard solutions are given in Figure 4.
Figure 4.
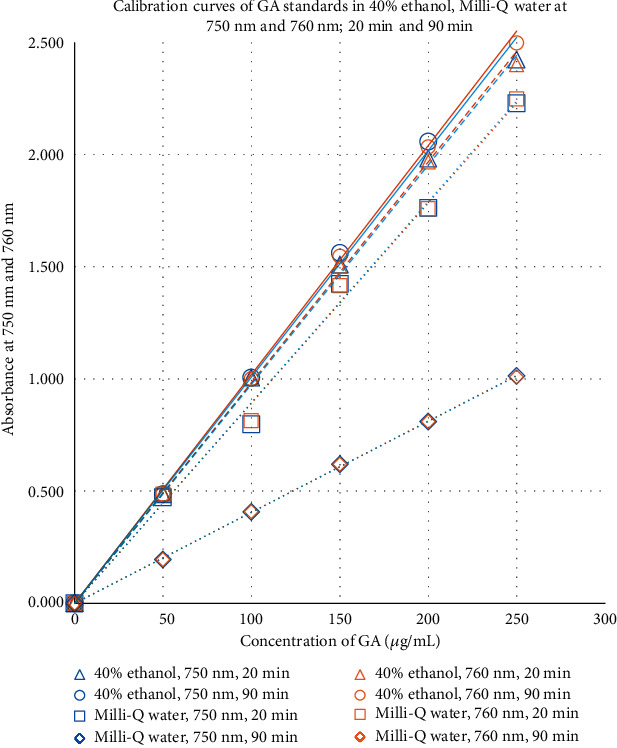
Calibration curves of GA standards in 40% ethanol and Milli-Q water, at 750 nm and 760 nm, after 20 min and 90 min of incubation.
The verification steps confirmed that the assay reagent and GA standards used in the current study were stable in 40% ethanol, as stipulated in the modified assay by Pereira et al. [12], as well as Milli-Q water used in the conventional method by Singleton and Rossi [7]. The mentioned verification also confirmed the suitability of a shorter assay incubation time of 20 min when compared to 90 min used in the conventional method. The comparability of the two different wavelengths of absorption reported [7, 12], i.e., 750 nm and 760 nm (Figure 4 and Table 5), was also noted. Due to the reliability of the shorter assay incubation time and similarity of the absorbance readings in both the reported wavelengths of absorption, a 20 min assay time and 750 nm were selected as the most appropriate conditions for consistency.
Table 5.
Slope of calibration curves (from Figure 4) in different solvents at different time points and at different wavelengths.
| Assay solvent and time | 750 nm | 760 nm |
|---|---|---|
| FC-TPC assay in 40% ethanol; 20 min |
y = 0.010200x R2 = 0.999822 |
y = 0.010088x R2 = 0.999826 |
|
| ||
| FC-TPC assay in 40% ethanol; 90 min |
y = 0.009841x R2 = 0.999763 |
y = 0.009770x R2 = 0.999709 |
|
| ||
| FC-TPC assay in Milli-Q water; 20 min |
y = 0.008917x R2 = 0.998418 |
y = 0.008956x R2 = 0.998708 |
|
| ||
| FC-TPC assay in Milli-Q water; 90 min |
y = 0.004062x R2 = 0.999916 |
y = 0.004053x R2 = 0.999901 |
A representative sample was assayed using both TPC methods [7, 12] and the results were compared (see Figure 5). It was confirmed from the results that the conventional hydrophilic extract in Milli-Q water produced much lower TPC assay results compared to the lipophilic extracts using 40% ethanol, presumably as the hydrophilic solvent was not able to extract the lipophilic polyphenols present in the sample [2–4, 6]. The standard calibration curve of pure gallic acid standard in Milli-Q water at 90 min was found to be different from that of at 20 min (Figure 4) possibly due to stability issues of such polyphenolic compounds at a higher pH over time. Thus, both the hydrophilic and lipophilic extraction solvents were selected for our studies and the results compared.
Figure 5.
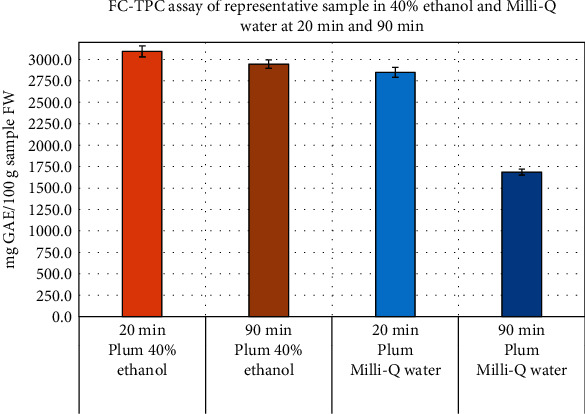
FC-TPC assay of a representative sample in 40% ethanol and Milli-Q water at 20 and 90 min.
3.3. Assay Results—Comparison with Reported Values
The results from the current study are provided in Table 2 and are expressed as μmol of reference standard equivalents per gram on a dry weight basis.
Direct comparisons were not possible between the results of our study and most of the previously published assay values due to the vast differences in the published literature, in terms of extracting solvents, assay incubation time, selection of different reference standards, presentation of results in different units, etc. (Tables 1 and 2). To facilitate a meaningful comparison, we have converted the units of the published assay values by taking molecular weights and correction factors into account and further applying appropriate correction for the moisture content (based on the data available from United States Department of Agriculture (USDA)–Nutritional Value of Foods, Home and Garden Bulletin No. 72 [52]) to convert from a fresh weight to a dry weight basis if necessary. The published values are also presented in Table 2 (ɸ: reported values after unit conversions; ∗: published reference).
It became apparent from Table 2 that, though the matching assays show some similarities in their range of results (after converting to similar units), the range of assay results obtained in the current study was not directly comparable with the previously reported values.
In addition to the challenges associated with direct comparison of the assay results in Table 2, it is worth considering other contributors that may make such comparisons inappropriate and problematic. These include agrienvironmental factors such as the varietal differences, ecological influences, growing conditions, degree of ripening, and the natural variation of these compounds in the sample matrix.
Fruit in general, especially berries, are very good sources of phenolic compounds including anthocyanins, ellagic acid, GA, quercetin, ferulic acid, catechin, and caffeic acid [21, 53, 54]. However, notable differences have been reported for TPC and AOX capacities between berry varieties; this is probably due to agrienvironmental factors and the natural variation of these compounds in the fruit [14, 15, 21].
The main reported polyphenolics in capsicums are GA, chlorogenic acid, various glucopyranosides, capsaicin, quercetins, myricetin, and carotenoids [55, 56]. β-Carotene, catechin, chlorogenic acid, caffeic acid, coumaric acid, kaempferol, ferulic acid, salicylic acid, and syringic acid are the main reported polyphenols in pumpkins [26, 27]. Various phenolic acids, flavonoids, lycopene, and rutin apioside are reported in tomatoes [22, 28, 57]. Similar to fruit, the levels of phenolic compounds and carotenoids in vegetables are extremely variable, with agrienvironmental factors again contributing. The differences in the concentration of these compounds in vegetables are the key reason for their varying TPC and AOX capacities [22, 26–28, 55–57].
The key polyphenolic compounds reported in papaya seed are various phenolic acids, anthocyanins, quercetins, catechins, GA, phenolic aldehydes, coumarin, and phenolic diterpenes [29, 30]. Pomegranate seed extracts contain catechins, sterols, tocopherols, polyphenolic acids, and fatty acids. Pomegranate husk is rich in ellagitannins, GA, fatty acids, catechin and epicatechin, quercetin, rutin and other flavonols, flavones, flavonones, proanthocyanidins, and anthocyanidins [34]. Very high levels of catechin and GA were detected in the husk compared to other parts of the pomegranate fruit including the seeds and reported to exhibit higher assay results [34, 36]. As for fruit and vegetables, the seeds and peel of fruit vary in their levels of phenolic composition depending upon the genotype, cultivar, varietal differences, etc., and the variation in the concentration of these compounds results in their varying TPC and AOX capacities.
The major bioactive constituents reported in turmeric samples are diarylheptanoids, diarylpentanoids, phenylpropenes, terpenes, terpenoids, sterols, alkaloids, and curcuminoids (mostly curcumin) [58]. The phenolic compounds in ginger are mainly gingerols, shogaols, and paradols, along with quercetin, zingerone, gingerenone A, and 6-dehydrogingerdione [59]. The main polyphenols reported in cocoa beans are catechins, which constitute about 37% of the polyphenol content, anthocyanidins (about 4%), and proanthocyanidins (about 58%) [60]. Green tea contains flavanols (mostly catechins), flavandiols, flavonoids, and phenolic acids which together contribute up to 30% of the dry weight [61]. Similar to investigations of fruit and vegetables, the differing levels of these phenolic compounds in the condiment samples tested are the most likely reason for the different AOX capacities.
Based on the recommendations in Apak et al. [11], we investigated dichloromethane and pure ethanol extractions of the capsicum, tomato, and pumpkin samples to understand the contributions from specific carotenoid molecules which are known to be insoluble in other solvent combinations. The dichloromethane and pure ethanol extracts were not fully compatible (stability of radical species [11] and turbidity issues [3, 8, 9, 12]) with ABTS and FC-TPC assays restricting our investigation to CUPRAC results alone (data not shown) and were therefore removed from any further comparison.
3.4. Assay Results—Comparison Within the Laboratory
Though the comparison of assay results in Table 2 shows some similarity with the published literature values, the validity of such comparisons remains questionable on the grounds of the recommendations presented in the IUPAC Technical report by Apak et al. [2], i.e., “results in any publications have to be carefully scrutinised as they may not be comparable as each AOX assay has a different mechanism, redox potential, reaction media, extracting solvent, etc. However, in one laboratory the results within one test system can be used for a ranking.” Based on this statement, the average results for each of the samples tested in the current study, utilising three independent assay systems (ABTS, CUPRAC, and TPC), were compared within each solvent system selected for the extractions (Milli-Q water, 40% ethanol, and 40% ethanol with mβCD).
The mutual correlation of these three assays was also analysed, and the results are given in Tables 3(a) and 3(b). The samples showed strong correlations between assay systems with R2 values as shown and further confirm the reliability and acceptance of these assay systems. A similar observation was described by Sariburun et al. [62] who noted a high positive correlation between the ABTS, CUPRAC, and TPC assays for water and methanol extracts of raspberry and blackberry cultivars and attributed that the AOX activity of fruit tested appears to be largely influenced by the phenolic compounds.
It is evident from the comparative graphs (Figures 6–8) that, in terms of their AOX capacity and TPC content, all the samples exhibited a similar trend and maintained their relative ranking for all three assays, regardless of the solvent system. This confirms the reliability and practical usefulness of these assays to be employed in any laboratory to introduce an internal ranking system for similar samples based on their AOX capacity and TPC assays.
Figure 6.
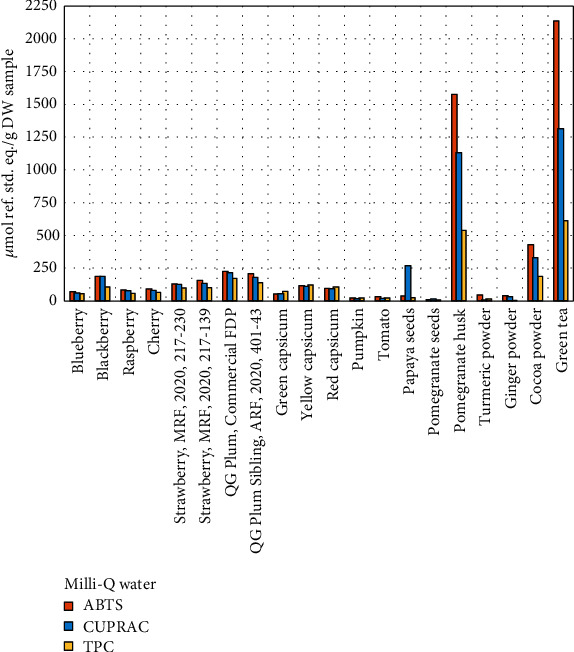
Comparison of ABTS, CUPRAC, and TPC assays in Milli-Q water.
Figure 7.
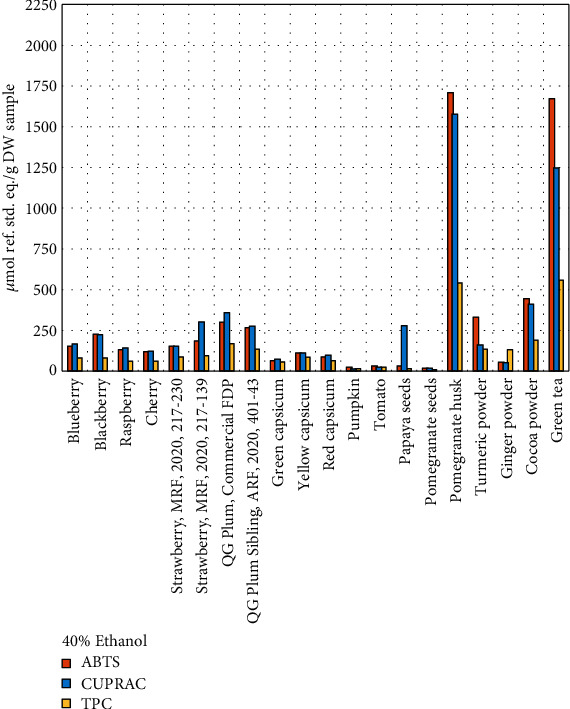
Comparison of ABTS, CUPRAC, and TPC assays in 40% ethanol.
Figure 8.
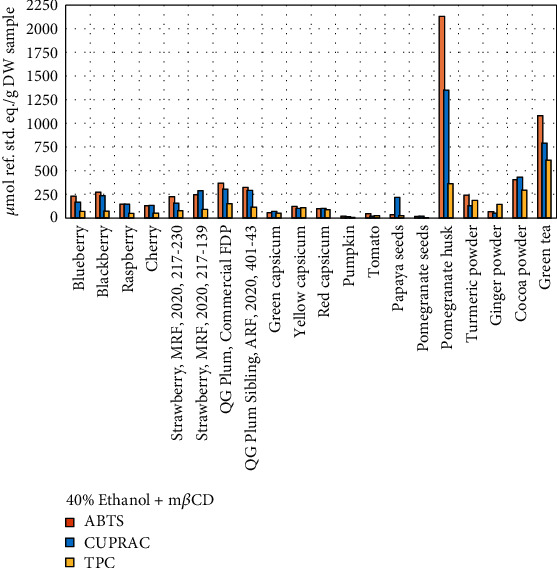
Comparison of ABTS, CUPRAC, and TPC assays in 40% ethanol modified with mβCD.
4. Conclusions
The current study employed two AOX capacity assays (ABTS and CUPARC) along with a FC-TPC assay, utilising both hydrophilic and lipophilic solvent systems, to measure the AOX properties of selected fruit, fruit waste, vegetables, and spices of commercial importance in Australia and worldwide. The assays were also verified within the laboratory to confirm their suitability
The modified ABTS assay (at pH 4.5) and the modified FC-TPC assay (in 40% ethanol) used in this study were applied for the first time to a range of fruit, vegetable, and spice samples and compared the results from three different solvent systems
The results obtained by this study utilising three independent assays in three different solvent systems exhibited good mutual correlation, confirming that the results are suitable to generate a reliable ranking system within the laboratory for similar sample matrices. This valuable information also enhances the usefulness of these assays
It is evident from the results obtained that the use of both hydrophilic and lipophilic solvents for extractions is vital and must be incorporated into any AOX capacity and/or TPC assay, as the solvents can produce widely differing results depending upon the properties of the polyphenolic molecules present in the sample matrices
Comparing the results of the current study with other published values proved problematic due to the vast differences in extraction solvents, assay systems, and the units of presentation presented in the literature. Even after converting the assay value units and applying the appropriate correction factors for moisture contents where appropriate, the current study revealed how challenging it is to make meaningful comparisons with previously reported AOX results
The unique approach employed in this study provided a strong platform to build an internal reference database for the food commodities commonly consumed and of commercial importance in Australia and worldwide. There is potential to broaden the scope and further develop the reference database to include an increased range of sample matrices
Acknowledgments
This research was financially supported by the Department of Agriculture and Fisheries, Queensland Government, Australia. Authors of this work also acknowledge the contributions of Dr. Dianna Liu (DAF).
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Prior R. L., Wu X., Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. Journal of Agriculture and Food Chemistry . 2005;53(10):4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- 2.Apak R., Gorinstein S., Böhm V., Schaich K. M., Özyürek M., Güçlü K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report) Pure and Applied Chemistry . 2013;85(5):957–998. doi: 10.1351/pac-rep-12-07-15. [DOI] [Google Scholar]
- 3.Huang D., Ou B., Prior R. L. The chemistry behind antioxidant capacity assays. Journal of Agriculture and Food Chemistry . 2005;53(6):1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 4.Santos-Sánchez N. F., Salas-Coronado R., Villanueva-Cañongo C., Hernández-Carlos B. Antioxidants . IntechOpen; 2019. Antioxidant compounds and their antioxidant mechanism. [Google Scholar]
- 5.Berker K. I., Ozdemir Olgun F. A., Ozyurt D., Demirata B., Apak R. Modified Folin−Ciocalteu antioxidant capacity assay for measuring lipophilic antioxidants. Journal of Agriculture and Food Chemistry . 2013;61(20):4783–4791. doi: 10.1021/jf400249k. [DOI] [PubMed] [Google Scholar]
- 6.Gulcin I. Antioxidant activity of food constituents: an overview. Archives of Toxicology . 2012;86(3):345–391. doi: 10.1007/s00204-011-0774-2. [DOI] [PubMed] [Google Scholar]
- 7.Singleton V. L., Rossi J. A., Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture . 1965;16(48):144–158. [Google Scholar]
- 8.Apak R., Ozyurek M., Guclu K., Capanoglu E. Antioxidant activity/capacity measurement. 1. classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. Journal of Agriculture and Food Chemistry . 2016;64(5):997–1027. doi: 10.1021/acs.jafc.5b04739. [DOI] [PubMed] [Google Scholar]
- 9.Olgun F. A., Ozyurt D., Berker K. I., Demirata B., Apak R. Folin–Ciocalteu spectrophotometric assay of ascorbic acid in pharmaceutical tablets and orange juice with pH adjustment and pre-extraction of lanthanum(III)-flavonoid complexes. Journal of the Science of Food and Agriculture . 2014;94(12):2401–2408. doi: 10.1002/jsfa.6569. [DOI] [PubMed] [Google Scholar]
- 10.Ozgen M., Reese R., Tulio A. Z., Jr., Scheerens J. C., Miller A. R. Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2’-diphenyl-1-picrylhydrazyl (DPPH) methods. Journal of Agriculture and Food Chemistry . 2006;54(4):1151–1157. doi: 10.1021/jf051960d. [DOI] [PubMed] [Google Scholar]
- 11.Apak R., Güçlü K., Özyürek M., Karademir S. E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. Journal of Agriculture and Food Chemistry . 2004;52(26):7970–7981. doi: 10.1021/jf048741x. [DOI] [PubMed] [Google Scholar]
- 12.Pereira G. A., Arruda H. S., Pastore G. M. Modification and validation of Folin-Ciocalteu assay for faster and safer analysis of total phenolic content in food samples. Brazilian Journal of Food Research, Campo Mourao . 2018;9(1):125–140. doi: 10.3895/rebrapa.v9n1.6062. [DOI] [Google Scholar]
- 13.Association of Official Analytical Chemists, Washington DC, AOAC. Official Methods of Analysis: Official Method for Moisture . 1995;925:p. 10. [Google Scholar]
- 14.Rodrigues E., Poerner N., Rockenbach I. I., Gonzaga L. V., Mendes C. R., Fett R. Phenolic compounds and antioxidant activity of blueberry cultivars grown in Brazil. Ciencia e Tecnologia de Alimentos . 2011;31(4):911–917. doi: 10.1590/s0101-20612011000400013. [DOI] [Google Scholar]
- 15.Kim J.-S. Antioxidant activities of selected berries and their free, esterified, and insoluble-bound phenolic acid contents. Preventive Nutrition and Food Science . 2018;23(1):35–45. doi: 10.3746/pnf.2018.23.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jara-Palacios M. J., Santisteban A., Gordillo B., Hernanz D., Heredia F. J., Escudero-Gilete M. L. Comparative study of red berry pomaces (blueberry, red raspberry, red currant and blackberry) as source of antioxidants and pigments. European Food Research and Technology . 2019;245(1):1–9. doi: 10.1007/s00217-018-3135-z. [DOI] [Google Scholar]
- 17.de Souza V. R., Pereira P. A. P., da Silva T. L. T., de Oliveira Lima L. C., Pio R., Queiroz F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chemistry . 2014;156:362–368. doi: 10.1016/j.foodchem.2014.01.125. [DOI] [PubMed] [Google Scholar]
- 18.Huang W.-y., Zhang H.-c., Liu W.-x., Li C.-y. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. Journal of Zhejiang University SCIENCE B . 2012;13(2):94–102. doi: 10.1631/jzus.b1100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Floegel A., Kim D.-O., Chung S.-J., Koo S. I., Chun O. K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. Journal of Food Composition and Analysis . 2011;24(7):1043–1048. doi: 10.1016/j.jfca.2011.01.008. [DOI] [Google Scholar]
- 20.Drozdz P., Seziene V., Pyrzynska K. Phytochemical properties and antioxidant activities of extracts from wild blueberries and lingonberries. Plant Foods for Human Nutrition . 2017;72:360–364. doi: 10.1007/s11130-017-0640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gundesli M. A., Korkmaz N., Okatan V. Polyphenol content and antioxidant capacity of berries: a review. International Journal of Agriculture, Forestry and Life Sciences . 2019;3(2):350–361. [Google Scholar]
- 22.Apak R., Güçlü K., Demirata B., et al. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules . 2007;12(7):1496–1547. doi: 10.3390/12071496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson J. B., Collins T., Mani J. S., Naiker M. Nutritional quality and bioactive constituents of six Australian plum varieties. International Journal of Fruit Science . 2021;21(1):115–132. doi: 10.1080/15538362.2020.1860863. [DOI] [Google Scholar]
- 24.Valle A. D., Dimmito M. P., Zengin G., et al. Exploring the nutraceutical potential of dried pepper Capsicum annuum L. on market from Altino in Abruzzo region. Antioxidants . 2020;9(5):p. 400. doi: 10.3390/antiox9050400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasli A. A., Yavuz-Düzgün M., Altuntas U., Altin G., Özçelik B., Firatligil E. In vitro bioaccessibility of phenolics and flavonoids in various dried vegetables, and the determination of their antioxidant capacity via different spectrophotometric assays. International Food Research Journal . 2019;26(3):793–800. [Google Scholar]
- 26.Kulczynski B., Gramza-Michałowska A., Krolczyk J. B. Optimization of extraction conditions for the antioxidant potential of different pumpkin varieties (Cucurbita maxima) Sustainability . 2020;12(4):p. 1305. doi: 10.3390/su12041305. [DOI] [Google Scholar]
- 27.Kostecka-Gugała A., Kruczek M., Ledwożyw-Smoleń I., Kaszycki P. Antioxidant s and health-beneficial nutrients in fruits of eighteen Cucurbita cultivars: analysis of diversity and dietary implications. Molecules . 2020;25(8):p. 1792. doi: 10.3390/molecules25081792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamiloglu S., Demirci M., Selen S., Toydemir G., Boyacioglu D., Capanoglu E. Home processing of tomatoes (Solanum lycopersicum): effects on in vitro bioaccessibility of total lycopene, phenolics, flavonoids, and antioxidant capacity. Journal of the Science of Food and Agriculture . 2014;94(11):2225–2233. doi: 10.1002/jsfa.6546. [DOI] [PubMed] [Google Scholar]
- 29.Kadiri O., Akanbi C. T., Olawoye B. T., Gbadamosi S. O. Characterization and antioxidant evaluation of phenolic compounds extracted from the protein concentrate and protein isolate produced from pawpaw (Carica papaya Linn.) seeds. International Journal of Food Properties . 2017;20(11):2423–2436. doi: 10.1080/10942912.2016.1230874. [DOI] [Google Scholar]
- 30.Rodrigues L. G. G., Mazzutti S., Vitali L., Micke G. A., Ferreira S. R. S. Recovery of bioactive phenolic compounds from papaya seeds agroindustrial residue using subcritical water extraction. Biocatalysis and Agricultural Biotechnology . 2019;22, article 101367:1–8. [Google Scholar]
- 31.Zhou K., Wang H., Mei W., Li X., Luo Y., Dai H. Antioxidant activity of papaya seed extracts. Molecules . 2011;16(8):6179–6192. doi: 10.3390/molecules16086179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gulsunoglu Z., Karbancioglu-Guler F., Raes K., Kilic-Akyilmaz M. Soluble and insoluble-bound phenolics and antioxidant activity of various industrial plant wastes. International Journal of Food Properties . 2019;22(1):1501–1510. [Google Scholar]
- 33.Balli D., Cecchi L., Khatib M., et al. Characterization of arils juice and peel decoction of fifteen varieties of Punica granatum L.: a focus on anthocyanins, ellagitannins and polysaccharides. Antioxidants . 2020;9(3):p. 238. doi: 10.3390/antiox9030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Surek E., Nilufer-Erdil D. Phenolic contents, antioxidant activities and potential bioaccessibilities of industrial pomegranate nectar processing wastes. International Journal of Food Science and Technology . 2016;51(1):231–239. doi: 10.1111/ijfs.13000. [DOI] [Google Scholar]
- 35.Qabaha K., Al-Rimawi F., Nusseibeh S., Abbadi J., Abu-Lafi S. Phenolic and flavonoids analysis of pomegranate peel extracts and their antiniflammatory and antioxidant activities. International Journal of Pharmaceutical Quality Assurance . 2019;10(1):60–65. [Google Scholar]
- 36.Magangana T. P., Makunga N. P., Fawole O. A., Opara U. L. Processing factors affecting the phytochemical and nutritional properties of pomegranate (Punica granatum L.) peel waste: a review. Molecules . 2020;25(20):p. 4690. doi: 10.3390/molecules2s5204690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cikrikci S., Mozioglu E., Yılmaz H. Biological activity of curcuminoids isolated from Curcuma longa. Records of Natural Products . 2008;2(1):19–24. [Google Scholar]
- 38.Li S., Li S. K., Gan R. Y., Song F. L., Kuang L., Li H. B. Antioxidant capacities and total phenolic contents of infusions from 223 medicinal plants. Industrial Crops and Products . 2013;51:289–298. [Google Scholar]
- 39.Mushtaq Z., Tahir Nadeem M., Arshad M. U., et al. Exploring the biochemical and antioxidant potential of ginger (Adric) and turmeric (Haldi) International Journal of Food Properties . 2019;22(1):1642–1651. [Google Scholar]
- 40.Nisar T., Iqbal M., Raza A., Safdar M., Iftikhar F., Waheed M. Estimation of total phenolics and free radical scavenging of turmeric (Curcuma longa) Environmental Sciences . 2015;15(7):p. 6. [Google Scholar]
- 41.Maizura M., Aminah A., Wan Aida W. M. Total phenolic content and antioxidant activity of kesum (Polygonum minus), ginger (Zingiber officinale) and turmeric (Curcuma longa) extract. International Food Research Journal . 2011;18(2):526–531. [Google Scholar]
- 42.Sepahpour S., Selamat J., Abdul Manap M. Y., Khatib A., Abdull Razis A. F. Comparative analysis of chemical composition, antioxidant activity and quantitative characterization of some phenolic compounds in selected herbs and spices in different solvent extraction systems. Molecules . 2018;23(2):p. 402. doi: 10.3390/molecules23020402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osae R., Essilfie G., Alolga R. N., Bonah E., Ma H., Zhou C. Drying of ginger slices - evaluation of quality attributes, energy consumption, and kinetics study. Journal of Food processing Engineering . 2019;43(2, article e13348) doi: 10.1111/jfpe.13348. [DOI] [Google Scholar]
- 44.Tohma H., Gülçin İ., Bursal E., Gören A. C., Alwasel S. H., Köksal E. Antioxidant activity and phenolic compounds of ginger (Zingiber officinale Rosc.) determined by HPLC-MS/MS. Journal of Food Measurement and Characterization . 2017;11(2):556–566. [Google Scholar]
- 45.Dat N. T. Antioxidant activity, total phenolics and metal contents of ginger powders in Hanoi, Vietnam. Nutrition and Food Toxicology . 2018;3(3):645–651. [Google Scholar]
- 46.Belščak A., Komes D., Horžić D., Ganić K. K., Karlović D. Comparative study of commercially available cocoa products in terms of their bioactive composition. Food Research International . 2009;42(5-6):707–716. [Google Scholar]
- 47.Apak R., Güçlü K., Özyürek M., Esin Karademir S., Erçağ E. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. International Journal of Food Sciences and Nutrition . 2006;57(5-6):292–304. doi: 10.1080/09637480600798132. [DOI] [PubMed] [Google Scholar]
- 48.Çelik S. E., Özyürek M., Güçlü K., Apak R. Solvent effects on the antioxidant capacity of lipophilic and hydrophilic antioxidants measured by CUPRAC, ABTS/persulphate and FRAP methods. Talanta . 2010;81(4-5):1300–1309. doi: 10.1016/j.talanta.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 49.Zhao C. N., Tang G. Y., Cao S. Y., et al. Phenolic profiles and antioxidant activities of 30 tea infusions from green, black, oolong, white, yellow and dark teas. Antioxidants . 2019;8(7):p. 215. doi: 10.3390/antiox8070215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cleverdon R., Elhalaby Y., Md M. A., Gittings W., Ward W. E. Total polyphenol content and antioxidant capacity of tea bags: comparison of black, green, red rooibos, chamomile and peppermint over different steep times. Beverages . 2018;4(1):p. 15. [Google Scholar]
- 51.Dutta A. K., Siddiquee M. A., Hossain S., Kabir Y. Finlay green tea possesses the highest in vitro antioxidant activity among the twenty commercially available tea brands of Bangladesh. Malaysian Journal of Pharmaceutical Sciences . 2013;11(2):11–20. [Google Scholar]
- 52.United States Department of Agriculture. Food Science and Technology . Brazilian Society of Food Science and Technology; 2002. [Google Scholar]
- 53.Stoner G. D., Seeram N. P. Berries and Cancer Prevention . Berlin/Heidelberg, Germany: Springer; 2011. [Google Scholar]
- 54.Skrovankova S., Sumczynski D., Mlcek J., Jurikova T., Sochor J. Bioactive compounds and antioxidant activity in different types of berries. International Journal of Molecular Sciences . 2015;16(10):24673–24706. doi: 10.3390/ijms161024673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hallmann E., Rembiałkowska E. Characterisation of antioxidant compounds in sweet bell pepper (Capsicum annuum L.) under organic and conventional growing systems. Journal of the Science of Food and Agriculture . 2012;92(12):2409–2415. doi: 10.1002/jsfa.5624. [DOI] [PubMed] [Google Scholar]
- 56.Materska M., Perucka I. Antioxidant activity of the main phenolic compounds isolated from hot pepper fruit (Capsicum annuum L.) Journal of Agriculture and Food Chemistry . 2005;53(5):1750–1756. doi: 10.1021/jf035331k. [DOI] [PubMed] [Google Scholar]
- 57.Chaudhary P., Sharma A., Singh B., Nagpal A. K. Bioactivities of phytochemicals present in tomato. Journal of Food Science and Technology . 2018;55(8):2833–2849. doi: 10.1007/s13197-018-3221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanvir E. M., Hossen M., Hossain M., et al. Antioxidant properties of popular turmeric (Curcuma longa) varieties from Bangladesh. Journal of Food Quality . 2017;2017:8. doi: 10.1155/2017/8471785.8471785 [DOI] [Google Scholar]
- 59.Mao Q. Q., Xu X. Y., Cao S. Y., et al. Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe) Foods . 2019;8(6):p. 185. doi: 10.3390/foods8060185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andújar I., Recio M. C., Giner R. M., Ríos J. L. Cocoa polyphenols and their potential benefits for human health. Oxidative Medicine and Cellular Longevity . 2012;2012:23. doi: 10.1155/2012/906252.906252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chacko S. M., Thambi P. T., Kuttan R., Nishigaki I. Beneficial effects of green tea: a literature review. Chinese Medicine . 2010;5(1):1–9. doi: 10.1186/1749-8546-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sariburun E., Şahin S., Demir C., Türkben C., Uylaşer V. Phenolic content and antioxidant activity of raspberry and blackberry cultivars. Journal of Food Science . 2010;75(4):328–335. doi: 10.1111/j.1750-3841.2010.01571.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


